Abstract
Objective.
To characterize the catchment area and patient profile of large cochlear implant (CI) centers in the United States.
Study Design.
Multi-institutional retrospective case series.
Setting.
Tertiary referral CI centers.
Methods.
Patients who underwent CI surgery at 7 participating CI centers between 2015 and 2020 were identified. Patients’ residential zip codes were used to approximate travel distances and urban vs rural residential areas.
Results.
Over the 6-year study period (2015–2020), 6313 unique CI surgical procedures occurred (4529 adult, 1784 pediatric). Between 2015 and 2019, CI procedures increased by 43%. Patients traveled a median 52 miles (interquartile range, 21–110) each way; patients treated at rural CI centers traveled greater distances vs those treated at urban centers (72 vs 46 miles, P <.001). Rural residents represented 61% of the patient population and traveled farther than urban residents (73 vs 24 miles, P <.001). Overall, 91% of patients lived within a 200-mile radius of the institution, while 71% lived within a 100-mile radius. In adults, multiple regression analysis redemonstrated an association between greater travel distances and (1) older age at the time of CI and (2) residential rural setting (both P <.001, r2 = 0.2).
Conclusions.
While large CI centers serve geographically dispersed populations, most patients reside within a 200-mile radius. Strategies to expand CI utilization may leverage remote programming, telemedicine, and strategic placement of new centers and satellite clinics to ameliorate travel burden.
Keywords: cochlear implant, catchment area, geographic, access to care, rural, urban, travel, barriers to care
Despite growing attention to the health and socioeconomic ramifications of untreated and undertreated hearing loss, hearing rehabilitation remains persistently low in the United States.1,2 Specifically, estimates of cochlear implant (CI) utilization among adults deemed to be audiologic candidates range from 2% to 13%, depending on the definition of audiologic candidacy.2–7 Prior work assessing barriers to care in this patient population has demonstrated considerable differences in access to care and utilization of hearing health resources across geographic regions.8 Greater geographic distance from a CI center has been linked to delayed implantation,9 reduced access to health care resources,10 and inconvenience for patients.11 As increasing emphasis has been placed on improving CI utilization and expanding hearing health services in the United States, a comprehensive understanding of the current CI geographic land-scape is warranted.
The relative scarcity of CI centers in certain areas of the United States has significant implications on locoregional variation in access to care. Strategies designed to improve access to care and CI utilization hinge on an understanding of the current status of the CI landscape and limitations posed by the variable geographic distribution of CI centers. While the implications of the relative scarcity of CI centers have been explored, the distances that patients are able and willing to travel for CI care have yet to be been quantified. Specifically, the catchment profiles of large CI centers, which contribute to a significant portion of CIs performed in the United States, has not been previously described. The objective of the present study was to characterize to the catchment profile and geographic reach of large CI centers in the United States.
Methods
Patient Population
Seven institutions participated in the study, representing a convenience sample of large CI centers in the United States that perform >100 adult and pediatric CI surgical procedures annually on average. A retrospective case review of all patients who underwent CI surgery between 2015 and 2020 was undertaken following Institutional Review Board approval per institution (Mayo Clinic, 20–011851; Vanderbilt University Medical Center, 192331; University of Miami, 20201389; University of North Carolina, 09–2328; Washington University in St Louis, 202012163; Johns Hopkins, 00188251; University of Michigan, HUM00191812). International patients as well as those undergoing revision CI surgery or explantation were excluded from the analysis. Patient demographics, including age at the time of surgery and residential zip code, were collected with clinical data, including year and type of CI surgery (ie, revision, bilateral simultaneous, or bilateral sequential). Adult patients were defined as ≥ 18 years of age. While patients who underwent implantation in 2020 were included for most analyses, the CI surgery number growth analysis included patients from 2015 to 2019 in an effort to avoid confounding growth patterns with the atypical surgical numbers during the COVID-19 pandemic in 2020.
US Census 2010 data were used to approximate the population density for each patient’s residential area.12 Population density information was unavailable for 45 (0.7%) patients; these individuals were excluded from analyses requiring population density data. Rural and urban residential categorization was determined for each patient according to population density by residential zip code: those with population density ≥ 1000 people per square mile were categorized as urban areas while others were categorized as rural, according to the 2010 Census Urban and Rural Classification Criteria.13 Similarly, institutions were categorized as being located in a rural or urban area based on the designation of the institutional zip code following the aforementioned criteria.
Geographic Analyses
All study patients were included for geographic analyses. Individuals undergoing bilateral simultaneous or sequential implantation were considered a single surgical event in an effort to avoid duplication of unique patients. For each patient, the distance from the institution in miles was calculated as that between the geographic latitude and longitude center point of each zip code (patient residential and institutional zip codes). Distance calculations were performed with the National Bureau of Economic Research Zip Code Distance Database 14 and Excel version 16.50 (Microsoft Corp). The distance between a patient’s home zip code and the institution served as a proxy for distance traveled for CI care. Patients were plotted on a US map according to the residential zip code in the medical record. Geographic maps used to depict patient location were generated through Tableau version 2020.4.1 (Tableau Software, LLC).
Statistical Analysis
Statistical analyses were performed with Prism version 9.1.0 (GraphPad Software, LLC) and Excel. Features following a normal distribution were summarized with means and standard deviations, while those without normal distribution were summarized with medians and interquartile ranges (IQRs). Wilcoxon rank sum test was used for variable comparison, while linear regression was used to test correlation between variables. All tests were 2-sided, and P values <.05 were considered statistically significant.
Results
Over the 6-year study period (2015–2020), 6313 unique adult and pediatric CI surgical procedures were performed across the 7 participating institutions. While the majority underwent unilateral implantation, 1269 (20.1%) received a second implant during the study period (265 bilateral simultaneous, 1004 bilateral sequential). Between 2015 and 2019, the number of CI surgical procedures performed across all participating institutions increased by 43.3%. Patient demographics are summarized in Table 1. The majority of patients (61%) resided in a rural area. Among all patients, the median distance between a patient’s residential location and the institution was 52 miles (IQR, 21–110). Adults lived farther away from the institution than pediatric patients (54 vs 49 miles, P = .005), although the mean difference was small (5 miles). Overall, 91% of individuals undergoing cochlear implantation during the study period lived within a 200-mile radius of the institution where they received care, while 71% lived within a 100-mile radius (Figure 1). There was no significant change in the travel distance over the 6-year study period (P = .4).
Table 1.
Patient Demographics and Large CI Center Catchment Areas.
| All | Adult | Pediatric | |
|---|---|---|---|
| Patients, No. (%) | 6313 | 4529 (71.7) | 1784 (28.3) |
| Age, y, median (IQR) | 56.2 (12.0–74.5) | 66.3 (52.7–76.0) | 4.0 (1.8–8.0) |
| Rural population, % | 61.0 | 63.1 | 55.1 |
| Distance from CI center, miles, median (IQR) | 52 (21–110) | 54 (21–114) | 49 (21–102) |
| Patients residing within distance radius of CI center, % | |||
| 200 miles | 91 | 90 | 94 |
| 150 miles | 86 | 84 | 89 |
| 100 miles | 71 | 70 | 74 |
Abbreviations: IQR, interquartile range; CI, cochlear implant.
Figure 1.
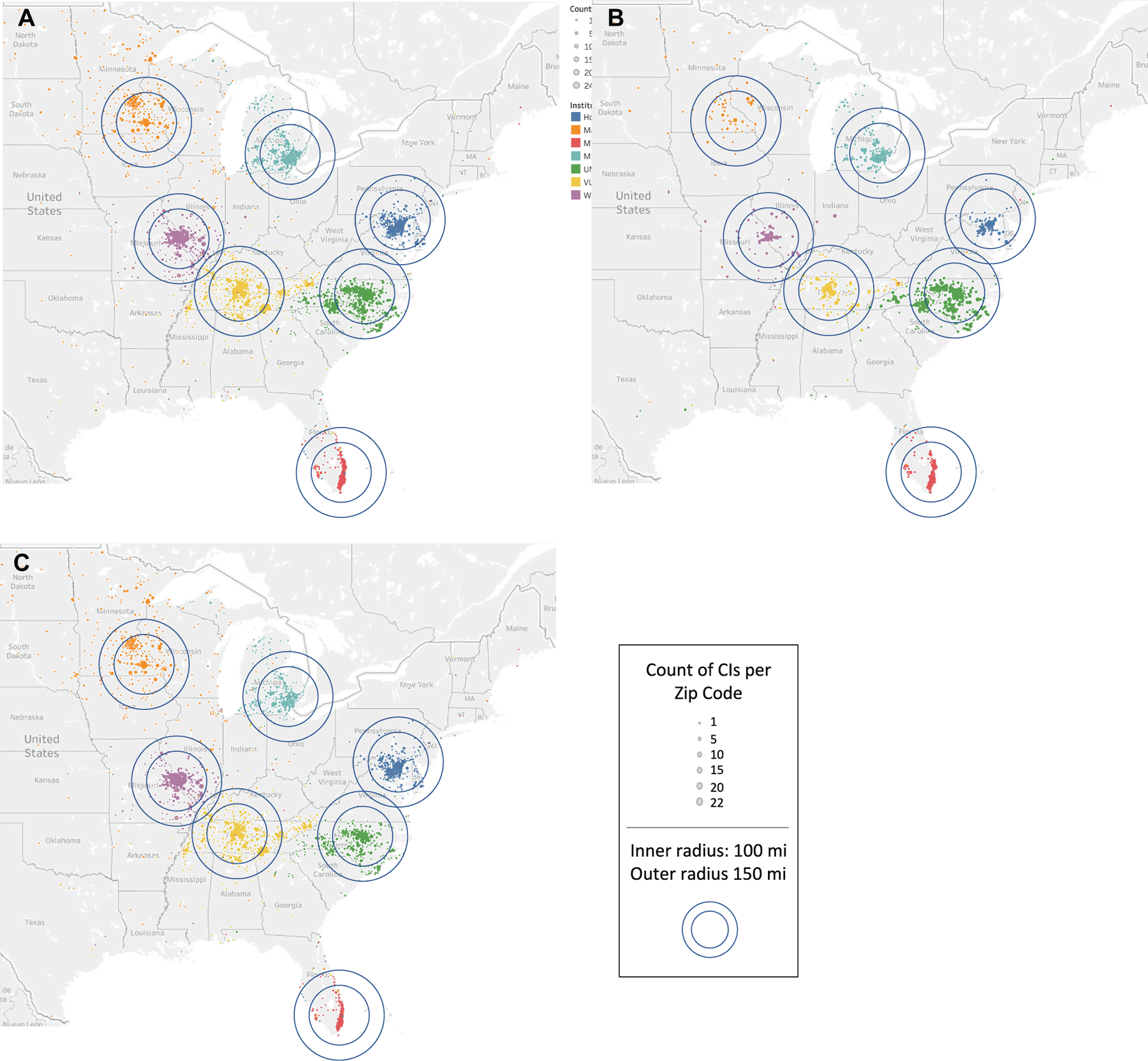
Large cochlear implant (CI) center catchment areas. Patients are represented geographically according to residential zip code and by institution where they received CI surgery (color): (A) all patients, (B) pediatric, and (C) adult.
The location of the institution, specifically within a rural or urban setting, was associated with patient location and setting. Of the 7 institutions participating in this study, 2 were in rural counties and 5 were in urban counties. CI centers in rural settings treated more patients in rural residential areas than centers in urban settings (70% vs 58%, P < .001; Figure 2). Patients treated at CI centers in rural settings traveled a greater distance to the institution than those treated in urban settings (72 miles [IQR, 34–164] vs 46 miles [IQR, 18–105], P <.001; Figure 3).
Figure 2.
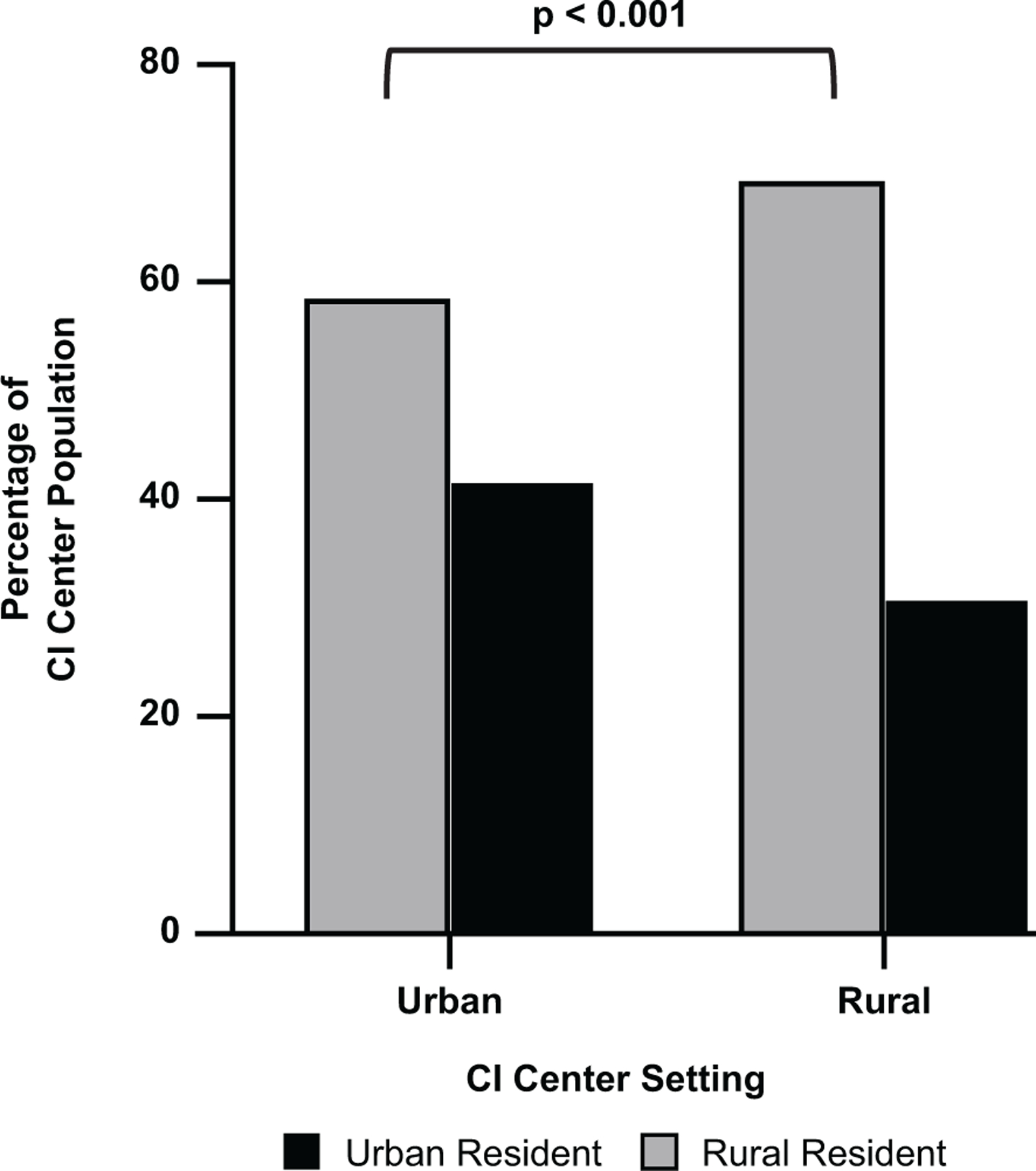
Cochlear implant (CI) centers in a rural setting treat a significantly greater percentage of patients from a rural residential area vs CI centers in an urban setting (70% vs 58%, P <.001).
Figure 3.
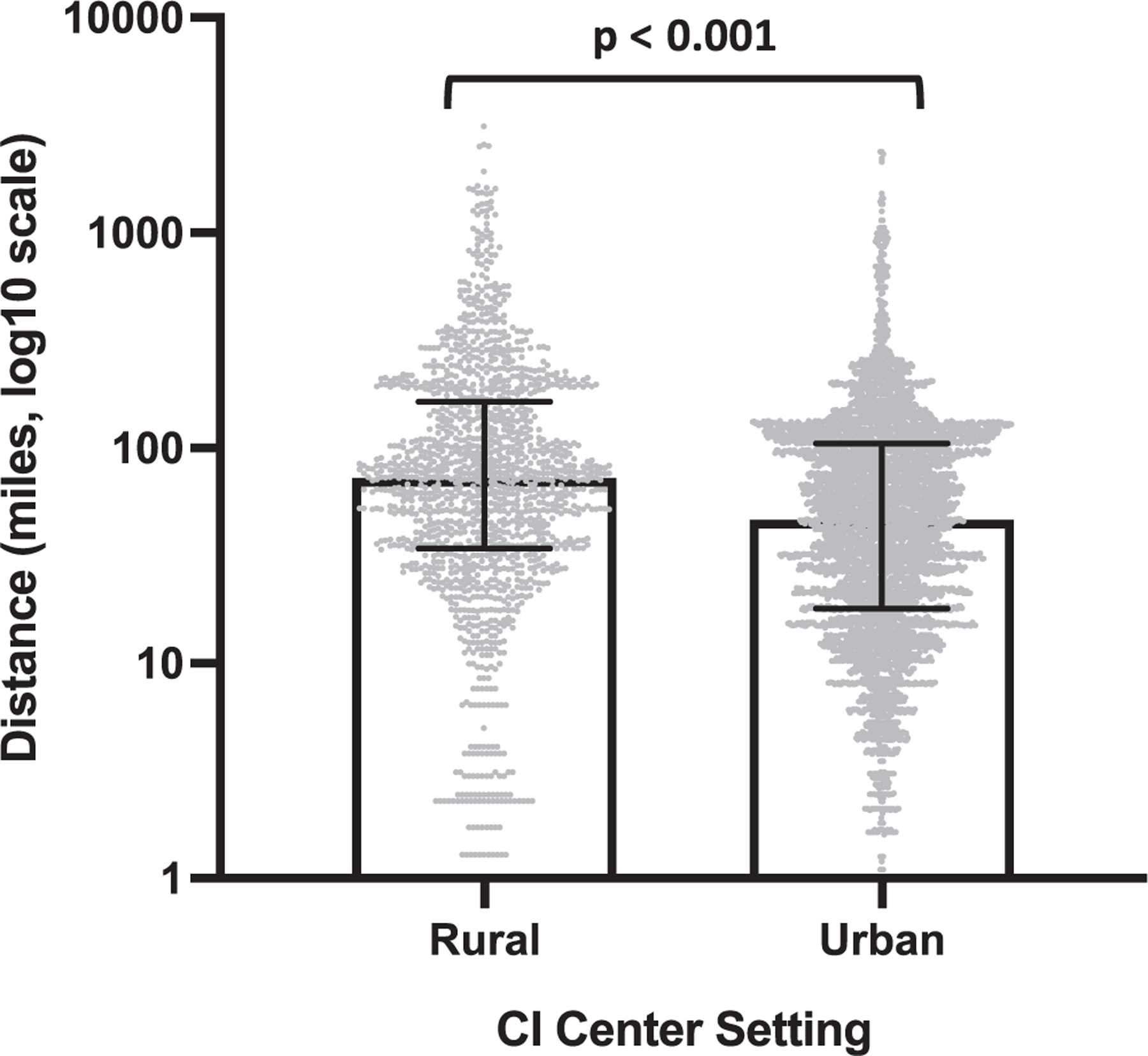
Patients receiving care at a cochlear implant (CI) center in a rural setting reside farther away from the institution than patients receiving care in an urban setting (72 vs 46 miles, P <.001). Values are presented as median (interquartile range).
Similarly, patient demographics and residential location, specifically within a rural or urban setting, played a role in geographic distance from large CI centers. Patients living in a rural zip code were more likely than urban residents to reside a farther distance from the CI center (73 miles [IQR, 36–125] vs 24 miles [IQR, 10–61], P <.001). A similar analysis evaluating zip code population density on a continuous scale confirmed the prior finding: patients living in areas with lower population density resided a greater distance from a CI center (P < .001, r2 = 0.18; Figure 4). Regarding adults, multiple regression analysis redemonstrated an association between distance from the institution and (1) age at the time of CI and (2) residential population density (both P < .001, r2 = 0.2). Specifically, older patients were more likely than younger patients to live in a rural residential area farther away from the institution.
Figure 4.
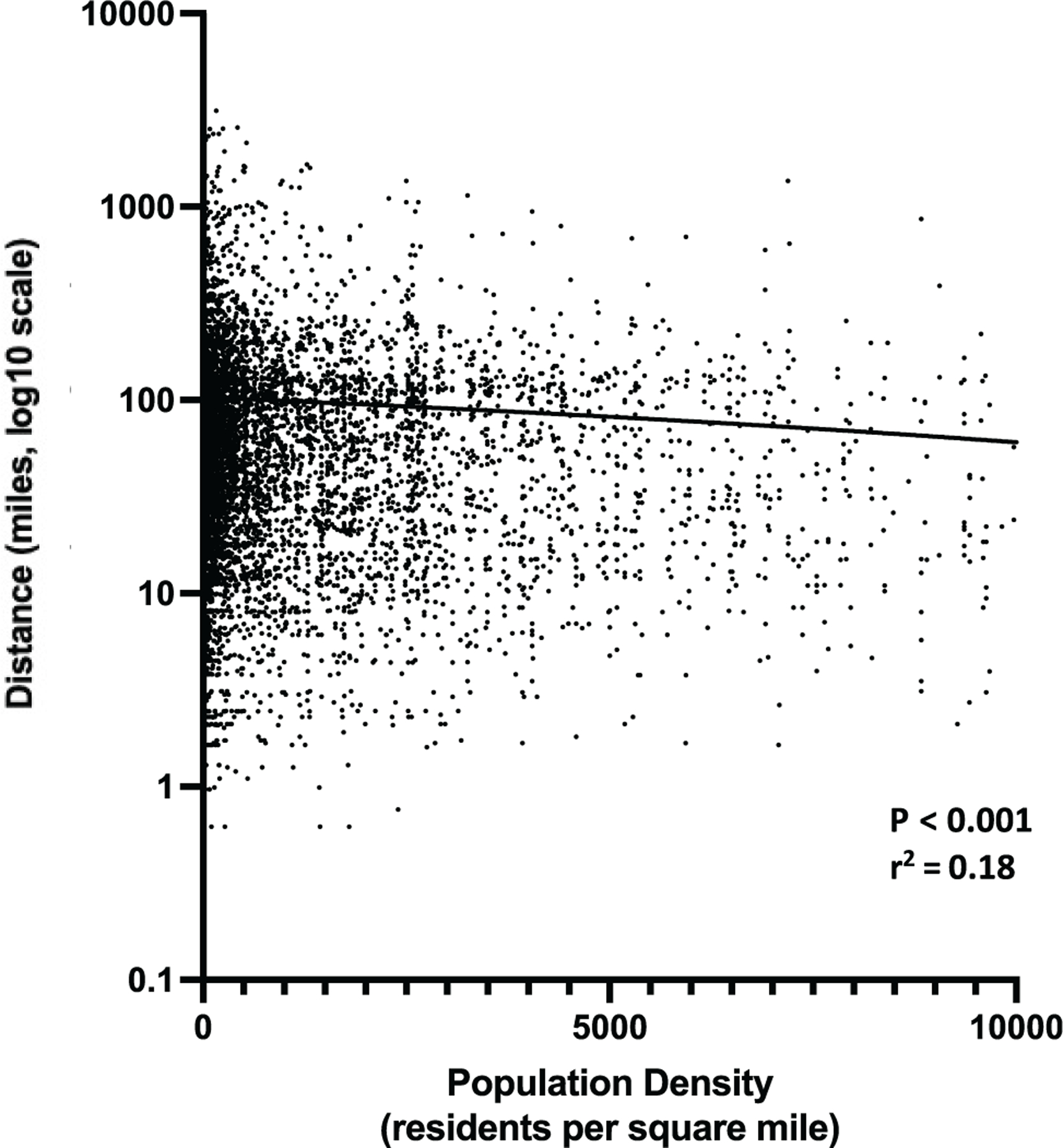
Residential areas with lower population density (ie, more rural areas) were associated with farther distance from cochlear implant centers (P <.001).
Discussion
While the large CI centers in this study demonstrated substantial growth during the study period, their catchment areas are generally limited to a 200-mile radius, which accounts for 91% of CI recipients receiving care at these institutions. Depending on the location of the institution, catchment areas may vary across institutions, with patients traveling greater distances to large CI centers in rural settings. This may be reflective of greater geographic dispersion of rural CI centers with fewer alternative care options for patients in these regions, although further work is required to describe this association. Similarly, the patients’ residential settings affect geographic barriers, as patients residing in rural zip codes travel a significantly greater distance to receive CI care (73 vs 24 miles, P < .001). Given that the majority of the adult CI patient population consists of older adults who live in rural residential areas, this geographic limitation may affect access to care for patients who do not live within the catchment area of a large or smaller CI center. Prior work has demonstrated travel time and distance, lost wages, and hotel costs as unique barriers for rural patients and those residing a greater distance from care centers.10,15,16 While outside the scope of this study, the role of socioeconomic status as it relates to patient residential geography may provide additional context for understanding the unique barriers to care faced by certain patient populations. For patients living in watershed areas, or areas outside the catchment areas for CI centers, these barriers may overwhelm efforts to seek and receive hearing health care. The quantification of large CI center catchment areas can be useful in designing strategies with thoughtful use and placement of local resources aimed to reduce geographic barriers to care.
Strategies aimed to improve CI utilization in the United States must consider health care accessibility from geographic and patient experience standpoints. Potential considerations include expanding the reach of large CI centers and increasing the number of smaller CI practices in watershed areas. When the expansion of large CI centers is considered as a potential strategy to reach additional patients, the ramifications associated with this strategy must also be considered, such as longer travel times and increased geographic distance for patients. At its current state in most practices, the CI care delivery model is a long and arduous process for patients, requiring up to 10 appointments from initial consultation to 1-year follow-up CI programming.11 One method to ameliorate the burden associated with travel is to coordinate appointments and utilize remote technology to minimize the number of trips required for CI care.17 While the COVID-19 pandemic has more recently encouraged an uptick in telemedicine, remote appointments and CI programming are not widely practiced at the time of this publication, which may in part be due to the lack of reimbursement for these services. A significant expansion of this type of highly coordinated care leveraging technology would be required to bridge gaps in geographic distribution of large CI centers.18–20 Along the same lines, satellite care sites (operated by otolaryngologists, audiologists, or industry partners) may be utilized to reduce geographic barriers and provide local outreach stations. Satellite centers have been successfully implemented to expand the reach of CI centers outside the United States, leading to reduced travel time for patients and fewer missed appointments.21 This may be an effective option for some large CI centers looking to have greater geographic impact while minimizing travel time for patients. While patients may not be able or willing to travel to large CI centers in the current state, strategies such as coordinated care, telemedicine, and satellite clinics may reduce barriers to care and increase the catchment area of these centers.
Smaller CI centers may play a significant role in improving access to care for patients. While the catchment areas of smaller CI centers have not been previously characterized, it can be assumed that smaller CI centers have variability in patient reach, depending on practice size, geographic location, and surrounding competition. Expanding the number of smaller CI centers, particularly into watershed areas, may be one strategy to overcome geographic barriers for patients. Unfortunately, the relative scarcity of smaller CI centers in certain areas in part reflects the financial difficulties faced by practices offering CI care. A survey of CI surgeons and audiologists revealed that Medicare and Medicaid reimbursement rates failed to cover the costs associated with surgery and aural rehabilitation, thus financially disincentivizing practices to provide CI care.22 While potential financial losses are better tolerated in larger institutions where costs can be distributed institutionally, smaller practices may not be able to tolerate a similar level of financial risk associated with CI care and therefore may not be able to treat such patients. Part of this risk may be mitigated through partnership across practices aimed to minimize costs through bulk implant purchasing.23 Partnership with CI manufacturers may provide an opportunity to reduce financial costs associated with CI care through shared resources. For longterm success, expansion of CI services will likely require a combination of strategies: coordinated care, utilization of telemedicine and remote programming, thoughtful selection of CI center locations and satellite clinics, and partnership across practices and with industry to maximize resources and mitigate financial risk.
While this study does characterize the catchment profile of large CI centers, it does not account for individual center variability. As evident in Figure 1, catchment profiles can be influenced by topography (nearby oceans and lakes), state and country borders, and surrounding competition. While this study was designed to quantify catchment profiles, information about market factors that affect catchment areas may be useful in future work aimed to expand programs. Additionally, this study included all patients treated at large CI centers, regardless of whether another center may have been more geographically convenient for the patient. In some cases, patients may have electively presented to the large CI center for reasons other than geographic convenience, such as program reputation, referral patterns, or institutional familiarity. Additionally, while our study was able to characterize distance traveled based on geographic locations, it was not statistically powered to identify a difference in rates of bilateral simultaneous or sequential implants based on patient geographic location. In the future, a broader collaboration across institutions may allow for adequate pooling of data to evaluate the association of bilateral implantation with geographic distribution of patients and CI centers. Moreover, the study period included a portion of time that was affected by the COVID-19 pandemic. While no major shifts in patient or institutional catchment profile characteristics were noted during that time, there was a temporary decrease in CI surgery case volume. Consequently, data from the year 2020 were excluded when program growth was calculated over time. Finally, 45 patients (0.7%) across all 7 institutions had residential zip codes for which population density was not available in the 2010 census database. While these missing data may allow for selection bias, the proportion of missing data is small and unlikely to influence the conclusions of the study.
Conclusion
Large CI centers serve geographically dispersed patient populations. As large CI centers continue to grow, significant consideration to geographic barriers faced by patients, particularly those residing in rural regions, is required. Strategies to expand CI utilization and catchment areas must take travel burden and access to local care into consideration.
Funding source:
Nick S. Andresen is funded by a National Institutes of Health grant (T32DC000027), and this study was consequently supported in part by funding from it.
Footnotes
This article was presented at the 2021 AAO-HNSF Annual Meeting & OTO Experience; October 5, 2021; Los Angeles, California.
Disclosures
Competing interests: Ashley M. Nassiri—research funding from Cochlear Americas. Meredith A. Holcomb—consultant for Advanced Bionics, Med-El, Cochlear; research funding from Cochlear; instructor for audiology course at the Institute of Cochlear Implant Training. Sandra M. Prentiss—research funding from Med-El Corporation; consultant for Pipeline Therapeutics. Cameron C. Wick—consultant for Stryker and Cochlear Americas. Andrea L. Bucker, Kevin D. Brown—Advisory Board for Med-El. Teresa A. Zwolan—Advisory Board and research support for Cochlear; Advisory Board for Envoy Medical. David S. Haynes— consultant for Cochlear, Med-El, Advanced Bionics, Anspach. Aniket A. Saoji—research funding from Cochlear Americas and Advanced Bionics; consultant for Advanced Bionics, Oticon Medical, and Envoy Medical.
Matthew L. Carlson—research funding from Cochlear Americas.
Sponsorships: None.
References
- 1.Cunningham LL, Tucci DL. Hearing loss in adults. N Engl J Med 2017;377(25):2465–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute on Deafness and Other Communication Disorders. Quick statistics about hearing Accessed November 1, 2020. https://www.nidcd.nih.gov/health/statistics/quick-statistics-hearing#10
- 3.Sorkin DL. Cochlear implantation in the world’s largest medical device market: utilization and awareness of cochlear implants in the United States. Cochlear Implants Int 2013;14(suppl 1):S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorkin DL, Buchman CA. Cochlear implant access in six developed countries. Otol Neurotol 2016;37(2):e161–e164. [DOI] [PubMed] [Google Scholar]
- 5.iData Research Inc. US Market for Hearing Aids and Audiology Devices iData Research Inc; 2010. [Google Scholar]
- 6.iData Research Inc. US Market Report Suite for Hearing Devices iData Research Inc; 2016. [Google Scholar]
- 7.Nassiri AM, Sorkin DL, Carlson ML. Current estimates of cochlear implant utilization in the United States Presented at: American Cochlear Implant Alliance Virtual Conference; April 30, 2021. [DOI] [PubMed] [Google Scholar]
- 8.Hixon B, Chan S, Adkins M, Shinn JB, Bush ML. Timing and impact of hearing healthcare in adult cochlear implant recipients: a rural-urban comparison. Otol Neurotol 2016;37(9):1320–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzpatrick EM, Ham J, Whittingham J. Pediatric cochlear implantation: why do children receive implants late? Ear Hear 2015;36(6):688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shayman CS, Ha YM, Raz Y, Hullar TE. Geographic disparities in US veterans’ access to cochlear implant care within the Veterans Health Administration system. JAMA Otolaryngol Head Neck Surg 2019;145(10):889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nassiri AM, Yawn RJ, Gifford RH, et al. Same-day patient consultation and cochlear implantation: innovations in patient-centered health care delivery. Otol Neurotol 2020;41(2):e223–e226. [DOI] [PubMed] [Google Scholar]
- 12.US Census Bureau. Zip code tabulation Published 2020. Updated August 26, 2020. Accessed January 11, 2021. www.census.gov/programs-surveys/geography/guidance/geo-areas/zctas.html
- 13.US Census Bureau. 2010 census urban and rural classification and urban area criteria Published 2019. Updated December 2, 2019. Accessed January 11, 2021. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- 14.National Bureau of Economic Research. Zip code distance database Published 2010. Updated 2016. Accessed January 11, 2021. https://www.nber.org/research/data/zip-code-distance-database
- 15.Brems C, Johnson ME, Warner TD, Roberts LW. Barriers to healthcare as reported by rural and urban interprofessional providers. J Interprof Care 2006;20(2):105–118. [DOI] [PubMed] [Google Scholar]
- 16.Elpers J, Lester C, Shinn JB, Bush ML. Rural family perspectives and experiences with early infant hearing detection and intervention: a qualitative study. J Community Health 2016; 41(2):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nassiri AM, Saoji AA, Haynes DS, Carlson ML. Complete cochlear implant care (CCIC) requiring a single on-site visit: patient-centered care using telehealth Presented at: American Cochlear Implant Alliance Virtual Conference; April 29, 2021. [Google Scholar]
- 18.Meeuws M, Pascoal D, Janssens de Varebeke S, De Ceulaer G, Govaerts PJ. Cochlear implant telemedicine: remote fitting based on psychoacoustic self-tests and artificial intelligence. Cochlear Implants Int 2020;21(5):260–268. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Jeon S, Kim D, Shin Y. A review of contemporary teleaudiology: literature review, technology, and considerations for practicing. J Audiol Otol 2021;25(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhutta MF, Swanepoel W, Fagan J. ENT from afar: opportunities for remote patient assessment, clinical management, teaching and learning. Clin Otolaryngol Published online April 19, 2021. doi: 10.1111/coa.13784 [DOI] [PMC free article] [PubMed]
- 21.Arumugam SV, Thirugnanam S, Paramasivan VK, Pradananga RB, Nithya, Kameswaran M. Satellite habilitation centres following cochlear implantation: are they the way ahead in improving outcomes in developing countries? Int J Pediatr Otorhinolaryngol 2021;144:110606. [DOI] [PubMed] [Google Scholar]
- 22.Garber S, Ridgely MS, Bradley M, Chin KW. Payment under public and private insurance and access to cochlear implants. Arch Otolaryngol Head Neck Surg 2002;128(10):1145–1152. [DOI] [PubMed] [Google Scholar]
- 23.Nassiri AM, Garrett CG, Dail TL, et al. Should I buy this? A decision-making tool for surgical value-based purchasing. Otolaryngol Head Neck Surg 2020;163(3):397–399. [DOI] [PubMed] [Google Scholar]


