Abstract
Study objective
The lockdown imposed on children due to the COVID-19 pandemic and their inability to attend school increased their exposure to indoor allergens by causing them to spend more time indoors. In this study, the aim was to reveal the effect of the pandemic and increased exposure to indoor aeroallergens on the symptom severity of school-age children with house dust mite-sensitized allergic rhinitis (AR).
Patients and methods
Patients between the ages of 6-18-years old, who were followed-up with the diagnosis of perennial AR sensitized to only mites were questioned about their sinonasal symptoms. The Total Nasal Symptom Score (TNSS) questionnaire was performed. The clinical findings, drug usage, frequency of infections and attacks were evaluated and compared during COVID-19 lockdown and the same time frame in 2019.
Results
Sixty-five patients had AR, and 33 patients (50.8%) had AR with asthma. TNSS of the patients improved during the pandemic (P < 0.001) and their medication scores decreased significantly (P < 0.001). The frequency of respiratory tract infections and asthma attacks decreased significantly (P < 0.001). In multivariate analysis, risk factors were evaluated for the ‘group with worsening TNSS’ and coal/wood burning was detected to be an independent risk factor (P = 0.006; OR = 10.09 (95% CI: 1.97–51.87)).
Conclusion
Although the increased stay at home, it is surprising that nasal symptoms improved in our patients. This result suggests that whereas allergen sensitivity is responsible for the pathogenesis of AR, exposure to pollution and viral infections which are reduced by masking and social distance may also play an important role in the pathogenesis.
Keywords: Allergic rhinitis, COVID-19, House dust mite, Indoor pollution, Lockdown, Pandemic
Résumé
Introduction
Le confinement imposé aux enfants en raison de la pandémie de COVID-19, l’impossibilité pour les enfants d’aller à l’école; ont augmenté leur exposition aux allergènes intérieurs en les obligeant à passer plus de temps à l’intérieur. Dans cette étude, il visait à révéler l’effet de la période pandémique et de l’exposition accrue aux aéroallergènes intérieurs sur la sévérité des symptômes des enfants d’âge scolaire atteints de rhinite allergique (RA) sensibilisée aux acariens.
Méthode
Patients âgés de 6 à 18 ans, qui ont été suivis avec le diagnostic de RA perannuelle avec seulement des acariens sensibilisés; ont été interrogés sur leurs symptômes naso-sinusiens. Le questionnaire Total Nasal Symptom Score (TNSS) a été réalisé. Leurs résultats cliniques, l’utilisation de médicaments, la fréquence des infections et des attaques ont été évalués et comparés pendant le verrouillage de COVID-19 et la même période de 2019.
Résultats
Soixante-cinq patients avaient une RA et 33 patients (50,8 %) avaient une RA avec asthme. Le TNSS des patients pendant la période pandémique s’est amélioré (p < 0,001) et leurs scores de médication ont diminué de manière significative (p < 0,001). La fréquence des infections des voies respiratoires et des crises d’asthme a diminué significativement (p < 0,001). En analyse multivariée, les facteurs de risque ont été évalués pour le « groupe d’aggravation du TNSS », la combustion de charbon/bois a été détectée comme étant un facteur de risque indépendant (p = 0,006 ; OR = 10,09 (IC95 % : 1,97–51,87)).
Conclusion
Malgré l’augmentation du séjour à domicile, il est surprenant que les symptômes nasaux de nos patients se soient améliorés. Ce résultat suggère que si la sensibilité aux allergènes est responsable de la pathogenèse de la RA, l’exposition à la pollution et aux infections virales qui sont réduites par le masque et la distance sociale peuvent également jouer un rôle important dans la pathogenèse.
Mots clés: Acariens, Confinement, COVID-19, Pandémie, Pollution intérieure, Rhinite allergique
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel type of human coronavirus that appeared in December 2019 in the city of Wuhan, China, and the disease it causes was named novel coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO) [1]. By March 2020, COVID-19 had reached pandemic status [2]. Since the beginning of the pandemic, some precautions such as wearing masks and social distance were taken regarding the (COVID-19) pandemic within the recommendations of infectious diseases scientific authorities. Many countries ordered their citizens to ‘stay-at-home’ or mandated them to stay at home except to work in an essential job or shop for essential needs.
In our country, the first case was seen on March 11, 2020 and lockdowns and some other restrictions began to be implemented after that [3]. Schools were closed on March 13 and online education was started [4]. Face-to-face education was optional and families generally preferred online education. This new era caused children to spend more time at home and increased their exposure to indoor allergens. As may be expected, exposure to indoor allergens and pollutants is a trigger for allergic patients [5]. House dust mite is one of the most common indoor allergens and previous studies showed that more than half of the houses in our city – Izmir- had detectable dust mite allergens due to high humidity, [6]. Therefore, we thought that lockdown would increase the burden of allergic disease by increasing house dust exposure. In this study, we aimed to reveal the effect of the COVID-19 pandemic lockdown and increasing exposure to indoor aeroallergens on the symptom severity of school-age children with allergic rhinitis who are sensitized to house dust mites.
2. Patients and methods
2.1. Study design and population
Patients aged between 6 and 18 years with perennial allergic rhinitis (AR) and only house dust mite (HDM) sensitization, who were followed up for at least three years in the outpatient clinic of Dr. Behçet Uz Children's Hospital, Department of Pediatric Allergy and Immunology were included in the study. Patients under the age of 6, who attended to face-to-face education, did not come to regular follow-up, with polysensitization and seasonal symptoms, and who underwent immunotherapy were excluded from the study. Patients who moved house and changed housing conditions in the last 3 years were also excluded. Ethical approval was obtained from the Turkish Ministry of Health and institutional local ethics committee (ID: 2020/527). The patients and their parents were informed about the study and their consent was obtained.
2.2. Data collection
Patient characteristics, clinical features, concomitant allergies, medications and laboratory findings were collected from the medical records and personal health record system. Patients’ allergic rhinitis diagnosis, severity and classifications were determined and graded according to the “Allergic Rhinitis and its Impact on Asthma” (ARIA) guidelines [7]. In our clinic, the skin prick test is routinely applied to patients with 14 standard aeroallergen solutions (ALK, Denmark) including Dermatophagoides pteronyssinus and Dermatophagoides farinae. Histamine is used as positive and 0.9% NaCl as negative control, respectively. A positive response is recorded if the mean diameter of the wheal is ≥ 3 mm, and the negative control is non-reactive at the same time. Patients with ≥ 3 mm induration in the skin prick test and/or spIgE ≥ 0.35 kU/L for the Dermatophagoides spp. were considered to be sensitized for house dust mites.
2.3. Symptoms and medication scores
A telephone survey was carried out asking data about the children's housing conditions, frequency of outdoor activities, individual habits of wearing a mask, environmental exposures such as indoor smoking and presence of pets, their current clinical status, symptom severity and the drug usage. The severity of symptoms (nose, eye and night symptoms), respiratory tract infections and asthma attacks of patients with asthma and their medications were evaluated by comparing a year during the pandemic period (1st April, 2020–31st March, 2021) with the same period of the previous year (1st April, 2019–31st March, 2020). The severity of symptoms of the patients were questioned according to a four-point scoring system (0 point: absent, 2 points: mild, 3 points: moderate, 4 points: severe) for each nasal (sneezing, nasal discharge, itching and nasal obstruction), ocular (redness, itching, tearing) and night symptoms. The total score for all rhinitis and conjunctivitis symptoms was named the total nasal symptom scores (TNSS) and total ocular symptom score (TOSS), respectively [8], [9]. The combination of TNSS and TOSS was calculated as total symptom score (TSS). Symptom scores were questioned twice at an interval of 6 months and the mean score value was obtained. Drug usage was examined from past medical records and determined as the total medication score (1 point: b-2 agonists and nasal/systemic antihistamines, 2 points: inhaled/intranasal steroids, 3 points: systemic corticosteroid) [9]. The frequency of asthma exacerbations, defined as progressive shortness of breath, cough and wheezing, was also questioned for patients with asthma for the pandemic and pre-pandemic period. Hospital visits due to respiratory infections were evaluated by looking at medical records.
2.4. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) Program (Version 22.0). The normality of the distribution of continuous variables was evaluated with the Kolmogorov-Smirnov test. Data that did not have normal distribution has the non-parametric analysis (Mann Whitney U test) used for continuous variables. Pearson's chi-square test or Fisher's exact test was used to compare the categoric data. The Wilcoxon signed-rank test was used to evaluate the difference between repeated measurements to assess whether the mean ranks differed. Logistic regression analysis was used for multivariate analysis in our study group.
3. Results
A total of 65 patients with allergic rhinitis were included in the study. Patient characteristics are given in Table 1 . Among patients, 21 (32.3%) were female. The mean age of the patients was 10.8 ± 2.8 (6–16.9) years. Forty-eight (73.8%) patients had a history of parental atopy. Patients with moderate to severe allergic rhinitis were in the majority among the patients. Among the study population, 33 (50.8%) patients were also diagnosed with asthma. Allergic conjunctivitis (41.5%), atopic dermatitis (13.8%), food allergies (7.7%), and drug allergies (7.7%) were the other concomitant allergic diseases, respectively. Frequency of symptoms related to allergic rhinitis of all patients are given in Table 2 . In the laboratory findings, absolute eosinophilia count was 468.7 ± 344.7 (0–1890), total IgE was 507.8 ± 594.6 (36.2–2852) and specific IgE for HDM 13.5 ± 17.2 (0–75).
Table 1.
Characteristics of children with HDM sensitized allergic rhinitis.
| All patients (n = 65) | |
|---|---|
| Age (year), mean ± SD (min–max) | 10.8 ± 2.8 (6–16.9) |
| Gender, n (%) | |
| Male | 44 (67.7) |
| Female | 21 (32.3) |
| Parental atopy history, n (%) | 48 (73.8) |
| AR classification, n (%) | |
| Mild intermitant | 15 (23.1) |
| Mild persistant | 8 (13.8) |
| Modarate to severe intermittent | 15 (23.1) |
| Moderate to severe persistent | 27 (41.5) |
| Concomitant allergic diseases, n (%) | |
| Asthma | 33 (50.8) |
| Allergic conjuctivitis | 27 (41.5) |
| Atopic dermatitis | 9 (13.8) |
| Food allergies | 5 (7.7) |
| Drug allergies | 5 (7.7) |
HDM: house dust mites.
Table 2.
Frequency of symptoms related to allergic rhinitis in patients.
| All patients (n = 65) | |
|---|---|
| Nasal symptoms, n (%) | |
| Nasal obstruction | 61 (93.8) |
| Nasal discharge | 55 (84.6) |
| Nasal itching | 55 (84.6) |
| Sneezing | 58 (89.2) |
| Eye symptoms, n (%) | |
| Redness | 27 (41.5) |
| Itching | 27 (41.5) |
| Tearing | 24 (36.9) |
| Nighttime symptoms, n (%) | 24 (36.9) |
| Cough, n (%) | 22 (33.9) |
| Snorring, n (%) | 14 (21.5) |
| Sleep difficulties, n (%) | 24 (36.9) |
| Irritability, n (%) | 31 (47.7) |
| Attention deficit, n (%) | 31 (47.7) |
| Headache, n (%) | 26 (40) |
The majority of patients lived in the city center (92.3%) and in apartments (70.8%). Fifty-seven (87.7%) patients were attending public school before the pandemic. Housing condition features and individual behaviors of the patients are given in Table 3 . When the indoor exposures are examined, 40% of patients had indoor smoking, 25% of patients had pets, and 32% of patients had mold exposure. While 60% of the patients had central heating, 25% of the patients lived in housing heated with coal/or wood stove. Frequency of outdoor activities of children was often two or less per a week. The majority of the patients declared that they always wore a mask outdoors.
Table 3.
Housing condition features and individual behaviours of the patients.
| All patients (n = 65) | |
|---|---|
| Living area, n (%) | |
| City center | 60 (92.3) |
| Rural area | 5 (7.7) |
| House characteristics, n (%) | |
| Apartment | 46 (70.8) |
| Single house | 17 (26.2) |
| Slum | 2 (3.1) |
| Parental education | |
| Primary and middle school | 15 (23.1) |
| High school and university | 50 (76.9) |
| School type of the patients | |
| Public school | 57 (87.7) |
| College | 8 (12.3) |
| Indoor exposures | |
| Indoor smoking | 27 (41.5) |
| Mold exposure | 21 (32.3) |
| Pet owner | 16 (24.6) |
| Heating system, n (%) | |
| Central heating | 39 (60) |
| Coal/wood-burning/biomass | 16 (24.6) |
| Air condition | 10 (15) |
| Family members number at home | |
| ≤ 4 person | 43 (66.2) |
| > 4 person | 22 (33.8) |
| Frequency of outdoor activities | |
| Once a week or less | 26 (40) |
| Twice a week | 18 (27.7) |
| 3 times a week or more | 21 (32.3) |
| Habits of wearing mask outdoor | |
| Always | 61 (93.8) |
| Sometimes | 3 (4.6) |
| Never | 1 (1.5) |
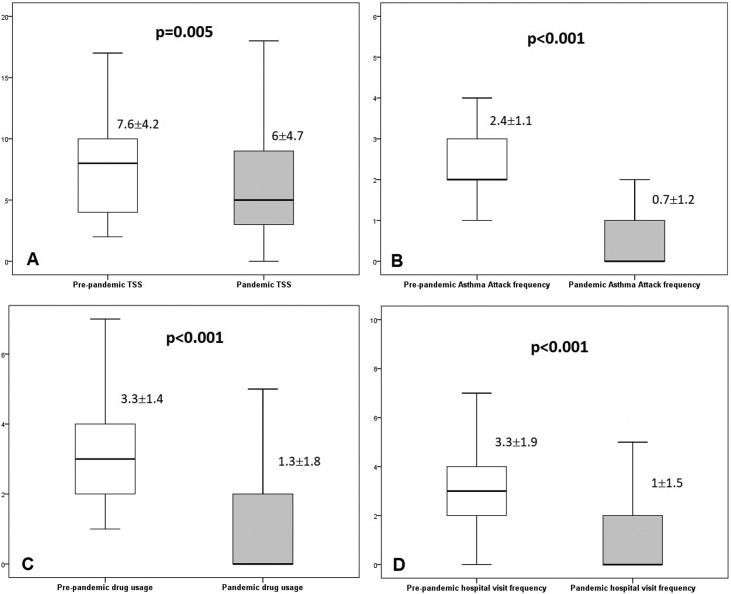
Symptom scores are given in Table 4 . The average TNSS and TSS scores significantly decreased in the pandemic period compared to the previous period (P < 0.05). Ocular symptoms (TOSS) also decreased but there was no statistical significance, except ocular itching. Asthma attack frequency for asthma patients significantly reduced (P < 0.001). Total medication score and hospital visit frequency for respiratory tract infections (RTI) also reduced (for both P < 0.001) (Fig. 1 ). More than half of the patients (34 patients; 52.3%) did not use any medication during the pandemic period.
Table 4.
Symptom scores of the pre-pandemic and pandemic periods.
| All scores | Pre-pandemic period | Pandemic period | P |
|---|---|---|---|
| TNSS | 5.6 ± 2.4 (1–10) | 4.3 ± 3.1 (0–10) | 0.001 |
| Nasal obstruction | 1.6 ± 0.7 (0–3) | 1.1 ± 0.9 (0–3) | < 0.001 |
| Nasal discharge | 1.3 ± 0.8 (0–3) | 1 ± 0.9 (0–3) | 0.006 |
| Nasal itching | 1.3 ± 0.7 (0–2) | 0.9 ± 0.9 (0–3) | 0.001 |
| Sneezing | 1.4 ± 0.8 (0–3) | 1.3 ± 0.9 (0–3) | 0.117 |
| TOSS | 2 ± 2.8 (0–9) | 1.7 ± 2.4 (0–9) | 0.111 |
| Redness | 0.7 ± 1 (0–3) | 0.6 ± 0.8 (0–3) | 0.061 |
| Itching | 0.7 ± 1 (0–3) | 0.6 ± 0.8 (0–3) | 0.022 |
| Tearing | 0.6 ± 0.9 (0–3) | 0.6 ± 0.8 (0–3) | 0.827 |
| TSS | 7.6 ± 4.2 (2–17) | 6 ± 4.7 (0–18) | 0.005 |
TNSS: Total Nasal Symptom Score; TOSS: Total Ocular Symptom Score; TSS: Total Symptom Score.
Fig. 1.
Pre-pandemic and pandemic periods (A) Total Symptom Scores (TSS), (B) Asthma attack frequency (for patients with asthma), (C) Total Medication Score and (D) Hospital visit frequency for respiratory tract infection.
When all patients are examined, TNSS were the same or improved for most of the patients (56 patients; 86.2%) named ‘same/improved TNSS group’. On the other hand, some of patients (9 patients; 13.8%) had increased TNSS named ‘worsened TNSS group’. In this context, all factors mentioned above were evaluated for the ‘worsened TNSS group’ in multivariate analysis and coal/wood burning was detected to be an independent risk factor (P = 0.006; OR = 10.09 (95% CI: 1.97–51.87)) (Table 5 ). In addition, outdoor activity frequency was also found to be less in this group. However, there was no statistical significance for the other parameters such as type of AR, concomitant allergic diseases, patient characteristics and laboratory findings, housing conditions or individual behaviors.
Table 5.
Multivariate analysis of the factors affect on TNSS.
| The worsened TNSS group | The same/improved TNSS group | P | OR (95% CI) | |
|---|---|---|---|---|
| Heating type coal/biomass | 6(66.6%) | 10(17.9%) | 0.006 | 10.09 (1.97–51.87) |
| Frequency of outdoor activities (< 1/week) | 6(66.6%) | 20(35.7%) | 0.083 | – |
TNSS: Total Nasal Symptom Score.
4. Discussion
Allergic rhinitis is the most common clinical manifestation of allergic disease. According to World Allergy Organization (WAO) estimates, the incidence of AR ranges from 10-30% worldwide [10]. Development of AR is controlled by a complex interaction of genetic, environmental and lifestyle factors. The burden of climate change and air pollution affects the development of allergic diseases [11]. The increase in the incidence in recent years emphasizes the importance of environmental factors in the emergence of the disease [12]. Children and adolescents stayed away from school, social life, and outdoor activities and sedentary daily life increased during the pandemic. The lockdown for children and transition to online education were restrictions applied in our country that caused children to spend their time at home. A four-year cross-sectional study conducted in southern China demonstrated the positive rates of indoor inhalant allergens increased during the COVID-19 pandemic and attributed this to lifestyle changes [13]. So far, only a few studies in pediatric populations demonstrated the effects of quarantine on allergic rhinitis. In our study population, nasal symptom scores were improved in the majority of patients, although most patients were diagnosed with moderate to severe AR and discontinued their maintenance therapy. Our patients consisted of low- and middle-income families and the majority of them attended crowded public school before the pandemic. The majority of them lived with nuclear families (≤ 4 people) and had high parental education levels. Due to the pandemic, increased adherence of parents with indoor allergy intervention recommendations may explain the clinical improvement. Poor symptom scores in the pre-pandemic period can be explained by exposure to more dusty environments at school and respiratory infection pathogens in crowded classes. A very small percentage of the population reported worse symptom scores, consistent with previous studies. In the study by Gelardi et al. in an adult group with allergic rhinitis sensitized to house dust mites (HDM), a telehealth questionnaire was conducted with the sinonasal outcome test (SNOT-22) and all SNOT-22 scores were found to be higher than in the previous year [14].
According to our study results, the frequency of asthma attacks and hospital visit frequency for respiratory tract infections significantly reduced in the pandemic compared to the pre-pandemic period. Although chronic respiratory diseases such as asthma were thought to be risk factors for COVID-19 disease at the outset of the pandemic, some adult studies reported fewer cases of asthma among COVID-19 patients [15]. A recent study from our country, in Istanbul during the 3 month lockdown period in asthmatic children population, patients experienced reduced numbers of upper respiratory tract infections and reduced asthma exacerbations, similar to our results [16]. However, they reported nasal symptoms were significantly worsened in the mite-sensitized group. In a recent study conducted by Sancakli et al., polysensitized asthmatic children were evaluated with the childhood asthma control test in the lockdown period and their C-ACT scores were found to be better [17].
In our study population, medication scores were also improved in the majority of patients. Brindisi et al. reported clinical improvement and reduction in the usage of on-demand and basal therapy in the pediatric asthma and/or allergic rhinitis population sensitized to grass pollen or dust mites during the lockdown, as in our study [18]. Moreover, Gallo et al. stated that the COVID-19 quarantine may improve symptoms and quality of life in patients with seasonal allergic symptoms, but worsen allergic symptoms in patients sensitized to dust mites [19].
At the beginning of the pandemic, patient fears of being infected with the SARS-CoV-2 virus resulted in a low number of outpatient and emergency room visits. However, afterwards it was observed that patients did not attend follow-up examinations and did not receive their medication prescriptions [20]. Moreover, during the pandemic, respiratory diseases such as asthma required less hospitalization than the previous years due to a reduction in respiratory tract infections (RTI) [21]. In our study, the frequency of RTI and hospital admissions were significantly decreased in most patients compared to the previous year. The frequency of acute exacerbations in patients with asthma was also reduced. This situation can be explained by many different reasons. Respiratory viral infections are triggers for exacerbations of allergic diseases. In a previous study we conducted in our clinic, at least one viral agent was identified in more than half of (56.3%) patients during asthma exacerbation [22]. It was reported that viral respiratory infectious agents, especially rhinoviruses, increase epithelial cells to produce IL-25 and IL-33 as in allergen exposure, thus promoting Th2 type inflammation [23]. In the lockdown era, there was a significant reduction in respiratory viruses, which are risk factors for allergic diseases [24]. In addition, the use of masks and social distance had positive effects in reducing the incidence of respiratory system infections. In the current literature, a study conducted by Dror et al. reported that mask usage decreased allergic rhinitis symptoms [25]. In our study, almost all of the study group wore masks outdoors.
Due to the pandemic that humanity is facing, human activities decreased significantly [26]. As a result of this, globally air pollution decreased in many countries including our country during the pandemic. Some authors hypothesized that breathing “cleaner” air with fewer pollutants, increased the inhalation of purified allergens; thus, causing worsening of symptoms in allergic populations [19]. On the other hand, they argued allergic patients would benefit from the reduction in air pollution and wearing a mask can also reduce the concentration of inhaled airborne allergens [26]. Exposure to allergens and pollutants may induce augmented inflammatory responses with the recruitment of cytokines and inflammatory cells, which results in worsening of symptoms [27]. Yucel et al. also reported improved outdoor air quality in Istanbul during the pandemic and associated this with improved asthma exacerbations and reduced upper RTI frequency [16]. The majority of our study group lived in the city center but their outdoor activity frequencies were significantly less. Before the pandemic, they were more exposed to outdoor pollution, which also contributed to their poor control. Indoor pollution is as important as outdoor pollution for allergic diseases. Some indoor activities such as smoking, cooking, and burning coal/wood cause fine particulate matter that can reach the deep airways and cause indoor pollution. According to ‘Global Atlas of Allergic Rhinitis’, indoor pollution may contribute to the rising prevalence of allergic rhinitis and asthma [11]. Staying at home for a long time increased exposure to indoor allergens and pollutants, while it reduced exposure to outdoor allergens, pollutants and upper RTI pathogens. The lockdown was expected to worsen asthma and AR symptoms in patients with indoor allergen sensitization, such as HDM, molds and pet allergens, due to exposure to aeroallergens, indoor smoking and pollution. Therefore, we questioned the causes of indoor pollution and housing conditions in our study. The patients had smoking exposure, mold exposure and pet presence, respectively. A quarter of the patients lived in homes heated with a coal or wood stove. Coal-burning, which we identified as a risk factor, reminded us that the importance of indoor air pollution is at least as much as outdoor air pollution. According to WHO data, approximately 2.6 billion people around the world cook using polluting open fires or simple stoves fueled by kerosene, biomass and coal. Nearly half of deaths due to pneumonia among children under 5 years of age are caused by particulate matter inhaled from household air pollution [28]. Unfortunately, due to the increasing prices, the usage of poor quality coal is increasing in our country and in many developing countries too.
This study involves some limitations. First of all, this study was conducted with a small number of patients who could be reached during the pandemic. Although our study group is limited, it is a homogeneous group with only HDM sensitivity. The study covered a one-year period and is important due to including all seasons and evaluating patients twice. It will be enlightening to conduct studies focusing on clinical progression in allergic children with different sensitivities and in different age groups during the pandemic. Furthermore, it is well known that symptoms in HDM-sensitized allergic patients correlate with the HDM allergen levels in their houses [29]. Due to the pandemic, we were not able to measure HDM concentration or indoor pollution in the patients’ houses. Our city, which is by the sea, is quite humid and we know that in a previous community-based study conducted with school-aged children in our region, the majority of skin prick tests had house dust mite susceptibility [30].
5. Conclusions
In conclusion, in spite of the increased stay at home nasal symptoms of our patients improved, contrary to expectations. This result suggests that whereas allergen sensitivity is responsible for the pathogenesis of allergic rhinitis, exposure to air pollution and viral infections, which are reduced by masking and social distance, may also play an important role in the pathogenesis. In addition to the direct effect of COVID-19, the indirect effect of the pandemic on the course of allergic diseases should be revealed with further studies.
Sources of funding
None.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
None.
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Director-General's Statement on IHR Emergency Committee on Novel Coronavirus (2019-nCoV). Available online at: https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov) (accessed June 1, 2020).
- 3.World Health Organization Global-Turkey. Available online at: https://covid19.who.int/region/euro/country/tr (accessed December 11, 2020).
- 4.Ministry of Education-Turkey. Available online at: http://covid19.meb.gov.tr/ (accessed December 11, 2020)
- 5.Campo P., Eguiluz-Gracia I., Plaza-Seron M.C., Salas M., José Rodríguez M., Pérez-Sánchez N., et al. Bronchial asthma triggered by house dust mites in patients with local allergic rhinitis. Allergy. 2019;74(8):1502–1510. doi: 10.1111/all.13775. [DOI] [PubMed] [Google Scholar]
- 6.Gulbahar O., Mete N., Kokuludag A., Sin A., Sebik F. House dust mite allergens in Turkish homes. Allergy. 2004;59(2):231. doi: 10.1046/j.1398-9995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J., Schünemann H.J., Togias A., Bachert C., Erhola M., Hellings P.W., et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145(1) doi: 10.1016/j.jaci.2019.06.049. [70-80.e3] [DOI] [PubMed] [Google Scholar]
- 8.Eifan A.O., Akkoc T., Yildiz A., Keles S., Ozdemir C., Bahceciler N.N., et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy. 2010;40(6):922–932. doi: 10.1111/j.1365-2222.2009.03448.x. [DOI] [PubMed] [Google Scholar]
- 9.Bielory L. Ocular symptom reduction in patients with seasonal allergic rhinitis treated with the intranasal corticosteroid mometasone furoate. Ann Allergy Asthma Immunol. 2008;100(3):272–279. doi: 10.1016/S1081-1206(10)60453-X. [DOI] [PubMed] [Google Scholar]
- 10.WAO White Book on Allergy: Update 2013. Executive Summary. Ruby Pawankar, Giorgio Walter Canonica, Stephen T. Holgate, Richard F. Lockey, Michael S. Blaiss. Copyright 2013 World Allergy Organization.
- 11.Agache I., Miller R., Gern J.E., Hellings P.W., Jutel M., Muraro A., et al. Emerging concepts and challenges in implementing the exposome paradigmin allergic diseases and asthma: a Practall document. Allergy. 2019;74:449–463. doi: 10.1111/all.13690. [DOI] [PubMed] [Google Scholar]
- 12.Akdis C.A., Hellings P.W., Agache I. Global atlas of allergic rhinitis and chronic rhinosinusitis. European Academy of Allergy and Clinical Immunology; Zurich, Switzerland: 2015. European Academy of Allergy and Clinical Immunology. [Google Scholar]
- 13.Yusi Li. Increase in Indoor Inhalant Allergen Sensitivity During the COVID-19 Pandemic in South China: A Cross-Sectional Study from 2017 to 2020. [DOI] [PMC free article] [PubMed]
- 14.Gelardi M., Trecca E.M.C., Fortunato F., Iannuzzi L., Marano P.G., Quaranta N.A.A., et al. COVID-19: When dust mites and lockdown create the perfect storm. Laryngoscope Investig Otolaryngol. 2020;5(5):788–790. doi: 10.1002/lio2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carli G., Cecchi L., Stebbing J., Parronchi P., Farsi A. Is asthma protective against COVID-19? Allergy. 2021;76(3):866–868. doi: 10.1111/all.14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yucel E., Suleyman A., Hizli Demirkale Z., Guler N., Tamay Z.U., Ozdemir C. “Stay at home”: Is it good or not for house dust mite sensitized children with respiratory allergies? Pediatr Allergy Immunol. 2021;32(5):963–970. doi: 10.1111/pai.13477. [DOI] [PubMed] [Google Scholar]
- 17.Sancaklı O., Tuncel T., Eren Akarcan S., Kanık A., Özyurt G. Evaluation of the impact of environmental changes on asthma control in children, access to health care and treatment adherence in early COVID-19 lockdown. Turk Arch Pediatr. 2022;57(2):228–234. doi: 10.5152/TurkArchPediatr.2022.21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brindisi G., De Vittori V., De Nola R., Pignataro E., Anania C., De Castro G., et al. Updates on children with allergic rhinitis and asthma during the COVID-19 Outbreak. J Clin Med. 2021;10(11):2278. doi: 10.3390/jcm10112278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallo O., Bruno C., Orlando P., Locatello L.G. The impact of lockdown on allergic rhinitis: What is good and what is bad? Laryngoscope Investig Otolaryngol. 2020;5(5):807–808. doi: 10.1002/lio2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angoulvant F., Ouldali N., Yang D.D., Filser M., Gajdos V., Rybak A., et al. Coronavirus disease 2019 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and nonviral infections-a time series analysis. Clin Infect Dis. 2021;72(2):319–322. doi: 10.1093/cid/ciaa710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelletier J.H., Rakkar J., Au A.K., Fuhrman D., Clark R.S.B., Horvat C.M. Trends in US pediatric hospital admissions in 2020 compared with the decade before the Covid-19 pandemic. JAMA Netw Open. 2021;4:e2037227. doi: 10.1001/jamanetworkopen.2020.37227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Özen S., Taşkırdı I., Akçal Ö., Kaya M.S., Akay Haci I., Soyoz O., et al. Prevalence of detected viruses in acute asthma exacerbations in children. Iran J Allergy Asthma Immunol. 2022;21(2):112–118. doi: 10.18502/ijaai.v21i2.9219. [DOI] [PubMed] [Google Scholar]
- 23.Beale J., Jayaraman A., Jackson D.J., Macintyre J.D.R., Edwards M.R., Walton R.P., et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3009124. [256ra134] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta A., Bush A., Nagakumar P. Asthma in children during the COVID-19 pandemic: Lessons from lockdown and future directions for management. Lancet Respir Med. 2020;8:1070–1071. doi: 10.1016/S2213-2600(20)30278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dror A.A., Eisenbach N., Marshak T., Layous E., Zigron A., Shivatzki S., et al. Reduction of allergic rhinitis symptoms with face mask usage during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3590–3593. doi: 10.1016/j.jaip.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navel V., Chiambaretta F., Dutheil F. Will environmental impacts of social distancing due to the pandemic caused by SARS-CoV-2 decrease allergic disease? J Allergy Clin Immunol. 2020;146(1):70–71. doi: 10.1016/j.jaci.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traina G., Barbalace A., Betti F., Bolzacchini E., Bonini M., Contini D., et al. What impact of air pollution in pediatric respiratory allergic diseases. Pediatr Allergy Immunol. 2020;31(Suppl 26):26–28. doi: 10.1111/pai.13362. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization Global. Available at: https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health (accessed December 1, 2021).
- 29.Zuiani C., Custovic A. Update on house dust mite allergen avoidance measures for asthma. Curr Allergy Asthma Rep. 2020;20(9):50. doi: 10.1007/s11882-020-00948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karaman O., Turgut C.S., Uzuner N., Olmez D., Babayigit A., Kose S., et al. The determination of asthma, rhinitis, eczema, and atopy prevalence in 9- to 11-year-old children in the city of Izmir. Allergy Asthma Proc. 2006;27(4):319–324. doi: 10.2500/aap.2006.27.2877. [DOI] [PubMed] [Google Scholar]