Abstract
Aims
In heart failure (HF), anaerobic threshold (AT) may be indeterminable but its value held a relevant prognostic role. AT is evaluated joining three methods: V‐slope, ventilatory equivalent, and end‐tidal methods. The possible non‐concordance between the V‐slope (met AT) and the other two methods (vent AT) has been highlighted in healthy individuals and named double threshold (DT).
Methods and results
We reanalysed 1075 cardiopulmonary exercise tests of HF patients recruited in the Metabolic Exercise test data combined with Cardiac and Kidney Indexes (MECKI) score database. We identified DT in 43% of cases. Met AT precedes vent AT being met–ventΔVO2 221 (interquartile range: 129–319) mL/min. Peak VO2, 1307 ± 485 vs. 1343 ± 446 mL/min (63 ± 17 vs. 63 ± 17 percentage of predicted), was similar between DT+ and DT− patients. Differently, DT+ showed a lower ventilatory vs. carbon dioxide production (VE/VCO2) slope (29.6 ± 6.1 vs. 31.0 ± 6.3), a lower peak exercise end‐tidal oxygen tension (PetO2) 115.3 (111.5–118.9) vs. 116.4 (112.4–120.2) mmHg, and a higher carbon dioxide tension (PetCO2) 34.2 (30.9–37.1) vs. 32.4 (28.7–35.5) mmHg. Vent AT showed a significant higher VO2, 957 ± 318 vs. 719 ± 252 mL/min, VCO2, 939 ± 319 vs. 627 ± 226 mL/min, ventilation, 31.0 ± 8.3 vs. 22.5 ± 6.3 L/min, respiratory exchange ratio, 0.98 ± 0.08 vs. 0.87 ± 0.07, PetO2, 108 (104–112) vs. 105 (101–109) mmHg, PetCO2, 37 (34–40) vs. 36 (33–39) mmHg, and VE/VO2 ratio, 33.5 ± 6.7 vs. 32.6 ± 6.9, but lower VE/VCO2 ratio, 33 (30–37) vs. 36 (32–41), compared with met AT. At 2 year survival by Kaplan–Meier analysis, even adjusted for confounders, DT resulted not associated with survival.
Conclusions
Double threshold is frequently observed in HF patients. DT+ is associated to a decreased ventilatory response during exercise.
Keywords: Cardiopulmonary exercise test, Anaerobic threshold, Heart failure prognosis
Introduction
Cardiopulmonary exercise testing (CPET) is an effective tool in heart failure (HF) patients. It fulfils a pivotal role in the evaluation of the functional capacity of these subjects, for the prognosis, eligibility for advanced HF therapy including heart transplant, the evaluation of surgical risk, and in cardiac rehabilitation. 1 , 2 , 3 , 4 , 5 Among the various parameters of CPET, the anaerobic threshold (AT) plays an important role. Of note, even the presence of an indefinable AT, despite a maximal effort, has a strong prognostic power. 3 , 6 , 7 , 8 During an incremental ramp exercise, AT indicates the transition from a predominantly aerobic metabolism to a metabolism, which combines aerobic and anaerobic pathways. AT is measured non‐invasively using three approaches: V‐slope method, the ventilatory equivalent method, and the end‐tidal method. 9 , 10 The first approach is independent from ventilation, while the other two are affected by the ventilatory response to the extra production of carbon dioxide (VCO2) that occurs during exercise above AT. The possibility of a divergence between the V‐slope method, and the other two methods, defined as double threshold (DT), has been reported initially by an anecdotal case report and recently in ≈10% of cases in healthy individuals. 11 , 12 A delay in the chemoreceptor response has been suggested as DT leading causes. 11 In HF, no information about prevalence of DT, its possible pathophysiological basis, and its potential prognostic role are available so far. The present study was therefore undertaken to assess the prevalence, the physiological meaning, and the prognostic role of DT in a sizable HF population belonging to the Metabolic Exercise test data combined with Cardiac and Kidney Indexes (MECKI) score data set. 13
Methods
We retrospectively analysed the data of HF patients enrolled in the MECKI score database by two centres: the Centro Cardiologico Monzino IRCCS and the Cardiothoracovascular Department of University of Trieste. The MECKI score database inclusion/exclusion criteria and the clinical, laboratory, echocardiographic, exercise collections, and patient follow‐up modalities have been previously described in details. 13 , 14 In brief, the MECKI score inclusion criteria were previous or present HF symptoms [New York Heart Association functional class I–III and stages B and C of the American College of Cardiology (ACC)/American Heart Association (AHA) classification], former documentation of left ventricular systolic dysfunction [left ventricular ejection fraction (LVEF) < 40%], stable clinical conditions with unchanged medications for at least 3 months, ability to perform a CPET, and no critical cardiovascular treatment or intervention scheduled. Exclusion criteria were history of pulmonary embolism, moderate‐to‐severe aortic and mitral stenosis, severe obstructive lung disease, pericardial disease, exercise‐induced angina, and significant electrocardiogram (ECG) alterations or presence of any clinical co‐morbidity interfering with exercise performance. 13 , 14 CPETs interrupted before AT or around AT [respiratory exchange ratio (RER) < 1.0], tests with exercise oscillatory ventilation (EOV) or with marked respiratory distress, which made AT undefinable, were excluded. MECKI score was calculated using the formula reported by Agostoni et al. 13 The considered outcome measure was the composite of cardiovascular death, left ventricular assist device (LVAD) implantation, and urgent heart transplantation.
Cardiopulmonary exercise test
In every case, a personalized ramp exercise protocol was performed with the aim of attaining the peak of the exercise in ~10 min. 15 Electronically braked cycle ergometer (Erg 800S, Sensor Medics, or Lode Corival) was used. Respiratory gases were collected and analysed on a breath‐by‐breath basis (229D Spectra Metabolic Cart, Sensor Medics). CPETs were analysed following a standard methodology by two experts. 8 Predicted values of VO2 were calculated according to the Hansen et al. formula. 16 AT was identified by three methods: the V‐slope, the ventilatory equivalent, and the end‐tidal methods. To be specific, AT was identified with the V‐slope method when the relationship between VO2 and VCO2, initially linear, showed an increased slope when plotted on equal axis scale squared graph. The AT assessment by the ventilatory equivalent method involved the simultaneous analysis vs. time (or work rate) of the ratio between ventilation and carbon dioxide (VE/VCO2) or oxygen uptake (VE/VO2), the so‐called ventilatory equivalents. The AT corresponds to the VO2 at which VE/VO2, after reaching the lowest point, starts to increase consistently, while VE/VCO2 remains unchanged. The previous approach, called the end‐tidal method, is in match with ventilatory equivalent method, and it identifies AT when end‐tidal oxygen tension (PetO2) begins to increase while end‐tidal carbon dioxide tension (PetCO2) is stable. We defined the presence of a divergence between the V‐slope and ventilation‐derived methods (>100 mL/min) with the term of DT. We formerly named the AT detected by V‐slope method as metabolic AT (met AT) and the AT identified by ventilation‐derived methods as ventilation AT (vent AT). 11
All other CPET parameters were calculated and reported as standard. 8 Specifically, peak VO2 was reported as the highest recorded VO2 values (average of 20 s), the oxygen pulse (O2‐pulse) as VO2 over heart rate, and the VE/VCO2 was determined as the slope of the linear relationship between VE and VCO2 between the second minute of loaded exercise and the respiratory compensation point. In the present study, we re‐evaluated the CPETs with a focus on finding the AT using the three above described methods.
All the MECKI score analyses were approved by the Ethics Committee of Centro Cardiologico Monzino IRCSS (R116/14‐CCM 127).
Statistical analysis
Continuous variables are presented as mean ± standard deviation or as median and interquartile range in case of non‐normally distributed variables. Categorical variables were reported as absolute values and percentages, and they were compared using the χ 2 test. We compared subjects with DT (DT+ subjects) with those with a single AT (DT− subjects) by ANOVA for normally distributed variables and the Kruskal–Wallis test for non‐normally distributed variables. Considering only the DT+ subjects, values at met AT and at vent AT were compared using paired t‐test for normally distributed variables and non‐parametric Wilcoxon–Mann–Whitney test for non‐normally distributed variables. We evaluated survival in DT+ and DT− subjects through Kaplan–Meier analysis, and we used the log‐rank test for comparison when adjusted for age, sex, haemoglobin (Hb), LVEF, kidney function, and peak VO2 (considered in absolute values, pro kg values, or as a percentage of predicted). A P‐value < 0.05 was used to define statistical significance. We collected all data in an Excel database and performed analyses using IBM SPSS Statistics 26.
Results
From a total of 1971 patients, 284 tests were interrupted before/around AT and 612 tests were characterized by EOV or respiratory disorders and therefore excluded from the present analysis leading to a study population of 1075 cases. Among these subjects, 83% were males and 465 out of 1075 HF cases had DT (43%). The presence of DT was more frequent in women (51% of women have DT vs. 42% of men, P‐value < 0.02). Demographic, laboratory, and CPET data at peak exercise are reported in Table 1 . Therapy was compliant with guidelines; to be specific, 89% of patients were taking a beta‐blocker, 66% was taking angiotensin‐converting enzyme (ACE) inhibitors, 18% angiotensin receptor blockers (ARB), 6% angiotensin receptor‐neprilysin inhibitor (ARNI), 53% K+‐sparing diuretics, 75% other diuretics, and 19% statins.
Table 1.
Demographic, laboratory, and cardiopulmonary exercise test data
| All (n = 1075) | DT− (n = 610) | DT+ (n = 465) | P‐value | |
|---|---|---|---|---|
| Age (years) | 62 ± 12 | 61 ± 12 | 62 ± 12 | 0.080 |
| Sex male (%) | 895 (83%) | 522 (86%) | 373 (80%) | 0.012 |
| BMI (kg/m2) | 26.6 ± 4.2 | 26.8 ± 4.1 | 26.4 ± 4.3 | 0.231 |
| Peak work rate measured (W) | 88 (65; 116) | 90 (67; 116) | 86 (63; 117) | 0.325 |
| Peak VO2 (mL/min) | 1328 ± 464 | 1343 ± 446 | 1307 ± 485 | 0.219 |
| Peak VO2 (mL/min/kg) | 16.9 ± 5.2 | 17.0 ± 5.1 | 16.8 ± 5.3 | 0.462 |
| Peak VO2 predicted (%) | 63 ± 17 | 63 ± 17 | 63 ± 17 | 0.911 |
| Peak VCO2 (mL/min) | 1538 ± 536 | 1556 ± 516 | 1515 ± 561 | 0.212 |
| Peak RER | 1.14 (1.09; 1.20) | 1.14 (1.09; 1.20) | 1.14 (1.08; 1.21) | 0.574 |
| Peak VE (L/min) | 55 (44; 67) | 56 (46; 69) | 53.1 (42.9; 64.8) | 0.001 |
| Peak RR (breath/min) | 33 ± 7 | 33 ± 7 | 32 ± 7 | 0.149 |
| Peak PetO2 (mmHg) | 115.8 (112.1; 119.8) | 116.4 (112.4; 120.2) | 115.3 (111.5; 118.9) | 0.001 |
| Peak PetCO2 (mmHg) | 33.3 (29.4; 36.2) | 32.4 (28.7; 35.5) | 34.2 (30.9; 37.1) | 0.000 |
| VE/VCO2 slope | 30.0 ± 6.3 | 31.0 ± 6.3 | 29.6 ± 6.1 | 0.000 |
| VE/VCO2 slope predicted (%) | 115.8 ± 23 | 118.3 ± 23.2 | 112.4 ± 22.4 | 0.000 |
| VO2/work rate slope (mL/min/W) | 10.1 (9.1; 11.3) | 10.1 (9.1; 11.3) | 10.1 (9.1; 11.2) | 0.803 |
| MECKI score (%) | 3.51 (1.68; 8.05) | 3.65 (1.78; 8.74) | 3.31 (1.58; 7.31) | 0.089 |
| LVEF (%) | 34 ± 9 | 34 ± 9 | 35 ± 9 | 0.137 |
| Hb (g/dL) | 13.8 ± 1.6 | 13.9 ± 1.6 | 13.8 ± 1.7 | 0.214 |
| Na+ (mmol/L) | 139.3 ± 3.0 | 139.2 ± 3.2 | 139.4 ± 2.8 | 0.454 |
| eGFR (mL/min) | 70.9 ± 23.5 | 71.0 ± 23.4 | 71.5 ± 23.6 | 0.703 |
| BNP (pg/mL) | 209.5 (84; 477.5) | 212 (91; 470) | 206 (82; 495) | 0.879 |
BMI, body mass index; BNP, B‐type natriuretic peptide; DT, double threshold; eGFR, glomerular filtration rate by Modification of Diet in Renal Disease (MDRD) formula; Hb, haemoglobin; LVEF, left ventricular ejection fraction; MECKI, Metabolic Exercise test data combined with Cardiac and Kidney Indexes; Na+, serum sodium; PetCO2, end‐tidal carbon dioxide tension; PetO2, end‐tidal oxygen tension; RER, respiratory exchange ratio; RR, respiratory rate; VCO2, carbon dioxide output; VE, ventilation; VE/VCO2 slope %, slope of ventilation to carbon dioxide output as percentage of predicted value; VE/VCO2 slope, slope of ventilation to carbon dioxide output; VO2%, oxygen uptake as percentage of predicted value; VO2, oxygen uptake.
Data are presented as mean ± standard deviation and as median and interquartile range. Statistically significant values are highlighted in bold.
No significant differences were detected between DT+ and DT− subjects for age and peak exercise performance (peak work load and peak VO2). DT+ patients showed a lower VE/VCO2 slope, a lower peak exercise PetO2, and a higher peak exercise PetCO2. In DT+ subjects, vent AT always followed met AT. Vent AT showed a significant higher VO2, VCO2, ventilation, RER, PetO2, PetCO2, and VE/VO2, but lower VE/VCO2 compared with met AT (Figure 1 ). Median ΔVO2 between met AT and vent AT was 221 (129; 319) mL/min and 2.9 (1.8; 4.3) mL/kg/min. Median ΔWatt between the two ATs was 20 (14; 29) W. Of note, presence of DT+ was not different among the various degrees of exercise performance limitation.
Figure 1.

Graphical representation of cardiopulmonary test parameters at the anaerobic threshold calculated with the V‐slope method (met AT) and at the anaerobic threshold detected with the equivalent ventilatory method and the end‐tidal method (vent AT) in heart failure with reduced ejection fraction patients with double threshold using box and whiskers plot. PetCO2, end‐tidal carbon dioxide tension; PetO2, end‐tidal oxygen tension; RER, respiratory exchange ratio; VCO2, carbon dioxide output; VE, ventilation; VE/VCO2, ventilatory equivalent for carbon dioxide; VE/VO2, ventilatory equivalent for oxygen; VO2, oxygen uptake.
As regards prognosis in the entire population, the median follow‐up was 1019 (361; 2437) days. The MECKI score between DT+ and DT− patients was not different [3.65 (1.78; 8.74) and 3.31 (1.58; 7.31), P = 0.089]. At 2 year follow‐up, a total of 63 events was observed, of which 38% in DT+ subjects. Considering 2 year survival by Kaplan–Meier analysis, DT was not associated with a worse survival, even after adjustment for age, sex, Hb, LVEF, kidney function, and peak VO2 (considered as a percentage of predicted) (Figure 2 ).
Figure 2.
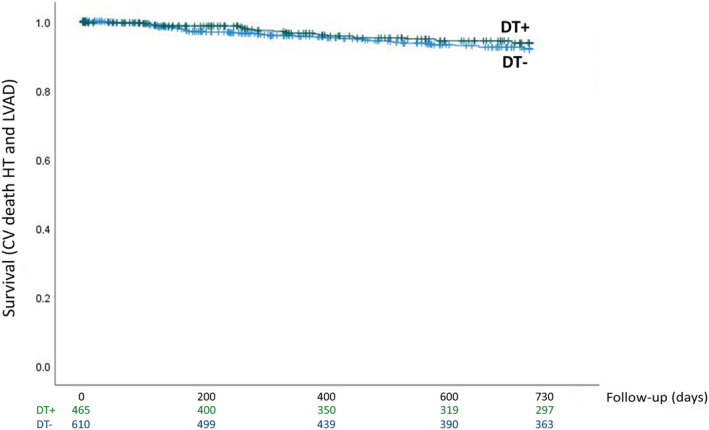
Kaplan–Meier survival curves of study endpoint [cardiovascular (CV) death, urgent heart transplant (HT), or left ventricular assist device (LVAD) implantation] according to the presence or absence of double threshold (DT+ and DT−) at 2 year follow‐up (P = 0.322; χ 2 = 0.979). Kaplan–Meier survival curves were adjusted for age, sex, haemoglobin, left ventricular ejection fraction, kidney function, and peak VO2.
Discussion
This study shows for the first time that a significant percentage, that is, 42%, of patients with HF have DT. The analysed population that belongs to the MECKI score data set consists of HF patients with reduced LVEF in stable medical condition. In this setting, DT was unrelated to exercise performance limitation but it was more frequent than in healthy subjects, which was identified in 11% of cases. 11 Additionally, there was a gender difference: specifically, in HF patients, DT is more frequent in women than in men, being 51% and 42%, respectively. This is different from that observed in healthy subjects, where DT was present in a similar percentage in both sexes, respectively, 10% of women and 11% of men. 11
As in normal subjects, vent AT always followed met AT. The gap between met AT and vent AT is significant both considering VO2 and Watts, respectively, 221 (129; 319) mL/min and 20 (14; 29) W. Of note, due to the intrinsic variability of VO2 changes during exercise in HF patients, to define DT, we utilized a minimum ∆VO2 100 mL/min between met AT and vent AT. It is recognized that this cut‐off value is completely arbitrary and that a smaller one, as that applied in normal subjects (∆VO2 15 mL), would likely increase the percentage of DT+ cases. However, with the 100 mL ∆VO2 between met AT and vent AT, we are confident that all the erroneous grouping due to the intrinsic VO2 recording variability was eliminated.
Albeit presence of DT did not affect peak exercise performance, in DT+ patients' peak exercise is characterized by a lower ventilation and PetO2 but higher PetCO2. Of note, DT+ patients had a lower VE/VCO2 slope. Altogether, these data suggest a reduced ventilatory response in DT+ patients compared with DT−. This feature is also confirmed by the comparison between met AT and vent AT. Indeed, between met AT and vent AT, VO2, VCO2, end‐tidal tensions, ventilation, RER, and VE/VO2 increase, while VE/VCO2 decreases, as if ventilation was not affected by the extra VCO2 produced by the buffering of the anaerobic metabolism. This finding is in line with our original hypothesis that DT is associated to a reduced chemoreflex activity. 11 In fact, as previously hypothesized in normal subjects, the delayed ventilatory response to exercise‐induced acidosis could be linked to a reduced chemoreflex activity albeit, in HF patients, also a greater circulatory time could play a role. Of note, as regards in reflex‐mediated regulation of ventilation, the chemoreflexes are likely to play a major role. We cannot exclude, however, that other reflexes such as metaboreflexes or lung stretch receptors have a role in DT genesis. Irrespectively of the causes, the ventilatory response to the extra CO2 production present above met AT is clearly delayed. 11 Of note, we recently observed that DT is affected by beta‐blocker therapy being more frequently observed during Carvedilol treatment than Bisoprolol 17 and Carvedilol reduces the chemoreflex response. 18 According to the hypothesis that DT+ is associated to a lower chemoreflex activity and thus, likely, to a lower overall sympathetic activation, we were keen to suggest that DT+ patients have a more favourable prognosis. However, the presence of DT is not associated with a better prognosis as shown by the 2 year Kaplan–Meier survival curves, after adjustment for some known important confounders such as age, sex, Hb, LVEF, and kidney function. However, the MECKI score presented a not significant trend vs. a more favourable prognosis in DT+ patients (P = 0.089), which needs to be confirmed by larger studies. Indeed, in order to evaluate the effective prognostic significance of DT, this is a preliminary study, albeit performed in a vast HF population, that paves the way for future research exploring larger populations and specific settings such as non‐ischaemic vs. ischaemic HF patients, either as HF with preserved ejection fraction (HFpEF) vs. HF with reduced ejection fraction (HFrEF) patients or as different treatment regimen.
In clinical practice, AT is measured by the V‐slope method and confirmed by ventilatory equivalent and end‐tidal methods. In the present study, we showed that in a relevant percentage of HF patients, DT is observed. It is questionable whether in case of DT only one AT value or both met AT and vent AT should be reported. We suggest to state in the CEPT report the presence of DT and report both met AT and vent AT albeit, as regards clinical use of AT, we believe that met AT should be considered.
Our study has some limitations, which need to be acknowledged. To begin with, this is a retrospective study performed in two expert centres in terms of HF patients' evaluation and follow‐up. Second, repeatability of DT, as well as day‐by‐day variability, was not assessable in the present setting. Third, we considered solely HF patients with history of a reduced LVEF, so we do not know if DT is present in different HF population phenotypes such as patients with preserved ejection fraction or valvular heart disease, or it is affected by treatment. Ultimately, it is acknowledged that a relevant number of patients, 896 out of 1971, were excluded because tests were interrupted before AT or because of EOV or respiratory disorders that impeded a proper AT definition. Albeit this selection was unavoidable, it represents a potential study bias.
In conclusion, DT is present in a considerable number of HF patients and it is more frequent in women. DT is not associated with reduced performance, but only with reduced ventilatory response throughout exercise. Its presence is likely associated to an overall reduced chemoreflex activity, that is, with a prolonged circulatory time or to a true reduced chemoreflex activity, or both, but it has no evident prognostic value. However, DT in specific HF settings needs to be carefully evaluated in the next future.
Conflict of interest
Nothing to declare.
Funding
None.
Rovai, S. , Zaffalon, D. , Cittar, M. , Felli, L. F. , Salvioni, E. , Galotta, A. , Mattavelli, I. , Carriere, C. , Mapelli, M. , Merlo, M. , Vignati, C. , Sinagra, G. , and Agostoni, P. (2022) The double anaerobic threshold in heart failure: MECKI score database overview. ESC Heart Failure, 9: 2119–2124. 10.1002/ehf2.13920.
References
- 1. Paolillo S, Agostoni P. Prognostic role of cardiopulmonary exercise testing in clinical practice. Ann Am Thorac Soc. 2017; 14: S53–S58. [DOI] [PubMed] [Google Scholar]
- 2. Corrà U, Piepoli MF, Adamopoulos S, Agostoni P, Coats AJS, Conraads V, Lambrinou E, Pieske B, Piotrowicz E, Schmid JP, Seferović PM, Anker SD, Filippatos G, Ponikowski PP. Cardiopulmonary exercise testing in systolic heart failure in 2014: the evolving prognostic role: a position paper from the committee on exercise physiology and training of the heart failure association of the ESC. Eur J Heart Fail. 2014; 16: 929–941. [DOI] [PubMed] [Google Scholar]
- 3. Mezzani A, Agostoni P, Cohen‐Solal A, Corrà U, Jegier A, Kouidi E, Mazic S, Meurin P, Piepoli M, Simon A, Laethem CV, Vanhees L. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: a report from the Exercise Physiology Section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2009; 16: 249–267. [DOI] [PubMed] [Google Scholar]
- 4. Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999; 116: 355–362. [DOI] [PubMed] [Google Scholar]
- 5. Levett DZ, Grocott MP. Cardiopulmonary exercise testing for risk prediction in major abdominal surgery. Anesthesiol Clin. 2015; 33: 1–16. [DOI] [PubMed] [Google Scholar]
- 6. Carriere C, Corrà U, Piepoli M, Bonomi A, Merlo M, Barbieri S, Salvioni E, Binno S, Mapelli M, Righini F, Sciomer S, Vignati C, Moscucci F, Veglia F, Sinagra G, Agostoni P. Anaerobic threshold and respiratory compensation point identification during cardiopulmonary exercise tests in chronic heart failure. Chest. 2019; 156: 338–347. [DOI] [PubMed] [Google Scholar]
- 7. Agostoni P, Corrà U, Cattadori G, Veglia F, Battaia E, la Gioia R, Scardovi AB, Emdin M, Metra M, Sinagra G, Limongelli G, Raimondo R, Re F, Guazzi M, Belardinelli R, Parati G, Magrì D, Fiorentini C, Cicoira M, Salvioni E, Giovannardi M, Mezzani A, Scrutinio D, di Lenarda A, Mantegazza V, Ricci R, Apostolo A, Iorio A, Paolillo S, Palermo P, Contini M, Vassanelli C, Passino C, Piepoli MF, MECKI Score Research Group . Prognostic value of indeterminable anaerobic threshold in heart failure. Circ Heart Fail. 2013; 6: 977–987. [DOI] [PubMed] [Google Scholar]
- 8. Agostoni P, Dumitrescu D. How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int J Cardiol. 2019; 288: 107–113. [DOI] [PubMed] [Google Scholar]
- 9. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985). 1986; 60: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 10. Sietsema K. E., Stringer W. W., Sue D. Y., Ward S., eds. Wasserman & Whipp's Principles of Exercise Testing and Interpretation, 6th ed. Philadelphia: Wolters‐Kluwer; 2020. [Google Scholar]
- 11. Rovai S, Magini A, Cittar M, Carriere C, Contini M, Vignati C, Sinagra G, Agostoni P, Agostoni P. Evidence of a double anaerobic threshold in healthy subjects. Eur J Prev Cardiol. 2021. [DOI] [PubMed] [Google Scholar]
- 12. Whipp BJ, Agostoni P. Noninvasive estimation of the lactate threshold in a subject with dissociated ventilatory and pulmonary gas exchange indices: a case report. Chest. 2007; 132: 1994–1997. [DOI] [PubMed] [Google Scholar]
- 13. Agostoni P, Corrà U, Cattadori G, Veglia F, la Gioia R, Scardovi AB, Emdin M, Metra M, Sinagra G, Limongelli G, Raimondo R, Re F, Guazzi M, Belardinelli R, Parati G, Magrì D, Fiorentini C, Mezzani A, Salvioni E, Scrutinio D, Ricci R, Bettari L, di Lenarda A, Pastormerlo LE, Pacileo G, Vaninetti R, Apostolo A, Iorio A, Paolillo S, Palermo P, Contini M, Confalonieri M, Giannuzzi P, Passantino A, Cas LD, Piepoli MF, Passino C, MECKI Score Research Group . Metabolic Exercise test data combined with Cardiac and Kidney Indexes, the MECKI score: a multiparametric approach to heart failure prognosis. Int J Cardiol. 2013; 167: 2710–2718. [DOI] [PubMed] [Google Scholar]
- 14. Agostoni P, Paolillo S, Mapelli M, Gentile P, Salvioni E, Veglia F, Bonomi A, Corrà U, Lagioia R, Limongelli G, Sinagra G, Cattadori G, Scardovi AB, Metra M, Carubelli V, Scrutinio D, Raimondo R, Emdin M, Piepoli M, Magrì D, Parati G, Caravita S, Re F, Cicoira M, Minà C, Correale M, Frigerio M, Bussotti M, Oliva F, Battaia E, Belardinelli R, Mezzani A, Pastormerlo L, Guazzi M, Badagliacca R, di Lenarda A, Passino C, Sciomer S, Zambon E, Pacileo G, Ricci R, Apostolo A, Palermo P, Contini M, Clemenza F, Marchese G, Gargiulo P, Binno S, Lombardi C, Passantino A, Filardi PP. Multiparametric prognostic scores in chronic heart failure with reduced ejection fraction: a long‐term comparison. Eur J Heart Fail. 2018; 20: 700–710. [DOI] [PubMed] [Google Scholar]
- 15. Agostoni P, Bianchi M, Moraschi A, Palermo P, Cattadori G, la Gioia R, Bussotti M, Wasserman K. Work‐rate affects cardiopulmonary exercise test results in heart failure. Eur J Heart Fail. 2005; 7: 498–504. [DOI] [PubMed] [Google Scholar]
- 16. Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984; 129: S49–S55. [DOI] [PubMed] [Google Scholar]
- 17. Rovai S, Contini M, Sciomer S, Vignati C, Agostoni P. The double anaerobic threshold in heart failure. Int J Cardiol. 2022, in press; 353: 68–70. [DOI] [PubMed] [Google Scholar]
- 18. Contini M, Apostolo A, Cattadori G, Paolillo S, Iorio A, Bertella E, Salvioni E, Alimento M, Farina S, Palermo P, Loguercio M, Mantegazza V, Karsten M, Sciomer S, Magrì D, Fiorentini C, Agostoni P. Multiparametric comparison of CARvedilol, vs. NEbivolol, vs. BIsoprolol in moderate heart failure: the CARNEBI trial. Int J Cardiol. 2013; 168: 2134–2140. [DOI] [PubMed] [Google Scholar]


