Abstract
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has led to the most severe global pandemic, which began in Wuhan, China. Angiotensin-converting enzyme 2 (ACE2) combines with the spike protein of SARS-CoV-2, allowing the virus to cross the membrane and enter the cell. SARS-CoV-2 is modified by the transmembrane protease serine 2 (TMPRSS2) to facilitate access to cells. Accordingly, ACE2 and TMPRSS2 are targets of vital importance for the avoidance of SARS-CoV-2 infection. Sanghuangporus sanghuang (SS) is a traditional Chinese medicine that has been demonstrated to have antitumor, antioxidant, anti-inflammatory, antidiabetic, hepatoprotective, neuroprotective and immunomodulatory properties. In this paper, we demonstrated that SS decreased ACE2 and TMPRSS2 expression in cell lines and a mouse model without cytotoxicity or organ damage. Liver and kidney sections were confirmed to have reduced expression of ACE2 and TMPRSS2 by immunohistochemistry (IHC) assessment. Then, hispidin, DBA, PAC, PAD and CA, phenolic compounds of SS, were also tested and verified to reduce the expression of ACE2 and TMPRSS2. In summary, the results indicate that SS and its phenolic compounds have latent capacity for preventing SARS-CoV-2 infection in the future.
Abbreviations: 293T, human embryonic kidney cell line; ACE2, Angiotensin-converting enzyme 2; AKI, acute kidney injury; ANOVA, one-way analysis Variance; CA, caffeic acid; COVID-19, Coronavirus disease; DBA, 3,4- dihydroxybenzalacetone; H&E, hematoxylin-eosin staining; HepG2, human hepatocellular carcinoma cell line; HRP, horseradish peroxidase; IHC, Immunohistochemistry; PAC, Protocatechuic acid; PAD, Protocatechualdehyde; S.E.M, standard error of the mean; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SS, Sanghuangporus sanghuang; TMPRSS2, Transmembrane proteases serine 2
Keywords: SARS-CoV-2; ACE2; TMPRSS2; Sanghuangporus sanghuang; Hispidin; 3,4- dihydroxybenzalacetone; Protocatechuic acid; Protocatechualdehyde; Caffeic acid
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has led to the most severe global pandemic, which began in Wuhan, China [1], [2]. The symptoms of infection in adults include a high temperature, continuous cough, muscle pain and tiredness. The symptoms of infection in younger people include headache, fainting and diarrhea. Pulmonary inflammation with severe lung injury and systemic immune dysregulation are the main components of the COVID-19 pathogenesis [3].
Angiotensin-converting enzyme 2 (ACE2) combines with the spike protein of SARS-CoV-2, allowing the virus to cross the membrane and enter the cell [4]. SARS-CoV-2 is modified by the transmembrane protease serine 2 (TMPRSS2) to facilitate access to cells [5]. When SARS-CoV-2 enters cells, it causes a strong innate immune response, producing inflammatory cytokines that cause cellular damage and a procoagulant state [6], [7], [8]. In a previous study, ACE2 was detected in the brain, heart, lung, colon, kidney and other organs. TMPRSS2 was detected in lung, intestines, kidney, liver and other tissues and organs [9], [10], [11], [12]. A major factor in determining the transmissibility of SARS-CoV-2 was found to be related to the efficiency of ACE2 and TMPRSS2 [13], [14]. The expression of ACE2 and TMPRSS2 in organs, may play a key role in SARS-CoV-2 prevention or treatment [15], [16], [17].
Macrofungi, such as “Donchong Xiacao” (Ophiocordyceps sinensis) and “Lingzhi” (Ganoderma lingzhi), are traditional Chinese medicines that have been used for more than 6000 years and can be traced back to Shen Nong Material Medica [18], [19]. Sanghuangporus sanghuang (SS) is a well-known medicinal polypore with a long history in China, Japan and Korea [19]. In previous pharmacological studies, SS has been demonstrated to have antitumor, antioxidant, anti-inflammatory, antidiabetic, hepatoprotective, neuroprotective and immunomodulatory properties [20], [21], [22], [23], [24], [25], [26], [27]. In this article, we investigated the impact of SS and its phenolic compounds on the protein expression of ACE2 and TMPRSS2 in vivo and in vitro.
2. Materials and methods
2.1. Materials
S. sanghuang mycelium was provided by Grape King Bio Ltd., Taoyuan, Taiwan. Dried SS powders were macerated in 70 % ethanol for 5 days and filtered. Then, the samples were concentrated under reduced pressure to remove the solvent in the filtrate. The above steps were repeated 4 times each concentrate was stored at − 80 °C for subsequent experiments [28]. In a previous study, we had determined the phenolic compounds of SS with HPLC. Protocatechuic acid (PAC), protocatechualdehyde (PAD), caffeic acid (CA), 3,4- dihydroxybenzalacetone (DBA) and hispidin were used for the subsequent experiments [28].
2.2. Cell culture and treatment
We purchased the human hepatocellular carcinoma cell line (HepG2) and human embryonic kidney cell line (293 T) from the Bioresource Collection and Research Center in Taiwan. Cells were grown in DMEM supplemented with 10 % FBS and cultured at 37 °C and 5 % CO2. The concentration of the compounds used in the follow-up experiments was diluted with medium. Cells were seeded at 2.5 × 104 cells per well in 6-well plates. After the designated treatment for 24 h, the collection was lysed with RIPA buffer. The supernatant was then purified by refrigerated centrifugation at 15,000 (×g) and 4 °C for 15 min. It was kept at − 20 °C and used for subsequent experiments.
2.3. MTT assay
We seeded cells in 96-well plates at a density of 2.5 × 104 cells per well in DMEM containing 10 % FBS. When the cells were attached, we replaced the waste medium and added fresh medium consisting of 10 % FBS. The medium was supplemented with the drugs at the respective concentrations for 24 h. After using an MTT assay kit (MedChemExpress, HY-15924) based on the descriptions of the manufacturer and incubating for at least 3 h, the ELISA reader (Molecular Devices) was used to calculate the cell viability by absorbance values at 570 nm.
2.4. Western blot analysis
After treatment with the designated concentration of drugs for 24 h, the cells were extracted and collected. RIPA buffer, the extraction buffer, was purchased from GENESTAR (Kaohsiung, Taiwan). A Bio-Rad protein assay kit (BioRad, Hercules, CA) was used to measure the concentration of total protein. For electrophoresis, 20 µg/well of the proteins was separated within a gel and transferred to a membrane. After a series of treatments, including primary (ACE2: GTX101395, 1:1500; TMPRSS2: GTX100743, 1:1500; Genetex, San Antonio, TX, USA) and secondary antibodies (goat anti-rabbit IgG antibody (HRP): ARG65351, 1:5000; Arigo, Hsinchu, Taiwan), horseradish peroxidase (HRP) conjugate and ECL substrate (201765; Merck, Branchburg, NJ, U.S.), the signals were identified by using Kodak Gel Logic 1500 Imaging Software (East-man Kodak Company, Rochester, NY, U.S.).
2.5. Mouse model
We purchased 12 C57BL/6 female mice aged 6–8 weeks and weighing 18–20 g from BioLASCO Taiwan Co. The two groups of mice (n = 6) were divided at random. The administration group was treated with 100 mg/kg SS by oral gavage for 10 days, and the control group was treated as usual. Mouse weights were measured on Day 0, Day 5 and Day 10. After 10 days, whole blood was collected from the mice, and they were sacrificed.
2.6. Histopathological analysis
Visceral tissues were embedded in paraffin, cut into 3 µm sections and subjected to hematoxylin-eosin staining (H&E) to stain the target. Liver and kidney tissue sections were observed with a microscope (Nikon, ECLIPSE, TS100, Japan) and photographed with a photomicrographic camera (Jenoptik, ProgRes CF Scan, CA).
2.7. Immunohistochemistry (IHC)
Visceral tissues were embedded in paraffin, cut into 3 µm sections and subjected to immunohistochemistry to stain the target. Liver and kidney tissue specimens from mice were stained with ACE2 primary antibody (bs-1004R, Bioss Inc, dilution 50×) or TMPRSS2 primary antibody (ab214462, Abcam, dilution 200×). A Polink-2 Plus HRP DAB Rabbit Bulk kit (D39, GBI LABS) was used for IHC assessment based on the descriptions of the manufacturer, observed with a microscope (Nikon, ECLIPSE, TS100, Japan) and photographed with a photomicrographic camera (Jenoptik, ProgRes CF Scan, CA).
2.8. Statistical analyses
All data are shown as the mean ± standard error of the mean (S.E.M.). Data were analyzed by SPSS software 21.0 (SPSS, Inc., Chicago, IL, USA). Two different groups were analyzed by unpaired two-tailed Student’s t test; more than two groups were analyzed by one-way analysis of variance (ANOVA) and Scheff’e test. The asterisk (*) represents the p value, the grade of significance according to < 0.05, < 0.01 and < 0.001.
3. Result
3.1. Effect of SS on the growth of HepG2 and 293T cell lines
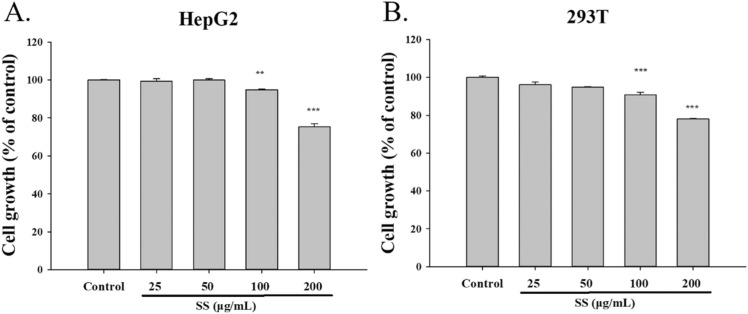
ACE2 combines with the spike protein of SARS-CoV-2, allowing the virus to cross the membrane and enter the cell [4]. SARS-CoV-2 is modified by TMPRSS2 to facilitate access to cells [5]. To research the effects of SS on ACE2 and TMPRSS2, we added each drug concentration (25–200 μg/mL) to HepG2 and 293T cells for the study. We performed an MTT assay to study the cytotoxicity of SS on cells for subsequent experiments. The results showed that 25, 50, and 100 μg/mL SS were not toxic to HepG2 and 293T cells, so we chose 50 and 100 μg/mL for subsequent experiments ( Fig. 1).
Fig. 1.
Effect of SS on HepG2 cells and 293T cells. (A) HepG2 cells and (B) 293T cells. SS (25–200 μg/mL) were added into the cells for 24 h and measured by MTT assay. All the data were performed at least 3 independent examinations and mean ± S.E.M. **p < 0.01 and ***p < 0.001 were contrasted with control group.
3.2. Effect of SS on ACE2 and TMPRSS2 expression in HepG2 and 293T cell lines
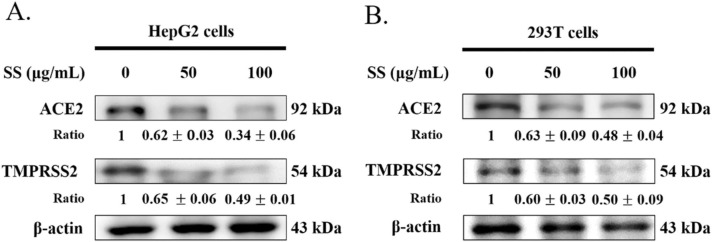
The effect of SS on cell lines was investigated. Subsequently, we demonstrated the role of SS on ACE2 and TMPRSS2 protein expression. These outcomes showed that SS significantly decreased ACE2 and TMPRSS2 protein expression in a dose-dependent manner in HepG2 cells and 293T cells after 24 h ( Fig. 2).
Fig. 2.
Effect of SS on ACE2 and TMPRSS2 expression in HepG2 cells and 293T cells. In (A) HepG2 cells and (B) 293T cells were added SS (50 and 100 μg/mL) and cultured for 24 h and analyzed ACE2 and TMPRSS2 expression by western blot. After densitometric analysis, the results were expressed as a ratio (SS/control). β-actin was chosen as an inner control.
3.3. Effect of phenolic compounds on the growth of HepG2 and 293T cell lines
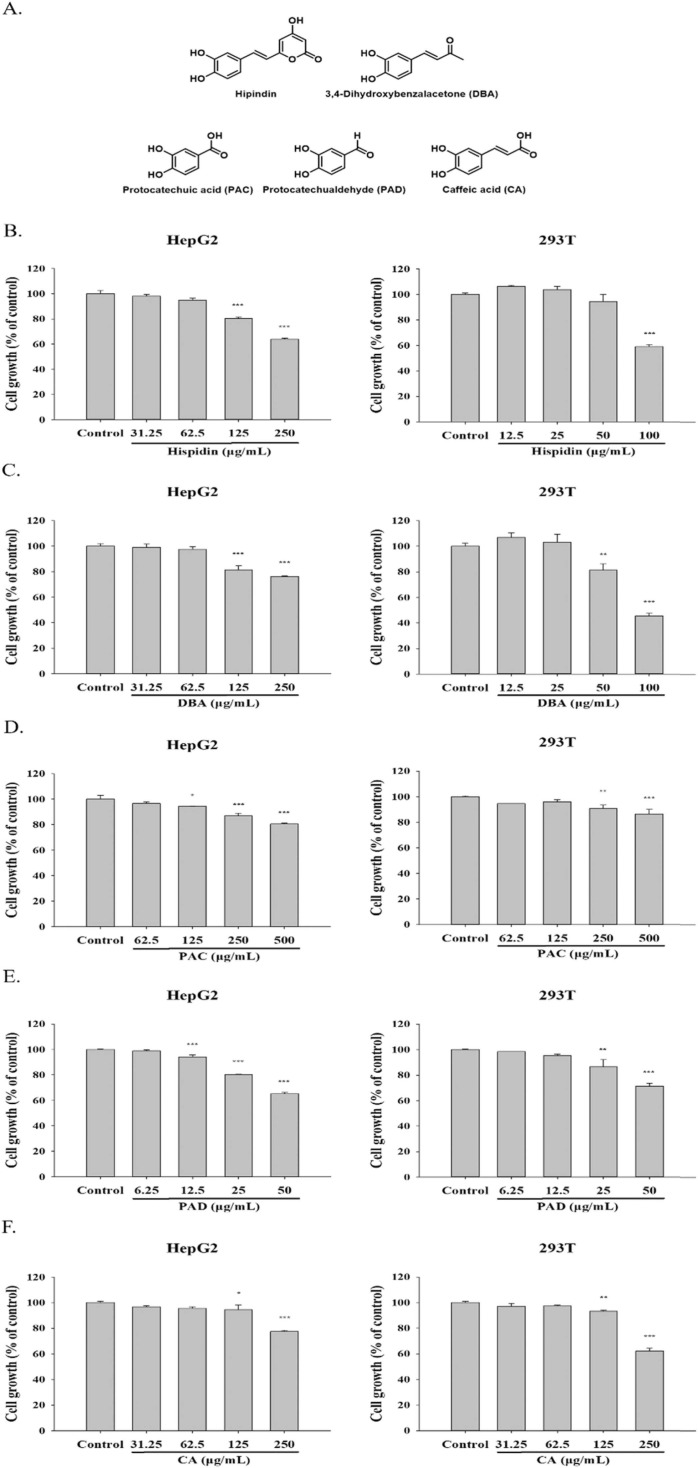
Hispidin, DBA, PAC, PAD and CA are phenolic compounds that are extracted from SS. To investigate the effect of phenolic compounds on different cells, we added each drug concentration to HepG2 and 293T cells for the study. HepG2 cells were treated with hispidin (31.25–250 μg/mL), DBA (31.25–250 μg/mL), PAC (62.5–500 μg/mL), PAD (6.25–50 μg/mL), and CA (31.25–250 μg/mL). 293T cells were treated with Hispidin (12.5–100 μg/mL), DBA (12.5–100 μg/mL), PAC (62.5–500 μg/mL), PAD (6.25–50 μg/mL), and CA (31.25–250 μg/mL). We performed an MTT assay to measure the cytotoxicity of phenolic compounds on cells and confirmed the concentration of each compound for subsequent experiments ( Fig. 3). Hispidin (62.5, 125 μg/mL), DBA (62.5, 125 μg/mL), PAC (250, 500 μg/mL), PAD (12.5, 25 μg/mL) and CA (62.5, 125 μg/mL) were chosen for HepG2 cells. Hispidin (25, 50 μg/mL), DBA (25, 50 μg/mL), PAC (250, 500 μg/mL), PAD (12.5, 25 μg/mL) and CA (62.5, 125 μg/mL) were chosen for 293T cells.( Fig. 4).
Fig. 3.
Effect of phenolic compounds on HepG2 cells and 293T cells. (A) The structure of phenolic compounds. The cell growth of (B) hispidin, (C) DBA, (D) PAC, (E) PAD and (F) CA in HepG2 and 293T cells. The concentration of the respective phenolic compounds was added into the cells for 24 h, and measured by MTT assay. All the data were performed at least 3 independent examinations and mean ± S.E.M. *p < 0.05, **p < 0.01 and ***p < 0.001 were contrasted with control group. (DBA: 3,4- dihydroxybenzalacetone; PAC: protocatechuic acid; PAD: protocatechualdehyde; CA: caffeic acid.).
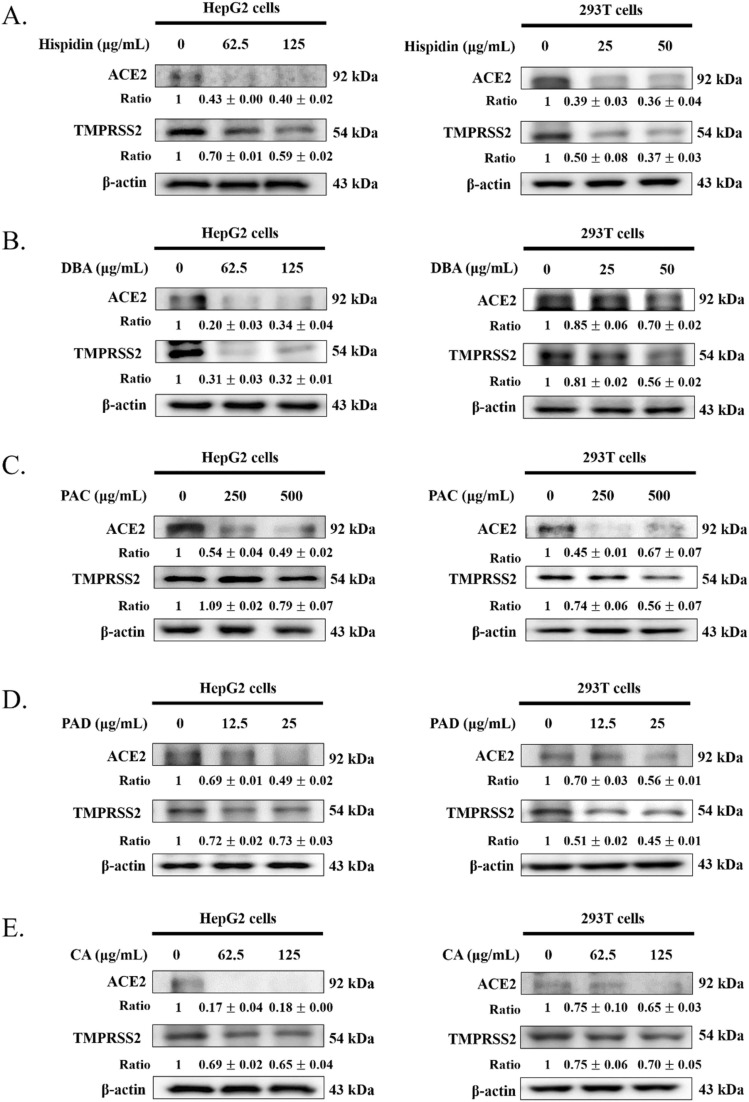
Fig. 4.
Effect of phenolic compounds on ACE2 and TMPRSS2 expression in HepG2 cells and 293T cells. In different cell lines, (A) hispidin, (B) DBA, (C) PAC, (D) PAD and (E) CA were added and cultured for 24 h and analyzed ACE2 and TMPRSS2 expression by Western blot. After densitometric analysis, the results were expressed as a ratio (SS/control). β-actin was chosen as an inner control. (DBA: 3,4- dihydroxybenzalacetone; PAC: protocatechuic acid; PAD: protocatechualdehyde; CA: caffeic acid.).
3.4. Effect of phenolic compounds on ACE2 and TMPRSS2 expression in HepG2 and 293T cell lines
The effect of phenolic compounds on HepG2 and 293T cell lines was investigated. Subsequently, we investigated the role of phenolic compounds on ACE2 and TMPRSS2 protein expression. These outcomes showed that ACE2 and TMPRSS2 protein expression were significantly decreased in HepG2 cells and 293T cells.
3.5. The effect of SS in a mouse model
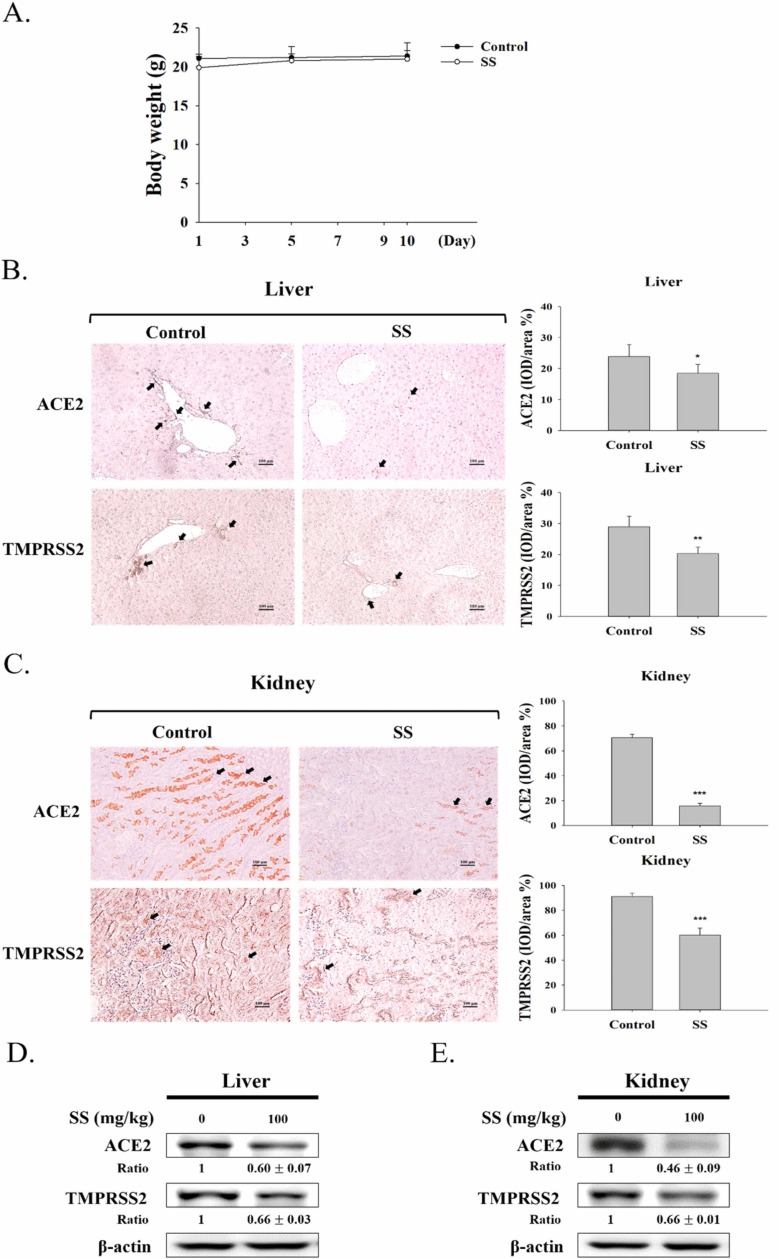
To investigate the effects of SS within living organisms, a mouse model experiment was conducted. The mice were treated with 100 mg/kg SS for 10 days. The body weight of the mice did not change much after ten days ( Fig. 5A).
Fig. 5.
Effect of SS on mouse model. (A) The mice were measured weight and counted after fed with 100 mg/kg SS by oral gavage. The images captured from IHC stained in (B) liver and (C) kidney tissue. Representative histological sections after IHC staining were zoomed in at 200 x and photographed for documentation. The results were presented as IOD/area (%). The values are reported as the mean ± S.E.M (n = 6). *p < 0.05, **p < 0.01 and ***p < 0.001 contrast with the control group. Arrows show the expression of ACE2 or TMPRSS2; scale bar = 100 µm. Protein levels of ACE2 and TMPRSS2 protein expression in the (D) liver and (E) kidney tissues were analyzed by Western blot after treated with 100 mg/kg SS. After densitometric analysis, the results were expressed as a ratio (SS/control). β-actin was chosen as an inner control.
3.6. Assessment of ACE2 and TMPRSS2 expression by immunohistochemistry (IHC) in a mouse model
As shown in Fig. 5, the control group showed many cells stained by IHC assessment, and the SS (100 mg/kg) group had significantly decreased expression of ACE2 and TMPRSS2 in the kidney (Fig. 5C) but slightly decreased expression in the liver (Fig. 5B). These studies demonstrated that SS could block the expression of ACE2 and TMPRSS2 in the liver and kidney without liver toxicity, renal toxicity or pulmonary toxicity.
3.7. ACE2 and TMPRSS2 expression by Western blot in a mouse model
To further confirm whether SS reduces ACE2 and TMPRSS2 protein expression, we performed Western blotting. As shown in Figs. 5D and 5E, SS significantly decreased the protein expression of ACE2 and TMPRSS2 in a mouse model, especially the ACE2 expression in the kidney.
4. Discussion
It was originally discovered in 2003 that the spike protein of SARS-CoV-2 could enter target cells by directly binding with ACE2 [29]. In late 2019, a mysterious outbreak of atypical pneumonia emerged in Wuhan, China [30]. To date, SARS-CoV-2 has infected 509,531,232 people, of whom 6,230,357 have died (last update: 28 April 2022). Pulmonary inflammation with severe lung injury and systemic immune dysregulation are the main components of the COVID-19 pathogenesis [3]. A common complication in gravely ill patients is acute kidney injury (AKI), which shows significant incidence and fatality [31], [32], [33]. ACE2 protein expression is one of the mechanisms that affects AKI in COVID-19 patients [31].
In a previous study, ACE2 was detected in the brain, heart, lung, colon, kidney and other organs. TMPRSS2 was detected in lung, intestinal enteroids, kidney, liver and other tissues and organs [9], [10], [11], [12]. A major factor in determining the transmissibility of SARS-CoV-2 was found to be related to the efficiency of ACE2 and TMPRSS2 [13], [14]. The expression of ACE2 and TMPRSS2 in organs may play a key role in the prevention or treatment of SARS-CoV-2 infection [15], [16], [17]. The HepG2 cell line is used extensively in cytotoxicity experiments [34]. In fact, HepG2 cells are more predictive of human than animal cell lines, including CHO-k1 and ECC-1 [35], [36]. HepG2 cells are rapidly dividing and retain the genotypic and phenotypic characteristics of normal cells even if they are cancer cells [37]. It was found that 93% of the compounds could be tested for toxicity by HepG2 because of the low metabolic capacity of HepG2 [36]. Human embryonic kidney 293T cells have been used in experiments to study apoptosis, glucose transporters, mitochondria, and antiviral drugs [38], [39], [40], [41]. Therefore, we chose these two cell lines for the subsequent experiments.
S. sanghuang is a traditional medicine that has been used to treat gastrointestinal, digestive, and gynecological diseases, cancer, and other diseases in Asian countries for many years [20], [42], [43]. In previous pharmacological studies, SS has been demonstrated to have antitumor, antioxidant, anti-inflammatory, antidiabetic, hepatoprotective, neuroprotective and immunomodulatory properties [20], [21], [22], [23], [24], [25], [26], [27]. ACE2 is the primary receptor of the COVID-19 virus, and its binding to the spike protein plays a critical role in viral entry into host cells and subsequent infection. Blocking this binding event reduces viral receptivity to the ACE2 receptor and represents a strategy to prevent infection with COVID-19 [44].
The function of ACE2 in a tissue or organ is a determinant of the maintenance of its activity and effect on the renin-angiotensin system (RAS) and thus the homeostasis of vasoconstriction, blood pressure, and heart, lung and kidney physiology [45]. Therefore, reducing the expression of ACE2 may be a strategy to reduce the entry of SARS-CoV-2. ACE2 downregulation may have beneficial effects prior to SARS-CoV-2 infection, as it may inhibit the cellular entry and replication of SARS-CoV-2. However, upon infection with SARS-CoV-2, the expression of ACE2 decreases [46], which may subsequently lead to elevated angiotensin II (AngII) levels and worsen clinical outcomes. Indeed, a small cohort of COVID-19 patients had significantly elevated circulating Ang II concentrations compared with healthy controls, which was associated with high viral load and lung injury, such as severe acute respiratory distress syndrome (ARDS) or severe acute respiratory syndrome(SARS) [47].
In addition, alveolar cells such as type 2 pneumocytes and macrophages express ACE2 abundantly and are particularly susceptible to SARS-CoV-2, and this combined with loss of ACE2 activity appears to be the most worrisome feature of COVID-19 in the respiratory system. These effects lead to pneumonia and pulmonary fibrosis [48]. The findings suggest that the virus can stimulate a terrible storm of cytokines, such as IL-6 and TNFα, in the lungs, followed by edema, air exchange dysfunction, acute respiratory distress syndrome, acute cardiac injury, and secondary infection [49], which may cause death. Therefore, avoiding cytokine storms may be the key to treating patients with COVID-19 infection. Several articles have suggested the use of ACE2 inhibitors to block viral infection of cells expressing this enzyme, but given their relevance for maintaining different organ functions, especially lung function, eliminating or reducing its physiological role does not seem to be a good idea [50]. In our previous study, the mycelium of S. sanghuang showed potential in the treatment and/or prevention of inflammation-related diseases such as acute lung injury [28], [51].
In vitro, this study showed that SS and its phenolic compounds (hispidin, DBA, PAC, PAD and CA) significantly decreased ACE2 and TMPRSS2 expression in a dose-related manner, except ACE2 of PAC treated 293T cells and TMPRSS2 of PAD and ACE2 of CA treated HepG2 cells. In vivo, there were no significant differences in body weight or H&E staining between the control and SS groups. The IHC staining data showed high expression of ACE2 and TMPRSS2 in the control group in the kidney, and both significantly decreased in the SS group in the liver and kidney. The data from the animal model showed that SS significantly decreased the expression of ACE2 and TMPRSS2 in liver and kidney tissue. The findings offer a justification that SS could be used as a possible therapeutic agent to inhibit COVID-19 infection.
5. Conclusion
In this article, we showed that S. sanghuang and its phenolic compounds (Hispidin, DBA, PAC, PAD, CA) can decrease the expression of ACE2 and TMPRSS2 in both cell lines and a mouse model. Thus, SS and its phenolic compounds have a latent capacity to prevent SARS-CoV-2 infection.
Funding
This research was funded by the National Science Council (MOST 108–2320-B-039–009-), China Medical University (CMU) (CMU108-MF-117), and Asia University (ASIA-110-CMUH-02, ASIA-109-CMUH-06).
Institutional Review Board Statement
This study was carried out in animals in accordance with approved guidelines (approval number: CMUIACUC-2019–215) by the Animal Management Committee of China Medical University, Taiwan.
Informed Consent Statement
Not applicable.
CRediT authorship contribution statement
L.-H.C., conducted majority of the experiments and prepared the first draft of the manuscript. C.-T.W., J.-S.D., W.-P.J. and W.-C.H., participated in data interpretation and helped to draft the manuscript. J.-G.L. and G.-J.H., interpreted the results, supervised the research work and proofread the manuscript. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded by the National Science Council (MOST 108–2320-B-039–009-), China Medical University (CMU) (CMU108-MF-117), and Asia University (ASIA-110-CMUH-02, ASIA-109-CMUH-06).
Data Availability
The data presented in this study are available on request from the corresponding author.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh M.K., Mobeen A., Chandra A., Joshi S., Ramachandran S. A meta-analysis of comorbidities in COVID-19: which diseases increase the susceptibility of SARS-CoV-2 infection? Comput. Biol. Med. 2021;130 doi: 10.1016/j.compbiomed.2021.104219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L.Q., Huang T., Wang Y.Q., Wang Z.P., Liang Y., Huang T.B., Zhang H.Y., Sun W., Wang Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glowacka I., Bertram S., Müller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., Niemeyer D., Schneider H., Drosten C., Pöhlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85(9):4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egilmezer E., Rawlinson W.D. Review of studies of severe acute respiratory syndrome related coronavirus-2 pathogenesis in human organoid models. Rev. Med. Virol. 2021;31(6) doi: 10.1002/rmv.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B., Ni C., Gao R., Wang Y., Yang L., Wei J., Lv T., Liang J., Zhang Q., Xu W., Xie Y., Wang X., Yuan Z., Liang J., Zhang R., Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salahudeen A.A., Choi S.S., Rustagi A., Zhu J., de la O.S., Flynn R.A., Margalef-Català M., Santos A.J.M., Ju J., Batish A., van Unen V., Usui T., Zheng G.X.Y., Edwards C.E., Wagar L.E., Luca V., Anchang B., Nagendran M., Nguyen K., Hart D.J., Terry J.M., Belgrader P., Ziraldo S.B., Mikkelsen T.S., Harbury P.B., Glenn J.S., Garcia K.C., Davis M.M., Baric R.S., Sabatti C., Amieva M.R., Blish C.A., Desai T.J., Kuo C.J. Progenitor identification and SARS-CoV-2 infection in long-term human distal lung organoid cultures. bioRxiv. 2020 doi: 10.1101/2020.07.27.212076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 14.Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S., Wong S.K., Huang I.C., Xu K., Vasilieva N., Murakami A., He Y., Marasco W.A., Guan Y., Choe H., Farzan M. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24(8):1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon Renin-Angiotensin system blockers. Hypertension. 2020;75(6):1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong N., Yang X., Ye L., Chen K., Chan E.W.-C., Yang M., Chen S. Genomic and protein structure modelling analysis depicts the origin and infectivity of 2019-nCoV, a new coronavirus which caused a pneumonia outbreak in Wuhan, China. bioRxiv. 2020 doi: 10.1101/2020.01.20.913368. [DOI] [Google Scholar]
- 18.Yuan Y., Wang Y., Sun G., Wang Y., Cao L., Shen Y., Yuan B., Han D., Huang L. Archaeological evidence suggests earlier use of Ganoderma in Neolithic China. Chin. Sci. Bull. 2018;63(13):1180–1188. [Google Scholar]
- 19.Wu P., Sun X., Sun F. Scientific and Technical Documentation Press,; Beijing: 2003. Shen nong ben cao jing. [Google Scholar]
- 20.Song M., Park H.-J. Anti-inflammatory effect of Phellinus linteus grown on germinated brown rice on dextran sodium sulfate-induced acute colitis in mice and LPS-activated macrophages. J. Ethnopharmacol. 2014;154(2):311–318. doi: 10.1016/j.jep.2013.12.059. [DOI] [PubMed] [Google Scholar]
- 21.Lin C.-J., Lien H.-M., Lin H.-J., Huang C.-L., Kao M.-C., Chen Y.-A., Wang C.-K., Chang H.-Y., Chang Y.-K., Wu H.-S. Modulation of T cell response by Phellinus linteus. J. Biosci. Bioeng. 2016;121(1):84–88. doi: 10.1016/j.jbiosc.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Kong S.-Z., Li J.-C., Li S.-D., Liao M.-N., Li C.-P., Zheng P.-J., Guo M.-H., Tan W.-X., Zheng Z.-H., Hu Z. Anti-aging effect of chitosan oligosaccharide on d-galactose-induced subacute aging in mice. Mar. Drugs. 2018;16(6):181. doi: 10.3390/md16060181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirahata T., Ino C., Mizuno F., Asada Y., Hirotani M., Petersson G.A., Ōmura S., Yoshikawa T., Kobayashi Y. γ-Ionylidene-type sesquiterpenoids possessing antimicrobial activity against Porphyromonas gingivalis from Ph ellinus linteus and their absolute structure determination. J. Antibiot. 2017;70(5):695–698. doi: 10.1038/ja.2017.35. [DOI] [PubMed] [Google Scholar]
- 24.Chandimali N., Huynh D.L., Jin W.Y., Kwon T. Combination effects of hispidin and gemcitabine via inhibition of stemness in pancreatic cancer stem cells. Anticancer Res. 2018;38(7):3967–3975. doi: 10.21873/anticanres.12683. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Wang C., Li J., Mei Y., Liang Y. Hypoglycemic and hypolipidemic effects of phellinus linteus mycelial extract from solid-state culture in a rat model of type 2 diabetes. Nutrients. 2019;11(2) doi: 10.3390/nu11020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S.-C., Wang P.-W., Kuo P.-C., Hung H.-Y., Pan T.-L. Hepatoprotective principles and other chemical constituents from the mycelium of Phellinus linteus. Molecules. 2018;23(7):1705. doi: 10.3390/molecules23071705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi D.J., Cho S., Seo J.Y., Lee H.B., Park Y.I. Neuroprotective effects of the Phellinus linteus ethyl acetate extract against H2O2-induced apoptotic cell death of SK-N-MC cells. Nutr. Res. 2016;36(1):31–43. doi: 10.1016/j.nutres.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Lin W.-C., Deng J.-S., Huang S.-S., Wu S.-H., Lin H.-Y., Huang G.-J. Evaluation of antioxidant, anti-inflammatory and anti-proliferative activities of ethanol extracts from different varieties of Sanghuang species. RSC Adv. 2017;7(13):7780–7788. [Google Scholar]
- 29.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabarre P., Dumas G., Dupont T., Darmon M., Azoulay E., Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46(7):1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peerapornratana S., Manrique-Caballero C.L., Gómez H., Kellum J.A. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083–1099. doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan L., Chaudhary K., Saha A., Chauhan K., Vaid A., Zhao S., Paranjpe I., Somani S., Richter F., Miotto R., Lala A., Kia A., Timsina P., Li L., Freeman R., Chen R., Narula J., Just A.C., Horowitz C., Fayad Z., Cordon-Cardo C., Schadt E., Levin M.A., Reich D.L., Fuster V., Murphy B., He J.C., Charney A.W., Böttinger E.P., Glicksberg B.S., Coca S.G., Nadkarni G.N. Center, AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. 2021;32(1):151–160. doi: 10.1681/ASN.2020050615. (o.b.o.t.M.S.C.I.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerets H.H., Hanon E., Cornet M., Dhalluin S., Depelchin O., Canning M., Atienzar F.A. Selection of cytotoxicity markers for the screening of new chemical entities in a pharmaceutical context: a preliminary study using a multiplexing approach. Toxicol. Vitr. 2009;23(2):319–332. doi: 10.1016/j.tiv.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Clemedson C., Barile F., Chesne C., Cottin M., Curren R., Ekwall B., Ferro M., Gomez-Lechon M., Imai K., Janus J. MEIC evaluation of acute systemic toxicity: part VII: prediction of human toxicity by results from testing the first 30 reference chemicals with 27 further in vitro assays. ATLA. 2000;28(suppl. 1):161–200. [Google Scholar]
- 36.Schoonen W.G., De Roos J.A., Westerink W.M., Débiton E. Cytotoxic effects of 110 reference compounds on HepG2 cells and for 60 compounds on HeLa, ECC-1 and CHO cells.: II Mechanistic assays on NAD (P) H, ATP and DNA contents. Toxicol. Vitr. 2005;19(4):491–503. doi: 10.1016/j.tiv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Sassa S., Sugita O., Galbraith R.A., Kappas A. Drug metabolism by the human hepatoma cell, Hep G2. Biochem. Biophys. Res. Commun. 1987;143(1):52–57. doi: 10.1016/0006-291x(87)90628-0. [DOI] [PubMed] [Google Scholar]
- 38.Yang Q., Hughes T.A., Kelkar A., Yu X., Cheng K., Park S., Huang W.C., Lovell J.F., Neelamegham S. Inhibition of SARS-CoV-2 viral entry upon blocking N- and O-glycan elaboration. eLife. 2020;9 doi: 10.7554/eLife.61552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Z., Huan C., Wang H., Liu Y., Liu X., Su X., Yu J., Zhao Z., Yu X.F., Zheng B., Zhang W. TRIM21-mediated proteasomal degradation of SAMHD1 regulates its antiviral activity. EMBO Rep. 2020;21(1) doi: 10.15252/embr.201847528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y., Hu M., Wang F., Tan H., Hu J., Wang X., Wang B., Hu J., Li Y. Quantification of 2-NBDG, a probe for glucose uptake, in GLUT1 overexpression in HEK293T cells by LC-MS/MS. Anal. Biochem. 2021;631 doi: 10.1016/j.ab.2021.114357. [DOI] [PubMed] [Google Scholar]
- 41.Wang J., Jiang Y., Soulixay S., Fu D., You Y. [Angiotensin Ⅱ induces apoptosis of HEK293T cells by up-regulating Cyr61 expression] Nan Fang. Yi Ke Da Xue Xue Bao. 2019;39(7):810–815. doi: 10.12122/j.issn.1673-4254.2019.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han S.B., Lee C.W., Jeon Y.J., Hong N.D., Yoo I.D., Yang K.-H., Kim H.M. The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology. 1999;41(2):157–164. doi: 10.1016/s0162-3109(98)00063-0. [DOI] [PubMed] [Google Scholar]
- 43.Hu T., Lin Q., Guo T., Yang T., Zhou W., Deng X., Yan J.-K., Luo Y., Ju M., Luo F. Polysaccharide isolated from Phellinus linteus mycelia exerts anti-inflammatory effects via MAPK and PPAR signaling pathways. Carbohydr. Polym. 2018;200:487–497. doi: 10.1016/j.carbpol.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 44.Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46(3):239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 45.Scialo F., Daniele A., Amato F., Pastore L., Matera M.G., Cazzola M., Castaldo G., Bianco A. ACE2: the major cell entry receptor for SARS-CoV-2. Lung. 2020;198(6):867–877. doi: 10.1007/s00408-020-00408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beyerstedt S., Casaro E.B., Rangel É B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40(5):905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahmudpour M., Roozbeh J., Keshavarz M., Farrokhi S., Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133 doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng H., Wang Y., Wang G.Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020;92(7):726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurz D.J., Eberli F.R. Cardiovascular aspects of COVID-19. Swiss Med. Wkly. 2020;150 doi: 10.4414/smw.2020.20417. [DOI] [PubMed] [Google Scholar]
- 50.Choudhary S., Sharma K., Silakari O. The interplay between inflammatory pathways and COVID-19: a critical review on pathogenesis and therapeutic options. Micro Pathog. 2021;150 doi: 10.1016/j.micpath.2020.104673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin W.C., Deng J.S., Huang S.S., Wu S.H., Chen C.C., Lin W.R., Lin H.Y., Huang G.J. Anti-inflammatory activity of sanghuangporus sanghuang mycelium. Int. J. Mol. Sci. 2017;18(2) doi: 10.3390/ijms18020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.