Abstract
Sustained high levels of activated polymorphonuclear leukocytes (PMNs) and PMN-derived proteases in the microenvironment of chronic venous leg ulcers (CVLUs) are linked to chronic inflammation and delayed healing. Uncontrolled PMN activity eventually destroys newly developed tissue and degrades critical growth factors. The bioactive components of fish oil (n-3 eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) have strong inflammation-resolving actions and have been shown to assuage PMN activity, but have not been tested in CVLU patients. This randomized controlled study compared the effectiveness of oral EPA + DHA therapy to a placebo for reducing PMN activation in CVLU microenvironments. At Days 0, 28, and 56, markers of PMNs (CD15) and activated PMNs (CD66b), and levels of PMN-derived proteases human neutrophil elastase and matrix metalloproteinase-8 were measured in CVLU fluid from patients receiving standard compression therapy and (1) EPA + DHA therapy (n = 16) or (2) placebo (n = 19). By Day 56, the EPA + DHA Group had a significantly lower percentage of CD66b+ cells in CVLU fluid compared to Day 0 (p = 0.02) and to Day 28 (p = 0.05). Importantly, there were downward trends in levels of both matrix metalloproteinase-8 and human neutrophil elastase over time in the EPA + DHA Group, which also demonstrated greater reductions in wound area by Day 28 (57% reduction) and Day 56 (76% reduction) than the Control Group (35% and 59%, respectively). Moreover, reductions in wound area had significant negative relationships with CD15+ cells in wound fluid at Days 28 (p = 0.008) and 56 (p < 0.001), and CD66b+ cells at Days 28 (p = 0.04) and 56 (p = 0.009). The collective findings provide supplemental evidence that high levels of activated PMNs in CVLU microenvironments inhibit healing, and suggest that EPA + DHA oral therapy may modulate PMN activity and facilitate healing of CVLUs when added to standard care regimens.
Chronic venous leg ulcers (CVLU) are common, costly conditions in the aging population that have high recidivism rates and cause considerable morbidity.1,2 In the US alone, the average annual incidence of venous leg ulcers in individuals aged 65+ is 2.2% with payer burden at nearly $15 billion, excluding out-of-pocket payments or other indirect costs such as lost productivity.1 Additionally, the incidence of CVLUs is increasing dramatically due to their association with obesity and aging.3–5 CVLUs are very distressing for patients because of pain, reduced mobility, social isolation, and high health care costs related to protracted treatments that collectively reduce quality of life.6 Current strategies for treating CVLUs generally involve locally applied therapies such as compression (the gold standard), oxygen therapy, growth factors, and various dressing types that are often ineffective or result in only short-term recovery.2,7 Novel adjuvant therapies aimed at the underlying etiology of CVLUs are greatly needed to improve healing outcomes and prevent recurrence of these recalcitrant wounds. The purpose of this study was to test the effectiveness of a low-risk, low-cost oral therapy containing the bioactive components of fish oil (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) to reduce numbers of activated polymorphonuclear leukocytes (PMNs) and PMN-derived proteases in the microenvironment of CVLUs associated with chronic inflammation and delayed healing.8,9
The pathogenesis of CVLUs involves venous hypertension and high numbers of activated PMNs in the wound microenvironment that foster unremitting inflammation.10–12 Sustained levels of activated PMNs lead to excessive quantities of PMN-derived proteases such as human neutrophil elastase (HNE) and matrix metalloproteinase-8 (MMP-8) that ultimately destroy growth factors, receptors and the extracellular matrix that are essential for wound healing.10–13 Therefore, reducing prolonged, excessive PMN activity at CVLU sites may facilitate healing progression. Studies involving cell cultures and animal models demonstrate that EPA + DHA are metabolized to lipid mediators (e.g., eicosanoids, resolvins) that inhibit PMN migration to inflamed sites and reduce cell synthesis and secretion of proinflammatory cytokines that are involved in recruiting and activating PMNs.14,15 Human studies report decreases in the chemotactic responsiveness of PMNs from whole blood after just 4 weeks of EPA + DHA supplementation in healthy subjects.16,17 Additionally, our early pilot work provides evidence that oral supplementation with EPA + DHA can push an EPA + DHA lipid mediator profile in acute human wound microenvironments that is associated with decreased levels of myeloperoxidase, a biomarker of PMN activity.18 Nevertheless, no studies have investigated the effects of EPA + DHA therapy on the elevated levels of activated PMNs and PMN-derived proteases typically found in the microenvironment of CVLUs linked to chronic inflammation and slow healing. For the current study, we hypothesized that boosting systemic levels of EPA + DHA in blood plasma using an oral supplement would aid healing by reducing PMN activation locally in CVLU wound fluid that “traps” the wound in a chronic inflammatory state.
The primary aim of this randomized, double-blind, controlled study was to assess the effectiveness of EPA + DHA oral supplementation vs. placebo in terms of reductions in numbers of PMNs and activated PMNs in blood and wound fluid, and levels of PMN-derived MMP-8 and HNE in wound fluid over time in patients with CVLUs. The impact of treatment vs. placebo on reduction in wound area was also evaluated.
MATERIALS AND METHODS
This prospective randomized double-blind controlled study was conducted at the Clinical Research Center (CRC) associated with a large Midwest University Medical Center between August 2012 and July 2015 after approval by the institutional review board and according to the 1975 Declaration of Helsinki Principles. Written informed consent was obtained from all participants prior to beginning the study.
A total of 40 participants were recruited over a period of 24 months from a pool of patients scheduled to begin receiving CVLU treatment with a silver-coated dressing beneath a four-layer compression dressing at the University-affiliated Comprehensive Wound Centers. Men and women between 18 and 81 years of age having at least one existing CVLU between the ankle and knee for ≥ 3 months and (1) prescribed compression therapy, (2) an ankle brachial pressure index of ≥ 0.8, (3) a target wound of at least 5 cm2 were eligible to participate in the study. Excluded were patients (1) allergic to fish or seafood, (2) having immunological related conditions or chronic inflammatory skin diseases, (3) receiving blood thinning therapy or corticosteroids, or (4) required to take anti-inflammatory drugs such as corticosteroids or ibuprofen more than twice a week. Study participants were randomly assigned to treatment with EPA + DHA supplementation (EPA + DHA Group) or placebo (Control Group) on Day 0 using a computer-generated table of random numbers after eligibility screening, informed consent, and enrollment to the study. The primary efficacy variables were reductions in activated PMN counts in blood plasma and CVLU fluid, reductions in levels of MMP-8 and HNE in CVLU fluid, and percent reduction in wound area on Day 28 and Day 56 compared to Day 0.
Participants in the EPA + DHA Group consumed five opaque EPA + DHA softgels each day of the study which provided a total of 2.5 g EPA and 0.5 g DHA. The US Federal Drug Administration evaluated the safety of EPA and DHA and concluded that a daily intake of up to 3.0 g/day is acceptable for the general public.19 A similar EPA + DHA dose significantly increased plasma levels in healthy human subjects after 4 weeks in our pilot work,20 and significantly reduced ex vivo proinflammatory cytokine production after 4 weeks.21
Participants in the Control Group consumed five opaque placebo softgels each day of the study which provided a total daily intake of 2.5 mL of mineral oil, which is well below the therapeutic dose of 10 mL for constipation. Mineral oil is chemically inert and on ingestion the majority (98%) remains unabsorbed in the feces. The same daily mineral oil dose has been used as the placebo in previous studies with no adverse events being reported.18,20 All softgels were identical in appearance, lemon-flavored to reduce the risk of “fish burps,” and compounded and packaged in like containers by J.R. Carlson Laboratories, Inc. (Arlington Heights, IL).
All participants also consumed 1 aspirin (ASA) tablet each day which provided a total daily intake of 81 mg. ASA enhances the action of the EPA-derived resolvin species reported to be particularly effective in reducing PMN recruitment to inflamed tissue sites.22 A general summary of the study design is presented in Figure 1.
Figure 1.
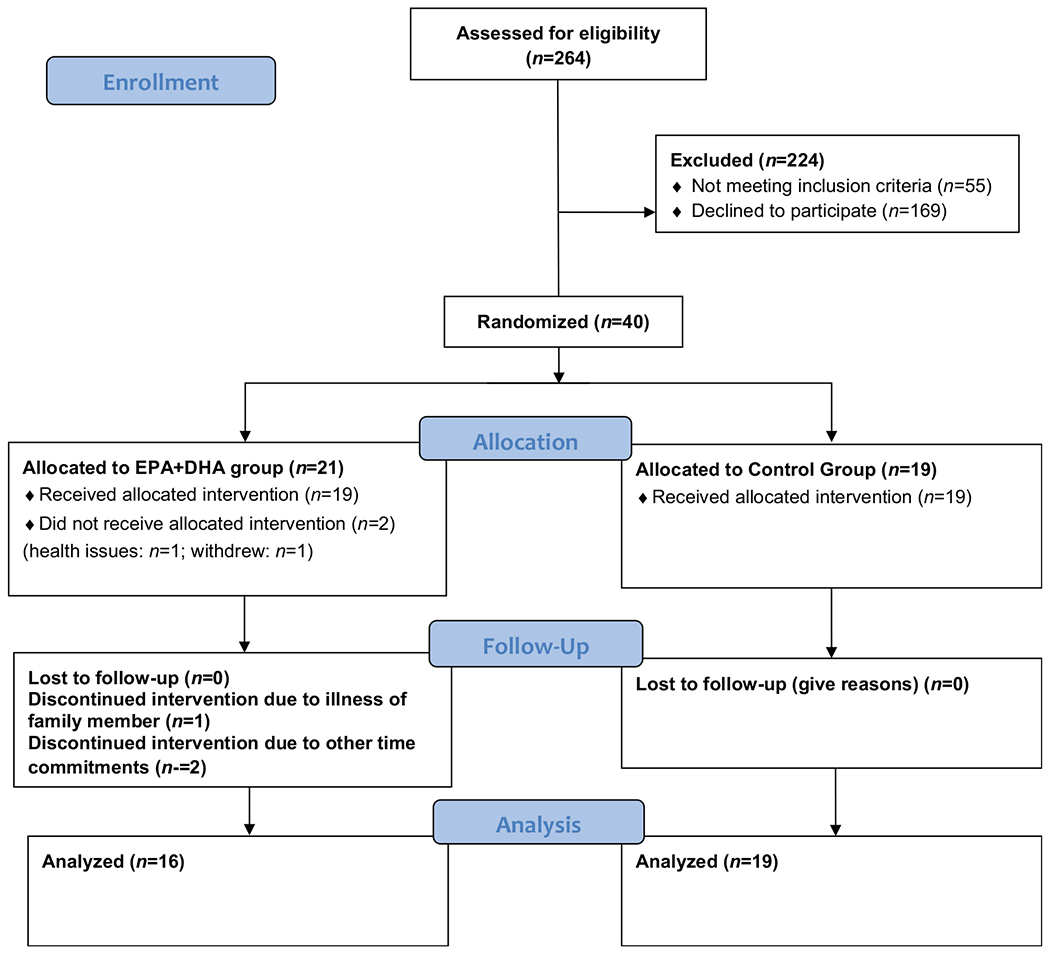
The CONSORT diagram illustrating the flow of patients who participated in the study.
Protocol
All participants in both groups were studied at 0, 28, and 56 days at the CRC. On Day 0 (baseline), participants completed an electronic health and lifestyle questionnaire wherein they self-reported sociodemographic data. They also reported the estimated age of their leg wounds. Blood and CVLU fluid were collected and the leg ulcer area yet to be healed was measured using a single digital camera photogrammetry (SCP) system. Verbal and written instructions were given to all participants to maintain their usual diets, but to exclude fish, seafood, algae, kelp, and other nutritional supplements containing fish oil until study completion to help isolate the EPA + DHA treatment effect. Participants were instructed to refrain from using anti-inflammatory drugs during the study interval if possible, other than the 81 mg/day of ASA. All participants received a pill bottle containing the subsequent month’s supply of softgels and one containing the subsequent month’s supply of ASA. They were instructed to store the softgels in the refrigerator and to take 5 softgels and 1 ASA tablet every day with their evening meal to reduce the incidence of “fish burps” and to encourage compliance. Participants began taking the softgels at the end of enrollment day (Visit 1—Day 0) and were instructed to bring empty bottles to Visit 2 (28 days later). At Visit 2, blood and wound fluid samples were collected, and wounds were measured. Pill bottles were collected to evaluate compliance and new bottles containing the subsequent month’s supply of softgels and ASA were provided. At Visit 3 (56 days after Visit 1), samples of blood and wound fluid were collected, pill bottles were collected and final wound measurements were obtained. Weekly phone calls were made to participants to assess for any treatmentrelated side effects.
Wound fluid was collected using a standard collection technique that has been used successfully in previous studies.23 Briefly, the CVLU was washed with sterile water, patted dry with sterile gauze, covered with a transparent occlusive film (Opsite, Smith & Nephew, London, United Kingdom) and wrapped with roll gauze. The participant’s leg was placed in a dependent position. Fluid that accumulated beneath the occlusive film was aspirated after 1–1½ hours with a 26G × 0.5” needle and syringe (Terumo Medical, Somerset, NJ) and transferred into plain collection tubes.
Measurements
Polymorphonuclear leukocytes
Differential counts and fluorescence-activated cell sorting of PMNs were accomplished by assessing aliquots of cells obtained from blood and CVLU fluid for total and differential leukocyte counts via light microscopy to identify individual cell types (i.e., neutrophil, monocyte, etc.). Blood and wound fluid samples were transported in plain collection tubes in a Styrofoam cooler within 30 minutes of collection to the laboratory where the analyses were completed. Cells and fluid were separated via centrifugation. Cells were fixed and analyzed using flow cytometry. Fluid was aliquoted in 200 μL portions then frozen and stored at −80 °C until further analysis. For flow cytometry analysis, cells were stained with CD45-FITC to gate for all leukocytes. In the total gated (CD45+) leukocyte population, PMNs can be easily distinguished in a forward scatter/side scatter plot. A dual staining approach using PE conjugated antibodies was also used to identify specific cell types: CD15 for human PMNs, CD14 for human monocytes. Activated PMNs were detected using CD66b.
Matrix metalloproteinase-8
MMP-8 is a PMN-specific protease that was measured as a biomarker of PMN activation in the CVLU fluid using the MMP-8, neutrophil collagenase, Biotrak enzyme-linked immunosorbent assay (ELISA) kit (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Standards, blanks, and samples (1.0 μg of wound fluid extracts or biopsy lysates in triplicate) were incubated in the microplate wells, precoated with anti-MMP-8 antibody, for 2 hours at 25 °C. Wells were washed and a peroxidase-labeled FAB’ antibody to MMP-8 were added. After further incubation and washing, the 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate was added to the wells and the plate was incubated for 30 minutes at 25 °C. The reaction was stopped and a standard curve was generated by plotting the optical density against a purified MMP-8 standard.
Human neutrophil elastase
HNE, another PMN-specific protease, was measured as a biomarker of PMN activation in the CVLU fluid using the InnoZyme Human Neutrophil Elastase Immunocapture Activity Assay Kit (Calbiochem, EMD Biosciences Inc., San Diego, CA). The assay uses a monoclonal antibody on a 96-well plate to specifically bind HNE.
Polyunsaturated fatty acids
Plasma polyunsaturated fatty acids (PUFAs) were analyzed by the well-established gas chromatography/mass spectrometry (GC/MS) method. Lipids were extracted from plasma samples with 2:1 (v/v) chloroform:methanol and 0.24 mL 0.88% KCL.24,25 Fatty acid methyl esters were prepared using tetramethylguanidine at 100 °C. Fatty acid methyl esters were analyzed by GS using a 30-m Omegawax™ 320 (Sigma-Aldrich, St. Louis, MO) capillary column. Oven temperature was started at 175 °C and increased at a rate of 3 °C/minute until reaching 220 °C. Flow rate of the carrier gas helium was 30 mL/minute. Retention times were compared to standards for fatty acid methyl esters (Sigma-Aldrich, and Matreya, LLC, Pleasant Gap, PA). The resultant values were expressed as % composition. This is routinely done to express fatty acid values and widely accepted in the field.24,25
Macroscopic analysis of wound healing
CVLU healing was quantified for all participants at each of the study’s three time points by the PI using Wound Zoom, a SCP system.26 Wound Zoom is a noncontact wound documentation system that uses a handheld imaging device with projected laser dots alongside a camera sensor to acquire a two-dimensional image with depth and skew correction. It provides precise objective information on wound size, shape, outline, area, color, and surrounding tissue changes. In this study, the PI captured each wound image and outlined the epithelial margins with the cursor. The system then calculated the exact area, length, and width using laser projections and advanced algorithms to correct for distance and skew. The Wound Zoom’s Analysis Software allowed the team to manage wound records, track wound healing, adjust reports, create measurements, and manage the data on the Wound Zoom Camera. Developed at Georgia Tech by Dr. Stephen Sprigle, Wound Zoom has been rigorously tested for accuracy, reliability, and repeatability.26,27 The percent reduction in wound area at Days 28 and 56 compared to Day 0 was calculated for each participant and then averages were calculated for each group. A larger percentage indicates greater healing.
Statistical analysis
As this study was a pilot study, formal statistical power calculations were not made for all measures and tests of interest. The primary limitation for performing power calculations was the lack of available information on what would constitute a clinically significant effect size for the measures of interest. Instead of identifying sample sizes a priori, we recruited as many subjects as possible during the first 24 months of the study and obtained 20 subjects in each of the (EPA + DHA and Control) groups. This sample size provided 80% power to detect an effect size of 0.6 at an alpha level of 0.05. Based on a previous study conducted by the PI, a significant effect of EPA + DHA on wound area for an acute wound was detected after only 5 days with only six subjects in each of two (EPA + DHA and Control) groups.18 In that study, the effect size was 1.36. Based on this effect size obtained over a short period, we believed a detectable effect size of 0.6 could reasonably be exceeded for most measures in the current study.
Categorical variables are reported as frequencies and percentages, and continuous variables as means and standard deviations (SD). For the dependent variables of PMN counts, MMP-8 and HNE levels and percent reduction in wound area, a linear censored data model was constructed. Treatment group was used as the independent variable and each response was classified as being either observed or interval censored (for values that fell between zero and the limit of detection). T-tests evaluated between-group comparisons of sociodemographic data (age, BMI) at baseline, and levels of PUFAs, PMNs, MMP-8, HNE, and percent reduction in wound area at Days 0, 28, and 56. Paired t-tests assessed within-group comparisons of levels of PUFAs, PMNs, MMP-8, HNE, and percent reduction in wound area at Days 28 and 56 compared to Day 0 and at Day 56 compared to Day 28. IBM’s Statistical Package for the Social Sciences version 21.0 for Windows was used for the data analysis (SPSS, Chicago, IL). Significance levels were set a priori at α = 0.05.
RESULTS
Sociodemographic and wound characteristics
Data describing the sociodemographic characteristics of the study participants and the wound attributes at Day 0 (baseline) are displayed in Table 1. There were no statistically significant differences in characteristics between the two groups.
Table 1.
Sociodemographic and wound characteristics of participants (N = 35)
| EPA+DHA* (n = 16) | Control* (n = 19) | |
|---|---|---|
| Age, mean years (SD) | 60.3 (12.6) | 60.9 (11.8) |
| Male (n, %) | 10 (62.5) | 11 (58) |
| Female (n, %) | 6 (37.5) | 8 (42) |
| White (n, %) | 12 (75) | 14 (75) |
| African American (n, %) | 4 (25) | 5 (26) |
| Education | ||
| Some high school (n, %) | 0 | 1 (5.3) |
| High school graduate (n, %) | 6 (37.5) | 5 (26.3) |
| Some college (n, %) | 4 (25) | 8 (42.1) |
| College graduate (n, %) | 6 (37.5) | 5 (26.3) |
| BMI (kg/m2)—mean (SD) | ||
| Baseline | 40.4 (8.2) | 42.7 (13.8) |
| 28 days | 40.9 (8.5) | 42.7 (8.5) |
| 56 days | 40.6 (8.9) | 42.1 (13.7) |
| Wound characteristics | ||
| Size, baseline (cm2)—mean (SD) | 15.6 (34.4) | 19.7 (23.2) |
| Estimated wound age | ||
| < 6 months (n, %) | 8 (50) | 7 (36.8) |
| > 6 months (n, %) | 8 (50) | 12 (63.2) |
| Healed by 56 days (n, %) | 5 (31) | 5 (26) |
No significant differences between groups.
BMI, body mass index; SD, standard deviation.
Plasma PUFA levels
The within-group analyses of the EPA + DHA Group showed that plasma PUFA levels were significantly higher for EPA at Day 28 (t = 5.9, p < 0.001) and at Day 56 (t = 7.6, p < 0.001) relative to Day 0 (baseline) (Table 2). Similarly, DHA levels were significantly higher at Day 28 (t = 5.3, p < 0.001) and Day 56 (t = 6.8, p < 0.001) when compared to Day 0. Conversely, levels of the n-6 PUFA arachidonic acid (AA) were significantly lower at 28 (t = 2.9, p = 0.01) and 56 days (t = 3.4, p < 0.001) than at baseline, as was the AA:EPA ratio (Day 28: t = 4.8, p < 0.001; Day 56: t = 7.1, p < 0.001), suggesting that the EPA study dose resulted in significantly increased proportions of EPA in the plasma that occurred partly at the expense of AA. No statistically significant changes in levels of EPA, DHA, AA, or AA:EPA ratios were detected within the Control Group at Day 28 or Day 56 relative to Day 0.
Table 2.
Plasma fatty acids
| Day 0 |
Day 28 |
Day 56 |
||||
|---|---|---|---|---|---|---|
| Fatty acids | EPA + DHA | Control | EPA + DHA | Control | EPA + DHA | Control |
| EPA | 0.52 (0.22) | 0.52 (0.26) | 2.21 (1.25)* | 0.46 (0.24)** | 2.33 (0.98)*,# | 0.48 (0.25)** |
| DHA | 1.47 (0.59) | 1.50 (0.44) | 2.77 (0.84)* | 1.46 (0.50)** | 3.09 (0.99)*,# | 1.49 (0.52)** |
| AA | 7.83 (2.45) | 8.38 (2.00) | 6.82 (1.83)* | 8.37 (2.49)** | 6.80 (2.16)*,# | 8.83 (2.61)** |
| Total | ||||||
| n-6 | 38.02 (4.32) | 37.46 (3.81) | 35.37 (4.78) | 36.51 (4.68) | 35.99 (5.77) | 37.27 (4.10) |
| n-3 | 2.89 (0.55) | 3.05 (0.71) | 6.16 (1.83)* | 2.88 (0.50)** | 6.55 (1.61)*,# | 2.99 (0.67)** |
| Ratios | ||||||
| AA:EPA | 13.75 (6.66) | 16.28 (8.05) | 4.19 (3.27)* | 15.39 (7.70)** | 3.46 (1.80)*,# | 15.81 (8.40)** |
| n-6:n-3 | 15.21 (6.96) | 12.69 (2.14) | 8.35 (8.62)* | 12.90 (2.29)** | 8.04 (8.83)*,# | 12.91 (2.62)** |
The table provides the amount of EPA, DHA, AA, total n-6, and total n-3 in percent (%) of all fatty acids in plasma and ratios of AA:EPA and n-6:n-3 in plasma at the different sampling time points in EPA + DHA Group and Control Group. Data are given in mean (SD). (Student’s t test, EPA + DHA group: n = 16; Control Group: n = 19).
Different from Day 0 (p < 0.05).
Different from Day 28 (p < 0.05).
Different between groups (p < 0.05).
As expected, the between-group comparisons showed that at Day 28 post enrollment, the EPA + DHA Group demonstrated significantly higher plasma levels of EPA (t = 5.9, p < 0.001) and DHA (t = 5.6, p < 0.001), and significantly lower ratios of AA:EPA (t = 5.4, p < 0.001) than there Control Group (Table 2). The same pattern was noted when comparing levels of EPA (t = 8.0, p < 0.001), DHA (t = 6.1, p < 0.001), and ratios of AA:EPA (t = 5.8, p < 0.001) between the two groups at Day 56 post enrollment.
Polymorphonuclear leukocytes
The within-group analysis of the EPA + DHA Group indicated a significantly lower percentage of CD15+ cells (PMNs) in both the blood at Day 56 (p = 0.05, Table 3) and in the wound fluid at Day 56 when compared to Day 0 (p = 0.04). Similarly, there was a significantly lower percentage of CD66b+ cells (activated PMNs) in the wound fluid at Day 56 compared to Day 0 (p = 0.02) and at Day 56 compared to Day 28 (p = 0.05). There was also a significant reduction in the ratio of CD66b+/CD15+ cells in the wound fluid at Day 56 compared to Day 0 (p = 0.01) and a lower ratio at Day 56 compared to Day 28 (p = 0.06, Table 3).
Table 3.
Dynamics of CD15+ and CD66b+ cells
| Day 0 |
Day 28 |
Day 56 |
||||
|---|---|---|---|---|---|---|
| PMN Markers | EPA + DHA | Control | EPA + DHA | Control | EPA + DHA | Control |
| CD15+ | ||||||
| Blood | 42.74 (15.42) | 46.05 (17.04) | 41.64 (12.68) | 42.77 (18.37) | 32.62 (24.44)* | 41.38 (11.99) |
| WF | 43.43 (32.58) | 48.46 (24.17) | 38.64 (31.26) | 22.72 (19.41)* | 21.17 (21.21)* | 33.76 (32.51)* |
| CD66b+ | ||||||
| Blood | 2.45 (7.12) | 8.87 (18.24) | 2.72 (6.76) | 8.25 (17.32) | 5.75 (16.35) | 5.60 (9.48) |
| WF | 19.89 (20.71) | 23.71 (25.82) | 7.88 (11.90) | 11.04 (18.90) | 4.23 (7.77)*,# | 15.49 (26.13) |
| CD66b+/CD15 | ||||||
| Blood | 57.45 (35.06) | 46.96 (35.98) | 50.27 (36.95) | 69.85 (28.50) | 47.57 (35.61) | 57.40 (34.01) |
| WF | 48.44 (41.57) | 58.71 (34.07) | 44.87 (38.17) | 43.26 (38.86) | 15.19 (28.21)* | 31.89 (41.00)* |
The tables provides the number of CD15+ or CD66b+ cells in percent (%) of all cells in blood or wound fluid at the different sampling time points in EPA + DHA Group and Placebo Group. Data are given in mean (SD).
CD15, marker of PMN; CD66b, marker of activated PMN; CD66b/PMN, ratio of activated PMNs to total PMNs; PMN, polymorphonuclear leukocytes; WF, wound fluid.
Different from Day 0 (p ≤ 0.05).
Different from Day 28 (p ≤ 0.05)
The within-group analysis of the Control Group detected no significant difference in percentage of CD15+ cells in the blood between any time points, but the mean percentage of CD15+ cells in wound fluid at Day 28 and Day 56 were significantly lower vs. Day 0 (p =0.004, p = 0.03, respectively, Table 3). However, in terms of the percentage of CD66B+ cells in the blood or wound fluid, no significant differences emerged between any of the time points. Finally, the ratio CD66b+/CD15+ cells in the blood actually increased from Day 0 to Day 28, however, in wound fluid, this ratio was significantly lower at Day 56 when compared to Day 0 (p = 0.03).
The between-group comparisons detected no significant differences in percentage of CD15+ cells, CD66b+ cells, or CD66b+/CD15+ cells in the blood or wound fluid between the two groups at Days 0, 28, or 56. However, the reductions in percentage of CD66b+ cells and in the ratio of CD66b+/CD15+ cells in the wound fluid that occurred by Day 56 compared to Day 0 were much more striking in the EPA + DHA Group when compared to the Control Group.
MMP-8 and HNE
Although the between-group analyses of levels of MMP-8 and HNE measured in wound fluid at Days 0, 28, and 56 detected no statistically significant differences at the study time points, the Cohen’s effect size values for MMP-8 at Day 56 (d = 0.63) and HNE at Day 56 (d = 0.80) indicate a medium to large treatment effect that may be clinically meaningful. Additionally, a downward trend in MMP-8 levels emerged within the EPA + DHA Group over the study interval after two participant outliers were removed from the analysis (Figure 2A). MMP-8 levels at Day 56 before removing the two outliers: M = 27.02, SD = 74.22; MMP-8 levels at Day 56 after removing the two outliers: M = 2.98, SD = 4.14. A similar downward trend was also noted in HNE levels in the EPA + DHA Group (Figure 2B).
Figure 2.
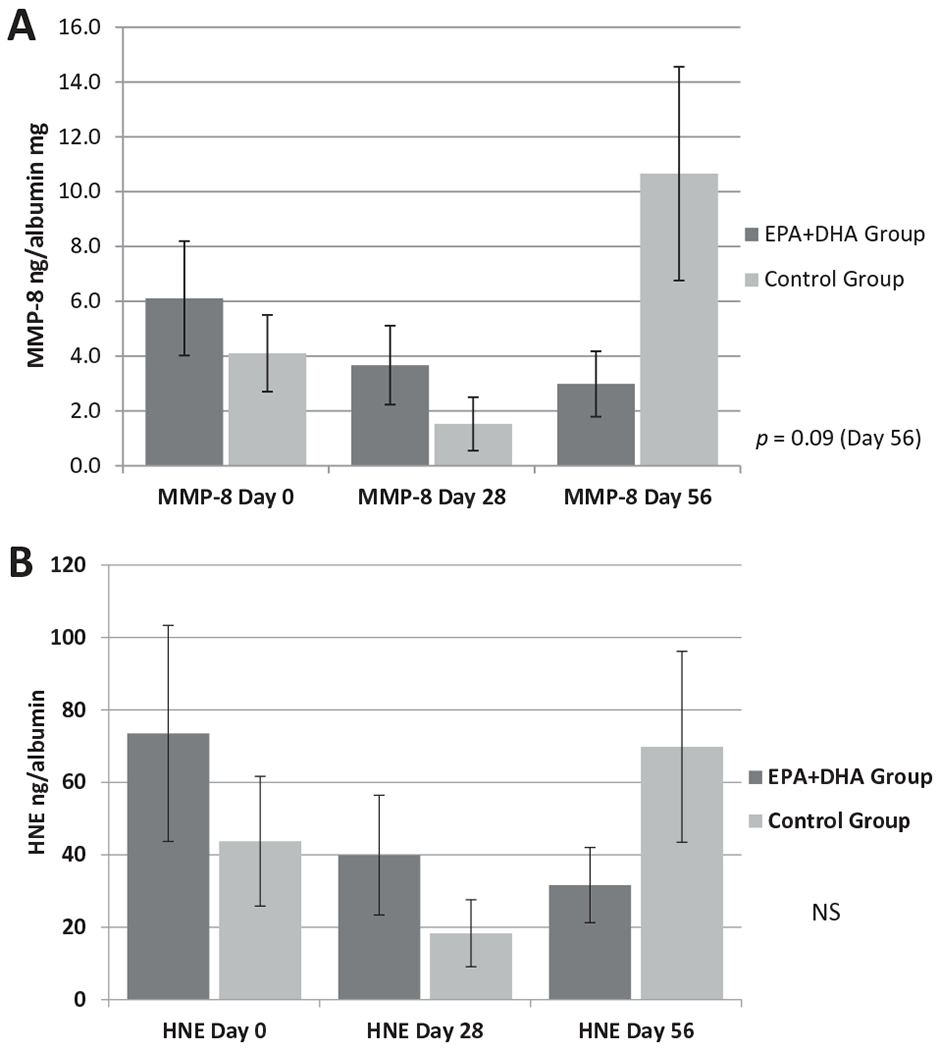
Comparison of levels of MMP-8 (A) and HNE (B) in wound fluid of venous leg ulcers at Days 0, 28, and 56 of the study between the EPA + DHA Group and the Control Group. The graph provides comparisons between the EPA + DHA Group and Control Group in terms of levels of MMP-8 and HNE (measured in ng per mg of albumin) at the study’s three time points. Each bar represents the mean ± SEM. (Student’s t test, EPA + DHA group: n = 16; Control Group: n = 19). MMP-8 = matrix metalloproteinase; HNE = human neutrophil elastase.
Wound healing
The within-group analysis of wound healing in the EPA + DHA Group detected a significant increase in percent reduction in wound area at Day 28 compared to Day 0 (t = 6.304, p < 0.001), at Day 56 compared to Day 0 (t = 9.228, p < 0.001) and at Day 56 compared to Day 28 (t = 2.951, p = 0.01). A similar pattern emerged in the Control Group; a significant increase in percent reduction of wound area at Day 28 compared to Day 0 (t = 4.005, p = 0.001), at Day 56 compared to Day 0 (t = 7.032, p < 0.001) and at Day 56 vs. Day 28 (t = 2.858, p = 0.01).
Between-group analyses indicated that the mean percent reduction in wound area was greater at both Days 28 and 56 for the EPA + DHA Group. Although the group differences were not significant relative to the standard alpha level of 0.05 (p = 0.09 at 28 days; p = 0.17 at 56 days), the Cohen’s effect size values (d = 0.60 at 28 days and d = 0.50 at 56 days) suggest a medium practical significance (Figure 3).
Figure 3.
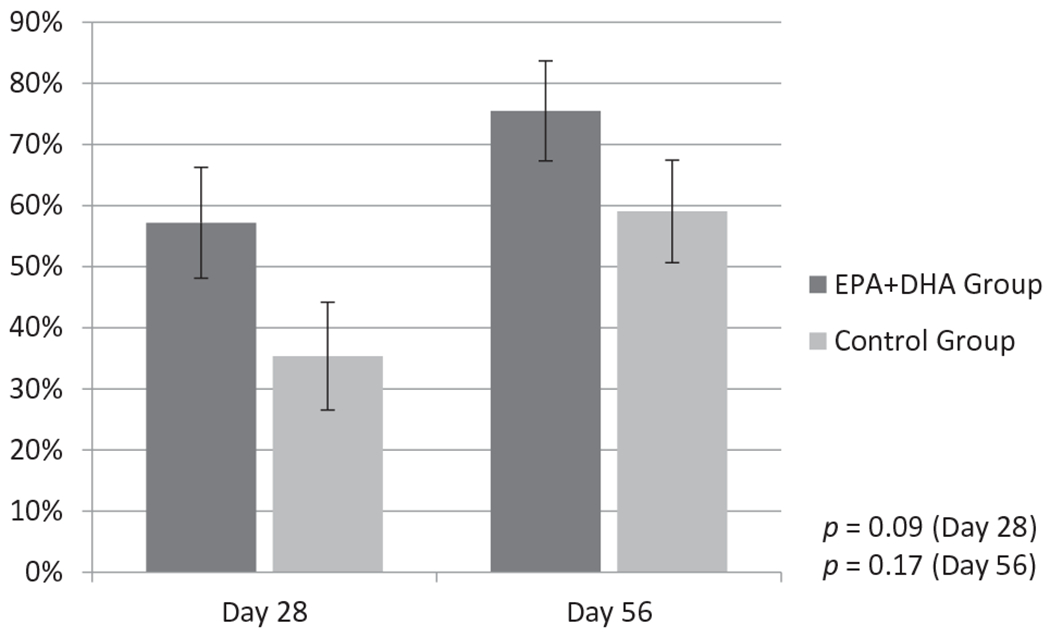
Comparison of percent reduction in wound area at Day 28 and Day 56 of the study between the EPA+DHA Group and the Control Group. The graph provides comparisons between the EPA+DHA and Control Group in terms of percent reduction in wound area at Day 28 and Day 56 compared to Day 0. Each bar represents the mean percent reduction ± SEM. (Student’s t test, EPA+DHA Group: n = 16; Control Group: n = 19).
Spearman’s rho correlation analyses assessed relationships among percent reductions in wound area at Days 28 and 56 and select PUFAs in plasma, and PMN markers, MMP-8 and HNE in wound fluid for the group as a whole. As expected, there were significant negative relationships between healing and several of the PMN markers and MMP-8, and positive relationships between healing and EPA, DHA, and total n-3 PUFAs (Table S1).
DISCUSSION
Persistent high levels of activated PMNs and PMN-derived proteases in the microenvironment of CVLUs contribute to the unremitting inflammation that prevents the move to subsequent healing stages.28–30 The findings from this randomized clinical study suggest that EPA + DHA oral therapy, as an adjuvant to compression therapy and standard wound management, may reduce PMN activation in the microenvironment of CVLUs and have a favorable effect on wound healing after just 4 weeks of therapy. This is the first study, to our knowledge, designed to answer specific questions about the utility of oral EPA + DHA therapy for the management of CVLUs. New therapeutic approaches are needed because the incidence of CVLUs is rising and many patients are not candidates for or opt not to undergo invasive surgical procedures to correct the venous incompetence usually involved in the development of CVLUs.8 Moreover, compression therapy (the gold standard of CVLU care) alone does not result in complete healing of these frequently recurring wounds for many people.8 Although the findings from this study should be interpreted with caution, given the small sample size, they suggest that EPA + DHA therapy may be effective as a low-risk systemic addition to conventional therapy for patients with elevated levels of activated PMNs and PMN-derived proteases in the CVLU fluid to improve healing outcomes.
The health benefits of diets high in EPA and DHA have been noted since the early studies of Greenland Eskimos who were found to consume relatively large quantities of fatty fish, a primary source of EPA and DHA, and have low rates of cardiovascular disease and diabetes, conditions with inflammatory components.31 Since then, numerous studies have shown that EPA and DHA are precursors to many lipid mediators (e.g., eicosanoids and resolvins) that have potent anti-inflammatory and inflammation-resolving actions, such as inhibiting PMN recruitment and migration into inflammatory sites and amplifying macrophage phagocytosis.32,33 For example, in vivo experiments by Dalli et al.34 indicated that DHA-derived resolvin D3 reduces PMN transmigration by 45% in a murine model of peritonitis after zymosam challenge. Other studies have shown that EPA + DHA slow chemotaxis of PMNs by reducing expression or antagonism of receptors for chemoattractants35 and by down-regulating the gene expression of proinflammatory cytokines that signal PMN activation.36,37
Although the dose-specific effects of EPA + DHA supplementation in humans are not entirely known, an intake greater than 2 g/day seems to be needed to induce anti-inflammatory actions.35 Our data show that oral supplementation with 2.5 g/day of EPA and 0.5 g/day of DHA results in a significant rise in the mean EPA and DHA plasma levels and a significant decrease in the mean AA to EPA ratio after just 4 week of therapy, with even greater changes occurring after 8 weeks of therapy. These data are in line with previous human studies indicating that EPA + DHA levels rise relatively quickly (1–4 weeks) with oral supplementation, and reduce high AA to EPA ratios in both plasma and membranes of cells such as neutrophils.18,38 For example, Sorensen et al.39 reported that after only 7 days of EPA + DHA therapy (3 g/day), there were threefold increases in the percentage of EPA and 22% increases in DHA in PMN membranes that occurred at the expense of AA.
While data from the current study do not show significant differences in levels of activated PMNs in the blood between the two groups, the ratio of activated PMNs to total PMNs declined after 4 weeks of therapy in the EPA + DHA Group, and then declined even more after 8 weeks of therapy. These findings are important because studies have reported increased PMN degranulation in the blood in all clinical stages of venous disease evident by ELISA testing for plasma neutrophil elastase 40,41 and lactoferrin.40 We also report that the EPA + DHA Group had significantly fewer activated PMNs in the wound fluid collected from CVLUs after 4 weeks and 8 weeks of therapy compared to baseline, and significantly fewer activated PMNs by 8 weeks when compared to 4 weeks. Further, there is a significant reduction in the ratio of activated PMNs to total PMNs in wound fluid after 8 weeks of EPA + DHA therapy compared to baseline, suggesting that 8 weeks of therapy is more effective than four in terms of reducing PMN activation in CVLU microenvironments. These collective findings are in line with previous in vitro and in vivo studies demonstrating that various EPA and DHA metabolites reduce PMN activation by inhibiting recruitment and accumulation during inflammatory responses.39,42
As hypothesized, the study analyses also show declining trends in levels of PMN-derived MMP-8 and HNE in CVLU fluid over the study interval in the EPA + DHA Group, which correspond to the significant reduction of activated PMNs (CD66+) noted in the wound fluid of this group. In contrast, the high levels of MMP-8 and HNE at Day 56 in the Control Group likely reflect the nonhealing nature of the majority of wounds in this group. Taken together these observations suggest that EPA + DHA therapy attenuates inflammation resulting in reduced PMN activation and MMP-8 and HNE production. These findings are important because previous studies have reported high levels and expression of MMP-8 and HNE in fluid and tissues biopsied from CVLUs,43–45 significantly higher levels of MMPs and HNE in chronic wounds compared with healing wounds,29,46,47 and stronger expression in chronic wounds than in acute wounds,28,29,47–49 indicating that sustained high levels of MMPs and HNE are factors in healing delays.
Finally, when comparing wound healing within and between groups, our data indicate significant CVLU healing after 4 weeks of EPA + DHA therapy and significantly more healing after 8 weeks of therapy compared to 4 weeks, indicating that EPA + DHA therapy may be needed for at least 8 weeks to achieve maximum healing effects. Although a similar healing pattern is noted for the Control Group, there is a more striking increase in percent reduction of wound area at both time points for the EPA + DHA Group. However, given the nonsignificant differences in healing between the groups it is important to consider that the greater reductions in wound area noted in the EPA ± DHA group may have been due to chance or some other factor.
There are currently two systemic agents, pentoxifylline and micronized purified flavanoid fraction (MPFF),50 included in the Wound Healing Society’s Guidelines for the Treatment of Venous Ulcers (Level I evidence)51 that have inhibitory effects on PMN activation. Pentoxifylline is thought to slow PMN infiltration into wound sites by reducing leukocyte adhesion to the vascular endothelium.50,51 However, there are potential side effects with pentoxifylline (e.g., nausea, headaches), and it is contraindicated in patients with some comorbidities such as angina, or marked liver and kidney disease.52 Although no side effects are associated with MPFF (Daflon), it may only be beneficial after 8 weeks of therapy for larger ulcers (5–10 cm2) that have been present for at least 6 months.50 Given that data from the current study indicate (1) EPA + DHA therapy works relatively quickly in terms of raising EPA + DHA plasma levels, (2) there are significantly lower levels of activated PMNs in CVLUs by 4 weeks of therapy that are associated with significant healing, and (3) there are no reported side effects or adverse events, EPA + DHA supplementation may prove to be another systemic adjuvant therapy to consider for patients with excessive, chronic PMN activation in CVLU sites.
This study provides new insights into the extent of PMN activation in the blood and wound fluid of CVLU patients, and the effect EPA + DHA therapy has on PMN activation and healing over time. It did not, however, assess bacterial counts at wounds sites, which can impact protease levels and healing, nor did it quantify all proteases and TIMPs that have been previously noted in CVLU fluid.28 We acknowledge that a balance between MMPs and TIMPs is important for efficient wound healing and recommend that total MMPs and TIMPs (in addition to HNE) be quantified in future studies testing the efficacy of EPA + DHA therapy for certain CVLU patients so that a more comprehensive profile can be generated. Other limitations of this study include a relatively small sample size, which hindered us from answering important questions such as whether certain thresholds of activated PMNs and/or MMP-8 and HNE could predict greater reductions in wound area over time.
In conclusion, our data demonstrate that EPA + DHA oral therapy results in significantly higher plasma levels of EPA and DHA, greater reductions in levels of activated PMNs and MMP-8 and HNE in the microenvironment, and more healing by 4 and 8 weeks of therapy when compared to a Control Group. Considering that Western diets contain much lower levels of n-3 PUFAs relative to n-6 PUFAs,33 and that many patients with chronic wounds have nutritional deficiencies such as lower than Recommended Daily Intakes of protein, vitamin C, and zinc,53 it is likely that patients in the US with CVLUs consume nominal amounts of EPA + DHA33 (which is confirmed in the current study sample). Further, the high AA to EPA ratios noted in our study’s sample indicate high levels of systemic inflammation33 that may increase the risk for developing CVLUs that fail to heal or recur. Since the sample size was small, the study findings must be interpreted with caution. Nonetheless, the results have significant implications for future clinical trials to further test EPA + DHA adjuvant therapy for people found to have high levels of PMN-derived proteases in the CVLU microenvironment to facilitate healing and perhaps help prevent ulcer recurrence. Potential advanced work could (1) evaluate “high” and “low” dose EPA + DHA therapy at weekly time points to determine the most effective dose and duration of therapy, (2) test a more personalized EPA + DHA dose based on certain genetic polymorphisms that may increase the rate of EPA + DHA conversion to inflammatory mediators, (3) test adjuvant EPA + DHA therapy for preventing ulcer recurrence in a prospective long-term study, and (4) include patients with other types of chronic wounds having high levels of PMN-derived proteases, such as diabetic foot ulcers.
Supplementary Material
Table 4.
Spearman’s rho correlations between select independent variables and dependent variable % reduction in wound area in 35 participants with CVLUs—total group
| % Reduction in wound area |
||
|---|---|---|
| Correlation coefficient (p value) |
||
| Day 28 | Day 56 | |
| EPA + DHA | ||
| Day 28 | 0.27 (0.13) | 0.20 (0.25) |
| Day 56 | 0.34 (0.05)* | 0.25 (0.16) |
| EPA | ||
| Day 28 | 0.18 (0.32) | 0.30 (0.09) |
| Day 56 | 0.28 (0.10) | 0.28 (0.11) |
| DHA | ||
| Day 28 | 0.35 (0.045)* | 0.18 (0.30) |
| Day 56 | 0.34 (0.046)* | 0.24 (0.16) |
| Total n-3 PUFAs | ||
| Day 28 | 0.31 (0.07) | 0.23 (0.18) |
| Day 56 | 0.36 (0.03)* | 0.28 (0.11) |
| CD15+ cells in WF | ||
| Day 28 | −0.18 (0.33) | −0.53 (0.003)** |
| Day 56 | −0.45 (0.008)** | −0.33 (<0.001)** |
| CD66b+cells in WF | ||
| Day 28 | −0.28 (0.14) | −0.45 (0.01)* |
| Day 56 | −0.35 (0.04)* | −0.45 (0.009)** |
| CD66b+/CD15+ cells in WF | ||
| Day 28 | −0.15 (0.43) | −0.44 (0.02)* |
| Day 56 | −0.35 (0.05)* | −0.43 (0.01)* |
| MMP-8 | ||
| Day 28 | −0.21 (0.30) | −0.36 (0.06) |
| Day 56 | −0.29 (0.17) | −0.41 (0.04)* |
| HNE | ||
| Day 28 | −0.28 (0.29) | −0.02 (0.96) |
| Day 56 | −0.28 (0.23) | −0.22 (0.34) |
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HNE, human neutrophil elastase; MMP-8, matrix metalloproteinase-8; PUFA, polyunsaturated fatty acids; WF, wound fluid.
p≤0.05,
p<0.01.
Source of Funding:
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number R21NR012803. Content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s web-site:
Table S1. Spearman’s rho correlations between select independent variables and dependent variable % reduction in wound area in 35 participants with chronic venous leg ulcers total group
REFERENCES
- 1.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ 2014; 17: 347–56. [DOI] [PubMed] [Google Scholar]
- 2.Gould LJ, Dosi G, Couch K, Gibbons GW, Howell RS, Brem H, Tomic M. Modalities to treat venous ulcers: compression, surgery, and bioengineered tissue. Plast Reconstr Surg 2016; 138 (3 Suppl.): 199S–208S. [DOI] [PubMed] [Google Scholar]
- 3.van Rij AM, De Alwis CS, Jiang P, Christie RA, Hill GB, Dutton SJ, Thomson IA. Obesity and impaired venous function. Eur J Vasc Endovasc Surg 2008; 35: 739–44. [DOI] [PubMed] [Google Scholar]
- 4.Alavi A, Sibbald RG, Phillips TJ, Miller OF, Margolis DJ, Marston W, et al. What’s new: management of venous leg ulcers: treating venous leg ulcers. J Am Acad Dermatol 2016; 74: 643–64; quiz 665–64. [DOI] [PubMed] [Google Scholar]
- 5.Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol 2002; 46: 381–6. [DOI] [PubMed] [Google Scholar]
- 6.Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients’ quality of life. Health Qual Life Outcomes 2007; 5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankaran V, Brooks M, Mostow E. Advanced therapies for chronic wounds: NPWT, engineered skin, growth factors, extracellular matrices. Dermatol Ther 2013; 26: 215–21. [DOI] [PubMed] [Google Scholar]
- 8.Chi YW, Raffetto JD. Venous leg ulceration pathophysiology and evidence based treatment. Vasc Med 2015; 20: 168–81. [DOI] [PubMed] [Google Scholar]
- 9.Pocock ES, Alsaigh T, Mazor R, Schmid-Schonbein GW. Cellular and molecular basis of venous insufficiency. Vasc Cell 2014; 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moor AN, Vachon DJ, Gould LJ. Proteolytic activity in wound fluids and tissues derived from chronic venous leg ulcers. Wound Repair Regen 2009; 17: 832–9. [DOI] [PubMed] [Google Scholar]
- 11.Smith PC. The causes of skin damage and leg ulceration in chronic venous disease. Int J Low Extrem Wounds 2006; 5: 160–8. [DOI] [PubMed] [Google Scholar]
- 12.International Consensus. The role of proteases in wound diagnostics. An expert working group review. London: Wounds International, 2011. [Google Scholar]
- 13.Yager DR, Kulina RA, Gilman LA. Wound fluids: a window into the wound environment? Int J Low Extrem Wounds 2007; 6: 262–72. [DOI] [PubMed] [Google Scholar]
- 14.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, et al. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol 2008; 181: 8677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood 2008; 112: 848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terano T, Seya A, Harai A. Effects of oral administration of highly purified EPA and DHA on eicosanoid formation and neutrophil function in healthy subjects. In Lands WEM (Ed.) Proceedings of the AOCS short course on polyunsaturated fatty acids and eicosanoids. Champaign, IL: American Oil Chemists’ Society, 1987; 133–8. [Google Scholar]
- 17.Sperling RI, Benincaso AI, Knoell CT, Larkin JK, Austen KF, Robinson DR. Dietary omega-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J Clin Invest 1993; 91: 651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDaniel J, Massey K, Nicolaou A. Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds. Wound Repair Regen 2011; 19: 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Nutritional Products Labeling and Dietary Supplements (FDA/CSFAN). Letter regarding dietary supplement health claim for omega-3 fatty acids and coronary heart disease (Docket No. 91N-0103). 2000. Silver Spriung, MD: US Food and Drug Administration. [Google Scholar]
- 20.McDaniel JC, Belury M, Ahijevych K, Blakely W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen 2008; 16: 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr 1996; 63: 116–22. [DOI] [PubMed] [Google Scholar]
- 22.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 2002; 196: 1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rayment EA, Dargaville TR, Shooter GK, George GA, Upton Z. Attenuation of protease activity in chronic wound fluid with bisphosphonate-functionalised hydrogels. Biomaterials 2008; 29: 1785–95. [DOI] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957; 226: 497–509. [PubMed] [Google Scholar]
- 25.Parinandi NL, Weis BK, Natarajan V, Schmid HH. Peroxidative modification of phospholipids in myocardial membranes. Arch Biochem Biophys 1990; 280: 45–52. [DOI] [PubMed] [Google Scholar]
- 26.Nemeth M, Sprigle S, Gajjala A. Clinical usability of a wound measurement device. 36th Annual American Spinal Injury Conference, Nashville, TN, 2010. [Google Scholar]
- 27.Bowling FL, Paterson J, Ndip A. Applying 21st century imaging technology to wound healing: an Avant-Gardist approach. J Diabetes Sci Technol 2013; 7: 1190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serena TE, Cullen BM, Bayliff SW, Gibson MC, Carter MJ, Chen L, et al. Defining a new diagnostic assessment parameter for wound care: elevated protease activity, an indicator of nonhealing, for targeted protease-modulating treatment. Wound Repair Regen 2016; 24: 589–95. [DOI] [PubMed] [Google Scholar]
- 29.Wiegand C, Schönfelder U, Abel M, Ruth P, Kaatz M, Hipler UC. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch Dermatol Res 2010; 302: 419–28. [DOI] [PubMed] [Google Scholar]
- 30.Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol 2008; 158: 951–61. [DOI] [PubMed] [Google Scholar]
- 31.Bang HO, Dyerberg J, Nielsen AB. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet 1971; 1: 1143–5. [DOI] [PubMed] [Google Scholar]
- 32.Lundström SL, Yang J, Brannan JD, Haeggstrom JZ, Hammock BD, Nair P, et al. Lipid mediator serum profiles in asthmatics significantly shift following dietary supplementation with omega-3 fatty acids. Mol Nutr Food Res 2013; 57: 1378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015; 1851: 469–84. [DOI] [PubMed] [Google Scholar]
- 34.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol 2013; 20: 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 2013; 75: 645–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daak AA, Elderdery AY, Elbashir LM, Mariniello K, Mills J, Scarlett G, et al. Omega 3 (n-3) fatty acids down-regulate nuclear factor-kappa B (NF-kappaB) gene and blood cell adhesion molecule expression in patients with homozygous sickle cell disease. Blood Cells Mol Dis 2015; 55: 48–55. [DOI] [PubMed] [Google Scholar]
- 37.Allam-Ndoul B, Guenard F, Barbier O, Vohl MC. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis 2016; 15: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faber J, Berkhout M, Vos AP, Sijben JW, Calder PC, Garssen J, van Helvoort A. Supplementation with a fish oil-enriched, high-protein medical food leads to rapid incorporation of EPA into white blood cells and modulates immune responses within one week in healthy men and women. J Nutr 2011; 141: 964–70. [DOI] [PubMed] [Google Scholar]
- 39.Sorensen LS, Thorlacius-Ussing O, Rasmussen HH, Lundbye-Christensen S, Calder PC, Lindorff-Larsen K, Schmidt EB. Effects of perioperative supplementation with omega-3 fatty acids on leukotriene B(4) and leukotriene B(5) production by stimulated neutrophils in patients with colorectal cancer: a randomized, placebo-controlled intervention trial. Nutrients 2014; 6: 4043–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shields DA, Andaz S, Abeysinghe RD, Porter JB, Scurr JH, Coleridge Smith PD. Plasma lactoferrin as a marker of white cell degranulation in venous disease. Phlebology 1994; 9: 55–8. [Google Scholar]
- 41.Shields DA, Andaz SK, Sarin S, Scurr JH, Coleridge Smith PD. Plasma elastase in venous disease. Br J Surg 1994; 81: 1496–99. [DOI] [PubMed] [Google Scholar]
- 42.Tull SP, Yates CM, Maskrey BH, O’Donnell VB, Madden J, Grimble RF, et al. Omega-3 Fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biol 2009; 7: e1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen 2008; 16: 642–8. [DOI] [PubMed] [Google Scholar]
- 44.Grinnell F, Zhu M. Fibronectin degradation in chronic wounds depends on the relative levels of elastase, alpha1-proteinase inhibitor, and alpha2-macroglobulin. J Invest Dermatol 1996; 106: 335–41. [DOI] [PubMed] [Google Scholar]
- 45.Smeets R, Ulrich D, Unglaub F, Wöoltje M, Pallua N. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J 2008; 5: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res 1999; 81: 189–95. [DOI] [PubMed] [Google Scholar]
- 47.Tarlton JF, Bailey AJ, Crawford E, Jones D, Moore K, Harding KD. Prognostic value of markers of collagen remodeling in venous ulcers. Wound Repair Regen 1999; 7: 347–55. [DOI] [PubMed] [Google Scholar]
- 48.Pirila E, Korpi JT, Korkiamäki T, Jahkola T, Gutierrez-Fernandez A, Lopez-Otin C, et al. Collagenase-2 (MMP-8) and matrilysin-2 (MMP-26) expression in human wounds of different etiologies. Wound Repair Regen 2007; 15: 47–57. [DOI] [PubMed] [Google Scholar]
- 49.Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999; 7: 442–52. [DOI] [PubMed] [Google Scholar]
- 50.Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg 2005; 30: 198–208. [DOI] [PubMed] [Google Scholar]
- 51.Marston W, Tang J, Kirsner RS, Ennis W. Wound Healing Society 2015 update on guidelines for venous ulcers. Wound Repair Regen 2016; 24: 136–44. [DOI] [PubMed] [Google Scholar]
- 52.Medline Plus. Pentoxifylline. US National Library of Medicine and National Institutes of Health. Available at https://medlineplus.gov/druginfo/meds/a685027.html (accessed September 1, 2010). [Google Scholar]
- 53.Tobón J, Whitney JD, Jarrett M. Nutritional status and wound severity of overweight and obese patients with venous leg ulcers: a pilot study. J Vasc Nurs 2008; 26: 43–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


