Abstract
Children with asthma who live in urban neighborhoods experience a disproportionately high asthma burden, with increased incident asthma and increased asthma symptoms, exacerbations, and acute visits and hospitalizations for asthma. There are multiple urban exposures that contribute to pediatric asthma morbidity, including exposure to pest allergens, mold, endotoxin, and indoor and outdoor air pollution. Children living in urban neighborhoods also experience inequities in social determinants of health, such as increased poverty, substandard housing quality, increased rates of obesity, and increased chronic stress. These disparities then in turn can increase the risk of urban exposures and compound asthma morbidity as poor housing repair is a risk factor for pest infestation and mold exposure and poverty is a risk factor for exposure to air pollution. Environmental interventions to reduce in‐home allergen concentrations have yielded inconsistent results. Population‐level interventions including smoking bans in public places and legislation to decrease traffic‐related air pollution have been successful at reducing asthma morbidity and improving lung function growth. Given the interface and synergy between urban exposures and social determinants of health, it is likely population and community‐level changes will be needed to decrease the excess asthma burden in children living in urban neighborhoods.
Keywords: air pollution, asthma disparities, childhood asthma, pediatric urban asthma, pest allergen exposure, social determinants of health, urban asthma, urban exposures
Key Message.
Multiple factors contribute to the excess asthma burden in children living in urban communities, including pest allergen, mold, and air pollution exposures as well as disparities in social determinants of health. It is likely that population‐level, rather than individual‐level, interventions will be needed to meaningfully decrease pediatric urban asthma risk and morbidity.
1. INTRODUCTION
Children living in urban areas are at higher risk of developing asthma and have increased asthma morbidity. Increased asthma prevalence and morbidity in urban communities have been observed across North America and Europe as well as in Asian, African, and South American countries. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 In the United States (US), the terms urban and inner city refer to centrally located neighborhoods, often in historic cities, characterized by concentrated poverty and predominantly racial and ethnic minority populations. In 1991, the National Institute of Allergy and Infectious Diseases (NIAID) began funding research aimed at addressing the increased asthma burden in inner cities. 9
Children living in urban neighborhoods are at increased risk of exposures known to be associated with asthma and asthma morbidity, such as pest allergens and mold as well as indoor and outdoor pollutants. 10 Additionally, children living in urban neighborhoods experience disadvantageous social determinants of health including increased poverty, poor housing quality, increased rates of obesity, and increased stress, all of which can contribute to asthma risk and morbidity. 11 The purpose of this manuscript is to review exposures which are unique to children with asthma living in urban neighborhoods and how these exposures contribute to the increased asthma burden (Figure 1), and discuss opportunities for intervention which could help to mitigate asthma disparities among urban children with asthma (Table 1).
FIGURE 1.
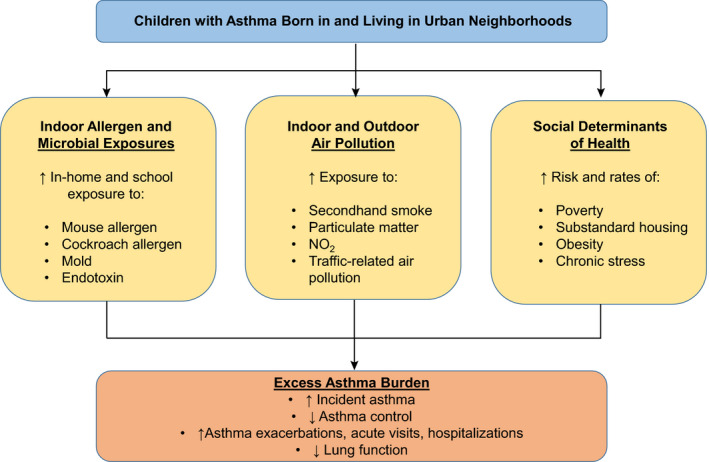
The relationship between urban risk factors and the excess asthma burden in children with asthma living in urban neighborhoods
TABLE 1.
Community‐level and population‐level opportunities for intervention to improve pediatric urban asthma
|
Improving the state of repair of public housing in an effort to decrease pest infestation and exposure as well as mold exposure |
|
Smoking bans in public places where children are at highest risk of SHS exposure |
|
Outdoor air quality measures to reduce TRAP and idling vehicles in urban neighborhoods and near urban schools |
|
Legislation, research, and public programs aimed at reducing urban, racial and ethnic disparities in income, housing, obesity rates, chronic stress exposure, and other SDoH |
Abbreviations: SDoH, social determinants of health; SHS, secondhand smoke; TRAP, traffic‐related air pollution.
2. INDOOR ALLERGENS AND MICROBES
Indoor allergen and microbial exposures have been recognized for decades as contributors to pediatric urban asthma. In particular, pest allergens, such as mouse and cockroach allergens, are major causes of urban pediatric asthma morbidity in the United States. 1 , 12 , 13 , 14 , 15 Low‐income children living in urban homes are disproportionately at risk of pest infestation and allergen exposure due to housing disrepair in inner cities. 16 , 17 , 18 Additionally, pest allergen concentrations are significantly higher in urban homes as compared with suburban homes. 19 In the United States, mouse allergen has been found in 95% of home dust samples from a multicenter asthma study, the National Cooperative Inner‐City Asthma Study, with higher concentrations of mouse allergen being found in homes with concomitant cockroach infestation. 20 Furthermore, urban children with asthma are often exposed to mouse allergen at school and day care, with one study out of Boston, Massachusetts detecting mouse allergen in 99.5% of schools sampled. 21 Cockroach allergen exposure is also very common in US urban centers, with the National Cooperative Inner‐City Asthma Study detecting cockroach allergen in >85% over homes, with concentrations considered to be “high” found in >50% of children's bedrooms. 13
Mouse allergen exposure in European homes is not as well‐studied or characterized and may be less clinically relevant. 10 , 22 A study out of Strasburg, France found in‐home rodent allergen concentrations to be lower than rodent allergen concentrations found in US homes, 23 with a recent Dutch study finding no association between self‐reported asthma and detectable mouse allergen. 22 However, cockroach allergen may be a more important urban pest allergen in Europe. A separate study out of Strasburg, France found high concentrations of cockroach allergen in low‐cost public housing. 24 Similarly, study out of Poland found higher cockroach concentrations in older homes, homes without central heat, and low‐income homes. 25
Pest allergen exposure, especially when combined with sensitization, has been repeatedly associated with asthma morbidity. Numerous studies in the United States have demonstrated the association between both mouse and cockroach allergen exposure and increased asthma morbidity, including increased asthma symptoms and exacerbations, increased acute visits and hospitalizations for asthma, and lower lung function in urban children with asthma. 12 , 13 , 14 , 26 , 27 , 28 The above‐mentioned study of Polish children with asthma found cockroach exposure to be associated with more severe asthma and lower lung function. 25
In addition to increased exposure to pest allergens, children in urban neighborhoods are also at increased risk of exposure to mold, which has also been associated with housing disrepair and low‐income housing. 29 Exposure to mold and dampness has been associated with childhood wheezing and childhood asthma 30 , 31 and mold sensitization and exposure has been associated with asthma symptoms, exacerbations, urgent visits for asthma, and lower lung function in urban asthmatic children. 32 , 33 , 34
While exposure to pest allergens in children with asthma is a clear risk factor for asthma morbidity in children with established disease, there is evidence suggesting that exposure to pest allergens in early life may in fact be protective against wheezing and childhood asthma development. In a US study of urban children who were at high risk of asthma development, the Urban Environment and Childhood Asthma (URECA) study, early‐life exposure to both mouse and cockroach was associated with a decreased risk of wheeze at age 3 35 and a decreased risk of asthma diagnosis at age 7. 36 Perhaps more interesting, not only did these children have high allergen exposures in the first year of life, but they were also exposed to higher levels and a more diverse bacterial content in house dust, 35 , 36 suggesting the microbiome associated with pest allergens may be the actual protective factor against wheeze and childhood asthma.
Endotoxin, a lipopolysaccharide (LPS) found in the outer membrane of gram‐negative bacteria, is often used as a marker of bacteria or microbial exposure. Endotoxin exposure in children to date has yielded mixed and complicated results. For example, a study of infants in New York City found endotoxin exposure was associated with an increased risk of wheezing, but a decreased risk of eczema. 37 However, in studies of rural, farming communities in both the United States and Europe, high endotoxin exposure is associated with decreased risk of asthma. 38 , 39 The difference in asthma outcomes in urban versus rural endotoxin exposure could partly be explained by higher exposure levels in rural areas, but likely is more complex owing to other exposures associated with urban versus rural residence.
Additionally, endotoxin exposure and its effects on asthma are often linked to and even modified by other exposures. In a national US survey, exposure to endotoxin was associated with wheezing, finding low income, cockroach sightings, pets in the homes, Mexican American race, and age less than 18 years to be predictors of higher endotoxin concentrations in house dust. 40 In a study of primarily low‐income, Black participants with asthma, the effect of endotoxin on asthma morbidity, including acute visits and oral steroids for asthma, was modified by air nicotine and nitrogen dioxide (NO2) concentrations. 41 For children living in homes with high air nicotine concentrations, endotoxin exposure was associated with increased acute visits for asthma. Conversely, children living in homes with lower NO2 concentrations, endotoxin exposure was associated with increased acute visits for asthma. 41 Lastly, endotoxin exposure has also been associated with childhood asthma morbidity in urban schools with increased exposure in school being associated with increased asthma symptoms. 42
As this fascinating story has developed over the past 30–40 years, it is clear that urban pest allergen and mold exposures are major risk factors for asthma morbidity in children with existing asthma, especially for children with high levels of exposure to allergens to which they are sensitized. It was, therefore, unexpected to find that high levels of pest allergen exposure in early life appear to be protective against asthma development, although this finding may be modified by, or even primarily driven by, the microbial exposures that coexist in these same households. This information, much like the data regarding protective effects of high levels of endotoxin exposure in early life, all lend support to the notion that the hygiene hypothesis may be just as relevant in urban areas as it was initially shown to be in farming communities.
3. INDOOR AND OUTDOOR URBAN POLLUTANTS
Children living in urban areas are exposed to high levels of both indoor and outdoor air pollution, both of which have been extensively linked to asthma risk and asthma morbidity. The primary and most well‐studied sources of indoor air pollution for urban children with asthma are secondhand tobacco smoke (SHS), particulate matter (PM), and nitrogen dioxide (NO2). 43
Children living in US urban centers are at risk for secondhand smoke exposure as multiple studies have found ≥50% of urban children are exposed to SHS. 10 , 44 , 45 , 46 This percentage is higher than expected, as currently 12.4% of US adults and 8.1% of US adolescents are active smokers, 47 illustrating both higher rates of tobacco use and overcrowding in urban households. Moreover, persons living in poverty, children under age 11, non‐Hispanic Blacks, persons living in rental housing, and those with less than a high school education are more likely to be exposed to SHS. 48 Following a public smoking ban, childhood SHS exposure has decreased considerably in England over the last 20 years. 49 However, SHS exposure remains high in the United Kingdom and many other European countries, with notable exposure at primary school entrances (46%) and outdoor playgrounds (41%) in multinational European studies, 50 , 51 with higher SHS exposure being associated with lower income areas in both studies. While some studies have failed to show an association between exposure to SHS and asthma, the majority of studies, including a 2012 systematic review and meta‐analysis, show a clear association between prenatal and childhood SHS exposure and an increased risk of wheezing and incident asthma. 52 The URECA study also showed prenatal smoke exposure was associated with increased asthma diagnosis in urban children at risk of asthma. 36 Additionally, SHS exposure may attenuate the effect of inhaled corticosteroids for the treatment of asthma in urban children, 53 making asthma controller medications less effective and contributing to increased asthma symptoms. Lastly, SHS exposure during childhood also has long‐term respiratory health effects beyond pediatric asthma, with parental smoking being associated with lower lung function at age 53 and an increased risk of adult obstructive lung disease. 54
Particulate matter is also a significant source of indoor air pollution for children with asthma living in urban centers. The primary source of indoor PM in urban homes is tobacco smoke, but other sources include cooking, heating, sweeping, and candle or incense burning. 55 , 56 Outdoor PM can also be a significant source of indoor PM 55 through open windows, doors, cracks, and poor housing repair. Urban indoor PM concentrations are significantly higher than those found in suburban homes 19 and can even be higher than outdoor urban PM. 57 Indoor PM exposure has been associated with increased asthma symptoms and exacerbations in urban children. 43 , 56 , 57 Nitrogen dioxide (NO2) is also an important component of indoor air pollution which has been associated with childhood asthma. Gas heating and gas stoves are the major sources of in‐home NO2, and urban families may use gas stoves as a source of heat during the winter when other heat sources are not available. 10 , 58 In‐home NO2 concentrations are often higher than outdoor NO2 concentrations, with higher in‐home NO2 concentrations being associated with increased asthma symptoms and decreased peak flows in urban children with asthma. 58 , 59
In addition to indoor air pollution, children living in urban communities also have higher exposure to outdoor air pollution. Traffic‐related air pollution (TRAP) and energy generation are the main sources of outdoor air pollution. 60 Multiple longitudinal studies, including birth cohort studies, have described a strong association between outdoor air pollution, particularly TRAP, and incident asthma, increased asthma symptoms, hospitalization for asthma, and lower lung function. 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 Urban children with asthma who live near major highways have been found to have increased exacerbations and poorer asthma control, 65 , 69 with idling cars and buses in dense urban traffic and near schools contributing to higher urban air pollution. 65 , 70 Poor indoor air quality in urban schools is also mostly owing to high levels of outdoor air pollution. 71 In the United States, low‐income racial and ethnic minority populations are at greater risk of being exposed to high pollution levels in urban areas, with historic redlining being associated with higher current air pollution levels. 72 In recent years, PM from wildfires has also become a significant source of outdoor air pollution. 73 In California, US, wildfires during the 2017 season led to an excess of asthma hospitalizations in the San Francisco Bay Area. 74
In summary, exposure to both indoor and outdoor air pollution are high in urban areas and contribute to excess asthma incidence and morbidity. Urban pollution exposure disparities represent important opportunities for intervention, which are discussed in a later section of this review.
4. SOCIAL DETERMINANTS OF HEALTH (SDOH) IN URBAN NEIGHBORHOODS
While indoor allergen exposures, microbial exposures, and air pollution are tangible and measurable urban risk factors for asthma, several other population‐level characteristics contribute to asthma disparities and the excess asthma burden in children living in urban communities. Social determinants of health (SDoH) are non‐medical influences that affect health outcomes 75 and include the conditions in which people are born, grow up, go to school and work, live, and age. SDoH are becoming increasingly recognized as important risk factors for pediatric urban asthma. 11 , 76 Here, we will discuss poverty, housing, obesity, and stress in the context of pediatric urban asthma.
In the United States, urban areas have high rates of poverty, with racial and ethnic minority populations having the highest rates of poverty in both urban and rural areas. 77 Income level in the United States is inversely related to asthma prevalence, with those living in the greatest degree of poverty having the highest asthma prevalence. 78 In a study of urban children with asthma in Baltimore, Maryland, there was an increase in odds of prevalent asthma per unit decrease in the household income to poverty ratio. 79 Low income is also associated with asthma exacerbations and asthma morbidity. 80 Poverty's effects on asthma are likely multifactorial, encompassing disparities in housing, education, employment, exposure to pests and pollution, limited health literacy, and access to health care. 11
Housing quality is an important SDoH, with racial and ethnic minority populations being more likely to reside in housing considered substandard or in poor repair, which contributes to environmental health disparities. 81 Substandard urban housing is a risk factor for mouse and cockroach exposure as poor housing conditions contribute to pest infestation, 16 , 17 , 18 which has been highlighted above as being associated with asthma risk and morbidity. Similarly, living in homes with visible mold in the main living areas has been associated with pediatric asthma risk, asthma symptoms, and persistent asthma. 30
Next, pediatric urban asthma patients have higher rates of obesity. 10 , 82 In the United States, Black children have the highest rates of early childhood (age 9 months to kindergarten entry) obesity. 83 Obesity in childhood has been linked to incident asthma and asthma morbidity 84 and lower lung function 85 in large‐scale studies. Of further interest, being overweight or obese has been associated with increased susceptibility to urban exposures such as pollution and SHS, 82 , 86 and may further increase asthma morbidity.
Lastly, children and caregivers living in low‐income, urban communities experience high levels of chronic stress, which is often multifactorial, involving high rates of income, job and food insecurity, exposure to violence, incarceration, and social disadvantage. 87 Early‐life exposure to stress and maternal stress have both been associated with childhood asthma diagnosis. 36 , 88 Moreover, chronic stress has been associated with poor asthma control and asthma exacerbations in Black and other racial and ethnic minority children with asthma. 89 , 90 Chronic stress may influence asthma through chronic hypothalamic‐pituitary‐adrenocortical activation and a decrease in β2 adrenergic and glucocorticoid receptors. 91 This chronic activation and receptor downregulation may then lead to a decrease in responsiveness to asthma medication and an increase in asthma symptoms. 91 Chronic stress may also mediate the effects of SDoH on asthma in urban children, but the degree to which SDoH influence or explain asthma disparities is not clear.
5. OPPORTUNITIES FOR INTERVENTION
Given the increased risk of asthma and increased asthma morbidity associated with children living in urban neighborhoods, interventions aimed at improving the urban environment in an effort to improve pediatric asthma health have been attempted for several decades. On the individual level, attempts at reducing indoor pest allergen exposure have produced mixed and often disappointing results. It can be difficult to achieve sustained allergen reduction in the urban setting. Multimodal approaches are necessary and have been successful at reducing cockroach allergen exposure and asthma symptoms in multicenter study of urban children with asthma. 92 Conversely, similar methods were not successful at reducing mouse allergen exposure in a separate study of urban pediatric patients with asthma; however, participants who did experience a reduction in mouse allergen exposure, regardless of group assignment, did have an improvement in asthma symptoms. 93 A secondary analysis of this study found that significant reduction in mouse allergen exposure was associated with improved lung function growth over 1 year. 94 Yet, a different multifaceted allergen reduction study of children with asthma in New York City reported reduction in allergen exposure, but no change in asthma controller medication. 95 It is unclear as to why some studies have been successful in reducing allergen exposure and impacting asthma outcomes, while others either did not reduce allergen exposure or impact clinical outcomes. It is possible that the poor state of housing repair and high levels of infestation are limiting factors in the success of allergen exposure reduction methods in certain urban neighborhoods and community‐level, rather than individual‐level, approaches to improve housing conditions and pest infestation should be considered.
Population‐level approaches to reduce urban pollutant exposures have been successful. As an example, in Scotland, the enactment of a smoking ban in public places has been associated with a decrease in asthma hospitalizations in children under age 15. 96 Follow‐up of the public smoking ban in the United Kingdom has found decreased SHS exposure in children, including in children living in rental housing. 49 Public smoking bans aimed at reducing SHS in areas with high rates of children, such as public housing, school entrances, and playgrounds, could meaningfully affect childhood SHS exposure and reduce asthma risk and morbidity.
Another example of a successful population‐level approach to reducing urban asthma risk and morbidity has been measures taken to reduce TRAP and improve outdoor air quality in California. Reduction in California air pollution has been associated with a decrease in incident pediatric asthma and an improvement in pediatric lung function growth. 97 , 98 Similar regulations in other densely populated urban centers with high TRAP could meaningfully reduce urban asthma disparities.
Lastly, making population‐level changes to help reduce inequities in SDoH, such as high poverty, housing disrepair, higher rates of obesity, and chronic stress, will be needed to help reduce the excess asthma burden in urban children.
6. CONCLUSION
Multiple environmental exposures and influences contribute to the increased incidence of asthma and excess asthma morbidity among children with asthma living in urban communities. Indoor pest allergen and mold exposures have been repeatedly linked to increased asthma diagnosis, symptoms, and exacerbations in urban children. However, data in high‐risk urban populations found early‐life pest allergen exposure, along with microbial and endotoxin exposures, to be associated with a decreased risk of wheezing and asthma, 35 , 36 illustrating that the association is more complex than previously thought. Individual‐level allergen exposure reduction in urban children with asthma has proven challenging and yielded inconsistent results in allergen and asthma outcomes. Community‐level interventions targeting housing disparities leading to pest infestation are likely needed to meaningfully change urban pest allergen exposure.
Population‐level interventions have been successful at reducing childhood SHS and TRAP exposure with associated improvements in incident asthma, pediatric asthma hospitalizations, and childhood lung function growth. 96 , 97 , 98 However, children living in urban neighborhoods, in particularly racial and ethnic minority children, continue to be exposed to high levels of indoor and outdoor air pollution. Similarly, urban children with asthma are disproportionately affected by disadvantageous social determinants of health, including poverty, poor housing, increased rates of obesity, and chronic stress. While these disparities have been described in the literature, the extent to which and how individual SDoH influence pediatric urban asthma is unknown.
The environmental exposures and influences affecting pediatric urban asthma are complex and intertwined. Ultimately, community‐level and population‐level changes targeting pest allergen, mold, and air pollution exposures in conjunction with community‐level and population‐level changes to decrease income, housing, and other social inequalities will be needed to meaningfully change pediatric urban asthma risk and morbidity.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13784.
Grant TL, Wood RA. The influence of urban exposures and residence on childhood asthma. Pediatr Allergy Immunol. 2022;33:e13784. doi: 10.1111/pai.13784
Editor: Ömer Kalayci
REFERENCES
- 1. Milligan KL, Matsui E, Sharma H. Asthma in Urban children: epidemiology, environmental risk factors, and the public health domain. Curr Allergy Asthma Rep. 2016;16(4):33. doi: 10.1007/s11882-016-0609-6 [DOI] [PubMed] [Google Scholar]
- 2. Lawson JA, Janssen I, Bruner MW, Madani K, Pickett W. Urban‐rural differences in asthma prevalence among young people in Canada: the roles of health behaviors and obesity. Ann Allergy Asthma Immunol. 2011;107(3):220‐228. doi: 10.1016/j.anai.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 3. Keet CA, Matsui EC, McCormack MC, Peng RD. Urban residence, neighborhood poverty, race/ethnicity, and asthma morbidity among children on Medicaid. J Allergy Clin Immunol. 2017;140(3):822‐827. doi: 10.1016/j.jaci.2017.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elholm G, Linneberg A, Husemoen LLN, et al. The Danish urban‐rural gradient of allergic sensitization and disease in adults. Clin Exp Allergy. 2016;46(1):103‐111. doi: 10.1111/cea.12583 [DOI] [PubMed] [Google Scholar]
- 5. Majkowska‐Wojciechowska B, Pełka J, Korzon L, et al. Prevalence of allergy, patterns of allergic sensitization and allergy risk factors in rural and urban children. Allergy. 2007;62(9):1044‐1050. doi: 10.1111/j.1398-9995.2007.01457.x [DOI] [PubMed] [Google Scholar]
- 6. Shimwela M, Mwita JC, Mwandri M, Rwegerera GM, Mashalla Y, Mugusi F. Asthma prevalence, knowledge, and perceptions among secondary school pupils in rural and urban coastal districts in Tanzania. BMC Public Health. 2014;14:387. doi: 10.1186/1471-2458-14-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu WJ, Ma HX, Cui HY, et al. Prevalence and treatment of children’s asthma in rural areas compared with urban areas in beijing. Chin Med J (Engl). 2015;128(17):2273‐2277. doi: 10.4103/0366-6999.163381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin M, Sauer T, Alarcon JA, et al. Prevalence and impact of asthma among school‐aged students in Lima, Peru. Int J Tuberc Lung Dis. 2017;21(11):1201‐1205. doi: 10.5588/ijtld.17.0282 [DOI] [PubMed] [Google Scholar]
- 9. Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol. 2010;125(3):540‐544. doi: 10.1016/j.jaci.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 10. Matsui EC. Environmental exposures and asthma morbidity in children living in urban neighborhoods. Allergy. 2014;69(5):553‐558. doi: 10.1111/all.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grant T, Croce E, Matsui EC. Asthma and the social determinants of health. Ann Allergy Asthma Immunol. 2022;128(1):5‐11. doi: 10.1016/j.anai.2021.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahluwalia SK, Peng RD, Breysse PN, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol. 2013;132(4):830‐835.e2. doi: 10.1016/j.jaci.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenstreich DL, Slavin RG, Lynn H. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner‐city children with asthma. N Engl J Med. 1997;336(19):1356‐1363. [DOI] [PubMed] [Google Scholar]
- 14. Torjusen EN, Diette GB, Breysse PN, Curtin‐Brosnan J, Aloe C, Matsui EC. Dose‐response relationships between mouse allergen exposure and asthma morbidity among urban children and adolescents. Indoor Air. 2013;23(4):268‐274. doi: 10.1111/ina.12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Exposure to cockroach allergen in the home is associated with incident doctor‐diagnosed asthma and recurrent wheezing. J Allergy Clin Immunol. 2001;107(1):41‐47. doi: 10.1067/mai.2001.111143 [DOI] [PubMed] [Google Scholar]
- 16. Chew GL, Perzanowski MS, Miller RL, et al. Distribution and determinants of mouse allergen exposure in low‐income New York city apartments. Environ Health Perspect. 2003;111(10):1348‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peters JL, Levy JI, Rogers CA, Burge HA, Spengler JD. Determinants of allergen concentrations in apartments of asthmatic children living in public housing. J Urban Health. 2007;84(2):185‐197. doi: 10.1007/s11524-006-9146-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rauh VA, Chew GR, Garfinkel RS. Deteriorated housing contributes to high cockroach allergen levels in inner‐city households. Environ Health Perspect. 2002;110(Suppl 2):323‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simons E, Curtin‐Brosnan J, Buckley T, Breysse P, Eggleston PA. Indoor environmental differences between inner city and suburban homes of children with asthma. J Urban Health. 2007;84(4):577‐590. doi: 10.1007/s11524-007-9205-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. I. The prevalence of mouse allergen in inner‐city homes. The National Cooperative Inner‐City Asthma Study. J Allergy Clin Immunol. 2000;106(6):1070‐1074. doi: 10.1067/mai.2000.110796 [DOI] [PubMed] [Google Scholar]
- 21. Sheehan WJ, Permaul P, Petty CR, et al. Association between allergen exposure in inner‐city schools and asthma morbidity among students. JAMA Pediatr. 2017;171(1):31‐38. doi: 10.1001/jamapediatrics.2016.2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burt SA, Parramon Dolcet LI, Wouters IM. Airborne rodent allergen levels in Dutch households: a pilot study. Int J Environ Res Public Health. 2019;16(19):3736. doi: 10.3390/ijerph16193736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muti D, Purohit A, Dazy A, Verot A, de Blay F. Mouse (Mus m1) and rat (Rat n1) allergen levels in dust from private and public houses in Strasbourg, France are lower than houses in the U.S.A. Eur Ann Allergy Clin Immunol. 2012;44(2):93‐95. [PubMed] [Google Scholar]
- 24. de Blay F, Sanchez J, Hedelin G, et al. Dust and airborne exposure to allergens derived from cockroach (Blattella germanica) in low‐cost public housing in Strasbourg (France). J Allergy Clin Immunol. 1997;99(1 Pt 1):107‐112. doi: 10.1016/s0091-6749(97)70307-5 [DOI] [PubMed] [Google Scholar]
- 25. Stelmach I, Jerzynska J, Stelmach W, et al. Cockroach allergy and exposure to cockroach allergen in Polish children with asthma. Allergy. 2002;57(8):701‐705. doi: 10.1034/j.1398-9995.2002.23561.x [DOI] [PubMed] [Google Scholar]
- 26. Matsui EC, Eggleston PA, Buckley TJ, et al. Household mouse allergen exposure and asthma morbidity in inner‐city preschool children. Ann Allergy Asthma Immunol. 2006;97(4):514‐520. doi: 10.1016/S1081-1206(10)60943-X [DOI] [PubMed] [Google Scholar]
- 27. Pongracic JA, Visness CM, Gruchalla RS, Evans R, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in inner‐city children. Ann Allergy Asthma Immunol. 2008;101(1):35‐41. doi: 10.1016/S1081-1206(10)60832-0 [DOI] [PubMed] [Google Scholar]
- 28. Rhee H, Love T, Harrington D, Grape A. Common allergies in urban adolescents and their relationships with asthma control and healthcare utilization. Allergy Asthma Clin Immunol. 2018;14:33. doi: 10.1186/s13223-018-0260-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hood E. Dwelling disparities: how poor housing leads to poor health. Environ Health Perspect. 2005;113(5):A310‐A317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karvonen AM, Hyvärinen A, Korppi M, et al. Moisture damage and asthma: a birth cohort study. Pediatrics. 2015;135(3):e598‐e606. doi: 10.1542/peds.2014-1239 [DOI] [PubMed] [Google Scholar]
- 31. Shorter C, Crane J, Pierse N, et al. Indoor visible mold and mold odor are associated with new‐onset childhood wheeze in a dose‐dependent manner. Indoor Air. 2018;28(1):6‐15. doi: 10.1111/ina.12413 [DOI] [PubMed] [Google Scholar]
- 32. Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness‐related agents: a review of the epidemiologic evidence. Environ Health Perspect. 2011;119(6):748‐756. doi: 10.1289/ehp.1002410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fisk WJ, Lei‐Gomez Q, Mendell MJ. Meta‐analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air. 2007;17(4):284‐296. doi: 10.1111/j.1600-0668.2007.00475.x [DOI] [PubMed] [Google Scholar]
- 34. Pongracic JA, O’Connor GT, Muilenberg ML, et al. Differential effects of outdoor vs indoor fungal spores on asthma morbidity in inner‐city children. J Allergy Clin Immunol. 2010;125(3):593‐599. doi: 10.1016/j.jaci.2009.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lynch SV, Wood RA, Boushey H, et al. Effects of early‐life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134(3):593‐601.e12. doi: 10.1016/j.jaci.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Connor GT, Lynch SV, Bloomberg GR, et al. Early‐life home environment and risk of asthma among inner‐city children. J Allergy Clin Immunol. 2018;141(4):1468‐1475. doi: 10.1016/j.jaci.2017.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perzanowski MS, Miller RL, Thorne PS, et al. Endotoxin in inner‐city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol. 2006;117(5):1082‐1089. doi: 10.1016/j.jaci.2005.12.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stein MM, Hrusch CL, Gozdz J, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375(5):411‐421. doi: 10.1056/NEJMoa1508749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Braun‐Fahrländer C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school‐age children. N Engl J Med. 2002;347(12):869‐877. doi: 10.1056/NEJMoa020057 [DOI] [PubMed] [Google Scholar]
- 40. Thorne PS, Mendy A, Metwali N, et al. Endotoxin exposure: predictors and prevalence of associated asthma outcomes in the United States. Am J Respir Crit Care Med. 2015;192(11):1287‐1297. doi: 10.1164/rccm.201502-0251OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsui EC, Hansel NN, Aloe C, et al. Indoor pollutant exposures modify the effect of airborne endotoxin on asthma in urban children. Am J Respir Crit Care Med. 2013;188(10):1210‐1215. doi: 10.1164/rccm.201305-0889OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lai PS, Sheehan WJ, Gaffin JM, et al. School endotoxin exposure and asthma morbidity in inner‐city children. Chest. 2015;148(5):1251‐1258. doi: 10.1378/chest.15-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, McCormack MC. Indoor air pollution and asthma in children. Proc Am Thorac Soc. 2010;7(2):102‐106. doi: 10.1513/pats.200908-083RM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu TD, Eakin MN, Rand CS, et al. In‐home secondhand smoke exposure among urban children with asthma: contrasting households with and without residential smokers. J Public Health Manag Pract. 2019;25(2):E7‐E16. doi: 10.1097/PHH.0000000000000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Butz AM, Halterman JS, Bellin M, et al. Factors associated with second‐hand smoke exposure in young inner‐city children with asthma. J Asthma. 2011;48(5):449‐457. doi: 10.3109/02770903.2011.576742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quinto KB, Kit BK, Lukacs SL, Akinbami LJ. Environmental tobacco smoke exposure in children aged 3–19 years with and without asthma in the United States, 1999–2010. NCHS Data Brief. 2013;126:1‐8. [PubMed] [Google Scholar]
- 47. Cigarette smoking and electronic cigarette use. 2022. https://www.cdc.gov/nchs/fastats/smoking.htm Accessed March 25, 2022
- 48. Tsai J, Homa DM, Gentzke AS, et al. Exposure to secondhand smoke among nonsmokers — United States, 1988–2014. MMWR Morb Mortal Wkly Rep. 2018;67(48):1342‐1346. doi: 10.15585/mmwr.mm6748a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tattan‐Birch H, Jarvis MJ. Children’s exposure to second‐hand smoke 10 years on from smoke‐free legislation in England: cotinine data from the Health Survey for England 1998–2018 Lancet Reg Health Eur. 2022;15:1998‐2018. doi: 10.1016/j.lanepe.2022.100315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Henderson E, Continente X, Fernández E, et al. Secondhand smoke exposure and other signs of tobacco consumption at outdoor entrances of primary schools in 11 European countries. Sci Total Environ. 2020;743:140743. doi: 10.1016/j.scitotenv.2020.140743 [DOI] [PubMed] [Google Scholar]
- 51. Henderson E, Continente X, Fernández E, et al. Secondhand smoke exposure in outdoor children’s playgrounds in 11 European countries. Environ Int. 2021;149:105775. doi: 10.1016/j.envint.2020.105775 [DOI] [PubMed] [Google Scholar]
- 52. Burke H, Leonardi‐Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta‐analysis. Pediatrics. 2012;129(4):735‐744. doi: 10.1542/peds.2011-2196 [DOI] [PubMed] [Google Scholar]
- 53. Lazarus SC, Chinchilli VM, Rollings NJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med. 2007;175(8):783‐790. doi: 10.1164/rccm.200511-1746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bui DS, Walters HE, Burgess JA, et al. Childhood respiratory risk factor profiles and middle‐age lung function: a prospective cohort study from the first to sixth decade. Annals ATS. 2018;15(9):1057‐1066. doi: 10.1513/AnnalsATS.201806-374OC [DOI] [PubMed] [Google Scholar]
- 55. Zhang L, Ou C, Magana‐Arachchi D, et al. Indoor particulate matter in urban households: sources, pathways, characteristics, health effects, and exposure mitigation. Int J Environ Res Public Health. 2021;18(21):11055. doi: 10.3390/ijerph182111055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grant T, Brigham EP, McCormack MC. Childhood origins of adult lung disease as opportunities for prevention. J Allergy Clin Immunol Pract. 2020;8(3):849‐858. doi: 10.1016/j.jaip.2020.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McCormack MC, Breysse PN, Matsui EC, et al. In‐home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117(2):294‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hansel NN, Breysse PN, McCormack MC, et al. A longitudinal study of indoor nitrogen dioxide levels and respiratory symptoms in inner‐city children with asthma. Environ Health Perspect. 2008;116(10):1428‐1432. doi: 10.1289/ehp.11349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kattan M, Gergen PJ, Eggleston P, Visness CM, Mitchell HE. Health effects of indoor nitrogen dioxide and passive smoking on urban asthmatic children. J Allergy Clin Immunol. 2007;120(3):618‐624. doi: 10.1016/j.jaci.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 60. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581‐1592. doi: 10.1016/S0140-6736(14)60617-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schultz ES, Hallberg J, Bellander T, et al. Early‐life exposure to traffic‐related air pollution and lung function in adolescence. Am J Respir Crit Care Med. 2016;193(2):171‐177. doi: 10.1164/rccm.201505-0928OC [DOI] [PubMed] [Google Scholar]
- 62. Brauer M, Hoek G, Smit HA, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007;29(5):879‐888. doi: 10.1183/09031936.00083406 [DOI] [PubMed] [Google Scholar]
- 63. Brauer M, Hoek G, Van Vliet P, et al. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med. 2002;166(8):1092‐1098. doi: 10.1164/rccm.200108-007OC [DOI] [PubMed] [Google Scholar]
- 64. Gehring U, Wijga AH, Koppelman GH, Vonk JM, Smit HA, Brunekreef B. Air pollution and the development of asthma from birth until young adulthood. Eur Respir J. 2020;56(1):2000147. doi: 10.1183/13993003.00147-2020 [DOI] [PubMed] [Google Scholar]
- 65. Patel MM, Quinn JW, Jung KH, et al. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environ Res. 2011;111(8):1222‐1229. doi: 10.1016/j.envres.2011.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sunyer J, Spix C, Quenel P, et al. Urban air pollution and emergency admissions for asthma in four European cities: the APHEA Project. Thorax. 1997;52(9):760‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pénard‐Morand C, Raherison C, Charpin D, et al. Long‐term exposure to close‐proximity air pollution and asthma and allergies in urban children. Eur Respir J. 2010;36(1):33‐40. doi: 10.1183/09031936.00116109 [DOI] [PubMed] [Google Scholar]
- 68. Keet CA, Keller JP, Peng RD. Long‐term coarse particulate matter exposure is associated with asthma among children in medicaid. Am J Respir Crit Care Med. 2018;197(6):737‐746. doi: 10.1164/rccm.201706-1267OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li S, Batterman S, Wasilevich E, Elasaad H, Wahl R, Mukherjee B. Asthma exacerbation and proximity of residence to major roads: a population‐based matched case‐control study among the pediatric Medicaid population in Detroit, Michigan. Environ Health. 2011;10:34. doi: 10.1186/1476-069X-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Richmond‐Bryant J, Bukiewicz L, Kalin R, Galarraga C, Mirer F. A multi‐site analysis of the association between black carbon concentrations and vehicular idling, traffic, background pollution, and meteorology during school dismissals. Sci Total Environ. 2011;409(11):2085‐2093. doi: 10.1016/j.scitotenv.2011.02.024 [DOI] [PubMed] [Google Scholar]
- 71. Majd E, McCormack M, Davis M, et al. Indoor air quality in inner‐city schools and its associations with building characteristics and environmental factors. Environ Res. 2019;170:83‐91. doi: 10.1016/j.envres.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lane HM, Morello‐Frosch R, Marshall JD, Apte JS. Historical redlining is associated with present‐day air pollution disparities in U.S. cities. Environ Sci Technol Lett. 2022;9(4):345‐350. doi: 10.1021/acs.estlett.1c01012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cascio WE. Wildland fire smoke and human health. Sci Total Environ. 2018;624:586‐595. doi: 10.1016/j.scitotenv.2017.12.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cleland SE, Serre ML, Rappold AG, West JJ. Estimating the acute health impacts of fire‐originated pm2.5 exposure during the 2017 California wildfires: sensitivity to choices of inputs. Geohealth. 2021;5(7):e2021GH000414. doi: 10.1029/2021GH000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Social determinants of health. https://www.who.int/westernpacific/health‐topics/social‐determinants‐of‐health Accessed June 17, 2021
- 76. Federico MJ, McFarlane AE, Szefler SJ, Abrams EM. The impact of social determinants of health on children with asthma. J Allergy Clin Immunol Pract. 2020;8(6):1808‐1814. doi: 10.1016/j.jaip.2020.03.028 [DOI] [PubMed] [Google Scholar]
- 77. Bureau UC. A Comparison of Rural and Urban America: Household Income and Poverty. The United States Census Bureau; 2016. https://www.census.gov/newsroom/blogs/random‐samplings/2016/12/a_comparison_of_rura.html Accessed March 25, 2022 [Google Scholar]
- 78. CDC . Most Recent National Asthma Data. CDC; 2021. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm Accessed June 15, 2021 [Google Scholar]
- 79. Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: rethinking the inner‐city asthma epidemic. J Allergy Clin Immunol. 2015;135(3):655‐662. doi: 10.1016/j.jaci.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cardet JC, Louisias M, King TS, et al. Income is an independent risk factor for worse asthma outcomes. J Allergy Clin Immunol. 2018;141(2):754‐760.e3. doi: 10.1016/j.jaci.2017.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jacobs DE. Environmental health disparities in housing. Am J Public Health. 2011;101(Suppl 1):S115‐S122. doi: 10.2105/AJPH.2010.300058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lu KD, Breysse PN, Diette GB, et al. Being overweight increases susceptibility to indoor pollutants among urban children with asthma. J Allergy Clin Immunol. 2013;131(4):1017‐1023.e3. doi: 10.1016/j.jaci.2012.12.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Isong IA, Rao SR, Bind MA, Avendaño M, Kawachi I, Richmond TK. Racial and ethnic disparities in early childhood obesity. Pediatrics. 2018;141(1):e20170865. doi: 10.1542/peds.2017-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Khalid F, Holguin F. A review of obesity and asthma across the life span. J Asthma. 2018;55(12):1286‐1300. doi: 10.1080/02770903.2018.1424187 [DOI] [PubMed] [Google Scholar]
- 85. Forno E, Han YY, Mullen J, Celedón JC. Overweight, obesity, and lung function in children and adults‐a meta‐analysis. J Allergy Clin Immunol Pract. 2018;6(2):570‐581.e10. doi: 10.1016/j.jaip.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wu TD, Brigham EP, Peng R, et al. Overweight/obesity enhances associations between secondhand smoke exposure and asthma morbidity in children. J Allergy Clin Immunol Pract. 2018;6(6):2157‐2159.e5. doi: 10.1016/j.jaip.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brondolo E, Byer K, Gianaros PJ, Liu C, Prather AA, Thomas K. Stress and Health Disparities: Contexts, Mechanisms, and Interventions among Racial/Ethnic Minority and low‐Socioeconomic Status Populations [Internet]. American Psychological Association (APA); 2017. doi: 10.1037/e500202018-001 [DOI] [Google Scholar]
- 88. Rosa M, Lee A, Wright R. Evidence establishing a link between prenatal and early‐life stress and asthma development. Curr Opin Allergy Clin Immunol. 2018;18:1. doi: 10.1097/ACI.0000000000000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jones BL, Staggs V, Woods‐Jaeger B. Chronic stress exposure among young African American children with asthma: Racism is a factor. Ann Allergy Asthma Immunol. 2019;123(5):507‐508. doi: 10.1016/j.anai.2019.08.023 [DOI] [PubMed] [Google Scholar]
- 90. Landeo‐Gutierrez J, Forno E, Miller GE, Celedón JC. Exposure to violence, psychosocial stress, and asthma. Am J Respir Crit Care Med. 2020;201(8):917‐922. doi: 10.1164/rccm.201905-1073PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Landeo‐Gutierrez J, Celedón JC. Chronic stress and asthma in adolescents. Ann Allergy Asthma Immunol. 2020;125(4):393‐398. doi: 10.1016/j.anai.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home‐based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068‐1080. doi: 10.1056/NEJMoa032097 [DOI] [PubMed] [Google Scholar]
- 93. Matsui EC, Perzanowski M, Peng RD, et al. Effect of an integrated pest management intervention on asthma symptoms among mouse‐sensitized children and adolescents with asthma: a randomized clinical trial. JAMA. 2017;317(10):1027‐1036. doi: 10.1001/jama.2016.21048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Grant T, Phipatanakul W, Perzanowski M, et al. Reduction in mouse allergen exposure is associated with greater lung function growth. J Allergy Clin Immunol. 2020;145(2):646‐653.e1. doi: 10.1016/j.jaci.2019.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. DiMango E, Serebrisky D, Narula S, et al. Individualized household allergen intervention lowers allergen level but not asthma medication use: a randomized controlled trial. J Allergy Clin Immunol Pract. 2016;4(4):671‐679.e4. doi: 10.1016/j.jaip.2016.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mackay D, Haw S, Ayres JG, Fischbacher C, Pell JP. Smoke‐free legislation and hospitalizations for childhood asthma. N Engl J Med. 2010;363(12):1139‐1145. doi: 10.1056/NEJMoa1002861 [DOI] [PubMed] [Google Scholar]
- 97. Garcia E, Berhane KT, Islam T, et al. Association of changes in air quality with incident asthma in children in California, 1993–2014. JAMA. 2019;321(19):1906. doi: 10.1001/jama.2019.5357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gauderman WJ, Urman R, Avol E, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015;372(10):905‐913. doi: 10.1056/NEJMoa1414123 [DOI] [PMC free article] [PubMed] [Google Scholar]


