Abstract
The present study aimed to investigate the potential probiotic properties of six lactic acid bacteria (LAB) intended for human use, Lactobacillus rhamnosus ATCC 53103, Lactobacillus casei Shirota, Lactobacillus bulgaricus, L. rhamnosus LC 705, Bifidobacterium lactis Bb12, and Lactobacillus johnsonii La1, and one for animal use, Enterococcus faecium Tehobak, for use as a fish probiotic. The strains for human use were specifically chosen since they are known to be safe for human use, which is of major importance because the fish are meant for human consumption. The selection was carried out by five different methods: mucosal adhesion, mucosal penetration, inhibition of pathogen growth and adhesion, and resistance to fish bile. The adhesion abilities of the seven LAB and three fish pathogens, Vibrio anguillarum, Aeromonas salmonicida, and Flavobacterium psychrophilum, were determined to mucus from five different sites on the surface or in the gut of rainbow trout. Five of the tested LAB strains showed considerable adhesion to different fish mucus types (14 to 26% of the added bacteria). Despite their adhesive character, the LAB strains were not able to inhibit the mucus binding of A. salmonicida. Coculture experiments showed significant inhibition of growth of A. salmonicida, which was mediated by competition for nutrients rather than secretion of inhibitory substances by the probiotic bacteria as measured in spent culture liquid. All LAB except L. casei Shirota showed tolerance against fish bile. L. rhamnosus ATCC 53103 and L. bulgaricus were found to penetrate fish mucus better than other probiotic bacteria. Based on bile resistance, mucus adhesion, mucus penetration, and suppression of fish pathogen growth, L. rhamnosus ATCC 53103 and L. bulgaricus can be considered for future in vivo challenge studies in fish as a novel and safe treatment in aquaculture.
Aeromonas salmonicida subsp. salmonicida is the causative agent of the fish disease called furunculosis. It is one of the most common fish diseases in Finland, along with vibriosis, caused by Vibrio anguillarum. These diseases may cause major economic losses in hatcheries. The port of entry of these pathogens has not been identified, but the gastrointestinal tract has been implicated as a site of colonization and a possible port of entry (8). The third major fish disease, cold water disease, is caused by Flavobacterium psychrophilum (4), which affects primarily juvenile salmonid fish (13).
Currently, either treatment with chemotherapeutic agents or vaccination is used to protect fish against different bacterial diseases in hatchery conditions. The former method may alter the profile of a healthy gut microflora, while the latter is stressful for fish; both methods may enable the access of some pathogens. The use of chemotherapeutic agents has also led to occurrence of resistant bacteria (22), and thus their use should be restricted. Both methods are also quite expensive. Preventing diseases in juvenile fish is of significant economic importance, since small fish have high mortality rates and are too small for vaccination.
Probiotics may provide an alternative way to reduce the use of antibiotics in aquaculture and simultaneously avoid the development of antibiotic-resistant bacteria. Probiotics are microbial cell preparations or components of microbial cells that have a beneficial effect on the health and well-being of the host (19). Selected probiotics have been shown to have significant health benefits for humans, and thus several well-characterized strains are available for human use to reduce the risk of gastrointestinal infections or to treat such infections (18, 19). Probiotics may provide a potential support or alternative to vaccinations and treatment with antibiotics in fish farming.
Adhesion to the intestinal mucosa is regarded a prerequisite for colonization (3) and is one of the main selection criteria for new probiotic strains (7, 18). Adhesion to and colonization of the mucosal surfaces are possible protective mechanisms against pathogens through competition for binding sites and nutrients (25), steric hindrance, or immune modulation (18). Other important characteristics for potential probiotics are tolerance to low pH and bile (7, 18, 19). The probiotic strains tested here are acid resistant. Resistance to fish bile has not been tested earlier.
New probiotic candidates for fish have been investigated in many studies; these include Vibrio alginolyticus (1), Carnobacterium sp. (17), Carnobacterium sp. strain K (9), and unidentified strains (15). The aim of the present study was to examine the potential of human probiotics for use in fish because these probiotics have documented health effects and have been shown to be safe for humans. The latter is of major importance since the fish are farmed mainly for human consumption. These extensively studied probiotics may also have applications in fish farming.
MATERIALS AND METHODS
Adhesion analysis.
V. anguillarum 1-284, F. psychrophilum T1-1, and A. salmonicida SN1 were isolated from rainbow trout (Oncorhynchus mykiss) at the Institute of Parasitology, Department of Biology, Åbo Akademi University, Turku, Finland, during a natural outbreak of the diseases. V. anguillarum and A. salmonicida were grown in tryptic soy broth (TSB; Difco) overnight at 20°C, while F. psychrophilum was grown in TYES broth (0.4% tryptone, 0.05% yeast extract, 0.05% MgSO4 · 7H2O, 0.02% CaCl2 · 2H2O [pH 7.2]) for 2 days at 15°C with agitation. The following probiotic strains were used: Lactobacillus rhamnosus ATCC 53103, Lactobacillus casei Shirota, Lactobacillus delbrueckii subsp. bulgaricus ATCC 11842, L. rhamnosus LC 705, Bifidobacterium lactis Bb12, and Lactobacillus johnsonii La1 (all intended for human use) plus Enterococcus faecium Tehobak (intended for animal use). The strains were a generous gift from M. Saxelin (Valio Ltd., Helsinki, Finland). All probiotic strains were grown under anaerobic conditions in MRS broth (Merck, Darmstadt, Germany) overnight at 37°C. To the medium, tritiated thymidine ([methyl-1,2-3H]thymidine; 10 μl/ml, 117 Ci/mmol) was added to metabolically radiolabel the bacteria. After incubation, the cells were harvested by centrifugation (2,000 × g), washed twice with phosphate-buffered saline (PBS; 10 mM phosphate [pH 7.2]), and resuspended in PBS. The absorbance at 600 nm was adjusted to 0.25 ± 0.05 in order to standardize the number of bacteria (107 to 108 CFU/ml). The relationship between absorbance at 600 nm and CFU per milliliter was established by flow cytometry (23).
Mucus preparation.
The fish used for the preparation of mucus and bile were obtained from the Finnish Game and Fisheries Research Institute, Laukaa, Finland. They were kept in quarantine in freshwater for 2 weeks without any signs of disease. Mucus samples were isolated from four 400-g healthy rainbow trout immediately after sacrifice according to the method of Cohen and Laux (6). The mucus was obtained by gently scraping the surfaces with a rubber spatula into a small amount of HEPES (10 mM; pH 7.4)-buffered Hanks' balanced salt solution (HH). The skin mucus was collected from the whole body, and gill mucus was isolated after removing the gills. For intestinal mucus, the intestine was separated from the internal organs and divided in three parts—esophagus, stomach, and intestine—from which mucus was collected separately. The mucus samples were stored in 1-ml aliquots at −70°C until use.
Mucus characterization.
Before use, the protein concentration was determined by a modification of the method of Lowry et al. (11) as described by Miller and Hoskins (14), using bovine serum albumin (BSA; Sigma, St. Louis, Mo.) as a standard. The mucus was used at a protein concentration of 0.5 mg/ml in HH.
In vitro adhesion assay.
Adhesion of the radioactively labeled bacteria was determined as described by Kirjavainen et al. (10). In brief, mucus was immobilized on microtiter plate wells by overnight incubation at 4°C. Excess mucus was removed by washing with HH. Radioactively labeled bacteria (see above) were added, and the wells were incubated for 1 h at 37°C. Nonbound bacteria were removed by washing with HH. Bound bacteria were released and lysed by incubation at 60°C for 1 h with 1% sodium dodecyl sulfate (SDS) in 0.1 M NaOH. Adhesion was assessed by quantitating the amount of radioactivity by liquid scintillation and was expressed as the percentage of radioactivity recovered after adhesion relative to the radioactivity in the bacterial suspension added to the immobilized mucus. Adhesion of the bacteria was determined in at least three independent experiments for each mucus type, and each assay was performed in triplicate to correct for intra-assay variation.
Nonspecific adhesion of probiotic bacteria.
To determine if the observed mucus adhesion of the probiotic bacteria was due to nonspecific adhesion, adhesion of the strains to BSA, gelatin, and polystyrene was assessed. Adhesion to BSA, gelatin, and polystyrene was determined as described above for mucus.
Competitive adhesion.
Labeling and culture conditions were as described above for the in vitro adhesion assay. Nonlabeled probiotic bacteria (100 μl; 107 to 108 CFU/ml) were allowed to bind to the immobilized mucus, for 1 h at 37°C. Nonbound probiotic bacteria were washed away with PBS. Subsequently, 100 μl of labeled A. salmonicida SN1 was added to the wells and incubated for 1 h at 20°C. After unbound labeled bacteria were washed away, bound bacteria were released and lysed by incubation at 60°C for 1 h with 1% SDS in 0.1 M NaOH. The adhesion of A. salmonicida was determined as described above for the in vitro adhesion assay. Since the mucus adhesion of F. psychrophilum and V. anguillarum was found to be very low (1% or less), no competitive exclusion by probiotic bacteria was assessed for these strains.
Growth inhibition by spent culture liquid.
To assess the production of possible antimicrobial substances, five of the probiotic bacteria which exhibited good adhesion to intestinal mucus, L. rhamnosus ATCC 53103, E. faecium Tehobak, L. bulgaricus, B. lactis Bb12, and L. johnsonii La1, were grown in 10 ml of MRS overnight at 37°C. The bacteria were removed by centrifugation (2,000 × g), and spent culture supernatants were sterilized by passage through 0.22-μm-pore-size filters. After sterilization, half (5 ml) of each spent culture supernatant was neutralized (pH 7.0) with 5 N NaOH.
The fish pathogens V. anguillarum and A. salmonicida were grown in 1 ml of TSB overnight at 20°C. The cells were harvested by centrifugation (2,000 × g), washed twice with PBS, and resuspended in 1 ml of PBS. The bacterial suspensions were transferred evenly on tryptic soy agar (TSA; Difco) plates. Four wells were made in each agar plate with a sterile pasteur pipette; 50 μl of neutralized and 50 μl of untreated spent culture supernatant from the five different lactic acid bacteria were added to the wells. In two wells, neutralized MRS and MRS (pH 5.59) were added to determine possible inhibitory activity of the medium. After aerobic incubation for 3 days at room temperature, the clearing zone was determined.
Growth inhibition by coculture.
Competition for nutrients is one of the mechanisms by which probiotics are thought to affect pathogenic microorganisms. This was assessed by growing each probiotic strain with either A. salmonicida or V. anguillarum in coculture. F. psychrophilum was not assessed in coculture because its optimal growth temperature is below the temperature range of the probiotic strains. The probiotic strains will thus not cause competition.
Overnight cultures of the fish pathogens A. salmonicida SN1 and V. anguillarum 1-284 were washed twice with PBS, and cell concentrations were adjusted to an absorbance at 600 nm of 0.5. Overnight cultures of probiotic bacteria L. rhamnosus ATCC 53103, L. johnsonii La1, L. casei Shirota, L. bulgaricus, L. rhamnosus LC 705, B. lactis Bb12, and E. faecium Tehobak were treated similarly. Of each probiotic strain and pathogen, 100 μl of bacterial suspension was mixed in 1 ml of TSB and incubated for 2 days at 20°C. As a control, 100 μl of PBS and pathogen suspension in TSB was used. After incubation, the number of cells in each sample was determined by spreading appropriate dilutions on MRS, TSA, and TCBS plates, for probiotic, A. salmonicida, and V. anguillarum enumeration, respectively. The results are expressed as percentage of pathogen growth in coculture with a probiotic strain compared to growth on its own (control).
Fish bile resistance.
Bacterial suspensions were prepared in PBS, and the absorbance at 600 nm was adjusted to 0.25 as described above. A 500-μl aliquot of each bacterial suspension was centrifuged and resuspended in sterile PBS or in sterile PBS with 10% fish bile. The bile was collected from rainbow trout by puncturing the gallbladder and stored at −20°C until use. Samples were incubated for 1.5 h at 37°C. After incubation, samples were serially diluted in sterile PBS, and viable counts were determined by plate counting using MRS agar and TSA for the probiotic strains and fish pathogens, respectively.
Mucus penetration.
The intestinal mucus was diluted to a protein concentration of 3 mg/ml with HH. After dilution, the penetration assay was performed as described by Cohen and Laux (6). In short, 50 μl of diluted mucus sample was added to microtiter plate wells. On top of the mucus samples, 20 μl of radiolabeled bacterial suspension was applied; the bacterial suspensions were prepared as described above. The wells of the microtiter plate (two wells for each bacterial sample and each incubation time) were incubated for 1.5, 3, 4.5, and 6 h at room temperature. After incubation, the wells were emptied. To lyse the penetrated bacteria, 200 μl of 1% SDS–0.1 M NaOH was added to each well, and the plate was incubated at 60°C for 1 h. The lysate was removed from the wells and mixed with scintillation liquid (OptiPhase HiSafe 3; Wallac, Loughborough, United Kingdom), and the radioactivity was measured by liquid scintillation counting. The proportion of penetrated bacteria was assessed as the percentage of radioactivity recovered from the wells compared to the radioactivity of the added bacterial suspension on top of the mucus.
Statistical analysis.
All results are shown as the average of at least three independent experiments; variation is expressed as standard deviation. Student's t test was used to determine the significant difference (P < 0.05) between different tested groups.
RESULTS
Adhesion of pathogens.
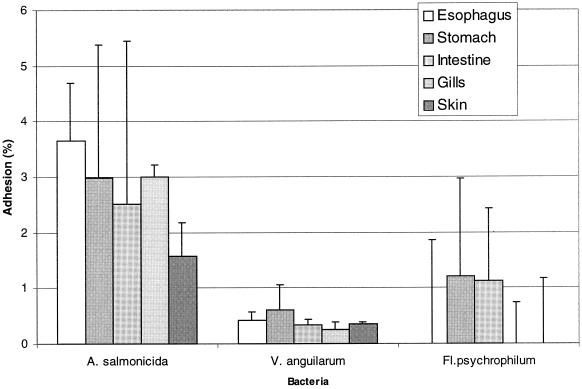
All three tested fish pathogen strains tended to adhere in relatively low numbers or not at all to the five different fish mucus preparations tested (0 to 3.7% adhesion [Fig. 1]). The lowest adhesion was observed for F. psychrophilum, which adhered below the detection limit to mucus isolated from the skin, esophagus, and gills. The tested fish pathogens did not show preferential binding to mucus preparations isolated from different sites on the surface or in the gut from rainbow trout (P > 0.05).
FIG. 1.
Adhesion of three radioactively labeled fish pathogens, A. salmonicida, V. anguillarum, and F. psychrophilum, to mucus preparations from five different sites on the surface or in the gut of rainbow trout. Adhesion is expressed as percentage of radioactivity recovered from the immobilized mucus compared to radioactivity added to the mucus; error bars indicate SD.
Adhesion of probiotics.
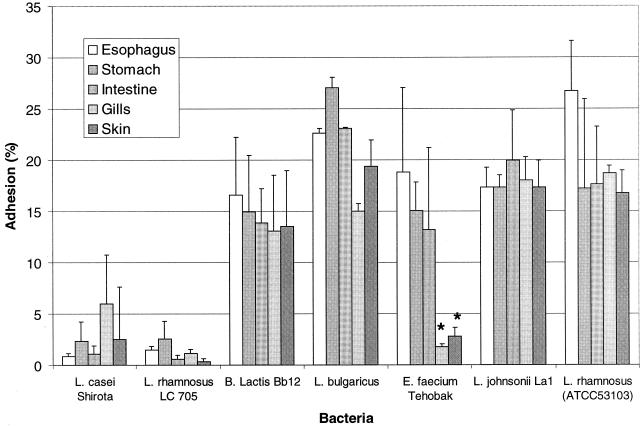
The probiotic bacteria strains L. rhamnosus ATCC 53103, L. bulgaricus, B. lactis Bb12, and L. johnsonii La1 tended to adhere in high numbers to the different fish mucus types (13.1 to 27.1% adhesion [Fig. 2]).
FIG. 2.
Adhesion of probiotic lactic acid bacteria to various fish mucus types. Adhesion is expressed as percentage of radioactivity recovered from the immobilized mucus compared to radioactivity added to the mucus; error bars indicate SD. Adhesion of E. faecium to mucus from gill and skin is significantly (∗, P < 0.0001) different from mucus from esophagus, stomach, and intestine.
L. casei Shirota and L. rhamnosus LC 705 adhered to fish mucus in low numbers (0.3 to 5.9% adhesion [Fig. 2]). E. faecium was found to adhere well to mucus from the intestine, stomach, and esophagus (around 15%) but significantly less (P < 0.0001) to the mucus from gills and skin (around 2.5%) (Fig. 2). For the other tested probiotic strains, no significant difference in adhesion to the five different mucus preparations was observed. The adhesion of L. casei Shirota to all tested mucus preparations was significantly (P < 0.05) different from that of B. lactis Bb12, L. bulgaricus, L. johnsonii La1, and L. rhamnosus ATCC 53103. The adhesion of L. rhamnosus LC 705 to all tested mucus preparations was significantly (P < 0.01) different from that of B. lactis Bb12, L. bulgaricus, L. johnsonii La1, L. rhamnosus ATCC 53103, and E. faecium.
Nonspecific adhesion.
Each tested strain adhered similar to BSA and gelatin (P > 0.05). B. lactis Bb12, L. bulgaricus, and L. rhamnosus ATCC 53103 adhered significantly better to BSA and gelatin than the other strains. With the exception of L. casei Shirota, all strains adhered well to polystyrene. The strains that were observed to bind well to intestinal mucus, L. bulgaricus, B. lactis Bb12, L. johnsonii La1, L. rhamnosus ATCC 53103, and E. faecium, adhered significantly less to gelatin and BSA (Table 1).
TABLE 1.
Nonspecific adhesion of probiotic bacteria to the gelatin, BSA, and polystyrene
| Probiotic bacterium | % Adhesiona (SD)
|
|||
|---|---|---|---|---|
| BSA | Gelatin | Polystyrene | Intestinal mucus | |
| L. rhamnosus LC 705 | 0.4 (0.3) | 0.2 (0.2) | 11.2 (3.6) | 0.6 (0.4) |
| B. lactis Bb12 | 5.6 (1.5) | 5.4 (0.6) | 29.5 (9.7) | 14.0 (3.3) |
| L. bulgaricus | 5.7 (0.9) | 6.3 (0.4) | 25.7 (6.4) | 23.1 (0.1) |
| E. faecium Tehobak | 1.6 (1.1) | 0.9 (0.6) | 19.3 (10.6) | 13.2 (8.0) |
| L. rhamnosus ATCC 53103 | 6.2 (0.8) | 6.3 (1.6) | 19.6 (12.5) | 17.6 (5.6) |
| L. johnsonii La1 | 3.0 (1.0) | 3.2 (2.4) | 23.6 (2.5) | 20.0 (4.9) |
| L. casei Shirota | 1.3 (1.1) | 1.6 (1.4) | 3.0 (0.6) | 1.1 (0.8) |
Percentage of radioactivity recovered from wells compared to radioactivity of the added bacteria.
Competitive adhesion.
The tested fish pathogen A. salmonicida tended to adhere in similar numbers to immobilized fish mucus preparations (0.5 to 2.4% adhesion) regardless of the prior binding of any of the tested probiotic bacteria (P > 0.05) (Table 2).
TABLE 2.
Competition of probiotic lactic acid bacteria toward A. salmonicida for adhesion to fish intestinal and skin mucus
| Probiotic bacteria | % A. salmonicida adhesiona (SD)
|
|
|---|---|---|
| Skin | Intestine | |
| None (buffer) | 1.6 (0.6) | 2.5 (2.9) |
| L. rhamnosus LC 705 | 0.8 (0.1) | 1.7 (0.7) |
| B. lactis Bb12 | 1.4 (0.4) | 2.4 (0.5) |
| L. bulgaricus | 0.4 (0.2) | 1.6 (0.4) |
| E. faecium Tehobak | 0.9 (0.1) | 1.5 (0.3) |
| L. rhamnosus ATCC 53103 | 2.0 (0.3) | 2.4 (0.9) |
| L. johnsonii La1 | 0.7 (0.1) | 1.1 (0.3) |
| L. casei Shirota | 1.4 (0.1) | 0.5 (0.1) |
Percentage of radioactivity recovered from immobilized mucus and compared to radioactivity of the added bacteria.
Growth inhibition by spent culture liquid.
After incubation of pathogens on TSA plates, no measurable clearing zones were detected around the wells filled with neutralized or nonneutralized spent culture liquid from the tested probiotic lactic acid bacteria.
Growth inhibition by coculture.
Upon 24 h growth in coculture, all tested probiotic bacteria were found to significantly inhibit the growth A. salmonicida (P < 0.05). However, only L. johnsonii La1 and L. casei Shirota significantly inhibited the growth of V. anguillarum (Table 3).
TABLE 3.
Effects of probiotics on the growth of A. salmonicida and V. anguillarum in coculture
| Probiotic bacterium | % Growth in coculturea (SD)
|
|
|---|---|---|
| A. salmonicida | Vibrio anguillarum | |
| L. johnsonii La1 | −59.0 (25.9) | −49.0 (13.7) |
| L. rhamnosus LC 705 | −61.3 (25.5) | −11.0 (30.7) |
| B. lactis Bb12 | −43.0 (13.4) | −26.0 (34.2) |
| L. rhamnosus ATCC 53103 | −42.5 (35.9) | 4.3 (67.4) |
| L. bulgaricus | −70.7 (5.9) | 13.0 (51.0) |
| L. casei Shirota | −42.8 (13.7) | −58.2 (28.3) |
| E. faecium Tehobak | −59.8 (21.6) | −34.3 (52.1) |
Percentage of pathogens (CFU per milliliter) grown in coculture compared to growth alone.
Fish bile resistance.
With the exception of L. casei Shirota, all strains, including the fish pathogens, tolerated 1.5 h of incubation in the presence of 10% fish bile; no significant changes in numbers of viable counts were observed compared to the control. The viable count of L. casei Shirota was reduced by 19.7% (SD = 5%; P < 0.001).
Mucus penetration.
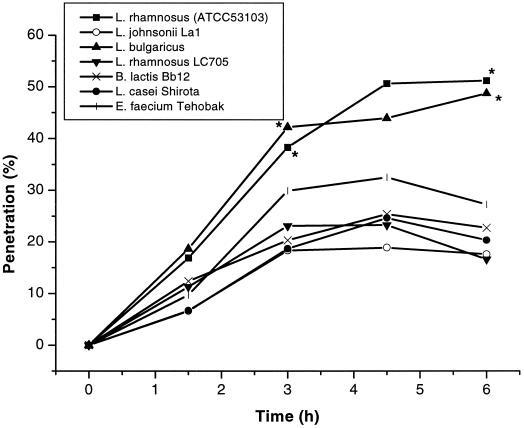
L. rhamnosus ATCC 53103 and L. bulgaricus were found penetrate intestinal mucus better than the other tested probiotic strains (P < 0.05) (Fig. 3). The differences between other probiotic strains were not significant.
FIG. 3.
Penetration of probiotic bacteria trough fish intestinal mucus. The mucus concentration was adjusted to 3 mg/ml with HH. Wells of the microtiter plate (two wells for each bacterial sample and each incubation time) were incubated for 1.5, 3, 4.5, and 6 h at room temperature. Penetrated bacteria were quantitated as percentage of radioactivity recovered from the wells compared to radioactivity of the added bacterial suspension on top of the mucus.
DISCUSSION
Interaction with mucus is the first step in adhesion of bacteria to the intestinal mucosa and other mucosal surfaces. This may provide competitive exclusion by probiotic microorganisms by blocking adhesion receptors, competition for nutrients, and production of antimicrobial substances. The result may be blockage of the ports of entry for fish pathogens. The ability of the tested fish pathogens to adhere to different mucus preparations of fish was low, and no clear preferential binding for mucus from a certain site of the body was observed. Horne and Baxendale (8) observed some differences between the binding ability of V. anguillarum to different mucus types, but these differences were small; Olsson and coworkers (15) obtained similar results. However, the differences observed in the present study did not reach statistical significance, which may be explained by the use of a different V. anguillarum strain. However, A. salmonicida was observed to adhere relatively well to all mucus types, which may help to explain its virulence. The results do not suggest the possible ports of entry of the pathogens in the fish, since no major differences in adhesion to the different mucus types were observed.
An interesting observation was that most of the adhesive human probiotic bacteria also bound well to fish mucus. Especially L. rhamnosus ATCC 53103, L. bulgaricus, L. johnsonii La1, and B. lactis Bb12 bound at similar levels to fish mucus as to human intestinal mucus (16). This also indicates that species specificity may not always be a factor in terms of initial adhesion properties. The adhesion to mucus appeared to be specific, since the adhesion of the above-mentioned strains to BSA and gelatin was significantly less. Only L. rhamnosus LC 705 and L. casei Shirota exhibited similar adhesion to BSA and gelatin as to intestinal mucus, suggesting that the observed low adhesion to intestinal mucus is due mainly to nonspecific adhesion. The high binding of most strains to polystyrene may indicate the importance of hydrophobic interactions in the adhesion to intestinal mucus, as suggested by other workers (24). In contrast to the other tested bacteria, E. faecium exhibited mucus specificity. It adhered to gill and skin mucus significantly less well than to mucus from the other tested organs.
Because of the low binding capacity of the tested fish pathogens, the effect of the tested probiotic strains on this adhesion was small or not detectable, despite the high adhesive capacity of some of the tested probiotic strains. Competition for nutrients was observed to significantly reduce the growth of A. salmonicida, but only two of the tested probiotic strains affected the growth of V. anguillarum. The probiotic strains were not observed to produce any significant antimicrobial activity against the fish pathogens as measured in spent culture liquid. This indicates that earlier reported antimicrobial substances produced by L. rhamnosus ATCC 53103 (21), L. bulgaricus (20), and L. johnsonii La1 (5) were either not produced or not effective against the tested pathogens. The antimicrobial activity of L. bulgaricus was tested by a slightly different method (20), which may also explain the observed differences. Other mechanisms by which probiotics may affect pathogens include binding of bacterial toxins, modulation of the immune system, and stabilization of a normal gut microflora. These mechanisms were, however, not assessed here. All probiotic strains tested have been reported to be acid and bile tolerant by the manufacturers. When the probiotics are less sensitive to acid and bile, they are more likely to survive passage through the gastrointestinal tract and may colonize, albeit transiently, both the intestinal and other mucosal surfaces of the fish. Of the tested probiotic strains, only L. casei Shirota was found to be sensitive to fish bile. It should, however, be noted that the bile concentration used is relatively high. In humans, the physiological concentration is estimated to be approximately 3% in the upper small intestine. However, the physiological concentration of bile for fish is not known.
Penetration through the mucus layer may be also an important property, since the intestinal mucus layer is constantly being synthesized and sloughed off. Organisms that can more easily penetrate and colonize deep within the mucus layer may have an advantage in colonizing the intestine (12). It remains to be explained how L. rhamnosus ATCC 53103 and L. bulgaricus penetrate the mucus quicker than the other tested strains, since lactic acid bacteria are, by definition, nonmotile (2).
The good adhesive ability, the ability of some strains to penetrate mucus well, and the ability of most strains to suppress pathogen growth in coculture, together with the observed stability against fish bile, may indicate that probiotics intended for human use may provide safe probiotics for use in the farming of fish for subsequent human consumption. The most effective mucus-binding and mucus-penetrating strains, L. bulgaricus and L. rhamnosus ATCC 53103, should be further studied in challenge experiments in fish to observe their potential protective effectiveness for fish against the opportunistic fish pathogens which cause economic losses in fish farming. This approach may provide safe novel treatment and feeding additives for fish farming.
ACKNOWLEDGMENTS
This study was supported by the Academy of Finland.
Satu Tölkkö and Pia Niemi are acknowledged for skillful technical assistance.
REFERENCES
- 1.Austin B, Stuckey L F, Robertson P A W, Effendi I, Griffith D R W. A probiotic strain of Vibrio alginolyticus effective in reducing diseases caused by Aeromonas salmonicida, V. anguillarum and V. ordalii. J Fish Dis. 1995;18:93–96. [Google Scholar]
- 2.Axellson L. Lactic acid bacteria: classification and physiology. In: Salminen S, von Wright A, editors. Lactic acid bacteria: microbiology and functional aspects. 2nd ed. New York, N.Y: Marcel Dekker, Inc; 1998. pp. 1–72. [Google Scholar]
- 3.Beachey E H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. J Infect Dis. 1981;143:325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 4.Bernardet J F, Segers P, Vancanneyt M, Berthe F, Kersters K, Vandamme P. Cutting a Gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (basonym, Cytophaga aquatilis Strohl and Tait 1978) Int J Syst Bacteriol. 1996;46:128–148. [Google Scholar]
- 5.Bernet-Camard M-F, Liévin V, Brassart D, Neeser J-R, Servin A L, Hudault S. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997;63:2747–2753. doi: 10.1128/aem.63.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen P S, Laux D C. Bacterial adhesion to and penetration of intestinal mucus in vitro. Methods Enzymol. 1995;253:309–314. doi: 10.1016/s0076-6879(95)53026-6. [DOI] [PubMed] [Google Scholar]
- 7.Havenaar R, ten Brink B, Huis in't Veld J H J. Selection of strains for probiotic use. In: Fuller R, editor. Probiotics, the scientific basis. 1st ed. London, England: Chapman and Hall; 1992. pp. 209–224. [Google Scholar]
- 8.Horne M T, Baxendale A. The adhesion of Vibrio anguillarum to host tissues and its role in pathogenesis. J Fish Dis. 1983;6:461–471. [Google Scholar]
- 9.Jöborn A. The role of the gastrointestinal microbiota in the prevention of bacterial infections in fish. Ph.D. thesis. Gothenburg, Sweden: Göteborg University; 1998. [Google Scholar]
- 10.Kirjavainen P E, Ouwehand A C, Isolauri E, Salminen S J. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol Lett. 1998;167:185–189. doi: 10.1111/j.1574-6968.1998.tb13226.x. [DOI] [PubMed] [Google Scholar]
- 11.Lowry O H, Rosebourgh N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;19:265–275. [PubMed] [Google Scholar]
- 12.Madsen L, Dalsgaard I. Reproducible methods for experimental infection with Flavobacterium psychrophilum in rainbow trout Oncorhynchus mykiss. Dis Aquat Org. 1999;36:169–176. doi: 10.3354/dao036169. [DOI] [PubMed] [Google Scholar]
- 13.McCormick B A, Stocker B A D, Laux D C, Cohen P S. Roles of motility, chemotaxis, and penetration through and growth in intestinal mucus in the ability of an avirulent strains of Salmonella typhimurium to colonize the large intestine of streptomycin-treated mice. Infect Immun. 1988;56:2209–2217. doi: 10.1128/iai.56.9.2209-2217.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller R S, Hoskins L C. Mucus degradation in human colon ecosystems. Faecal population densities of mucus degrading bacteria estimated by a ‘most probable number’ method. Gastroenterology. 1981;81:759–765. [PubMed] [Google Scholar]
- 15.Olsson J C, Westerdahl A, Conway P L, Kjelleberg S. Intestinal colonization potential of turbot (Scophthalmus maximus)- and dab (Limanda limanda)-associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol. 1992;58:551–556. doi: 10.1128/aem.58.2.551-556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouwehand A C, Kirjavainen P V, Grönlund M-M, Isolauri E, Salminen S. Adhesion of probiotic micro-organisms to intestinal mucus. Int Dairy J. 1999;9:623–630. [Google Scholar]
- 17.Robertson P A W, O'Dowd C, Burrells C, Williams P, Austin B. Use of Carnobacterium sp. as a probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss, Walbaum) Aquaculture. 2000;185:235–243. [Google Scholar]
- 18.Salminen S, Bouley C, Boutron-Ruault M-C, Cummings J H, Franck A, Gibson G R, Isolauri E, Moreau M-C, Roberfroid M, Rowland I. Functional food science and gastrointestinal physiology and function. Br J Nutr. 1998;80:S147–S171. doi: 10.1079/bjn19980108. [DOI] [PubMed] [Google Scholar]
- 19.Salminen S, Ouwehand A C, Benno Y, Lee Y K. Probiotics: how should they be defined? Trends Food Sci Technol. 1999;10:107–110. [Google Scholar]
- 20.Shahani K M, Vakil J R, Kilara A. Natural antibiotic activity of Lactobacillus acidophilus and bulgaricus. I. Cultural conditions for the production of antibiosis. Cult Dairy Prod J. 1976;11:14–17. [Google Scholar]
- 21.Silva M, Jacobus N V, Deneke C, Gorbach S L. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987;31:1231–1233. doi: 10.1128/aac.31.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith P, Hiney M P, Samuelsen O B. Bacterial resistance to antimicrobial agents used in fish farming: a critical evaluation of method and meaning. Annu Rev Fish Dis. 1994;4:273–313. [Google Scholar]
- 23.Virta M, Lineri S, Kankaanpää P, Karp M, Peltonen K, Nuutila J, Lilius E-M. Determination of the complement-mediated killing of the bacteria by viability staining and bioluminescence. Appl Environ Microbiol. 1998;2:515–519. doi: 10.1128/aem.64.2.515-519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadström T, Andersson K, Sydow M, Axelsson L, Lindgren S, Gullmar B. Surface properties of lactobacilli isolated from the small intestine of pigs. J Appl Bacteriol. 1987;62:513–520. doi: 10.1111/j.1365-2672.1987.tb02683.x. [DOI] [PubMed] [Google Scholar]
- 25.Westerdahl A, Olsson J C, Kjelleberg S, Conway P L. Isolation and characterization of turbot (Scophthalmus maximus)-associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol. 1991;57:2223–2228. doi: 10.1128/aem.57.8.2223-2228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]