Abstract
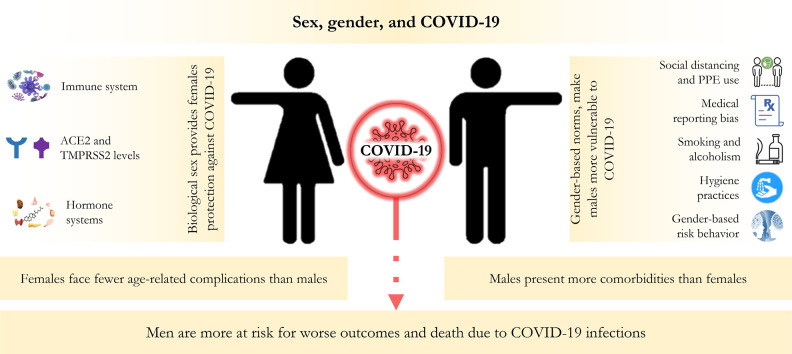
Multiple lines of evidence indicate that the male sex is a significant risk factor for severe disease and mortality due to coronavirus disease 2019 (COVID-19). However, the precise explanation for the discrepancy is currently unclear. Immunologically, the female-biased protection against COVID-19 could presumably be due to a more rapid and robust immune response to viruses exhibited by males. The female hormones, e.g., estrogens and progesterone, may have protective roles against viral infections. In contrast, male hormones, e.g., testosterone, can act oppositely. Besides, the expression of the ACE-2 receptor in the lung and airway lining, which the SARS-CoV-2 uses to enter cells, is more pronounced in males. Estrogen potentially plays a role in downregulating the expression of ACE-2, which could be a plausible biological explanation for the reduced severity of COVID-19 in females. Comorbidities, e.g., cardiovascular diseases, diabetes, and kidney disorders, are considered significant risk factors for severe outcomes in COVID-19. Age-adjusted data shows that males are statistically more predisposed to these morbidities—amplifying risks for males with COVID-19. In addition, many sociocultural factors and gender-constructed behavior of men and women impact exposure to infections and outcomes. In many parts of the world, women are more likely to abide by health regulations, e.g., mask-wearing and handwashing, than men. In contrast, men, in general, are more involved with high-risk behaviors, e.g., smoking and alcohol consumption, and high-risk jobs that require admixing with people, which increases their risk of exposure to the infection. Overall, males and females suffer differently from COVID-19 due to a complex interplay between many biological and sociocultural factors.
Keywords: COVID-19, Gender disparities, Risk factors, Biological difference, Sociocultural effects
Graphical abstract
1. Introduction
Understanding the degree to which outbreaks of infectious diseases affect people across sex, gender, ethnicity, race, and age distinctively is a fundamental step to understanding the primary and secondary effects of any health emergency (Wenham et al., 2020). Such knowledge is also vital for creating equitable and effective mitigation strategies. The response to the ‘once in a century’ outbreak of coronavirus disease 2019 (COVID-19) is no different (Wenham et al., 2020; Gates, 2020). Sex-disaggregated infection and mortality data of COVID-19 do not explicitly show whether people of different socio-demographic backgrounds, especially across biological sex and gender, are more likely to become infected. However, existing data indicate that biology and gender norms shape the disease burden differently between males and females (Pinheiro et al., 2020; Jin et al., 2020; Liu et al., 2017). Emerging evidence hints that COVID-19 results in more adverse effects on males than females. This could be related to intrinsic differences imparted by the males and females, specific characteristics of the SARS-CoV-2 infectious process, or gender norms (Klein and Flanagan, 2016a; Gebhard et al., 2020; Spagnolo et al., 2020). Nevertheless, currently available data are still inadequate to draw any conclusions on the gendered effects of COVID-19 (Global Health 50/50, 2022).
Biological sex and gender differences are directly associated with epidemiology, symptoms, and management of diseases and hence are essential concepts in the comprehensive study of public health (van Hagen et al., 2021; Lips, 2020). Sex is the biological construct depending on the genetic, reproductive, and endocrine organization that differentiates between males and females. As human response toward pathogens is strongly influenced by their genetic, immunological, and hormone systems, the sex difference results in disparity in how males and females are affected by the condition these pathogens cause. On the other hand, gender is the continuum of socially constructed roles, behaviors, expressions, and identities of men, women, and gender-diverse people. Gender norms impact how men or women are exposed to certain pathogens, especially when it comes to infectious diseases, and thus can cause differences in how men and women are susceptible to diseases. Sex (i.e., males and females) and gender (i.e., men and women) are inherently intertwined and entangled in complex ways, shaping how we respond to pathogens and the diseases caused by them (Springer et al., 2012).
Experience from previous outbreaks of infectious diseases shows that sex and gender differences, along with other socio-demographic factors, affect males/men and females/women differently (Mauvais-Jarvis et al., 2020; Ingersoll, 2017; vom Steeg and Klein, 2016). COVID-19, similarly, emphasizes the gendered nature of the risk that predominantly male/men individuals incur, conceivably due to sex-based physiological and immunological differences, e.g., X-linked immunity genes, sex hormone influenced immune cell function, and gendered variations, e.g., tobacco smoking and alcoholism (Wenham et al., 2020; Takahashi et al., 2020; Scully et al., 2020). Unfortunately, the interaction between biological sex and corresponding gender norms, sex-biased susceptibility to pathogens, and the direct gendered impacts remain among the underexplored topics in current public health and medicine. Efforts do not focus much on the interaction between sex and gender roles and exposure to pathogens or direct gendered impacts (Wenham et al., 2020; Muurlink and Taylor-Robinson, 2020; Smith, 2019; Klein and Flanagan, 2016b). Studies on sex and gender differences would critically shape our current understanding of vaccine and therapeutic strategy development, optimization, and administration. They can potentially fill the gap in the traditional therapeutic approach. Also, it will promote gender-specific management of post-traumatic stress symptoms (Spagnolo et al., 2020).
It has been repeatedly reported that the infection, hospitalization, and mortality rate due to COVID-19 are higher among males/men (Wenham et al., 2020; Global Health 50/50, 2022). However, there is a dearth of studies on the underlying factors precisely responsible for such differences. The socio-demographic, genetic, physiological, immunological, and cultural practice-related differences among males/men and females/women potentially have a strong influence on the sex- and gender-biased variation of COVID-19 related burden, and there has been a push to dig deeper into the pandemic's differential impacts. A clear understanding of the interaction of age, sex, gender, and risk factors, including genetic epidemiology and epigenetics, would unequivocally allow public health authorities to tailor their management strategies to better serve the people at most risk. In this write-up, we shed light upon the possible biological and physiological backgrounds and cultural norms related to the differential dimensions of COVID-19. While we recognize that gender is not a binary identity, this manuscript focused only on men and women as it sought to drill into how biological sex (male and female) and their corresponding gender (man and woman) cooperates and results in the disproportionate impact of COVID-19 in the males and females.
2. Difference in COVID-19 infection rate and mortality varies markedly by sex, gender, and age
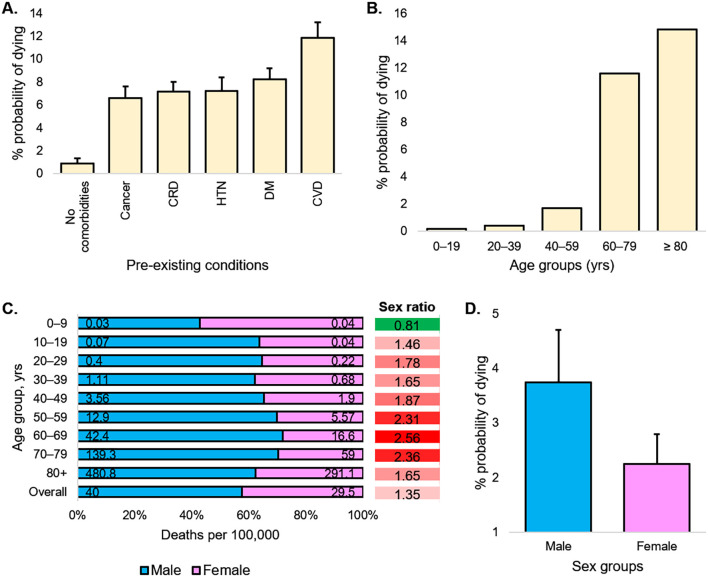
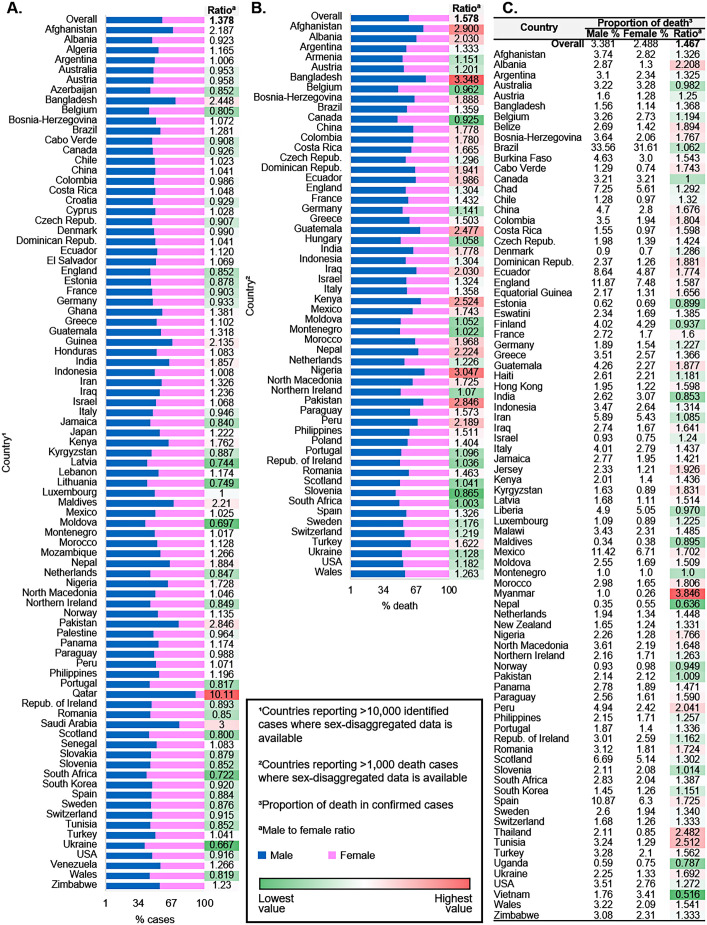
Institutionalized gender and culturally established roles and norms play roles in determining who is most at risk of acquiring an infection or receiving a test. To date, many studies have indicated that sex and gender potentially influence infectious disease risk and outcomes. Looking at the publicly available data on sex-disaggregated case identification rates and death rates, COVID-19 appears to be no exception as males seem more disadvantaged to it. According to the global health 50/50's data, males' global case identification rate and share in the death toll due to COVID-19 are ~40% and ~ 60% higher, respectively, compared to their female counterparts (Global Health 50/50, 2022). Likewise, the proportion of death in confirmed cases is also significantly higher among males (Fig. 1 ). These data indicate that institutionalized gender inequality is associated with the male to female ratio of reported cases of COVID-19 across the world.
Fig. 1.
Age, sex, existing conditions of COVID-19 cases and deaths. (a) Percent probability of dying for a patient with a given pre-existing condition. (b) Percent probability of dying depending on the age group. (c) In 10-year age bands by sex, deaths per 100,000 population and sex ratio. (d) Percent probability of dying for a patient by sex. . The percentages shown in panels a, b, and d do not add up to 100%, as they do not represent share of deaths by condition. CRD = chronic respiratory diseases, HTN = hypertension, DM = diabetes, CVD = cardiovascular disorders.
Also, data from other sources show that the death rate is not equal across sex-disaggregated age groups (Fig. 1). There was some variation across countries, although roughly, the pattern was comparable, and often the population sizes were not large enough to draw a concrete conclusion at a country-specific level (Global Health 50/50, 2022). Besides, pre-existing conditions that put patients at higher risk of dying from a COVID-19 infection include cardiovascular diseases, diabetes, chronic respiratory diseases, hypertension, and cancer, usually affecting older people and affecting males and females differently (Fig. 1).
3. Sex difference due to immunity
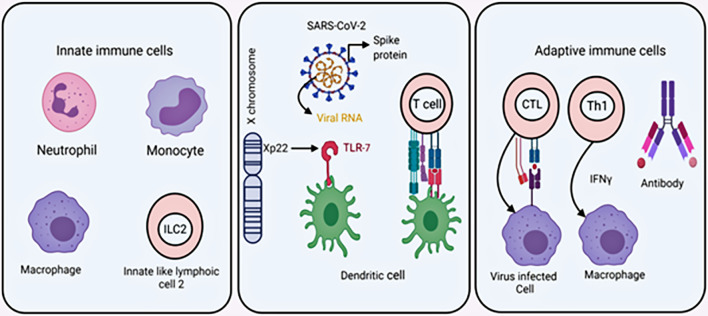
Male and female responds differently against viral infections (Fig. 2 ). Innate biological factors, e.g., genetic, epigenetic, and hormonal factors, help females exhibit a stronger immunity against these infections (Klein and Flanagan, 2016b; Klein, 2012). Components of the cellular and extracellular systems also contribute to a stronger immunity against a vast array of pathogens.
Fig. 2.
A partial overview of the immune system. Left panel: innate immune cells, middle: dendritic cell (DC) as a sensor cell, it recognizes virus RNA through PRR and DC act as antigen-presenting cell, found higher in the females, right panel: Cytotoxic T cell (CTL), Th1 can kill virus-infected cells present higher in female and basal antibody is also higher.
Humans, like other multicellular eukaryotes, the initial defense against pathogens is mediated by a diverse class of ubiquitous molecules called antimicrobial peptides (AMPs). AMPs are 10–100 residue long positively charged peptides with potent antibacterial, antifungal, and especially antiviral activities, expressed by the epithelial and professional host defense cells, e.g., macrophages or neutrophils (McCann et al., 2003). LL37, a cationic peptide derived from cathelicidin, is such an AMP that creates pores on coronavirus envelope protein and causes the virus to be destroyed (Steinstraesser et al., 2005). Vitamin D is a fat-soluble essential vitamin that upregulates AMP synthesis by regulating the NF-kB signaling pathway (Subramanian et al., 2017). This vitamin helps reduce microbial infections by generating physical barriers and boosting natural immunity and adaptive immunity (Beard et al., 2011; Abhimanyu, 2017; Rondanelli et al., 2018). Several meta-analyses generated from a considerable number of observational and clinical trial studies have indicated that vitamin D deficiency is associated with greater severity of COVID-19; however, a recent Mendelian randomization study did not observe evidence to support the association (Butler-Laporte et al., 2021; Wang et al., 2021; Liu et al., 2021; Szarpak et al., 2021). It was also reported that the serum levels of vitamin D were consistently lower in males than females, especially in older ages (Sanghera et al., 2017; Orwoll et al., 2009; Mosekilde, 2005; La Vignera et al., 2020). These indicate the synthesis of antimicrobial peptide LL37 is comparatively higher in females, which might protect females from viral infection (Sanghera et al., 2017; La Vignera et al., 2020). In addition, the elevated vitamin D level may also help the females to suppress the inflammatory cytokines, leukocyte infiltration, interaction with polymorphonuclear leukocytes, complement component C3, and enhance serological response and CD8+ T cell performance after being infected by SARS-CoV-2 (Teymoori-Rad et al., 2019; Grant et al., 2020; Xu et al., 1993; Risitano et al., 2020; Zheng et al., 2020; Diao, 2020). When viruses breach the initial AMP mediated defense mechanism, innate immune cells, e.g., neutrophils, and alveolar macrophages, are recruited in the infectious site. It has been reported that females have more dendritic cells (DCs), neutrophils, monocytes, and macrophages (Klein and Flanagan, 2016b). Also, phagocytic cells exhibit more vigorous activity in females (Spitzer, 1999). The DCs serve as a bridge between innate and adaptive immunity and function as an antigen-presenting cell. They promote the antigen-presenting capacity of adaptive immune cells, which is also reported to function better in females (Weinstein et al., 1984).
The innate immune cells, macrophages, and dendritic cells express pattern recognition receptors (PRRs), e.g., toll-like receptors 7 and 8 (TLR-7 and TLR-8), and act as sensor cells. Using these PRRs, they recognize molecular patterns present on the pathogens, e.g., bacterial lipopolysaccharide and viral genetic material. The expression of TLR-7 and TLR-8 is prominent in plasmacytoid DCs, and myeloid lineage cells, and they both can recognize single-strand RNA viruses (Diebold et al., 2004; Boehme and Compton, 2004). It is reported that plasmacytoid DCs expressing TLR-7 produces type 1 interferon against the influenza virus (Kaisho and Akira, 2006; Di Domizio et al., 2009). The expression of PRRs varies from male to female due to genetic differences (Berghöfer et al., 2006; Marriott and Huet-Hudson, 2006). Interestingly, studying several viral diseases, Klein et al. 2010 found ten times more interferon I responses in cell lines obtained from the females (Klein et al., 2010). The gene coding for TLR-7 is present in the X-chromosome, and as females have an additional copy of the X-chromosome than males, it provides them a female with more TLR-7 mediated immune response. This additional copy of the X-chromosome usually becomes condensed and forms an inactive Barr body to maintain proper gene dose in the females (Barr and Bertram, 1949). During the inactivation process, 10% of genes located in the non-recombinant region escape inactivation. It is reported that TLR-7 escapes the X-chromosome inactivation mechanism (Schurz et al., 2019). The inactivation of the X-chromosome's escaped genes compensates for any mutational loss in other chromosomes. Other than TLR-7, X-chromosomes contain nearly 60 immune genes, contributing to their better immunity against many pathogens (Tukiainen et al., 2017).
Innate-like lymphoid cells (ILC) originates from lymphoid progenitor also show a sex-biased response. There are three types of ILCs, such as ILC1, ILC2, ILC3, and these are prominent in the lung (De Grove et al., 2016). These ILCs recognize pathogens and provide signals to other immune cells to release cytokines. Interleukin-13 (IL-13), produced from ILC-2, has a protective role in influenza-induced lung infection in mice (Monticelli et al., 2011). The presence of an elevated number of ILC-2 cells was previously reported in female murine lung models, indicating the potential role of ILC-2 in female immunity (Cephus et al., 2017). Also, recently reported studies on human subjects suggested an age- and sex-dependent reduction in ILC-2 cell numbers in individuals over 10 years of age, and adult females, in general, possess more ILC-2 than males (Darboe et al., 2020).
The adaptive immune system also functions better in females. The females express more CD3+ and CD4+ T cells, which are the primary components of cellular immunity. Several clinical studies have shown that the ratio of CD4+ to CD8+ T cells is higher in females. The T helper 1 cell-, anti-inflammatory T helper 2 cell-, and regulatory T cell-mediated response are lower in males (Frisancho-Kiss et al., 2007). Additionally, the cytotoxic T cell level and the expression of antiviral and proinflammatory genes are higher in females due to their estrogen response element in the promoter region (Hewagama et al., 2009). The humoral immunity, basal immunoglobulin, and antibody response to viruses are higher in females (Abdullah et al., 2012; Teixeira et al., 2011).
Sex hormones, the environmental factors, can also regulate immune cells and upregulate lymphocytes, macrophages, and dendritic cells. Carreras et al. 2008 showed that dendritic cell differentiation could be stimulated by estradiol (Carreras et al., 2008). Estradiol binds with specific receptors expressed in the immune cells. It activates the NF-kB signaling pathway and interferon regulatory factors that modulate cytokine and chemokine production (Carreras et al., 2010). Estrogen, abundant in females, can foster innate and adaptive immunity, and, oppositely, testosterone, prominent in males, suppresses the immune response (Bartz et al., 2020). Robinson and colleagues reported that estrogen stimulates higher T cell response in female murine models (Robinson et al., 2014). Also, estrogen contributes to vasodilation and inhibition of viral replication by promoting the catalysis of nitric oxide (NO) from L-arginine (Wink et al., 2011; Rimmelzwaan et al., 1999). Estrogen activates the estrogen receptor, which helps initiate the transcription of the eNOS gene by binding to the estrogen response element in the gene's promoter, therefore contributing to the generation of NO (Wink et al., 2011; MacRitchie et al., 1997). The impact of estrogen on NO may provide an additional layer of defense against COVID-19. Besides, the testosterone level decreases with age, and the hormone deficiency upregulates the inflammatory cytokine level, e.g., TNF-α, IL1β, IL2 (Musabak et al., 2003; Bobjer et al., 2013). This might be another possible explanation of higher mortality due to COVID-19 among the older males, compared to the females of the same age group.
Growing evidence suggests that non-coding RNAs can also regulate the immune system. The expression of microRNA and long non-coding RNA varies from female to male. Viral microRNAs, for instance, modulate immune evasion and maintain chronic infection and viral diseases (Mishra et al., 2020). The X-chromosome contains nearly 10% microRNA of the human genome, whereas the Y-chromosome contains only two microRNAs. MicroRNAs are known to modulate antiviral immunity during viral infection. The X-chromosome inactivation escape might play a role in microRNA-mediated immune regulation. Besides, the immune system can also be influenced by host genetics like allelic polymorphism and copy number variation (CNV). Human leukocyte antigen (HLA) encodes MHC class I and class II molecular and other essential factors vital for antigen presentation. These HLA alleles are highly polymorphic and reported critical factors in disease severity. A recent study by Nguyen et al. 2020 also suggests that HLA polymorphism is responsible for COVID-19 severity (Nguyen et al., 2020). In addition, growing evidence indicates that CNV is associated with the pathogenesis of SARS-CoV disease (Manry and Quintana-Murci, 2013; Wang et al., 2014). A genetic variant of TLR-7 with impaired interferon I and interferon II response are reported for severe COVID-19 male patients (van der Made et al., 2020).
A couple of recent studies have explored the sex difference in immune responses to SARS-CoV-2 infection and predictors of disease progression, endeavoring to illustrate the underlying mechanism for why males are disproportionately affected by COVID-19 (Takahashi et al., 2020; Ursin et al., 2020; Lieberman et al., 2020). These reports provide evidence that males infected with SARS-CoV-2 develop elevated levels of innate immune cytokines, e.g., IL-8 and IL-18, along with robust induction of non-classical monocytes. Females infected with the virus, on the other hand, present an elevated level of activated CD38+HLA-DR+ and terminally differentiated PD-1+TIM-3+ T cells, enabling a more robust T-cell mediated immune reaction. Also, the poor T cell response is associated with worse disease outcomes of COVID-19 in the males but not in the females (Takahashi et al., 2020). RNA sequencing data from COVID-19 infected individuals identified at least 19 genes' expressions that could be attributed to SARS-CoV-2 infection (Lieberman et al., 2020). Expressions of these genes are related to the activities of B-cell, natural killer cell-activating receptors, and inhibitors of NF-κB signaling in males (Lieberman et al., 2020). Circulating autoantibodies neutralizing IFN-α and/or IFN-ω are detectable in one out of every seven patients who experience a severe disease outcome (Bastard et al., 2020). Strikingly, 94% of these patients were male (Bastard et al., 2020). The disproportionate presence of these neutralizing IgG autoantibodies in males suggests another possible mechanism for the observed male bias in severe outcomes of COVID-19 (Ursin et al., 2020; Bastard et al., 2020).
4. Role of sex hormone in virus entry
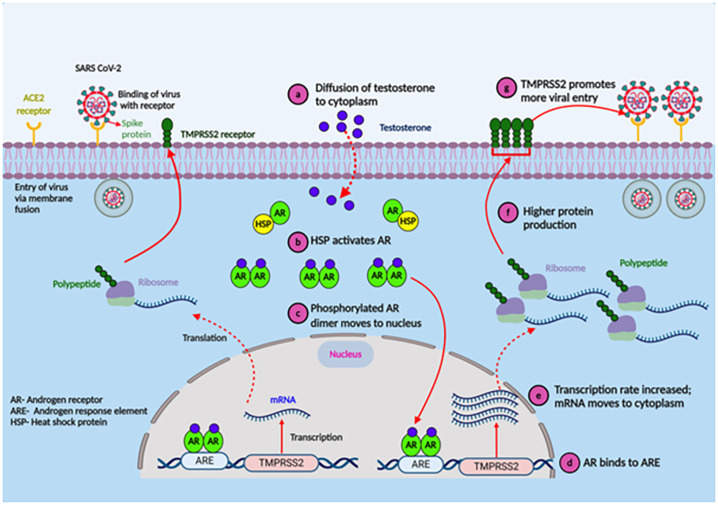
The spike protein of SARS-CoV-2 attaches to the human angiotensin-converting enzyme 2 (ACE2) receptor—resulting in the receptor's downregulation and ultimately impairing the renin-angiotensin system and potentially leading to acute respiratory problems (Hoffmann et al., 2020; Glowacka et al., 2010). Reports suggest that ACE2 expression in pneumocytes was higher in males than in females (Song et al., 2020; Salah and Mehta, 2021). Estradiol, a major female sex hormone, presumably regulates ACE2 expression in airway epithelial cells, kidneys, cardiac, and adipose tissues (Stelzig et al., 2020; Gupte et al., 2012; Dalpiaz et al., 2015). The polymorphism of the ACE2 gene correlates with diabetes mellitus, cerebral stroke, and hypertension—a plausible reason these patients carry elevated risk due to COVID-19 (Fang et al., 2020). Other than the ACE2 receptor, SARS-CoV-2 requires another serine protease, transmembrane serine protease 2 (TMPRSS2), to enter the airway epithelial cells [52]. TMPRSS2, primarily expressed in the airway epithelial cells, primes the spike protein followed by androgenic ligands and androgen receptor-regulated transcription of TMPRSS2 (Tomlins et al., 2005; Ko et al., 2015). The transcription of TMPRSS2 is directly regulated by the androgen receptors (AR) (Lucas et al., 2014). Located on the long arm of the X-chromosome, ARs function as steroid hormones and activated transcription factors in humans. Because females have two copies of the X-chromosome, they may have better regulation systems for TMPRSS2 than males with only one copy of the chromosome (Mjaessab et al., 2020; Brown et al., 1989). Besides, the expression of ARs is low in prepubertal stages, which may explain the low incidence of severe COVID-19 infection in children (Roehrborn et al., 1987; Kashon et al., 1995). On the other hand, the weaker TMRPSS2 regulation in males resulting in the stronger receptor priming might be a plausible reason for COVID-19's progression going worse and contributing to an elevated mortality rate (Gebhard et al., 2020). Also, the male hormone testosterone helps the upregulation of TMPRSS2 and promotes viral entry (Fig. 3 ). This hypothesis is strengthened by higher hospitalization among bald males due to COVID-19, as androgenetic alopecia (APA; male-pattern baldness) is induced by hyperactivated androgenic receptors (Wambier and Goren, 2020; Goren et al., 2020). Sexual dimorphism in ACE2 and TMPRSS2 expression likely guides the clinical progression of COVID-19 (Wray and Arrowsmith, 2021).
Fig. 3.
Testosterone promotes viral entry via upregulating TMPRSS2 transcription (Gebhard et al., 2020). The entry of androgen (a) stimulates heat shock protein (HSP) to activates androgen receptor (AR) (b), and phosphorylated AR homodimer translocate to the nucleus to (c) bind to androgen response element (ARE). This (d) upregulates the transcription of TMPRSS2. Upregulation of TMPRSS2 protein (f) promotes virus entry (g) to the cells.
5. Role of age
The case fatality rate due to COVID-19 is higher among older individuals than middle age and young adults. Human metabolism and body composition change with aging; insulin resistance, growth, and sex hormones depreciate over time (Barzilai et al., 2012). A recent study has reported that D-dimer, immune-related metabolic index, and neutrophil to lymphocyte ratio are several risk factors for COVID-19 (Zhao et al., 2021). They also found that interferon atmotherapy and activity of metabolic pathways are associated with elderly patients' survival (Zhao et al., 2021; Lipkowitz and Navarra, 2002). Similarly, aged people are vulnerable to other infectious diseases, e.g., influenza. The worst influenza outcome is reported in older adults having comorbidities, including coronary complications and pneumonia (Keilich et al., 2019). Studies indicate that several types of malignancies, e.g., lung cancer, diabetes, obesity, hypertension, vitamin D deficiency, and many cardiovascular diseases, all of which are significant risk factors for severe outcomes in COVID-19, are associated with older age and male sex (Orwoll et al., 2009; Mosekilde, 2005; Ghasemian et al., 2021). Adult females usually are reported to experience fewer comorbid conditions than males of the same age group (Roth et al., 2018). As comorbidity contributes to the severe outcomes of COVID-19, this also adds to the gendered effects of the infection. Sex steroids and immunosenescence also impact the susceptibility to seasonal influenza, as it interferes with the B-cell receptors (BCR), and T-cell receptors (TCR) repertoires (Dugan et al., 2020). Aging significantly alters the endocrine system, particularly in the sex steroids and immunosenescence systems. It gives immune responses to viral infection a sex- and gendered dimension, which helps older females better defend against diseases (Gomez et al., 2019; Zarulli et al., 2018). These have helped females survive better from previous famines and epidemics and probably play a role in causing the male-biased effects of COVID-19.
Children are usually at a lower risk of developing COVID-19 and also have a milder disease than adults (Ding et al., 2020; Carsetti et al., 2020; Falahi et al., 2021). The sex-based disproportionate impact of COVID-19 is also much less pronounced among children (Falahi et al., 2021; Brizuela et al., 2021; Saatci et al., 2021; Parcha et al., 2021). Most studies, except some small cohort studies, report that the clinical outcomes of COVID-19 were similar across sex groups (Ding et al., 2020; Brizuela et al., 2021; Saatci et al., 2021; Parcha et al., 2021). The possible reasons for children not experiencing a sex-biased impact of COVID-19 could be due to many factors, e.g., stronger immune response than adults and different endothelial system functions or physiology (Falahi et al., 2021; Brodin, 2020; Lingappan et al., 2020; Mallapaty, 2021). The expression of many age-dependent genes, e.g., ACE2 (expression increases with age), contributes to the more robust immunity presented by the children. Moreover, children present less susceptibility to cytokine storms and inflammations (i.e., inflammaging) (Brodin, 2020; Lingappan et al., 2020). These contribute to a better immune response to infections by the children. Besides, while prior infections and vaccination give the adults advantages, their immune system is more trained to deal with similar pathogens. However, when it comes to dealing with a novel infection, children are naturally better and more adaptable at controlling them (Mallapaty, 2021). With the novelty of SARS-CoV-2, the children presented a better ability to combat the infection than the adults. With age, the adult immune system becomes more trained in dealing with similar infections but at the cost of its adaptability to combat new infections, perhaps in a sex-biased manner. In addition, the weakly imposed gender norms may play a role in the less disproportionate impact of COVID-19 in children. However, the precise mechanism underneath is currently unclear (Falahi et al., 2021).
6. Comorbidities
Along with the sex chromosome and hormonal difference between gender and age groups, comorbidities might be responsible for the higher death rate among males. Patients with preexisting heart conditions, diabetes, and other inflammatory diseases are more prone to SARS-CoV-2 infection. These complications are usually more prevalent among males and may contribute to elevated death incidences (Roth et al., 2018). In contrast, some pre-existing conditions, for example, thalassemia, might protect females from SARS-CoV-2 infection (Chowdhury and Anwar, 2020).
6.1. Heart disease
Many epidemiological studies have indicated that the mean age of developing a cardiovascular condition is 7–10 years lower among females (Maas and Appelman, 2010). The female hormone, estrogen, protects against coronary diseases (Merz et al., 2003), which is why females often exhibit a lower burden of cardiac diseases than males. Also, male sex is an independent risk factor for atrial fibrillation and Brugada syndrome in cardiac arrhythmias and thrombotic events, e.g., myocardial infarction, venous thromboembolism, and thrombotic stroke (Ehdaie et al., 2018; Wong et al., 2008). Consequently, the prevalence of cardiovascular diseases is considerably higher in males than in females.
People with preexisting cardiovascular conditions do not seem to be more susceptible to get COVID-19; however, acute myocardial injury, cardiac arrhythmias, microvascular dysfunction, and thrombosis are reported to contribute to a considerable proportion of COVID-19-related deaths (Huang et al., 2020; Zhou et al., 2020; Wang et al., 2020; Aggarwal et al., 2020; Iba et al., 2020; Shen and Ge, 2018). In addition, COVID-19 itself can induce myocardial injury, arrhythmia, and several other coronary complications (Nishiga et al., 2020). A considerable number of reports indicate that preexisting cardiovascular diseases are among the most common risk factors associated with severe outcomes in COVID-19, e.g., hospitalizations, intensive care support, and mortality (Zhou et al., 2020; Li et al., 2020; Deng et al., 2020). Preexisting cardiovascular conditions, thus, are thought to exacerbate the disease severity and contribute to the elevated mortality in COVID-19 patients by promoting cardiovascular injury (Medzikovic et al., 2020). However, COVID-19-induced cardiac injury is moderately modulated by sex hormones, ACE2 expression, and systemic inflammation, with the latter being less noticeable in females (Medzikovic et al., 2020; Shi et al., 2020a; Shi et al., 2020b). Also, as per a general understanding of preexisting cardiovascular conditions, sex chromosomes and hormones, ACE2 expression, drug interactions, obesity, and smoking habits may modulate disease conditions (Kaptoge et al., 2019; Ruan et al., 2018). These disease-modulating factors can predispose males and females differently to COVID-19 severity. Obesity and smoking, along with cardiovascular diseases, are risk factors with a higher burden in male patients with COVID-19, as compared to females. Altogether, these factors may explain why male COVID-19 patients seem to be at higher risk for severe disease progression and cardiovascular injury than females.
6.2. Diabetes
Diabetes does not seem to increase susceptibility to COVID-19; however, existing data show that the people with diabetes are more likely to require intensive care units (ICU) or to die due to COVID-19 (Apicella et al., 2020; Shi et al., 2020c). The precise link underlying such a worse prognosis due to COVID-19 in people with diabetes is currently unclear.
The association between diabetes and COVID-19, in general, is thought to be biunivocal, and the poorer prognosis is the likely outcome of the syndromic nature of the disease (Apicella et al., 2020). The prevalence of diabetes increases with age, and it reaches a peak in people of >65 years (Sinclair et al., 2020). People of >65 years of age are more likely to have a longer duration of diabetes and, thus, are prone to develop more diabetes-associated complications. Also, diabetes and older age often correlate with comorbidities, e.g., cardiovascular disease and hypertension. In the case of COVID-19, people with diabetes, nevertheless, have worse outcomes because of the associated conditions, e.g., hyperglycemia, older age, comorbidities, hypertension, and cardiovascular diseases, enhancing the risk (Apicella et al., 2020). Older males with diabetes are also more likely to experience diabetes-associated complications than their female counterparts (Corriere et al., 2013). On the other hand, SARS-CoV-2, because of its tropism for pancreatic ß-cells, can potentially lead to cell damage and impairment in insulin secretion, resulting in diabetes-like or hyperglycemic conditions. Impaired ß-cell function along with the inflammatory cytokine storm and counter-regulatory hormonal responses can result in more acute metabolic conditions, e.g., diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome, which is also more pronounced among males (Gannon et al., 2018). New-onset diabetes-like conditions, hyperglycemia, and acute metabolic conditions, in turn, can further worsen COVID-19 outcomes. In addition, the ACE2 receptor level is upregulated in people with type 1 and type 2 diabetes who uses ACE inhibitors and angiotensin II type-1 receptor blockers (ARBs) as treatment (Wan et al., 2020). Besides, people with diabetes often experience low vitamin D levels than healthy individuals (Abudawood et al., 2018). Lower vitamin D downregulates the antimicrobial peptide production, hence reducing the innate immune response. Alongside, diabetes-associated immune dysregulation, alveolar and endothelial dysfunction, and increased systemic coagulation may cause individuals to be more susceptible to COVID-19. Androgens play a sex-dimorphic role in diabetes, presumably through the involvement of visceral fat cells. Hyperandrogenism in females and hypogonadism in males are reported to be risk factors for diabetes. Consequently, the male sex is regarded as a risk factor for the development of diabetes (Chen et al., 2012; Yang et al., 2010; Tracey et al., 2016; Wändell and Carlsson, 2014). Now, because males are more prone to developing diabetes and diabetes-associated complications (Nordström et al., 2016), this also partly explains why COVID-19 hits males harder.
6.3. Thalassemia
Patients with blood disorders, e.g., thalassemia, anemia, and sickle cell disease, are generally more prone to viral and bacterial infections. Many patients with blood disorders have immunocompromised conditions, e.g., splenectomized or post-transplant patients and those who receive hydroxyurea, making them more vulnerable to highly infectious diseases, e.g., COVID-19 (Chowdhury and Anwar, 2020; Lederman et al., 2014). However, blood indices evaluations in individuals with COVID-19 infection showed that most patients had a normal complete blood count and lactate dehydrogenase (LDH), and they do not usually present moderate or severe thrombocytopenia, a common finding in other viral illnesses, e.g., dengue fever (Lippi and Mattiuzzi, 2020). Hemoglobin concentration is reportedly substantially reduced in severe cases of COVID-19, which aligns with the previous evidence documented from patients with other types of pneumonia (Shang et al., 2007). Hemoglobin carries almost two-thirds of the total iron in the human body. Usually, the hemoglobin level in females is 12% lower than their male counterparts, and as such, they have less amount of iron circulating in the blood (Murphy, 2014). While this causes the females to be affected by iron deficiency complications, this also helps them stay away from complications related to excessive iron. On the other hand, transfusion-related excess iron load in the blood plasma of thalassemia patients causes the non-transferrin-bound iron to enter some cells and convert to ferritin, which becomes hemosiderin over time. Excess iron can also affect the hypothalamus or adrenal glands, potentially causing adrenal hypofunction (Nakavachara and Viprakasit, 2013; Powars et al., 1991), and contribute to a compromised ability to fight infections. It was reported that, iron overload, e.g., in the form of hemosiderin in close contact with damaged mitochondria, was associated with the severe outcomes in COVID-19 patients, e.g., hepatic failure, suggesting iron overload as a pathogenic trigger for the severe outcomes (Del Nonno et al., 2021). It is well established that females are generally less efficient than males in absorbing iron (Saljoughian, 2007); they technically are less prone to excess iron in the blood.
7. Racial and ethnic disparities
Over the last couple of decades, many reports have stressed that ethnic background and racial disparities potentially lead to poorer health in specific populations (Jackson et al., 2010). Especially in some racial/ethnicity groups, people with higher socioeconomic status sometimes pay a disproportionate toll on health due to biological disparities. The COVID-19 pandemics' impact on racialized and marginalized communities appears no different as it seems to hit some ethnic/racial groups harder than others.
Fig. 4 provides a global view of the sex-disaggregated data on identified cases and deaths due to COVID-19 (Global Health 50/50, 2022). Initially, the concerns about a possible association between ethnic background and outcome of COVID-19 raised after the observational analysis that a third of the patients admitted to critical care units in London were from an ethnic minority background (Siddique, 2020; Public Health England, 2020; Intensive Care National Audit and Research Centre, 2020). The report showed that the death rate due to COVID-19, adjusted for age, gender, ethnicity, and deprivation, was six times higher among people with a Bangladeshi (South Asian) background than the British White people Also, the all-cause mortality was 3–4 times higher than expected among the males in the Black and Asian population, compared to 1.7 times higher among the British White population. Similarly, in females, mortality was 2–3 times higher than expected among the Black and Asian population compared to 1.6 times in the British White population. A comparable disproportionate infection rate was reported in the US (Resnick et al., 2020). The areas where a majority of the population were Black people have three times more COVID-19 cases and over five times more death rates (Thebault et al., 2020). However, in another observational study on the COVID-19 mortality data from the Georgia and Michigan, two ethnically diverse states of the US, found that the sex disparity does not hold across racial groups while males had overall higher rates than females and Black people experienced the highest case fatality rates due to COVID-19 (Rushovich et al., 2021). In a large cohort study held at Bronx Montefiore Health System, the Black people had significantly higher mortality with COVID-19 (Golestaneh et al., 2020). Similar findings were observed in Canada: Black people and other people of color made up over two-thirds of reported cases and hospitalized patients in Toronto (Toronto Public Health, 2020). In contrast, both the case and death rate of COVID-19 was significantly higher among general Australians than Australia's Aboriginal and Torres Strait Islanders, more than one in three of whom have either cardiovascular disease, diabetes, or renal disease (Kirby, 2020). The racial disparity in COVID-19 outcomes was also reported among the children. Several large-scale studies indicate that race may plan an essential role in childhood COVID-19 outcomes as more Asian, Black, Hispanic, and mixed-race children faced adverse clinical outcomes than White children (Saatci et al., 2021; Parcha et al., 2021).
Fig. 4.
Sex disaggregated data on identified cases and deaths due to COVID-19 across countries included in the Global Health 50/50 tracker (as of 30 Nov 2020) (Global Health 50/50, 2022). (a) Male to female case identification rate in countries reporting >10,000 identified cases (N = 41,840,331) where the sex-disaggregated data is available. (b) Male to female death rate among male and female in countries reporting >1000 COVID-19 related death incidences (N = 1,155,509) where the sex-disaggregated data is available. The name of the countries is ordered alphabetically. (c) The proportion of death in male and female confirmed cases.
The fact that COVID-19 has disproportionately affected the racial and ethnic minority groups and race and ethnicity have an association with the worse outcomes of the infection was established by several large meta-analyses (Sze et al., 2020; Mude et al., 2021; Magesh et al., 2021). These studies revealed that individuals of Black, Hispanic, and Asian ethnicity were most likely to test positive for the infection and had the highest risk of ICU admission. By contrast, White people were the least likely to test positive for and require an ICU admission due to COVID-19. It was also indicated that, social determinants, e.g., area deprivation index and reduced access to clinical care, were positively correlated with the COVID-19 outcomes in racial and ethnic minority groups (Magesh et al., 2021).
The analysis of the US cohort of the COVID-19 patients incompletely explained the association between race and COVID-19 outcome by age, multiple reported comorbidities, and sociodemographic metrics (Golestaneh et al., 2020). Many experts have so far emphasized that the elevated rates of cases and severity in some specific ethnic groups may link with the sociodemographic, cultural, and genetic predisposition and physiological differences in infection susceptibility or response (Muurlink and Taylor-Robinson, 2020; Razaq et al., 2020; Pareek et al., 2020). An increased risk for acute respiratory tract infections, higher incidence of Vitamin D deficiency, obesity, diabetes, acute kidney diseases, the tendency of developing inflammations, and response to it, and predispositions of cardiovascular diseases in certain ethnic groups may lead to susceptibility to infections like COVID-19 (Simpson et al., 2015; Martineau et al., 2017; Miller et al., 2020; Tillin et al., 2012; Hernandez-Romieu et al., 2021; Garg et al., 2020). Evidence also shows that ethnicity/race can significantly affect the circulating 25(OH)D levels and vitamin D absorption by cells (Ghasemian et al., 2021; Correia et al., 2014). An increased interest has focused on the role of ethnically differential expression of angiotensin-converting enzyme 2 (ACE2) and serine protease 2 (TMPRSS2), the host receptor for SARS-CoV-2, as an increased incidence of cardiovascular risk factors may present in many ethnic groups (Asselta et al., 2020; Devaux et al., 2020; Cao et al., 2020; Darbani, 2020). A recent report indicated that Asian males might have a higher expression of ACE2 (Zhao et al., 2020). However, comparative genetic analyses could not identify ACE2 mutants' presence that inhibits coronavirus binding to ACE2 among different populations (Cao et al., 2020). It also showed that the frequencies of alleles associated with higher ACE2 expression were higher among East Asian people, suggesting different susceptibility or responses to SARS-CoV-2 from diverse populations. On the other hand, preliminary studies on the role of TMPRSS2 revealed that the variants linked with reduced expression of TMPRSS2 might bestow less individual susceptibility to COVID-19 and favor a better clinical outcome (Russo et al., 2020).
Additionally, many disadvantaged socioeconomic and lifestyle-related features of some ethnic groups, e.g., low income, extended cohabiting families in many Southeast Asian and West African countries, may contribute to the spread of the virus. Furthermore, many Black, Asian, Western African, Indian subcontinental, and ethnic minority people, whether in their own countries or in a different country where they have migrated, face several disadvantages, including inadequate housing, overcrowding, and low income. As a result, the preventive practices to reduce the spread of COVID-19 were challenging for them, contributing to the increased case rates and death rates among these populations (Rossouw et al., 2020; Anwar et al., 2020a; Bong et al., 2020).
The overall significance of the racial and ethnic disparities of COVID-19, however, remains to be deciphered precisely. Besides, many experts argue that the racial and ethnic disparities are not due to intrinsic biological makeup but socio-cultural differences for hundreds of years, which have influenced their physiology through social determinants of health and embodiment (Racism and Genetics, 2020; Non et al., 2012; Montoya, 2011; Das, 2013). Therefore, it is crucial to take it cautiously when interpreting the data on racial disparities of COVID-19, as this disparity—as appalling as it is—may still understate the actual situation due to the incompleteness of our knowledge (Milner et al., 2020; Gravlee, 2020).
8. Gender-specific practices and cultural backgrounds
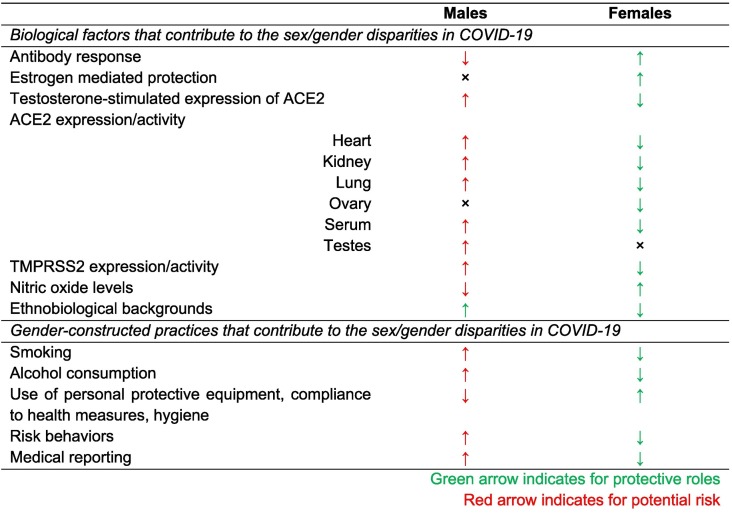
Alongside biological and ethnobiological factors, COVID-19 disease progression, severity depends on behavioral and cultural variables (Table 1 ). Some cultures allow men to smoke, where women smoking is treated as a social taboo. The tendency of harmful behavior such as smoking and taking alcohol is higher among men than women. According to WHO, globally men's smoking rate is 4.5 times higher than women smokers, accounting to 40% and 9%, respectively (World Health Organization, 2010). As the virus causes severe damage to the lung, the impact of smoking might be a potent cause of elevated death rate among men. Also, it was reported that middle-aged women practice more hand hygiene than men (Suen et al., 2019). Besides, air pollution impacts the outcome due to COVID-19, and men are exposed more to air pollution as more men work outside (Wu et al., 2020). A hypothesis is that postulated fine particles in air interfere with LL-37, an antimicrobial peptide (Crane-Godreau et al., 2020).
Table 1.
Potential factors influencing sex- and gender-specific outcomes in COVID-19 infections.
The difference between men's and women's infection rates and fatality for COVID-19 can also be related to cultural factors. A multinational study held in 2011 reports that the prevalence of an arboviral disease was remarkably high among Asian men only when over 15 years of age, when the cultural differences in work patterns, social behavior, activities, and dress come to apply (Anker and Arima, 2011). This inequality may reasonably be explained as a variation in exposure to the infection source. Also, it can be considered a plausible explanation for why the gendered effects of COVID-19 is less evident among the children. This may have a connection with the dress convention in these countries. In the countries where the definition of modesty of clothing is equivalent between men and women, the gendered effects of the infections are often disappear (Muurlink and Taylor-Robinson, 2020; da Glória et al., 2002; Hussain, 2022). For instance, in Brazil, where the definition of clothing modesty is equivalent between men and women, the gendered difference in dengue infection rate fades away (da Glória et al., 2002). However, in a recent study in Bangladesh, which belongs to a semiconservative social structure, women were more affected by Chikungunya, another arboviral disease (Anwar et al., 2020b). Nevertheless, arboviral diseases have a different transmission mode than COVID-19, and gender risks and vulnerabilities may differ based on how diseases are transmitted (Muurlink and Taylor-Robinson, 2020). There is still a dearth of studies that systematically and comprehensively evaluating the role of clothing in the transmission efficiency of COVID-19. In addition, the public perception, knowledge, and attitude towards infectious diseases, e.g., COVID-19, is often comparable among men and women of these countries (Anwar et al., 2020c; Alberta Health Services COVID-19 Scientific Advisory Group, 2020). What this means is that the differences in knowledge, attitude, and perception of risk between men and women cannot be regarded as a major contributor to the gender disparities among these population groups. The culturally biased gendered dimension of infectious diseases is yet to be established solidly.
One significant indicator of conservative or semiconservative societies is the extent of long or modest clothing worn by both young and older people, especially women. This so-called clothing convention may involuntarily determine the extent of rapidity and degree of the spread of infectious diseases, e.g., COVID-19. Cultures that limit women's movement and clothing freedom may contribute to the fewer possibilities for pathogen transmission for women than men. One such example could be wearing a burqa (veils) and niqab (face coverings) by the women in conservative Muslim communities. This practice helps the women in these communities restrict them from touching their face, mouth, nose, and eyes. Frequent touching of the face, mouth, nose, and eyes have been a well-perceived risk factor for infection with SARS-CoV-2. The use of burqa and niqab inadvertently helps them avoid this risk factor, while many who does not wear burqa and niqab may find it challenging not to touch the face, mouth, and nose as this is instinctive and habitual (Muurlink and Taylor-Robinson, 2020; Sobh et al., 2012; Benning et al., 2020). Also, facial coverings, like the niqab, may contribute to a very low-level filtration of air-borne droplets, potentially carrying pathogenic agents (Rengasamy et al., 2010). However, the level of barrier niqab provides may not be highly significant in terms of controlling viral spread as compared to P2/N95 standard particulate filtering systems or even regular surgical masks. Besides wearing a burqa and niqab, covering the hairs also has an unintended value from the perspective of public health microbiology (Sobh et al., 2012). In contrast, the cultural predilection for mustache and beard among males of different cultures is likely to increase male exposure to pathogens like SARS-CoV-2, particularly amongst health professionals where facial hair compromises the seal P2/N95-standard particulate filtering respirators and surgical masks (Sandaradura et al., 2020).
Gender apartheid in communities influences the women's engagement in activities and roles outside the home, e.g., occupation or profession (Timmis et al., 2005; Berecki-Gisolf et al., 2008). While the reduced active engagement of women in social and professional activities beyond household and babysitting functions reduces their exposure to disease outbreaks, it, in turn, reduces the likelihood of women visiting a healthcare facility, leading to underreporting of disease incidence among them.
In the rural and remote regions where medical care facilities are not widely accessible in most cases, there is often a gender imbalance in favor of the men (Wainer et al., 2001). Often, these facilities lack workforces, and male practitioners dominate the limited workforce they have. Women originating from conservative families used not to seek medical care from male practitioners as religious and cultural factors disallow them from interacting with men outside their family and close relatives (Chaudhury and Hammer, 2003). As a result, women in these communities tend not to visit the medical care centers and remain marginalized with scarcer diagnoses using qualified methods.
Conceivably, the cultural norms and practices may have strongly influenced the reported COVID-19 infection rates' gendered dimensions and the death rates. Women may have achieved some protection by social, professional, and clothing practices related to the conservative, religious and traditional norms. Additionally, their hesitation in visiting a male medical practitioner may have led them to be underreported in the infection and fatality data. Scientifically rigorous and large-scale studies involving subjects from culturally diverse backgrounds could help establish the role of cultural practices and norms on infection rates and mortality due to outbreaks. As the COVID-19 pandemic has hit (almost) every community of the world, from a researcher's point of view, it offers an opportunity to conduct such studies to provide insights into the role of cultural factors on the rates of infection, morbidity, and mortality due to outbreaks. Indeed, such a study's findings would contribute hugely to redesigning the public health policies to combat any future outbreaks.
9. Females are more likely to suffer from long COVID-19
Several studies suggest that females are more likely to suffer from long COVID-19 (Rubin, 2020). Female sex and 40–50 years of age are considered risk factors for Long COVID (Sigfrid et al., 2021; Torjesen, 2021; Mahmud et al., 2021). While the long-lasting symptoms of most other diseases are primarily associated with adults with severe illness who spend weeks in intensive care and have older age, and comorbidities, interestingly, most of the sufferers of long COVID-19 initially face mild to moderate symptoms without requiring lengthy hospitalization and are largely unaffected by preexisting conditions (Rubin, 2020; Sigfrid et al., 2021; Stewart et al., 2021). The underlying reason behind this ‘inverse sex’ effect of long COVID-19 is currently unclear. Hypothetically, the disparity in risk and outcomes of long COVID-19 between sexes could be related to the effects of sex hormones (Stewart et al., 2021; Zoltán and István, 2021). The symptoms of long COVID-19, e.g., fatigue, myalgia, palpitations, cognitive impairment, and sleep disturbance, mostly overlap with those of perimenopause and menopause (Zoltán and István, 2021). Besides, the risk factors, e.g., female sex and age between 40 and 50 years, indicate the effect of the difference between pre- and postmenopausal time. As with perimenopause and menopause, the levels of estrogen and estradiol change, and so is the estradiol-stimulated immune cell activity. This change in the activity of the immune cells may have a role behind females being more affected by long COVID-19. In addition, the overlapping symptoms create diagnostic uncertainty, leading to the possibility that females with symptoms of perimenopause and menopause are misdiagnosed with long COVID-19 or the opposite (Stewart et al., 2021). More systematic studies are warranted to drill down into the mechanism causing females to suffer more from long COVID-19.
10. Need for the implementation of gender medicine
The dearth of sex and gender-specific approaches that consider sex as an analytical variable a priori in epidemiological and clinical trial studies is well recognized (Brady et al., 2021). However, the failure to explore the sex- and gender-specific risks and outcomes during outbreaks, especially during a pandemic like COVID-19, is unacceptable and linked to manifold risks (Stewart et al., 2021; Brady et al., 2021). It fails the research endeavors exploring the pathobiology and therapeutic strategy that satisfies the sex-specific biology. Besides, it endangers global women's health due to the risk of misdiagnosing, mismanagement, and in a broader sense, slipping to provide them with appropriate health care. In addition, it impairs the global post-pandemic recovery efforts and future preparedness due to the highly gendered nature of many sectors, e.g., health and social care (Wenham et al., 2020). The COVID-19 pandemic and its disproportionate impact on males and females is an awakening call for the urgent need for robust efforts to help understand the epidemiological background and the biological mechanisms underneath for sex differences in infectious diseases and other human diseases (Wenham et al., 2020). It also calls for the systematic and sustainable application of sex-specific methodologies. In this context, several groups are working toward developing plans for implementing gender medicine, and some of them have already come out with recommendations for implementing gender medicine in the post-COVID-19 age (Organisation for Economic Co-operation and Development, 2021; Klinge and Oertelt-Prigione, 2021; Machluf et al., 2020; Jagsi et al., 2021). It is suggested that developing tailored guidelines and raising awareness of gender-specific medicine could reduce disparities and facilitate understanding of certain subpopulations' health dynamics and needs within each gender (Machluf et al., 2020; Jagsi et al., 2021). However, these studies mostly fit the context of European, American, and other high-income and upper-middle-income counties. To achieve universal sex- and gender-inclusive recovery, low- and lower-middle-income countries must develop effective and malleable plans to implement gender medicine in their regional context. Importantly, the proper implementation of these gender medicine plans across the globe without regard to the racial and economic boundaries is needed.
11. Conclusions and future perspectives
The sex and gender-based difference in the impact of the COVID-19 pandemic is not understood precisely, but it could be multifactorial as genetic, racial, certain immunological factors, and the communal psychosocial practices by men and women contribute to how they respond to infections. Females are genetically and immunologically able to mount a more robust defense against infections which serves them better in managing viral load and viral clearance than males. The sex genotype of males and females could be considered a major determinant since the X-chromosome carries a good number of immune genes in humans. Also, the hormone system in females involving estrogen and estradiol can have immune-enhancing effects, while the male system involving testosterone secreted can have immune-suppressive effects against viruses. Furthermore, comorbidity is considered a risk factor for severe outcomes in COVID-19, and the prevalence of comorbid conditions is naturally different among males and females. In addition, the aging and stress endurance of males and females may have a role to play. Besides, the gender-constructed roles of men and women put them at different levels of exposure to pathogens. A better understanding of the factors behind the sex- and gender differences in COVID-19 is necessary to tailor therapies and vaccine strategies in a step toward sex-based personalized medicine, and further research is warranted to dig deeper into the precise mechanism for these differences.
Sex and gender are strongly inter-related and to respond to infectious disease outbreaks, e.g., COVID-19, effectively and equitably, it is essential that gender norms, roles, and relations that influence women's and men's differential vulnerability to infection, exposure to pathogens, and treatment received, and how these may differ among different groups of women and men, be cautiously considered and appropriately addressed as a core component of all the efforts. The governments and health service providers need to recognize the sex and gender effects of the COVID-19 outbreak. Also, the voice of men and women need to be considered separately depending on their intrinsic immunological and genetic backgrounds, particularly on the front line of the response to COVID-19. Advocacy efforts to diminish the sex-based and gendered barriers in health service-seeking practices must be initiated. Overall, special efforts tailored and customized to meet the gender-specific and cultural demands of pandemic-affected populations, including men, women, seniors, and children, are strongly recommended to ensure that pre-existing gender-constructed social, political, and cultural differences do not amplify the impacts of the pandemic.
Authors' contributions
MH conceptualized the study. JT and SA contributed to the methodology, data collection, data visualization, and drafting the manuscript. SA revised and edited the manuscript. All authors critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
No specific grant was received for this study. JT is supported by the COSMOS Scholars Program and the American Association of University Women (AAUW). SA is supported by the Maternal and Child Health (MatCH) Scholarship program, the Women and Children’s Health Research Institute (WCHRI) Graduate Studentship, and the Alberta Innovates Graduate Students Scholarship (AIGSS). MH is supported by the SUST Research Centre, the University Grant Commission (UGC) of Bangladesh, the Ministry of Science and Technology, and the Bangladesh Bureau of Educational Information and Statistics (BANBEIS), Ministry of Education, Government of Bangladesh.
Declaration of Competing Interest
The author reports no conflicts of interest in this work.
Acknowledgements
The authors wholeheartedly acknowledge Sabrina Sharmin (Wesleyan University, CT, USA) and Md. Abu Bakar Siddique (St. John's University, NY, USA). Figures 2 and 3 are created with BioRender.com.
References
- Abdullah M., Chai P.-S., Chong M.-Y., et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell. Immunol. 2012;272(2):214–219. doi: 10.1016/j.cellimm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Abhimanyu Coussens A.K. The role of UV radiation and Vitamin D in the seasonality and outcomes of infectious disease. Photochem. Photobiol. Sci. 2017;16(3):314–338. doi: 10.1039/c6pp00355a. [DOI] [PubMed] [Google Scholar]
- Abudawood M., Tabassum H., Ansar S., et al. Assessment of gender-related differences in vitamin D levels and cardiovascular risk factors in Saudi patients with type 2 diabetes mellitus. Saudi J. Biol Sci. 2018;25(1):31–36. doi: 10.1016/j.sjbs.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S., Garcia-Telles N., Aggarwal G., Lavie C., Lippi G., Henry B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis. 2020;7(2):91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- Alberta Health Services COVID-19 Scientific Advisory Group COVID-19 Scientific Advisory Group Rapid Evidence Report. 2020. https://www.albertahealthservices.ca/assets/info/ppih/if-ppih-covid-19-sag-rapid-evidence-report-attitudes-and-adherence-to-covid-19-guidelines.pdf
- Anker M., Arima Y. Male-female differences in the number of reported incident dengue fever cases in six Asian countries. West Pacific Surveill Response. 2011;2(2):17–23. doi: 10.5365/wpsar.2011.2.1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar S., Nasrullah M., Hosen M.J. COVID-19 and Bangladesh: challenges and how to address them. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar S., Taslem Mourosi J.T., Khan M.F., Ullah M.O., Vanakker O.M., Hosen M.J. Chikungunya outbreak in Bangladesh (2017): clinical and hematological findings. PLoS Negl. Trop. Dis. 2020;14(2) doi: 10.1371/journal.pntd.0007466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar S., Araf Y., Newaz Khan A., et al. Women’s knowledge, attitude, and perceptions toward COVID-19 in lower-middle-income countries: a representative cross-sectional study in Bangladesh. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.571689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselta R., Paraboschi E.M., Mantovani A., Duga S. 12(11) Aging; 2020. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy; pp. 10087–10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr M.L., Bertram E.G. A morphological distinction between neurones of the male and female, and the behaviour of the nucleolar satellite during accelerated nucleoprotein synthesis. Nature. 1949;163(4148):676–677. doi: 10.1038/163676a0. [DOI] [PubMed] [Google Scholar]
- Bartz D., Chitnis T., Kaiser U.B., et al. Clinical advances in sex-and gender-informed medicine to improve the health of all: a review. JAMA Intern. Med. 2020;180(4):574–583. doi: 10.1001/jamainternmed.2019.7194. [DOI] [PubMed] [Google Scholar]
- Barzilai N., Huffman D.M., Muzumdar R.H., Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard P., Rosen L.B., Zhang Q., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J.A., Bearden A., Striker R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning S.D., Labus B., Barchard K.A. How to stop touching your face to minimize spread of. Coronavirus Other Germs. 2020:1–4. https://theconversation.com/how-to-stop-touching-your-face-to-minimize-spread-of-coronavirus-and-other-germs-133683 [Google Scholar]
- Berecki-Gisolf J., Lucke J., Hockey R., Dobson A. Transitions into informal caregiving and out of paid employment of women in their 50s. Soc. Sci. Med. 2008;67(1):122–127. doi: 10.1016/j.socscimed.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Berghöfer B., Frommer T., Haley G., Fink L., Bein G., Hackstein H. TLR7 ligands induce higher IFN-α production in females. J. Immunol. 2006;177(4):2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- Bobjer J., Katrinaki M., Tsatsanis C., Giwercman Y.L., Giwercman A. Negative association between testosterone concentration and inflammatory markers in young men: a nested cross-sectional study. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme K.W., Compton T. Innate sensing of viruses by toll-like receptors. J. Virol. 2004;78(15):7867–7873. doi: 10.1128/JVI.78.15.7867-7873.2004. Doi: 10.1128%2FJVI.78.15.7867-7873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bong C.-L., Brasher C., Chikumba E., McDougall R., Mellin-Olsen J., Enright A. The COVID-19 pandemic: effects on low- and middle-income countries. Anesth. Analg. 2020;131(1):86–92. doi: 10.1213/ANE.0000000000004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady E., Nielsen M.W., Andersen J.P., Oertelt-Prigione S. Lack of consideration of sex and gender in COVID-19 clinical studies. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-24265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela M., Lenzi J., Ulloa-Gutiérrez R., et al. Influence of sex on disease severity in children with COVID-19 and Multisystem Inflammatory Syndrome in Latin America. Int. J. Gend. Specif. Med. 2021;7(3):128–133. doi: 10.1723/3673.36590. [DOI] [Google Scholar]
- Brodin P. Why is COVID-19 so mild in children? Acta Paediatr. Int. J. Paediatr. 2020;109(6):1082–1083. doi: 10.1111/apa.15271. [DOI] [PubMed] [Google Scholar]
- Brown C.J., Goss S.J., Lubahn D.B., et al. Androgen receptor locus on the human X chromosome: regional localization to Xq11-12 and description of a DNA polymorphism. Am. J. Hum. Genet. 1989;44(2):264. [PMC free article] [PubMed] [Google Scholar]
- Butler-Laporte G., Nakanishi T., Mooser V., et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 Host Genetics Initiative: a Mendelian randomization study. PLoS Med. 2021;18(6) doi: 10.1371/journal.pmed.1003605. e1003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Li L., Feng Z., et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6(1):1–4. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras E., Turner S., Paharkova-Vatchkova V., Mao A., Dascher C., Kovats S. Estradiol acts directly on bone marrow myeloid progenitors to differentially regulate GM-CSF or Flt3 ligand-mediated dendritic cell differentiation. J. Immunol. 2008;180(2):727–738. doi: 10.4049/jimmunol.180.2.727. [DOI] [PubMed] [Google Scholar]
- Carreras E., Turner S., Frank M.B., et al. Estrogen receptor signaling promotes dendritic cell differentiation by increasing expression of the transcription factor IRF4. Blood, J. Am. Soc. Hematol. 2010;115(2):238–246. doi: 10.1182/blood-2009-08-236935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsetti R., Quintarelli C., Quinti I., et al. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? Lancet. Child Adolesc. Heal. 2020;4(6):414–416. doi: 10.1016/S2352-4642(20)30135-8MJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cephus J.-Y., Stier M.T., Fuseini H., et al. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21(9):2487–2499. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury N., Hammer J. The World Bank; Washington DC: 2003. Ghost Doctors: Absenteeism in Bangladeshi Health Facilities. [Google Scholar]
- Chen L., Magliano D.J., Zimmet P.Z. The worldwide epidemiology of type 2 diabetes mellitus - Present and future perspectives. Nat. Rev. Endocrinol. 2012;8(4):228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- Chowdhury S.F., Anwar S. Management of hemoglobin disorders during the COVID-19 pandemic. Front. Med. 2020;7:306. doi: 10.3389/fmed.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia A., Azevedo M., do S, Gondim F, Bandeira F. Ethnic aspects of vitamin D deficiency. Arq. Bras Endocrinol. Metabol. 2014;58(5):540–544. doi: 10.1590/0004-2730000003320. [DOI] [PubMed] [Google Scholar]
- Corriere M., Rooparinesingh N., Kalyani R.R. Epidemiology of diabetes and diabetes complications in the elderly: an emerging public health burden. Curr. Diab. Rep. 2013;13(6):805–813. doi: 10.1007/s11892-013-0425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane-Godreau M.A., Clem K.J., Payne P., Fiering S. Vitamin D Deficiency and Air Pollution Exacerbate COVID-19 Through Suppression of Antiviral Peptide LL37. Front. Public Health. 2020;8:232. doi: 10.3389/fpubh.2020.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Glória Teixeira M., da Conceição Nascimento Costa M., Guerra Z., Barreto M.L. Dengue in Brazil: situation-2001 and trends. Dengue Bull. 2002;26:70–76. [Google Scholar]
- Dalpiaz P.L.M., Lamas A.Z., Caliman I.F., et al. Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0127515. e0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbani B. The expression and polymorphism of entry machinery for COVID-19 in human: juxtaposing population groups, gender, and different tissues. Int. J. Environ. Res. Public Health. 2020;17(10):3433. doi: 10.3390/ijerph17103433. Doi: 10.3390%2Fijerph17103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darboe A., Nielsen C.M., Wolf A.-S., et al. Age-related dynamics of circulating innate lymphoid cells in an african population. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. How does race get “under the skin”?: Inflammation, weathering, and metabolic problems in late life. Soc. Sci. Med. 2013;77:75–83. doi: 10.1016/j.socscimed.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grove K.C., Provoost S., Verhamme F.M., et al. Characterization and quantification of innate lymphoid cell subsets in human lung. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Nonno F., Nardacci R., Colombo D., et al. Hepatic failure in COVID-19: is iron overload the dangerous trigger? Cells. 2021;10(5):1103. doi: 10.3390/cells10051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q., Hu B., Zhang Y., et al. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.-M., Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020;53(3):425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domizio J., Blum A., Gallagher-Gambarelli M., Molens J.-P., Chaperot L., Plumas J. TLR7 stimulation in human plasmacytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood. 2009;114(9):1794–1802. doi: 10.1182/blood-2009-04-216770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B. Fighting COVID-19 exhausts T cells. Nat. Rev. Immunol. 2020;20(6) doi: 10.1038/s41577-020-0304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold S.S., Kaisho T., Hemmi H., Akira S., e Sousa CR. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Ding Y., Yan H., Guo W. Clinical characteristics of children with COVID-19: a meta-analysis. Front. Pediatr. 2020;8 doi: 10.3389/fped.2020.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan H.L., Henry C., Wilson P.C. Aging and influenza vaccine-induced immunity. Cell. Immunol. 2020;348 doi: 10.1016/j.cellimm.2019.103998. [DOI] [PubMed] [Google Scholar]
- Ehdaie A., Cingolani E., Shehata M., Wang X., Curtis A.B., Chugh S.S. Sex differences in cardiac arrhythmias. Circ. Arrhythm. Electrophysiol. 2018;11(3) doi: 10.1161/CIRCEP.117.005680. [DOI] [PubMed] [Google Scholar]
- Falahi S., Abdoli A., Kenarkoohi A. Claims and reasons about mild COVID-19 in children. New Microbes New Infect. 2021;41 doi: 10.1016/j.nmni.2021.100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respiratory Med. 2020;8(4) doi: 10.1016/s2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisancho-Kiss S., Davis S.E., Nyland J.F., et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J. Immunol. 2007;178(11):6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- Gannon M., Kulkarni R.N., Tse H.M., Mauvais-Jarvis F. Sex differences underlying pancreatic islet biology and its dysfunction. Mol. Metab. 2018;15:82–91. doi: 10.1016/j.molmet.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S., Kim L., Whitaker M., et al. Vol. 69. MMWR Morb Mortal Wkly Rep; 2020. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 — COVID-NET, 14 States, March 1–30, 2020; pp. 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates B. Responding to Covid-19 — a once-in-a-century pandemic? N. Engl. J. Med. 2020;382(18):1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020;11(1):1–13. doi: 10.1186/s13293-020-00304-9. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemian R., Shamshirian A., Heydari K., et al. The role of vitamin D in the age of COVID-19: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021;75(11) doi: 10.1111/ijcp.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Health 50/50 The COVID-19 Sex-Disaggregated Data Tracker. 2022. globalhealth5050.orghttps://globalhealth5050.org/the-sex-gender-and-covid-19-project/ Accessed February 21, 2021.
- Glowacka I., Bertram S., Herzog P., et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010;84(2):1198–1205. doi: 10.1128/jvi.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestaneh L., Neugarten J., Fisher M., et al. The association of race and COVID-19 mortality. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez C.R., Nomellini V., Kovacs E.J. Sex hormones and immunosenescence. Handb Immunosenescence Basic Underst. Clin. Appl. 2019;9781402090:799–831. doi: 10.1007/978-1-4020-9063-9_42. [DOI] [Google Scholar]
- Goren A., Vaño-Galván S., Wambier C.G., et al. A preliminary observation: Male pattern hair loss among hospitalized COVID-19 patients in Spain–a potential clue to the role of androgens in COVID-19 severity. J. Cosmet. Dermatol. 2020;19(7):1545–1547. doi: 10.1111/jocd.13443. [DOI] [PubMed] [Google Scholar]
- Grant W.B., Lahore H., McDonnell S.L., et al. Evidence that vitamin d supplementation could reduce risk of influenza and covid-19 infections and deaths. Nutrients. 2020;12(4) doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravlee C.C. Systemic racism, chronic health inequities, and COVID-19: a syndemic in the making? Am. J. Hum. Biol. 2020;32(5) doi: 10.1002/ajhb.23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte M., Thatcher S.E., Boustany-Kari C.M., et al. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler. Thromb. Vasc. Biol. 2012;32(6):1392–1399. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Romieu A.C., Leung S., Mbanya A., et al. Health care utilization and clinical characteristics of nonhospitalized adults in an integrated health care system 28–180 days after COVID-19 diagnosis — Georgia, May 2020–March 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70(17) doi: 10.15585/mmwr.mm7017e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewagama A., Patel D., Yarlagadda S., Strickland F.M., Richardson B.C. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009;10(5):509–516. doi: 10.1038/npre.2008.2690.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A. 2022. Carnival Reveals Much About Gender, Sexuality, and Culture in Brazil. Berkley Center for Religion, Peace & World Affairs. Published 2013. https://berkleycenter.georgetown.edu/posts/carnival-reveals-much-about-gender-sexuality-and-culture-in-brazil. [Google Scholar]
- Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18(9):2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll M.A. Sex differences shape the response to infectious diseases. PLoS Pathog. 2017;13(12) doi: 10.1371/journal.ppat.1006688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intensive Care National Audit and Research Centre ICNARC Report on COVID-19 in Critical Care. 2020. https://www.icnarc.org/DataServices/Attachments/Download/42ceb4d2-3dd3-ea11-9128-00505601089b
- Jackson J.S., Knight K.M., Rafferty J.A. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am. J. Public Health. 2010;100(5):933–939. doi: 10.2105/AJPH.2008.143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagsi R., Fuentes-Afflick E., Higginbotham E. Promoting equity for women in medicine — seizing a disruptive opportunity. N. Engl. J. Med. 2021;384(24):2265–2267. doi: 10.1056/nejmp2104228. [DOI] [PubMed] [Google Scholar]
- Jin J.M., Bai P., He W., et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho T., Akira S. Toll-like receptor function and signaling. J. Allergy Clin. Immunol. 2006;117(5):979–987. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Kaptoge S., Pennells L., De Bacquer D., et al. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob. Health. 2019;7(10):e1332–e1345. doi: 10.1016/S2214-109X(19)30318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashon M.L., Hayes M.J., Shek P.P., Sisk C.L. Regulation of brain androgen receptor immunoreactivity by androgen in Prepubertal male ferrets. Biol. Reprod. 1995;52(5):1198–1205. doi: 10.1095/biolreprod52.5.1198. [DOI] [PubMed] [Google Scholar]
- Keilich S.R., Bartley J.M., Haynes L. Diminished immune responses with aging predispose older adults to common and uncommon influenza complications. Cell. Immunol. 2019;345 doi: 10.1016/j.cellimm.2019.103992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T. Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. Lancet Respir. Med. 2020;8(6):547–548. doi: 10.1016/S2213-2600(20)30228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. BioEssays. 2012;34(12):1050–1059. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16(10):626. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Klein S.L., Jedlicka A., Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010;10(5):338–349. doi: 10.1016/s1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge I., Oertelt-Prigione S. The impact of the covid-19 pandemic on sex-and gender-sensitive research policymaking. Ital. J. Gender-Specific Med. 2021;7(2):63–68. doi: 10.1723/3600.35809. [DOI] [Google Scholar]
- Ko C.-J., Huang C.-C., Lin H.-Y., et al. Androgen-induced TMPRSS2 activates matriptase and promotes extracellular matrix degradation, prostate cancer cell invasion, tumor growth, and metastasis. Cancer Res. 2015;75(14):2949–2960. doi: 10.1158/0008-5472.can-14-3297. [DOI] [PubMed] [Google Scholar]
- La Vignera S., Cannarella R., Condorelli R.A., Torre F., Aversa A., Calogero A.E. Sex-specific SARS-CoV2 mortality: among hormone-modulated ace2 expression, risk of venous thromboembolism and hypovitaminosis D. Int. J. Mol. Sci. 2020;21(8) doi: 10.3390/ijms21082948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman H.M., Connolly M.A., Kalpatthi R., et al. Immunologic effects of hydroxyurea in sickle cell anemia. Pediatrics. 2014;134(4):686–695. doi: 10.1542/peds.2014-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-W., Han T.-W., Woodward M., et al. The impact of 2019 novel coronavirus on heart injury: a systematic review and meta-analysis. Prog. Cardiovasc. Dis. 2020;63(4):518–524. doi: 10.1016/j.pcad.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman N.A.P., Peddu V., Xie H., et al. In vivo antiviral host transcriptional response to SARS-CoV-2 by viral load, sex, and age. PLoS Biol. 2020;18(9) doi: 10.1371/JOURNAL.PBIO.3000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappan K., Karmouty-Quintana H., Davies J., Akkanti B., Harting M.T. Understanding the age divide in COVID-19: why are children overwhelmingly spared? Am. J. Phys. Lung Cell. Mol. Phys. 2020;319(1):L39–L44. doi: 10.1152/ajplung.00183.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowitz M.A., Navarra T. 2nd ed. Vol 39. Facts On File, Inc; 2002. The Encyclopedia of Allergies. [DOI] [Google Scholar]
- Lippi G., Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42(2):116–117. doi: 10.1016/j.htct.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips H.M. 7th ed. Waveland Press Inc; 2020. God, Sex, and Gender: An Introduction. [Google Scholar]
- Liu S., Zhang M., Yang L., et al. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J. Epidemiol. Community Health. 2017;71(2):154–161. doi: 10.1136/jech-2016-207805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Sun J., Wang X., Zhang T., Zhao M., Li H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int. J. Infect. Dis. 2021;104:58–64. doi: 10.1016/j.ijid.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J.M., Heinlein C., Kim T., et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4(11):1310–1325. doi: 10.1158/2159-8290.cd-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A.H.E.M., Appelman Y.E.A. Gender differences in coronary heart disease. Neth. Hear. J. 2010;18(12):598–603. doi: 10.1007/s12471-010-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machluf Y., Chaiter Y., Tal O. Gender medicine: Lessons from COVID-19 and other medical conditions for designing health policy. World J. Clin. Cases. 2020;8(17):3645–3668. doi: 10.12998/wjcc.v8.i17.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRitchie A.N., Jun S.S., Chen Z., et al. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ. Res. 1997;81(3):355–362. doi: 10.1161/01.RES.81.3.355. [DOI] [PubMed] [Google Scholar]
- Magesh S., John D., Li W.T., et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status. JAMA Netw. Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.34147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud R., Rahman M.M., Rassel M.A., et al. Post-COVID-19 syndrome among symptomatic COVID-19 patients: a prospective cohort study in a tertiary care center of Bangladesh. Zivkovic AR, ed. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0249644. e0249644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. Kids and COVID: why young immune systems are still on top. Nature. 2021;597(7875):166–168. doi: 10.1038/d41586-021-02423-8. [DOI] [PubMed] [Google Scholar]
- Manry J., Quintana-Murci L. A genome-wide perspective of human diversity and its implications in infectious disease. Cold Spring Harb. Perspect. Med. 2013;3(1) doi: 10.1101/cshperspect.a012450. Doi: 10.1101%2Fcshperspect.a012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott I., Huet-Hudson Y.M. Sexual dimorphism in innate immune responses to infectious organisms. Immunol. Res. 2006;34(3):177–192. doi: 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- Martineau A.R., Jolliffe D.A., Hooper R.L., et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. bmj. 2017;23(2):1–44. doi: 10.3310/hta23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F., Bairey Merz N., Barnes P.J., et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565–582. doi: 10.1016/S0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann K.B., Lee A., Wan J., Roginski H., Coventry M.J. The effect of bovine lactoferrin and lactoferricin B on the ability of feline calicivirus (a norovirus surrogate) and poliovirus to infect cell cultures. J. Appl. Microbiol. 2003;95(5):1026–1033. doi: 10.1046/j.1365-2672.2003.02071.x. [DOI] [PubMed] [Google Scholar]
- Medzikovic L., Cunningham C.M., Li M., et al. Sex differences underlying preexisting cardiovascular disease and cardiovascular injury in COVID-19. J. Mol. Cell. Cardiol. 2020;148:25–33. doi: 10.1016/j.yjmcc.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz C.N.B., Johnson B.D., Sharaf B.L., et al. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J. Am. Coll. Cardiol. 2003;41(3):413–419. doi: 10.1016/S0735-1097(02)02763-8. [DOI] [PubMed] [Google Scholar]
- Miller A., Reandelar M.J., Fasciglione K., Roumenova V., Li Y., Otazu G.H. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. MedRxiv. 2020 doi: 10.1101/2020.03.24.20042937. Published online. [DOI] [Google Scholar]
- Milner A., Franz B., Braddock J.H. We need to talk about racism-in all of its forms-to understand COVID-19 disparities. Heal Equity. 2020;4(1):397–402. doi: 10.1089/heq.2020.0069. https://pubmed.ncbi.nlm.nih.gov/32999950/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R., Kumar A., Ingle H., Kumar H. The interplay between viral-derived miRNAs and host immunity during infection. Front. Immunol. 2020;10 doi: 10.3389/fimmu.2019.03079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaessab G., Karamb A., Aounbc F., Albisinnia S., Roumeguèreac T. COVID-19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog. Urol. 2020;30(10):484–487. doi: 10.1016/j.purol.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli L.A., Sonnenberg G.F., Abt M.C., et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011;12(11):1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya M.J. 2011. Making the Mexican Diabetic: Race, Science, and the Genetics of Inequality. [DOI] [Google Scholar]
- Mosekilde L. Vitamin D and the elderly. Clin. Endocrinol. 2005;62(3):265–281. doi: 10.1111/j.1365-2265.2005.02226.x. [DOI] [PubMed] [Google Scholar]
- Mude W., Oguoma V.M., Nyanhanda T., Mwanri L., Njue C. Racial disparities in COVID-19 pandemic cases, hospitalisations, and deaths: a systematic review and meta-analysis. J. Glob. Health. 2021;11:05015. doi: 10.7189/jogh.11.05015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W.G. The sex difference in haemoglobin levels in adults - Mechanisms, causes, and consequences. Blood Rev. 2014;28(2):41–47. doi: 10.1016/j.blre.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Musabak U., Bolu E., Ozata M., et al. Gonadotropin treatment restores in vitro interleukin-1β and tumour necrosis factor-α production by stimulated peripheral blood mononuclear cells from patients with idiopathic hypogonadotropic hypogonadism. Clin. Exp. Immunol. 2003;132(2):265–270. doi: 10.1046/j.1365-2249.2003.02141.x. Doi: 10.1046%2Fj.1365-2249.2003.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muurlink O.T., Taylor-Robinson A.W. COVID-19: cultural predictors of gender differences in global prevalence patterns. Front. Public Health. 2020;8:174. doi: 10.3389/fpubh.2020.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakavachara P., Viprakasit V. Adrenal insufficiency is prevalent in HbE/β-thalassaemia paediatric patients irrespective of their clinical severity and transfusion requirement. Clin. Endocrinol. 2013;79(6):776–783. doi: 10.1111/cen.12235. [DOI] [PubMed] [Google Scholar]
- Nguyen A., David J.K., Maden S.K., et al. Human leukocyte antigen susceptibility map for SARS-CoV-2. J. Virol. 2020;94(3):e00510–e00520. doi: 10.1128/jvi.00510-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17(9):543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Non A.L., Gravlee C.C., Mulligan C.J. Education, genetic ancestry, and blood pressure in African Americans and whites. Am. J. Public Health. 2012;102(8):1559–1565. doi: 10.2105/AJPH.2011.300448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström A., Hadrévi J., Olsson T., Franks P.W., Nordström P. Higher prevalence of type 2 diabetes in men than in women is associated with differences in visceral fat mass. J. Clin. Endocrinol. Metab. 2016;101(10):3740–3746. doi: 10.1210/jc.2016-1915. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development Towards Gender-Inclusive Recovery. 2021. https://www.oecd.org/coronavirus/policy-responses/covid-19-and-the-aviation-industry-impact-and-policy-responses-26d521c1/
- Orwoll E., Nielson C.M., Marshall L.M., et al. Vitamin D deficiency in older men. J. Clin. Endocrinol. Metab. 2009;94(4):1214–1222. doi: 10.1210/jc.2008-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcha V., Booker K.S., Kalra R., et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci. Rep. 2021;11(1):10231. doi: 10.1038/s41598-021-89553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek M., Bangash M.N., Pareek N., et al. Ethnicity and COVID-19: an urgent public health research priority. Lancet. 2020;395(10234):1421–1422. doi: 10.1016/S0140-6736(20)30922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro D.S., Santos R.S., Veiga Jardim P.C.B., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(2):94–103. doi: 10.1016/j.cell.2020.02.058%0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powars D.R., Elliott-Mills D.D., Chan L., et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann. Intern. Med. 1991;115(8):614–620. doi: 10.7326/0003-4819-115-8-614. [DOI] [PubMed] [Google Scholar]
- Public Health England Disparities in the Risk and Outcomes of About Public Health England. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/908434/Disparities_in_the_risk_and_outcomes_of_COVID_August_2020_update.pdf
- Racism Gravlee C., Genetics Not. Explains why black americans are dying of COVID-19 - Scientific American Blog Network. Sci. Am. 2020:1–7. https://blogs.scientificamerican.com/voices/racism-not-genetics-explains-why-black-americans-are-dying-of-covid-19/ [Google Scholar]
- Razaq A., Harrison D., Karunanithi S., Barr B., Asaria M., Khunti K. 2020. Bame Covid-19 Deaths - What Do We Know? Rapid Data & Evidence Review: ‘Hidden in Plain Sight’. [Google Scholar]
- Rengasamy S., Eimer B., Shaffer R.E. Simple respiratory protection—evaluation of the filtration performance of cloth masks and common fabric materials against 20–1000 nm size particles. Ann. Occup. Hyg. 2010;54(7):789–798. doi: 10.1093/annhyg/meq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick A., Galea S., Sivashanker K. Covid-19: The painful price of ignoring health inequities. BMJ Opin. 2020;18 [Google Scholar]
- Rimmelzwaan G.F., Baars M.M.J.W., de Lijster P., Fouchier R.A.M., Osterhaus A.D.M.E. Inhibition of influenza virus replication by nitric oxide. J. Virol. 1999;73(10):8880–8883. doi: 10.1128/jvi.73.10.8880-8883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano A.M., Mastellos D.C., Huber-Lang M., et al. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.P., Hall O.J., Nilles T.L., Bream J.H., Klein S.L. 17β-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J. Virol. 2014;88(9):4711–4720. doi: 10.1128/jvi.02081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrborn C.G., Lange J.L., George F.W., Wilson J.D. Changes in amount and intracellular distribution of androgen receptor in human foreskin as a function of age. J. Clin. Invest. 1987;79:44–47. doi: 10.1172/jci112805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondanelli M., Miccono A., Lamburghini S., et al. Self-care for common colds: the pivotal role of Vitamin D, Vitamin C, Zinc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds - practical advice on dosages. Evid. Based Complement Altern Med. 2018;2018 doi: 10.1155/2018/5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw T.M., Boswell M.T., Nienaber A.G., Moodley K. Comorbidity in context: Part 1. Medical considerations around HIV and tuberculosis during the COVID-19 pandemic in South Africa. S. Afr. Med. J. 2020;110(7):621–624. doi: 10.7196/SAMJ.2020.v110i7.14856. [DOI] [PubMed] [Google Scholar]
- Roth G.A., Abate D., Abate K.H., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y., Guo Y., Zheng Y., et al. Cardiovascular disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: results from SAGE Wave 1. BMC Public Health. 2018;18(1):778. doi: 10.1186/s12889-018-5653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA - J. Am. Med. Assoc. 2020;324(14):1381–1383. doi: 10.1001/jama.2020.17709. [DOI] [PubMed] [Google Scholar]
- Rushovich T., Boulicault M., Chen J.T., et al. Sex disparities in COVID-19 mortality vary across US racial groups. J. Gen. Intern. Med. 2021;36(6):1696–1701. doi: 10.1007/s11606-021-06699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R., Andolfo I., Lasorsa V.A., Iolascon A., Capasso M. Genetic analysis of the coronavirus SARS-CoV-2 host protease TMPRSS2 in different populations. Front. Genet. 2020;11 doi: 10.3389/fgene.2020.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatci D., Ranger T.A., Garriga C., et al. Association between race and COVID-19 outcomes among 2.6 million children in England. JAMA Pediatr. 2021;175(9):928. doi: 10.1001/jamapediatrics.2021.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah H.M., Mehta J.L. Hypothesis: sex-related differences in ACE2 activity may contribute to higher mortality in men versus women with COVID-19. J. Cardiovasc. Pharmacol. Ther. 2021;26(2):114–118. doi: 10.1177/1074248420967792. [DOI] [PubMed] [Google Scholar]
- Saljoughian M. Iron deficiency anemia: a closer look. US Pharm. 2007;32(8) https://www.uspharmacist.com/article/iron-deficiency-anemia-a-closer-look [Google Scholar]
- Sandaradura I., Goeman E., Pontivivo G., et al. A close shave? Performance of P2/N95 respirators in health care workers with facial hair: results of the BEARDS (Adequate Respiratory DefenceS) study. J. Hosp. Infect. 2020;104(4):529–533. doi: 10.1016/j.jhin.2020.01.006. [DOI] [PubMed] [Google Scholar]
- Sanghera D.K., Sapkota B.R., Aston C.E., Blackett P.R. Vitamin D status, gender differences, and cardiometabolic health disparities. Ann. Nutr. Metab. 2017;70(2):79–87. doi: 10.1159/000458765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz H., Salie M., Tromp G., Hoal E.G., Kinnear C.J., Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum. Genom. 2019;13(1) doi: 10.1186/s40246-018-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020;20(7):442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang G., Biggerstaff B.J., Yang B., Shao C., Farrugia A. Theoretically estimated risk of severe acute respiratory syndrome transmission through blood transfusion during an epidemic in Shenzhen, Guangdong, China in 2003. Transfus. Apher. Sci. 2007;37(3):233–240. doi: 10.1016/j.transci.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Ge J. Epidemic of cardiovascular disease in China. Circulation. 2018;138(4):342–344. doi: 10.1161/CIRCULATIONAHA.118.033484. [DOI] [PubMed] [Google Scholar]
- Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Qin M., Cai Y., et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q., Zhang X., Jiang F., et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- Siddique H. UK government urged to investigate coronavirus deaths of BAME doctors. The Guardian. 2020;2020;10 https://www.theguardian.com/society/2020/apr/10/uk-coronavirus-deaths-bame-doctors-bma Published online. [Google Scholar]
- Sigfrid L., Drake T.M., Pauley E., et al. Long Covid in adults discharged from UK hospitals after Covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Heal - Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C.R., Steiner M.F.C., Cezard G., et al. Ethnic variations in morbidity and mortality from lower respiratory tract infections: a retrospective cohort study. J. R. Soc. Med. 2015;108(10):406–417. doi: 10.1177/0141076815588321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair A., Saeedi P., Kaundal A., Karuranga S., Malanda B., Williams R. Diabetes and global ageing among 65–99-year-old adults: findings from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020;162:108078. doi: 10.1016/j.diabres.2020.108078. [DOI] [PubMed] [Google Scholar]
- Smith J. Overcoming the ‘tyranny of the urgent’: integrating gender into disease outbreak preparedness and response. Gend. Dev. 2019;27(2):355–369. doi: 10.1080/13552074.2019.1615288. [DOI] [Google Scholar]
- Sobh R., Belk R.W., Gressel J. Modest seductiveness: reconciling modesty and vanity by reverse assimilation and double resistance. J. Consum. Behav. 2012;11(5):357–367. doi: 10.1002/cb.1379. [DOI] [Google Scholar]
- Song H., Seddighzadeh B., Cooperberg M.R., Huang F.W. Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells. Eur. Urol. 2020;78(2):296–298. doi: 10.1016/j.eururo.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo P.A., Manson J.A.E., Joffe H. Sex and gender differences in health: what the COVID-19 pandemic can teach us. Ann. Intern. Med. 2020;173(5):385–386. doi: 10.7326/M20-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer J.A. Gender differences in some host defense mechanisms. Lupus. 1999;8(5):380–383. doi: 10.1177/096120339900800510. [DOI] [PubMed] [Google Scholar]
- Springer K.W., Mager Stellman J., Jordan-Young R.M. Beyond a catalogue of differences: a theoretical frame and good practice guidelines for researching sex/gender in human health. Soc. Sci. Med. 2012;74(11):1817–1824. doi: 10.1016/j.socscimed.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Steinstraesser L., Tippler B., Mertens J., et al. Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides. Retrovirology. 2005;2(1):2. doi: 10.1186/1742-4690-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzig K.E., Canepa-Escaro F., Schiliro M., Berdnikovs S., Prakash Y.S., Chiarella S.E. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am. J. Phys. Lung Cell. Mol. Phys. 2020;318(6) doi: 10.1152/AJPLUNG.00153.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S., Newson L., Briggs T.A., Grammatopoulos D., Young L., Gill P. Long COVID risk - a signal to address sex hormones and women’s health. Lancet Reg Heal - Eur. 2021;11 doi: 10.1016/j.lanepe.2021.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K., Bergman P., Henriques-Normark B. Vitamin D promotes pneumococcal killing and modulates inflammatory responses in primary human neutrophils. J. Innate Immun. 2017;9(4):375–386. doi: 10.1159/000455969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen L.K.P., So Z.Y.Y., Yeung S.K.W., Lo K.Y.K., Lam S.C. Epidemiological investigation on hand hygiene knowledge and behaviour: a cross-sectional study on gender disparity. BMC Public Health. 2019;19 doi: 10.1186/s12889-019-6705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarpak L., Rafique Z., Gasecka A., et al. A systematic review and meta-analysis of effect of vitamin D levels on the incidence of COVID-19. Cardiol. J. 2021;28(5):647–654. doi: 10.5603/CJ.a2021.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze S., Pan D., Nevill C.R., et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29-30 doi: 10.1016/j.eclinm.2020.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Ellingson M.K., Wong P., et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira D., Longo-Maugeri I.M., Santos J.L.F., Duarte Y.A.O., Lebrão M.L., Bueno V. Evaluation of lymphocyte levels in a random sample of 218 elderly individuals from Sao Paulo city. Rev. Bras. Hematol. Hemoter. 2011;33(5):367–371. doi: 10.5581/1516-8484.20110100. Doi: 10.5581%2F1516-8484.20110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teymoori-Rad M., Shokri F., Salimi V., Marashi S. The interplay between vitamin D and viral infections. Rev. Med. Virol. 2019;29 doi: 10.1002/rmv.2032. [DOI] [PubMed] [Google Scholar]
- Thebault R., Tran A.B., Williams V. The coronavirus is infecting and killing black Americans at an alarmingly high rate. Wash. Post. 2020 Published online. [Google Scholar]
- Tillin T., Forouhi N.G., McKeigue P.M., Chaturvedi N. Southall And Brent REvisited: Cohort profile of SABRE, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African Caribbean origins. Int. J. Epidemiol. 2012;41(1):33–42. doi: 10.1093/ije/dyq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis A.D., Baker C., Banerjee S., et al. Women in UK cardiology: report of a Working Group of the British Cardiac Society. Heart. 2005;91(3):283–289. doi: 10.1136/hrt.2004.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins S.A., Rhodes D.R., Perner S., et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- Torjesen I. Covid-19: Middle aged women face greater risk of debilitating long term symptoms. BMJ. 2021 doi: 10.1136/bmj.n829. [DOI] [PubMed] [Google Scholar]
- Toronto Public Health COVID-19 Cases in Toronto. 2020. https://www.toronto.ca/home/covid-19/covid-19-latest-city-of-toronto-news/covid-19-status-of-cases-in-toronto/
- Tracey M.L., Mchugh S.M., Buckley C.M., Canavan R.J., Fitzgerald A.P., Kearney P.M. The prevalence of Type 2 diabetes and related complications in a nationally representative sample of adults aged 50 and over in the Republic of Ireland. Diabet. Med. 2016;33(4):441–445. doi: 10.1111/dme.12845. [DOI] [PubMed] [Google Scholar]
- Tukiainen T., Villani A.-C., Yen A., et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550(7675):244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin R.L., Shapiro J.R., Klein S.L. Sex-biased immune responses following SARS-CoV-2 infection. Trends Microbiol. 2020;28(12):952–954. doi: 10.1016/j.tim.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Made C.I., Simons A., Schuurs-Hoeijmakers J., et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324(7):663–673. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hagen L.J., Muntinga M., Appelman Y., Verdonk P. Sex- and gender-sensitive public health research: an analysis of research proposals in a research institute in the Netherlands. Women Health. 2021;61(1):109–119. doi: 10.1080/03630242.2020.1834056. [DOI] [PubMed] [Google Scholar]
- vom Steeg L.G., Klein S.L. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016;12(2) doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer J., Bryant L., Strasser R. Sustainable rural practice for female general practitioners. Aust. J. Rural Health. 2001;9:43. doi: 10.1046/j.1440-1584.9.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Wambier C.G., Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J. Am. Acad. Dermatol. 2020;83(1):308–309. doi: 10.1016/j.jaad.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wändell P.E., Carlsson A.C. Gender differences and time trends in incidence and prevalence of type 2 diabetes in Sweden-A model explaining the diabetes epidemic worldwide today? Diabetes Res. Clin. Pract. 2014;106(3):e90–e92. doi: 10.1016/j.diabres.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Wang C.-M., Chang S.-W., Wu Y.-J.J., et al. Genetic variations in Toll-like receptors (TLRs 3/7/8) are associated with systemic lupus erythematosus in a Taiwanese population. Sci. Rep. 2014;4(1) doi: 10.1038/srep03792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Joshi A., Leopold K., et al. Association of vitamin D deficiency with COVID-19 infection severity: Systematic review and meta-analysis. Clin. Endocrinol. 2021 doi: 10.1111/cen.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Ran S., Segal S. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J. Immunol. 1984;132(2):656–661. [PubMed] [Google Scholar]
- Wenham C., Smith J., Morgan R. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395(10227):846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D.A., Hines H.B., Cheng R.Y.S., et al. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011;89(6):873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.H., Dukes J., Levy R.E., et al. Sex differences in thrombosis in mice are mediated by sex-specific growth hormone secretion patterns. J. Clin. Invest. 2008 doi: 10.1172/JCI34957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization 10 Facts on Gender and Tobacco. 2010. https://www.who.int/gender/documents/10facts_gender_tobacco_en.pdf
- Wray S., Arrowsmith S. The physiological mechanisms of the sex-based difference in outcomes of COVID19 infection. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.627260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci. Adv. 2020;6(45) doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Soruri A., RKH Gieseler, Peters J.H. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand. J. Immunol. 1993;38(6):535–540. doi: 10.1111/j.1365-3083.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- Yang W., Lu J., Weng J., et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010;362(12):1090–1101. doi: 10.1056/nejmoa0908292. [DOI] [PubMed] [Google Scholar]
- Zarulli V., Barthold Jones J.A., Oksuzyan A., Lindahl-Jacobsen R., Christensen K., Vaupel J.W. Women live longer than men even during severe famines and epidemics. Proc. Natl. Acad. Sci. 2018;115(4):E832–E840. doi: 10.1073/pnas.1701535115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;202(5):756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Q., Wang A.Y., Bryant A., Yang Y., Li M., Wang F., Du S., Kurts C., Wu P., Ma K., Wu L., Chen H., Luo J., Li Y., Hu G., Yuan X., Li J. Survival Factors and Metabolic Pathogenesis in Elderly Patients (≥65) With COVID-19: A Multi-Center Study. Front. 2021;7:595503. doi: 10.3389/fmed.2020.595503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltán S., István V.N. Post-acute COVID-19 Syndrome. Orv. Hetil. 2021;62(27):1067–1078. doi: 10.1556/650.2021.32282. [DOI] [PubMed] [Google Scholar]