Abstract
Aims
The cardiac injury and sequelae of Delta Variant of coronavirus disease 2019 (COVID‐19) remain unknown. This study aimed to evaluate the presence of cardiac involvement in patients recovering from Delta Variant of COVID‐19 based on multi‐parametric cardiac magnetic resonance imaging (MRI).
Methods and results
We prospectively assessed patients recovering from Delta Variant of COVID‐19 using multi‐parametric cardiac magnetic resonance imaging (MRI) between June 2021 and July 2021. Comparison was made with 25 healthy controls. Forty‐four patients (median age 51 years, 28 women) recovering from Delta Variant were recruited and had a median time of 35 days between diagnosis and cardiac MRI. There were no patients with chest pain (0/44, 0%) and high sensitivity cardiac troponin T troponin elevation (median levels 2.20 pg/mL, IQR levels 0.85–4.40 pg/mL). Regarding the cardiac imaging findings, a total of 14 (32%) patients presented cardiac tissue feature abnormalities, and a total of 9 (20%) patients had a myocarditis‐like injury based on cardiac MRI 2018 Lake Louise criteria. When we further assessed the T1 and T2 mapping values for of patients' individual, abnormal raised global native T1, T2, and extracellular volume were seen in 6 (14%), 6 (14%), and 4 (9%) patients, respectively. Comparing with controls, the patients had lower LV global longitudinal strain and (−22.2 ± 2.8% vs. −24.6 ± 2.0%, P < 0.001) and global circumferential strain (−20.7 ± 6.8% vs. −24.3 ± 2.9%, P = 0.014), but higher global native T1 (1318.8 ± 55.5 ms vs. 1282.9 ± 38.1 ms, P = 0.006). Four (9%) patients presented myocardial late gadolinium enhancement with subepicardial pattern mostly common seen, and two (5%) patients presented pericardial enhancement.
Conclusions
The cardiac MRI could detect subclinical functional and myocardial tissue characteristic abnormalities in individuals who were recovering from Delta Variant without cardiac‐related clinical findings. The native T1 mapping and strain imaging may be a sensitive tool for the noninvasive detection of a subset of patients who are at risk for cardiac sequelae and more prone to myocardial damage in survivors with Delta Variant.
Keywords: coronavirus disease 2019, Delta Variant, T1 mapping, T2 mapping, feature tracking
Introduction
The Delta (B.1.617.2) Variant, first identified in India in December 2020, transmitted easily and spread rapidly worldwide in 2021, which caused considerable number of cases, hospitalizations, and deaths. 1 , 2 , 3 In China, the Delta Variant was first identified in May 2021 at Guangzhou and had caused a wave of infection. Previous studies among hospitalized or recovered coronavirus disease 2019 (COVID‐19) patients suggested that it could cause cardiac injury, which mainly manifested as myocarditis or pericarditis, by autopsy cases 4 , 5 , 6 or cardiac imaging. 7 , 8 , 9 , 10 , 11 , 12 , 13 However, the related cardiac involvement of the Delta Variant of COVID‐19 remains unknown. And there remains poor insight into the cardiovascular sequelae about this variant of COVID‐19.
Cardiac magnetic resonance imaging (MRI) has shown to be the preferred imaging modality in COVID‐19 with excellent tissue characterization, 14 including T2‐weighted imaging (T2WI), quantitative parametric mapping of T1 and T2 relaxation times, and late gadolinium enhancement (LGE). These cardiac MRI parameters could use to see the underlying pathophysiologic changes from oedema and hyperaemia to fibrosis during the course of disease, which can be a significant contribution in this challenging clinical setting. 15 In addition, feature tracking strain analysis based on cine images has been proven as an effective method to evaluate both regional and global myocardial systolic dysfunction in myocarditis. 16 Therefore, to better understand the prevalence, extent, and type of cardiac injury and sequelae of this variant, this study aimed to recruit patients recovering from the Delta Variant of COVID‐19 and assess comprehensively the cardiac involvement and sequelae in participants by using multi‐parametric cardiac MRI techniques.
Methods
Study population
Patients recovering from the Delta Variant of COVID‐19 were prospectively recruited from June 2021 to July 2021 in a single tertiary centre, Guangzhou Eighth People's Hospital, Guangzhou, China. The inclusion criteria for the patients were as follows: (i) previously confirmed to have Delta Variant of SARS‐CoV‐2 (i.e. COVID‐19) infection with a positive reverse transcription polymerase chain reaction (RT‐PCR) on nasopharyngeal swab test about B.1.617.2 gene; (ii) age ≥ 18 years with no clinical symptoms, no chest radiology positive findings, and a 48 h RT‐PCR negative for SARS‐CoV‐2 on nasopharyngeal swab test now at the time of cardiac MRI scanning. The exclusion criteria were general contraindications to cardiac MRI scanning (e.g. anaphylaxis to contrast agent, implanted metal devices of the heart, or claustrophobia), severe renal insufficiency (estimated glomerular filtration rate <30 mL/min/1.73 m2), and MRI images quality not sufficient for analysis. Baseline assessment for all patients included gender, age, symptoms, clinical type of disease severity, co‐morbidities, laboratory data, treatment, and chest computed tomography (CT) findings. This study complied with the 2013 Declaration of Helsinki and was approved by local ethics committee (No. 202115202). All patients gave written informed consent.
A total of 25 healthy volunteers without cardiovascular disease were selected retrospectively as control from a database (March 2018 to May 2019) who had previously undergone cardiac MRI scanning with the same protocol and field strength for imaging systems.
Cardiac magnetic resonance imaging scan
Cardiac MRI examinations were performed with a 3.0‐T Philips Ingenia system (Ingenia, Philips Medical Systems, Best, the Netherlands) using a standard scan protocol. 17 For morphological and functional analyses, a stack of short‐axis slices of cine images from the apex to base were collected along with long‐axis planes (two‐chamber, three‐chamber, and four‐chamber views), using the single‐shot breath‐hold steady‐state free‐precession (SSFP) sequence. Myocardial oedema imaging was performed using breath hold black blood T2WI or T2‐weighted short‐tau inversion recovery (STIR) sequences in short axis views and four‐chamber long axis views. Resting perfusion images were acquired using a saturation‐recovery sequence in horizontal long‐ and short‐axis orientations.
Late‐gadolinium enhancement was performed 10–15 min after the administration of an intravenous bolus of gadolinium, using phase sensitive inversion recovering (PSIR) sequences, in a stack of short‐axis slices and two‐chamber, three‐chamber, and four‐chamber long‐axis views. T2 mapping with a multi‐echo mGraSE Black‐Blood sequence were performed before injection of gadolinium at basal, middle, and apical level of short‐axis view. Pre‐contrast T1 mapping with a modified look‐locker inversion‐recovery (MOLLI) 5s(3s)3s scheme was performed at basal, middle, and apical level of short‐axis view. For post‐contrast T1 mapping, a MOLLI 4s(1s)3s(1s)2s scheme was performed 15–17 min after a total dose of 0.2 mmol/kg gadopentetate dimeglumine injection (Consun Pharmaceutical CO, LTD.). All these cardiac MRI sequences parameters are reported in Supporting Information, Table S1 .
Cardiac magnetic resonance imaging analysis
All cardiac MRI images analysis was performed by a commercial software (Version 3.0, Medis, Leiden, the Netherlands) by two experienced radiologists (with 6 years and 13 years of cardiac MRI experience) blinded to clinical data through consensus. The left ventricular (LV) and right ventricular (RV) general function parameters (end‐diastolic volume index [EDVi], end‐systolic volume index [ESVi], and ejection fraction [EF]) and LV structure (maximal wall thickness [MWT]) were obtained by a stack of short‐axis cine images. Visual analysis of perfusion images was made to compare the relative hypoperfusion region between endocardial and epicardial regions, between segments of the same slice and between slices. To analysis the T2WI and/or STIR images, the region of interest (ROI) was drawn in the affected area and divided the signal intensity by that of the skeletal muscle. The abnormal T2 high‐intensity signal (i.e. myocardial oedema) was defined as the ratio between myocardial to skeletal muscle, which the ratio ≥2.0 was considered as abnormal.
Feature tracking strain was measured by loading cine images into the tissue tracking module (QStrain 3.0, Medis, Leiden, the Netherlands) using two‐dimensional (2D) technique. The LV's endocardial and epicardial borders were manually sketched in the end‐diastolic and end‐systolic phases, respectively. Trabeculations were all excluded from the endocardial borders. Global longitudinal strain (GLS) were obtained from two‐chamber, three‐chamber, and four‐chamber views for LV. Global circumferential strain (GCS) and global radial strain (GRS) were obtained from the basal, middle, and apical levels in the short‐axis view. The reduced strain values for individuals were defined as <2 standard deviations (SDs) above the respective means in the healthy controls group.
Late gadolinium enhancement images were assessed visually to analyse the presence, location (16 segments of American Herat Association), and pattern (subendocardial, mid‐wall, epicardial or pericardial). LGE of <1% of the myocardium at the inter‐ventricular insertion points only was ignored. For T1 mapping and T2 mapping, the endocardial and epicardial borders were drawn respectively at basal, middle, and apical short‐axis images of LV with care taken to avoid blood pool and epicardial fat. Meanwhile, for T1 mapping, an ROI was drawn in LV cavity, avoiding the papillary muscle to measure blood pool T1 time. Areas with LGE were not excluded from T1 and T2 mapping analysis. The global T1, T2, and extracellular volume (ECV) values of LV were average across the three slices. Abnormal raised native T1, T2, and ECV values for individuals were defined as >2 SDs above the respective means in the healthy controls group according to previous studies and the Society for Cardiovascular Magnetic Resonance expert consensus of COVID‐19 and/or classical myocarditis. 6 , 18 , 19 , 20 Individual myocardial inflammation injury was assessed in accordance with the cardiac MRI 2018 Lake Louise Criteria (LLC). 21
Pericardial effusion (≥5 mm) and enhancement were assessed visually at cine images and PSIR sequence, respectively. The positive finding was considered if there was involvement of the pericardial layers and confirmed by two observers. T1 mapping and cine images were used to differentiate fat tissue carefully from pericardial enhancement.
Statistical analysis
Statistical analyses were conducted using the SPSS version 22.0 software (IBM Inc, IL, USA). Normality was checked using the Shapiro–Wilk test. Continuous variables were expressed as mean ± standard deviation or median, interquartile range (IQR). The comparison between the patients and controls was tested by using the unpaired‐sample t‐test or the Mann–Whitney U‐test, as appropriate. Categorical variables were expressed as numbers and percentages. The χ 2 and Fisher's exact tests were used to compare the differences between two groups. A two‐sided P value <0.05 was considered significant.
Results
Population characteristics
Sixty patients recovering from Delta Variant of COVID‐19 were admitted to the MRI scanning, and 16 were excluded owing to no informed consent for cardiac MRI scanning (n = 11), contraindications for cardiac MRI scanning (n = 4), and image quality not sufficient for analysis (n = 1). Finally, a total of 44 patients (median age 51 years, IQR age 39–62 years; 28 women) were enrolled in this study (Figure 1 ). The baseline characteristics of study population are summarized in Table 1 . Patients were evaluated with a median of 35 days (IQR 30–39 days) between the diagnosis and cardiac MRI scanning. The cough (23/44, 52%), fever (22/44, 50%), and sore throat (18/44, 41%) were the most common symptoms on admission. No patients had chest pain (0/44, 0%). The mild, moderate, and severe of disease severity of COVID‐19 were assigned in 12 (27%), 30 (68%), and 2 (5%) individuals, respectively. Overall, only the IL‐6 was mildly elevated in 23 patients (52%) with a median of 6.95 pg/mL (IQR 2.17–18.17 pg/mL) (upper reference limit, 5.30 pg/mL) among all laboratory parameters. The high sensitivity cardiac troponin T troponin (Hs‐cTNT) with a median of 2.20 pg/mL (IQR 0.85–4.40 pg/mL) and N‐terminal pro‐B‐type brain natriuretic peptide (Nt‐proBNP) with a median of 17.00 ng/mL (IQR 11.25–33.00 ng/mL) were within normal range. CT showed lung parenchymal abnormalities in 26 (59%) patients and pleural effusion in 1 patient at admission.
Figure 1.
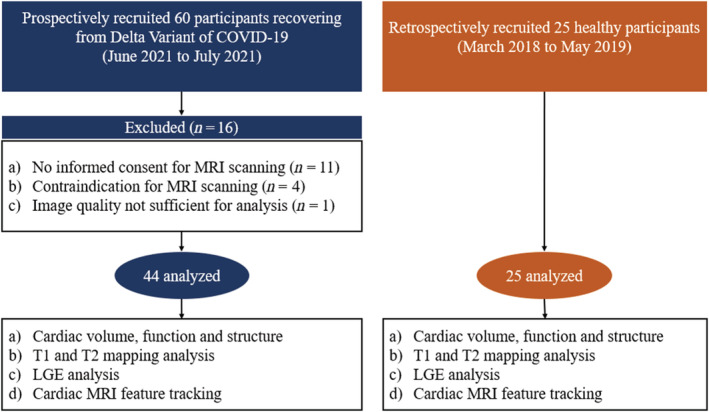
The flowchart of patient recruitment. COVID‐19, coronavirus disease 2019; MRI, magnetic resonance imaging; LGE, late gadolinium enhancement.
Table 1.
Demographics and baseline characteristics of study patients
| Characteristic | Patients with Delta Variant (n = 44) |
|---|---|
| Age, years | 51 (39–62) |
| Male sex | 16 (36) |
| BSA, m2* | 1.65 ± 0.2 |
| BMI, kg/m2* | 23.3 ± 2.8 |
| Heart rate, b.p.m* | 93.0 ± 14.5 |
| RT‐PCR positive to cardiac MRI, days | 35 (30–39) |
| Admission to cardiac MRI, days | 35 (30–38) |
| Mild/moderate/severe † | 12 (27) /30 (68) /2 (5) |
| Symptoms on admission | |
| Cough | 23 (52) |
| Fever | 22 (50) |
| Sore throat | 18 (41) |
| Muscle ache | 4 (9) |
| Headache | 3 (7) |
| Joint pain | 0 (0) |
| Chest pain | 0 (0) |
| Co‐morbidities | |
| Hyperlipidaemia | 8 (18) |
| Hypertension | 5 (11) |
| Diabetes mellitus | 4 (9) |
| COPD | 1 (2) |
| Coronary heart disease | 0 (0) |
| Laboratory results ‡ | |
| White blood cell count (×109/L) | 6.80 (5.35–7.99) |
| Hs‐cTNT, pg/mL | 2.20 (0.85–4.40) |
| Nt‐proBNP, ng/L | 17.00 (11.25–33.00) |
| LDH (U/L) | 170.50 (151.0–216.5) |
| CK‐MB (U/L) | 12.15 (9.78–14.75) |
| CRP, mg/L | 6.95 (2.17–18.17) |
| D‐dimer, mg/L | 0.36 (0.15–0.68) |
| IL‐6, pg/mL | 6.95 (2.17–18.17) |
| Treatment during hospitalization | |
| Traditional Chinese medicines | 40 (91) |
| Antibiotic therapy | 5 (11) |
| Corticosteroids | 2 (5) |
| Chest CT findings on admission | |
| Lung parenchymal abnormalities | 26 (59) |
| Pleural effusion | 1 (2) |
Unless otherwise specified, data are median, with the interquartile range in parentheses, and number and percentage for categorical variable.
BSA, body surface area; CK‐MB, creatine kinase‐MB; COPD, chronic obstructive pulmonary; COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; CT, computed tomography; Hs‐cTNT, high sensitivity cardiac troponin T; LDH, lactic dehydrogenase; NT‐proBNP, N‐terminal pro‐B‐type brain natriuretic peptide; RT‐PCR, reverse transcription polymerase chain reaction.
Data are expressed as the means ± standard deviation for continuous variables.
The clinical type of disease severity of Delta Variant of COVID‐19 was assessed according to the Chinese Center of Disease Control (CDC) criteria (32). The mild, moderate, and severe categories were assigned accordingly.
The white blood cell count, Hs‐cTNT, Nt‐pro BNP, LDH, CK‐MB, CRP, D‐dimer, and IL‐6 normal ranges below 9.5 × 109/L, 17.5 pg/mL, 100 ng/L, 250 U/L, 24 U/L, 10 mg/L, 0.55 mg/L, and 5.30 pg/mL, respectively, in our laboratory.
Cardiac magnetic resonance imaging findings
Cardiac MRI findings are summarized in Table 2 . Comparing the controls, the patients with Delta Variant had lower LV GLS and LV GCS values (LV GLS: −22.2 ± 2.8% vs. −24.6 ± 2.0%, P < 0.001; LV GCS: −20.7 ± 6.8% vs. −24.3 ± 2.6%, P = 0.014) but had no difference in the LVEF (61.5 ± 3.3% vs. 63.0 ± 6.1%, P = 0.051) and RVEF (55.5 ± 4.0% vs. 57.2 ± 7.2%, P = 0.305). When we further assessed the abnormal strain values for individuals, reduced LV GLS (<−20.6%) was seen in 10 (23%) patients, and reduced LV GCS (<−19.1%) was seen in 8 (18%) patients. There were no differences in LV maximal wall thickness and end‐diastolic diameter between patients group and controls group (all P values >0.05).
Table 2.
Cardiac MRI findings of study patients and controls
| Characteristic | Patients with Delta Variant (n = 44) | Healthy controls (n = 25) | P value |
|---|---|---|---|
| Female sex | 28 (64) | 11 (44) | 0.135 |
| Age, years | 51 (39–62) | 44 (39–51) | 0.058 |
| Haematocrit, % | 38 ± 4 | 42 ± 3 | <0.001 |
| Function and structure | |||
| LV EDVi, mL/m2 | 66.5 ± 11.5 | 79.6 ± 9.0 | <0.001 |
| LV ESVi, mL/m2 | 26.3 ± 6.2 | 28.2 ± 5.2 | 0.195 |
| LV EF, % | 61.5 ± 3.3 | 63.0 ± 6.1 | 0.051 |
| LV MWT, mm | 9.6 ± 1.8 | 9.7 ± 1.4 | 0.797 |
| LV EDD, mm | 46.0 ± 4.7 | 47.8 ± 3.7 | 0.089 |
| RV EDVi mL/m2 | 68.8 ± 13.1 | 81.9 ± 11.1 | <0.001 |
| RV ESVi, mL/m2 | 30.6 ± 6.4 | 35.0 ± 7.4 | 0.012 |
| RV EF, % | 55.5 ± 4.0 | 57.2 ± 7.2 | 0.305 |
| 2D‐strain | |||
| LV GLS, % | −22.2 ± 2.8 | −24.6 ± 2.0 | <0.001 |
| <−20.6% (2 SDs from control mean) | 10 (23%) | 0 (0) | NA |
| LV GCS, % | −20.7 ± 6.8 | −24.3 ± 2.6 | 0.014 |
| <−19.1 % (2 SDs from control mean) | 8 (18%) | 0 (0) | NA |
| LV GRS, %* | 100.6 ± 34.3 | 107.0 ± 30.2 | 0.440 |
| LV tissue feature | |||
| Late gadolinium enhancement | |||
| Positive | 4 (9) | 0 (0) | NA |
| Subendocardial pattern | 0 (0) | 0 (0) | NA |
| Mid‐wall pattern | 1 (2) | 0 (0) | NA |
| Subepicardial pattern | 3 (7) | 0 (0) | NA |
| T1 mapping | |||
| Global native T1, ms | 1318.8 ± 55.5 | 1282.9 ± 38.1 | 0.006 |
| >1359.1 ms (2 SDs from control mean) | 6 (14) | 0 (0) | NA |
| Global ECV, % | 26.2 ± 4.0 | 26.7 ± 1.9 | 0.516 |
| >29.7% (2 SDs from control mean) | 4 (9) | 1 (2) | NA |
| T2 mapping and T2WI | |||
| Global T2, ms | 47.6 ± 4.2 | 47.7 ± 2.2 | 0.936 |
| >52.1 ms (2 SDs from control mean) | 6 (14) | 0 (0) | NA |
| Abnormal T2 SI | 5 (11) | 0 (0) | NA |
| Resting perfusion defect | 0 (0) | 0 (0) | |
| Pericardial effusion (≥5 mm) | 4 (9) | 0 (0) | NA |
| Pericardial enhancement | 2 (5) | 0 (0) | 0 (0) |
Unless otherwise specified, data are means ± standard deviation, and number and percentage for categorical variable. P < 0.05 is considered to indicate a significant difference.
COVID‐19 = coronavirus disease 2019, EDVi = indexed end diastolic volume, ESVi = indexed systolic volume, EF = ejection fraction, EDD = end diastolic diameter, ECV = extracellular volume, GLS = global longitudinal strain, GCS = global circumferential strain, GRS = global radial strain, LV = left ventricular, LGE = late gadolinium enhancement, MWT = maximal wall thickness, NA = not applicable, RV = right ventricular, SI = signal intensity, SDs = standard deviations, T2WI = T2 weighted image, 2D = two‐dimensional.
Data are expressed as the median, with the interquartile range in parentheses for continuous variables.
Overall, a total of 14 (32%) patients presented tissue feature abnormalities in our participants, and a total of 9 (20%) patients had a myocarditis injury. Regarding the T1 and T2 mapping values, the global native T1 raised in patients compared with controls (1318.8 ± 55.5 ms vs. 1282.9 ± 38.1 ms, P = 0.006), but the global ECV (26.2 ± 4.0% vs. 26.7 ± 1.9%, P = 0.516) and global T2 (47.6 ± 4.2 ms vs. 47.7 ± 2.2 ms, P = 0.936) showed no differences between the two groups. When we further assessed the abnormal native T1/T2 and ECV values for individuals, raised native T1 (>1359.1 ms) was seen in 6 (14%) patients, raised T2 (>52.1 ms) seen in 6 (14%) patients, and raised ECV (>29.7%) seen in 4 (9%) patients. In addition, five (11%) patients manifested as the myocardial hyperintensity at T2‐STIR images and 4 (9%) patients as the pericardial effusion (≥5 mm) at cine images. Four (9%) patients had myocardial LGE with subepicardial pattern mostly common seen at the basal‐mid level of LV in inferior or inferoseptal segments (Figure 2 ). In addition, two (5%) patients presented pericardial enhancement at LV free wall (Figure 3 ). A perfusion defect was not found in any patients after assessing the first‐pass perfusion images. Figure 4 showed the typical images of the multi‐parametric cardiac MRI findings of patients and controls.
Figure 2.
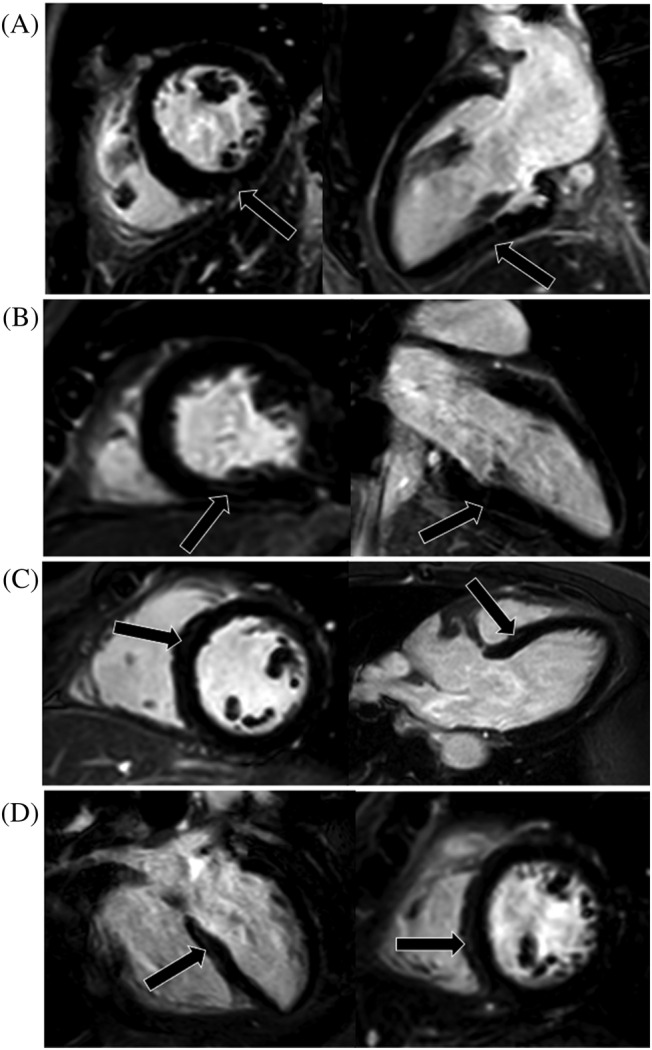
The illustration of all four LGE positive patients' myocardial injury. (A–D) represents cases 1–4, respectively. One short axis and orthogonal long‐axis PSIR images showed focal LGE positive (black arrows) for each patient at the basal‐mid level of the left ventricular in inferior or inferoseptal segments. LGE was most commonly seen in the subepicardial location (A, C, and D). LGE, late gadolinium enhancement; PSIR, phase sensitive inversion recovery.
Figure 3.
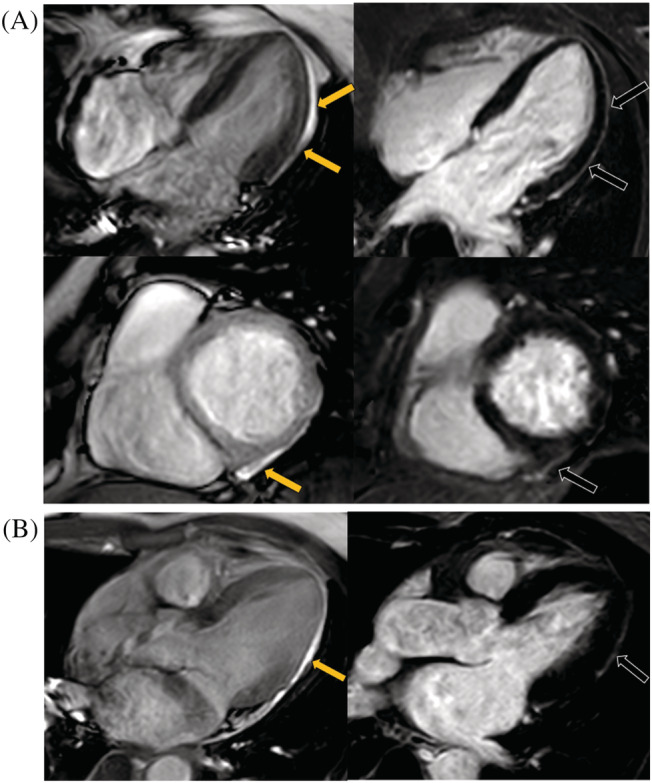
The illustration of all two LGE positive patients' pericardium injury. (A, B) represents cases 1–2, respectively. Cine images showed pericardial effusion at LV free wall (yellow arrows), and PSIR images showed the corresponded pericardial enhancement (black arrows) for each patient. LV, left ventricular; PSIR, phase sensitive inversion recovery.
Figure 4.
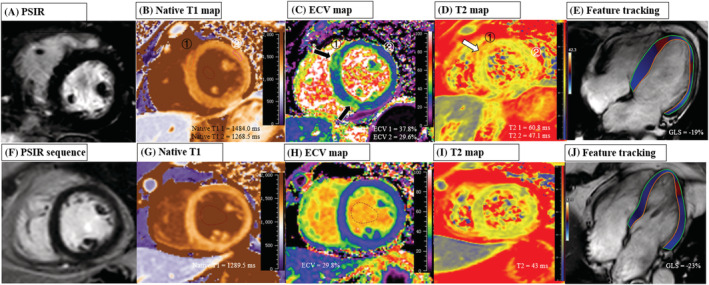
The typical images of the multi‐parameter cardiac MRI findings of patients and controls. Top row: Images in a 63‐year‐old female (patient subject) with severe clinical type of Delta Variant, showing negative LGE, elevated global native T1 of 1405.7 ms, elevated ECV of 33.4%, elevated T2 of 57.1 ms, and reduced GLS of −19.0%. In addition, the ECV map (C) and T2 map (D) showed higher ECV (black arrow) and T2 values (white arrow) at the mid‐level of LV in septal segments (ROI 1: ECV = 37.8%, T2 = 60.8 ms) compared with remote normal myocardium (ROI 2: ECV = 29.6%, T2 = 47.1 ms), but the corresponding LGE location was negative; Bottom row: Images in a 57‐year‐old female (control subject) with negative LGE, normal global native T1 (1289.5 ms), normal ECV of 29.8%, normal global T2 (43 ms), and a GLS of −22.7%. ECV, extracellular volume; GLS, global longitudinal strain; LGE, late gadolinium enhancement; PSIR, phase sensitive inversion recovery; SSFP, steady‐state free‐precession.
Discussion
To our knowledge, this is first prospective study reporting the cardiac involvement and sequelae in patient with Delta Variant of coronavirus 2019 using the multi‐parametric cardiac MRI techniques. The main findings of our study are summarized as follows: (i) in our participants recovering from Delta Variant without cardiac related clinical findings, a total of 32% patients presented cardiac tissue feature abnormalities based on the cardiac MRI findings, and the most common imaging diagnosis was the myocarditis‐like injury (20%); (ii) these abnormal MRI findings included the raised global native T1 (14%), T2 (14%), and ECV (9%) values in a proportion of patients when further individually analysed the patients with Delta Variant. Moreover, localized myocardial LGE with subepicardial pattern mostly common seen (9%) and pericardial enhancement (5%) were also detected in patients with Delta Variant; (iii) although there was no difference in LVEF, small portion of patients had reduced LV GLS and GRS compared with healthy controls.
As the most common imaging diagnosis in our population, MRI possible myocarditis as an underlying mechanism for COVID‐19‐associated myocardial injury has been widely reported. Previous studies 12 , 13 , 22 , 23 , 24 , 25 , 26 showed that the incidence of myocarditis in patients with COVID‐19 ranged 0% from 60% based on the 2019 and/or 2018 LLC. Among these cohort study, the Ng et al. 12 and Eiros et al. 23 found that the identified myocarditis in 18.8% (3/16) and in 26% (36/139) patients with recovered from COVID‐19, similar to our study with an incidence of 20% (9/44). It should be noted that 20% of patients in convalescence had MRI possible myocarditis‐like injury but no patient with chest pain in our cohort, which was in line with previous studies regarding the recovered patients from COVID‐19 (the prevalence of myocarditis injury varied between 32% and 60% vs. of chest pain was 0%). 6 , 10 , 27 However, there was low incidence (0–2.3%) of myocarditis injury in those studies which recruited the athlete participants recovered from COVID‐19. In addition, echoing with the most common of cardiac imaging diagnosis (i.e. myocarditis), the most common imaging findings included raised T2 (14%) and raised native T1(14%) mapping abnormalities, and myocardial oedema (11%) on T2WI in our participants. Of the studies that evaluated both T1 and T2 mapping, Clark et al. 26 and Rajpal et al. 13 detected the similar incidence of increased T2 to our study with 13.5% (3/22) and 15.4% (4/26), respectively, and Kotecha et al. 10 reported the similar incidence (13%) of increased native T1 to our study. The remaining studies reported rates of increased T2 ranging from 0% to 73% and increased native T1 ranging from 0% to 60%. Interestingly, regarding T1 and T2 mapping parameters, we found that only native T1 significantly increased in patients compared with controls. The similar research results are also reported in the Ferreira et al. 21 and Lurz et al. 28 study which showed the native T1 values had the best diagnostic performance for the detection of suspected myocarditis compared with T2 and ECV. Of the all seven studies that evaluated ECV mapping at 1.5 T 13 , 24 , 26 , 29 Siemens or 3.0 T 30 , 31 , 32 Siemens systems, the ECV values in patents with COVID‐19 ranged 22.1 ± 2.2% from 30.4% (28.3–31.3%). The ECV vales in our participants was 26.2 ± 4.0% at 3.0 T Philips system. Regarding myocardial fibrosis, in agreement with the rare myocyte necrosis on endomyocardial biopsy sample of COVID‐19, 5 , 6 , 27 the small amount of LGE with non‐ischemic pattern was detected in our cases. However, of the studies that evaluated LGE, the detected LGE ranged from 0% to 51.7%. The varied rates of myocardial tissue injury among these reported studies may be explained by the differences in the participants in terms of disease severity, course of disease at the time of evaluation, imaging equipment, and so on. The underlying pathophysiology of these varied imaging findings will need to be addressed in further studies with biopsy sample at patients with Delta Variant.
To explore the impact of Delta Variant of COVID‐19 on cardiac function in detail, we performed feature tracking based on cine images. Although LV/RV EF was normal in our patients, a more subtle cardiac dysfunction using feature tracking strain showed deteriorated LV systolic function assessed by GLS and GCS in a small portion of recovering patients. The reduced GLS has been reported previously in patients with COVID‐19 infection using speckle tracking echocardiography (STE) 11 , 27 or MRI feature tracking. 33 , 34 Weckbach et al. 27 revealed that moderately to severely reduced LV GLS of −11.2% (−7.6% to −15.1%) occurred in patents with COVID‐19‐associated myocardial injury even if the LVEF was within normal range, and that the LV GLS significantly improved after a median of 52.0 days at follow‐up (11.2% vs. −15.6%). However, unlike Weckebach's study which enrolled the COVID‐19 patients at acute phase of disease course, only a small portion of participants had mildly reduced LV GLS and LV GCS in our study. We speculated that the different severity of strain imaging values among studies might be affected by the disease course, clinical type of COVID‐19, or post‐processing method of cardiac MRI images. So far, there is no study of myocardial strain analysis on Delta Variant of COVID‐19 using STE or MRI feature tracking. Further studies, especially concerning longitudinal follow‐up, are needed to study the evolution of this slight adverse dysfunction and its remodelling in Delta Variant infection.
As described above, our study demonstrated that cardiac MRI‐derived native T1 and feature‐tracking global longitudinal and circumferential strain could as serve non‐invasive imaging markers of cardiac involvement in individuals who were recovering from Delta Variant of COVID‐19 with preserved LVEF and without cardiac symptoms or troponin elevation, highlighting the cardiac injury present beyond the acute phase. This emphasizes the importance of closely follow‐up monitoring after several weeks and months of recovery in survivors with Delta Variant of COVID‐19.
Study limitations
This study had several limitations. First, this was a single‐centre cohort study with a small sample size, and our results need to be evaluated by larger‐scale cohorts. Second, we are not able to include all recovering patients with Delta Variant of COVID‐19 due to various exclusion criteria, which can cause selection bias in this small cohort study. Third, the present study lacked follow‐up and prognostic information. Studies with long‐term follow‐up are required to validate and expand the findings of this study in the future. Finally, no baseline cardiac MRI examinations as comparison for the investigated participants. Therefore, we are not able to clarify whether partially tissue abnormalities (e.g. focal LGE) at cardiac imaging represented baseline or persistent impairment of cardiac tissue due to Delta Variant‐related infection.
Conclusions
In conclusion, we demonstrated subclinical functional and myocardial tissue characteristic abnormalities in individuals based on cardiac MRI who were recovering from Delta Variant of COVID‐19 without cardiac related clinical findings. The native T1 mapping and global longitudinal and circumferential strain may be a sensitive tool for the noninvasive detection of a subset of patients who are at risk for cardiac sequelae and more prone to myocardial damage in survivors with Delta Variant of COVID‐19. The clinical implications of these imaging findings need more short‐ and long‐term follow up research in survivors with Delta Variant of COVID‐19 to validate.
Conflict of interest
None declared.
Supporting information
Table S1. The cardiac MRI sequences parameters of this study.
Acknowledgement
This study was funded by the National Natural Science Foundation of China (Grant No. 81974262) and Natural Science Foundation of Guangdong Province (Grant No. 2020A1515010650).
Zhang, L. , Wei, X. , Wang, H. , Jiang, R. , Tan, Z. , Ouyang, J. , Li, X. , Lei, C. , Liu, H. , and Liu, J. (2022) Cardiac involvement in patients recovering from Delta Variant of COVID‐19: a prospective multi‐parametric MRI study. ESC Heart Failure, 9: 2576–2584. 10.1002/ehf2.13971.
Contributor Information
Hui Liu, Email: liuhuijiujiu@gmail.com.
Jinxin Liu, Email: liujx83710378@126.com.
References
- 1. Del Rio C, Malani PN, Omer SB. Confronting the Delta Variant of SARS‐CoV‐2, summer 2021. JAMA. 2021. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 2. Dyer O. Covid‐19: Indonesia becomes Asia's new pandemic epicentre as delta variant spreads. BMJ. 2021; 374: n1815. [DOI] [PubMed] [Google Scholar]
- 3. CDC . COVID‐19: SARS‐CoV‐2 variant classifications and definitions. Atlanta, GA: US Department of Health and Human Services. https://www.cdc.gov/coronavirus/2019‐ncov/cases‐updates/variant‐surveillance/variant‐info.html (2021; date last accessed).
- 4. Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, et al. Association of Cardiac Infection with SARS‐CoV‐2 in confirmed COVID‐19 autopsy cases. JAMA Cardiol. 2020; 5: 1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020; 5: 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luetkens JA, Isaak A, Zimmer S, Nattermann J, Sprinkart AM, Boesecke C, et al. Diffuse myocardial inflammation in COVID‐19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2020; 13: e010897. [DOI] [PubMed] [Google Scholar]
- 8. Chen BH, Shi NN, Wu CW, An DA, Shi YX, Wesemann LD, et al. Early cardiac involvement in patients with acute COVID‐19 infection identified by multiparametric cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2021; 22: 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galea N, Marchitelli L, Pambianchi G, Catapano F, Cundari G, Birtolo LI, et al. T2‐mapping increase is the prevalent imaging biomarker of myocardial involvement in active COVID‐19: A cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2021; 23: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kotecha T, Knight DS, Razvi Y. Patterns of myocardial injury in recovered troponin‐positive COVID‐19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021; 42: 1866–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brito D, Meester S, Yanamala N, Patel HB, Balcik BJ, Casaclang‐Verzosa G, et al. High prevalence of pericardial involvement in college student athletes recovering from COVID‐19. J Am Coll Cardiol Img. 2021; 14: 541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ng MY, Ferreira VM, Leung ST, Yin Lee JC, Ho‐Tung Fong A, To Liu RW, et al. Patients recovered from COVID‐19 show ongoing subclinical myocarditis as revealed by cardiac magnetic resonance imaging. J Am Coll Cardiol Img. 2020; 13: 2476–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID‐19 infection. JAMA Cardiol. 2021; 6: 116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanghvi SK, Schwarzman LS. Cardiac MRI and myocardial injury in COVID‐19: Diagnosis, risk stratification and prognosis. Diagnostics (Basel). 2021; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cosyns B, Lochy S, Luchian ML, Gimelli A, Pontone G, Allard SD, et al. The role of cardiovascular imaging for myocardial injury in hospitalized COVID‐19 patients. Eur Heart J Cardiovasc Imaging. 2020; 21: 709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luetkens JA, Schlesinger‐Irsch U, Kuetting DL, Dabir D, Homsi R, Doerner J, et al. Feature‐tracking myocardial strain analysis in acute myocarditis: Diagnostic value and association with myocardial oedema. Eur Radiol. 2017; 27: 4661–4671. [DOI] [PubMed] [Google Scholar]
- 17. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013; 15: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Breitbart P, Koch A, Schmidt M, Magedanz A, Lindhoff‐Last E, Voigtländer T, et al. Clinical and cardiac magnetic resonance findings in post‐COVID patients referred for suspected myocarditis. Clin Res Cardiol. 2021; 110: 1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Radunski UK, Lund GK, Säring D, Bohnen S, Stehning C, Schnackenburg B, et al. T1 and T2 mapping cardiovascular magnetic resonance imaging techniques reveal unapparent myocardial injury in patients with myocarditis. Clin Res Cardiol. 2017; 106: 10–17. [DOI] [PubMed] [Google Scholar]
- 20. Puntmann VO, Valbuena S, Hinojar R, Petersen SE, Greenwood JP, Kramer CM, et al. Society for Cardiovascular Magnetic Resonance (SCMR) expert consensus for CMR imaging endpoints in clinical research: Part I ‐ analytical validation and clinical qualification. J Cardiovasc Magn Reson. 2018; 20: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferreira VM, Schulz‐Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J Am Coll Cardiol. 2018; 72: 3158–3176. [DOI] [PubMed] [Google Scholar]
- 22. Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS‐CoV‐2 infection: Results from the big ten COVID‐19 cardiac registry. JAMA Cardiol. 2021: e212065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eiros R, Barreiro‐Perez M, Martin‐Garcia A, Almeida J, Villacorta E, Perez‐Pons A, et al. Pericarditis and myocarditis long after SARS‐CoV‐2 infection: A cross‐sectional descriptive study in health‐care workers. medRxiv. 2020. [Google Scholar]
- 24. Starekova J, Bluemke DA, Bradham WS, Eckhardt LL, Grist TM, Kusmirek JE, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021; 6: 945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Małek ŁA, Marczak M, Miłosz‐Wieczorek B, Konopka M. Cardiac involvement in consecutive elite athletes recovered from Covid‐19: A magnetic resonance study. J Magn Reson Imaging. 2021; 53: 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clark DE, Parikh A, Dendy JM, Diamond AB, George‐Durrett K, Fish FA, et al. COVID‐19 myocardial pathology evaluated through scrEening cardiac magnetic resonance (COMPETE CMR). medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weckbach LT, Curta A, Bieber S, Kraechan A, Brado J, Hellmuth JC, et al. Myocardial inflammation and dysfunction in COVID‐19‐associated myocardial injury. Circ Cardiovasc Imaging. 2021; 14: e012220. [DOI] [PubMed] [Google Scholar]
- 28. Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: The MyoRacer‐trial. J Am Coll Cardiol. 2016; 67: 1800–1811. [DOI] [PubMed] [Google Scholar]
- 29. Joy G, Artico J, Kurdi H, Seraphim A, Lau C, Thornton GD, et al. Prospective case‐control study of cardiovascular abnormalities 6 months following mild COVID‐19 in healthcare workers. J Am Coll Cardiol Img. 2021; S1936‐878X 00356‐9. [Google Scholar]
- 30. Wu X, Deng KQ, Li C, Yang Z, Hu H, Cai H, et al. Cardiac involvement in recovered patients from COVID‐19: A preliminary 6‐month follow‐up study. Front Cardiovasc Med. 2021; 8: 654405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro‐Almagro F, et al. Medium‐term effects of SARS‐CoV‐2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post‐hospital discharge. EClinicalMedicine. 2021; 31: 100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, et al. Cardiac involvement in patients recovered from COVID‐2019 identified using magnetic resonance imaging. J Am Coll Cardiol Img. 2020; 13: 2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li X, Wang H, Zhao R, Wang T, Zhu Y, Qian Y, et al. Elevated extracellular volume fraction and reduced global longitudinal strains in participants recovered from COVID‐19 without clinical cardiac findings. Radiology. 2021; 299: E230–E240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang H, Li R, Zhou Z, Jiang H, Yan Z, Tao X, et al. Cardiac involvement in COVID‐19 patients: Mid‐term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021; 23: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The cardiac MRI sequences parameters of this study.


