Abstract
Aims
In a hypertensive population with optimal blood pressure control with a long‐term follow‐up, we aimed at analysing possible predictors of left ventricular (LV) ejection fraction (LVEF) reduction, including indexed mechano‐energetic efficiency (MEEi), a well‐recognized echo‐derived parameter of LV performance.
Methods and results
The study population included 5673 hypertensive patients from the Campania Salute Network with a long‐term follow‐up, normal baseline LVEF (≥50%), and no prevalent cardiovascular (CV) disease. Patients developing LVEF impairment (LVEF < 50% or a reduction of at least 10 percentage points compared with baseline) were compared with patients with persistently normal LVEF. Optimal blood pressure control was achieved in about 80% of patients. Patients who experienced LVEF reduction were 2.41% during a long‐term follow‐up (mean duration 5.6 ± 3.9 years). At baseline, they were older (59.46 ± 11.58 vs. 53.40 ± 11.41, P < 0.0001) and showed higher LV mass index (53.3 ± 12.83 vs. 47.56 ± 9.58, P < 0.0001), left atrial (LA) volume index (14.4 ± 4.2 vs. 13.1 ± 2.8, P < 0.0001) and carotid intima–media thickness (1.99 ± 0.86 vs. 1.61 ± 0.73, P < 0.0001), lower MEEi (0.32 ± 0.08 vs. 0.34 ± 0.07, P = 0.037), and higher prevalence of CV events during follow‐up (13.9% vs. 3%, P < 0.0001) compared with patients with persistently normal LVEF. A logistic regression analysis, performed after running univariate analyses and selecting parameters significantly associated with LVEF reduction, showed that having a CV event [odds ratio (OR) 7.57, P < 0.0001], being in the lowest MEEi quartile (OR 2.43, P = 0.003), and having a larger LA volume index (OR 1.08, P = 0.028) were all parameters independently associated with the development of LV systolic dysfunction. A further logistic regression model, performed by excluding patients experiencing CV events, demonstrated that the lowest MEEi quartile was independently associated with the evolution towards LVEF reduction (OR 2.35, P = 0.004), despite significant impact of LA volume index (OR 1.08, P = 0.023) and antiplatelet therapy (OR 1.89, P < 0.01). Receiver operating characteristic curves showed that the model including MEEi had higher accuracy than the model without MEEi in predicting LVEF reduction (areas under the curve 0.68 vs. 0.63, P = 0.046).
Conclusions
Lower values of MEEi at baseline identify hypertensive patients more liable to develop LVEF reduction. In hypertensive setting, MEEi evaluation improves risk stratification for development of LV systolic dysfunction during long‐term follow‐up.
Keywords: Arterial hypertension, Systolic dysfunction, Mechano‐energetic efficiency, Heart failure
Introduction
According to the American College of Cardiology Foundation/American Heart Association classification, arterial hypertension, even in absence of myocardial functional and/or structural changes, should be considered as stage A of heart failure (HF), identifying patients at high risk of decompensation. 1 Chronic pressure overload related to hypertension initially leads to left ventricular (LV) diastolic impairment and LV hypertrophy (LVH) and eventually to ischaemic dilated cardiomyopathy associated with both diastolic and systolic dysfunction. 2 , 3 , 4 The evolution of hypertensive cardiomyopathy towards systolic HF is usually monitored by measuring left ventricular ejection fraction (LVEF), assessed by ultrasounds. 5 However, LVEF reduction develops when cardiac damage and dysfunction are already established, both often being irreversible. 6 In addition, there is a lack of well‐established echo parameters, allowing HF prediction specifically in the hypertensive setting. Because the identification of hypertensive patients with high‐risk phenotype for developing LV systolic dysfunction could allow more aggressive treatments in order to prevent disease progression from stages A to D of HF, it is emerging the need to collect easily obtainable parameters for early assessment of myocardial dysfunction at a subclinical stage. 7 , 8
Thus, we sought to analyse the possible parameters associated with the development of reduced LVEF and their clinical implications in a large cohort of hypertensive patients, with normal LVEF at baseline, derived from the Campania Salute Network (CSN). In particular, we considered well‐known determinants of LV systolic function and focused on mechano‐energetic efficiency indexed for myocardial mass (MEEi), which previous studies suggested as a predictor of HF onset in the general population. 9 MEEi estimates LV performance by defining the magnitude of LV work developed for a given unit of energetic consumption, thus representing the ratio between stroke work and oxygen consumption, indexed for LV mass. 9 , 10
Methods
Patients' population
The study population included hypertensive patients enrolled in the CSN who showed normal LVEF at the baseline echocardiogram. The CSN has been previously described in detail. 11 Briefly, CSN is an electronic register that collects all data related to cardiologic visits, ultrasound exams, and laboratory tests of hypertensive patients, involving 23 community hospitals, 60 general practitioners (peripheral units), and the Hypertension Outpatient Clinic of the Federico II University in Naples (coordinating centre). The CSN was approved by the Federico II University Hospital Ethic Committee (ClinicalTrials.gov Identifier: NCT02211365). All participants gave signed written informed consent. 11 , 12
From CSN, we selected all hypertensive patients with the following inclusion criteria (Figure 1 ):
Age more than 18 years;
Available follow‐up with echo examination ≥ 12 months;
No history of prevalent CV disease;
Available baseline echocardiography and carotid ultrasound;
Normal LVEF at first echocardiographic exam;
Ability to give informed consent.
Figure 1.
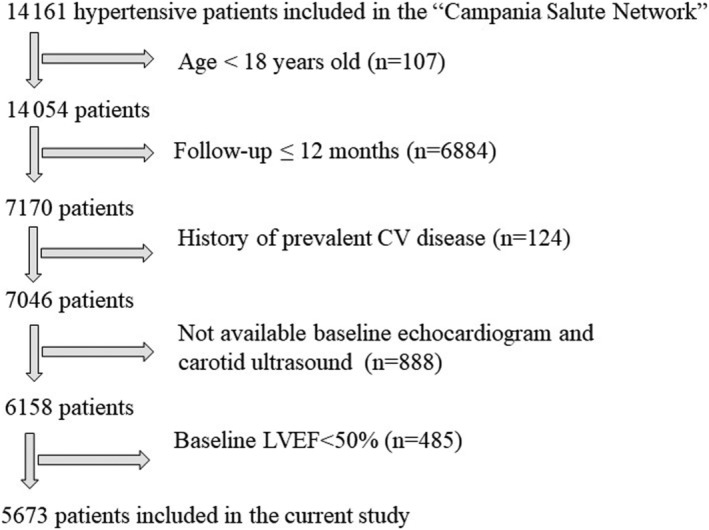
Flow chart showing how hypertensive patients of the current study were selected from the ‘Campania Salute Network’. CV, cardiovascular; LVEF, left ventricular ejection fraction.
The study population thus included 5673 hypertensive patients evaluated during a follow‐up of about 5 years.
Definition and outcomes of interest
Prevalent cardiovascular (CV) disease was defined as any of the following cases: (i) myocardial infarction, (ii) angina pectoris, (iii) coronary or carotid revascularization procedures, (iv) stroke, (v) transitory ischaemic attack, (vi) congestive HF, (vii) clinically relevant heart valvular disease (more than mild valve regurgitations and any stenosis), and (viii) chronic kidney disease (CKD) more than stage 3.
Glomerular filtration rate was calculated with the chronic kidney disease epidemiology collaboration equation. 13
Diabetes was defined as a fasting plasma glucose > 126 mg/dL or specific antidiabetic treatment. 14
Cardiovascular events were defined as the occurrence of myocardial infarction, coronary or carotid revascularization procedures, stroke, and transitory ischaemic attack during the follow‐up.
Diagnosis of arterial hypertension was established on the basis of current ESC/ESH guidelines. 15 For all patients, heart rate (HR), systolic, and diastolic blood pressures were collected in the sitting position, after 5 min of rest, using an oscillometric semiautomatic sphygmomanometer with cuffs of appropriate size. All measurements were repeated in the supine position after the echocardiographic examination. Optimal office blood pressure control was defined according to ESC/ESH guidelines for management of arterial hypertension. 15
Echocardiography
All the echocardiographic exams were performed at the Hypertension Outpatient Clinic of the Federico II University in Naples, using a standardized protocol. After the ultrasound examination, images were stored and read offline, using a workstation, by an expert reader, under the supervision of a senior member. Echocardiograms were performed at first visit and repeated during the follow‐up.
All measurements were assessed according to the latest consolidated convention. 5 , 16 , 17 LVEF was measured by four and two chamber views, tracing the LV endocardial border in end‐diastole and end‐systole with the Simpson biplane method. 5 , 16 , 17 , 18 In our study, normal LVEF was defined as LVEF values greater than or equal 50% at first echocardiogram. Conversely, LVEF was considered reduced at final echo examination for values less than 50% or for a LVEF reduction of 10 percentage points compared with basal value. 19 , 20
Left ventricular mass was estimated from a necropsy‐validated formula and normalized for height in metres to the power of 2.7 [LV mass index (LVMi)]. LVH was defined for values of LVMi > 47 g/m2.7 in women and >50 g/m2.7 in men. 5 , 16 , 17 , 21
Stroke volume was calculated as the difference between LV end‐diastolic and end‐systolic volume by the z‐derived method and indexed for height to the power of 2.04. 22 Pulse pressure was estimated as the difference between systolic blood pressure and diastolic blood pressure. 15 , 23
As previously described, LV myocardial mechano‐energetic efficiency was estimated as the ratio between stroke volume and HR and normalized per grammes of LV mass, and finally expressed in mL/s/g (MEEi). 10 , 24
Left atrial (LA) volume was estimated according to previously validated formula and indexed for height powered to 2. 25
Carotid ultrasound
Carotid ultrasound was performed in supine position. The data were archived and read offline, as previously described. The intima–media thickness (IMT) was measured as the distance between lumen‐intima and media‐adventitia interface in up to two arterial walls, on both near and far walls of distal common carotid (1 cm), bifurcation and proximal internal carotid artery of both sides. A carotid IMT > 1.5 mm has been reported as a plaque. 26
Statistical analysis
Categorical variables were expressed as number (percentage) and continuous variables as mean ± standard deviation. Chi‐square test and Student's t‐test were used to assess differences in dichotomous/categorical and continuous covariates, respectively.
In logistic regression models, MEEi, HR, and LVMi were entered in quartiles. Quartiles of LVMi were computed as follows: first quartile ≤ 41.0 g/m2.7, second quartile ranging from 41.0 to ≤46.3 g/m2.7, third quartile from 46.3 to <52.8 g/m2.7, and fourth (highest) quartile ≥ 52.8 g/m2.7. HR quartiles were assessed as follows: first quartile ≤ 66 bpm, second quartile ranging from 66 to ≤72 bpm, third quartile from 72 to <80 bpm, and fourth (highest) quartile ≥ 80 bpm. MEEi quartiles were measured as follows: first (lowest) quartile ≤ 0.29 mL/s/g, second quartile ranging from 0.30 to ≤0.33 mL/s/g, third quartile from 0.34 to <0.38 mL/s/g, and fourth quartile ≥ 0.38 mL/s/g. Univariate logistic regression analyses were assessed to verify important variables for LVEF reduction and those significantly related to the development of LV systolic dysfunction, in terms of LVEF reduction, were tested in multivariate logistic regression models. Calculation of tolerance and variance inflation was performed by linear modelling, and collinearity was considered acceptable for variance inflation factor less than 3.
In addition, two models were built for the evaluation of predicted probability of LVEF reduction with and without MEEi. For each model, individual hazard functions were generated and compared using receiving operating characteristic curves, and the areas under the curve (AUC) were calculated and compared by the De Long method. Detection of a significant difference between two AUC indicates significant difference in the overall ability of the prediction with the largest area indicating the best predictive model.
In all analyses, a P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics 26 software (IBM Corp).
Results
The study population included 5673 hypertensive patients, with a mean age of 53.5 ± 11.4 years, 58% of whom were male patients, 10% of patients were affected by diabetes mellitus and 24.4% by obesity.
During the follow‐up (mean duration 5.6 ± 3.9 years), optimal blood pressure control was achieved in 79% (4483/5673) of the hypertensive population.
According to the previously defined reduction in LVEF, LV systolic dysfunction occurred in 137 (2.41%) patients. Clinical characteristics and echo data of patients who developed a reduction in LVEF were compared with those of patients (n = 5536) not showing a reduction of LVEF at the end of the follow‐up (Table 1 ). Patients who experienced a reduction in LVEF were older and more often diabetic, showed higher baseline values of fasting plasma glucose, serum creatinine, uric acid than patients who maintained a normal value of LVEF. In addition, patients who reduced LVEF had higher values of HR, LVMi, LA volume index, LV end‐diastolic diameter and carotid IMT, while lower MEEi at baseline examination, as compared with patients who maintained a normal LVEF.
Table 1.
Baseline clinical and echo characteristics of the study population
| Normal LVEF during follow‐up (n = 5536) | Low LVEF during follow‐up (n = 137) | P value | |
|---|---|---|---|
| Parameters | |||
| Age, years, mean (SD) | 53.40 (11.41) | 59.46 (11.58) | <0.0001 |
| Female sex, n (%) | 2.332 (42.1) | 52 (38) | 0.329 |
| Basal LVEF, %, mean (SD) | 65.82 (3.86) | 65.23 (11.53) | 0.104 |
| Follow‐up period, median (IQR) | 5.64 (3.96) | 5.30 (4.00) | 0.323 |
| Diabetes, n (%) | 543 (9.8) | 25 (18.2) | 0.001 |
| Smoker, n (%) | 1060 (19.1) | 26 (19) | 0.960 |
| Obesity, n (%) | 1347 (24.3) | 37 (27) | 0.471 |
| CKD < 3, n (%) | 483 (9.3) | 25 (20.2) | <0.0001 |
| Fasting plasma glucose, mg/dL, mean (SD) | 98.83 (22.96) | 104.67 (29.84) | 0.004 |
| Serum creatinine, mg/dL, mean (SD) | 0.97 (0.32) | 1.08 (0.44) | <0.0001 |
| Serum uric acid, mg/dL, mean (SD) | 5.18 (1.48) | 5.46 (1.64) | 0.026 |
| Serum Triglycerides, mg/dL, mean (SD) | 134.10 (73.37) | 136.33 (77.97) | 0.725 |
| Serum total cholesterol, mg/dL, mean (SD) | 204.62(38.74) | 205.02 (45.62) | 0.909 |
| Serum HDL cholesterol, mg/dL, mean (SD) | 50.46 (12.89) | 49.43 (13.78) | 0.359 |
| Basal body weight, kg, mean (SD) | 77.68 (13.97) | 78.92 (13.91) | 0.304 |
| Systolic BP, mmHg, mean (SD) | 142.73 (17.87) | 144.64 (19.77) | 0.216 |
| Diastolic BP, mmHg, mean (SD) | 88.04 (10.78) | 88.56 (13.27) | 0.609 |
| Heart rate, bpm, mean (SD) | 74.09 (11.44) | 76.14 (11.89) | 0.041 |
| LV mass index, g/m2.7, mean (SD) | 47.56 (9.58) | 53.30 (12.83) | <0.0001 |
| Intima–media thickness, mm, mean (SD) | 1.61 (0.73) | 1.99 (0.86) | <0.0001 |
| Pulse pressure, mmHg, mean (SD) | 61.65 (15.95) | 63.90 (16.39) | 0.103 |
| MEEi, mL/s/g | 0.34 (0.07) | 0.32 (0.08) | 0.037 |
| SVi, mL/m2, mean (SD) | 41.25 (5.92) | 44.13 (9.34) | <0.0001 |
| LV end‐diastolic diameter, mm (SD) | 49.9 (3.6) | 51.1 (4.6) | <0.001 |
| LA volume index, mL/m2 (SD) | 13.1 (2.8) | 14.4 (4.2) | <0.0001 |
BP, blood pressure; bpm, beats per minutes; CKD, chronic kidney disease; HDL, high‐density lipoprotein; LA, left atrial, LV, left ventricular; LVEF, left ventricular ejection fraction; MEEi, mechano‐energetic efficiency indexed for myocardial mass; SVi, stroke volume index.
As shown in Table 2 , patients who developed a reduction in LVEF received a greater number of medications during the follow‐up and were more often treated with diuretics, anti‐renin‐angiotensin system and antiplatelet drugs, as compared with the stable LVEF group.
Table 2.
Follow‐up data and treatments
| Normal LVEF during follow‐up (n = 5536) | Low LVEF during follow‐up (n = 137) | P value | |
|---|---|---|---|
| Number of medications in at least 50% of control visits, mean (SD) | 1.63 (1.01) | 1.96 (0.98) | <0.0001 |
| BP control at the last available visit, n (%) | 4.373 (79.7) | 110 (80.90) | 0.728 |
| CV events, n (%) | 167 (3.0) | 19 (13.9) | <0.0001 |
| Final LVEF, %, mean | 66.25 (3.73) | 58.83 (8.60) | <0.0001 |
| Treatments during follow‐up | |||
| Anti‐RAS, n (%) a | 4.531 (82.4) | 123 (89.8) | 0.024 |
| Antiplatelet therapy, n (%) a | 995 (18.3) | 45 (33.6) | <0.0001 |
| Ca++ channel blockers, n (%) a | 1.340 (24.4) | 34 (24.8) | 0.901 |
| Beta‐blockers, n (%) a | 1.430 (26) | 41 (29.9) | 0.300 |
| Statin, n (%)a | 1.073 (19.8) | 32 (23.9) | 0.239 |
| Diuretics, n (%) a | 2.390 (43.4) | 79 (57.7) | 0.001 |
BP, blood pressure; CI, confidence interval; CV, cardiovascular event; LVEF, left ventricular ejection fraction; OR, odds ratio; RAS, renin‐angiotensin system.
Medications used for more than 50% of control visits.
During the follow‐up, CV events occurred in 19 patients (13.9%) in the group which reduced LVEF and 167 patients (3.0%) in the group with persistently normal LVEF (P < 0.001).
Indexed mechano‐energetic efficiency was significantly correlated with both LVMi (r = 0.31, P < 0.0001) and HR (r = −0.64, P < 0.0001).
Univariate logistic regression analyses showed that age, baseline pulse pressure, diabetes, CKD, baseline LA volume index, baseline highest LVMi quartile, baseline IMT, baseline lowest MEEi quartile, medication in at least 50% of control visit, antiplatelet therapy, diuretics in at least 50% of control visit, and the occurrence of CV events were significantly related to LVEF reduction (Supporting Information, Table S1 ).
A logistic regression analysis showed that having a CV event, being in the lowest MEEi quartile and having a larger LA volume index were all parameters independently associated with the occurrence of LV systolic dysfunction (Table 3 ). When the lowest MEEi quartile was replaced by the highest LVMi quartile in a subsequent analysis, the latter did not enter the model (Table S2 ).
Table 3.
Multivariate logistic regression model performed in hypertensive patients experiencing CV events to test parameters associated with LV systolic dysfunction
| Parameter | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.00 | 0.97–1.02 | 0.805 |
| Baseline pulse pressure (mmHg) | 1.01 | 0.99–1.02 | 0.288 |
| Baseline diabetes | 1.00 | 0.99–1.01 | 0.729 |
| Baseline CKD | 1.56 | 0.86–2.87 | 0.144 |
| Baseline LA volume index, mL/m2 | 1.08 | 1.01–1.16 | 0.028 |
| Baseline intima–media thickness, mm | 1.10 | 0.81–1.49 | 0.553 |
| Baseline lowest MEEi quartile | 2.43 | 1.35–4.39 | 0.003 |
| Medication in at least 50% of control visit | 1.06 | 0.80–1.40 | 0.677 |
| Antiplatelet therapy | 1.71 | 1.04–2.82 | 0.036 |
| Diuretics in at least 50% of control visit | 1.26 | 0.72–2.20 | 0.414 |
| CV events | 7.57 | 2.84–20.2 | <0.0001 |
CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; LA, left atrial, LV, left ventricular; MEEi, mechano‐energetic efficiency indexed for myocardial mass; OR, odds ratio.
A further logistic regression analysis, performed by excluding hypertensive patients who experienced CV events, confirmed that the lowest MEEi quartile, LA volume index, and the use of antiplatelet drugs were associated with the development of LVEF reduction (Table 4 ).
Table 4.
Multivariate logistic regression model performed in hypertensive patients without CV events to test parameters associated with LV systolic dysfunction
| Parameter | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.00 | 0.97–1.02 | 0.820 |
| Baseline pulse pressure (mmHg) | 1.01 | 0.99–1.02 | 0.468 |
| Baseline diabetes | 1.00 | 0.99–1.01 | 0.541 |
| Baseline CKD | 1.58 | 0.87–2.86 | 0.129 |
| Baseline LA volume index, mL/m2 | 1.08 | 1.01–1.16 | 0.023 |
| Baseline intima–media thickness, mm | 1.13 | 0.74–1.52 | 0.417 |
| Baseline lowest MEEi quartile | 2.35 | 1.31–4.22 | 0.004 |
| Medication in at least 50% of control visit | 1.07 | 0.81–1.42 | 0.615 |
| Antiplatelet therapy | 1.89 | 1.16–3.08 | 0.01 |
| Diuretics in at least 50% of control visit | 1.22 | 0.70–2.14 | 0.481 |
CI, confidence interval; CKD, chronic kidney disease; LA, left atrial, LV, left ventricular; MEEi, mechano‐energetic efficiency indexed for myocardial mass; OR, odds ratio.
The hazard function of the regression models with and without MEEi was compared. As shown in Figure 2 , the model that included MEEi predicted LVEF reduction better than the model without this parameter (AUC 0.68 vs. AUC 0.63, P = 0.046).
Figure 2.
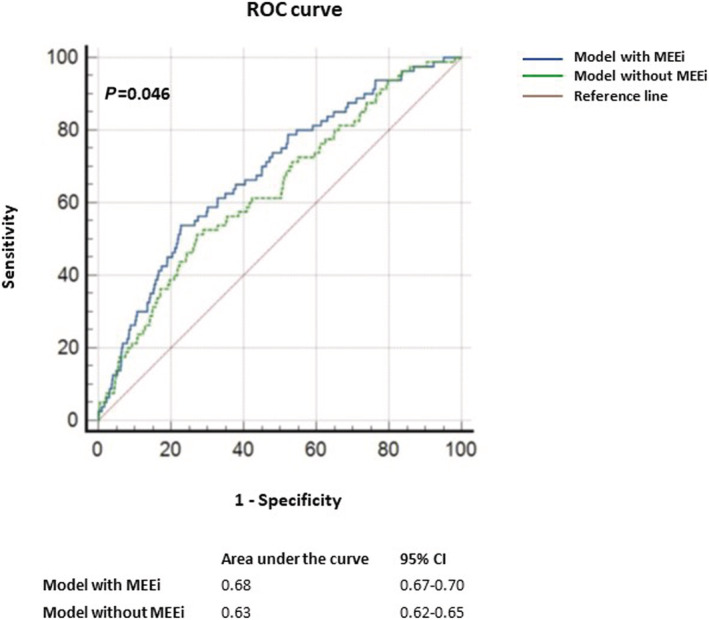
ROC curves for predicted probability assessed according to two models, the first one including parameters in Table 4 including MEEi and the second one including the same parameters without MEEi. The model including MEEi predicted LVEF reduction better than the model without this parameter. MEEi, indexed mechano‐energetic efficiency; ROC, receiver operating characteristic.
Discussion
To the best of our knowledge, this is the largest study performed on a treated hypertensive population showing that MEEi could represent an early parameter associated with the development of future LV systolic dysfunction. In the present study, we demonstrated that (i) in a cohort of hypertensive patients without prevalent CV disease, despite a large rate of optimal blood pressure control, 2.41% of patients developed LV systolic dysfunction, defined as LVEF < 50% or 10 percentage point reduction as compared with the basal value 19 ; (ii) the association between baseline LA volume index and future reduction of LVEF underlines early LV diastolic dysfunction which precedes systolic impairment; and (iii) a value of MEEi below 0.29 is independently associated with the evolution towards LVEF reduction in hypertensive patients without CV events.
It is well‐known that arterial hypertension is a major risk factor for the development of CV events, especially when it is combined with other conditions, such as diabetes mellitus, CKD, dyslipidaemia, LVH, and vascular atherosclerosis, that could exert their impact in a cumulative way. 27 , 28 The results of the present study corroborate this assumption, because hypertensive patients developing LVEF reduction, had also a higher prevalence of diabetes mellitus and CKD, and higher basal values of LVMi and IMT and showed a higher rate of CV events during the follow‐up. However, CV events only occurred in 13.9% of patients who developed LVEF reduction during the follow‐up, thus implying that additional mechanisms could underline the evolution towards HF in hypertensive patients. Therefore, we searched for additional possible early altered parameters associated with development of LVEF reduction, also after excluding patients experiencing CV events. 6
A quite large variability in the prevalence of LV systolic dysfunction has been reported, from 3% to 6% in the general population and from 3.6% to 14% in hypertensive patients, related to differences in patients' clinical characteristics and CV comorbidities 29 , 30 , 31 , 32 and in part to the technical variability of measuring LVEF making it difficult to perform comparisons among different settings. 5 Thus, the exact incidence of reduction in LVEF in the specific setting of arterial hypertension is still unclear. In our study population, the prevalence of fall in LVEF during the follow‐up was 2.41%, which is lower than reported in literature, but it has to be considered that optimal blood pressure control was achieved in about 80% of our hypertensive patients, and this could, at least in part, account for the lower rate of reduction in LVEF we found.
Recent evidence demonstrated that low hyperaemic coronary flow, measured by positron emission tomography, related to myocardial mass predicted hospitalization for HF in hypertensive patients with no obstructive coronary artery disease. 33 In addition, cardiac magnetic resonance and 31P spectroscopy measured dysfunction in adenosine triphosphate delivery were observed in obese patients with a high prevalence of arterial hypertension. 34 These studies support the speculation that arterial hypertension, especially when combined with other CV risk factors, determines metabolic alterations by an increased myocardial demand and/or reduced efficiency of energy utilization, this impairment identifying a subclinical damage with an elevated risk of HF development. However, the use of positron emission tomography, cardiac magnetic resonance, and 31P spectroscopy would not be feasible or advisable into routine clinical decision‐making algorithms for patients with hypertension, because hypertensive patients are widely present in the global adult population, reaching a prevalence of more than 20%. Thus, the possibility to investigate a subclinical LV impairment by a simple method easily derived from standard echocardiography is of relevant interest, considering its accessible approach. 35
In this panorama, MEEi, as the ratio between stroke work and oxygen consumption for gramme of LV mass, appears a simply obtainable index of LV systolic dysfunction for risk‐stratification in hypertensive patients. 9 , 10
Reduced MEEi was identified as a predictor of HF onset in a cohort of 1912 American Indian patients, derived from the Strong Heart Study, who had normal LVEF at baseline, no CV events and only 27% of whom was affected by arterial hypertension. 9 In addition, it was demonstrated to be associated with subclinical systolic dysfunction in a general population study. 36 MEEi was also demonstrated to be a powerful prognosticator of adverse CV events in multiple different settings, including the hypertensive one. 37 , 38
In the present study, hypertensive patients who experienced LVEF reduction had lower values of MEEi at baseline thus demonstrating subclinical LV impairment present in these patients, when LVEF was still normal. In addition, patients in the lowest MEEi quartile developed a higher rate of LVEF reduction, thus showing an increased risk to develop LV systolic dysfunction.
Indexed mechano‐energetic efficiency gathers in one parameter info about three different aspects, possibly present at the same time in the hypertensive heart: functional impairment with alteration in LV stroke work, morphological changes with increased LV mass, and metabolic dysfunction with increased oxygen consumption. The correlation of both HR, as main determinant of oxygen consumption, and LVMi, as structural change, with LV systolic dysfunction has been previously reported. 32 , 39
On the other hand, the present study suggests that MEEi, including in its computation multiple aspects, could represent a more sensitive parameter for the identification of LV systolic dysfunction than HR or LVMi alone, because both did not reach significance in univariate and multivariate models respectively. It has to be considered that previous studies recognize LV systolic dysfunction as a drop of LVEF below a defined threshold, whereas we also included in this definition patients who experienced a reduction of at least 10 percentage points compared with baseline. Thus, besides patients with LVEF drop below 50%, we included in the evaluation of LVEF reduction also patients with a possible early systolic impairment (with a drop of at least 10 percentage points compared with baseline but above the cut‐off of 50%); this could explain the need of a more complex and sensitive parameter as MEEi for the evaluation of LVEF impairment.
Logistic regression analyses provided further information. After adjusting for several clinical and echo parameters, CV events were highly associated with the development of LV systolic dysfunction; nonetheless, the association between LVEF reduction and low MEEi remained significant despite the impact of CV events.
A subsequent logistic regression model, run after excluding patients experiencing CV events during the long‐term follow‐up, showed that low MEEi remained independently associated with the occurrence of LVEF reduction together with LA volume index and antiplatelet therapy (Figure 3 ).
Figure 3.
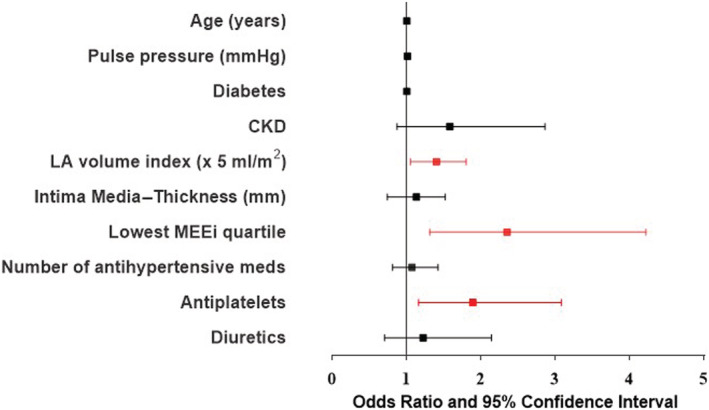
Odds ratio and 95% confidence interval of clinical and echocardiographic parameters highlighting parameters associated with the development of LV systolic dysfunction. LA, left atrial; LV, left ventricular; MEEi, indexed mechano‐energetic efficiency.
The greater use of antiplatelet drugs could just be a consequence of the worse clinical conditions in these patients, due to exacerbated atherosclerotic process and endothelial dysfunction. 28 , 40 It was reported that arterial hypertension can induce coronary vascular remodelling and microvascular impairment that could contribute to LVEF decline. 40 , 41
Left atrial volume, reflecting diastolic function and LV filling pressure level, was demonstrated to be a predictor of HF with both preserved and reduced LVEF. 42 , 43 In the present study, we confirmed those findings, because baseline LA volume index was associated with the subsequent occurrence of LV systolic dysfunction and it resulted significantly increased in hypertensive patients experiencing LVEF reduction, revealing an early diastolic dysfunction.
Furthermore, the presence of MEEi in the model for the predicted probability of LV systolic impairment, provided an increased AUC as compared with the model without this parameter, thus demonstrating the higher accuracy of the model including MEEi.
Therefore, the use of MEEi could help to characterize the HF risk profile of hypertensive patients and could be an important parameter for defining future development of LV systolic dysfunction in this setting.
Additional studies are needed to explore which type of drugs could have a greater impact on MEEi and could possibly have an effect in preventing progression towards LVEF reduction in hypertensive patients.
Limitations
The main limitation of the study is linked to the lack of sensitive additional parameters for the evaluation of subclinical LV systolic dysfunction, such as global longitudinal strain and strain derived myocardial work components, in order to analyse their ability to detect LVEF reduction in hypertensive patients. Nonetheless, MEEi is a well‐established parameter for the evaluation of LV performance, and its capability to predict a future LV systolic dysfunction corroborates the fact that standard echocardiography is a simple, but accurate method for LV assessment and its derived parameters can provide very useful predictors in the hypertensive setting.
In addition, it has to be considered that CSN is an observational registry and thus possibly influenced by selection bias. However, all patients underwent the same echocardiographic and visit assessment and followed the same standardized protocol.
Conclusions
In a population of hypertensive patients achieving optimal blood pressure control, incident LV systolic dysfunction, assessed by reduction of LVEF, was limited to 2.41%. Lower values of MEEi at baseline significantly contributed to identify patients more prone to develop LV systolic dysfunction.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by Ministero della Salute (grant 00014Prin_2017 ID43237).
Supporting information
Table S1. Univariate regression analyses.
Table S2. Multivariate logistic regression model performed in hypertensive patients experiencing CV events replacing the lowest MEEi quartile with the highest LV mass index quartile.
Acknowledgements
Dr M. Lembo participates in the PhD programme in Cardiovascular Pathophysiology and Therapeutics (CardioPaTh).
Manzi, M. V. , Mancusi, C. , Lembo, M. , Esposito, G. , Rao, M. A. E. , de Simone, G. , Morisco, C. , Trimarco, V. , Izzo, R. , and Trimarco, B. (2022) Low mechano‐energetic efficiency is associated with future left ventricular systolic dysfunction in hypertensives. ESC Heart Failure, 9: 2291–2300. 10.1002/ehf2.13908.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology F , American Heart Association Task Force on Practice G . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 2. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 3. Sorrentino R, Esposito R, Santoro C, Vaccaro A, Cocozza S, Scalamogna M, Lembo M, Luciano F, Santoro A, Trimarco B, Galderisi M. Practical impact of new diastolic recommendations on noninvasive estimation of left ventricular diastolic function and filling pressures. J Am Soc Echocardiogr. 2020; 33: 171–181. [DOI] [PubMed] [Google Scholar]
- 4. Lembo M, Manzi MV, Mancusi C, Morisco C, Rao MAE, Cuocolo A, Izzo R, Trimarco B. Advanced imaging tools for evaluating cardiac morphological and functional impairment in hypertensive disease. J Hypertens. 2021; 40: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perrone‐Filardi P, Coca A, Galderisi M, Paolillo S, Alpendurada F, de Simone G, Donal E, Kahan T, Mancia G, Redon J, Schmieder R, Williams B, Agabiti‐Rosei E. Non‐invasive cardiovascular imaging for evaluating subclinical target organ damage in hypertensive patients: a consensus paper from the European Association of Cardiovascular Imaging (EACVI), the European Society of Cardiology Council on Hypertension, and the European Society of Hypertension (ESH). Eur Heart J Cardiovasc Imaging. 2017; 18: 945–960. [DOI] [PubMed] [Google Scholar]
- 6. Galderisi M, Trimarco B. Global longitudinal strain: a novel hallmark of cardiac risk in arterial hypertension. J Hypertens. 2016; 34: 1050–1051. [DOI] [PubMed] [Google Scholar]
- 7. Lembo M, Esposito R, Santoro C, Lo Iudice F, Schiano‐Lomoriello V, Fazio V, Grimaldi MG, Trimarco B, de Simone G, Galderisi M. Three‐dimensional echocardiographic ventricular mass/end‐diastolic volume ratio in native hypertensive patients: relation between stroke volume and geometry. J Hypertens. 2018; 36: 1697–1704. [DOI] [PubMed] [Google Scholar]
- 8. Lembo M, Santoro C, Sorrentino R, Trimarco B, Galderisi M, Esposito R. Impact of left ventricular mass/end‐diastolic volume ratio by three‐dimensional echocardiography on two‐dimensional global longitudinal strain and diastolic function in native hypertensive patients. J Hypertens. 2019; 37: 2041–2047. [DOI] [PubMed] [Google Scholar]
- 9. Losi MA, Izzo R, Mancusi C, Wang W, Roman MJ, Lee ET, Howard BV, Devereux RB, de Simone G. Depressed myocardial energetic efficiency increases risk of incident heart failure: the strong heart study. J Clin Med. 2019; 8: 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mancusi C, Losi MA, Izzo R, Canciello G, Manzi MV, Sforza A, De Luca N, Trimarco B, de Simone G. Effect of diabetes and metabolic syndrome on myocardial mechano‐energetic efficiency in hypertensive patients. The campania salute network. J Hum Hypertens. 2017; 31: 395–399. [DOI] [PubMed] [Google Scholar]
- 11. Stabile E, Izzo R, Rozza F, Losi MA, De Luca N, Trimarco B. Hypertension survey in Italy: novel findings from the campania salute network. High Blood Pressure Cardiovasc Prevent. 2017; 24: 363–370. [DOI] [PubMed] [Google Scholar]
- 12. Manzi MV, Mancusi C, Trimarco V, Izzo R, Franco D, Barbato E, Morisco C, Trimarco B. The intergated approach to the management of arterial hypertension: the campania salute network. Panminerva Med. 2021; 63: 451–457. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American DA. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2019. Diabetes Care. 2019; 42: S13–S28. [DOI] [PubMed] [Google Scholar]
- 15. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, Group ESCSD . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018; 39: 3021–3104. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 17. de Simone G, Mancusi C, Esposito R, De Luca N, Galderisi M. Echocardiography in arterial hypertension. High Blood Pressure Cardiovasc Prevent. 2018; 25: 159–166. [DOI] [PubMed] [Google Scholar]
- 18. Lembo M, Santoro C, Sorrentino R, Canonico ME, Fazio V, Trimarco B, Tadic M, Galderisi M, Esposito R. Interrelation between midwall mechanics and longitudinal strain in newly diagnosed and never‐treated hypertensive patients without clinically defined hypertrophy. J Hypertens. 2020; 38: 295–302. [DOI] [PubMed] [Google Scholar]
- 19. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Group ESCSD . 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016; 37: 2768–2801. [DOI] [PubMed] [Google Scholar]
- 20. Cadeddu Dessalvi C, Deidda M, Mele D, Bassareo PP, Esposito R, Santoro C, Lembo M, Galderisi M, Mercuro G. Chemotherapy‐induced cardiotoxicity: new insights into mechanisms, monitoring, and prevention. J Cardiovasc Med (Hagerstown). 2018; 19: 315–323. [DOI] [PubMed] [Google Scholar]
- 21. Lembo M, Santoro C, Sorrentino R, Fazio V, Canonico ME, Chiariello L, Galderisi M, Esposito R. Prominent basal and middle strain longitudinal involvement in newly‐diagnosed and never treated hypertensive patients without clear‐cut hypertrophy. Int J Cardiol. 2020; 304: 179–184. [DOI] [PubMed] [Google Scholar]
- 22. De Marco M, Gerdts E, Mancusi C, Roman MJ, Lonnebakken MT, Lee ET, Howard BV, Devereux RB, de Simone G. Influence of left ventricular stroke volume on incident heart failure in a population with preserved ejection fraction (from the strong heart study). Am J Cardiol. 2017; 119: 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lembo M, Esposito R, Lo Iudice F, Santoro C, Izzo R, De Luca N, Trimarco B, de Simone G, Galderisi M. Impact of pulse pressure on left ventricular global longitudinal strain in normotensive and newly diagnosed, untreated hypertensive patients. J Hypertens. 2016; 34: 1201–1207. [DOI] [PubMed] [Google Scholar]
- 24. de Simone G, Izzo R, Losi MA, Stabile E, Rozza F, Canciello G, Mancusi C, Trimarco V, De Luca N, Trimarco B. Depressed myocardial energetic efficiency is associated with increased cardiovascular risk in hypertensive left ventricular hypertrophy. J Hypertens. 2016; 34: 1846–1853. [DOI] [PubMed] [Google Scholar]
- 25. Canciello G, de Simone G, Izzo R, Giamundo A, Pacelli F, Mancusi C, Galderisi M, Trimarco B, Losi MA. Validation of left atrial volume estimation by left atrial diameter from the parasternal long‐axis view. J Am Soc Echocardiogr. 2017; 30: 262–269. [DOI] [PubMed] [Google Scholar]
- 26. Izzo R, Stabile E, Esposito G, Trimarco V, Laurino FI, Rao MA, De Marco M, Losi MA, De Luca N, Trimarco B, de Simone G. Development of new atherosclerotic plaque in hypertensive patients: an observational registry study from the Campania‐Salute network. J Hypertens. 2015; 33: 2471–2476. [DOI] [PubMed] [Google Scholar]
- 27. Reinikainen J, Laatikainen T, Karvanen J, Tolonen H. Lifetime cumulative risk factors predict cardiovascular disease mortality in a 50‐year follow‐up study in Finland. Int J Epidemiol. 2015; 44: 108–116. [DOI] [PubMed] [Google Scholar]
- 28. Lembo M, Sicari R, Esposito R, Rigo F, Cortigiani L, Lo Iudice F, Picano E, Trimarco B, Galderisi M. Association between elevated pulse pressure and high resting coronary blood flow velocity in patients with angiographically normal epicardial coronary arteries. J Am Heart Assoc. 2017; 6: e005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of "asymptomatic" left ventricular systolic dysfunction: implications for screening. Ann Intern Med. 2003; 138: 907–916. [DOI] [PubMed] [Google Scholar]
- 30. Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003; 108: 977–982. [DOI] [PubMed] [Google Scholar]
- 31. Devereux RB, Bella JN, Palmieri V, Oberman A, Kitzman DW, Hopkins PN, Rao DC, Morgan D, Paranicas M, Fishman D, Arnett DK, Hypertension Genetic Epidemiology Network Study G . Left ventricular systolic dysfunction in a biracial sample of hypertensive adults: the hypertension genetic epidemiology network (HyperGEN) study. Hypertension. 2001; 38: 417–423. [DOI] [PubMed] [Google Scholar]
- 32. Verdecchia P, Angeli F, Gattobigio R, Sardone M, Porcellati C. Asymptomatic left ventricular systolic dysfunction in essential hypertension: prevalence, determinants, and prognostic value. Hypertension. 2005; 45: 412–418. [DOI] [PubMed] [Google Scholar]
- 33. Brown JM, Zhou W, Weber B, Divakaran S, Barrett L, Bibbo CF, Hainer J, Taqueti VR, Dorbala S, Blankstein R, Di Carli MF. Low coronary flow relative to myocardial mass predicts heart failure in symptomatic hypertensive patients with no obstructive coronary artery disease. Eur Heart J. 2021; ehab610. 10.1093/eurheartj/ehab610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rayner JJ, Peterzan MA, Clarke WT, Rodgers CT, Neubauer S, Rider OJ. Obesity modifies the energetic phenotype of dilated cardiomyopathy. Eur Heart J. 2021; 43: 868–877. 10.1093/eurheartj/ehab663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Collaboration NCDRF . Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population‐based measurement studies with 19.1 million participants. Lancet. 2017; 389: 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mancusi C, Midtbø H, De Luca N, Halland H, de Simone G, Gerdts E. Association of myocardial energetic efficiency with circumferential and longitudinal left ventricular myocardial function in subjects with increased body mass index (the FATCOR study). J Clin Med. 2021; 10: 1581. 10.3390/jcm10081581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bahlmann E, Einarsen E, Cramariuc D, Midtbo H, Mancusi C, Rossebo A, Willems S, Gerdts E. Low myocardial energetic efficiency is associated with increased mortality in aortic stenosis. Open Heart. 2021; 8: e001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cioffi G, Mancusi C, de Simone G, Ognibeni F, Orsolini G, Dalbeni A, Gatti D, Fassio A, Adami G, Rossini M, Viapiana O, Giollo A. Predictors and prognostic role of low myocardial mechano‐energetic efficiency in chronic inflammatory arthritis. J Hypertens. 2021; 39: 53–61. [DOI] [PubMed] [Google Scholar]
- 39. Opdahl A, Ambale Venkatesh B, Fernandes VRS, Wu CO, Nasir K, Choi EY, Almeida ALC, Rosen B, Carvalho B, Edvardsen T, Bluemke DA, Lima JAC. Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (multi‐ethnic study of atherosclerosis). J Am Coll Cardiol. 2014; 63: 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou W, Brown JM, Bajaj NS, Chandra A, Divakaran S, Weber B, Bibbo CF, Hainer J, Taqueti VR, Dorbala S, Blankstein R, Adler D, O'Gara P, Di Carli MF. Hypertensive coronary microvascular dysfunction: a subclinical marker of end organ damage and heart failure. Eur Heart J. 2020; 41: 2366–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamasaki S, Al Suwaidi J, Higano ST, Miyauchi K, Holmes DR Jr, Lerman A. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy. J Am Coll Cardiol. 2000; 35: 1654–1660. [DOI] [PubMed] [Google Scholar]
- 42. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015; 8: 295–303. [DOI] [PubMed] [Google Scholar]
- 43. Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TS. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well‐preserved left ventricular systolic function. Am J Cardiol. 2005; 96: 832–836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate regression analyses.
Table S2. Multivariate logistic regression model performed in hypertensive patients experiencing CV events replacing the lowest MEEi quartile with the highest LV mass index quartile.


