Key Teaching Points.
-
•
Presence of B symptoms (fever, cough, fatigue, night sweats) with bradyarrhythmia should prompt consideration of cardiac lymphoma in the differential diagnosis.
-
•
Absence of macroscopic evidence of cardiac tumor should not rule out cardiac lymphoma.
-
•
Early initiation of therapy can potentially resolve bradyarrhythmia in cardiac lymphoma.
Introduction
Cardiac tumors have been a point of interest and controversy since the earliest attempt to categorize them in 1931 by Yater.1,2 Secondary cardiac tumors are 30 times more common than primary cardiac tumors, with an incidence of 1.7%–14% vs 0.001%–0.03% on autopsy.3 Primary cardiac tumors are most commonly benign (70%–80%).3,4 However, of the primary malignant cardiac tumors, lymphoma has been shown to account for 1%–1.6%.5,6 Up to 20% of patients with noncardiac primary lymphoma will exhibit cardiac metastases.7 The majority of primary cardiac lymphomas are from B-cell origins and are typically found in immunosuppressed adults.8 Lymphoma involving the heart or its alternative name, reticulum cell sarcoma, has been described in the literature as early as 1942 to be associated with cardiac arrhythmias, including atrial flutter and fibrillation.1,2 In this report we describe 2 cases of cardiac lymphoma in immunocompetent patients presenting with cardiac bradyarrhythmia as their initial manifestation. The first is a presentation of secondary cardiac lymphoma invading the anterior interatrial septum and with potential extension into the area of the atrioventricular (AV) node with resulting AV block, that resolves with prednisone therapy alone. The second is a presentation of a primary cardiac lymphoma presenting as sinus node dysfunction with progressive atrial myopathy.
Case report
Case 1
Presentation
A 62-year-old woman with history only significant for tobacco abuse and depression presented to the emergency department (ED) with 1 month of abdominal pain and diarrhea and days of chest discomfort associated with cough, dyspnea, and malaise. On further questioning she reported palpitations, 2 episodes of syncope, and 2 months of fatigue, weakness, night sweats, and unexplained 10-pound weight loss.
Investigation and course of disease
In the ED, an electrocardiogram demonstrated complete heart block with junctional escape at a rate of 40 beats per minute (Figure 1A). Blood pressure was reduced with mean arterial pressure 50 mm Hg. She had an elevated serum lactate of 6.0. Echocardiogram showed a small pericardial effusion without tamponade and chest radiography showed evidence of atelectasis. Ongoing abdominal pain prompted computed tomography (CT) of the abdomen, which revealed a 4.5 × 7.2 cm mass invading the right ilium and pubic ramus with a 1.2 cm retroperitoneal nodule and T6 vertebral body lesion. In addition, CT chest revealed an infiltrative mass along the free wall of the right atrium, right ventricle, and right-sided AV groove surrounding the right coronary artery without compression. There was extension into the interatrial septum and left atrium (Figure 2A).
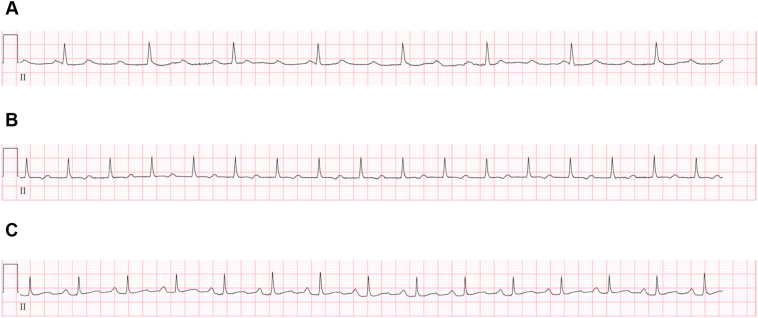
Figure 1.
Atrioventricular (AV) conduction abnormalities normalized after steroids for cardiac large B-cell lymphoma. A: Electrocardiogram (ECG) at time of presentation, showing complete heart block and AV dissociation. This rhythm was associated with cardiogenic shock and lactic acidosis. B: ECG showing first-degree AV block, obtained on hospital day 3, after 48 hours pre-phase prednisone that was started immediately following histologic diagnosis of large B-cell lymphoma. Shock resolved less than 24 hours after starting prednisone. C: ECG showing return to normal sinus rhythm with normal PR interval, obtained 28 days after initial presentation and after patient underwent first cycle of R-CHOP.
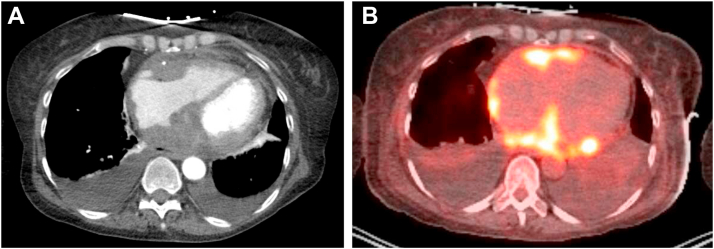
Figure 2.
Case 1: Cross-sectional imaging demonstrating infiltrative soft tissue mass along AV groove and free walls of atria. A: Computed tomography angiography (CTA) chest revealed an infiltrative soft tissue mass along the free wall of right atrium, right ventricle, right-sided atrioventricular groove, interatrial septum, and left atrium with trace pericardial effusion. B: Fluorodeoxyglucose (FDG)–positron emission tomography imaging shows avid FDG uptake in the soft tissue cardiac masses described in the CTA chest.
Management
The patient was volume resuscitated and placed on dopamine. She was admitted to the cardiac intensive care unit for monitoring with plan to consider implantation of a dual-chamber pacemaker for complete heart block. Medical oncology was consulted and recommended core biopsy of the abdominal mass. She developed signs of tumor lysis syndrome with hyperuricemia, elevated lactate dehydrogenase, hyperkalemia, and hyperphosphatemia, which was treated with rasburicase. Core biopsy of the right iliac lesion demonstrated diffuse large B-cell lymphoma. Positron emission tomography–CT, obtained for staging of lymphoma, further confirmed cardiac involvement (Figure 2B). Prednisone was started with ongoing treatment for worsening tumor lysis syndrome. Electrolytes were normalized by the second day in hospital. Within 3 days, she had continued improvement in conduction, transitioning from complete heart block to sinus rhythm with first-degree AV block (Figure 1B). She began R-CHOP chemotherapy on the tenth day after admission and has subsequently recovered normal AV conduction in follow-up (Figure 1C).
Case 2
Presentation
A 76-year-old man with history of chronic obstructive pulmonary disease, asthma, gout, hyperlipidemia, and obstructive sleep apnea presented to the ED with 8 weeks of nonproductive cough and dyspnea and 4 weeks of decreased appetite, weakness, and fatigue. He denied angina, dizziness, syncope, palpitations, and edema. Labs were significant for NT-proBNP of 984. A chest CT demonstrated a small pericardial effusion and borderline cardiomegaly. In-hospital cardiac telemetry was significant for multiple sinus pauses up to 5.8 seconds long, and a dual-chamber pacemaker was implanted. One month remote follow-up was notable for decreased right atrial lead sensing from 4.9 to 0.4 mV with stable lead impedance (780 to 839 ohms). There was associated loss of atrial capture. Echocardiogram showed persistent mild pericardial effusion, and chest radiography showed stable right atrial lead position. Right atrial lead dysfunction was felt to be most consistent with possible microdislodgement or microperforation.
Investigation and course of disease
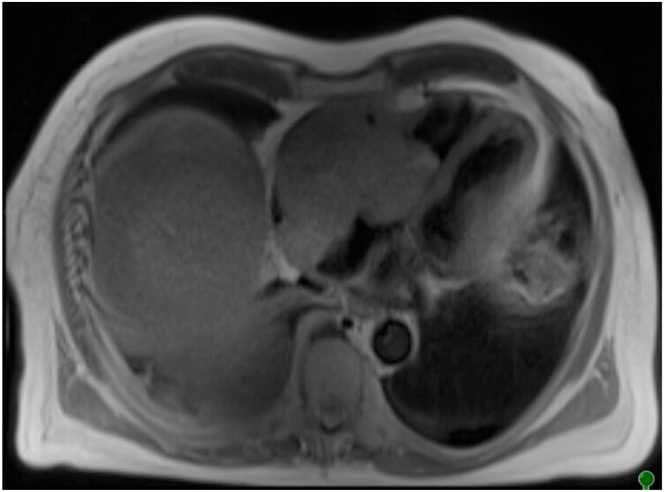
The patient was referred for atrial lead revision, which was notable for no sensed P waves, but revised atrial capture was 0.5 V at 0.4 ms. Over the next 12 weeks he developed intermittent atrial flutter and had intermittent loss of atrial sensing and capture. Stable atrial lead placement was confirmed by chest radiography and CT. Repeat lead revision was deferred. Over the subsequent weeks, the patient had increasing fluid retention and was referred for right and left heart catheterization. Initial hemodynamic assessment was consistent with tricuspid inflow obstruction. A follow-up echocardiogram with contrast and subsequent cardiac magnetic resonance imaging revealed a 10 × 8 cm mass in the right atrium, obliterating the chamber and extending through the tricuspid valve into the right ventricle with further extension into the epicardial fat, compression of the inferior vena cava and superior vena cava, and extension across the left atrial roof (Figure 3). The patient was referred for urgent surgical tumor debulking, but intraoperative macroscopic examination revealed that tumor removal was not possible owing to the extent of invasion of cardiac and surrounding structures. The patient was then placed on extracorporeal membrane oxygenation and evaluated for chemotherapy. Pathology identified a peripheral mature T-cell lymphoma unlikely to be responsive to chemotherapeutic regimens. On further discussion with family, the decision was made to not pursue additional aggressive measures and he was transitioned to comfort care.
Figure 3.
Case 2: Cardiac magnetic resonance demonstrating large aggressive mass centered at right atrium and obliterating the lumen and with significant local invasion into inferior vena cava, superior vena cava, and left atrium.
Discussion
In this report we have presented 2 cases of cardiac lymphoma that presented with bradyarrhythmia. These cases encompass 2 ends of the spectrum of cardiac lymphoma and demonstrate the unusual fashion that patients may present, including metastatic cardiac lymphoma with large B-cell phenotype and primary cardiac lymphoma with T-cell morphology. By definition, these are both cardiac lymphomas because they presented with cardiac symptoms and in both cases, significant tumor bulk was localized to the heart and pericardium. The most common presenting symptoms and signs of cardiac lymphoma are dyspnea (64%), abdominal and chest discomfort (26% and 24%), acute heart failure (47%), and arrhythmia (61%: 23% atrial arrhythmia, 22% AV block, 11% bundle branch block, and 5% ventricular arrhythmias). The most common chamber of origin is the right atrium, and 58% will have pericardial effusions.9
The first of these cases is an example of the more common metastatic disease of the heart with evidence of metastatic tumor involvement throughout the body (typically B cell, as in this case9). This case presented as complete heart block with a junctional escape and lactic acidosis. A similar presentation was reported in a 66-year-old AIDS patient in 2014, who was also found to have diffuse large B-cell lymphoma with cardiac involvement.10 The patients in most previously reported cases of cardiac lymphoma with lactic acidosis did not survive.10 Other cases of diffuse large B-cell lymphoma resulting in complete heart block have shown restoration of AV nodal conduction with as little as 3 cycles of R-CHOP.7,11,12 Caramelli and Tellini13 reported cases of resolution of intermittent complete heart block with prednisone therapy. Here we describe a case of persistent complete heart block owing to cardiac lymphoma resolving with prednisone therapy alone. Tumor-associated inflammation and edema were likely responsible for AV block such that the anti-inflammatory effects of prednisone were enough to reverse this conduction disease. Alternatively, prednisone has a toxic effect on the lymphocyte population, and it may have been enough to reverse the tumor infiltration into the conduction system for this patient. In either case, prednisone therapy has the potential benefit of enabling treatment initiation prior to histologic diagnosis, as it did in this case.
The second of these cases is an example of an extremely rare primary cardiac lymphoma with T cell–specific phenotype. There are rare reports of primary cardiac T-cell lymphoma since 1949 and even more rare reports in an immunocompetent patient.6,14,15 In 1 case a 70-year-old woman presented with cardiac tamponade with a 500 mL pericardial effusion and subsequently developed complete AV block and torsades de pointes. There was recovery of conduction with CHOP chemotherapy.6 To our knowledge, our patient is the first reported case of cardiac lymphoma presenting with sinus node dysfunction. Macroscopic evidence of cardiac tumor was not evident until >4 months following initial pacemaker implant and subsequent lead revision despite transthoracic echocardiogram and CT scan. Although a small pericardial effusion was present with initial presentation, there was no increase in size, and therefore pericardiocentesis was not performed. Unlike previous case reports of primary cardiac lymphoma, our patient presented with sinus node dysfunction in addition to progressive atrial myopathy and associated paroxysmal atrial flutter. Furthermore, atrial myopathy preceded the presence of macroscopic cardiac tumor. Echocardiography has been the gold standard for diagnosing cardiac lymphoma since it was first reported in 1981, and its use increased the percentage of premortem cardiac lymphoma diagnoses from 46% to 85%.9 However, this case is an example of the limitations in this diagnostic modality with reported sensitivity of 73% for right atrial tumors.9 Unfortunately, the early indolent but rapid late presentation delayed diagnosis until his disease had progressed too far. Most likely microscopic, localized tissue changes associated with tumor infiltration resulted in progressive disruption of atrial electrical activity.
Though it is a rare diagnosis, patients presenting with B symptoms (fever, weight loss, fatigue, night sweats) with bradyarrhythmia should have cardiac lymphoma considered in the differential diagnosis. Cardiac imaging (including echocardiography and possibly cardiac magnetic resonance imaging) should be performed. Presence of an unexplained pericardial effusion should raise suspicion. Levels of lactate dehydrogenase and B2 microglobulins, which are often elevated in cardiac lymphoma, should be included in the evaluation when there is suspicion for cardiac lymphoma. Once the diagnosis is made, an immunohistochemistry evaluation is essential to determine overall prognosis and guide therapy.
Conclusion
Cardiac lymphoma, an extremely rare disease, is categorized as either primary or secondary cardiac lymphoma, of which secondary cardiac lymphoma is the most common. Of the secondary cardiac lymphoma, diffuse large B cell is the most common subtype.
We report 2 cases of cardiac lymphoma presenting with bradyarrhythmia. The first is a female patient with secondary cardiac lymphoma of B-cell origin who presented with complete heart block, cardiogenic shock, and lactic acidosis. With prompt diagnosis and therapy, the patient’s AV conduction improved within 3 days. The second case is a male patient with primary cardiac lymphoma of T-cell origin who presented with an acute exacerbation of heart failure and was subsequently found to have sinus node dysfunction followed by progressive atrial myopathy and paroxysmal atrial flutter. The diagnosis of lymphoma was delayed by 6 months as visible cardiac tumor on multiple imaging modalities was delayed and followed bradyarrhythmia presentation by 6 months. It is important to consider the spectrum of pathophysiology and presentation of these 2 types of cardiac lymphoma. The first case suggests that tumor-associated inflammation and interstitial edema could be responsible for the AV block, and that the anti-inflammatory effects of prednisone might be enough to reverse this conduction disease. The second case illustrates how microscopic localized tissue changes associated with tumor growth could lead to bradyarrhythmia but precede visible tumor burden.
Though it is a rare diagnosis, patients who present with B symptoms (fever, weight loss, fatigue, and night sweats) with an abnormal electrocardiogram finding should have cardiac lymphoma in the differential diagnosis.
Footnotes
Disclosures: Dr Gehi reports research funding from Bristol Myers Squibb Foundation and speaker’s honoraria from Zoll Medical, Abbott, and Biotronik.
References
- 1.Yater W.M. Tumors of the heart and pericardium. Arch Intern Med. 1931;48:627. [Google Scholar]
- 2.Brick I.B., Greenfield M. Reticulum cell sarcoma with cardiac metastasis; report of two cases with ante-mortem diagnosis of one. Am Heart J. 1947;34:599–611. doi: 10.1016/0002-8703(47)90536-x. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt D.L., Bonow R.O., Braunwald E., et al. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Elsevier; Philadelphia, PA: 2022. Ch 85: Tumors affecting the cardiovascular system; pp. 1863–1875. [Google Scholar]
- 4.Blondeau P. Primary cardiac tumors—French studies of 533 cases. Thorac Cardiovasc Surg. 1990;38:192–195. doi: 10.1055/s-2007-1014065. [DOI] [PubMed] [Google Scholar]
- 5.Grantomo J., Pratita J., Rachmat J., Saraswati M. A rare case of primary cardiac lymphoma and the role of early surgical debulking: a case report. Eur Heart J Case Rep. 2018;2:yty116. doi: 10.1093/ehjcr/yty116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang T.L., Lai C.H., Liou J.Y., Lo H.M., Shyu K.G. Complete AV block and torsades de pointes in a case of primary cardiac t-cell lymphoma. Acta Cardiol Sin. 2015;31:245–248. doi: 10.6515/ACS20141117A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giudici M.C., Sadler R.L., Robken J.A., Ahearn M.A., Sekharan R., Kovach G. Complete atrioventricular block due to large cell lymphoma: resolution with chemotherapy. Clin Cardiol. 1996;19:262–264. doi: 10.1002/clc.4960190326. [DOI] [PubMed] [Google Scholar]
- 8.Ceresoli G.L., Ferreri A.J., Bucci E., Ripa C., Ponzoni M., Villa E. Primary cardiac lymphoma in immunocompetent patients: diagnostic and therapeutic management. Cancer. 1997;80:1497–1506. doi: 10.1002/(sici)1097-0142(19971015)80:8<1497::aid-cncr18>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Petrich A., Cho S.I., Billet H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer. 2011;117:581–589. doi: 10.1002/cncr.25444. [DOI] [PubMed] [Google Scholar]
- 10.Ijaz M., Tariq H., Niazi M., Lovovsky D. Complete heart block and persistent lactic acidosis as an initial presentation of non-Hodgkin lymphoma in a critically ill newly diagnosed AIDS patient. Case Rep Crit Care. 2014;2014:214970. doi: 10.1155/2014/214970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho S.W., Kang Y.J., Kim T.H., et al. Primary cardiac lymphoma presenting with atrioventricular block. Korean Circ J. 2010;40:94–98. doi: 10.4070/kcj.2010.40.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crisel R.K., Knight B.P., Kim S.S. Reversible, complete atrioventricular block caused by primary cardiac lymphoma in a nonimmunocompromised patient. J Cardiovasc Electrophysiol. 2012;23:1386–1389. doi: 10.1111/j.1540-8167.2012.02343.x. [DOI] [PubMed] [Google Scholar]
- 13.Caramelli Z., Tellini R.R. Treatment of atrioventricular block with prednisone. Am J Cardiol. 1960;5:263–265. doi: 10.1016/0002-9149(60)90207-1. [DOI] [PubMed] [Google Scholar]
- 14.Motomatsu Y., Oishi Y., Matsunaga S., et al. Primary cardiac T-cell lymphoma localized in the mitral valve. Ann Thorac Surg. 2016;101:2363–2365. doi: 10.1016/j.athoracsur.2015.08.091. [DOI] [PubMed] [Google Scholar]
- 15.Sherkat R., Sabri M.R., Dehghan B., et al. EBV lymphoproliferative-associated disease and primary cardiac T-cell lymphoma in a STK4 deficient patient: a case report. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000008852. [DOI] [PMC free article] [PubMed] [Google Scholar]