Abstract
Aims
This study aimed to examine the prognostic significance of a history of cancer and atrial fibrillation (AF) in antithrombotic therapy for patients with chronic heart failure (CHF).
Methods and results
We enrolled consecutive 4876 CHF patients (69 ± 12 years; women, 31.9%) in our multicentre, hospital‐based cohort study, the Chronic Heart Failure Analysis and Registry in the Tohoku District‐2 (CHART‐2), with a median follow‐up of 8.7 years. Among them, 14% and 41% had a history of cancer and AF, respectively. AF patients with a history of cancer were older, more frequently men. History of cancer was not statistically associated with higher rate of composite of stroke, systemic thrombosis, and major bleeding defined by International Society on Thrombosis and Haemostasis [Fine–Gray sub‐distribution hazard ratio (sHR) accounting for the competing risk of all‐cause death, 0.91; 95% confidence interval (CI), 0.56–1.48; P = 0.715]. The patients with history of cancer and AF had a heightened risk for the composite of stroke, systemic thrombosis, and major bleeding (sHR, 1.64; 95% CI, 1.04–2.60; P = 0.033), especially in those aged >75 years (sHR, 2.14; 95% CI, 1.01–4.53; P = 0.046) and those with ischaemic heart disease (IHD; 2.48; 1.30–4.72; P = 0.006). Furthermore, 36% of AF patients with a history of cancer did not receive anticoagulant therapy.
Conclusions
The CHF patients with history of cancer and AF had higher risk for stroke, systemic thrombosis, and major bleeding, especially in the elderly and those with IHD, but considerable number of the patients did not receive anticoagulant therapy, indicating the need for better optimal anticoagulation strategy.
Keywords: Heart failure, Cancer, Atrial fibrillation, Anticoagulant, Antiplatelet
Introduction
Cardiovascular disease and cancer are leading causes of death worldwide. 1 Both disorders share the common risk factors and pathophysiology that activate neurohumoral pathway, leading to the development and progression of cancer. 2 Previous studies showed that patients with chronic heart failure (CHF) have an increased risk of cancer 3 and cancer death 4 and conversely that a history of cancer is associated with increased risk of HF hospitalization, all‐cause death, and cardiac death in HF patients. 1 Furthermore, in both cancer and HF, atrial fibrillation (AF) is frequently associated with these disorders (14% in cancer and 4–50% in HF), suggesting an overlap in the pathophysiological processes between the two disorders. 2 , 3 For the treatment of AF, anticoagulation is recommended to reduce the risk of stroke and mortality. 4 However, the benefits of antithrombotic therapy are occasionally offset by bleeding complications, especially when patients with AF are taking antiplatelets for other comorbidities, such as coronary artery disease, peripheral artery disease, and internal carotid artery stenosis. 5 , 6 Furthermore, limited data exist regarding the predictive value of a history of cancer in HF patients with AF, particularly among those with anticoagulants and/or antiplatelets at high risk for bleeding.
In the present study, we thus aimed to examine the prognostic impact of a history of cancer and AF for stroke, thrombosis, and bleeding according to the use of anticoagulants and antiplatelets in the large‐scale, hospital‐based, prospective CHF cohort, named the Chronic Heart Failure Analysis and Registry in the Tohoku District‐2 (CHART‐2) Study. 7 , 8 , 9 , 10 , 11
Methods
CHART‐2 Study
The CHART‐2 Study is a hospital‐based prospective observational study with 23 hospitals in 6 prefectures in Japan. The design and methods have been previously described in detail. 7 In brief, from October 2006 and March 2010, we enrolled consecutive patients older than 20 years with significant coronary artery disease and those in Stage B (structural heart disease but without signs or symptoms of HF), Stage C (structural heart disease with earlier or current symptoms of HF), and Stage D enumerated by the professional guidelines. 12 , 13 Subjects in Stage B must meet at least one of the following structural disorders and must not have signs, symptoms, or history of hospitalization for heart failure: (i) enlarged left ventricular end‐diastolic dimension (≥55 mm) measured on echocardiography, (ii) impaired left ventricular ejection fraction (≤50%) measured on echocardiography, (iii) thickened interventricular septum (>12 mm) and/or thickened left ventricular posterior wall (>12 mm) measured on echocardiography, (iv) significant valvular stenosis/insufficiency, (v) significant myocardial abnormalities, (vi) congenital abnormalities, and (vii) previous cardiac surgery. In Stage C, the diagnosis of HF was made by attending cardiologists based on the criteria of the Framingham study. 14 Enrolment began in October 2006 and ended in March 2010. All information, including medical history, laboratory data, and echocardiography data, were recorded in a computer database at the time of enrolment. Annual follow‐up was made by clinical research coordinators by means of review of medical records, surveys, and telephone interviews. The present study conformed to the Declaration of Helsinki. The study protocol was approved by institutional review boards at each field centre, and all participants provided written informed consent. 11
History of cancer
Experienced clinical research coordinators of the CHART‐2 Study collected baseline data from the hospital databases. History of cancer was documented at baseline assessment as part of medical history. Baseline medications were reviewed to identify patients receiving cancer treatment with hormonal or chemotherapeutic agents. These patients were defined as actively treated cancer and were excluded from the group of a history of cancer. No additional information on cancer stage or treatment was collected.
Outcomes
The primary outcome was composite of stroke, thrombosis, and major bleeding. Stroke was defined as a loss of neurological function caused by an ischaemic or haemorrhagic event with residual symptoms at least 24 h after onset based on the imaging (computed tomographic scan or magnetic resonance imaging). 15 Major bleeding was defined by the International Society on Thrombosis and Haemostasis criteria. 16
All events were reviewed and adjudicated by consensus of three independent physicians, the members of the Tohoku Heart Failure Association. They reviewed case reports, death certificates, medical records, and summaries provided by the investigators.
Statistical methods
Clinical characteristics are described by a history of cancer and AF. We then evaluated the association of a history of cancer and AF, and a composite of stroke, systemic thrombosis, and major bleeding according to antithrombotic therapy, using Kaplan–Meier curves with log‐rank tests. Based on literature search, we considered the following variables as the potential confounders: age, sex, left ventricular ejection fraction, New York Heart Association (NYHA) functional class, systolic blood pressure, heart rate, body mass index, serum sodium, smoking status, aetiology of ischaemic heart disease (IHD), diabetes, history of previous HF hospitalization, history of stroke, brain natriuretic peptide (BNP), and estimated glomerular filtration rate. 17 , 18 Then, we tested for inclusion in the regression models by stepwise selection with significance level at <0.05. When assessing prognostic impacts of a history of cancer for stroke, systemic thrombosis, and major bleeding, we employed Fine–Gray competing risk model considering all‐cause death as the competing risk. 19 Differences in the incidence rate of the composite of stroke, systemic thrombosis, and major bleeding stratified by a history of cancer and AF according to antithrombotic therapy were tested by median‐unbiased estimation (Mid‐P) method. 20 A two‐sided P value of <0.05 was considered to be statistically significant. All analyses were performed with STATA 17 (College Station, TX, USA).
Results
Baseline patient characteristics
Mean age of the 4876 study participants was 67 ± 13 years and 32% were women (Table 1 ). Among them, 14% and 41% had a history of cancer and AF, respectively. The type of cancer was as follows: colorectum (N = 141, 21%), stomach (N = 139, 21%), prostate (N = 75, 11%), breast (N = 48, 7%), lung (N = 40, 6%), oesophagus (N = 19, 3%), cervix uteri and body (N = 17, 3%), liver (N = 16, 2%), pancreas (N = 14, 2%), other cancer (N = 156, 23%), and unknown (N = 3, 1%). Median BNP level was 104.0 [interquartile range (IQR) 41.3, 239.0] pg/mL. History of HF and stroke was noted in 53% and 20.5% of overall subject, respectively. AF patients with a history of cancer were older, more frequently men, and more likely to have higher BNP levels (Table 1 ). Among the four categories of patients by a history of cancer and AF, patients with both of them had a highest prevalence of history of HF hospitalization compared with other three groups (Table 1 ).
Table 1.
Baseline patient characteristics stratified by history of cancer and atrial fibrillation
| Total | W/o Hx cancer and w/o AF | W/o Hx cancer and w/ AF | W/ Hx cancer and w/o AF | W/ Hx cancer and w/ AF | P value | |
|---|---|---|---|---|---|---|
| n = 4876 | n = 2496 | n = 1709 | n = 375 | n = 296 | ||
| Age, years | 69.0 ± 12.3 | 66.7 ± 13.3 | 70.2 ± 11.0 | 73.7 ± 9.5 | 75.3 ± 7.6 | <0.001 |
| Women, n (%) | 1556 (31.9%) | 775 (31.0%) | 596 (34.9%) | 108 (28.8%) | 77 (26.0%) | 0.003 |
| Systolic BP, mmHg | 126.2 ± 19.1 | 127.6 ± 19.1 | 124.4 ± 18.9 | 127.0 ± 20.1 | 123.1 ± 18.7 | <0.001 |
| Diastolic BP, mmHg | 72.2 ± 11.9 | 72.6 ± 11.7 | 72.1 ± 12.1 | 70.8 ± 12.1 | 70.0 ± 12.4 | <0.001 |
| Heart rate, b.p.m. | 72.4 ± 14.9 | 70.8 ± 13.4 | 74.5 ± 16.7 | 70.9 ± 12.1 | 74.6 ± 16.2 | <0.001 |
| Type of AF | <0.001 | |||||
| Paroxysmal | 651 (13.4%) | 0 | 556 (32.5%) | 0 | 95 (32.1%) | |
| Persistent | 1354 (27.8%) | 0 | 1153 (67.5%) | 0 | 201 (67.9%) | |
| Body mass index, kg/m2 | 23.8 ± 3.9 | 24.2 ± 3.9 | 23.5 ± 3.8 | 22.8 ± 3.8 | 22.9 ± 3.2 | <0.001 |
| NYHA, n (%) | <0.001 | |||||
| I | 1153 (23.8%) | 670 (27.0%) | 325 (19.1%) | 105 (28.2%) | 53 (17.9%) | |
| II | 3169 (65.3%) | 1568 (63.2%) | 1176 (69.0%) | 224 (60.1%) | 201 (67.9%) | |
| III | 494 (10.2%) | 226 (9.1%) | 189 (11.1%) | 38 (10.2%) | 41 (13.9%) | |
| IV | 38 (0.8%) | 16 (0.6%) | 15 (0.9%) | 6 (1.6%) | 1 (0.3%) | |
| Hypertension, n (%) | 4406 (90.4%) | 2271 (91.0%) | 1532 (89.6%) | 332 (88.5%) | 271 (91.6%) | 0.26 |
| Diabetes, n (%) | 1970 (40.4%) | 1086 (43.5%) | 605 (35.4%) | 147 (39.2%) | 132 (44.6%) | <0.001 |
| Dyslipidaemia, n (%) | 4014 (82.3%) | 2180 (87.3%) | 1308 (76.5%) | 310 (82.7%) | 216 (73.0%) | <0.001 |
| History of HF hospitalization, N (%) | 2586 (53.0%) | 1104 (44.2%) | 1122 (65.7%) | 161 (42.9%) | 199 (67.2%) | <0.001 |
| History of stroke, n (%) | 1001 (20.5%) | 423 (16.9%) | 431 (25.2%) | 78 (20.8%) | 69 (23.3%) | <0.001 |
| Ischaemic heart disease, n (%) | 2479 (50.8%) | 1578 (63.2%) | 541 (31.7%) | 258 (68.8%) | 102 (34.5%) | <0.001 |
| CHADS2 score | <0.001 | |||||
| 1 | 236 (4.8%) | 121 (4.8%) | 89 (5.2%) | 21 (5.6%) | 5 (1.7%) | |
| 2 | 1532 (31.4%) | 843 (33.8%) | 536 (31.4%) | 84 (22.4%) | 69 (23.3%) | |
| 3 | 1832 (37.6%) | 974 (39.0%) | 577 (33.8%) | 164 (43.7%) | 117 (39.5%) | |
| 4 | 658 (13.5%) | 287 (11.5%) | 261 (15.3%) | 51 (13.6%) | 59 (19.9%) | |
| 5 | 441 (9.0%) | 194 (7.8%) | 185 (10.8%) | 37 (9.9%) | 25 (8.4%) | |
| 6 | 177 (3.6%) | 77 (3.1%) | 61 (3.6%) | 18 (4.8%) | 21 (7.1%) | |
| Previous MI, n (%) | 1699 (34.8%) | 1157 (46.4%) | 314 (18.4%) | 175 (46.7%) | 53 (17.9%) | <0.001 |
| PCI, n (%) | 1568 (32.2%) | 1083 (43.4%) | 263 (15.4%) | 166 (44.3%) | 56 (18.9%) | <0.001 |
| CABG, n (%) | 440 (9.0%) | 286 (11.5%) | 94 (5.5%) | 49 (13.1%) | 11 (3.7%) | <0.001 |
| PMI, n (%) | 386 (7.9%) | 110 (4.4%) | 213 (12.5%) | 31 (8.3%) | 32 (10.8%) | <0.001 |
| Valve surgery, n (%) | 422 (8.7%) | 130 (5.2%) | 246 (14.4%) | 15 (4.0%) | 31 (10.5%) | <0.001 |
| Haemoglobin, g/dL | 13.2 ± 2.0 | 13.3 ± 1.9 | 13.3 ± 2.1 | 12.3 ± 1.9 | 12.6 ± 2.0 | <0.001 |
| eGFR, mL/min/1.73 m2 | 60.7 ± 21.3 | 62.5 ± 22.1 | 59.6 ± 19.8 | 57.9 ± 22.1 | 55.4 ± 20.3 | <0.001 |
| LVEF, % | 56.6 ± 15.3 | 56.4 ± 15.7 | 56.6 ± 14.8 | 57.3 ± 15.8 | 58.1 ± 14.3 | 0.22 |
| Classification by LVEF | <0.001 | |||||
| HFrEF (LVEF < 40%) | 730 (21.9%) | 392 (26.6%) | 249 (18.1%) | 55 (22.7%) | 34 (14.1%) | |
| HFmEF (LVEF 40–49%) | 556 (16.7%) | 261 (17.7%) | 225 (16.3%) | 44 (18.2%) | 26 (10.8%) | |
| HFpEF (LVEF > 50%) | 2052 (61.5%) | 823 (55.8%) | 905 (65.6%) | 143 (59.1%) | 181 (75.1%) | |
| LVDd, mm | 52.1 ± 9.2 | 52.4 ± 9.4 | 51.8 ± 9.2 | 51.3 ± 9.0 | 52.1 ± 8.1 | 0.1 |
| BNP, pg/mL | 104.0 [41.3, 239.0] | 68.3 [27.0, 180.0] | 144.0 [73.8, 273.0] | 104.0 [37.8, 267.0] | 190.8 [91.6, 333.5] | <0.001 |
| PT‐INR a | 1.7[1.5, 2.1] | 1.7[1.3, 2.1] | 1.8 [1.5, 2.2] | 1.6 [1.3, 2.1] | 1.8 [1.4, 2.1] | <0.001 |
| Beta‐blocker, n (%) | 2402 (49.3%) | 1230 (49.3%) | 876 (51.3%) | 159 (42.4%) | 137 (46.3%) | 0.013 |
| RASI, n (%) | 3591 (73.6%) | 1865 (74.7%) | 1237 (72.4%) | 270 (72.0%) | 219 (74.0%) | 0.33 |
| Ca blocker, n (%) | 1889 (38.7%) | 1045 (41.9%) | 573 (33.5%) | 158 (42.1%) | 113 (38.2%) | <0.001 |
| Diuretics, n (%) | 2787 (57.2%) | 1197 (48.0%) | 1191 (69.7%) | 191 (50.9%) | 208 (70.3%) | <0.001 |
| Statin, n (%) | 1866 (38.3%) | 1203 (48.2%) | 438 (25.6%) | 157 (41.9%) | 68 (23.0%) | <0.001 |
| Anticoagulants, n (%) | 1894 (38.8%) | 491 (19.7%) | 1151 (67.3%) | 64 (17.1%) | 188 (63.5%) | <0.001 |
| Antiplatelets, n (%) | 2969 (60.9%) | 1663 (66.6%) | 909 (53.2%) | 252 (67.2%) | 145 (49.0%) | <0.001 |
AF, atrial fibrillation; BNP, brain natriuretic peptide; BP, blood pressure; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; HFmEF, heart failure with mildly reduced ejection fraction; HFpEF heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVDd, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PCI, Percutaneous coronary intervention; PMI, pacemaker implantation; PT‐INR, prothrombin time and international normalized ratio; RASI, renin‐angiotensin system inhibitor.
PT‐INR was obtained in 491, 1151, 64, and 188 cases with warfarin in w/o Hx cancer and w/o AF, w/o Hx cancer and w/ AF, w/ Hx cancer and w/o AF, and w/ Hx cancer and w/ AF, respectively.
Association between history of cancer and atrial fibrillation in long‐term prognosis in chronic heart failure patients
Overall, patients with AF had a higher event rate for the composite of stroke, systemic thrombosis, and major bleeding (Figure 1 and Supporting Information, Table S1 ). When a history of cancer and AF coexisted, patients had the highest event rate (Figure 1A ), mainly driven by stroke (Figure 1B ) or bleeding (Figure 1C ). Patients with AF also had higher event rates for all‐cause mortality and highest event rate when associated with a history of cancer (Supporting Information, Figure S1A ). Consistent findings were also noted for cardiovascular death (Supporting Information, Figure S1B ), cancer death (Supporting Information, Figure S1C ), and the composite of myocardial infarction, stroke, systemic thrombosis, and HF hospitalization (Supporting Information, Figure S1D ). Of note, patients with AF but without a history of cancer had a similar event rate of cancer death compared with those without AF but without a history of cancer (Supporting Information, Figure S1C ).
Figure 1.
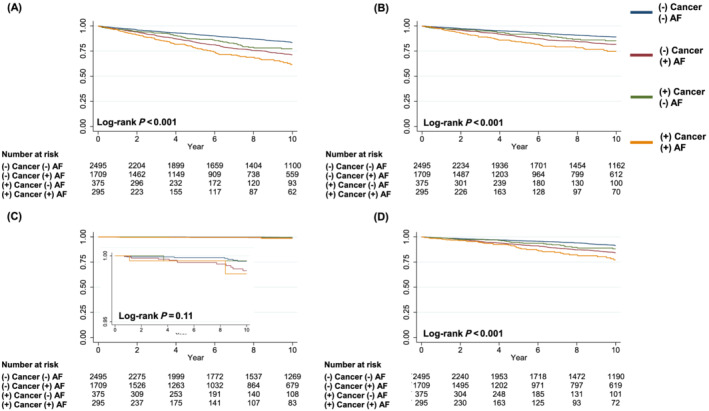
Kaplan–Meier curves representing association of AF and a history of cancer with (A) composite of stroke, systemic thrombosis, and major bleeding, (B) stroke, (C) systemic thrombosis, and (D) major bleeding. AF, atrial fibrillation.
After adjusting for age, sex, NYHA functional class, BNP, heart rate, haemoglobin, history of stroke, and IHD, considering all‐cause death as a competing risk, history of cancer was not associated with an increased risk of the composite of stroke, systemic thrombosis, and major bleeding [sub‐distribution hazard ratio (sHR), 0.94; 95% confidence interval (CI), 0.55–1.62; P = 0.823] (Figure 2 ). The patients with history of cancer and AF had a heightened risk for the composite outcome (sHR, 1.64; 95% CI, 1.00–2.69; P = 0.049) (Figure 2 ). When using the Cox regression model, we observed similar trend between history of cancer, AF, and the composite outcome. These observations were evident especially in patients aged >75 years (sHR, 2.65; 95% CI, 1.25–5.63; P = 0.011 in those with history of cancer and AF) and those with IHD (2.42; 1.17–5.00; P = 0.018).
Figure 2.
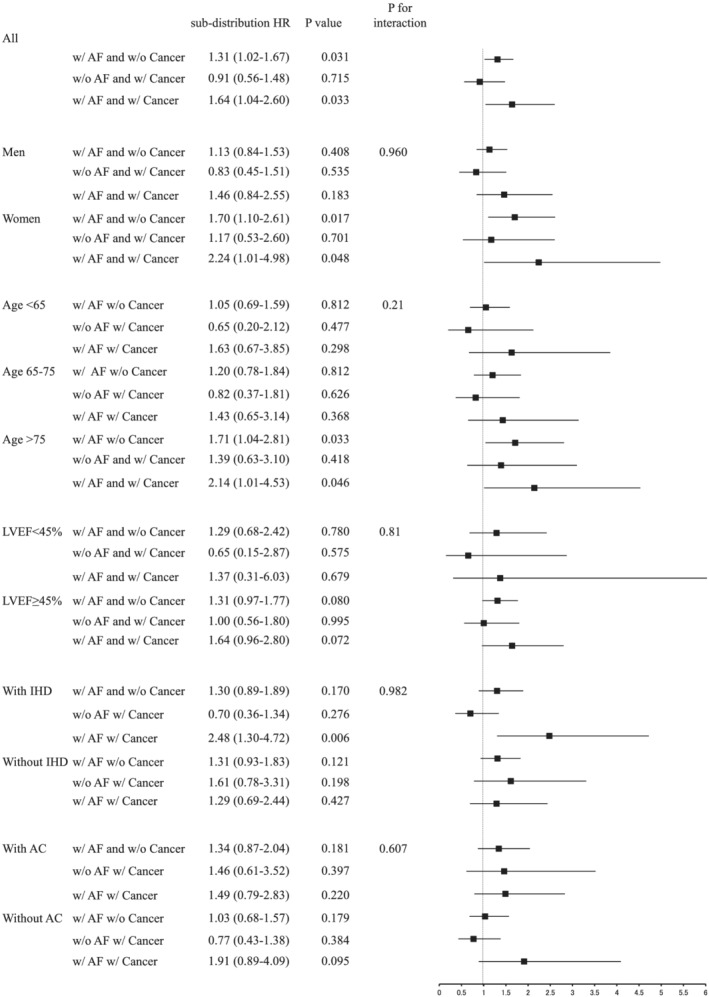
Association of a history of cancer and AF with composite of systemic thrombosis and major bleeding in overall and subgroups. Considering all‐cause death as the competing risk, the Fine and Gray model was adjusted for age, sex, New York Heart Association functional class, brain natriuretic peptide, heart rate, haemoglobin, history of stroke, and ischaemic heart disease (IHD). AC, anticoagulant; AF, atrial fibrillation; HR, hazard ratio; LVEF, left ventricular ejection fraction.
Antithrombotic therapy and the risks of stroke, thrombosis, and bleeding in chronic heart failure patients
In overall population, 38.8% and 60.9% received anticoagulant and antiplatelet, respectively, and median prothrombin time and international normalized ratio (PT‐INR) level was 1.7 [IQR 1.5, 2.1]. Between the AF patients with and without a history of cancer, median [IQR] PT‐INR levels were 1.8 [1.5, 2.2] and 1.8 [1.4, 2.1], respectively (Table 1 ). Of note, 36% of the AF patients with a history of cancer (n = 296) and 33% of those without a history of cancer (n = 1709) did not receive anticoagulant (Table 1 ). When stratifying the AF patients by a history of cancer, anticoagulant and/or antiplatelet use, median PT‐INR [IQR] levels were similar: 1.8 [1.5, 2.2] in (−) cancer/(+) AF/(+) anticoagulant/(−) antiplatelet, 1.8 [1.5, 2.1] in (−) cancer/(+) AF/(+) anticoagulant/(+) antiplatelet, 1.8 [1.4, 2.1] in (+) cancer/(+) AF/(+) anticoagulant/(−) antiplatelet, and 1.8 [1.4, 2.2] in (+) cancer/(+) AF/(+) anticoagulant/(+) antiplatelet, P = 0.77, among four groups (Supporting Information, Table S1 ).
In AF patients without a history of cancer, those with anticoagulant therapy had a significantly lower event rate for the composite of stroke, systemic thrombosis, and major bleeding compared with those with both anticoagulant and antiplatelet (28.9 vs. 38.7/1000 person‐years: P = 0.02) (Figure 3 ). In AF patients with a history of cancer, anticoagulant monotherapy had also a lowest composite event rate compared with those with both therapy (37.1 vs. 58.3/1000 person‐years; P = 0.13). Notably, in AF patients with a history of cancer, patients without anticoagulant nor antiplatelet had a higher event rate (64.2/1000 person‐years). When assessing the risk of bleeding in cancer patients with anticoagulant, history of cancer and AF was associated with heightened risk of bleeding (sHR, 1.76; 95% CI, 1.11–2.77; P = 0.015) (Supporting Information, Table S3 ).
Figure 3.
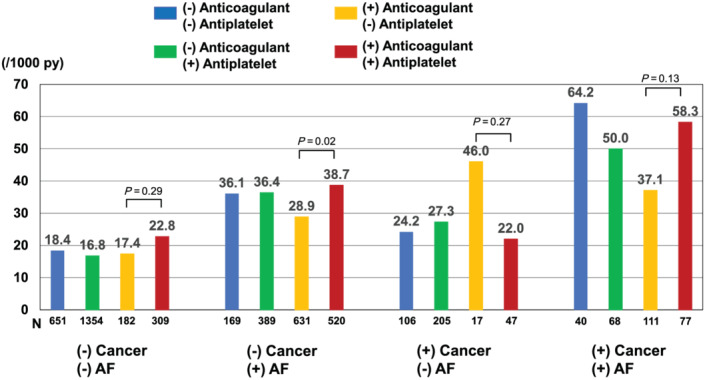
Event rates (/1000 person‐years) for the composite of stroke, systemic thrombosis, and major bleeding stratified by history of cancer and AF according to antithrombotic therapy. The rate differences were tested by median‐unbiased estimation method. AF, atrial fibrillation.
Discussion
The present study is one of the largest studies with long‐term prognosis to assess the prognostic significance of a history of cancer and AF in CHF patients, providing the important novel insights into a history of cancer as a predictor, and its relationship to anticoagulants and antiplatelets for clinical events.
We here report two novel findings. First, a history of cancer and AF increase the risk for the composite of stroke, systemic thrombosis, and major bleeding even after adjusting for potential confounders in CHF patients, especially in the elderly and those with IHD. Second, 36% of AF patients with a history of cancer did not receive anticoagulant therapy even though they had lower composite event rate compared with those who received it.
Heart failure and AF are closely inter‐related with similar risk factors and shared pathophysiology. Patients with concomitant HF and AF suffer from even worse symptoms and poorer prognosis, as we previously reported. 21 Furthermore, recent studies acknowledged emerging evidence on the association between AF and malignant cancers. 22 , 23 Indeed, patients with cancer are also at increased risk of developing AF, suggesting that AF and cancer may interact with each other on pathophysiological grounds. Cancer‐related inflammation, anti‐cancer treatment, and other cancer‐related comorbidities are proposed to affect atrial remodelling, increasing the susceptibility of cancer patients for developing AF. Previous registry study in Denmark reported that patients with AF (n = 269 742) had a 2.5% (95% CI, 2.4–2.5%) absolute risk of cancer in the first 3 months after diagnosis of AF, which represented a five‐fold increased risk. 22 In this study, the authors found that the cases of cancer were more likely to be metastatic (57%) at the time of diagnosis, suggesting that AF was unlikely to have caused by cancer. 22 In the present study, patients with AF but without a history of cancer had a similar event rate of cancer death compared with those without AF or a history of cancer, suggesting that AF itself is not oncogenic.
To date, attention to a history of cancer has been paid mainly for patients with IHD or AF. 24 , 25 In the Coronary REvascularization Demonstrating Outcome Study in Kyoto (CREDO‐Kyoto) percutaneous coronary intervention (PCI)/coronary artery bypass grafting (CABG) Registry Cohort‐2, the investigators examined the impacts of a history of cancer on clinical outcomes in patients with coronary artery disease who underwent PCI. 26 They found that patients with a history of cancer at the time of PCI had an increased risk of cardiac events, such as cardiac death and HF hospitalization, as well as non‐cardiac events, such as non‐cardiac death, major bleeding, and non‐CABG surgery. 24 In the present study, we now extend the prognostic impact of a history of cancer to CHF patients with AF, especially in the elderly and those with IHD. Indeed, the previous study with 8962 AF patients including 4130 elderlies (age ≥ 75 years) showed that event rates of death, stroke/thromboembolism, the composite of stroke/thromboembolism/death, and major bleeding increased with age, 27 suggesting that elderly patients with AF have a higher risk of stroke and bleeding. We also observed that the association among history of cancer, AF, and the composite of stroke, systemic thrombosis, and major bleeding was evident in women. However, these findings should be interpreted with caution. Even though our main findings were consistent in different statistical analyses, the multiplicity of study sample should be considered. Indeed, the impact of female gender on the risk of stroke and bleeding among patients with AF is controversial and is not well known in those with HF. 26 , 28 For example, a previous study reported that female gender has altered impact on ischaemic stroke or systemic embolism in AF patients with different CHA2DS2 VASc scores, and hence, gender may modify the risk of stroke. 28 However, another study reported that there were no differences in the risk of stroke between women and men with AF taking warfarin and risk of bleeding was lower in women, suggesting female gender may not be a risk factor for the outcome. 26
Treating AF in patients with malignancies is a challenge, especially in terms of antithrombotic therapy, because cancer may result in an increased risk of either thrombosis or haemorrhage and an unpredictable anticoagulation response. Indeed, in the present study, in AF patients with a history of cancer, 36% did not receive anticoagulant, whereas 26% received both anticoagulant and antiplatelet. A previous retrospective study demonstrated that a fourth of AF patients with ‘active’ cancer were not prescribed anticoagulation therapy. 29 Figure 2 shows that anticoagulant monotherapy had numerically lower composite event rate compared with those with both anticoagulant and antiplatelet. There has been accumulating evidence showing that ‘less is more’ concept regimen of anticoagulant monotherapy is advantageous for AF patients with multi‐mobility. 30 For example, the ELDERCARE‐AF trial has shown that in very elderly Japanese patients (≥80 years of age) with non‐valvular AF who were not appropriate candidates for standard doses of oral anticoagulants including IHD patients (26% of the total participants) and/or current use of an antiplatelet drug, low‐dose direct oral anticoagulant (DOAC) (a once‐daily 15 mg dose of edoxaban) was superior to placebo in preventing stroke or systemic embolism and did not increase the incidence of major bleeding than placebo. 31 Currently, no trials have been conducted to compare the efficacy of novel oral anticoagulants to vitamin K antagonists in AF patients with cancer. Future studies should focus on optimal anticoagulation therapy in specific situations.
Study limitations
Several limitations should be mentioned for the present study. First, the number of patients with a history of cancer and AF was relatively small, and we did not collect information on staging and grading of cancer, limiting the analysis for association between a history of cancer and antithrombotic therapy. Second, since the CHART‐2 Study launched in 2006, anticoagulant at enrolment was warfarin alone, the present results may be limited for patients who received DOACs thereafter. However, the recent sub‐analysis of the ROCKET AF showed that the relative efficacy of rivaroxaban vs. warfarin for prevention of stroke/systemic embolism is comparable between patients with non‐valvular AF with and those without a history of cancer including ~63% of congestive HF (interaction P value = 0.21). 25 Third, we did not consider adherence, discontinuation, changes, or crossover of antithrombotic therapy after enrolment in our study. However, PT‐INR levels in patients with anticoagulants were within therapeutic range, suggesting acceptable adherence levels in the present study. Fourth, we did not have information to evaluate the association between bleeding risk score (e.g. HAS‐BLED score) and outcomes in the study. Finally, because the CHART‐2 Study is an observational study in Japan, caution is needed when generalizing the data to other populations.
Conclusions
In a large CHF cohort, we were able to demonstrate that a history of cancer and AF increase the risk for the composite of stroke, systemic thrombosis, and major bleeding in CHF patients, especially in the elderly and those with IHD, but considerable number of the patients did not receive anticoagulant therapy. Further studies with longitudinal multidisciplinary approach are needed for better optimal anticoagulation strategy in CHF patients with AF and cancer.
Conflict of interest
Nothing to disclose.
Funding
This study was supported in part by the research grant from Daiichi‐Sankyo Company.
Supporting information
Table S1. Outcomes at 10 years.
Table S2. Baseline patient characteristics stratified by a history of cancer, anticoagulant and antiplatelet use in AF patients.
Table S3. The results of Fine‐Gray competing risk model among history of cancer, AF and bleeding events.
Figure S1. Type of cancer in the study sample.
Figure S2. Association of AF and a history of cancer with (A) all‐cause death, (B) CV death, (C) cancer death, and (D) composite of MI, stroke, systemic thrombosis, and HF hospitalization.
Acknowledgements
The authors wish to thank the staff and participants of the CHART‐2 Study for their important contributions.
Nochioka, K. , Yasuda, S. , Sakata, Y. , Shiroto, T. , Hayashi, H. , Takahashi, J. , Takahama, H. , Miyata, S. , and Shimokawa, H. (2022) Prognostic impact of a history of cancer and atrial fibrillation in antithrombotic therapy for chronic heart failure. ESC Heart Failure, 9: 2445–2454. 10.1002/ehf2.13941.
References
- 1. Yoshihisa A, Ichijo Y, Watanabe K, Sato Y, Kanno Y, Takiguchi M, Yokokawa T, Abe S, Misaka T, Sato T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Ishida T, Takeishi Y. Prior history and incidence of cancer impacts on cardiac prognosis in hospitalized patients with heart failure. Circ J. 2019; 83: 1709–1717. [DOI] [PubMed] [Google Scholar]
- 2. Lateef N, Kapoor V, Ahsan MJ, Latif A, Ahmed U, Mirza M, Anwar F, Holmberg M. Atrial fibrillation and cancer; understanding the mysterious relationship through a systematic review. J Commun Hosp Intern Med Perspect. 2020; 10: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol. 2014; 64: 710–721. [DOI] [PubMed] [Google Scholar]
- 4. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 146: 857–867. [DOI] [PubMed] [Google Scholar]
- 5. Fiedler KA, Maeng M, Mehilli J, Schulz‐Schupke S, Byrne RA, Sibbing D, Hoppmann P, Schneider S, Fusaro M, Ott I, Kristensen SD, Ibrahim T, Massberg S, Schunkert H, Laugwitz KL, Kastrati A, Sarafoff N. Duration of triple therapy in patients requiring oral anticoagulation after drug‐eluting stent implantation: the ISAR‐TRIPLE trial. J Am Coll Cardiol. 2015; 65: 1619–1629. [DOI] [PubMed] [Google Scholar]
- 6. Hansen ML, Sorensen R, Clausen MT, Fog‐Petersen ML, Raunso J, Gadsboll N, Gislason GH, Folke F, Andersen SS, Schramm TK, Abildstrom SZ, Poulsen HE, Kober L, Torp‐Pedersen C. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010; 170: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 7. Shiba N, Nochioka K, Miura M, Kohno H, Shimokawa H, Investigators C . Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan—first report from the CHART‐2 study. Circ J. 2011; 75: 823–833. [DOI] [PubMed] [Google Scholar]
- 8. Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, Shiroto T, Takahashi J, Miyata S, Shimokawa H, Investigators C . Characterization of heart failure patients with mid‐range left ventricular ejection fraction—a report from the CHART‐2 Study. Eur J Heart Fail. 2017; 19: 1258–1269. [DOI] [PubMed] [Google Scholar]
- 9. Yamanaka S, Sakata Y, Nochioka K, Miura M, Kasahara S, Sato M, Aoyanagi H, Fujihashi T, Hayashi H, Shiroto T, Sugimura K, Takahashi J, Miyata S, Shimokawa H, Investigators C . Prognostic impacts of dynamic cardiac structural changes in heart failure patients with preserved left ventricular ejection fraction. Eur J Heart Fail. 2020; 22: 2258–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015; 17: 884–892. [DOI] [PubMed] [Google Scholar]
- 11. Takada T, Sakata Y, Miyata S, Takahashi J, Nochioka K, Miura M, Tadaki S, Shimokawa H, Investigators C . Impact of elevated heart rate on clinical outcomes in patients with heart failure with reduced and preserved ejection fraction: a report from the CHART‐2 Study. Eur J Heart Fail. 2014; 16: 309–316. [DOI] [PubMed] [Google Scholar]
- 12. Writing Committee M, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice G . ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013. 2013; 128: e240–e327. [DOI] [PubMed] [Google Scholar]
- 13. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016. 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 14. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 15. Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, Van De Werf FJ, Weintraub WS, Mitchell KR, Morrisson SL, Brindis RG, Anderson HV, Cannom DS, Chitwood WR, Cigarroa JE, Collins‐Nakai RL, Ellis SG, Gibbons RJ, Grover FL, Heidenreich PA, Khandheria BK, Knoebel SB, Krumholz HL, Malenka DJ, Mark DB, McKay CR, Passamani ER, Radford MJ, Riner RN, Schwartz JB, Shaw RE, Shemin RJ, Van Fossen DB, Verrier ED, Watkins MW, Phoubandith DR, Furnelli T. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol. 2001; 38: 2114–2130. [DOI] [PubMed] [Google Scholar]
- 16. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 17. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN, Meta‐Analysis Global Group in Chronic Heart F . Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013; 34: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 18. Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, Woodward M, Patel A, McMurray J, MacMahon S. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014; 2: 440–446. [DOI] [PubMed] [Google Scholar]
- 19. Jason P, Fine RJG. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94: 496–509. [Google Scholar]
- 20. Kulkarni PM, Tripathi RC, Michalek JE. Maximum (Max) and Mid‐P confidence intervals and p values for the standardized mortality and incidence ratios. Am J Epidemiol. 1998; 147: 83–86. [DOI] [PubMed] [Google Scholar]
- 21. Yamauchi T, Sakata Y, Miura M, Onose T, Tsuji K, Abe R, Oikawa T, Kasahara S, Sato M, Nochioka K, Shiroto T, Takahashi J, Miyata S, Shimokawa H, Investigators C . Prognostic impact of atrial fibrillation and new risk score of its onset in patients at high risk of heart failure—a report from the CHART‐2 Study. Circ J. 2017; 81: 185–194. [DOI] [PubMed] [Google Scholar]
- 22. Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sorensen HT. Atrial fibrillation as a marker of occult cancer. PLoS One. 2014; 9: e102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farmakis D, Parissis J, Filippatos G. Insights into onco‐cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014; 63: 945–953. [DOI] [PubMed] [Google Scholar]
- 24. Nakatsuma K, Shiomi H, Morimoto T, Watanabe H, Nakagawa Y, Furukawa Y, Kadota K, Ando K, Ono K, Shizuta S, Kimura T, Investigators CR‐KPCRC . Influence of a history of cancer on long‐term cardiovascular outcomes after coronary stent implantation (an Observation from Coronary Revascularization Demonstrating Outcome Study‐Kyoto Registry Cohort‐2). Eur Heart J Qual Care Clin Outcomes. 2018; 4: 200–207. [DOI] [PubMed] [Google Scholar]
- 25. Chen ST, Hellkamp AS, Becker RC, Berkowitz SD, Breithardt G, Fox KAA, Hacke W, Halperin JL, Hankey GJ, Mahaffey KW, Nessel CC, Piccini JP, Singer DE, Patel MR, Melloni C. Efficacy and safety of rivaroxaban vs. warfarin in patients with non‐valvular atrial fibrillation and a history of cancer: observations from ROCKET AF. Eur Heart J Qual Care Clin Outcomes. 2019; 5: 145–152. [DOI] [PubMed] [Google Scholar]
- 26. Penttila T, Lehto M, Niiranen J, Mehtala J, Khanfir H, Lassila R, Raatikainen P. Differences in the risk of stroke, bleeding events, and mortality between female and male patients with atrial fibrillation during warfarin therapy. Eur Heart J Cardiovasc Pharmacother. 2019; 5: 29–36. [DOI] [PubMed] [Google Scholar]
- 27. Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke. 2015; 46: 143–150. [DOI] [PubMed] [Google Scholar]
- 28. Nielsen PB, Skjoth F, Overvad TF, Larsen TB, Lip GYH. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA2DS2‐VA score rather than CHA2DS2‐VASc? Circulation. 2018; 137: 832–840. [DOI] [PubMed] [Google Scholar]
- 29. Malavasi VL, Fantecchi E, Gianolio L, Pesce F, Longo G, Marietta M, Cascinu S, Lip GYH, Boriani G. Atrial fibrillation in patients with active malignancy and use of anticoagulants: under‐prescription but no adverse impact on all‐cause mortality. Eur J Intern Med. 2019; 59: 27–33. [DOI] [PubMed] [Google Scholar]
- 30. Kawakami S, Yasuda S, Ogawa H. Antithrombotic therapy in atrial fibrillation patients with coronary artery disease: shifting paradigm to a “less is more” concept regimen. J Cardiol. 2020; 76: 35–43. [DOI] [PubMed] [Google Scholar]
- 31. Okumura K, Akao M, Yoshida T, Kawata M, Okazaki O, Akashi S, Eshima K, Tanizawa K, Fukuzawa M, Hayashi T, Akishita M, Lip GYH, Yamashita T, Committees E‐A, Investigators . Low‐dose edoxaban in very elderly patients with atrial fibrillation. 2020; 383: 1735–1745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Outcomes at 10 years.
Table S2. Baseline patient characteristics stratified by a history of cancer, anticoagulant and antiplatelet use in AF patients.
Table S3. The results of Fine‐Gray competing risk model among history of cancer, AF and bleeding events.
Figure S1. Type of cancer in the study sample.
Figure S2. Association of AF and a history of cancer with (A) all‐cause death, (B) CV death, (C) cancer death, and (D) composite of MI, stroke, systemic thrombosis, and HF hospitalization.


