Abstract
Aims
In this prospective, placebo‐controlled, double‐blind, exploratory study, we examined early and more delayed effects of empagliflozin treatment on haemodynamic parameters (primary endpoint: cardiac output) and kidney function including parameters of acute kidney injury (AKI) in patients with acute decompensated heart failure (HF).
Methods and results
Patients with acute decompensated HF with or without diabetes were randomized to empagliflozin 10 mg or placebo for 30 days. Haemodynamic, laboratory, and urinary parameters were assessed after 6 h, 1 day, 3 days, 7 days, and 30 days of treatment. Median time between hospital admission and randomization was 72 h. Baseline characteristics were not different in the empagliflozin (n = 10) and placebo (n = 9) groups. Empagliflozin led to a significant increase in urinary glucose excretion throughout the study (baseline: 37 ± 15 mg/24 h; Day 1: 14 565 ± 8663 mg/24 h; P = 0.001). Empagliflozin did not affect the primary endpoint of cardiac index or on systemic vascular resistance index at any time point. However, empagliflozin significantly reduced parameters of AKI (urinary TIMP‐2 and IGFBP7 by NephroCheck® as indicators of tubular kidney damage), which became significant after 3 days of treatment [placebo: 1.1 ± 1.1 (ng/mL)2/1000; empagliflozin: 0.3 ± 0.2 (ng/mL)2/1000; P = 0.02] and remained significant at the 7 day time point [placebo: 2.5 ± 3.8 (ng/mL)2/1000; empagliflozin: 0.3 ± 0.2 (ng/mL)2/1000; P = 0.003].
Conclusions
In this study, empagliflozin treatment did not affect haemodynamic parameters but significantly reduced markers of tubular injury in patients with acute decompensated HF.
Keywords: SGLT2 inhibitors, Empagliflozin, Acute decompensated heart failure, Acute kidney injury, Haemodynamic parameters
Background
Sodium‐glucose cotransporter‐2 (SGLT2) inhibitors reduce heart failure (HF) hospitalization or cardiovascular (CV) death in patients with HF [HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF), respectively], type 2 diabetes mellitus (T2D), and/or chronic kidney disease (CKD). Inhibition of the SGLT2 transporter, which is expressed in the proximal tubule of the kidney, induces glucosuria and osmotic diuresis. Recent evidence suggests that SGLT2 inhibition also improves outcome in patients with acute decompensated HF—a situation often combined with impaired kidney function.
Aims
In this prospective, placebo‐controlled, double‐blind, exploratory pilot study, we randomized patients with acute decompensated HF with and without T2D to empagliflozin or placebo. We investigated haemodynamic and kidney parameters including markers of kidney tubular stress indicating acute kidney injury (AKI) at different time points.
Methods
This study had to be terminated prematurely due to the COVID‐19 pandemic. The endpoints were exploratively evaluated in the recruited collective of 19 study participants received empagliflozin 10 mg or placebo for a period of 30 days in addition to their concomitant medication. The primary endpoint of the study was cardiac output (CO) as determined by the ClearSight System®. Power calculation was based on detecting a difference in CO between the empagliflozin and placebo groups. A multivariate analysis of variance was considered with baseline and five follow‐up measurements of CO with treatment group and diabetes effect and testing for a treatment–time interaction. The following assumptions were considered: power 0.8, alpha 0.05, an autoregressive correlation structure across time, rho 0.5, standard deviation of CO 1 L/min based on Bubenek‐Turconi et al., 1 a mean difference of zero between treatments at baseline and first two follow‐up measurements, and a constant difference thereafter. Based on our assumptions, a total sample size of 48 would allow a detection of a 1 L/min minimal difference in CO.
Secondary endpoints included additional haemodynamic parameters and vital signs as well as several laboratory and urinary values including the AKI Risk Score by NephroCheck®.
For categorical variables, non‐missing sample size, frequencies, and percentages were tabulated. Continuous variables were summarized using means, medians, quartiles, standard deviations, and non‐missing sample size. Distributions of continuous variables were assessed using graphical methods (box‐plots).
Inclusion criteria were age ≥ 18 years, presence of acute HF requiring hospital admission, and associated signs and symptoms regardless of ejection fraction or diabetes status as well as a serum level of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) > 1000 pg/mL. Non‐invasive haemodynamic measurement, transthoracic echocardiography, blood pressure, blood, and urine chemistry were performed at baseline and 6 h, 1 day, 3 days, 7 days, and 30 days following treatment initiation. We used the ClearSight System® (Edwards Lifesciences, Irvine, USA) as a validated 2 non‐invasive tool to explore effects of empagliflozin on haemodynamic parameters including CO, stroke volume index (SVI), blood pressure, heart rate (HR), and systemic vascular resistance index (SVRI). Serum chemistry including haematology, lipid profile, glucose metabolism, estimated glomerular filtration rate (eGFR) [Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) formula], and serum cystatin C, NT‐proBNP, aldosterone, and erythropoietin was performed at each visit, and we collected 24 h urines at different time points to measure excretion of glucose and sodium. Additionally, we used NephroCheck® to assess tubular kidney damage. NephroCheck® is an in vitro diagnostic device that quantifies urinary concentrations of tissue inhibitors of metalloproteinases‐2 (TIMP‐2) and insulin‐like growth factor‐binding protein 7 (IGFBP7) by fluorescence immunoassay on the Astute 140® Meter. The test has been established to assess the risk of moderate or severe AKI. 3
Results
Baseline characteristics
From June 2018 to May 2020, a total of 19 patients underwent randomization. The median time between hospital admission and randomization was 72 h. Baseline characteristics were not different between empagliflozin and placebo‐treated patients. The mean age of study participants was 72.1 ± 11.7 years, 47% were male, with a mean left ventricular ejection fraction (LVEF) of 36%, a history of CV disease in 42%, and diabetes in 26%. Mean NT‐proBNP was 3420 pg/mL, mean eGFR was 58 mL/min/1.73 m2, and patients had a baseline blood pressure of 114/70 mmHg with an HR of 80/min. No difference in baseline medication was present between treatment groups including renin‐angiotensin‐aldosterone system (RAAS) inhibition, beta‐blockers, and oral diuretics (Table 1 ).
Table 1.
Baseline characteristics of the study population
| Placebo (N = 9) | Empagliflozin (N = 10) | P‐value | |
|---|---|---|---|
| Age, years | 72.3 ± 9.9 | 71.8 ± 13.4 | 0.93 |
| Male, no. (%) | 3 (33.3) | 6 (60) | 0.48 |
| Systolic blood pressure, mmHg | 126 ± 24 | 139 ± 23 | 0.19 |
| Diastolic blood pressure, mmHg | 67 ± 13 | 80 ± 24 | 0.46 |
| Heart rate, b.p.m. | 73 ± 10 | 75 ± 17 | 0.41 |
| LVEF, % | 38 ± 11 | 34 ± 11 | 0.64 |
| Type 2 diabetes, no. (%) | 1 (11) | 4 (40) | 0.36 |
| History of coronary heart disease, no. (%) | 3 (33) | 5 (50) | 0.79 |
| Medication, no. (%) | |||
| Antiplatelets | 5 (55.6) | 3 (30) | 0.79 |
| Oral anticoagulants | 6 (66.7) | 6 (60) | >0.999 |
| Diuretics | 8 (88.9) | 8 (80) | >0.999 |
| Calcium channel blockers | 3 (33.3) | 1 (10) | 0.66 |
| Beta‐blockers | 8 (88.9) | 8 (80) | >0.999 |
| RAAS inhibitors | 7 (77.8) | 8 (80) | 0.66 |
| eGFR, mL/min/1.73 m2 | 56 ± 16 | 63 ± 22 | 0.57 |
| NT‐proBNP, pg/mL | 3996 ± 6293 | 3562 ± 2527 | 0.74 |
Note: Values are mean ± standard deviation for normally distributed data and median and interquartile range for non‐normally distributed data, or no. (%); P‐values for continuous variables were calculated using t‐test; P‐values for categorical variables were calculated using χ 2 test; P‐values ≤ 0.05 were categorized as statistically significant.
Abbreviations: eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; RAAS, renin‐angiotensin‐aldosterone system.
Effect of empagliflozin on haemodynamic parameters
Cardiac output was not significantly different between empagliflozin and placebo‐treated patients at any time point (Table 2 ). No treatment‐dependent difference in SVRI, SVI, HR, and blood pressure was observed between both groups (Table 2 ).
Table 2.
Comparison of laboratory values, 24 h urine, haemodynamics, blood pressure, and echocardiography during the study
| Baseline | 6 h | Day 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Empagliflozin | Placebo | Empagliflozin | P‐value | Placebo | Empagliflozin | P‐value | |
| Cardiac index (CI), L/min/m2 | 2.4 ± 0.8 | 3.0 ± 1.0 | 2.5 ± 1.0 | 3.0 ± 0.6 | 0.70 | 2.6 ± 0.6 | 3.1 ± 0.6 | 0.22 |
| Systemic vascular resistance index (SVRI), dyne*s*cm−5*m−2 | 2588 ± 993 | 2735 ± 780 | 2197 ± 651 | 2077 ± 716 | 0.27 | 2806 ± 1084 | 2221 ± 658 | 0.30 |
| Systolic blood pressure, mmHg | 123 ± 23 | 138 ± 19 | 114 ± 21 | 143 ± 23 | 0.04 | 126 ± 23 | 126 ± 19 | 0.27 |
| Diastolic blood pressure, mmHg | 70 ± 11 | 74 ± 20 | 67 ± 11 | 77 ± 19 | 0.17 | 68 ± 13 | 69 ± 7 | 1.00 |
| Heart rate, b.p.m. | 82 ± 16 | 77 ± 12 | 81 ± 21 | 68 ± 16 | 0.20 | 78 ± 17 | 70 ± 13 | 0.36 |
| AKI risk markers (NephroCheck®), (ng/mL)2/1000 | 0.6 ± 0.7 | 0.9 ± 1.1 | ‐ | ‐ | ‐ | 0.5 ± 1.1 | 0.6 ± 0.6 | 0.07 |
| Oxygen saturation, % | 94 ± 2.6 | 95 ± 1.7 | 94 ± 3.1 | 95 ± 1.6 | 0.43 | 94 ± 3.4 | 95 ± 1.5 | 0.64 |
| 24 h glucose excretion, mg/24 h | 76 ± 110 | 37 ± 15 | ‐ | ‐ | ‐ | 43 ± 29 | 14 565 ± 8663 | 0.001 |
| 24 h urinary volume, mL | 1750 ± 1278 | 1215 ± 640 | ‐ | ‐ | ‐ | 2037 ± 1386 | 2794 ± 2223 | 0.27 |
| 24 h sodium excretion, mmol/24 h | 143 ± 118 | 130 ± 92 | ‐ | ‐ | ‐ | 245 ± 229 | 309 ± 311 | 0.78 |
| Cumulative doses of diuretics, mg/day furosemide equivalent | ‐ | ‐ | ‐ | ‐ | ‐ | 121.7 ± 42.5 | 99.3 ± 87.9 | 0.47 |
| eGFR, mL/min/1.73 m2 | 56 ± 16 | 60 ± 20 | 54 ± 19 | 55 ± 19 | 0.50 | 55 ± 18 | 52 ± 17 | 0.17 |
| Cystatin C, mg/L | 1.5 ± 0.3 | 1.5 ± 0.5 | 1.5 ± 0.3 | 1.7 ± 0.4 | 0.03 | 1.8 ± 0.8 | 1.7 ± 0.4 | 0.53 |
| NT‐proBNP, pg/mL | 3435 ± 5256 | 3404 ± 3031 | 3398 ± 4578 | 3703 ± 3454 | ‐ | 2635 ± 4062 | 2754 ± 2574 | ‐ |
| Glucose, mg/dL | 100 ± 16 | 109 ± 26 | 117 ± 23 | 146 ± 53 | 0.28 | 102 ± 11 | 102 ± 33 | 0.26 |
| HbA1c, % | 5.8 ± 0.5 | 6.0 ± 0.6 | 5.8 ± 0.5 | 6.0 ± 0.5 | ‐ | 5.7 ± 0.5 | 5.9 ± 0.6 | ‐ |
| Insulin, mU/L | 10.5 ± 5.6 | 11.4 ± 9.1 | 51.9 ± 39.6 | 32.0 ± 28.2 | 0.11 | 14.8 ± 9.3 | 12.0 ± 10.1 | 0.78 |
| Haemoglobin, g/dL | 12 ± 2.3 | 12 ± 2.6 | 12 ± 2.4 | 12 ± 2.7 | 0.39 | 12 ± 2.3 | 12 ± 2.6 | 0.55 |
| Haematocrit, % | 38 ± 5.2 | 37 ± 7.7 | 37 ± 6.3 | 37 ± 8.0 | 0.52 | 36 ± 5.8 | 36 ± 7.6 | 0.77 |
| Erythropoetin, mU/mL | 56 ± 56 | 44 ± 37 | 56 ± 62 | 41 ± 28 | 0.56 | 61 ± 72 | 42 ± 49 | 0.59 |
| Aldosterone, pg/mL | 122 ± 78 | 143 ± 121 | 126 ± 98 | 149 ± 123 | 0.81 | 145 ± 121 | 144 ± 129 | 0.38 |
| Day 3 | Day 7 | |||||
|---|---|---|---|---|---|---|
| Placebo | Empagliflozin | P‐value | Placebo | Empagliflozin | P‐value | |
| Cardiac index (CI), L/min/m2 | 2.8 ± 0.8 | 2.8 ± 0.7 | 0.71 | 2.5 ± 0.7 | 2.8 ± 0.6 | 0.68 |
| Systemic vascular resistance index (SVRI), dyne*s*cm−5*m−2 | 2093 ± 304 | 2211 ± 627 | 0.37 | 2121 ± 460 | 2256 ± 543 | 0.82 |
| Systolic blood pressure, mmHg | 112 ± 13 | 121 ± 17 | 0.32 | 116 ± 29 | 125 ± 17 | 0.77 |
| Diastolic blood pressure, mmHg | 67 ± 8 | 69 ± 12 | 0.73 | 61 ± 15 | 76 ± 17 | 0.08 |
| Heart rate, b.p.m. | 75 ± 15 | 71 ± 17 | 0.96 | 73 ± 16 | 63 ± 10 | 0.27 |
| AKI risk markers (NephroCheck®), (ng/mL)2/1000 | 1.0 ± 1.1 | 0.3 ± 0.2 | 0.02 | 2.5 ± 3.8 | 0.3 ± 0.2 | 0.003 |
| Oxygen saturation, % | 95 ± 2.4 | 96 ± 1.9 | 0.79 | 95 ± 1.9 | 96 ± 1.1 | 0.17 |
| 24 h glucose excretion, mg/24 h | 52 ± 69 | 15 993 ± 18 713 | 0.04 | 34 ± 32 | 13 428 ± 10 433 | 0.04 |
| 24 h urinary volume, mL | 1650 ± 851 | 1936 ± 818 | 0.6 | 1720 ± 1579 | 1867 ± 792 | 0.84 |
| 24 h sodium excretion, mmol/24 h | 119 ± 69 | 190 ± 123 | 0.28 | 179 ± 205 | 166 ± 79 | 0.35 |
| Cumulative doses of diuretics, mg/day furosemide equivalent | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| eGFR, mL/min/1.73 m2 | 54 ± 12 | 51 ± 20 | 0.15 | 49 ± 8 | 53 ± 24 | 0.64 |
| Cystatin C, mg/L | 1.7 ± 0.4 | 1.8 ± 0.5 | 0.21 | 1.8 ± 0.4 | 1.8 ± 0.5 | 0.16 |
| NT‐proBNP, pg/mL | 2622 ± 5028 | 1544 ± 2821 | ‐ | 2202 ± 5807 | 2050 ± 3243 | ‐ |
| Glucose, mg/dL | 112 ± 18 | 119 ± 40 | 0.92 | 120 ± 34 | 111 ± 30 | 0.23 |
| HbA1c, % | 5.8 ± 0.6 | 6.0 ± 0.6 | ‐ | 5.8 ± 0.6 | 6.0 ± 0.6 | ‐ |
| Insulin, mU/L | 34.8 ± 42.1 | 21.5 ± 23.4 | 0.40 | 24.6 ± 24.2 | 12.0 ± 9.3 | 0.16 |
| Haemoglobin, g/dL | 11 ± 1.9 | 12 ± 2.2 | 0.71 | 10 ± 1.8 | 12 ± 2.5 | 0.64 |
| Haematocrit, % | 36 ± 5.3 | 38 ± 6.0 | 0.74 | 33 ± 5.0 | 37 ± 6.6 | 0.58 |
| Erythropoetin, mU/mL | 59 ± 74 | 49 ± 77 | 0.96 | 43 ± 49 | 61 ± 89 | 0.20 |
| Aldosterone, pg/mL | 94 ± 61 | 147 ± 90 | 0.31 | 109 ± 63 | 137 ± 89 | 0.76 |
Note: All significant p‐values are in bold.
Abbreviations: AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; HbA1c, haemoglobin A1c; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Effect of empagliflozin on metabolic parameters and kidney function
As expected, empagliflozin treatment significantly increased urinary glucose excretion throughout the study period [from 37 ± 15 mg/24 h at baseline to 14 565 ± 8663 mg/24 h at Day 1 (P = 0.001) and 8890 ± 10 872 mg/24 h at Day 30 (P = 0.06)] (Figure 1 , Table 2 ). In parallel with glucosuria, 24 h urinary volume increased in empagliflozin‐treated patients without reaching statistical significance, most likely due to the small sample size. No difference in serum blood glucose, diuretic requirement, urinary sodium excretion, or body weight was found between both groups throughout the study (Table 2 ).
Figure 1.
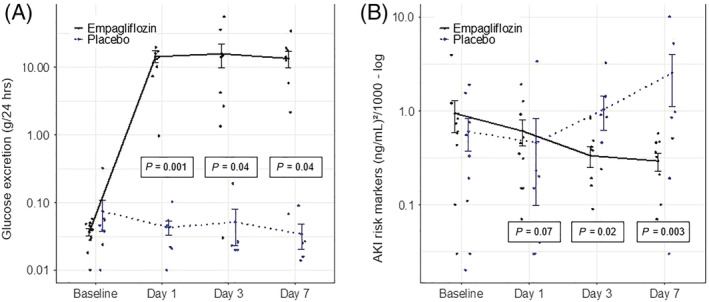
Effect of empagliflozin on markers of acute kidney injury (AKI). Urinary glucose excretion (A) and AKI risk markers (B) in patients with acute decompensated heart failure treated with empagliflozin (n = 10; black line) or placebo (n = 9; blue dotted line). Data are shown as mean ± standard error at baseline, after 1, 3, and 7 days. P‐values are calculated from the Wald tests for the intervention effect at each visit.
Empagliflozin had no significant effect on eGFR in comparison with placebo at any time point while serum cystatin C was transiently increased after 6 h but not at later time points in patients receiving empagliflozin (Table 2 ). TIMP‐2 and IGFBP7 (by NephroCheck®) were significantly reduced with empagliflozin treatment [from 0.9 ± 1.1 (ng/mL)2/1000 at baseline to 0.3 ± 0.2 (ng/mL)2/1000 at Day 3 (P = 0.02) and 0.3 ± 0.2 (ng/mL)2/1000 at Day 7 (P = 0.003)] suggesting nephroprotective effects of empagliflozin (Figure 1 , Table 2 ).
Discussion/conclusions
Our study in acute decompensated HF patients demonstrates a significant reduction of risk markers of AKI by empagliflozin suggesting that SGLT2 inhibitor treatment may prevent AKI in patients with acute decompensated HF. Acute decompensated HF frequently causes kidney impairment and treatment of decompensated HF may lead to AKI. 4 SGLT2 inhibitors have been shown to reduce both cardiac (CV death or HF hospitalization) and kidney endpoints in chronic HF patients, 5 , 6 , 7 but concerns have been raised that the agents could lead to AKI in patients with acute decompensated HF. Recent data from the EMPULSE trial, enrolling patients with acute decompensated HF on average within 3.4 days after hospital admission, did not show an increased risk for AKI in empagliflozin‐treated patients. Our data, enrolling patients within the same period, extend these results by demonstrating that empagliflozin significantly decreases the urinary AKI risk markers TIMP‐2 and IGFBP7, previously established as a sensitive tool to assess AKI risk before a decrease of kidney function becomes evident. 8 , 9 Renoprotective effects of SGLT2 inhibitors have been attributed to kidney tubular‐glomerular feedback with subsequent reduction of glomerular filtration pressure as the driving mechanism. In our study, this was evidenced by a transient elevation of serum cystatin C and a trend towards a lower eGFR with empagliflozin. Beyond tubuloglomerular feedback, other mechanisms such as reduced tubular energy consumption may also contribute to the nephroprotective effects of SGLT2 inhibitors.
Taken together, our data in patients with acute decompensated HF suggest that empagliflozin treatment does not affect haemodynamic parameters but significantly reduces markers of AKI.
This study has certain limitations. The main limitation is that the reduction of important validated markers of acute kidney damage is an exploratory finding in a limited number of patients and warrants confirmation in a larger study with changes in renal function defined as primary outcome. Still, the present study was randomized, blinded, and placebo‐controlled, and changes in renal function assessed by laboratory measurement and AKI Risk Score measured by NephroCheck® were predefined exploratory endpoints. Additional studies using larger populations will be required to investigate these effects of SGLT2 inhibition on structural changes of the kidney.
Conflict of interest
K.T., M.R., N.U.K.H., M.M., J.M., and A.K. report no potential conflict of interest. J.J. has given lectures for Bayer and Fresenius Medica Care. In addition, he holds four patents in the topic of the manuscript and is inventor of an additional, already sold patent to Baxter. M.B. received speaker honoraria from Astra‐Zeneca and Boehringer Ingelheim and was national coordinator of DAPA‐HF and EMPEROR‐Reduced. J.F. has received consultancy fees from AstraZeneca, Bayer, Boehringer, and Vifor and is a member of the data safety monitoring committee in NovoNordisk trials. N.M. has received support for clinical trial leadership from Boehringer Ingelheim and Novo Nordisk and served as a consultant to Boehringer Ingelheim, Merck, Novo Nordisk, and AstraZeneca. B.M.S. received grant support from Boehringer Ingelheim, Merck, and Novo Nordisk and served as a speaker for Boehringer Ingelheim, Merck, Novo Nordisk, Lilly, BMS, and Astra Zeneca. M.L. received grants and personal fees from Boehringer Ingelheim, MSD, and Novo Nordisk and personal fees from Amgen, Sanofi, Astra Zeneca, Bayer, and Lilly.
Funding
This investigator‐initiated trial was supported by a research grant provided by Boehringer Ingelheim Pharma GmbH & Co. KG. J.J., M.B., J.F., N.M., and M.L. are supported by the Deutsche Forschungsgemeinschaft [German Research Foundation; TRR 219; Project ID 322900939 (C01, C04, M02, M03, M05, S01, S03)].
Thiele, K. , Rau, M. , Hartmann, N.‐U. K. , Möller, M. , Möllmann, J. , Jankowski, J. , Keszei, A. P. , Böhm, M. , Floege, J. , Marx, N. , and Lehrke, M. (2022) Empagliflozin reduces markers of acute kidney injury in patients with acute decompensated heart failure. ESC Heart Failure, 9: 2233–2238. 10.1002/ehf2.13955.
Kirsten Thiele and Matthias Rau have equal contribution.
Trial registration: EudraCT Number: 2017‐002695‐45; date of registration: 2018‐06‐13 (clinicaltrials.gov).
References
- 1. Bubenek‐Turconi SI, Craciun M, Miclea I, Perel A. Noninvasive continuous cardiac output by the Nexfin before and after preload‐modifying maneuvers: a comparison with intermittent thermodilution cardiac output. Anesth Analg. 2013; 117: 366–372. [DOI] [PubMed] [Google Scholar]
- 2. Broch O, Renner J, Gruenewald M, Meybohm P, Schöttler J, Caliebe A, Steinfath M, Malbrain M, Bein B. A comparison of the Nexfin® and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia. 2012; 67: 377–383. [DOI] [PubMed] [Google Scholar]
- 3. Nalesso F, Cattarin L, Gobbi L, Fragasso A, Garzotto F, Calo LA. Evaluating Nephrocheck® as a predictive tool for acute kidney injury. Int J Nephrol Renovasc Dis. 2020; 13: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Filippatos G, Farmakis D, Parissis J. Renal dysfunction and heart failure: things are seldom what they seem. Eur Heart J. 2014; 35: 416–418. [DOI] [PubMed] [Google Scholar]
- 5. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐la Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 6. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner–la Rocca HP, Choi DJ, Chopra V, Chuquiure‐Valenzuela E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 7. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 8. Kashani K, Al‐Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong M, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EAJ, Joannes‐Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmelé T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013; 17: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bihorac A, Chawla LS, Shaw AD, al‐Khafaji A, Davison DL, DeMuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K, Heung M, Jortani S, Kleerup E, Koyner JL, Krell K, LeTourneau J, Lissauer M, Miner J, Nguyen HB, Ortega LM, Self WH, Sellman R, Shi J, Straseski J, Szalados JE, Wilber ST, Walker MG, Wilson J, Wunderink R, Zimmerman J, Kellum JA. Validation of cell‐cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014; 189: 932–939. [DOI] [PubMed] [Google Scholar]


