Abstract
Purpose
To evaluate the effect of wearing facemasks on dry eye symptoms and on the tear film while comparing surgical face masks to N95 particulate respirators.
Methods
A prospective observational study was conducted at Ain Shams University Hospitals in the period from September 2020 to January 2021. Two hundred volunteers were recruited, and the daily number of hours spent by each participant wearing a facemask was recorded. Recruits were divided into two groups: 100 volunteers were allocated to Group A to use the surgical mask, and 100 participants to Group B to use the N95 particulate respirator. The tear film parameters were assessed at baseline by answering the Ocular Surface Disease Index (OSDI) questionnaire and performing tear break-up time (TBUT), corneal fluorescein staining, and Schirmer-I test Subjects then wore a facemask for 60 min and then the tear film parameters were reassessed by repeating TBUT, corneal staining and Schirmer-I test
Results
Facemask use for 60 min significantly worsened all tear film parameters in both groups (P-value <0.0001). The deterioration was significantly larger in Group A subjects (P < 0.0001). The daily number of hours spent wearing a facemask correlated strongly with the OSDI and corneal staining. There was a strong negative correlation between the daily number of hours spent wearing a facemask and Schirmer test, and a weak negative correlation with TBUT.
Conclusions
Wearing facemasks during the COVID-19 pandemic is a risk factor for worsening tear film parameters. This deterioration is significantly greater with surgical masks than with N95 particulate respirators and increases with the duration of facemask use.
Keywords: Dry eye, tear film, COVID-19, facemasks
Introduction
Dry eye disease (DED) is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.1 It is a significant cause of ocular discomfort and visual impairment that could affect patient quality of life.2 The prevalence of DED is highly variable among different studies3–7 and is known to stress the healthcare system with a huge economic burden.8
At the end of 2019, an outbreak of severe respiratory illness occurred in Wuhan City, China. On January 9, 2020, the Chinese Center for Disease Control and Prevention declared the identification of a novel Coronavirus, that was later declared responsible for the outbreak.9 The newly discovered COVID-19 virus has quickly spread across the planet and the number of global cases has now surpassed 100 million with over two million mortalities as of February 4th, 2021.10
Wearing facemasks is being strongly encouraged to help combat the spread of COVID-19.11 As people are adapting to the wearing of face masks, there is a rising concern about Mask-Associated Dry Eye (MADE), caused by poor fitting masks and air leak.12 Studies indicate that air convection does have ocular complications. For example, air currents around the face from powered air-purifying respirators have been shown to aggravate dry eye symptoms13 and air regurgitation through the nasolacrimal system from continuous positive airway pressure masks may irritate the ocular surface.14 An additional example is exposure keratopathy in the Intensive Care Unit, which was recorded at 54.3% among mechanically ventilated patients compared to 5.1% in non-ventilated patients.15 There are two main types of facemasks: surgical masks which are poorly-fitting, disposable masks that filter out droplets, and tight-fitting N95 particulate respirator masks which filter out airborne particles.16 Pre-COVID-19 studies reveal that both types reduce clinical respiratory disease in health-care professionals by 41% and influenza-like illness by 66%.17
The purpose of this study is to demonstrate the effects of wearing facial masks on dry eye symptoms and on tear film parameters, and to compare two types of masks that are being used to combat the spread of COVID-19 regarding their effect on the ocular surface.
Methods
This prospective observational clinical study was conducted at Ain Shams University Hospitals after gaining approval of Ain Shams University Faculty of Medicine ethical committee and was in accordance with the 1964 Helsinki declaration. The study was carried out from September 2020 to January 2021. Informed consents were taken from all subjects after explanation of the nature and possible consequences of the study. We included 200 healthy volunteers recruited from relatives of patients presenting at Ain Shams University Hospitals outpatient clinics. Exclusion criteria were age less than 18 years, Meibomian gland dysfunction, conjunctivitis, contact lens users, smoking, any history of significant ocular or systemic pathology, history of previous ocular surgery, eyelid disorders, patients on systemic or topical medications that could affect the ocular surface, and patients with any respiratory disease who would be exhausted by wearing a facial mask. Subjects were randomly allocated to two subgroups: Group A included 100 volunteers assigned to use the fluid resistant surgical mask, and Group B included 100 participants assigned to use the N95 particulate respirator.
All subjects were subjected to detailed medical and ophthalmological history taking, with special emphasis on the daily number of hours each participant spends wearing a facemask. Ophthalmological examination included best-corrected visual acuity (BCVA) by decimal scale, anterior segment examination with slit-lamp biomicroscopy, and tear film assessment.
Evaluation of the tear film was performed both subjectively and objectively. Since this study was performed during the COVID-19 pandemic and most volunteers were already wearing facemasks, we asked participants to remove their masks for 120 min (to restore ocular surface) before taking baseline measurements (while strictly adhering to the rules of social distancing). DED symptoms were assessed using the Ocular Surface Disease Index (OSDI) questionnaire.18,19 The OSDI was measured on a scale from 0 to 100, with higher scores indicating more dryness. For clinical signs of dryness, objective tests were employed in the following sequence with 5 min breaks in between: the tear break-up time (TBUT), corneal fluorescein staining, and the Schirmer-I test without anaesthesia. Examination was done between 9am and 12pm to avoid the influence of diurnal variation on the tear film.20 For TBUT, fluorescein strips were applied to the lower conjunctival fornix, and the subject was requested to blink a few times and then keep their eyes open. While being monitored by the slit-lamp under cobalt-blue light illumination, the time interval between the last blink and the appearance of the first dry black spot was recorded in seconds using a stopwatch. The test was performed three times, and their mean was calculated. For corneal fluorescein staining, the dye was instilled using fluorescein strips and staining was observed using a slit-lamp under cobalt-blue illumination. Scoring of the corneal staining was done using the Oxford grading scheme.21 Schirmer-I test was performed by applying Schirmer strips (Tear Flo; Sigma Pharmaceuticals, Monticello, IA) to the lower conjunctival fornix away from the cornea and near the lateral canthus. Subjects were requested to keep their eyes shut for 5 min, and then the moistened segment of the strip was measured and recorded in millimetres.22
At this point, each participant was asked to wear a facemask and wait for 60 min in a room with no air currents and humidity level between 40% and 60% and temperature level between 20°C and 25°C. Group A subjects wore a fluid resistant surgical mask (3M™ High Fluid Resistant Mask, Earloop 1840), while Group B subjects wore an N95 particulate respirator (3M™ Health Care Particulate Respirator and Surgical Mask 1860, N95).
After the 60 min period, the TBUT, corneal staining and Schirmer-I test were repeated, and the results recorded.
Statistical analysis
An unpaired Student's t-test was used to compare parametric variables between the two groups, and a chi-square test was used to compare categorical variables. Paired Student's t-test was used to compare measurements before and after the facemask wearing. Pearson correlation coefficient was used to evaluate the correlation between the daily number of hours spent wearing a facemask and both subjective and objective tear film parameters at baseline (where r = 1 is total positive linear correlation, r = 0 is no linear correlation and r = -1 is total negative linear correlation). Values of P < 0.05 were considered statistically significant. All statistical analyses were performed using Microsoft Excel 2016.
Results
Demographic and clinical data for the two groups at baseline are shown in Table 1. There was no significant difference between the two groups as regards age, gender, BCVA, daily number of hours spent wearing a facemask, OSDI score, TBUT, corneal fluorescein staining or Schirmer test
Table 1.
Demographic and clinical data at baseline for groups A and B (SD: standard deviation).
| Group A | Group B | P-value | ||
|---|---|---|---|---|
| Mean age in years ± SD (range) | 30.26 ± 6.51 (18 to 40) | 29.43 ± 6.44 (18 to 40) | 0.37 | |
| Gender | Males | 54 (54%) | 52 (52%) | 0.78 |
| Females | 46 (46%) | 48 (48%) | ||
| Mean BCVA in decimals ± SD (range) | 0.76 ± 0.16 (0.1 to 1.0) | 0.76 ± 0.15 (0.2 to 1.0) | 1.0 | |
| Mean daily number of hours spent wearing a facemask ± SD (range) | 3.29 ± 2.3 (0 to 11) | 3.2 ± 2.33 (0 to 9) | 0.78 | |
| Mean OSDI score ± SD (range) | 22.53 ± 9.55 (8 to 46) | 21.58 ± 9.6 (9 to 45) | 0.48 | |
| Mean TBUT in seconds ± SD (range) | 10.07 ± 1.94 (6 to 14) | 9.9 ± 1.78 (6 to 14) | 0.52 | |
| Mean Oxford score for corneal fluorescein staining ± SD (range) | 0.94 ± 1.35 (0 to 5) | 1.04 ± 1.1 (0 to 4) | 0.57 | |
| Mean Schirmer in mm ± SD (range) | 12.82 ± 4.62 (4 to 21) | 12.88 ± 4.17 (4 to 20) | 0.92 | |
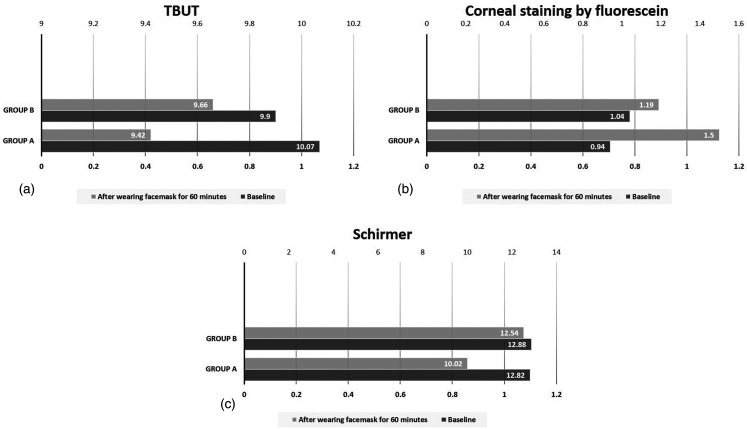
After 60 min of wearing a facemask, all tear film parameters worsened in both groups. These changes from baseline were statistically significant in both Group A (P-value < 0.0001 for each of TBUT, corneal fluorescein staining and Schirmer test) and Group B (P-value < 0.0001 for each of TBUT, corneal fluorescein staining and Schirmer test). This is shown in Table 2 and Figure 1.
Table 2.
Tear film parameters at baseline and after 60 min of wearing a facemask in groups a and b (SD: standard deviation).
| At Baseline | After 60 min facemask | P-value | ||
|---|---|---|---|---|
| Group A | Mean TBUT ± SD (range) | 10.07 ± 1.94 (6 to 14) | 9.42 ± 1.96 (5 to 14) | < 0.0001 |
| Mean Corneal fluorescein staining (Oxford scheme) ± SD (range) | 0.94 ± 1.35 (0 to 5) | 1.5 ± 1.41 (0 to 5) | < 0.0001 | |
| Mean Schirmer test ± SD (range) | 12.82 ± 4.62 (4 to 21) | 10.02 ± 3.87 (2 to 17) | < 0.0001 | |
| Group B | Mean TBUT ± SD (range) | 9.9 ± 1.78 (6 to 14) | 9.66 ± 1.78 (5 to 14) | < 0.0001 |
| Mean Corneal fluorescein staining (Oxford scheme) ± SD (range) | 1.04 ± 1.1 (0 to 4) | 1.19 ± 1.1 (0 to 4) | < 0.0001 | |
| Mean Schirmer test ± SD (range) | 12.88 ± 4.17 (4 to 20) | 12.54 ± 4.11 (4 to 19) | < 0.0001 | |
Figure 1.
Tear film parameters at baseline and after 60 min of wearing a facemask in groups A and B.
a: TBUT (tear break-up time), b: Corneal staining by fluorescein, c: Schirmer test.
Table 3 illustrates a comparison between Groups A and B as regards the magnitude of change (measurements at baseline – measurements after 60 min of wearing a facemask) in each of the tear film parameters. It is apparent that the magnitude of change in Group A subjects was significantly larger for all parameters than the magnitude of change in Group B (P < 0.0001 for TBUT, corneal staining by fluorescein and Schirmer test).
Table 3.
Comparison between changes in tear film parameters in groups a and b.
| Group A | Group B | P-value | |
|---|---|---|---|
| Mean change in TBUT from baseline in seconds ± SD (range) | −0.65 ± 0.78 (0 to −4) | −0.24 ± 0.47 (−1 to + 1) | < 0.0001 |
| Mean change in corneal fluorescein staining by Oxford scheme from baseline ± SD (range) | + 0.56 ± 0.5 (0 to + 1) | + 0.15 ± 0.36 (0 to 1) | < 0.0001 |
| Mean change in Schirmer test from baseline in mm ± SD (range) | −2.8 ± 1.46 (0 to −7) | −0.34 ± 0.48 (0 to −1) | < 0.0001 |
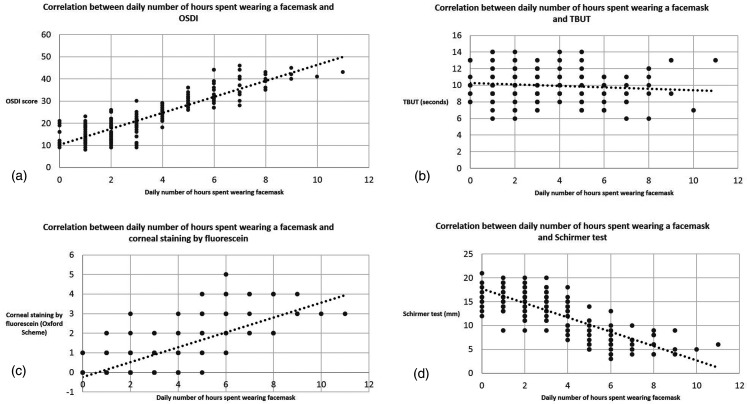
There was a strong positive correlation between the daily number of hours spent wearing a facemask and the OSDI and corneal staining by fluorescein (measured at baseline). On the other hand, there was a strong negative correlation between the daily number of hours spent wearing a facemask and Schirmer test, and a weak negative correlation with TBUT (measured at baseline). This is shown in Table 4 and Figure 2.
Table 4.
Correlation between daily number of hours spent wearing a facemask and subjective and objective tear film parameters.
| Pearson correlation coefficient “r” for daily number of hours spent wearing a facemask | Pearson correlation coefficient P-value for daily number of hours spent wearing a facemask | |
|---|---|---|
| OSDI | + 0.872 | < 0.0001 |
| TBUT | −0.1056 | 0.139 |
| Corneal staining by fluorescein | + 0.7139 | < 0.0001 |
| Schirmer test | −0.793 | < 0.0001 |
Figure 2.
Correlation between daily number of hours spent wearing a facemask and subjective and objective tear film parameters.
a: OSDI (Ocular Surface Disease Index), b: TBUT (tear break-up time), c: Corneal staining by fluorescein, c: Schirmer test.
Discussion
DED is a multifactorial disease of the ocular surface, in which the tear film stability and homeostasis are compromised, leading to variable symptoms and signs.1 Tear film evaporation is known to be a major contributing element in the pathogenesis of DED,23,24 and air currents, whether environmental as in windy weather25 or artificial as with continuous positive airway pressure14 have been shown to aggravate the condition. To our knowledge, there are no previous studies that assess the effect of wearing facemasks on the ocular surface objectively. Therefore, this is a novel study which provides an understanding on how objective tear film parameters are affected by using masks during the COVID-19 pandemic.
Our results indicate that both types of facemasks induced a significant deterioration of all objective tear film parameters (TBUT, corneal fluorescein staining and Schirmer test). This is similar to the findings of Giannaccare et al.,26 who conducted a survey among medical students and postulated that wearing facemasks during the COVID-19 pandemic has exacerbated dry eye symptoms. They attributed this to either mask displacement or improper fitting which scatters air around the eyes, causing accelerated tear evaporation. Likewise, Moshirfar et al.12 observed a proportional increase in symptoms of eye dryness among mask wearers, including those who had never previously complained of DED symptoms before. Such patients reported symptomatic deterioration on evaluation by the OSDI questionnaire, in addition to augmented postoperative dry eye sensation. Most were even conscious of air flowing from the mask into their eyes.
Similarly, Hadayer et al.27 used thermal and infrared cameras to monitor air flow from three types of facemasks during normal breathing, speech, and deep breathing. Air currents were recorded arising from the upper edges of the masks and flowing toward the ocular surface in 81% of cases. They therefore suggested that wearing facemasks by patients receiving intravitreal injections during the COVID-19 pandemic may pose as a risk for developing endophthalmitis. Another study28 described a recent surge of postoperative infections (including infectious keratitis and endophthalimitis) after corneal collagen cross-linking, cataract surgery and vitrectomy in patients wearing facemasks during and/or after surgery. Cultures detected bacterial flora of the oral cavity, which lead them to suggest that wearing facemasks during ophthalmic surgery could increase the incidence of infection by blowing bacterial flora from the oral cavity toward the ocular surface. Additionally, Chadwick and Lockington29 reported a case of early postoperative painful loss of vision after uneventful phacoemulsification and referred it the ill-fitting facemask directing the patient's exhalation to the eye leading to excessive dehydration of the corneal surface. Unlike normal breathing, which is directed away from the face, facemasks divert the exhaled air towards the ocular surface, therefore rendering it prone to dryness.
Furthermore, our findings reveal that the deterioration in objective tear film parameters (TBUT, corneal fluorescein staining and Schirmer test) after wearing a facemask for 60 min was significantly larger in Group A subjects than in Group B. Therefore, surgical masks could have a greater drying effect on tear film parameters compared to N95 particulate respirators. This could be due to the tighter fitting provided by the N95 particulate respirators, which partially impediments airflow from the facemask toward the eyes. Again, this is in concord with the findings of Hadayer et al.,27 who monitored air leak from three types of surgical facemasks. The facemask with four tying strips showed a leak in 83% of subjects, while the facemask with elastic ear loops produced a leak in 93% of cases. Seepage of air was detected in only 67% of subjects wearing the N95 tuberculosis particulate facemask. Therefore, the snug fit provided by the N95 particulate respirators could somewhat act as a barrier to airflow from the facemask to the corneal surface.
In addition, we found a strong positive correlation between the daily number of hours spent wearing a facemask and the OSDI and corneal staining by fluorescein, and a strong negative correlation with Schirmer test The correlation with TBUT was weak. Therefore, the longer the facemask is worn, the greater the tear film dehydration. Similarly, Moshirfar et al.12 noticed that those wearing facemasks for longer periods show symptoms of DED more frequently, particularly the elderly, immunocompromised, and healthcare professionals.
Chen et al.30 have described symptoms of DED in COVID-19 patients. Although dry eye is mentioned as a sequel of the disease itself, it is possible that this dryness may be due to the necessary, continual wearing of facemasks by such patients.
However, we are aware of our study limitations, including the absence of other objective tests such as tear osmolarity and other ocular surface staining methods such as Rose Bengal and lissamine green. These shortcomings may be addressed in future studies, in addition to the potential of evaluating the effect of taping the superior edges of facemasks.
Conclusion
To conclude, wearing facemasks during the COVID-19 pandemic could be a risk factor for DED. This risk is significantly greater with surgical masks than with N95 particulate respirators and increases with the duration of facemask use. Nonetheless, we do not discourage facemask use to prevent viral spread during the pandemic. Mask manufacturers could provide better fitting designs and taping the upper edge of the mask may reduce air leaks. Wearing protective glasses has been shown to reduce DED symptoms in medical staff.31 This is particularly important during the COVID-19 pandemic. The tear film has a vital antimicrobial function32 and viral transmission through the ocular surface has been proven possible.33 Consequently, excessive dryness could pose as a risk factor for COVID-19 spread.
Acknowledgements
The authors indicate no relationships/conditions/circumstances that present a potential financial conflict of interest Costs were the responsibility of the authors and instruments used in the study belong to Faculty of Medicine, a part of Ain Shams University, which is a public governmental organization.
Footnotes
animal research: No animal subjects were included in this study.
authors' contributions: All named authors meet the ICMJE requirement of authorship and have made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content. All authors read and approved the final manuscript.
consent to participate: Informed consent was obtained from all individual participants included in the study (or their legal guardians for minors).
consent to publish: The authors affirm that all participants provided informed consent for publication of their data.
data availability: The manuscript has no associated data in a data repository.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments. The study gained approval of Ain Shams University Faculty of Medicine ethics committee.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Hisham Samy Shalaby https://orcid.org/0000-0002-5483-9974
References
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017; 15: 276–283. [DOI] [PubMed] [Google Scholar]
- 2.Miljanović B, Dana R, Sullivan DA, et al. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol 2007; 143: 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaumberg DA, Dana R, Buring JE, et al. Prevalence of dry eye disease among US men: estimates from the Physicians’ health studies. Arch Ophthalmol 2009; 127: 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viso E, Rodriguez-Ares MT, Gude F. Prevalence of and associated factors for dry eye in a Spanish adult population (the Salnes eye study). Ophthalmic Epidemiol 2009; 16: 15–21. [DOI] [PubMed] [Google Scholar]
- 5.Uchino M, Schaumberg DA, Dogru M, et al. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology 2008; 115: 1982–1988. [DOI] [PubMed] [Google Scholar]
- 6.McCarty CA, Bansal AK, Livingston PM, et al. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology 1998; 105: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 7.Lu P, Chen X, Liu X, et al. Dry eye syndrome in elderly Tibetans at high altitude: a population-based study in China. Cornea 2008; 27: 545–551. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea 2011; 30: 379–387. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet 2020; 395: 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Worldometer COVID-19 Coronavirus Pandemic 2020. https://www.worldometers.info/coronavirus/ (Accessed February 2021)
- 11.Greenhalgh T, Schmid MB, Czypionka T, et al. Face masks for the public during the COVID-19 crisis. Br Med J 2020; 369: m1435. Published 2020 Apr 9. [DOI] [PubMed] [Google Scholar]
- 12.Moshirfar M, West WB, Jr, Marx DP. Face mask-associated ocular irritation and dryness. Ophthalmol Ther 2020; 9: 397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell JB, Kim JH, Roberge RJ. Powered air-purifying respirator use in healthcare: effects on thermal sensations and comfort. J Occup Environ Hyg 2017; 14: 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh NP, Walker RJE, Cowan F, et al. Retrograde air escape via the nasolacrimal system. Ann Otol Rhinol Laryngol 2014; 123: 321–324. [DOI] [PubMed] [Google Scholar]
- 15.Kousha O, Kousha Z, Paddle J. Exposure keratopathy: incidence, risk factors and impact of protocolised care on exposure keratopathy in critically ill adults. J Crit Care 2018; 44: 413–418. [DOI] [PubMed] [Google Scholar]
- 16.Isaacs D, Britton P, Howard-Jones A, et al. Do facemasks protect against COVID-19? J Paediatr Child Health 2020; 56: 976–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Offeddu V, Yung CF, Low MSF, et al. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clin Infect Dis 2017; 65: 1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozcura F, Aydin S, Helvaci MR.Ocular surface disease index for the diagnosis of dry eye syndrome. Ocul Immunol Inflamm 2007; 15: 389–393. [DOI] [PubMed] [Google Scholar]
- 19.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease Index. Arch Ophthalmol 2000; 118: 615–621. [DOI] [PubMed] [Google Scholar]
- 20.Ayaki M, Tachi N, Hashimoto Y, et al. Diurnal variation of human tear meniscus volume measured with tear strip meniscometry self-examination. PLoS One 2019; 14: e0215922. Published 2019 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bron A, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003; 22: 640–650. [DOI] [PubMed] [Google Scholar]
- 22.Schirmer O. Studien zur Physiologie und Pathologie der Tränenabsonderung und Tränenabfuhr. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1903; 56: 197–291. [Google Scholar]
- 23.Rolando M, Refojo MF, Kenyon KR. Increased tear evaporation in eyes with keratoconjunctivitis sicca. Arch Ophthalmol 1983; 101: 557–558. [DOI] [PubMed] [Google Scholar]
- 24.Mathers WD, Binarao G, Petroll M. Ocular water evaporation and the dry eye. A new measuring device. Cornea 1993; 12: 335–340. [DOI] [PubMed] [Google Scholar]
- 25.van Setten G, Labetoulle M, Baudouin C, et al. Evidence of seasonality and effects of psychrometry in dry eye disease. Acta Ophthalmol 2016; 94: 499–506. [DOI] [PubMed] [Google Scholar]
- 26.Giannaccare G, Vaccaro S, Mancini A, et al. Dry eye in the COVID-19 era: how the measures for controlling pandemic might harm ocular surface. Graefes Arch Clin Exp Ophthalmol 2020; 258: 2567–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadayer A, Zahavi A, Livny E, et al. Patients wearing face masks during intravitreal injections may be at a higher risk of endophthalmitis. Retina 2020; 40: 1651–1656. [DOI] [PubMed] [Google Scholar]
- 28.Khalili MR, Jahanbani-Ardakani H. A surge in ocular infection amid COVID-19 pandemic: a reality or a co-incidence? [Une flambée d’infections oculaires en pleine pandémie de COVID-19 : une réalité ou une coïncidence ?]. J Fr Ophtalmol. 2021;44(2):143-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chadwick O, Lockington D. Addressing post-operative mask-associated dry eye (MADE) Eye (Lond) 2021;35(6):1543-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Deng C, Chen X, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in wuhan, China: a cross-sectional study. Acta Ophthalmol 2020; 98: e951–e959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long Y, Wang X, Tong Q, et al. Investigation of dry eye symptoms of medical staffs working in hospital during 2019 novel coronavirus outbreak. Medicine (Baltimore) 2020; 99: e21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDermott AM. Antimicrobial compounds in tears. Exp Eye Res 2013; 117: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun C, Wang Y, Liu G, et al. Role of the eye in transmitting human coronavirus: what we know and what we do not know. Front Public Health 2020; 8: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]