Abstract
Aims
The aim of this study was to compare the diagnostic performance of the nutritional indicators, the mini nutritional assessment‐short form (MNA‐SF), the geriatric nutritional risk index (GNRI), and the controlling nutritional status (CONUT), in heart failure (HF) patients.
Methods and results
Nutritional status was prospectively assessed by the aforementioned three nutritional indicators in 150 outpatients with HF who were then followed for 1 year. The prevalence of patients with the nutritional risk as assessed by the MNA‐SF, GNRI, and CONUT scores was 50.0%, 13.3%, and 54.0%, respectively. There was slight agreement of nutritional risk assessment between the MNA‐SF and GNRI scores (κ coefficient = 0.16), as well as the GNRI and CONUT scores (κ = 0.11), but poor agreement between the MNA‐SF and CONUT scores (κ = −0.09). The CONUT score had the lowest area under the curve (AUC) for the identification of low body weight, low muscle mass, and low physical function among the three indicators (all P < 0.05). Compared with the MNA‐SF score, both the GNRI and CONUT scores had lower AUCs for the identification of reduced dietary intake and weight loss (all P < 0.05). There was no significant difference in predicting all‐cause mortality or HF rehospitalization among the three indicators. The prescription of statins reduced the diagnostic performance of the CONUT score, as the CONUT score includes cholesterol level assessment.
Conclusions
Of the three indicators, the diagnostic ability of the MNA‐SF score was the highest, and that of the CONUT score was the lowest, for the assessment of HF patient nutritional status. The CONUT score may misrepresent nutritional status, particularly in patients receiving statins.
Keywords: Malnutrition, MNA‐SF, GNRI, CONUT, Statin
Introduction
As society ages, the number of older and frail patients with heart failure (HF) is increasing worldwide. 1 , 2 Malnutrition is common in HF patients with frailty and is associated with an increased risk of functional decline and mortality. 2 , 3 Thus, it is important to properly assess the nutritional status of patients with HF, which may lead to appropriate nutritional intervention in malnourished HF patients. 4 , 5 , 6 Although the nutritional assessment of HF patients has not been well established, 7 recent studies have reported that various types of nutritional indicators, such as the mini nutritional assessment‐short form (MNA‐SF), geriatric nutritional risk index (GNRI), and controlling nutritional status (CONUT) scores, are useful for predicting the prognosis of HF patients. 7 , 8 , 9 , 10 , 11 However, most reports have only examined the association between these nutritional indicators and prognosis as a surrogate marker of nutritional status. 7 Few reports have directly evaluated the association of these nutritional indicators with body composition or physical function, which can both reflect actual nutritional status and its related functional disorders. Additionally, few reports have compared the diagnostic performance of each nutritional indicator in patients with HF. The aim of this study was to compare the diagnostic performance of the three representative nutritional indicators, MNA‐SF, GNRI, and CONUT, for assessing the nutritional status, physical function, and prognosis of HF patients.
Methods
Patients
This study was a sub‐analysis of a multicentre, prospective, observational study that assessed the nutritional status and clinical outcomes in chronic HF, as previously reported. 12 A total of 156 stable patients with chronic HF who regularly visited an outpatient ward for >1 month were enrolled between December 2012 and September 2014. These patients had a history of hospitalization because of worsening HF at least once within 5 years before enrolment. The exclusion criteria included nephrotic syndrome, liver cirrhosis, cancer, a history of gastrointestinal surgery within the 3 months prior, or poorly controlled diabetes, that is, glycosylated haemoglobin > 7.0%. We excluded patients who were taking steroids or antidepressants, which may influence appetite. Of 156 patients who were enrolled in this study, six patients were excluded because of missing data of the nutritional assessments for the MNA‐SF, GNRI, and CONUT scores. A total of 150 patients were included in the final analysis. At baseline, the patients performed the questionnaires, nutritional assessments, physical measurements, blood testing, and echocardiography. The patients were then followed up for 1 year to evaluate adverse clinical events including all‐cause death and hospitalization due to worsening HF.
Assessment of the nutritional indicators
Each patient's nutritional status was assessed by the MNA‐SF, GNRI, and CONUT scores. These nutritional indicators are representative nutritional screening methods that have been validated in several studies in HF patients. 4 , 5 , 7 , 9 , 10 The MNA‐SF is a simple nutritional screening tool, consisting of six questionnaire items. The MNA‐SF score ranges from 0 to 14 points, and patients with a score of 12 or less were defined as at nutritional risk, as previously described. 7 The GNRI score was calculated from the patient's body mass index (BMI) and albumin concentrations according to the following calculation: . 13 For patients with a BMI higher than 22, the BMI was determined uniformly as 22, as previously reported. 14 Patients with a GNRI score of <98 were defined as at nutritional risk, as previously described. 14 The CONUT score was calculated based on serum albumin levels, total peripheral lymphocyte count, and total cholesterol levels. The CONUT score ranges from 0 to 12 points, and patients with a score of 2 or more were defined as at nutritional risk, as previously described. 7
Assessment of the diagnostic performance of each nutritional indicator
To assess the diagnostic performance of the three nutritional indicators, the following components were applied to the diagnostic performance measures of each nutritional indicator: inflammation, reduced dietary intake, body weight loss, low body weight, and low muscle mass, which were recommended nutritional assessments by the Global Leadership Initiative on Malnutrition (GLIM) criteria that is an international standard for nutrition assessment. 15 We also included exercise performance and clinical events of all‐cause mortality and HF rehospitalization into the diagnostic performance measure because they were generally applied to outcome measures for functional disorders caused by malnutrition. 7 The inflammatory condition was assessed by albumin levels according to the GLIM criteria, 15 and patients with albumin levels < 3.2 mg/dL were defined as having an inflammatory condition. 16 Patients were asked about their dietary intake and changes in body weight. Because the GLIM criteria had not been proposed when this study was conducted, the information about reduced oral intake and weight loss defined by the GLIM criteria was not available for this study. Therefore, using the available data from the MNA‐SF score, we defined a decrease in food intake during the past 3 months as having reduced food intake, and a weight loss of 1 kg or more during the past 3 months as having weight loss. 7 The definition of underweight was based on Asian criteria, in which a BMI < 18.5 kg/m2 for patients under 70 years old and a BMI < 20 kg/m2 for patients 70 years or older were considered underweight. 15 To assess the patients' upper limb muscle mass, we measured the arm circumference (AC) and the triceps skinfold (TSF), as previously described. 7 The arm muscle circumference (AMC) was calculated as follows: . 7 We calculated %AMC and %TSF from the ratio of each value of AMC and TSF to the median anthropometric reference value for Japanese individuals of the same age and sex. A low muscle mass was defined as %AMC < 90%. 17 We also measured calf circumference (CC) to assess lower limb muscle mass. According to the definition indicated by the Asian Working Group for Sarcopenia, low muscle mass is defined as a CC < 34 cm in men and 33 cm in women. 18 Exercise performance was measured by the 6 min walk test. Low physical function was defined as a 6 min walk distance (6‐MWD) of 300 m or less. 19
Ethical considerations
The study was approved by the ethics committees of Hokkaido University Hospital (Approval No. 012‐0224) and the other nine participating research institutes—Hakodate National Hospital, Hikone Municipal Hospital, Kitami Red Cross Hospital, Keiwakai Ebetsu Hospital, Kushiro City General Hospital, Obihiro Kyokai Hospital, Otaru Kyokai Hospital, Saiseikai Fukuoka General Hospital, and Tottori University Hospital. The study was conducted in accordance with the ethical principles described in the Declaration of Helsinki. Written informed consent was obtained from each patient before his or her participation in the study.
Statistical analysis
Continuous variables are expressed as median and interquartile range. Differences in continuous variables were compared using the Mann–Whitney U test. Categorical variables are expressed as numbers (percentages) and were compared by the Fisher test. Cox proportional hazards models were used to assess the effect of nutritional status on the primary outcome. Agreement between diagnoses of nutritional indicators was assessed using the κ coefficient as previously described. 20 The diagnostic performance of each nutritional indicator for nutritional status, physical function, and clinical outcomes were assessed by the receiver operating curves, and the differences in diagnostic performance were compared by the area under the curve (AUC). Multiple differences between pairs were assessed using the Bonferroni test. A P‐value < 0.05 was considered statistically significant. All analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (Version 2.13.0; www.r‐project.org).
Results
Characteristics of patients
Table 1 shows the characteristics of the patients enrolled in this study. The median age was 67 (61–77) years and 69.5% of the patients were male. Ischaemic heart disease (31.8%) was the most common cause of HF, followed by dilated cardiomyopathy (30.5%). The median left ventricular ejection fraction was 45 (30–57)% and 29.8% and 61.6% of patients had a New York Heart Association (NYHA) classification of I and II, respectively. The prevalence of low BMI, low %AMC, low CC, and low physical function was 13.9%, 15.6%, 50.3%, and 15.7%, respectively.
Table 1.
Characteristic of patients with or without nutritional risk as assessed by mini nutritional assessment‐short form score
| Overall (n = 150) | MNA‐SF > 11 (n = 75) (nutritional risk [−]) | MNA‐SF ≤ 11 (n = 75) (nutritional risk [+]) | P‐value | |
|---|---|---|---|---|
| Age (years) | 67.5 [60.3, 77.0] | 67.0 [61.0, 74.0] | 68.0 [59.5, 77.5] | 0.463 |
| Male (%) | 104 (69.3) | 55 (73.3) | 49 (65.3) | 0.376 |
| SBP (mmHg) (n = 102) | 113.0 [96.0, 129.8] | 115.0 [97.0, 129.0] | 112.0 [96.0, 130.0] | 0.904 |
| NYHA (I/II/III), n (%) | 45 (30.0)/92 (61.3)/13 (8.7) | 30 (40.0)/41 (54.7)/4 (5.3) | 15 (20.0)/51 (68.0)/9 (12.0) | 0.017 |
| MLHFQ | 14.5 [5.0, 26.0] | 13.0 [4.0, 23.0] | 18.0 [6.0, 30.0] | 0.066 |
| PHQ‐9 | 2.0 [0.0, 4.0] | 2.0 [0.0, 3.5] | 2.0 [0.0, 6.0] | 0.132 |
| LVEF (%) (n = 147) | 45.0 [30.0, 57.0] | 48.0 [33.0, 56.0] | 43.5 [26.5, 57.9] | 0.395 |
| Aetiology of HF | ||||
| Ischaemic, n (%) | 48 (32.0) | 27 (36.0) | 21 (28.0) | 0.382 |
| Valve, n (%) | 38 (25.3) | 16 (21.3) | 22 (29.3) | 0.348 |
| DCM, n (%) | 46 (30.7) | 23 (30.7) | 23 (30.7) | 1.000 |
| Comorbidities | ||||
| Hypertension, n (%) | 83 (55.3) | 48 (64.0) | 35 (46.7) | 0.048 |
| DM, n (%) | 39 (26.0) | 23 (30.7) | 16 (21.3) | 0.264 |
| Dyslipidaemia, n (%) | 83 (55.3) | 49 (65.3) | 34 (45.3) | 0.021 |
| Atrial fibrillation, n (%) | 38 (25.3) | 19 (25.3) | 19 (25.3) | 1.000 |
| Medications | ||||
| ACE‐I/ARB, n (%) | 114 (76.0) | 51 (68.0) | 63 (84.0) | 0.035 |
| Beta‐blockers, n (%) | 129 (86.0) | 63 (84.0) | 66 (88.0) | 0.639 |
| MRA, n (%) | 85 (56.7) | 43 (57.3) | 42 (56.0) | 1.000 |
| Loop diuretics, n (%) | 117 (78.0) | 56 (74.7) | 61 (81.3) | 0.431 |
| Statins, n (%) | 72 (48.0) | 41 (54.7) | 31 (41.3) | 0.141 |
| Laboratory data | ||||
| Sodium (mEq) | 140.0 [139.0, 142.0] | 140.0 [139.0, 142.0] | 140.0 [139.0, 142.0] | 0.912 |
| Haemoglobin (g/dL) | 13.2 [11.9, 14.3] | 13.5 [12.1, 14.5] | 12.9 [11.6, 14.0] | 0.035 |
| BUN (mg/dL) (n = 149) | 20.0 [16.1, 27.0] | 20.0 [16.5, 25.0] | 20.0 [16.0, 31.7] | 0.313 |
| eGFR (mL/min/1.73 m2) (n = 149) | 54.2 [38.4, 67.6] | 54.6 [40.9, 64.7] | 52.7 [35.8, 69.8] | 0.752 |
| BNP (pg/mL) (n = 148) | 153.6 [72.9, 371.0] | 146.0 [58.7, 292.6] | 170.0 [87.6, 414.0] | 0.147 |
| Total cholesterol (mg/dL) | 172.5 [147.5, 193.0] | 173.0 [143.0, 192.0] | 172.0 [153.0, 194.5] | 0.257 |
| Albumin (g/dL) | 4.2 [3.9, 4.4] | 4.2 [3.9, 4.3] | 4.2 [4.0, 4.4] | 0.866 |
| Pre‐albumin (mg/dL) | 26.4 [21.9, 31.2] | 26.5 [22.9, 31.7] | 26.3 [21.4, 31.0] | 0.835 |
| Lymphocyte count (μL) | 1510.0 [1210.8, 1938.8] | 1550.0 [1224.0, 1909.5] | 1500.0 [1199.5, 1977.0]| | 0.728 |
| Nutritional indicators | ||||
| BMI | 22.9 [20.7, 25.7] | 24.7 [23.1, 26.6] | 20.8 [19.0, 22.9] | <0.001 |
| MNA‐SF | 11.5 [10.0, 13.0] | 13.0 [12.0, 13.0] | 10.0 [9.0, 11.0] | <0.001 |
| GNRI | 108.2 [103.7, 111.0] | 109.6 [105.2, 111.1] | 105.6 [100.3, 109.9] | 0.004 |
| CONUT | 2.0 [1.0, 2.0] | 2.0 [1.0, 2.0] | 1.0 [1.0, 2.5] | 0.508 |
| Reduced dietary intake, n (%) | 10 (6.7) | 0 (0.0) | 10 (13.5) | 0.001 |
| Body weight loss, n (%) | 33 (22.0) | 7 (9.3) | 26 (34.7) | <0.001 |
| %AMC (%) | 101.0 [94.0, 112.0] | 108.0 [97.0, 114.0] | 97.0 [88.0, 105.0] | <0.001 |
| %TSF (%) | 103.0 [78.0, 139.0] | 112.0 [94.0, 148.0] | 86.0 [65.0, 136.0] | 0.003 |
| CC (cm) (n = 149) | 33.8 [31.5, 36.5] | 35.1 [32.9, 37.0] | 32.0 [30.3, 34.4] | <0.001 |
| Physical function | ||||
| 6‐MWD (m) (n = 128) | 431.5 [349.5, 498.3] | 432.0 [364.0, 494.5] | 430.0 [332.0, 498.0] | 0.673 |
6‐MWD, 6 min walk distance; ACE‐I, angiotensin‐converting enzyme inhibitor; AMC, arm muscle circumference; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; BUN, blood urea nitrogen; CC, calf circumference; CONUT, controlling nutrition status; DCM, dilated cardiomyopathy; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; GNRI, geriatric nutritional risk index; HF, heart failure; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living Heart Failure Questionnaire; MNA‐SF, mini nutritional assessment‐short form; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; PHQ‐9, Patient Health Questionnaire‐9; SBP, systolic blood pressure; TSF, triceps skinfold.
Continuous variables are expressed as medians [interquartile ranges].
Agreement of nutritional risk assessment between the nutritional indicators
Figure 1 shows the agreement between each nutritional indicator for the assessment of nutritional risk. The prevalence of patients with nutritional risk as assessed by the MNA‐SF, GNRI, and CONUT scores was 50.0%, 13.3%, and 54.0%, respectively. There was slight agreement for the assessment of nutritional risk between the MNA‐SF and GNRI scores (κ = 0.16), as well as the GNRI and CONUT scores (κ = 0.11), but poor agreement between the MNA‐SF and CONUT scores (κ = −0.09).
Figure 1.
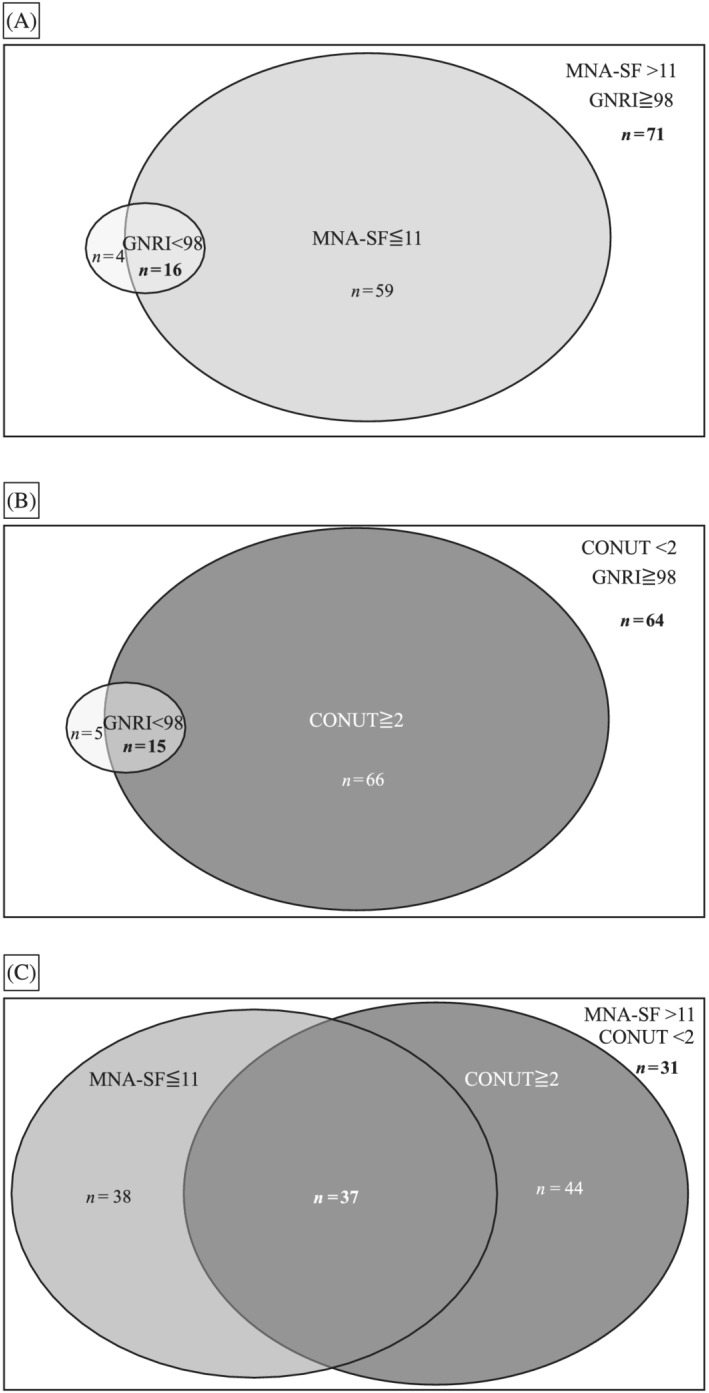
Agreement of the assessment of nutritional risk between the three nutritional indicators: MNA‐SF, GNRI, and CONUT. (A) Agreement between the GNRI and MNA‐SF scores for the judgement of nutritional risk. Scores of <98 and ≤11 are defined as patients at nutritional risk for the GNRI and MNA‐SF scores, respectively. GNRI vs. MNA‐SF: κ coefficient = 0.16. (B) Agreement between the GNRI and CONUT scores for the judgement of nutritional risk. Scores of <98 and ≥2 are defined as nutritional risk for the GNRI and CONUT scores, respectively. GNRI vs. CONUT: κ coefficient = 0.11. (C) Agreement between the MNA‐SF and CONUT scores for the judgement of nutritional risk. Scores of ≤11 and ≥2 are defined as nutritional risk for the MNA‐SF and CONUT scores, respectively. MNA‐SF vs. CONUT: κ coefficient = −0.09. CONUT, controlling nutritional status; GNRI, geriatric nutritional risk index; MNA‐SF, mini nutritional assessment‐short form.
Characteristics of patients with nutritional risk as assessed by mini nutritional assessment‐short form
Table 1 compares the two patient groups divided by the MNA‐SF score: patients with nutritional risk (MNA‐SF ≤ 11) and without nutritional risk (MNA‐SF > 11). Patients with nutritional risk judged by the MNA‐SF score had significantly higher NYHA class, higher prescription rates of angiotensin‐converting enzyme inhibitor/angiotensin II receptor blockers, lower prevalence of hypertension and dyslipidaemia, and lower levels of haemoglobin than those without nutritional risk. Regarding nutritional indicators and physical function, BMI, GNRI score, muscle mass (%AMC, CC), subcutaneous fat (%TSF), and the prevalence of reduced dietary intake and body weight loss were significantly lower in patients with nutritional risk than in those without nutritional risk, but the CONUT score and 6‐MWD did not differ between the two groups.
Characteristics of patients with nutritional risk as assessed by geriatric nutritional risk index
Table 2 compares the two patient groups evaluated by the GNRI score: patients with nutritional risk (GNRI < 98) and without nutritional risk (GNRI ≥ 98). Patients with nutritional risk judged by the GNRI score were significantly older and had lower levels of haemoglobin, albumin, and pre‐albumin and higher levels of B‐type natriuretic peptide (BNP) than those without nutritional risk. Regarding nutritional indicators and physical function, BMI, MNA‐SF and CONUT scores, muscle mass (%AMC, CC), subcutaneous fat (%TSF), and 6‐MWD were significantly lower in patients with nutritional risk than in those without nutritional risk, but the prevalence of reduced dietary intake and body weight loss did not differ between the two groups.
Table 2.
Characteristic of patients with or without nutritional risk as assessed by geriatric nutritional risk index score
| GNRI ≥ 98 (n = 130) (nutritional risk [−]) | GNRI < 98 (n = 20) (nutritional risk [+]) | P‐value | |
|---|---|---|---|
| Age (years) | 66.5 [59.3, 74.0] | 77.5 [67.5, 83.2] | 0.001 |
| Male (%) | 93 (71.5) | 11 (55.0) | 0.191 |
| SBP (mmHg) (n = 102) | 112.0 [96.0, 129.0] | 125.0 [114.5, 133.5] | 0.124 |
| NYHA (I/II/III), n (%) | 42 (32.3)/78 (60.0)/10 (7.7) | 3 (15.0)/14 (70.0)/3 (15.0) | 0.185 |
| MLHFQ | 15.5 [5.0, 26.8] | 13.0 [5.0, 24.0] | 0.664 |
| PHQ‐9 | 2.0 [0.0, 4.0] | 3.5 [0.8, 6.8] | 0.205 |
| LVEF (%) (n = 147) | 45.0 [30.0, 56.5] | 46.5 [37.8, 61.4] | 0.501 |
| Aetiology of HF | |||
| Ischaemic, n (%) | 41 (31.5) | 7 (35.0) | 0.799 |
| Valve, n (%) | 30 (23.1) | 8 (40.0) | 0.164 |
| DCM, n (%) | 41 (31.5) | 5 (25.0) | 0.615 |
| Comorbidities | |||
| Hypertension, n (%) | 71 (54.6) | 12 (60.0) | 0.810 |
| DM, n (%) | 33 (25.4) | 6 (30.0) | 0.784 |
| Dyslipidaemia, n (%) | 76 (58.5) | 7 (35.0) | 0.057 |
| Atrial fibrillation, n (%) | 32 (24.6) | 6 (30.0) | 0.590 |
| Medications | |||
| ACE‐I/ARB, n (%) | 98 (75.4) | 16 (80.0) | 0.784 |
| Beta‐blockers, n (%) | 112 (86.2) | 17 (85.0) | 1.000 |
| MRA, n (%) | 71 (54.6) | 14 (70.0) | 0.232 |
| Loop diuretics, n (%) | 98(75.4) | 19 (95.0) | 0.077 |
| Statins, n (%) | 64 (49.2) | 8 (40.0) | 0.480 |
| Laboratory data | |||
| Sodium (mEq) | 140.0 [139.0, 142.0] | 140.0 [139.0, 141.3] | 0.696 |
| Haemoglobin (g/dL) | 13.5 [12.2, 14.4] | 11.8 [11.1, 12.3] | <0.001 |
| BUN (mg/dL) (n = 149) | 19.9 [16.0, 26.0] | 22.0 [18.3, 32.0] | 0.105 |
| eGFR (mL/min/1.73 m2) (n = 149) | 54.2 [39.1, 68.5] | 51.6 [34.5, 62.1] | 0.245 |
| BNP (pg/mL) (n = 148) | 146.8 [59.5, 342.8] | 245.1 [121.1, 602.6] | 0.028 |
| Total cholesterol (mg/dL) | 174.5 [151.0, 195.0] | 159.0 [134.8, 186.3] | 0.075 |
| Albumin (g/dL) | 4.2 [4.0, 4.4] | 3.7 [3.2, 3.9] | <0.001 |
| Pre‐albumin (mg/dL) | 26.9 [23.4, 31.2] | 19.9 [16.3, 25.5] | 0.002 |
| Lymphocyte count (μL) | 1561.5 [1230.0, 1939.5] | 1297.0 [1113.8, 1624.8] | 0.098 |
| Nutritional indicators | |||
| BMI | 23.7 [21.7, 26.5] | 18.1 [16.4, 20.3] | <0.001 |
| MNA‐SF | 12.0 [10.0, 13.0] | 10.0 [9.0, 11.0] | 0.001 |
| GNRI | 108.5 [105.2, 111.1] | 94.7 [91.8, 97.2] | <0.001 |
| CONUT | 2.0 [1.0, 2.0] | 3.0 [1.8, 4.3] | 0.002 |
| Reduced dietary intake, n (%) | 8 (6.2) | 2 (10.0) | 0.625 |
| Body weight loss, n (%) | 28 (21.5) | 5 (25.0) | 0.773 |
| %AMC (%) | 103.0 [95.0, 112.0] | 93.0 [86.0, 99.0] | 0.004 |
| %TSF (%) | 105.0 [84.0, 140.0] | 78.0 [59.0, 117.0] | 0.021 |
| CC (cm) (n = 149) | 34.2 [31.9, 36.7] | 31.1 [27.6, 33.0] | <0.001 |
| Physical function | |||
| 6‐MWD (m) (n = 128) | 435.0 [361.3, 502.0] | 376.5 [260.5, 432.0] | 0.010 |
Abbreviations as Table 1 .
Characteristics of patients with nutritional risk as assessed by controlling nutritional status
Table 3 compares the two patient groups evaluated by the CONUT score: patients with nutritional risk (CONUT ≥ 2) and without nutritional risk (CONUT < 2). Patients with nutritional risk judged by the CONUT score were significantly older and had a higher prevalence of coronary artery disease, hypertension, dyslipidaemia, and statin prescription, higher levels of BNP, and lower levels of haemoglobin, albumin, pre‐albumin, lymphocyte count, and reduced estimated glomerular filtration rate than those without nutritional risk. Regarding nutritional indicators and physical function, BMI, MNA‐SF score, body weight loss, muscle mass (%AMC, CC), subcutaneous fat (%TSF), and 6‐MWD did not differ between the two groups, although GNRI score was significantly lower in patients with nutritional risk than in those without nutritional risk. The prevalence of reduced dietary intake was significantly lower in patients with nutritional risk than in those without nutritional risk, demonstrating that the CONUT score failed to identify patients with reduced dietary intake as nutritionally compromised.
Table 3.
Characteristic of patients with or without nutritional risk as assessed by controlling nutritional status score
| CONUT < 2 (n = 69) (nutritional risk [−]) | CONUT ≥ 2 (n = 81) (nutritional risk [+]) | P‐value | |
|---|---|---|---|
| Age (years) | 65.0 [55.0, 73.0] | 69.0 [63.0, 78.0] | 0.002 |
| Male (%) | 50 (72.5) | 54 (66.7) | 0.481 |
| SBP (mmHg) (n = 102) | 112.0 [100.8, 130.0] | 113.0 [96.0, 129.0] | 0.718 |
| NYHA (I/II/III), n (%) | 22 (31.9)/40 (58.0)/7 (10.1) | 23 (28.4)/52 (64.2)/6 (7.4) | 0.717 |
| MLHFQ | 11.0 [4.0, 23.0] | 19.0 [6.0, 27.0] | 0.142 |
| PHQ‐9 | 1.0 [0.0, 3.0] | 2.0 [1.0, 5.0] | 0.075 |
| LVEF (%) (n = 147) | 47.5 [34.5, 57.1] | 45.0 [27.5, 56.5] | 0.446 |
| Aetiology of HF | |||
| Ischaemic, n (%) | 12 (17.4) | 36 (44.4) | <0.001 |
| Valve, n (%) | 14 (20.3) | 24 (29.6) | 0.258 |
| DCM, n (%) | 30 (43.5) | 16 (19.8) | 0.002 |
| Comorbidities | |||
| Hypertension, n (%) | 31 (44.9) | 52 (64.2) | 0.021 |
| DM, n (%) | 17 (24.6) | 22 (27.2) | 0.852 |
| Dyslipidaemia, n (%) | 31 (44.9) | 52 (64.2) | 0.021 |
| Atrial fibrillation, n (%) | 12 (17.4) | 26 (32.1) | 0.059 |
| Medications | |||
| ACE‐I/ARB, n (%) | 56 (81.2) | 58 (71.6) | 0.185 |
| Beta‐blockers, n (%) | 60 (87.0) | 69 (85.2) | 0.817 |
| MRA, n (%) | 37 (53.6) | 48 (59.3) | 0.512 |
| Loop diuretics, n (%) | 50 (72.5) | 67 (82.7) | 0.167 |
| Statins, n (%) | 25 (36.2) | 47 (58.0) | 0.009 |
| Laboratory data | |||
| Sodium (mEq) | 140.0 [139.0, 142.0] | 140.0 [139.0, 142.0] | 0.386 |
| Haemoglobin (g/dL) | 13.8 [12.2, 14.7] | 12.5 [11.5, 13.9] | 0.001 |
| BUN (mg/dL) (n = 149) | 19.0 [15.0, 25.2] | 20.2 [17.0, 27.9] | 0.069 |
| eGFR (mL/min/1.73 m2) (n = 149) | 60.1 [42.5, 72.4] | 48.9 [37.1, 63.8] | 0.016 |
| BNP (pg/mL) (n = 148) | 107.3 [42.9, 204.7] | 208.2 [91.9, 418.0] | 0.001 |
| Total cholesterol (mg/dL) | 188.0 [171.0, 203.0] | 155.0 [131.0, 176.0] | <0.001 |
| Albumin (g/dL) | 4.2 [4.1, 4.4] | 4.1 [3.9, 4.3] | 0.002 |
| Pre‐albumin (mg/dL) | 28.4 [24.6, 32.4] | 25.4 [21.1, 29.7] | 0.005 |
| Lymphocyte count (μL) | 1898.0 [1603.0, 2180.0] | 1240.0 [969.0, 1456.0] | <0.001 |
| Nutritional indicators | |||
| BMI | 23.2 [20.7, 26.6] | 22.7 [20.6, 25.5] | 0.332 |
| MNA‐SF | 11.0 [10.0, 13.0] | 12.0 [10.0, 13.0] | 0.294 |
| GNRI | 109.6 [105.2, 111.1] | 105.6 [102.4, 109.6] | 0.007 |
| CONUT | 1.0 [0.0, 1.0] | 2.0 [2.0, 3.0] | <0.001 |
| Reduced dietary intake, n (%) | 8 (11.6) | 2 (2.5) | 0.044 |
| Body weight loss, n (%) | 18 (25.7) | 15 (18.5) | 0.324 |
| %AMC (%) | 102.0 [96.0, 112.0] | 100.0 [92.0, 110.0] | 0.123 |
| %TSF (%) | 100.0 [78.0, 132.0] | 111.0 [78.0, 150.0] | 0.577 |
| CC (cm) (n = 149) | 34.5 [32.0, 36.7] | 32.9 [31.3, 35.6] | 0.076 |
| Physical function | |||
| 6‐MWD (m) (n = 128) | 443.5 [365.0, 505.1] | 427.0 [346.5, 467.5] | 0.164 |
Abbreviations as Table 1 .
All‐cause death or heart failure hospitalization over 1 year
During the 1 year follow‐up, all‐cause death and HF readmissions occurred in 7 and 14 of the enrolled patients, respectively. Cox hazard analysis shows that there were no significant differences in composite event rates between patients with and without nutritional risk, as assessed by the MNA‐SF, GNRI, and CONUT scores; MNA‐SF: hazard ratio [95% confidence interval] = 0.637 [0.264–1.537], P = 0.316; GNRI: 0.658 [0.221–1.957], P = 0.452; CONUT: 2.172 [0.843–5.599], P = 0.108.
Diagnostic performance of controlling nutritional status in patients by statin use
The CONUT score is based on cholesterol levels, and patients with nutritional risk as assessed by the CONUT score had a significantly higher prescription rate of statins. Because statins may affect the diagnostic performance of the CONUT score, we evaluated the AUC of the CONUT score for assessing nutritional status, physical function, and prognosis in patients by statin use. Figure 2 shows the forest plot of the AUC of the CONUT score. The diagnostic ability of presence of inflammation is not shown because the number of patients with inflammatory conditions was too small to be divided into subgroups in this study. In patients not receiving statins, the CONUT score had significant diagnostic ability to identify low BMI; AUC [95% confidence interval] = 0.656 [0.509–0.802]. In contrast, in patients receiving statins, the CONUT score had no significant diagnostic ability to identify this condition; AUC = 0.600 [0.398–0.802]. Furthermore, in patients receiving statins, the AUC of the CONUT score for predicting reduced dietary intake was much <0.5 (AUC = 0.150 [0.030–0.270]), indicating that the CONUT score significantly overlooked the presence of low dietary intake. Thus, the prescription of statins decreased the diagnostic performance of the CONUT score.
Figure 2.
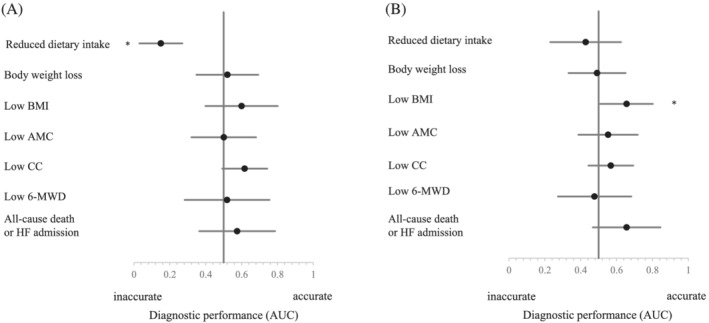
Forest plot of the AUC for the CONUT score in assessing nutritional status, physical function, and prognosis. (A) The AUC of the CONUT score in patients receiving statins. (B) The AUC of the CONUT score in patients not receiving statins. AUC is an indicator of diagnostic performance. An AUC of 0.5 indicates that the diagnostic performance is neutral; an AUC higher than 0.5 indicates that the diagnostic performance is high; and an AUC lower than 0.5 indicates that the diagnosis is incorrect. *P < 0.05. 6‐MWD, 6 min walk distance; AMC, arm muscle circumference; AUC, area under the curve; BMI, body mass index; CC, calf circumference; CONUT, controlling nutritional status; HF, heart failure.
Comparison of the diagnostic performance of the mini nutritional assessment‐short form, geriatric nutritional risk index, and controlling nutritional status scores
Figure 3 compares the diagnostic performance of the nutritional indicators for the assessment of nutritional status, physical function, and prognosis. Figure 3 A shows the forest plot of the AUC of each nutritional indicator and Figure 3 B shows their corresponding radar chart. The CONUT score had the lowest AUC for identifying low BMI, low muscle mass, and low physical function among the three indicators (all P < 0.05). Compared with the MNA‐SF score, both the GNRI and CONUT scores had significantly lower AUCs for identifying reduced dietary intake and weight loss (all P < 0.05). In contrast, the MNA‐SF score had a significantly lower AUC for diagnosing inflammatory conditions compared with both the GNRI and CONUT scores (both P < 0.05). There was no significant difference in predicting all‐cause mortality or HF rehospitalization among the three indicators. Taken together, among the eight diagnostic performance measures, the MNA‐SF had superior diagnostic performance in four items, the GNRI had superior performance in three items, and the CONUT had superior performance in one item than other nutritional indicators. Thus, the diagnostic ability of the MNA‐SF score was the highest, and that of the CONUT score was the lowest, for the assessment of nutritional status in HF patients.
Figure 3.
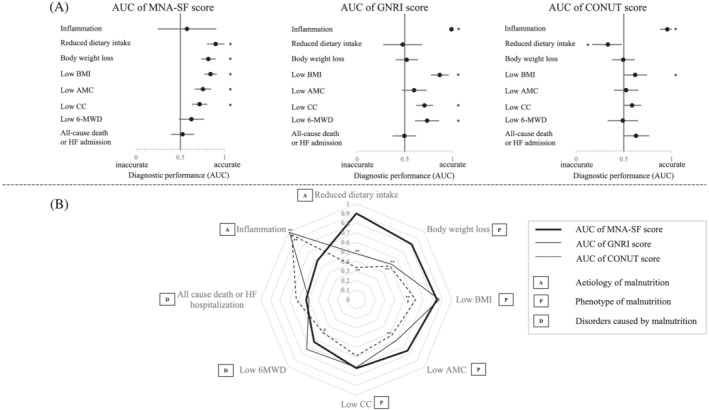
Forest plot and radar chart of the AUC for the three nutritional indicators in assessing nutritional status, physical function, and prognosis. (A) Forest plot of the AUC for the MNA‐SF, GNRI, and CONUT scores. AUC is an indicator of diagnostic performance. An AUC of 0.5 indicates that the diagnostic performance is neutral; an AUC higher than 0.5 indicates that the diagnostic performance is high; and an AUC lower than 0.5 indicates that the diagnosis is incorrect. (B) The radar chart of the AUC for the MNA‐SF, GNRI, and CONUT scores. *P < 0.05 (A). **P < 0.05 for MNA‐SF, # P < 0.05 for GNRI (B). 6‐MWD, 6 min walk distance; AMC, arm muscle circumference; AUC, area under the curve; BMI, body mass index; CC, calf circumference; CONUT, controlling nutritional status; GNRI, geriatric nutritional risk index; HF, heart failure; MNA‐SF, mini nutritional assessment‐short form.
Discussion
The current study showed that, of the three nutritional indicators, the MNA‐SF, GNRI, and CONUT scores, the diagnostic performance of the MNA‐SF score was the highest, whereas the diagnostic performance of the CONUT score was the lowest for the assessment of nutritional status in outpatients with HF. In addition, the diagnostic performance of the CONUT score was influenced by statin use.
In 2018, the GLIM criteria were proposed as the international standard for nutritional assessment. The GLIM criteria aim to diagnose the aetiology and phenotype of malnutrition. The aetiology of malnutrition includes reduced food intake or assimilation, and the presence of disease burden with inflammation. Malnutrition phenotypes can include non‐volitional weight loss, low BMI, and reduced muscle mass. 15 Analysis of these assessments takes time and effort, and it is difficult to apply these criteria to all HF patients. Therefore, patient screening is recommended using the established and validated nutritional screening methods. 15 The MNA‐SF, GNRI, and CONUT scores are representative nutritional screening methods that have been validated in several studies in HF patients. 4 , 5 , 7 , 9 , 10 , 11 The present study suggests that the MNA‐SF score is the most appropriate first choice for nutritional screening of HF patients because it had the highest diagnostic performance for the assessment of nutritional status in accordance with the GLIM criteria in our study (Figure 3 ). One possible reason for the inferiority of the GNRI and CONUT scores is that these indicators are only assessed by objective measures such as blood tests or BMI at a single point and do not include temporal or subjective nutritional information. 7 In fact, the GNRI and CONUT scores have very low diagnostic performance for the identification of patients with reduced food intake or weight loss (Figure 3 ). These indicators may be inadequate for identifying patients who are not currently undernourished but are at high risk of becoming undernourished. In contrast, the GNRI and CONUT scores were superior for the diagnosis of inflammatory conditions than the MNA‐SF score because the calculation of these scores requires information on albumin levels. 15 Therefore, each nutritional indicator has its own strengths and weaknesses for determining a diagnosis and the aetiology of malnutrition, and a comprehensive assessment rather than an evaluation with a single indicator is required in patients with HF. 7
The CONUT score had significantly lower diagnostic performance for the assessment of muscle mass and physical function than the MNA‐SF and GNRI scores (Figure 3 ). Because the CONUT score includes an assessment of cholesterol levels, the use of lipid‐lowering therapies can influence the CONUT score. 7 In the present study, patients judged to have nutritional risk by the CONUT score had a significantly higher rate of statin use, and statin use was associated with decreased diagnostic performance of the CONUT score. In particular, the CONUT score misjudged nutritional risk due to reduced oral intake in patients receiving statins (Figure 2 ). Most previous studies reported that the CONUT score was useful for predicting clinical events in patients with HF, 11 but few reports have directly evaluated the diagnostic performance of the CONUT score for the assessment of nutritional status, including body composition and physical function. The CONUT score may be a useful prognostic marker but may be inappropriate as a nutritional indicator.
Although the GNRI score had a comparable diagnostic performance compared with the MNA‐SF score for the assessment of muscle mass and physical function (Figure 3 ), there was slight agreement for the judgement of nutritional risk between the two indicators (Figure 1 ). In fact, the number of patients with nutritional risk diagnosed by the GNRI score (n = 20) was smaller than that diagnosed by the MNA‐SF score (n = 75) (Figure 1 ). Furthermore, patients with nutritional risk by the GNRI score had low muscle mass and a short 6‐MWD as compared with the nutritional risk group according to the MNA‐SF score (Tables 1 and 2 ). Therefore, patients with nutritional risk by the GNRI score may be at a more severe malnourished status than those deemed at nutritional risk by the MNA‐SF score. Conversely, the GNRI score is likely to fail to identify patients at an early stage of malnutrition that do not yet indicate a decline in body composition or physical function, even though these patients may have future risk of muscle wasting and functional decline. These aspects should be considered when applying the GNRI score over the MNA‐SF score.
There were several limitations in this study. The current study assessed muscle mass from anthropometric measurements. More precise evaluation of body composition using the impedance method or dual‐energy X‐ray absorptiometry is desirable for future studies. Most patients enrolled in this study were NYHA I–II class and had the median 6‐MWD of 431.5 [349.5, 498.3] m and the median BNP levels of 153.6 [72.9, 371.0] pg/mL. Thus, their HF severity was mild, and patient selection bias needs to be considered. All three indicators evaluated in this study had no significant prognostic value, probably because patients enrolled in the study were relatively young and well‐nourished and had lower event rates of all‐cause mortality or hospitalization for HF. In addition, follow‐up period was relatively short. Further investigations with long‐term follow‐up are necessary to assess the diagnostic performance of the three indicators in elderly HF patients with poorer nutritional status.
In conclusion, the MNA‐SF score is recommended as the first choice for nutritional screening of outpatients with HF. Conversely, the CONUT score may misrepresent nutritional status, particularly in patients receiving statins, and is not recommended as a nutritional indicator in patients with cardiovascular diseases who are frequently prescribed statins.
Conflict of interest
The other authors declare no conflict of interest relevant to this article.
Funding
This study was supported by a Grant‐in‐Aid for Scientific Research from KAKENHI (no. JP24614001 to M.T.‐M.).
Acknowledgements
The authors thank all investigators, clinical research coordinators, data managers, and laboratory technicians. We also thank Georgia Lenihan‐Geels, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Kinugasa, Y. , Sota, T. , Kamitani, H. , Nakayama, N. , Nakamura, K. , Hirai, M. , Yanagihara, K. , Kato, M. , Ono, T. , Takahashi, M. , Matsuo, H. , Matsukawa, R. , Yoshida, I. , Kakinoki, S. , Yonezawa, K. , Himura, Y. , Yokota, T. , Yamamoto, K. , Tsuchihashi‐Makaya, M. , and Kinugawa, S. (2022) Diagnostic performance of nutritional indicators in patients with heart failure. ESC Heart Failure, 9: 2096–2106. 10.1002/ehf2.13886.
References
- 1. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail 2015; 17: 884–892. [DOI] [PubMed] [Google Scholar]
- 2. Kinugasa Y, Yamamoto K. The challenge of frailty and sarcopenia in heart failure with preserved ejection fraction. Heart 2017; 103: 184–189. [DOI] [PubMed] [Google Scholar]
- 3. Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J 2013; 77: 705–711. [DOI] [PubMed] [Google Scholar]
- 4. Habaybeh D, de Moraes MB, Slee A, Avgerinou C. Nutritional interventions for heart failure patients who are malnourished or at risk of malnutrition or cachexia: a systematic review and meta‐analysis. Heart Fail Rev 2021; 26: 1103–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan MS, Khan F, Fonarow GC, Sreenivasan J, Greene SJ, Khan SU, Usman MS, Vaduganathan M, Fudim M, Anker SD, Butler J. Dietary interventions and nutritional supplements for heart failure: a systematic appraisal and evidence map. Eur J Heart Fail 2021; 23: 1468–1476. [DOI] [PubMed] [Google Scholar]
- 6. Kinugasa Y, Sota T, Ishiga N, Nakamura K, Kamitani H, Hirai M, Yanagihara K, Kato M, Yamamoto K. l‐Carnitine supplementation in heart failure patients with preserved ejection fraction; a pilot study. Geriatr Gerontol Int 2020; 20: 1244–1245. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto K, Tsuchihashi‐Makaya M, Kinugasa Y, Iida Y, Kamiya K, Kihara Y, Kono Y, Sato Y, Suzuki N, Takeuchi H, Higo T, Miyazawa Y, Miyajima I, Yamashina A, Yoshita K, Washida K, Kuzuya M, Takahashi T, Nakaya Y, Hasebe N, Tsutsui H, Japanese Heart Failure Society ECWC . Japanese Heart Failure Society 2018 scientific statement on nutritional assessment and management in heart failure patients. Circ J 2020; 84: 1408–1444. [DOI] [PubMed] [Google Scholar]
- 8. Lin H, Zhang H, Lin Z, Li X, Kong X, Sun G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail Rev 2016; 21: 549–565. [DOI] [PubMed] [Google Scholar]
- 9. Lv S, Ru S. The prevalence of malnutrition and its effects on the all‐cause mortality among patients with heart failure: a systematic review and meta‐analysis. PLoS One 2021; 16: e0259300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dong CH, Chen SY, Zeng HL, Yang B, Pan J. Geriatric nutritional risk index predicts all‐cause mortality in patients with heart failure: a systematic review and meta‐analysis. Clinics 2021; 76: e2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li H, Zhou P, Zhao Y, Ni H, Luo X, Li J. Prediction of all‐cause mortality with malnutrition assessed by controlling nutritional status score in patients with heart failure: a systematic review and meta‐analysis. Public Health Nutr 2021. Jun; 30: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Obata Y, Kakutani N, Kinugawa S, Fukushima A, Yokota T, Takada S, Ono T, Sota T, Kinugasa Y, Takahashi M, Matsuo H, Matsukawa R, Yoshida I, Yokota I, Yamamoto K, Tsuchihashi‐Makaya M. Impact of inadequate calorie intake on mortality and hospitalization in stable patients with chronic heart failure. Nutrients 2021; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, Kumagai H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr 2008; 87: 106–113. [DOI] [PubMed] [Google Scholar]
- 14. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–783. [DOI] [PubMed] [Google Scholar]
- 15. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R, Blaauw R, Coats A, Crivelli A, Evans DC, Gramlich L, Fuchs‐Tarlovsky V, Keller H, Llido L, Malone A, Mogensen KM, Morley JE, Muscaritoli M, Nyulasi I, Pirlich M, Pisprasert V, de van der Schueren MAE, Siltharm S, Singer P, Tappenden K, Velasco N, Waitzberg D, Yamwong P, Yu J, Van Gossum A, Compher C, Committee GCL , Group GW . GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr 2019; 38: 1–9. [DOI] [PubMed] [Google Scholar]
- 16. Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar‐Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 2008; 27: 793–799. [DOI] [PubMed] [Google Scholar]
- 17. Hosoya NOT, Muto Y. Japanese anthropometric reference data 2001 (JARD 2001). Japanese Journal of Nutritional Assessment 2002; 19: 1–18. [Google Scholar]
- 18. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020; 21: 300–307 e302. [DOI] [PubMed] [Google Scholar]
- 19. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, Group ESCSD . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]


