Tens of millions of patients worldwide use anticoagulant medications to prevent or treat thrombotic conditions, including atrial fibrillation and venous thromboembolism. With the introduction of direct oral anticoagulants (DOACs) as preferred therapy over vitamin K antagonists (eg, warfarin), overall use of anticoagulants has increased markedly. 1 Concurrently, there has also been a rise in anticoagulant‐related adverse drug events (ADEs), including life‐threatening bleeding and thrombotic complications. 2 , 3 To address this growing health crisis, anticoagulation stewardship programs have been instituted in several health systems.
The concept of anticoagulation stewardship builds on 25 years of antibiotic “stewardship” experience. Since antibiotic stewardship was first proposed in 1996, 4 the Centers for Disease Control and Prevention has issued Core Elements for Antibiotic Stewardship Programs for US health care institutions (2014), 5 the Joint Commission has established antibiotic stewardship standards for hospitals in the US (2017), 6 and the Centers for Medicare and Medicaid services now require antibiotic stewardship in all US acute care hospitals as of July 1, 2019. 7 In tandem, the incidence of US emergency department (ED) visits for antibiotic‐associated ADEs has fallen from 19.2% in 2005‐2006 8 to 12.8% in 2017‐2019 9 (Figure 1). Indeed, in 2014, anticoagulant medications eclipsed antibiotics as the leading medication class associated with ED visits for ADEs. 8 In addition to outpatient ADEs, anticoagulants are the cause of at least 8% to 10% of hospital medication errors. 10 , 11 With roughly 33 million hospital admissions annually in the United States, 12 this represents a minimum of 3 million inpatient anticoagulant‐associated medication errors each year. Estimates further suggest that at least half of these inpatient errors occur during the prescribing phase, 10 , 13 underscoring the importance of appropriate anticoagulant selection and initiation before hospital discharge. Collectively, these ubiquitous challenges with safe prescribing and use of anticoagulant medications represents a growing health crisis in the United States and abroad.
FIGURE 1.
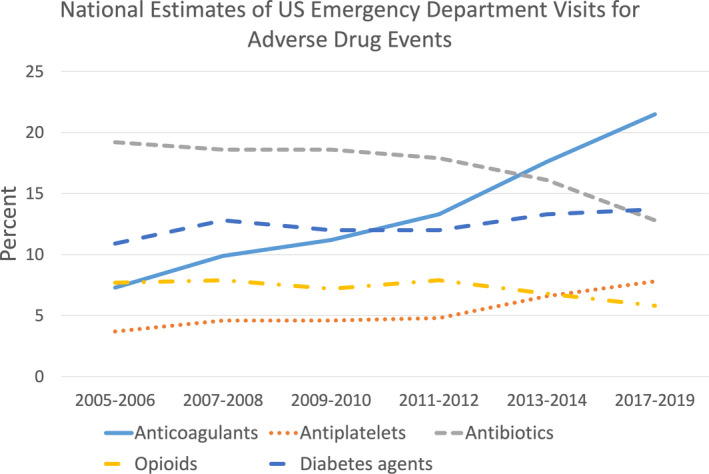
Top five medication classes associated with emergency department visits for adverse drug events in the United States 8 , 9
The marked rise in DOAC‐associated ADEs 2 is fueled by a multitude of issues. First, many patients who are prescribed a DOAC experience medication access issues related to high medication costs and variable insurance coverage that can potentially lead to gaps in therapy and thromboembolic events. Second, many clinicians lack familiarity with practical management aspects of DOACs, such as using vitamin K antagonist protocols in the periprocedural period despite DOACs having vastly different pharmacokinetic properties. This leads to increased thrombotic risk from inappropriately prolonged hold times and/or increased bleeding risk through inappropriate use of bridging therapy. Third, while DOACs have fewer drug interactions than warfarin, they are not devoid of interactions that may preclude their use or warrant dose adjustment. Limited and often conflicting information in this area puts patients at risk of an ADE or being unnecessarily relegated to potentially inferior warfarin therapy. 13 Fourth, off‐label DOAC dosing is one of the most prevalent issues, occurring in ≈25% of real‐world patients. 14 Recent metanalyses have confirmed the harm associated with such off‐label dosing practices, with a 22% increase in thrombotic events with underdosing and 30% increase in major bleeding with overdosing, relative to on‐label dosing. 3 Moreover, off‐label underdosing is associated with 24% to 27% increased relative risk of all‐cause mortality compared to on‐label dosing. 3 , 15 This is sobering proof of marginal progress in overall anticoagulation safety and optimization to date.
In this issue of RPTH, Koolian et al 16 describe the development and implementation of an inpatient pharmacist‐led, physician‐supported anticoagulation stewardship program using core elements set forth by the Anticoagulation Forum 17 (Figure 2). The primary objective of this single‐center, retrospective study was to assess the impact of the stewardship program on appropriate prescribing of any therapeutic‐intensity anticoagulant(s) according to Canadian best‐practice recommendations. The primary outcome of interest was the proportion of accepted stewardship recommendations leading to a change in anticoagulant prescription. Over a 6‐month postimplementation period, 381 patients underwent a total of 553 reviews by the anticoagulation stewardship program. Nearly two‐thirds (355, 64%) of these reviews generated a recommendation, most commonly dose adjustment (31%), drug interactions (19%), and laboratory monitoring (13%). A total of 299 of 355 (84%) stewardship recommendations were accepted by the treating team. The high proportion and varied nature of reviews requiring intervention in this inpatient study underscores the need for focused stewardship around these high‐risk yet essential therapies. Additionally, it demonstrates feasibility of implementation and suggests that such programs are well received by most prescribing physicians. Although retrospective in nature and noncomparative, this report adds to the growing body of literature that dedicated stewardship programs can optimize anticoagulant prescribing in hospitalized patients.
FIGURE 2.
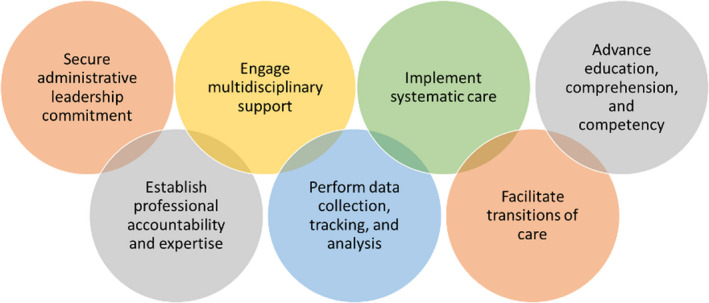
Core elements of anticoagulation stewardship programs 17
In another recent study, Sylvester et al 18 describe experiences and outcomes associated with evolving their pharmacist‐run outpatient anticoagulation clinic to provide stewardship over DOACs. Over a 4‐year period, the anticoagulation management service (AMS) completed 3154 DOAC follow‐up encounters in 1622 patients. Pharmacists made interventions in 35% (1113/3154) of DOAC encounters for a multitude of issues, including barriers to procurement of the DOAC at initial visit or follow‐up (11.1%), not taking the DOAC as prescribed (4%), required DOAC dose adjustment (5.4%), and/or development of a periprocedural plan (19.1%). This important study demonstrates the feasibility and value of incorporating DOACs into outpatient anticoagulation services and provides helpful details and pragmatic insights for other warfarin‐centric clinics that are exploring ways to evolve with the changing therapeutic landscape.
Changing the trajectory of anticoagulant‐related ADEs requires intentional, resolute, and broadly targeted efforts. Fortunately, a successful blueprint is readily available from the antibiotic stewardship realm, with immense potential to facilitate wide‐scale implementation of anticoagulation stewardship. Recognizing this, the Anticoagulation Forum, a national nonprofit whose organizational mission is education and empowerment of multidisciplinary clinicians in optimized use of anticoagulants, has partnered with the Food and Drug Administration to develop seven core elements specific to anticoagulation stewardship 17 (Figure 2), marking the first milestone in a very important journey.
Anticoagulation stewardship is defined as coordinated, efficient, and sustainable system‐level initiatives designed to achieve optimal anticoagulant‐related health outcomes and minimize avoidable ADEs through (i) the application of optimal evidence‐based care; (ii) appropriate prescribing, dispensing, and administration of anticoagulants and related agents; and (iii) provision of appropriate patient monitoring and clinical responsiveness. 17 Unlike conventional anticoagulation management, which is often siloed and focused solely on drug management, anticoagulation stewardship provides a holistic approach that spans the continuum of care, is focused on continuous quality improvement, and addresses patient‐level, clinician level, and system‐level barriers and opportunities to improve patient care. To address the ongoing pervasiveness of inappropriate anticoagulant prescribing and management, a growing number of health care institutions across the United States and globally have successfully implemented anticoagulation stewardship initiatives and assessed their impact in both inpatient and outpatient clinical settings. 19 , 20 , 21
Koolian et al 16 and Sylvester et al 18 are to be commended for publishing their experiences and providing meaningful momentum to advance anticoagulation stewardship. As evidenced by their program descriptions, there is no one‐size‐fits‐all approach, and program setting, size, structure, and scope will be heavily dependent on local resources and organizational needs. Nevertheless, as anticoagulant‐related patient harm increases, momentum will continue to swell around anticoagulation stewardship, with potential eventual culmination in regulatory and/or accreditation requirements for these programs, similar to the trajectory of antibiotic stewardship. Health care administrators and clinicians can best ready themselves by supporting development and implementation of anticoagulation stewardship programs and recruiting advanced‐trained clinical specialists to serve as champions and leaders of these programs.
AUTHOR CONTRIBUTIONS
AEB and GDB conceived of the manuscript. AEB drafted the manuscript. GDB provided critical revisions.
RELATIONSHIP DISCLOSURE
The authors report consulting fees for Abiomed, advisory board for Pfizer, Board of Directors National Board Certification for Anticoagulation Providers, Board of Directors for Anticoagulation Forum.
Burnett AE, Barnes GD. A call to action for anticoagulation stewardship. Res Pract Thromb Haemost. 2022;6:e12757. doi: 10.1002/rth2.12757
Handling Editor: Dr Lana Castellucci
Contributor Information
Allison E. Burnett, @aburnett_PharmD.
Geoffrey D. Barnes, Email: gbarnes@umich.edu, @GBarnesMD.
REFERENCES
- 1. Grymonprez M, Simoens C, Steurbaut S, De Backer TL, Lahousse L. Worldwide trends in oral anticoagulant use in patients with atrial fibrillation from 2010 to 2018: a systematic review and meta‐analysis. Europace. 2021;euab303. doi: 10.1093/europace/euab303 [DOI] [PubMed] [Google Scholar]
- 2. Geller AI, Shehab N, Lovegrove MC, et al. Emergency visits for oral anticoagulant bleeding. J Gen Intern Med. 2020;35:371‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang X‐L, Zhang XW, Wang TY, et al. Off‐label under‐ and overdosing of direct oral anticoagulants in patients with atrial fibrillation: a meta‐analysis. Circ Cardiovasc Qual Outcomes. 2021;14:e007971. [DOI] [PubMed] [Google Scholar]
- 4. McGowan JE, Gerding DN. Does antibiotic restriction prevent resistance? New Horiz. 1996;4:370‐376. [PubMed] [Google Scholar]
- 5. CDC . Core elements of hospital antibiotic stewardship programs. Antibiotic use. https://www.cdc.gov/antibiotic‐use/core‐elements/hospital.html (2021). Accessed May 17, 2022.
- 6. The Joint Commission . Antimicrobial stewardship – understanding the requirements. Critical access hospital. Medication Management MM. https://www.jointcommission.org/standards/standard‐faqs/critical‐access‐hospital/medication‐management‐mm/000002045/. Accessed May 18, 2022.
- 7. CMS . Omnibus burden reduction (conditions of participation) final rule CMS‐3346‐F. https://www.cms.gov/newsroom/fact‐sheets/omnibus‐burden‐reduction‐conditions‐participation‐final‐rule‐cms‐3346‐f. Accessed May 18, 2022.
- 8. Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013‐2014. JAMA. 2016;316:2115‐2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Budnitz DS, Shehab N, Lovegrove MC, Geller AI, Lind JN, Pollock DA. US emergency department visits attributed to medication harms, 2017‐2019. JAMA. 2021;326:1299‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dreijer AR, Diepstraten J, Bukkems VE, et al. Anticoagulant medication errors in hospitals and primary care: a cross‐sectional study. Int J Qual Health Care. 2019;31:346‐352. [DOI] [PubMed] [Google Scholar]
- 11. Koh H. National Action Plan for adverse drug event prevention. US Department of health and human services, Office of Disease Prevention and Health Promotion 190 (2014). https://health.gov/our‐work/national‐health‐initiatives/health‐care‐quality/adverse‐drugevents/national‐ade‐action‐plan. Accessed May 18, 2022.
- 12. AHA . Fast facts on U.S. hospitals, 2022. https://www.aha.org/statistics/fast‐facts‐us‐hospitals. Accessed May 18, 2022.
- 13. Vazquez SR, Barnes GD. Anticoagulant drug‐drug interactions: highlighting the need for antithrombotic stewardship and shared decision making. Res Pract Thromb Haemost. 2022;6:e12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen N‐N, Zhang C, Hang Y, et al. Real‐world prevalence of direct oral anticoagulant off‐label doses in atrial fibrillation: an epidemiological meta‐analysis. Front Pharmacol. 2021;12:581293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pereira MQ, David C, Almeida AG, Brito D, Pinto FJ, Caldeira D. Clinical effects of off‐label reduced doses of direct oral anticoagulants: a systematic review and meta‐analysis. Int J Cardiol. 2022;0:76‐82. [DOI] [PubMed] [Google Scholar]
- 16. Koolian M, Wiseman D, Mantzanis H, Kampouris N, Kerzner RS, Khan SR. Anticoagulation stewardship: Descriptive analysis of a novel approach to appropriate anticoagulant prescription. Res Pract Thromb Haemost. doi: 10.1002/rth2.12758, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anticoagulation forum. https://acforum.org/web/education‐stewardship.php. Accessed May 18, 2022.
- 18. Sylvester KW, Chen A, Lewin A, Fanikos J, Goldhaber SZ, Connors JM. Optimization of DOAC management services in a centralized anticoagulation clinic. Res Pract Thromb Haemost. 2022;6:e12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willeford A, Leiman V, Noel ZR. Impact of a pharmacist‐to‐dose direct oral anticoagulant protocol on medication errors at an academic medical center. J Am Coll Clin Pharm. 2021;4:1392‐1400. [Google Scholar]
- 20. Dane KE, Naik RP, Streiff MB, et al. Hemostatic and antithrombotic stewardship programs: a toolkit for program implementation. J Am Coll Clin Pharm. 2022;5(6):622‐631. [Google Scholar]
- 21. Dreijer AR, Kruip MJHA, Diepstraten J, et al. Effect of antithrombotic stewardship on the efficacy and safety of antithrombotic therapy during and after hospitalization. PLOS One. 2020;15:e0235048. [DOI] [PMC free article] [PubMed] [Google Scholar]


