Abstract
Introduction
The lifetime risk of women undergoing surgery for the presence of benign ovarian pathology in the UK is 5%–10%. Despite minimally invasive surgical techniques, evidence suggests a number of healthy ovarian follicles and tissues are resected intraoperatively, resulting in subsequent decline of ovarian reserve. As such, there is an increasing demand for the implementation of fertility preservation surgery (FPS). This study will evaluate the effect on ovarian reserve following two different surgical interventions for the management of benign ovarian cysts.
Methods and analysis
We will conduct a two-armed randomised controlled trial comparing laparoscopic ovarian cystectomy, considered gold standard treatment as per the Royal College of Obstetricians and Gynaecologists (RCOG) Green Top guidelines for the management of benign ovarian cysts, with ultrasound-guided laparoscopic ovarian cystectomy (UGLOC), a novel method of FPS. The study commencement date was October 2021, with a completion date aimed for October 2024. The primary outcome will be the difference in anti-Müllerian hormone (AMH) (pmol/L) and antral follicle count (AFC) measured 3 and 6 months postoperatively from the preoperative baseline. Secondary outcomes include assessment of various surgical and histopathological findings, including duration of hospital stay (days), duration of surgery (minutes), presence of intraoperative cyst rupture (yes/no), presence of ovarian tissue within the resected specimen (yes/no) and the grade of follicles excised within the specimen (grade 0–4). We aim to randomise 94 patients over 3 years to achieve power of 80% at an alpha level of 0.05.
Ethics and dissemination
Findings will be published in peer-reviewed journals and presented at national and international conferences and scientific meetings. The Chelsea NHS Research and Ethics Committee have awarded ethical approval of the study (21/LO/036).
Trial registration number
Keywords: ultrasonography, minimally invasive surgery, reproductive medicine
Strengths and limitations of this study.
This is the only reported prospective, randomised controlled trial to assess the use of intraoperative ultrasound as a method of fertility preservation surgery.
The trial will provide an evaluation of two different surgical interventions for the management of benign ovarian cysts.
The intervention is non-blinded.
Follow-up of women is limited to 6 months.
Background
Within the field of reproductive medicine, advancements over the last few decades have facilitated the rapidly emerging area of expertise, referred to as fertility preservation. This includes various methods to preserve reproductive tissue or gametes such as medical, surgical or laboratory techniques, thus empowering women to preserve their fecundity with a view to achieving pregnancy at a later date.1 Such techniques were initially considered for women of reproductive age diagnosed with cancer, embarking on gonadotoxic treatment regimens including chemotherapy or radiotherapy, or undergoing radical surgery to remove gynaecological organs, consequently rendering them infertile. Thus, in the context of surgical management of gynaecological cancers, there has been an increasing demand for less radical procedures, with a shift towards conservative surgical methods, in order to preserve the reproductive organs. In appropriately selected women, this enables the opportunity to balance the risks of recurrence from disease, whilst reserving the ability to conceive in the future. As such, the mainstay treatment of Borderline Ovarian Tumours (BOTs), for example, in women with early-stage disease, non-invasive implants or for those who wish to conceive, is fertility preservation surgery (FPS). Such procedures include performing a unilateral salpingo-oophorectomy or ovarian cystectomy, compared with previously adopted surgical methods of radical debulking, which required bilateral salpingo-oophorectomy. Evidence suggests that in this context, FPS is both safe and feasible.2
Consideration of fertility, however, is no longer limited to women undergoing treatment for cancer, as evidence suggests one in six women now experience infertility.3 Although there are a number of causes, it is also prevalent amongst women diagnosed with benign pathology, such as endometriosis or ovarian cysts. Infertility can be caused either by the underlying pathology itself, or indirectly associated with the surgical intervention required to treat.4 The latter is attributed to the fact that ovarian surgery, despite minimally invasive techniques, results in the resection of a number of healthy ovarian follicles and tissue.5 This is exemplified from a study demonstrating that anti-Müllerian hormone (AMH), a reliable marker of ovarian reserve, is reduced postoperatively following surgery for endometriosis.6 Considering the lifetime risk of women undergoing surgery for the presence of benign ovarian pathology is 5%–10%,7 it is perhaps understandable why there is an increasing demand for fertility preserving surgical techniques for women with benign pathology.8 Such demand is further exacerbated by the increasing age of motherhood observed over the last few decades.9 Increasing age is associated with poorer oocyte quality and yield, thereby inadvertently increasing the risk of involuntary childlessness as a direct consequence of age-related fertility decline.10 If women delay attempting pregnancy to a later age, in addition to the risks of surgically induced impairment of ovarian tissue, overall chances of achieving pregnancy in the future maybe significantly reduced. It is imperative, therefore, that fertility preserving techniques are implemented, where possible, in women of reproductive age in order to optimise the chances of future successful conception.
Intraoperative ultrasound
The use of intraoperative ultrasound has been widely implemented, with frequent use observed in the resection of hepatic metastatic disease, neuroendocrine tumours from the pancreas and renal cell carcinoma.11 12 However, within gynaecological surgery, it is not as commonly recognised, despite evidence that it can be used as an adjunct to improving minimally invasive surgical techniques.13 This is primarily due to the improved visualisation of the operative field, which can assist more technically difficult surgical procedures, thus minimising intraoperative complications and injury to surrounding vessels and organs.14 The application of ultrasound guidance within gynaecological procedures has included predominantly ovarian cyst aspiration, in vitro fertilisation, removal or insertion of intrauterine devices and in fertility preservation surgery for BOTs.11 15 Although preoperative imaging provides procedural planning, it cannot compare to the information gained from real-time imaging. For example, in previous studies, intraoperative ultrasound detected more myomas during myomectomy than preoperative transvaginal imaging.16 Furthermore, it provides the potential to assess lesion margins, ensuring resection of pathology is complete with negligible damage to surrounding healthy tissues.13 This is consistent with a recent systematic review, which also demonstrated that although a novel technique, among various case series, pathology can be safely resected without incurring injury to healthy reproductive tissue, as differentiation between pathology and healthy ovarian tissue could clearly be defined on scan.13 The application of intraoperative ultrasound as an adjunct to FPS has not been widely researched, with only a few case series reporting surgical outcomes on patients undergoing treatment for pre-malignant or malignant pathology.17
Aim
This study aims to compare the effect of two different surgical interventions, including either laparoscopic ovarian cystectomy (control group) or ultrasound-guided laparoscopic ovarian cystectomy (UGLOC) (experimental group) for the management of benign ovarian cysts, on the ovarian reserve measured 3 and 6 months postoperatively.
Primary objective
The primary objective is to compare the difference in serum AMH level and AFC number at 3 and 6 months postoperatively in women who have undergone UGLOC to the control group.
Secondary objectives
The secondary objective will include assessment of various surgical and histopathological findings.
Hypothesis
The difference in serum AMH level and AFC number measured at 3 and 6 months postoperatively will be significantly less following UGLOC when compared with laparoscopic ovarian cystectomy.
Methods
Trial design
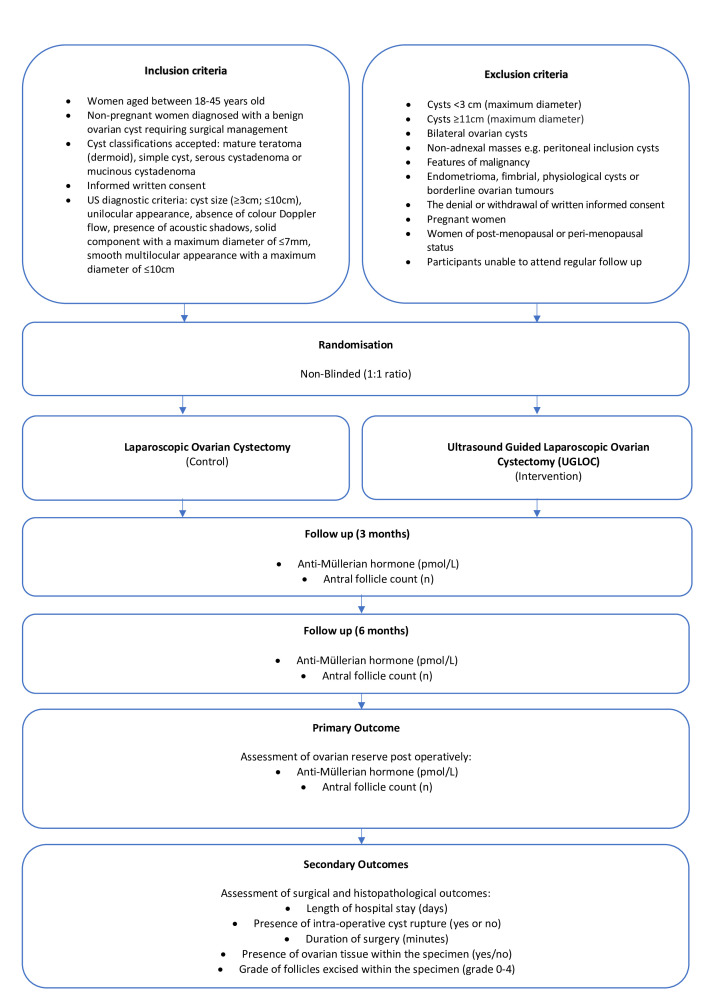
This is a single-centre randomised controlled trial (RCT) with two parallel arms, comparing laparoscopic ovarian cystectomy with UGLOC for the management of benign ovarian cysts. Women will be recruited from one tertiary gynaecology centre in the United kingdom (UK). They will be followed up at 3 and 6 months postoperatively to assess markers of ovarian reserve. Recruitment began in October 2021, with follow-up and assessment expected to conclude in October 2024. Figure 1 summarises the trial design.
Figure 1.
Summary of trial design.
Inclusion criteria
Women aged between 18 and 45 years old.
Non-pregnant women diagnosed with a benign ovarian cyst requiring surgical management.
Cyst classifications accepted: mature teratoma (dermoid), simple cyst, serous cystadenoma or mucinous cystadenoma.
Informed written consent.
A strict criterion of the ultrasound diagnostic features will include the following:
Cyst size ≥3 cm; ≤10 cm.
-
Presence of International Ovarian Tumour Analysis Benign (IOTA B) features:
Unilocular appearance.
Solid components (maximum diameter ≤7 mm).
Acoustic shadows.
No colour Doppler flow.
Smooth multilocular appearance (maximum diameter ≤10 cm).
Exclusion criteria
Cysts deemed to be clearly physiological and <3 cm (maximum diameter).
Cysts ≥11 cm (maximum diameter).
Bilateral ovarian cysts.
Non-adnexal masses, e.g. peritoneal inclusion cysts.
Cysts with features of malignancy.
Endometrioma, fimbrial cysts or BOTs.
The denial or withdrawal of written informed consent.
Pregnant women.
Women of post or peri-menopausal status.
Women unable to attend regular follow-up.
Outcome measures
The primary outcome of the study will be the assessment of ovarian reserve at 3 and 6 months postoperatively. This will be assessed by measuring the AMH (pmol/L) level and AFC (n). The secondary outcomes will include length of hospital stay (days), presence of intraoperative cyst rupture (yes or no), duration of surgery (minutes), presence of ovarian tissue (yes/no) and the grade of follicles excised within the specimen resected (grade 0–4).
Enrolment
All women referred to the outpatient gynaecology clinic with a suspected ovarian cyst will have a pelvic transvaginal ultrasound scan (two-dimensional (2D) and three-dimensional (3D)) as part of routine clinical care. If an ovarian cyst is diagnosed, it will be assessed according to local protocols based on simple descriptors and international ovarian tumour analysis (IOTA) simple rules. Depending on the severity of symptoms, nature of the cyst and whether surgical management is indicated to treat, should the woman fulfil aspects of the inclusion criteria, she will be invited to participate in the study. Any woman at the upper age limit for inclusion in the study who also presents with a history of climacteric symptoms, irregular periods or has an AFC ≤4,18 should be considered of peri-menopausal or menopausal status, and thus not eligible for recruitment to the study.
The study coordinator will be responsible for managing the registration of each participant to the trial and their allocation to either treatment arm. All participants will sign a written consent form, witnessed by a member of the research team, at least 24 hours after the participant information sheet has been read. All consent forms will be scanned into the electronic medical notes. It will not be possible to carry out any tasks pertaining to the trial, until written consent from the participant has been obtained.
Randomisation
A separate research team within Imperial College London Healthcare Trust, Department of Cancer and Surgery will be responsible for the allocation process, by producing randomisation sealed envelopes in a ratio of 1:1. The team will be asked to print labels with the allocated group and fold them to conceal the group name. They will then return the folded labels back to the study coordinator, who will be asked to place them into opaque sealed envelopes, which will be numbered in ascending order. Thus, the study coordinator will not be able to see the content of the labels. Given that it is necessary for the surgeon to know which operation to perform, both the participant and the research team will not be blinded. The allocated arm of the RCT will be recorded on the trial subject enrolment log. The principal investigator (PI) will be responsible for keeping the randomisation list.
Procedures
During the recruitment process, once consent to participate in the trial has been obtained, a member of the research team will select a sealed randomisation envelope as numbered in ascending order, which will then assign the participant to a treatment arm. The ascending order of envelopes will prevent the member of the research team performing the randomisation themselves, or from selecting another envelope, should they be dissatisfied by the treatment arm assigned to the participant. The participant will then undergo a blood test to record their baseline preoperative AMH level and a transvaginal scan to confirm their AFC. The same surgeons will operate on all participants, regardless of treatment arm assigned. This will aim to exclude bias, which may otherwise attribute findings to the surgeon operating. Only experienced surgeon’s with advanced competencies in gynaecological laparoscopy and pelvic ultrasound will perform the surgery at Imperial Healthcare NHS Trust. All participants will attend preoperative assessment with an anaesthetist as standard practice of care. All participants undergoing surgery will be required to have an overnight stay in hospital.
Surgical intervention
Laparoscopic ovarian cystectomy (control)
Laparoscopic entry will be performed according to basic laparoscopic principles from the British Society of Gynaecological Endoscopy.7 Pneumoperitoneum will be achieved through infiltration of carbon dioxide into the pelvis, in order to provide sufficient insufflation and visualisation of the pelvic organs. Participants will undergo laparoscopic ovarian cystectomy in the absence of ultrasound guidance. Following removal of the ovarian cyst through laparoscopic specimen retrieval bags, routine surgical closure of the abdomen will be performed.
Ultrasound-guided laparoscopic ovarian cystectomy (intervention)
Laparoscopic entry and peritoneal insufflation of carbon dioxide will be performed to achieve a pneumoperitoneum. Following laparoscopic entry and assessment of the operating field, 500 mL of normal saline (0.9%) will be infiltrated into the pelvis, for enhancement of the ultrasound image quality and transmission of the ultrasound. This remains within the pelvis during the course of the operation. An assistant with competencies in pelvic ultrasound scanning will insert a transrectal probe intraoperatively and provide real-time ultrasound images of the ovary and cyst to be resected. A non-traumatic instrument will be used to help stabilise the ovarian cyst, whilst correlating between the laparoscopic and ultrasound images. The cystectomy will be performed under continuous ultrasound guidance, above the level of the saline solution, ensuring the surgeon is able to differentiate between healthy ovarian tissue and cyst content. Following removal of the ovarian cyst through laparoscopic specimen retrieval bags, routine closure of the abdomen will be performed.
Follow-up
Postoperatively, participants will return to the outpatient gynaecology clinic for follow-up at 3 and 6 months. During the follow up appointment an AMH level will be taken. The blood samples will be processed by Imperial College Healthcare Trust laboratories and discarded as per local protocol once the AMH has been determined. There are no specific storage or transfer requirements outside of normal practice. In addition, a transvaginal ultrasound scan will be performed during the follow-up appointment, to measure AFC and assess volume of preserved ovarian tissue. Following the second follow-up attendance at 6 months, no further input is required from the participant in the study.
Discontinuation or withdrawal of participants
Participants who give consent to their recruitment to the trial agree to the intervention, compliance with follow-up assessments and data collection. Participants are free to withdraw at any time from the protocol treatment without reason. Furthermore, participants may be withdrawn from the trial by members of the research team, should participation in the trial no longer be deemed within the participant’s best interest. All data captured in relation to their participation may be destroyed at their request. Any decision to withdraw a participant from the study will be recorded in the electronic clinical record files (eCRFs) and medical notes. Reasons for stopping the trial include non-compliance by members of the research team to adherence of the study protocol, participants withdrawing consent, or adverse outcomes reported following the intervention.
Patient and public involvement
Ten women were approached by members of the research team from outpatient gynaecology clinics, to request assistance reviewing participant information resources applicable to the study. If agreeable, they were provided with a copy of the patient information leaflet and consent form to review over the course of a week. They then voluntarily attended a virtual research focus meeting, whereby their feedback regarding aspects of the study protocol were sought, including feasibility and acceptability of the study design, methods of monitoring ovarian reserve postoperatively and acceptability of the intervention arm of the trial. They also participated in revising patient information leaflets and GP letter templates.
Modification of the protocol
Any amendments to the research protocol will be first agreed by the PI and the study coordinators. This may include aspects of the study design, participant recruitment, sample size, interventions or ethics documents, including participant information sheet or consent form. Implementation of changes made will depend on subsequent approval from the Research Ethics Committee. Any amendments made will be updated on the clinialtrials.gov website accordingly.
Data and trial management
Data collection will include completion of eCRFs by members of the research team. The PI will ensure the accuracy of all data reported. Members of the research team and all aspects of the study protocol will adhere to the principles of the General Data Protection Regulation (2016/679) and the Data Protection Act (2018). All personal data will be password protected and held on a database, accessible only from a registered NHS Trust computer. Following data collection, all information will be anonymised during the data analysis stage, whereby access will be restricted to the PI and study coordinator only.
The sponsor of the study reserves the right to store all anonymised data for 10 years after the study has finished, in relation to data subject consent forms and 10 years after the study has completed in relation to primary research data. Following this, the sponsor will adhere to the confidential information trust destruction procedures for disposal of data. A trial management group (TMG) has been designed including the PI, two study coordinators and trial staff. They are responsible for the day-to-day running of the trial. The TMG will meet every 6 weeks to discuss recruitment numbers, adverse events (AEs) encountered or potential amendments to the study protocol, if required. The study may be subject to inspection and audit by the sponsor and other regulatory bodies to ensure adherence to Good Clinical Practice (GCP) and the UK Policy Framework for Health and Social Care Research. Direct access to source data/documents as requested will be permitted. A data safety monitoring board is not deemed necessary, as the study is associated with extremely low risks.
Safety
Any questions concerning AEs reporting will be directed to the PI in the first instance. For non-serious AEs, whether expected or not, a brief description of associated clinical symptoms with date and duration of onset will be documented in the medical notes. For serious adverse events (SAEs), an SAE form will be completed and emailed to the PI within 24 hours. Specifically, relapse and death due to other pathology, and hospitalisations for elective treatment of a pre-existing condition do not need reporting as SAEs. All SAEs will be reported to the Chelsea Research and Ethics Committee, where in the opinion of the PI the event was deemed as follows:
‘Related’, that is, resulted from the administration of any of the research procedures.
‘Unexpected’, that is, an event that is not listed in the protocol as an expected occurrence.
Reports of related and unexpected SAEs will be submitted within 15 days of the PI becoming aware of the event, using the NRES SAE form for Non Investigational Medicinal Product (NIMP) studies. The PI will notify the sponsor of all related and unexpected SAEs.
Sample size and power calculation
The impact of laparoscopic ovarian cystectomy performed for the management of benign ovarian cysts has been investigated by Kwon et al,19 whereby AMH and AFC was measured at 3 and 6 months postoperatively. We considered this study most applicable for determining the power calculation of our RCT, based on the following principles: pathology of the ovarian cysts were benign in nature and the sample size (n=100) is one of the largest reported. Kwon et al deduced that AMH levels decreased, on average, by 30.58% (±29.66%) between the preoperative value to the level assessed 3 months following surgery.19 Specifically, the group of women who underwent laparoscopic ovarian cystectomy for benign ovarian pathology had a mean ± standard deviation (SD) serum AMH of 1.59 (±1.92) (ng/mL), equivalent to 3.57 (±4.31) (pmol/L).
At present, there are no reported studies assessing the difference in AMH levels following ultrasound-guided ovarian cystectomy. We have performed a small pilot study consisting of five women who have undergone ultrasound-guided ovarian cystectomy for BOTs. In order to calculate the sample size, we used our pilot data to derive an estimate of the mean and SD (table 1).
Table 1.
Pilot data compared with control study
| AMH differences (pmol/L) | ||
| Treatment group (Pilot) | Control group (Kwon et al) | |
| Mean | 17.06 | 3.57 |
| SD | 22.28 | 4.31 |
| Coefficient of variation (CV)=(SD/mean) | 1.31 | 1.21 |
| Participants (n) | 5 | 100 |
AMH, anti-Müllerian hormone.
A power calculation was performed using the TrialSize package for a two sample mean for superiority or non-inferiority trials with R Statistical Programming (V.4.2.0). We assumed a power of 80% at an alpha level of 0.05 (two tailed), based on a superiority margin of 2.0 pmol/L in AMH, considered to be a significant difference between the two treatment groups in the primary outcome.
Referring to table 1, the true mean AMH difference between the groups is 13.49 pmol/L, and the pooled SD of the two groups is 22.44 pmol/L. Thus, the total number of participants required for each treatment arm is 47, or 94 in total. This is based on a 1:1 randomisation of participants, which should also consider for variation in baseline characteristics. To account for a 5% dropout and 10% loss to follow-up rate, we will recruit 108 participants into the study, or 54 per group.
It will be necessary to perform an interim analysis to re-assess the SD, given the low number of participants available for the power calculation.
Secondary analysis
We will exclude participants with missing data, non-compliance of follow-up or adherence to the study protocol, or those who withdraw from the study. The primary outcome will be compared across treatment groups using univariate and multivariate analysis. Confidence intervals of 99% for dichotomous outcomes and risk ratio will be assessed. A p value of less than 0.05 will be used to determine statistical significance.
Ethics and dissemination
This study will be conducted in accordance with the principles of GCP. This protocol was submitted to the Health Research Authority and Chelsea Research Ethics Committee, whereby a favourable ethical opinion was granted. The reference number is 21/LO/036. Subsequent approval by an individual ethical committee and competent authority was granted. Any modification to the protocol will be updated on the ClinicalTrials.gov website and disseminated to all relevant parties. The results will be published in peer-reviewed journals and disseminated at national and international conferences.
Data sharing
For the purpose of this publication, data sharing is not applicable as no datasets have been generated and/or analysed as yet.
Potential biases within the study
Evidence suggests the type of ovarian cyst resected determines the magnitude of decline in ovarian reserve. For example, surgical resection of endometriomas are associated with the greatest degree of decline in AMH, compared with cysts of other pathology.20 This is most likely because the underlying pathogenesis of endometriosis itself causes adhesions, which complicates the procedure and increases the volume of healthy ovarian stroma resected within the cyst contents.19 21 For this reason, we have excluded endometriomas from the study.
Furthermore, certain participant characteristics may lead to bias in the study, such as the ethnicity and age of the participant recruited. These are potential confounding variables, particularly as AMH levels are influenced by both age related and racial disparities.22 Therefore, it is appropriate during data analysis to perform a separate subgroup analysis to determine whether certain demographics are associated with degree of decline of AMH, between the control and experimental groups.
Discussion
Fertility preservation surgery has evolved immensely since it was first introduced for the management of gynaecological cancers in women of reproductive age. As evidence continues to propagate, it is considered a safe and feasible option in women of reproductive age diagnosed with BOTs or early stage ovarian carcinoma. Few studies, however, assess the use of FPS for the management of benign ovarian pathology. Considering the causes of infertility include a number of benign pathologies, alongside the increasing risk of age-related fertility decline associated with delayed childbearing, the demand for fertility preserving techniques is imminently growing among women undergoing surgery for benign pathology during their reproductive years.
Recent advancements within ultrasound technology have facilitated the enhancement in diagnostic accuracy, as evidenced by the detection of smaller ovarian lesions or pathology on ultrasound scan.17 Furthermore, the ability to delineate cyst content from healthy ovarian tissue also reduces the risk of cyst rupture, which depending on the contents may have detrimental effects on the pelvis. The ability to preserve optimal healthy ovarian tissue is therefore a benefit of intraoperative ultrasound, providing an apt alternative to the otherwise blind resection of healthy ovarian tissue during ovarian cystectomy.
The PI and coinvestigators of this study have previously published the outcomes of ultrasound-guided laparoscopic ovarian wedge resection for the management of recurrent serous BOTs,17and in the context of treatment for anti-NMDA receptor encephalitis.23 While both surgical and oncological outcomes reported were successful, there was no measurable effect on the ovarian reserve assessed postoperatively. This prospective trial therefore, will evaluate the effectiveness of this method of FPS and provide real-time evidence for the postoperative effects on ovarian reserve. The findings from the study will allow clinicians to provide informative counselling, to ensure women can make well-informed decisions regarding their future fertility before deciding to undergo surgery. Furthermore, the technique is readily available and considered a low cost treatment option for the management of benign ovarian cysts. Considering transvaginal ultrasound scanning is a competency acquired by all UK trainees, many specialists already attain the skills to implement this method of FPS. The procedure is therefore considered to be widely applicable nationally.
Supplementary Material
Footnotes
Twitter: @jan_verbakel
Contributors: JY and LSK conceived and designed the study. LSK drafted the trial protocol. JY, JV and ME-B provided methodological and statistical expertise. JY and SG-M provided expertise in clinical outcomes following ultrasound. LSK and BPJ drafted the manuscript. LSK and SS with the support of the trial principal investigator, have responsibilities for day-to-day running of the trial including participant recruitment, data collection and liaising with other sites. All authors critically reviewed and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Dolmans M-M, Manavella DD. Recent advances in fertility preservation. J Obstet Gynaecol Res 2019;45:266–79. 10.1111/jog.13818 [DOI] [PubMed] [Google Scholar]
- 2.Park J-Y, Kim D-Y, Kim J-H, et al. Surgical management of borderline ovarian tumors: the role of fertility-sparing surgery. Gynecol Oncol 2009;113:75–82. 10.1016/j.ygyno.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 3.Oakley L, Doyle P, Maconochie N. Lifetime prevalence of infertility and infertility treatment in the UK: results from a population-based survey of reproduction. Hum Reprod 2008;23:447–50. 10.1093/humrep/dem369 [DOI] [PubMed] [Google Scholar]
- 4.Rustamov O, Krishnan M, Roberts SA, et al. Effect of salpingectomy, ovarian cystectomy and unilateral salpingo-oopherectomy on ovarian reserve. Gynecol Surg 2016;13:173–8. 10.1007/s10397-016-0940-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muzii L, Bianchi A, Crocè C, et al. Laparoscopic excision of ovarian cysts: is the stripping technique a tissue-sparing procedure? Fertil Steril 2002;77:609–14. 10.1016/S0015-0282(01)03203-4 [DOI] [PubMed] [Google Scholar]
- 6.Victoria M, Labrosse J, Krief F, et al. Anti Müllerian hormone: more than a biomarker of female reproductive function. J Gynecol Obstet Hum Reprod 2019;48:19–24. 10.1016/j.jogoh.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 7.RCOG B. Management of Suspected Ovarian Masses in Premenopausal Women 2011;Green-top Guideline No.62.
- 8.G-tG N. Management of suspected ovarian masses in premenopausal women. last accessed 28thMay 2022 online.
- 9.Baldwin K. Motivations for social egg freezing. egg freezing, fertility and reproductive choice (Emerald studies in reproduction, culture and society) 2019:69–85.
- 10.Jones BP, Saso S, Mania A, et al. The dawn of a new ice age: social egg freezing. Acta Obstet Gynecol Scand 2018;97:641–7. 10.1111/aogs.13335 [DOI] [PubMed] [Google Scholar]
- 11.Grewal K, Jones B, L'Heveder A. The use of intra-operative ultrasound in gynecological surgery: a review. Future Science OA 2020;0:FSO678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lubner MG, Mankowski Gettle L, Kim DH, et al. Diagnostic and procedural intraoperative ultrasound: technique, tips and tricks for optimizing results. Br J Radiol 2021;94:20201406. 10.1259/bjr.20201406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galazis N, Saso S, Sorbi F, et al. Intraoperative ultrasound during fertility-sparing surgery: a systematic review and practical applications. Gynecol Obstet Invest 2020;85:127–48. 10.1159/000505689 [DOI] [PubMed] [Google Scholar]
- 14.Criniti A, Lin PC. Applications of intraoperative ultrasound in gynecological surgery. Curr Opin Obstet Gynecol 2005;17:339–42. 10.1097/01.gco.0000175349.10684.e8 [DOI] [PubMed] [Google Scholar]
- 15.Mascilini F, Quagliozzi L, Bolomini G, et al. Intraoperative ultrasound through laparoscopic probe in fertility‐sparing surgery for borderline ovarian tumor recurrence. Ultrasound Obstet Gynecol 2019;54:280–2. 10.1002/uog.20138 [DOI] [PubMed] [Google Scholar]
- 16.Levine DJ, Berman JM, Harris M, et al. Sensitivity of Myoma imaging using laparoscopic ultrasound compared with magnetic resonance imaging and transvaginal ultrasound. J Minim Invasive Gynecol 2013;20:770–4. 10.1016/j.jmig.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 17.Jones BP, Saso S, Farren J, et al. Ultrasound-Guided laparoscopic ovarian wedge resection in recurrent serous borderline ovarian tumours. International Journal of Gynecologic Cancer 2017;27:1813–8. 10.1097/IGC.0000000000001096 [DOI] [PubMed] [Google Scholar]
- 18.Wellons MF, Bates GW, Schreiner PJ. Antral follicle count predicts natural menopause in a population-based sample: the CARDIA Women’s Study. Menopause 2013;20:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon SK, Kim SH, Yun S-C, et al. Decline of serum antimüllerian hormone levels after laparoscopic ovarian cystectomy in endometrioma and other benign cysts: a prospective cohort study. Fertil Steril 2014;101:435–41. 10.1016/j.fertnstert.2013.10.043 [DOI] [PubMed] [Google Scholar]
- 20.Lind T, Hammarström M, Lampic C, et al. Anti‐Müllerian hormone reduction after ovarian cyst surgery is dependent on the histological cyst type and preoperative anti‐Müllerian hormone levels. Acta Obstet Gynecol Scand 2015;94:183–90. 10.1111/aogs.12526 [DOI] [PubMed] [Google Scholar]
- 21.Perlman S, Kjer JJ. Ovarian damage due to cyst removal: a comparison of endometriomas and dermoid cysts. Acta Obstet Gynecol Scand 2016;95:285–90. 10.1111/aogs.12841 [DOI] [PubMed] [Google Scholar]
- 22.Kotlyar AM, Seifer DB. Ethnicity/race and age-specific variations of serum AMH in women—a review. Frontiers in Endocrinology 2021;1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones BP, Rees R, Saso S, et al. Ultrasound-Guided laparoscopic ovarian preserving surgery to treat anti-NMDA receptor encephalitis. BJOG: Int J Obstet Gy 2017;124:337–41. 10.1111/1471-0528.14214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.