Abstract
Adverse remodelling following an initial insult is the hallmark of heart failure (HF) development and progression. It is manifested as changes in size, shape, and function of the myocardium. While cardiac remodelling may be compensatory in the short term, further neurohumoral activation and haemodynamic overload drive this deleterious process that is associated with impaired prognosis. However, in some patients, the changes may be reversed. Left ventricular reverse remodelling (LVRR) is characterized as a decrease in chamber volume and normalization of shape associated with improvement in both systolic and diastolic function. LVRR might occur spontaneously or more often in response to therapeutic interventions that either remove the initial stressor or alleviate some of the mechanisms that contribute to further deterioration of the failing heart. Although the process of LVRR in patients with new‐onset HF may take up to 2 years after initiating treatment, there is a significant portion of patients who do not improve despite optimal therapy, which has serious clinical implications when considering treatment escalation towards more aggressive options. On the contrary, in patients that achieve delayed improvement in cardiac function and architecture, waiting might avoid untimely implantable cardioverter‐defibrillator implantation. Therefore, prognostication of successful LVRR based on clinical, imaging, and biomarker predictors is of utmost importance. LVRR has a positive impact on prognosis. However, reverse remodelled hearts continue to have abnormal features. In fact, most of the molecular, cellular, interstitial, and genome expression abnormalities remain and a susceptibility to dysfunction redevelopment under biomechanical stress persists in most patients. Hence, a distinction should be made between reverse remodelling and true myocardial recovery. In this comprehensive review, current evidence on LVRR, its predictors, and implications on prognostication, with a specific focus on HF patients with non‐ischaemic cardiomyopathy, as well as on novel drugs, is presented.
Keywords: Heart failure, Non‐ischaemic cardiomyopathy, Predictors, Reverse remodelling, Left ventricular reverse remodelling, Cardiac remodelling
Introduction
Adverse remodelling following an initial insult is the hallmark of heart failure (HF) development and progression that leads to clinical deterioration, impaired prognosis, and, ultimately, death. It is defined as abnormalities in genome expression, molecular, cellular, and interstitial transformations that are manifested as changes in size, shape, and function of the myocardium. 1 While it has been initially described in patients in whom ventricular dilation after transmural myocardial infarction was observed, 2 similar observations could be made in non‐ischaemic cardiomyopathy (NICM) irrespective of aetiology, for example, genetic mutations in hereditary dilated cardiomyopathy (DCM), myocarditis, alcohol or cardiotoxic exposure, and tachycardia induced. 3 In the past two decades, it has been shown that this deleterious process driven by further neurohumoral activation and haemodynamic overload is not irreversible and that it could be reversed. Despite recent advances in HF therapies with a possible effect on cardiac remodelling, many issues remain unresolved. Here, we review the current evidence on left ventricular reverse remodelling (LVRR), its predictors, and implications on prognostication in patients with NICM.
Reverse remodelling
The term ‘reverse remodelling’ was first introduced by Kass et al., who observed reductions in end‐diastolic volumes (EDV) and increased ejection fraction of the left ventricle (LVEF) in a series of patients with idiopathic DCM following a cardiomyoplasty procedure. 4 In general, LVRR can be characterized as a decrease in chamber volume and normalization of shape associated with improvement in both systolic and diastolic function. There is a large heterogeneity in the definition of LVRR (for summary, see Table 1 ). Many of the studies rely on echocardiography 5 , 6 , 7 , 8 or cardiac magnetic resonance (CMR), 9 and LVRR is most often defined as an increase in LVEF > 10% 5 , 7 , 9 , 10 and/or its normalization (≥50%) accompanied by indexed left ventricular diameter reduction > 10% 5 , 9 , 10 and/or its normalization (≤33 mm/m2).
TABLE 1.
Examples of echocardiographic and cardiac magnetic resonance definitions of left ventricular reverse remodelling in selected studies
| Reference | Number of patients | Time of evaluation (months) | LVRR definition |
|---|---|---|---|
| Echocardiography | |||
| Merlo et al. 5 | 361 patients with idiopathic DCM | 24 |
LVEF increase ≥ 10% or LVEF ≥ 50% iLVEDD decrease ≥ 10% or iLVEDD ≤ 33 mm/m2 |
| Chung et al. 6 | 498 HFrEF patients (267 with NICM) | 6 | LVESV reduction ≥ 15% |
| Wilcox et al. 7 | 3994 HFrEF patients (1421 with NICM) | 24 | LVEF increase > 10% |
| Brenyo et al. 8 | 612 HFrEF patients (283 with NICM) | 12 | LVESV reduction ≥ 15% |
| Cardiac magnetic resonance | |||
| Masci et al. 9 | 58 patients with idiopathic DCM | 24 |
LVEF increase ≥ 10% LVEDV decrease ≥ 10% |
DCM, dilated cardiomyopathy; HFrEF, heart failure with reduced ejection fraction; iLVEDD, indexed left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVRR, left ventricular reverse remodelling; NICM, non‐ischaemic cardiomyopathy.
It is important to note that most changes take place at the microscopic level and manifest as a reversal in increased fibrosis of extracellular matrix, restoration of cellular energetics, and metabolic properties, as well as a shift in gene expression of the myocardium 11 —changes that are challenging to observe and study in detail (for summary, see Table 2 ). Limited data are available from tissue samples of patients undergoing left ventricular support device explantation during heart transplant or following sufficient recovery. 12 , 13 , 14 , 15 In one of the landmark studies of transcriptome analysis, Margulies et al. have shown that while expression of as many as 3000 genes is changed in advanced HF, the expression of only 5% of those genes tends to normalize in association with left ventricular assist device (LVAD) unloading. 14 Similar persistent transcriptional abnormalities were observed in 16 paired tissue samples before and after LVAD support (eight of which were from patients with NICM). 16 In addition, Yang et al. have shown that long non‐coding RNA expression profiles show significant differences in ischaemic and non‐ischaemic HF and when compared with mRNA and microRNA profiles, they also show the most apparent shift in response to mechanical unloading. 16 All these data suggest that reverse remodelled hearts continue to have abnormal features. Therefore, it is important to distinguish between reverse remodelling and true myocardial recovery. As Mann et al. suggested, the term myocardial recovery represents a successful and complete reversal of HF phenotype that is associated with freedom from future HF events and that could, they speculate, only be achieved in the myocardium that has not yet suffered irreversible damage 17 (Figure 1 ). This statement is supported by the observation from the Interagency Registry of Mechanically Assisted Circulatory Support (INTERMACS) where the highest rate of myocardial recovery allowing LVAD explantation was observed in patients with myocarditis and post‐partum cardiomyopathy 18 —aetiologies of injury that are classically considered transient in comparison with long‐lasting, permanent abnormality seen in genetically mediated cardiomyopathy. In fact, <1.5% of patients with DCM recover enough to undergo LVAD explant. 19 Taken together, reverse remodelled hearts continue to show susceptibility for more profound malfunction when undergoing mechanical or biological stress, one that would normally be well tolerated. 17
TABLE 2.
Summarized molecular and cellular changes, and structural and functional phenotypes in response to different therapies
| Initial insult | LVRR induced by | Molecular and cellular changes | Structural and functional phenotypes |
|---|---|---|---|
| Genetic mutation | Spontaneously | Reversal of cell hypertrophy 117 , 118 | Normalization of chamber geometry |
| Inflammation | Pharmacotherapy | Improved contractility 115 | Increased ejection fraction |
| Cardiotoxicity | Resynchronization therapy | Changes in collagen content 13 | Reduced volumes and diameters |
| Abnormal energetics | Mitral valve repair | Ca2+ metabolism 115 , 116 | Leftward shifts in EDPVR |
| Mechanical/biological stress | LVAD | β‐Adrenergic responsiveness 13 , 114 | ↓ Functional mitral regurgitation |
| Reduced apoptosis/necrosis 122 |
EDPVR, end‐diastolic pressure–volume ratio; LVAD, left ventricular assist device; LVRR, left ventricular reverse remodelling.
Figure 1.
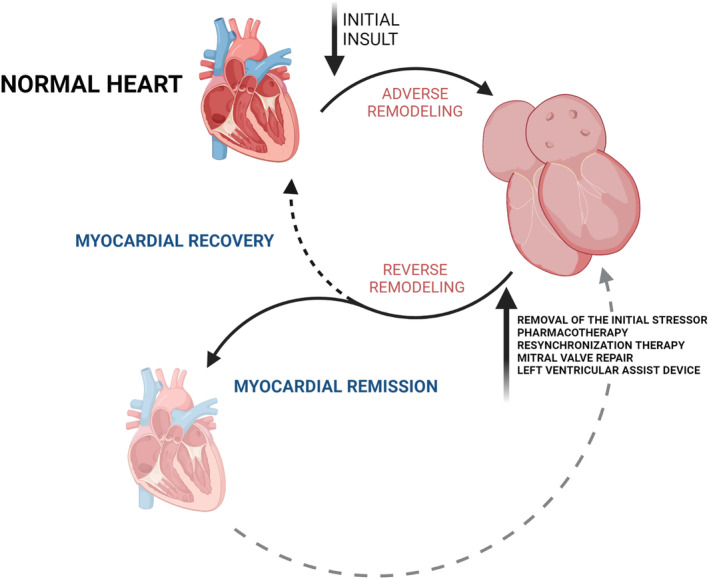
This figure represents the cycle of cardiac adverse and reverse remodelling. Spontaneous or therapy‐driven left ventricular reverse remodelling might lead to complete myocardial recovery, but most often leads to the phenotype of myocardial remission, in which many abnormal characteristics of the myocardium persist. Susceptibility to future heart failure events is illustrated by a dashed grey arrow. (Created with BioRender.com.)
Pharmacological reverse remodelling
Left ventricular reverse remodelling might occur spontaneously or more often in response to therapeutic interventions that either remove the initial stressor or alleviate some of the mechanisms that contribute to further deterioration of the failing heart, such as activation of the renin‐angiotensin‐aldosterone and adrenergic nervous systems. 1 Numerous large placebo‐controlled trials of drugs that are the cornerstone of guideline‐directed HF therapy 20 have demonstrated a positive effect on mortality. A portion of these studies that provide serial measurements of LVEF and diameters indicating the effect of pharmacotherapy on LVRR will be discussed below. In contrast with previous reviews on this topic, 21 , 22 , 23 we will specifically focus on NICM as well as on novel drugs that have been recently introduced.
Beta‐blockers
Beta‐adrenergic blocking agents are strongly associated with LVRR. In a meta‐analysis of 88 randomized control trials (RCTs) including 19 741 patients, which evaluated the effect of 25 drug or device therapies on LVRR, changes in left ventricular volumes and LVEF were apparent the most in groups treated with various beta‐blockers (BB). 24 After 6 months of treatment, metoprolol succinate was associated with an 8.8% increase in LVEF, as Goldstein et al. have shown in a small trial of 61 patients, of whom more than 60% presented with NICM. 25 Anti‐remodelling effects of metoprolol were demonstrated in the CMR substudy of MERIT‐HF where significant reductions in indexed left ventricular end‐systolic volume (ESV) and EDV were found. 26 Comparable results with no significant differences between non‐ischaemic and ischaemic groups were presented in the RESOLVD pilot II study 27 and the REVERT trial observed akin findings in 149 asymptomatic patients with only mild systolic dysfunction. 28 Moreover, the amount of remodelling appeared to be dose dependent. 28 Studies with other BB have brought similar results. A small RCT of 48 highly symptomatic patients with idiopathic DCM compared treatment with carvedilol vs. placebo over 12 months. Carvedilol significantly reduced end‐diastolic diameter (EDD) (40 ± 4 to 41 ± 4 mm/m2 in placebo arm vs. 38 ± 5 to 36 ± 4 mm/m2, P = 0.001) as well as end‐systolic diameter (ESD) (31 ± 5 to 31 ± 4 mm/m2 in placebo arm vs. 30 ± 3 to 28 ± 4 mm/m2, P = 0.01) of the left ventricle, with observed improvement in LVEF (29 ± 5% to 30 ± 6% in placebo arm vs. 27 ± 8% to 35 ± 6%, P = 0.01). 29 These data were confirmed in the PRECISE 30 and MOCHA trials, 31 two large multicentre double‐blind, placebo‐controlled studies of carvedilol. In addition, observations from the MOCHA trial suggest a dose‐dependent effect on LVEF (5% vs. 6% vs. 8% in low‐dose, medium‐dose, and high‐dose carvedilol groups, respectively). 31 There are less robust data on LVRR during bisoprolol treatment. Serial echocardiographic measurements from 160 patients (of whom 72 presented with idiopathic DCM) included in the CIBIS echocardiography substudy are available. When compared with placebo, after 5 months of treatment with bisoprolol, there was a significant change in left ventricular ESD (−4.3 ± 7.4 vs. 0.2 ± 7.5 mm, P = 0.0002), but no significant change in EDD (−1.57 ± 6.4 vs. 0.07 ± 7.6 mm, P = 0.14). 32 However, it is possible that the short study period could have limited the extent of LVRR observed. As Dubach et al. indicated in a small double‐blind RCT of 28 patients treated with bisoprolol for 1 year, most changes in EDV (252.1 ± 78 to 231.4 ± 85 mL during the first 6 months and 231.4 ± 85 to 197.8 ± 105 mL between 6 and 12 months), ESV (190.9 ± 68 to 191.2 ± 94 mL during the first 6 months and 191.2 ± 94 to 129.2 ± 85 mL between 6 and 12 months), and LVEF (25.0 ± 7% to 29.2 ± 8% during the first 6 months and 29.2 ± 8% to 36.2 ± 9% between 6 and 12 months) occurred during the latter treatment period, as assessed by CMR. 33 Therefore, the effect of BB therapy in DCM seems to be time dependent and only long‐term therapy may lead to adequate LVRR. 33 , 34 To sum up, a meta‐analysis of 18 BB trials that included 1513 patients with idiopathic DCM (roughly a half of the entire study populations) showed a significant and consistent increase in LVEF. 35
Renin‐angiotensin‐aldosterone inhibition
The role of angiotensin‐converting enzyme (ACE) inhibitors in LVRR is far less clear. The SOLVD trial was a large multi‐centre, randomized, placebo‐controlled RCT of 2569 patients treated either with enalapril or placebo. 36 Both the echocardiography substudy 37 and the radionuclide ventriculography and catheterization substudy 38 showed significant differences in change of left ventricular volumes during serial measurements between active treatment and placebo groups (P = 0.025 and P = 0.008 for EDV and P = 0.019 and P = 0.002 for ESV, respectively). However, while Konstam et al. observed significant reductions in EDV (140 ± 44 to 127 ± 37 mL/m2, P = 0.02) and ESV (106 ± 42 to 93 ± 37 mL/m2, P = 0.01), as well as an increase in LVEF (25 ± 7% to 29.8%, P = 0.01) after 1 year study period, 38 no significant change in the aforementioned parameters was seen in the echo substudy. 37 Also, no change in EDV was observed after 12 months of treatment with captopril in a small, single‐centre RCT of 50 patients followed up by echocardiography. 39 Finally, when effects of captopril and carvedilol in 57 patients (mainly with ICM) were directly compared, treatment with captopril increased LVEF to a significantly lesser extent (4.74% vs. 1.46%, P = 0.01) with no discernible change in ESV. 40 When combined, a further increase in LVEF was achieved. 40 To make these observations more relevant to the NICM population, the MOCHA trial showed a trend towards greater efficacy of carvedilol on left ventricular function in ACE inhibitor‐treated HF patients with NICM compared with ICM. 31 These data suggest that ACE inhibition prevents further remodelling (as previously demonstrated in numerous studies following patients after myocardial infarction), although it may not drive the reversal of such a process in developed HF.
Evidence for the anti‐remodelling effect of angiotensin II receptor antagonists appears to be more promising. Val‐HeFT echocardiographic substudy examined the effect of valsartan vs. placebo in addition to treatment with BB and/or ACE inhibitor in 5010 symptomatic HF patients (of whom more than 40% had NICM) and found out that valsartan lead to a significant decrease in left ventricular EDD (−1.2 ± 4 vs. −0.5 ± 4 mm/m2, P = 0.00001), which was accompanied by a significant increase in LVEF (4.5 ± 8.9% vs. 3.2 ± 8.6%, P = 0.00001). 41 Matsumori et al. investigated 6 month treatment with candesartan in a multi‐centre, double‐blind RCT of 305 patients (including 166 patients with DCM). There was a significant change in left ventricular ESD (−6.2 ± 11.7% vs. −2.0 ± 12.2%, P = 0.07) and LVEF (23.8 ± 46.0% vs. 8.4 ± 34.4%) and no difference in left ventricular EDD (−2.4 ± 9.2% vs. −0.8 ± 9.0%, P = 0.15). 42 However, the study was terminated prematurely and based on the observations that more profound LVRR occurs after a longer time period, it is possible that the effect was not fully expressed at the end of follow‐up.
The first study that investigated the anti‐remodelling effect of a mineralocorticoid receptor antagonist was conducted by Tsutamoto et al., who showed improvement in left ventricular volumes and function after 4 month treatment with spironolactone in patients with NICM. 43 These results were confirmed in a larger RCT of a mixed HF population, in which serial echocardiographic measurements were available at baseline and 12 months after randomization in 93 patients (P < 0.01 for both increase of LVEF and reduction in left ventricular volumes). 44 Whether a combination therapy of candesartan and spironolactone over a longer time induces further LVRR was studied by Chan et al. in an RCT of 52 ambulatory HF patients (of whom 21 had NICM) using serial CMR imaging. After a 12 month treatment period, there was a significant decrease in both indexed left ventricular EDV (154.68 ± 14.21 to 121.10 ± 15.76 mL/m2 in the combination group vs. 138.03 ± 10.29 to 135.13 ± 10.60 mL/m2 in the candesartan‐only group, P = 0.01) and ESV (120.30 ± 14.74 to 88.14 ± 17.10 mL/m2 vs. 101.96 ± 9.42 to 97.51 ± 10.16 mL/m2, P = 0.01), accompanied by an increase in LVEF (26 ± 2% to 35 ± 3% vs. 28 ± 2% to 31 ± 2, P = 0.03). 45 Only one study assessing the effect of eplerenone on ventricular remodelling in chronic HF is available. Udelson et al. conducted a double‐blind RCT of 226 patients assigned to either eplerenone or placebo and showed no significant change in function or volumes of the left ventricle. 46 All of these data were encompassed in a meta‐analysis of 19 trials including 2053 HF patients, which found a beneficial effect of mineralocorticoid receptor antagonists (spironolactone, eplerenone, or canrenoate) on reduction of left ventricular EDV and ESV as well as on the improvement of LVEF. 47
Angiotensin receptor‐neprilysin inhibitor
The introduction of angiotensin receptor‐neprilysin inhibitor sacubitril/valsartan into chronic HF management is one of the most important milestones in the past decade. In the PARADIGM‐HF trial, when compared with enalapril, sacubitril/valsartan reduced mortality and risk of HF hospitalizations in patients with guideline‐directed optimal medical therapy. 48 Whether this effect is conveyed by LVRR was evaluated in several studies. After sacubitril/valsartan initiation, Martens et al. observed a significant improvement in LVEF (29.6 ± 6% to 34.8 ± 6, P < 0.001) as well as a decrease in left ventricular volumes (147 ± 57 to 129 ± 55 mL in ESV, P < 0.001 and 206 ± 71 to 197 ± 72 mL in EDV, P = 0.027) in 125 symptomatic HF patients (of whom 45% presented with NICM) over a median follow‐up of 4 months. 49 Similar findings were reported in 654 patients with HF with reduced ejection fraction (HFrEF) and 12 month echocardiographic follow‐up. LVEF increased by 5.2% [95% confidence interval (CI), 4.8% to 5.6%, P < 0.001] at 6 months to a total increase of 9.4% (95% CI, 8.8% to 9.9%, P < 0.001) with a corresponding decrease in indexed left ventricular volumes at 1 year. 50 Furthermore, 25% of patients achieved an absolute increase in LVEF of more than 13%, all being treated by sacubitril–valsartan on top of contemporary guideline‐directed therapy. 50 Among the 371 consecutive patients with HF [mean baseline LVEF of 27.8% (22.9–33.1), 67.5% with non‐ischaemic origin] treated with sacubitril/valsartan, 60.9% achieved LVRR defined as a composite of an improvement in LVEF to ≥45% or a reduction in ESV by ≥15% from baseline. 51 Only one randomized, double‐blind remodelling study comparing sacubitril/valsartan in addition to standard medical therapy of HF is available. In the PRIME study, in which 118 patients with severe HF and chronic functional mitral regurgitation were assigned to sacubitril/valsartan or valsartan alone and followed for 12 months, there was a significant difference in indexed left ventricular EDV change (−11.8 ± 17.3 to −4.8 ± 19.9, P = 0.04) in the sacubitril/valsartan group while there was no significant change in LVEF in both groups. 52 These data confirm the beneficial role of angiotensin receptor‐neprilysin inhibitor therapy in the LVRR process, and similar to other aforementioned studies, 28 , 31 , 44 this effect seems to be dose dependent. 49
Sodium‐glucose cotransporter 2 inhibition
Sodium‐glucose cotransporter 2 (SGLT‐2) inhibitors reduce the risk of HF development in patients with type 2 diabetes, 53 but, more importantly, they diminish the risk of HF hospitalizations and death in HF patients both with and without diabetes. 48 , 54 The underlying mechanism of action is not fully understood and whether it includes the induction of LVRR is not known. Currently, there is a paucity of evidence regarding this topic. We identified only two trials, both assessing the potential anti‐remodelling effect by CMR. The REFORM trial, a small RCT of 56 patients with type 2 diabetes and HF, investigated the effect of 12 month treatment with dapagliflozin on LVRR and observed no significant difference in volumes or function of the left ventricle. 55 A larger RCT studied empagliflozin in 105 patients with HFrEF (of whom 31 were of non‐ischaemic origin) and observed significant reductions in indexed left ventricular ESV (80.8 ± 37.2 to 72.9 ± 37.0 mL/m2 in the empagliflozin group vs. 76.7 ± 29.3 to 75.2 ± 29.2 mL/m2 in the placebo group, P = 0.015) as well as in EDV (114.7 ± 37.0 to 105.7 ± 37.6 mL/m2 vs. 111.4 ± 29.2 to 110.9 ± 28.3 mL/m2, P = 0.004), with no significant change in LVEF at 9 months. 56 These data are inconclusive and insufficient both in terms of sample size and the length of follow‐up.
Non‐pharmacological reverse remodelling
Left bundle branch block (LBBB) is prevalent in around one‐third of patients with DCM. 57 Dyssynchrony affects both systolic and diastolic function and worsens prognosis. 58 , 59 In a recently published paper, Sze et al. showed an association of LBBB and lower LVRR response in patients with HF receiving guideline‐directed optimal medical therapy. 60 Cardiac resynchronization therapy (CRT) improves survival in patients with symptomatic HFrEF and prolonged QRS duration 44 , 61 ; there is also a sufficient evidence of a positive impact on LVRR. In the CARE‐HF trial, at 18 month echocardiographic follow‐up, CRT reduced indexed left ventricular ESV by 26.0 mL/m2 (95% CI, −31.5 to −20.4 mL/m2, P < 0.001) and increased LVEF by 6.9% (95% CI, 5.6% to 8.1%, P < 0.001). 44 Even more profound anti‐remodelling effect of resynchronization therapy was found in the MADIT‐CRT trial, in which serial echocardiographic measurements at baseline and 1 year were available in 1366 symptomatic HF patients (of whom 45.1% had NICM) receiving either CRT plus implantable cardioverter‐defibrillator (ICD) or ICD alone. 62 There was a significant decrease in left ventricular EDV (52 mL vs. 15 mL, P < 0.001) accompanied by an increase in LVEF (11% vs. 3%, P < 0.001) in the resynchronization group. 62 The LVRR effect of CRT is greater among patients with HF of non‐ischaemic origin. 44 , 63 , 64 Importantly, improvement of cardiac function occurs early during resynchronization therapy. As Sutton and Keane pointed out in their review, significant change is observable as early as 1 month after treatment initiation. 65 In the MIRACLE study, a double‐blind RCT of 323 patients with completed echocardiographic follow‐up, reductions in left ventricular EDV were apparent at 3 months and further continued between 3 and 6 months [−22.6 mL (95% CI, −33.3 to −5.8 mL, P < 0.001) and −27.2 mL (95% CI, −37.1 to −16.9 mL, P < 0.05), respectively] in the resynchronization group, with no observed changes in the control group. 66 The same was observed in changes of ESV [−21.8 mL (95% CI, −29.7 to −13.9 mL, P < 0.001) and −25.6 mL (95% CI, −37.4 to −17.7 mL, P < 0.05), respectively] and LVEF [2.3% (95% CI, 1.5% to 3.2%, P < 0.01) and 3.6% (95% CI, 2.5% to 5.8%, P < 0.001), respectively]. 66 Whether this transforms into long‐term anti‐remodelling benefit was studied by Verhaert et al., who used serial echocardiographic data of 313 patients undergoing CRT implantation (mean follow‐up of 1301 ± 573 days) and showed that after initial decrease of indexed left ventricular ESV during the first 6 months, any additional changes were much less pronounced. 67 Again, the non‐ischaemic aetiology of HF was associated with the greater initial response to CRT. 67 In the pre‐planned analysis from the REVERSE study, in which echocardiographic measurements of 360 patients 5 years after CRT initiation were available, reduction in indexed left ventricular ESV continued over the first 2 years (−14.9 ± 27.5, −3.5 ± 18.3, and −5.2 ± 18.8 mL/m2 between baseline to 6 months, 6 to 12 months, and 12 to 24 months, P < 0.001, respectively). 68 Similar changes in indexed left ventricular EDV were present. Moreover, no evidence of subsequent mitigation of the LVRR effect until the end of follow‐up was found. 68
Apart from the previously mentioned changes in cardiac structure and function, CRT also positively affects the magnitude of secondary mitral regurgitation, which stands as an independent predictor of mortality in patients with DCM. 69 In fact, functional mitral regurgitation could be viewed as another adverse remodelling phenotype actively contributing to further functional worsening rather than a simple result of left ventricular dilation, as the recent concept of proportionate vs. disproportionate mitral regurgitation hypothesized. 70 Therefore, functional mitral regurgitation could be a rational therapeutic target. Surgical mitral valve repair of severe mitral regurgitation in patients with DCM was associated with LVRR. 71 A percutaneous edge‐to‐edge mitral valve repair using the MitraClip device (Abbott Vascular, Menlo Park, CA, USA) is used as an alternative treatment for patients with advanced HF and high surgical risk. However, the COAPT and MITRA‐FR, two large RCTs, showed mixed results in regard to a mortality benefit. 72 , 73 Also, the effect of MitraClip device implantation on LVRR seemed uncertain. While in COAPT a significant decrease in left ventricular EDV (−3.7 ± 5.1 mL vs. 17.1 ± 5.1 mL, P = 0.004) 12 months after device implantation was observed, 72 no change in volumes at 1 year was observed in MITRA‐FR. 73 Few remodelling trials investigated HFrEF patients with functional mitral regurgitation. One of the first remodelling studies of the MitraClip device in these patients observed a significant decrease in indexed left ventricular EDV {100 mL [interquartile range (IQR), 84–134 mL] to 85 mL [IQR, 86–116 mL], P < 0.05} and ESV [64 mL (IQR, 49–93 mL) to 51 mL (IQR, 36–69 mL), P < 0.05], accompanied by an increased LVEF [35% (IQR, 31–41%) to 42% (IQR, 36–48%), P < 0.05] 6 months after device implant in a cohort of 26 subjects. 74 Reductions in left ventricular volumes (219 ± 74 to 193 ± 66 mL, P = 0.001 and 152 ± 68 to 136 ± 43 mL, P = 0.004 for EDV and ESV, respectively) were observed 1 year after MitraClip implantation in 58 patients (mean LVEF 31.9 ± 8.4%, 33% with DCM). 75 Bristow et al. observed a temporary increase in left ventricular volumes immediately after the procedure followed by a subsequent, significant decrease (95 ± 39 to 100 ± 40 to 82 ± 35 mL in indexed EDV and 60 ± 31 to 65 ± 34 to 52 ± 29 mL in indexed ESV, baseline, 1 month after procedure, and 6 months after procedure, respectively, P < 0.001 for all values). Interestingly, 40 out of 79 patients who achieved LVRR showed no early increase in left ventricular EDV (1.2 ± 10% vs. 8 ± 15%, P < 0.001). 61 In a meta‐analysis of 16 MitraClip trials (trials including only patients with primary mitral regurgitation were excluded) with 1266 patients and echocardiographic follow‐up, MitraClip implantation was associated with significant reductions in EDV [−14.24 mL (95% CI, −22.53 to −5.94 mL), P < 0.001] as well as in ESV [−7.67 mL (95% CI, −12.30 to −3.03 mL), P = 0.001], accompanied by increased LVEF [2.78% (0.91% to 4.66%), P = 0.004]. 76 Similar to CRT, LVRR following MitraClip is more frequent in patients with non‐ischaemic aetiology of mitral regurgitation. 77 However, in comparison with the aforementioned therapies, these changes are modest and as many of these studies have shown, only around half of the patients achieved LVRR despite significant mitral regurgitation severity reductions. 44 , 61 , 74 , 77 It is yet to be distinguished which of the HF‐associated mitral regurgitation represent irreversible and therapeutically unmodifiable remodelling phenotype.
Predictors of reverse remodelling
The process of LVRR in patients with DCM and new‐onset HF may take up to 2 years after initiating treatment. 68 , 78 However, there is a significant portion of patients who do not improve despite optimal therapy, and based on previously published data, LVRR occurs only in about one‐third of cases, ranging from 19% to 45%. 5 , 10 , 79 , 80 , 81 This fact has serious clinical implications when considering treatment escalation towards more aggressive options, such as CRT, LVAD, or heart transplant. Current guidelines recommend ICD implantation for primary prevention reasons if severe systolic dysfunction persists >3 months on optimal medical therapy 20 despite the fact that only one‐third of patients with DCM still fulfil these criteria 6 months after optimization of pharmacotherapy. 82 Therefore, the identification of predictors of LVRR is of utmost importance (Table 3 ).
TABLE 3.
Predictors of reverse remodelling
| Predictors of left ventricular reverse remodelling | |
|---|---|
| Clinical | |
| Female sex 15 , 83 , 85 | |
| Non‐ischaemic aetiology 15 , 31 , 44 , 63 , 64 , 81 , 83 , 86 | |
| Higher baseline systolic blood pressure 5 , 15 , 79 , 80 , 85 , 87 | |
| Shorter disease duration 79 , 83 | |
| Absence of LBBB 5 , 15 , 60 , 80 , 86 , 87 | |
| Idiopathic DCM 79 | |
| Imaging | |
| Absence of LGE on CMR 9 , 10 , 90 , 91 | |
| Biochemical | |
| NT‐proBNP < 1000 pg/mL 93 | |
| sST‐2 < 48 ng/mL 86 |
CMR, cardiac magnetic resonance; DCM, dilated cardiomyopathy; LBBB, left bundle branch block; LGE, late gadolinium enhancement; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; sST‐2, soluble ST2.
It seems that patients with idiopathic DCM have a lower probability of improvement of cardiac function in comparison with ‘phenocopies’ in which aetiology is identified, such as alcohol induced and tachycardia mediated. Cicoira et al. showed that only 19 out of 98 thoroughly evaluated patients with DCM with excluded secondary causes remodelled reversely, 79 a proportion much lower than in other, mixed populations of NICM. 5 , 79 , 80 , 81 , 83 Moreover, in those with known genetic profile, identification of pathogenic mutations in structural cytoskeleton Z‐disc proteins (desmin, dystrophin, and filamin C) show a strong association with low susceptibility to LVRR under optimal medical therapy [odds ratio (OR) 0.080 (95% CI, 0.010 to 0.623), P = 0.016]. 84 Female sex is another independent clinical predictor of LVRR. 15 , 83 , 85 In a study of 927 HF patients (mean LVEF 35%, 48% with non‐ischaemic origin), followed up for 1 year, LVRR was more frequent among women [hazard ratio 1.54 (95% CI, 1.11 to 2.14), P = 0.011], regardless of the severity of left ventricular dysfunction. 83 Furthermore, non‐ischaemic aetiology was predicting LVRR as well—a consistent finding among many general HF populations. 15 , 31 , 44 , 63 , 64 , 81 , 83 , 86 Several other clinical parameters, such as a higher baseline systolic blood pressure, 5 , 15 , 79 , 80 , 85 , 87 shorter duration of HF symptoms, 79 , 83 and an absence of LBBB, 5 , 15 , 60 , 80 , 86 , 87 are associated with LVRR.
Cardiac imaging is used not only for remodelling assessment, but different imaging modalities were also studied in the prognostication of successful LVRR. For example, baseline LVEF or chamber dimensions did not show a consistent association with LVRR 15 , 81 , 86 , 87 and perhaps should not be used routinely for this purpose. More advanced echocardiographic techniques, such as three‐dimensional echocardiography, speckle tracking, and estimation of wasted myocardial work using strain delay index across left ventricular segments, were studied in the prediction of LVRR mostly among patients with CRT. 88 , 89 However, considering image acquisition inconsistencies, high interindividual measurement variability, and unknown long‐term association with LVRR, none of these parameters are currently suitable for clinical application as well. Unlike echocardiography, CMR is capable of better tissue characterization. Detection of fibrosis, both focal using late gadolinium enhancement (LGE) and diffuse by T1‐weighted mapping, as well as tissue oedema as a marker of inflammation has been studied in recent years. Kubanek et al. studied 44 consecutive patients with recent‐onset DCM using CMR and found out that a lower extent of LGE [OR 0.67 (95% CI, 0.5 to 0.9), P = 0.008] and higher myocardial oedema ratio [OR 1.45 (95% CI, 1.04 to 2.02), P = 0.027] at baseline were independent predictors of LVRR at 12 months. 10 Similar results were seen by Masci et al. who demonstrated that the absence of LGE on CMR predicted LVRR at 2 year follow‐up in 58 patients with idiopathic DCM [OR 10.86 (95% CI, 1.84 to 63.91), P = 0.008]. 9 These data were later confirmed in a larger study of 71 NICM patients with a 5 year follow‐up. 90 Furthermore, among those with LGE, a cut‐off value of 7% (expressed as a percentage of left ventricular mass) predicted those with LVEF > 35% at the end of follow‐up. 90 In a prospective study of 48 mixed HF patients (of whom 44% had NICM), quantification of focal as well as diffuse fibrosis was assessed using T1‐weighted mapping and LGE before undergoing CRT implantation. 91 Resynchronization responders had a significantly smaller focal scar burden compared with non‐responders (9 ± 7% vs. 16 ± 7%, P = 0.002, expressed as a percentage of left ventricular mass), irrespective of HF aetiology. The extent of diffuse fibrosis was not different in between the groups. 91 We have not found any other trials evaluating T1‐weighted mapping in relation to LVRR. Despite the significance of tissue fibrosis in the pathogenesis of myocardial remodelling, future studies are needed to confirm the importance of its quantification (Figure 2 ).
Figure 2.
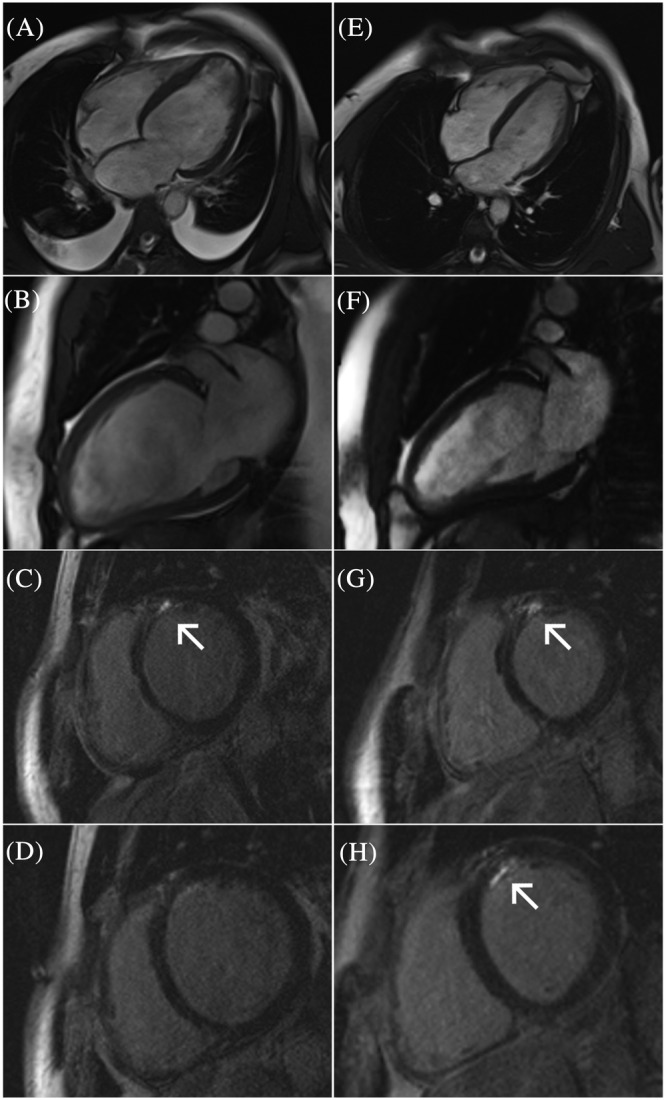
Cardiac magnetic resonance study of the same patient at the time of the diagnosis (A–D) and 14 months after treatment initiation (E–H). Chamber dilation, ventricular remodelling (A and B), and late gadolinium enhancement (arrows) are present (C and D). Despite substantial left ventricular reverse remodelling (E and F), focal fibrosis persists (G and H) and its extent seems to be greater on the follow‐up study (H).
The clinically most relevant biomarker in the HF population is N‐terminal pro‐brain natriuretic peptide (NT‐proBNP). Several studies of pharmacological and non‐pharmacological LVRR linked responders to therapies with reductions in NT‐proBNP levels. 43 , 44 , 50 Januzzi et al. showed in a comparison of NT‐proBNP‐guided vs. standard of care HF treatment that significant reductions of NT‐proBNP in the active treatment arm at 10 months were associated with a greater reduction in indexed left ventricular volumes and improvement of LVEF. 92 In the echocardiographic substudy of the GUIDE‐IT trial, patients achieving NT‐proBNP levels < 1000 pg/mL showed a greater increase in LVEF (9.9 ± 8.8% vs. 2.9 ± 7.9%, P < 0.001) and reductions in indexed left ventricular systolic and diastolic diameters (−24.6 ± 28.8 mL/m2 vs. −8.9 ± 17.3 mL/m2, P < 0.001 and −22.0 ± 31.8 mL/m2 vs. −8.0 ± 15.9 mL/m2, P = 0.006, respectively) regardless of treatment strategy (biomarker guided vs. usual care). 93 The extent of natriuretic peptide level reductions correlated with the amount of LVRR, and among patients achieving NT‐proBNP levels < 1000 pg/mL, there was a significantly higher portion of NICM (76.1% vs. 37.5%, P < 0.05). 93 Several other biomarkers were investigated. Lupón et al. studied NT‐proBNP, high‐sensitivity troponin T, galectin‐3, and soluble ST2 as possible predictors of LVRR in 304 consecutive HF patients (of whom 43.8% were of non‐ischaemic origin) with 12 month echocardiographic follow‐up. In a multivariable analysis, soluble ST2 was the only biomarker associated with LVRR; a cut‐off value of <48 ng/mL was then used in ST2‐R2 predictive score, which includes soluble ST2, non‐ischaemic aetiology, absence of LBBB, HF duration of <12 months, baseline LVEF < 24%, and BB treatment. 86 The score was validated externally proving the prediction of LVRR and its magnitude reasonable. 94 Implementation of these predictors in the routine clinical management (for early treatment escalation in therapy non‐responders, for ICD indication postponement, etc.) will require more robust evidence.
Impact of reverse remodelling on prognosis
In a meta‐analysis of 88 remodelling trials, Kramer et al. have demonstrated the association of the LVRR with lower long‐term mortality. 24 As mentioned above, a distinction should be made between reverse remodelling and cardiac recovery. Structural and functional abnormalities, with a susceptibility to dysfunction redevelopment under biomechanical stress, 17 persist in most patients. In a prospective study of 1160 consecutive HF patients followed up for 15 years, Lupón et al. have shown long‐term LVEF trajectories under guideline‐directed therapy. In patients with NICM, there was a typical inverted U‐shape pattern with marked improvement in the first year, which was maintained during the first decade with a subsequent decline thereafter. 95 Interestingly, these dynamics were related to outcomes as patients who died showed less initial improvement and greater declines afterward. Similar temporal trends were seen in 747 patients with DCM from the Heart Muscle Disease registry of Trieste. 96
Resulting from the fact that there is still not enough understanding of the recovery process, there has been some ambiguity in the clinical classification and nomenclature of patients who achieve LVRR and who form a distinct group within the general HF population—HF with mid‐range ejection fraction, 20 HF with improved ejection fraction, 97 and HF with recovered ejection fraction, 98 being just some of the terms used. Recently, an expert panel consensus on clinical management of HF with recovered left ventricular ejection fraction was published and comprehensibly differentiates this patient population by documentation of LVEF < 40% at baseline and ≥10% absolute improvement in LVEF with the second measurement of LVEF > 40%. 99 While this paper proves that the topic is eventually being discussed, still, several issues with important implications remain to be studied.
One of the clinical concerns is the timing of the evaluation of the prescribed therapy effect on reverse remodelling. As mentioned above, while the improvement in cardiac function and architecture often occurs relatively fast after treatment optimization, in some patients, the process of LVRR might take longer. The PROLONG study showed that while 65 (41.7%) out of 156 newly diagnosed HFrEF patients achieved adequate LVRR as early as 3 months after initiating treatment, additional 26 (16.6%) patients showed delayed improvement that avoided untimely ICD implantation during 12 month follow‐up. 100 Moreover, even the most aggressive up‐titration regimen using the whole pharmacotherapy armamentarium takes at least 6 (but more realistically 12) months to fully optimize the guideline‐directed treatment. 101 Next, in patients that improve profoundly, there is a paucity of data regarding possible treatment discontinuation. In the TRED‐HF, a small RCT with a single‐arm crossover design, which examined the effect of withdrawal of HF medication in patients with DCM that achieved LVRR, 40% (20 out of 50) subjects met the primary endpoint of relapse at 6 months, defined by a reduction in LVEF > 10% to <50%, an increase in left ventricular EDV > 10% to higher than a normal range, a two‐fold rise in NT‐proBNP to >400 pg/mL, or a manifestation of HF symptoms, compared with none of those who continued the treatment (P = 0.0001). 102 Similar observations were seen in super‐responders to CRT 12 months after pacing deactivation, with a significant decline in LVEF (55 ± 3% to 36 ± 12%, P = 0.001) accompanied by a profound dilation of the left ventricle (40 ± 3 to 53 ± 8 mm, P = 0.001 in ESD and 53 ± 3 to 61 ± 6 mm, P = 0.001 in EDD). 103 Based on these results, until more is known about the distinction between substantial LVRR and myocardial recovery phenotypes, treatment de‐escalation should not be advised.
Despite many advances in the transcriptional analysis of reverse remodelled hearts, it is not fully understood which of the mechanisms are linked to LVRR, which are directly affected by medical and device therapy and why do genetic, transcriptional, and metabolic profiles of reversely remodelled myocardium differ distinctly from the normal state. Although paired tissue samples of HF patients before and after LVAD implant represent an invaluable opportunity to study the biology of reverse remodelling in vivo, research will always be restricted with the limited availability of human hearts specimens. Several animal models (both small and large) that simulate LVRR in NICM are available. Apart from the ischaemic injury to induce HF, 104 , 105 , 106 surgical techniques causing volume overload, 107 , 108 genetic engineering using gene overexpression or knockout, 109 as well as different pharmacologic agents such as anthracyclines 110 , 111 or isoproterenol 112 and tachycardia‐mediated 113 HF models have been used to study structural, molecular, and transcriptional components of LVRR. Rodents used as small animal models share a high degree of homology to the human genome, but they often arise from a similar genetic background that does not reflect the genetic variety of the patient population. Because NICM can be caused by different insults (genetic mutations, inflammation, long‐standing impaired energetics, etc.), information gained from a specific animal model might not be uncritically translatable to heterogeneous underlying aetiologies of HF. In animal models, stressors that cause HF are often of rapid induction and occur in early life, whereas in human, insults are long‐lasting, disease progression is usually slow and HF often manifests at a later time. Studies that investigate the potential effect of a new therapy on LVRR in animal models use single‐drug regimens while in humans, the effect has to be observed on top of the optimal medical therapy. Despite these limitations, animal models are adjustable, controllable, and free of background confounder factors, thus providing mechanistic insight into the pathogenesis of HF and LVRR. They are incremental in the identification of important, yet currently unaffected, modulating pathways and drivers of further remodelling that could represent a target for future development of HF therapies. Examples of such pathways that might be involved in the process of LVRR include beta‐adrenergic, 13 , 114 calcium handling, 115 , 116 cardiomyocyte hypertrophy, 117 , 118 as well as integrin 119 , 120 signalling pathways and various microRNAs that regulate gene expression and proteome on the post‐translational level. 14 , 16 , 121 Nevertheless, if we could quantify the extent of initial myocardial injury irrespective of aetiology by obtainable clinical, biochemical, or imaging parameters, earlier implementation of more advanced treatment strategies in patients at risk of rapid deterioration would be possible.
In conclusion, reverse remodelling poses an essential objective and an important marker of successful treatment of patients with NICM. Appropriate timing of LVRR evaluation, identification of reliable predictors of positive and negative outcome, and introduction of novel HF therapies present an unmet challenge.
Conflict of interest
None declared.
Funding
The authors have no funding to disclose.
Hnat, T. , Veselka, J. , and Honek, J. (2022) Left ventricular reverse remodelling and its predictors in non‐ischaemic cardiomyopathy. ESC Heart Failure, 9: 2070–2083. 10.1002/ehf2.13939.
References
- 1. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000; 35: 569–582. [DOI] [PubMed] [Google Scholar]
- 2. Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990; 81: 1161–1172. [DOI] [PubMed] [Google Scholar]
- 3. Florea VG, Mareyev VY, Samko AN, Orlova IA, Coats AJS, Belenkov YN. Left ventricular remodelling: common process in patients with different primary myocardial disorders. Int J Cardiol. 1999; 68: 281–287. [DOI] [PubMed] [Google Scholar]
- 4. Kass DA, Baughman KL, Pak PH, Cho PW, Levin HR, Gardner TJ, Halperin HR, Tsitlik JE, Acker MA. Reverse remodeling from cardiomyoplasty in human heart failure: external constraint versus active assist. Circulation. 1995; 91: 2314–2318. [DOI] [PubMed] [Google Scholar]
- 5. Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Di LA, Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011; 57: 1468–1476. [DOI] [PubMed] [Google Scholar]
- 6. Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, Sutton MSJ, De SJ, Murillo J. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008; 117: 2608–2616. [DOI] [PubMed] [Google Scholar]
- 7. Wilcox JE, Fonarow GC, Yancy CW, Albert NM, Curtis AB, Heywood JT, Inge PJ, McBride ML, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Gheorghiade M. Factors associated with improvement in ejection fraction in clinical practice among patients with heart failure: findings from IMPROVE HF. Am Heart J. 2012; 163: 163–56.e2. [DOI] [PubMed] [Google Scholar]
- 8. Brenyo A, Barsheshet A, Kutyifa V, Ruwald AC, Rao M, Zareba W, Pouleur AC, Knappe D, Solomon SD, McNitt S, Huang DT, Moss AJ, Goldenberg I. Predictors of spontaneous reverse remodeling in mild heart failure patients with left ventricular dysfunction. Circ Hear Fail. 2014; 7: 565–572. [DOI] [PubMed] [Google Scholar]
- 9. Masci PG, Schuurman R, Andrea B, Ripoli A, Coceani M, Chiappino S, Todiere G, Srebot V, Passino C, Aquaro GD, Emdin M, Lombardi M. Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: a contrast‐enhanced cardiovascular magnetic study. Circ Cardiovasc Imaging. 2013; 6: 790–799. [DOI] [PubMed] [Google Scholar]
- 10. Kubanek M, Sramko M, Maluskova J, Kautznerova D, Weichet J, Lupinek P, Vrbska J, Malek I, Kautzner J. Novel predictors of left ventricular reverse remodeling in individuals with recent‐onset dilated cardiomyopathy. J Am Coll Cardiol. 2013; 61: 54–63. [DOI] [PubMed] [Google Scholar]
- 11. Kim GH, Uriel N, Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol. 2018; 15: 83–96. [DOI] [PubMed] [Google Scholar]
- 12. Barbone A, Holmes JW, Heerdt PM, The' AH, Naka Y, Joshi N, Daines M, Marks AR, Oz MC, Burkhoff D. Comparison of right and left ventricular responses to left ventricular assist device support in patients with severe heart failure. Circulation. 2001; 104: 670–675. [DOI] [PubMed] [Google Scholar]
- 13. Klotz S, Barbone A, Reiken S, Holmes JW, Naka Y, Oz MC, Marks AR, Burkhoff D. Left ventricular assist device support normalizes left and right ventricular beta‐adrenergic pathway properties. J Am Coll Cardiol. 2005; 45: 668–676. [DOI] [PubMed] [Google Scholar]
- 14. Margulies KB, Matiwala S, Cornejo C, Olsen H, Craven WA, Bednarik D. Mixed messages: transcription patterns in failing and recovering human myocardium. Circ Res. 2005; 96: 592–599. [DOI] [PubMed] [Google Scholar]
- 15. Binkley PF, Lesinski A, Ferguson JP, Hatton PS, Yamokoski L, Hardikar S, Cooke GE, Leier CV. Recovery of normal ventricular function in patients with dilated cardiomyopathy: predictors of an increasingly prevalent clinical event. Am Heart J. 2008; 155: 69–74. [DOI] [PubMed] [Google Scholar]
- 16. Yang KC, Yamada KA, Patel AY, Topkara VK, George I, Cheema FH, Ewald GA, Mann DL, Nerbonne JM. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation. 2014; 129: 1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart: myth, magic, or molecular target? J Am Coll Cardiol. 2012; 60: 2465–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Topkara VK, Garan AR, Fine B, Godier‐Furnémont AF, Breskin A, Cagliostro B, Yuzefpolskaya M, Takeda K, Takayama H, Mancini DM, Naka Y, Colombo PC. Myocardial recovery in patients receiving contemporary left ventricular assist devices. Circ Hear Fail. 2016; 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hetzer R, Müller J, Weng Y, Wallukat G, Spiegelsberger S, Loebe M. Cardiac recovery in dilated cardiomyopathy by unloading with a left ventricular assist device. Ann Thorac Surg. 1999; 68: 742–749. [DOI] [PubMed] [Google Scholar]
- 20. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members, Document Reviewers . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution. Eur J Heart Fail 2016. 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 21. Saraon T, Katz SD. Reverse remodeling in systolic heart failure. Cardiol Rev. 2015; 23: 173–181. [DOI] [PubMed] [Google Scholar]
- 22. Pieske B. Reverse remodeling in heart failure—fact or fiction? Eur Heart Journal, Suppl. 2004; 6: D66–D78. [Google Scholar]
- 23. Hellawell JL, Margulies KB. Myocardial reverse remodeling. Cardiovasc Ther. 2012; 30: 172–181. [DOI] [PubMed] [Google Scholar]
- 24. Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta‐analytic approach. J Am Coll Cardiol. 2010; 56: 392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldstein S, Kennedy HL, Hall C, Anderson JL, Gheorghiade M, Gottlieb S, Jessup M, Karlsberg RP, Friday G, Haskell L. Metoprolol CR/XL in patients with heart failure: a pilot study examining the tolerability, safety, and effect on left ventricular ejection fraction. Am Heart J. 1999; 138: 1158–1165. [DOI] [PubMed] [Google Scholar]
- 26. Groenning BA, Nilsson JC, Sondergaard L, Fritz‐Hansen T, Larsson HBW, Hildebrandt PR. Antiremodeling effects on the left ventricle during beta‐blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol. 2000; 36: 2072–2080. [DOI] [PubMed] [Google Scholar]
- 27. White M, Yusuf S, McKelvie RS, Pericak D, Young J, Latini R, Pogue J, Burns RJ, Probstfield J, Tsuyuki RT, Maggioni AP, Avezum AJ, Rouleau JL. Effects of metoprolol CR in patients with ischemic and dilated cardiomyopathy: the randomized evaluation of strategies for left ventricular dysfunction pilot study. Circulation. 2000; 101: 378–384. [DOI] [PubMed] [Google Scholar]
- 28. Colucci WS, Kolias TJ, Adams KF, Armstrong WF, Ghali JK, Gottlieb SS, Greenberg B, Klibaner MI, Kukin ML, Sugg JE. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the REversal of VEntricular remodeling with Toprol‐XL (REVERT) trial. Circulation. 2007; 116: 49–56. [DOI] [PubMed] [Google Scholar]
- 29. Palazzuoli A, Bruni F, Puccetti L, Pastorelli M, Angori P, Pasqui AL, Auteri A. Effects of carvedilol on left ventricular remodeling and systolic function in elderly patients with heart failure. Eur J Heart Fail. 2002; 4: 765–770. [DOI] [PubMed] [Google Scholar]
- 30. Packer M, Colucci WS, Sackner‐Bernstein JD, Liang CS, Goldscher DA, Freeman I, Kukin ML, Kinhal V, Udelson JE, Klapholz M, Gottlieb SS, Pearle D, Cody RJ, Gregory JJ, Kantrowitz NE, LeJemtel TH, Young ST, Lukas MA, Shusterman NH. Double‐blind, placebo‐controlled study of the effects of carvedilol in patients with moderate to severe heart failure: the PRECISE trial. Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise. Circulation. 1996; 94: 2793–2799. [DOI] [PubMed] [Google Scholar]
- 31. Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, Kubo SH, Narahara KA, Ingersoll H, Krueger S, Young S, Shusterman N. Carvedilol produces dose‐related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation. 1996; 94: 2807–2816. [DOI] [PubMed] [Google Scholar]
- 32. Léchât P, Escolano S, Golmard JL, Lardoux H, Witchitz S, Henneman JA, Maisch B, Hetzel M, Jaillon P, Boissel JP, Mallet A. Prognostic value of bisoprolol‐induced hemodynamic effects in heart failure during the Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation. 1997; 96: 2197–2205. [DOI] [PubMed] [Google Scholar]
- 33. Dubach P, Myers J, Bonetti P, Schertler T, Froelicher V, Wagner D, Scheidegger M, Stuber M, Luchinger R, Schwitter J, Hess O. Effects of bisoprolol fumarate on left ventricular size, function, and exercise capacity in patients with heart failure: analysis with magnetic resonance myocardial tagging. Am Heart J. 2002; 143: 676–683. [DOI] [PubMed] [Google Scholar]
- 34. Hall SA, Cigarroa CG, Marcoux L, Risser RC, Grayburn PA, Eichhorn EJ. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta‐adrenergic blockade. J Am Coll Cardiol. 1995; 25: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 35. Lechat P, Packer M, Chalon S, Cucherat M, Arab T, Boissel JP. Clinical effects of beta‐adrenergic blockade in chronic heart failure: a meta‐analysis of double‐blind, placebo‐controlled, randomized trials. Circulation. 1998; 98: 1184–1191. [DOI] [PubMed] [Google Scholar]
- 36. Yusuf S. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. Ann Intern Med. 1991; 115: 67. [DOI] [PubMed] [Google Scholar]
- 37. Greenberg B, Quinones MA, Koilpillai C, Limacher M, Shindler D, Benedict C, Shelton B. Effects of long‐term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction results of the SOLVD echocardiography substudy. Circulation. 1995; 91: 2573–2581. [DOI] [PubMed] [Google Scholar]
- 38. Konstam MA, Rousseau MF, Kronenberg MW, Udelson JE, Melin J, Stewart D, Dolan N, Edens TR, Ahn S, Kinan D, Howe DM, Kilcoyne L, Metherall J, Benedict C, Yusuf S, Pouleur H. Effects of the angiotensin converting enzyme inhibitor enalapril on the long‐term progression of left ventricular dysfunction in patients with heart failure. Circulation. 1992; 86: 431–438. [DOI] [PubMed] [Google Scholar]
- 39. Keren G, Pardes A, Eschar Y, Koifman B, Scherez J, Geleranter I, Laniado S. One‐year clinical and echocardiographic follow‐up of patients with congestive cardiomyopathy treated with captopril compared to placebo. Isr J Med Sci. 1994; 30: 90–98. [PubMed] [Google Scholar]
- 40. Khattar RS, Senior R, Soman P, Van Der Does R, Lahiri A. Regression of left ventricular remodeling in chronic heart failure: comparative and combined effects of captopril and carvedilol. Am Heart J. 2001; 142: 704–713. [DOI] [PubMed] [Google Scholar]
- 41. Wong M, Staszewsky L, Latini R, Barlera S, Volpi A, Chiang YT, Benza RL, Gottlieb SO, Kleemann TD, Rosconi F, Vandervoort PM, Cohn JN. Valsartan benefits left ventricular structure and function in heart failure: Val‐HeFT echocardiographic study. J Am Coll Cardiol. 2002; 40: 970–975. [DOI] [PubMed] [Google Scholar]
- 42. Matsumori A, Assessment of Response to Candesartan in Heart Failure in Japan (ARCH‐J) Study Investigators . Efficacy and safety of oral candesartan cilexetil in patients with congestive heart failure. Eur J Heart Fail. 2003; 5: 669–677. [DOI] [PubMed] [Google Scholar]
- 43. Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Matsui T, Kinoshita M. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol. 2001; 37: 1228–1233. [DOI] [PubMed] [Google Scholar]
- 44. Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Marino P, Zardini P. Long‐term, dose‐dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 2002; 40: 304–310. [DOI] [PubMed] [Google Scholar]
- 45. Chan AKY, Sanderson JE, Wang T, Lam W, Yip G, Wang M, Lam YY, Zhang Y, Yeung L, Wu EB, Chan WWM, Wong JTH, So N, Yu CM. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol. 2007; 50: 591–596. [DOI] [PubMed] [Google Scholar]
- 46. Udelson JE, Feldman AM, Greenberg B, Pitt B, Mukherjee R, Solomon HA, Konstam MA. Randomized, double‐blind, multicenter, placebo‐controlled study evaluating the effect of aldosterone antagonism with eplerenone on ventricular remodeling in patients with mild‐to‐moderate heart failure and left ventricular systolic dysfunction. Circ Hear Fail. 2010; 3: 347–353. [DOI] [PubMed] [Google Scholar]
- 47. Li X, Qi Y, Li Y, Zhang S, Guo S, Chu S, Gao P, Zhu D, Wu Z, Lu L, Shen W, Jia N, Niu W. Impact of mineralocorticoid receptor antagonists on changes in cardiac structure and function of left ventricular dysfunction a meta‐analysis of randomized controlled trials. Circ Hear Fail. 2013; 6: 156–165. [DOI] [PubMed] [Google Scholar]
- 48. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 49. Martens P, Beliën H, Dupont M, Vandervoort P, Mullens W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc Ther. 2018; 36: 36. [DOI] [PubMed] [Google Scholar]
- 50. Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Pinã IL, Rocha RA, Shah AM, Williamson KM, Solomon SD. Association of change in n‐terminal pro‐b‐type natriuretic peptide following initiation of sacubitril‐valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA ‐ J Am Med Assoc. 2019; 322: 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moon MG, Hwang IC, Choi W, Cho GY, Yoon YE, Park JB, Lee SP, Kim HK, Kim YJ. Reverse remodelling by sacubitril/valsartan predicts the prognosis in heart failure with reduced ejection fraction. ESC Hear Fail. 2021; 8: 2058–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, Yun SC, Song JM, Park SW, Kim JJ. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation: PRIME study. Circulation. 2019; 139: 1354–1365. [DOI] [PubMed] [Google Scholar]
- 53. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019; 393: 31–39. [DOI] [PubMed] [Google Scholar]
- 54. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi D‐J, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca H‐P, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 55. Singh JSS, Mordi IR, Vickneson K, Fathi A, Donnan PT, Mohan M, Choy AMJ, Gandy S, George J, Khan F, Pearson ER, Graeme Houston J, Struthers AD, Lang CC. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the Reform trial. Diabetes Care. 2020; 43: 1356–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, Dreisbach JG, Labinjoh C, Lang NN, Lennie V, McConnachie A, Murphy CL, Petrie CJ, Petrie JR, Speirits IA, Sourbron S, Welsh P, Woodward R, Radjenovic A, Mark PB, McMurray JJV, Jhund PS, Petrie MC, Sattar N. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR‐DM‐HF). Circulation. 2021; 143: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aleksova A, Carriere C, Zecchin M, Barbati G, Vitrella G, Di LA, Sinagra G. New‐onset left bundle branch block independently predicts long‐term mortality in patients with idiopathic dilated cardiomyopathy: data from the Trieste Heart Muscle Disease Registry. Europace. 2014; 16: 1450–1459. [DOI] [PubMed] [Google Scholar]
- 58. Witt CM, Wu G, Yang D, Hodge DO, Roger VL, Cha YM. Outcomes with left bundle branch block and mildly to moderately reduced left ventricular function. JACC Hear Fail. 2016; 4: 897–903. [DOI] [PubMed] [Google Scholar]
- 59. Lund LH, Jurga J, Edner M, Benson L, Dahlström U, Linde C, Alehagen U. Prevalence, correlates, and prognostic significance of QRS prolongation in heart failure with reduced and preserved ejection fraction. Eur Heart J. 2013; 34: 529–539. [DOI] [PubMed] [Google Scholar]
- 60. Sze E, Samad Z, Dunning A, Campbell KB, Loring Z, Atwater BD, Chiswell K, Kisslo JA, Velazquez EJ, Daubert JP. Impaired recovery of left ventricular function in patients with cardiomyopathy and left bundle branch block. J Am Coll Cardiol. 2018; 71: 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De MT, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004; 350: 2140–2150. [DOI] [PubMed] [Google Scholar]
- 62. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NAM, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med. 2009; 361: 1329–1338. [DOI] [PubMed] [Google Scholar]
- 63. Duncan A, Wait D, Gibson D, Daubert JC. Left ventricular remodelling and haemodynamic effects of multisite biventricular pacing in patients with left ventricular systolic dysfunction and activation disturbances in sinus rhythm: sub‐study of the MUSTIC (Multisite Stimulation in Cardiomyopathies) trial. Eur Heart J. 2003; 24: 430–441. [DOI] [PubMed] [Google Scholar]
- 64. St. John Sutton M, Ghio S, Plappert T, Tavazzi L, Scelsi L, Daubert C, Abraham WT, Gold MR, Hassager C, Herre JM, Linde C. Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association class I/II heart failure. Circulation. 2009; 120: 1858–1865. [DOI] [PubMed] [Google Scholar]
- 65. Sutton MSJ, Keane MG. Reverse remodelling in heart failure with cardiac resynchronisation therapy. Heart. 2007; 93: 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. St. John Sutton M, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, Loh E, Kocovic DZ, Fisher WG, Ellestad M, Messenger J, Kruger K, Hilpisch KE, Hill MRS. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003; 107: 1985–1990. [DOI] [PubMed] [Google Scholar]
- 67. Verhaert D, Grimm RA, Puntawangkoon C, Wolski K, De S , Wilkoff BL, Starling RC, Tang WHW, Thomas JD, Popović ZB. Long‐term reverse remodeling with cardiac resynchronization therapy. Results of extended echocardiographic follow‐up. J Am Coll Cardiol. 2010; 55: 1788–1795. [DOI] [PubMed] [Google Scholar]
- 68. Linde C, Gold MR, Abraham WT, St John Sutton M, Ghio S, Cerkvenik J, Daubert C. Long‐term impact of cardiac resynchronization therapy in mild heart failure: 5‐year results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Eur Heart J. 2013; 34: 2592–2599. [DOI] [PubMed] [Google Scholar]
- 69. Rossi A, Dini FL, Faggiano P, Agricola E, Cicoira M, Frattini S, Simioniuc A, Gullace M, Ghio S, Enriquez‐Sarano M, Temporelli PL. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non‐ischaemic dilated cardiomyopathy. Heart. 2011; 97: 1675–1680. [DOI] [PubMed] [Google Scholar]
- 70. Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA‐FR and COAPT trials. JACC Cardiovasc Imaging. 2019; 12: 353–362. [DOI] [PubMed] [Google Scholar]
- 71. De BM, Lapenna E, Verzini A, La CG, Grimaldi A, Torracca L, Maisano F, Alfieri O. Recurrence of mitral regurgitation parallels the absence of left ventricular reverse remodeling after mitral repair in advanced dilated cardiomyopathy. Ann Thorac Surg. 2008; 85: 932–939. [DOI] [PubMed] [Google Scholar]
- 72. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med. 2018; 379: 2307–2318. [DOI] [PubMed] [Google Scholar]
- 73. Obadia J‐F, Messika‐Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefèvre T, Piot C, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Leclercq F, Saint EC, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu J‐N, Cormier B, Armoiry X, Boutitie F, Maucort‐Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018; 379: 2297–2306. [DOI] [PubMed] [Google Scholar]
- 74. Gripari P, Tamborini G, Bottari V, Maffessanti F, Carminati MC, Muratori M, Vignati C, Bartorelli AL, Alamanni F, Pepi M. Three‐dimensional transthoracic echocardiography in the comprehensive evaluation of right and left heart chamber remodeling following percutaneous mitral valve repair. J Am Soc Echocardiogr. 2016; 29: 946–954. [DOI] [PubMed] [Google Scholar]
- 75. Papadopoulos K, Ikonomidis I, Chrissoheris M, Chalapas A, Kourkoveli P, Parissis J, Spargias K. MitraClip and left ventricular reverse remodelling: a strain imaging study. ESC Hear Fail. 2020; 7: 1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Megaly M, Khalil C, Abraham B, Saad M, Tawadros M, Stanberry L, Kalra A, Goldsmith SR, Bart B, Bae R, Brilakis ES, Gössl M, Sorajja P. Impact of transcatheter mitral valve repair on left ventricular remodeling in secondary mitral regurgitation: a meta‐analysis. Struct Hear. 2018; 2: 541–547. [Google Scholar]
- 77. Adamo M, Godino C, Giannini C, Scotti A, Liga R, Curello S, Fiorina C, Chiari E, Chizzola G, Abbenante A, Visco E, Branca L, Fiorelli F, Agricola E, Stella S, Lombardi C, Colombo A, Petronio AS, Metra M, Ettori F. Left ventricular reverse remodelling predicts long‐term outcomes in patients with functional mitral regurgitation undergoing MitraClip therapy: results from a multicentre registry. Eur J Heart Fail. 2019; 21: 196–204. [DOI] [PubMed] [Google Scholar]
- 78. Masè M, Merlo M, Vitrella G, Stolfo D, Sinagra G. Left ventricular reverse remodeling prediction in non‐ischemic cardiomyopathy: present and perspectives. Expert Rev Cardiovasc Ther. 2018; 16: 771–773. [DOI] [PubMed] [Google Scholar]
- 79. Cicoira M, Zanolla L, Latina L, Rossi A, Golia G, Brighetti G, Zardini P. Frequency, prognosis and predictors of improvement of systolic left ventricular function in patients with ‘classical’ clinical diagnosis of idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2001; 3: 323–330. [DOI] [PubMed] [Google Scholar]
- 80. Choi JO, Kim EY, Lee GY, Lee SC, Park SW, Kim DK, Oh JK, Jeon ES. Predictors of left ventricular reverse remodeling and subsequent outcome in nonischemic dilated cardiomyopathy. Circ J. 2013; 77: 462–469. [DOI] [PubMed] [Google Scholar]
- 81. D'Auria F, Polito MV, Vitulano G, Ciccarelli M, De RR, Gigantino A, Piscione F, Galasso G. Predictors of left ventricular reverse remodeling in patients with chronic heart failure. J Cardiovasc Med. 2018; 19: 465–469. [DOI] [PubMed] [Google Scholar]
- 82. Merlo M, Cannatà A, Gobbo M, Stolfo D, Elliott PM, Sinagra G. Evolving concepts in dilated cardiomyopathy. Eur J Heart Fail. 2018; 20: 228–239. [DOI] [PubMed] [Google Scholar]
- 83. Aimo A, Vergaro G, Castiglione V, Barison A, Pasanisi E, Petersen C, Chubuchny V, Giannoni A, Poletti R, Maffei S, Januzzi JL, Passino C, Emdin M. Effect of sex on reverse remodeling in chronic systolic heart failure. JACC Hear Fail. 2017; 5: 735–742. [DOI] [PubMed] [Google Scholar]
- 84. Dal Ferro M, Stolfo D, Altinier A, Gigli M, Perrieri M, Ramani F, Barbati G, Pivetta A, Brun F, Monserrat L, Giacca M, Mestroni L, Merlo M, Sinagra G. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart. 2017; 103: 1704–1710. [DOI] [PubMed] [Google Scholar]
- 85. Brenyo A, Barsheshet A, Kutyifa V, Ruwald A‐C, Rao M, Zareba W, Pouleur A‐C, Knappe D, Solomon SD, McNitt S, Huang DT, Moss AJ, Goldenberg I. Predictors of spontaneous reverse remodeling in mild heart failure patients with left ventricular dysfunction. Circ Heart Fail. 2014; 7: 565–572. [DOI] [PubMed] [Google Scholar]
- 86. Lupón J, Gaggin HK, De AM, Domingo M, Galán A, Zamora E, Vila J, Peñafiel J, Urrutia A, Ferrer E, Vallejo N, Januzzi JL, Bayes‐Genis A. Biomarker‐assist score for reverse remodeling prediction in heart failure: the ST2‐R2 score. Int J Cardiol. 2015; 184: 337–343. [DOI] [PubMed] [Google Scholar]
- 87. Amorim S, Campelo M, Martins E, Moura B, Sousa A, Pinho T, Silva‐Cardoso J, Maciel MJ. Prevalence, predictors and prognosis of ventricular reverse remodeling in idiopathic dilated cardiomyopathy. Rev Port Cardiol. 2016; 35: 253–260. [DOI] [PubMed] [Google Scholar]
- 88. Marsan NA, Breithardt OA, Delgado V, Bertini M, Tops LF. Predicting response to CRT. The value of two‐ and three‐dimensional echocardiography. Europace. 2008; 10: iii73–iii79. [DOI] [PubMed] [Google Scholar]
- 89. Lim P, Donal E, Lafitte S, Derumeaux G, Habib G, Rant P, Thivolet S, Lellouche N, Grimm RA, Gueret P. Multicentre study using strain delay index for predicting response to cardiac resynchronization therapy (MUSIC study). Eur J Heart Fail. 2011; 13: 984–991. [DOI] [PubMed] [Google Scholar]
- 90. Barison A, Aimo A, Ortalda A, Todiere G, Grigoratos C, Passino C, Camici PG, Aquaro GD, Emdin M. Late gadolinium enhancement as a predictor of functional recovery, need for defibrillator implantation and prognosis in non‐ischemic dilated cardiomyopathy. Int J Cardiol. 2018; 250: 195–200. [DOI] [PubMed] [Google Scholar]
- 91. Chen Z, Sohal M, Sammut E, Child N, Jackson T, Claridge S, Cooklin M, O'Neill M, Wright M, Gill J, Chiribiri A, Schaeffter T, Carr‐White G, Razavi R, Rinaldi CA. Focal but not diffuse myocardial fibrosis burden quantification using cardiac magnetic resonance imaging predicts left ventricular reverse modeling following cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2016; 27: 203–209. [DOI] [PubMed] [Google Scholar]
- 92. Januzzi JL, Rehman SU, Mohammed AA, Bhardwaj A, Barajas L, Barajas J, Kim HN, Baggish AL, Weiner RB, Chen‐Tournoux A, Marshall JE, Moore SA, Carlson WD, Lewis GD, Shin J, Sullivan D, Parks K, Wang TJ, Gregory SA, Uthamalingam S, Semigran MJ. Use of amino‐terminal ProB‐type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol. 2011; 58: 1881–1889. [DOI] [PubMed] [Google Scholar]
- 93. Daubert MA, Adams K, Yow E, Barnhart HX, Douglas PS, Rimmer S, Norris C, Cooper L, Leifer E, Desvigne‐Nickens P, Anstrom K, Fiuzat M, Ezekowitz J, Mark DB, O'Connor CM, Januzzi J, Felker GM. NT‐proBNP goal achievement is associated with significant reverse remodeling and improved clinical outcomes in HFrEF. JACC Hear Fail. 2019; 7: 158–168. [DOI] [PubMed] [Google Scholar]
- 94. Lupón J, Sanders‐Van Wijk S, Januzzi JL, De Antonio M, Gaggin HK, Pfisterer M, Galán A, Shah R, Brunner‐La Rocca HP, Bayes‐Genis A. Prediction of survival and magnitude of reverse remodeling using the ST2‐R2 score in heart failure: a multicenter study. Int J Cardiol. 2016; 204: 242–247. [DOI] [PubMed] [Google Scholar]
- 95. Lupón J, Gavidia‐Bovadilla G, Ferrer E, de Antonio M, Perera‐Lluna A, López‐Ayerbe J, Domingo M, Núñez J, Zamora E, Moliner P, Díaz‐Ruata P, Santesmases J, Bayés‐Genís A. Dynamic trajectories of left ventricular ejection fraction in heart failure. J Am Coll Cardiol. 2018; 72: 591–601. [DOI] [PubMed] [Google Scholar]
- 96. Aleksova A, Sabbadini G, Merlo M, Pinamonti B, Barbati G, Zecchin M, Bussani R, Silvestri F, Iorio AM, Stolfo D, Dal Ferro M, Dragos AM, Meringolo G, Pyxaras S, Lo GF, Perkan A, di LA, Sinagra G. Natural history of dilated cardiomyopathy: from asymptomatic left ventricular dysfunction to heart failure—a subgroup analysis from the Trieste Cardiomyopathy Registry. J Cardiovasc Med. 2009; 10: 699–705. [DOI] [PubMed] [Google Scholar]
- 97. Florea VG, Rector TS, Anand IS, Cohn JN. Heart failure with improved ejection fraction: clinical characteristics, correlates of recovery, and survival. Circ Hear Fail. 2016; 9: 9. [DOI] [PubMed] [Google Scholar]
- 98. Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang JC. Heart failure with recovered ejection fraction. Circulation. 2014; 129: 2380–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wilcox JE, Fang JC, Margulies KB, Mann DL. Heart failure with recovered left ventricular ejection fraction: JACC Scientific Expert Panel. J Am Coll Cardiol. 2020; 76: 719–734. [DOI] [PubMed] [Google Scholar]
- 100. Duncker D, König T, Hohmann S, Bauersachs J, Veltmann C. Avoiding untimely implantable cardioverter/defibrillator implantation by intensified heart failure therapy optimization supported by the wearable cardioverter/defibrillator—the PROLONG study. J Am Heart Assoc. 2017; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. DeFilippis EM, Butler J, Vaduganathan M. Waiting period before implantable cardioverter‐defibrillator implantation in newly diagnosed heart failure with reduced ejection fraction: a window of opportunity. Circ Hear Fail. 2017; 10: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, Jackson R, Rahneva T, Wage R, Smith G, Venneri L, Tayal U, Auger D, Midwinter W, Whiffin N, Rajani R, Dungu JN, Pantazis A, Cook SA, Ware JS, Baksi AJ, Pennell DJ, Rosen SD, Cowie MR, Cleland JGF, Prasad SK. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED‐HF): an open‐label, pilot, randomised trial. Lancet. 2019; 393: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cay S, Ozeke O, Ozcan F, Aras D, Topaloglu S. Mid‐term clinical and echocardiographic evaluation of super responders with and without pacing: the preliminary results of a prospective, randomized, single‐centre study. Europace. 2016; 18: 842–850. [DOI] [PubMed] [Google Scholar]
- 104. Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ Res. 1979; 44: 503–512. [DOI] [PubMed] [Google Scholar]
- 105. Patten RD, Aronovitz MJ, Deras‐Mejia L, Pandian NG, Hanak GG, Smith JJ, Mendelsohn ME, Konstam MA. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol. 1998; 274: H1812–H1820. [DOI] [PubMed] [Google Scholar]
- 106. Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol. 1995; 269: H2147–H2154. [DOI] [PubMed] [Google Scholar]
- 107. Hutchinson KR, Guggilam A, Cismowski MJ, Galantowicz ML, West TA, Stewart JA Jr, Zhang X, Lord KC, Lucchesi PA. Temporal pattern of left ventricular structural and functional remodeling following reversal of volume overload heart failure. J Appl Physiol (1985). 2011; 111: 1778–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kala P, Miklovic M, Jichova S, Skaroupkova P, Vanourkova Z, Maxova H, Gawrys O, Kompanowska‐Jezierska E, Sadowski J, Imig JD, Falck JR, Veselka J, Cervenka L, Aiglova R, Vicha M, Gloger V, Taborsky M. Effects of epoxyeicosatrienoic acid‐enhancing therapy on the course of congestive heart failure in angiotensin II‐dependent rat hypertension: from mRNA analysis towards functional in vivo evaluation. Biomedicine. 2021; 9: 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chu G, Haghighi K, Kranias EG. From mouse to man: understanding heart failure through genetically altered mouse models. J Card Fail. 2002; 8: S432–S449. [DOI] [PubMed] [Google Scholar]
- 110. Robert J. Long‐term and short‐term models for studying anthracycline cardiotoxicity and protectors. Cardiovasc Toxicol. 2007; 7: 135–139. [DOI] [PubMed] [Google Scholar]
- 111. Kala P, Bartuskova H, Pitha J, Vanourkova Z, Kikerlova S, Jichova S, Melenovsky V, Hoskova L, Veselka J, Kompanowska‐Jezierska E, Sadowski J, Gawrys O, Maxova H, Cervenka L. Deleterious effects of hyperactivity of the renin‐angiotensin system and hypertension on the course of chemotherapy‐induced heart failure after doxorubicin administration: a study in Ren‐2 transgenic rat. Int J Mol Sci. 2020; 21: 9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Teerlink JR, Pfeffer JM, Pfeffer MA. Progressive ventricular remodeling in response to diffuse isoproterenol‐induced myocardial necrosis in rats. Circ Res. 1994; 75: 105–113. [DOI] [PubMed] [Google Scholar]
- 113. Prabhu SD, Freeman GL. Effect of tachycardia heart failure on the restitution of left ventricular function in closed‐chest dogs. Circulation. 1995; 91: 176–185. [DOI] [PubMed] [Google Scholar]
- 114. Akhter SA, D'Souza KM, Malhotra R, Staron ML, Valeroso TB, Fedson SE, Anderson AS, Raman J, Jeevanandam V. Reversal of impaired myocardial beta‐adrenergic receptor signaling by continuous‐flow left ventricular assist device support. J Heart Lung Transplant. 2010; 29: 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Heerdt PM, Holmes JW, Cai B, Barbone A, Madigan JD, Reiken S, Lee DL, Oz MC, Marks AR, Burkhoff D. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end‐stage heart failure. Circulation. 2000; 102: 2713–2719. [DOI] [PubMed] [Google Scholar]
- 116. Terracciano CM, Harding SE, Adamson D, Koban M, Tansley P, Birks EJ, Barton PJ, Yacoub MH. Changes in sarcolemmal Ca entry and sarcoplasmic reticulum Ca content in ventricular myocytes from patients with end‐stage heart failure following myocardial recovery after combined pharmacological and ventricular assist device therapy. Eur Heart J. 2003; 24: 1329–1339. [DOI] [PubMed] [Google Scholar]
- 117. Zafeiridis A, Jeevanandam V, Houser SR, Margulies KB. Regression of cellular hypertrophy after left ventricular assist device support. Circulation. 1998; 98: 656–662. [DOI] [PubMed] [Google Scholar]
- 118. Hall JL, Grindle S, Han X, Fermin D, Park S, Chen Y, Bache RJ, Mariash A, Guan Z, Ormaza S, Thompson J, Graziano J, de Sam Lazaro SE, Pan S, Simari RD, Miller LW. Genomic profiling of the human heart before and after mechanical support with a ventricular assist device reveals alterations in vascular signaling networks. Physiol Genomics. 2004; 17: 283–291. [DOI] [PubMed] [Google Scholar]
- 119. Birks EJ, Hall JL, Barton PJ, Grindle S, Latif N, Hardy JP, Rider JE, Banner NR, Khaghani A, Miller LW, Yacoub MH. Gene profiling changes in cytoskeletal proteins during clinical recovery after left ventricular‐assist device support. Circulation. 2005; 112: I57–I64. [DOI] [PubMed] [Google Scholar]
- 120. Lara‐Pezzi E, Terracciano CM, Soppa GK, Smolenski RT, Felkin LE, Yacoub MH, Barton PJ. A gene expression profile of the myocardial response to clenbuterol. J Cardiovasc Transl Res. 2009; 2: 191–197. [DOI] [PubMed] [Google Scholar]
- 121. Matkovich SJ, Van Booven DJ, Youker KA, Torre‐Amione G, Diwan A, Eschenbacher WH, Dorn LE, Watson MA, Margulies KB, Dorn GW 2nd. Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation. 2009; 119: 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Francis GS, Anwar F, Bank AJ, Kubo SH, Jessurun J. Apoptosis, Bcl‐2, and proliferating cell nuclear antigen in the failing human heart: observations made after implantation of left ventricular assist device. J Card Fail. 1999; 5: 308–315. [DOI] [PubMed] [Google Scholar]


