Abstract
Flavobacterium psychrophilum is a fish pathogen that commonly affects salmonids. This bacterium produced an extracellular protease with an estimated molecular mass of 55 kDa. This enzyme, designated Fpp1 (F. psychrophilum protease 1), was purified to electrophoretic homogeneity from the culture supernatant by using ammonium sulfate precipitation, ion-exchange chromatography, hydrophobic chromatography, and size exclusion chromatography. On the basis of its biochemical characteristics, Fpp1 can be included in the group of metalloproteases that have an optimum pH for activity of 6.5 and are inhibited by 1,10-phenanthroline, EDTA, or EGTA but not by phenylmethylsulfonyl fluoride. Fpp1 activity was dependent on calcium ions not only for its activity but also for its thermal stability. In addition to calcium, strontium and barium can activate the protein. The enzyme showed typical psychrophilic behavior; it had an activation energy of 5.58 kcal/mol and was more active at temperatures between 25 and 40°C, and its activity decreased rapidly at 45°C. Fpp1 cleaved gelatin, laminin, fibronectin, fibrinogen, collagen type IV, and, to a lesser extent, collagen types I and II. Fpp1 also degraded actin and myosin, basic elements of the fish muscular system. The presence of this enzyme in culture media was specifically dependent on the calcium concentration. Fpp1 production started early in the exponential growth phase and reached a maximum during this period. Addition of calcium during the stationary phase did not induce Fpp1 production at all. Besides calcium and the growth phase, temperature also seems to play a role in production of Fpp1. In this study we found that production of Fpp1 depends on factors such as calcium concentration, growth phase of the culture, and temperature. The combination of these parameters corresponds to the combination in the natural host during outbreaks of disease caused by F. psychrophilum. Consequently, we suggest that environmental host factors govern Fpp1 production.
Microorganisms belonging to the Cytophaga-Flavobacterium-Bacteroides group constitute a large and diverse class of free-living organisms frequently found in terrestrial and marine environments. One of the most interesting features of most of the members of the genus Flavobacterium is their ability to degrade different polysaccharides, such as cellulose, agar, starch, pectin, chitin, etc., which contributes to aerobic degradation of organic material in nature and complements the global carbon cycle. By contrast, a small group of species, mainly fish pathogens, are actively proteolytic and are able to degrade casein, gelatin, etc. One of these species is Flavobacterium psychrophilum (4) (formerly Flexibacter psychropilus or Cytophaga psycrhophila), a yellow-pigmented psychrophilic bacterium responsible for systemic infections such as cold water disease (CWD) and rainbow trout fry syndrome in salmon and trout farms worldwide (9, 24). This disease causes mortality among fingerlings of rainbow trout in farms during the winter and spring with important economic consequences. However, the pathogenicity of F. psychrophilum is poorly understood, and little information about its physiology, genetic and biochemical aspects, is available, probably due to the difficulty of culturing of this bacterium in vitro (42). Virulence determinants similar to those found in other fish pathogens could contribute to the pathogenic potential of this bacterium. Thus, F. psychrophilum virulence has been associated with lipopolysaccharides (9), with the ability to lyse dead bacterial cells (48), and with the capacity of different strains to degrade chondroitin sulfate, collagen, or fibrinogen (24). The few studies done to date have related pathogenesis to the production of exocellular enzymes that degrade casein or gelatin (5) and elastin (38). Electrophoretic detection of proteases by substrate sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for different F. psychrophilum strains showed that a relationship between proteolytic profile and virulence can be established (5). Additionally, an association between elastin degradation and virulence was found by Madsen and Dalsgaard (38). In some cases, CWD-affected fish display erosion of external tissue and F. psychrophilum can be cultured from them, suggesting that extracellular proteases are virulence factors in the infection (13). However, until now, none of these proteases has been purified.
Although it is likely that the main role of bacterial proteases is to provide peptides as nutrients for the microorganism, they could also be pathogenic by facilitating bacterial erosion of host tissues. Thus, bacterial enzymes that degrade connective and muscular tissues, such as collagenases, elastases, gelatinases, etc., may play an important role. In fact, some authors regard proteases as the main virulence factors among all of the extracellular factors. It has been suggested that proteolytic enzymes of fish pathogens, such as Flavobacterium columnare (17), Aeromonas salmonicida (19), Vibrio anguillarum (47), Vibrio vulnificus (27), Yersinia ruckeri (50), and Aeromonas hydrophila (31), participate in causing massive tissue damage in the host and contribute to the invasion characteristic of the pathology.
Several reports have indicated that protease production is dependent on environmental conditions. Extracellular proteases have been shown to be sensitive to repression by different carbohydrate and nitrogen sources (22, 32). Additionally, in many cases expression of virulence factors is dependent on a single environmental factor, whereas in other cases virulence factors are coordinately regulated and their expression is mediated by a combination of two or more signals (for reviews, see references 18 and 41). Representative examples include production of the Shigella dysenteriae Shiga toxin, which is regulated by temperature and iron (55), and induction of several virulence factors in Yersinia spp. that are controlled by temperature and calcium (2, 3, 20). Other factors that affect virulence gene expression are the growth phase (40) and pH (43, 44).
In this study, we purified and characterized a 55-kDa psychrophilic protease from the fish pathogen F. psychrophilum that we designated Fpp1. This protein was thermally stabilized by calcium and was found to be a potent enzyme with broad specificity for degrading protein constituents of connective and muscular tissues. We also defined a variety of environmental conditions that stimulate expression of Fpp1 in vitro. Thus, production of this protease was controlled by the calcium concentration in the culture medium and the growth phase. Moreover, the incubation temperature also seemed to play a role in production of the protease.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Reference strain F. psychrophilum 1947 was obtained from the National Collection of Industrial, Food and Marine Bacteria. This bacterium was routinely cultured in nutrient broth (NB) (5 g of peptone from gelatin per liter, 3 g of beef extract per liter) or on NB agar (Pronadisa) at 12°C. Growth of F. psychrophilum was monitored by determining the absorbance at 525 nm of a culture with a Perkin-Elmer spectrophotometer at different times during incubation. For proteolytic activity studies, the microorganism was grown at pH 6 in NB containing 10 mM CaCl2 (NBF medium); 250-ml flasks containing 50 ml of NBF medium were each inoculated with 0.5 ml of a stationary-phase culture and incubated at 12°C and 250 rpm in a Gallenkamp orbital incubator. Aliquots were removed at different times and processed to measure the proteolytic activity and to determine presence of Fpp1 by Western blot analysis (see below).
Assay used to determine proteolytic activity and protein content.
Proteolytic activity was assayed by using azocasein (Sigma) as the substrate. Briefly, 250 μl of a suitable dilution of an enzyme solution was added to 350 μl of azocasein (1%, wt/vol) in reaction buffer containing 25 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] (pH 6.5) and 5 mM CaCl2. The mixture was incubated at 30°C for 2 h, and the reaction was terminated by adding 600 μl of 10% (wt/vol) trichloroacetic acid and leaving the preparation on ice for 30 min. The mixture was then centrifuged at 15,000 × g and 4°C for 10 min and 800 μl of the supernatant was neutralized by adding 200 μl of 1.8 N NaOH. Finally, absorbance at 420 nm was measured with a spectrophotometer (lambda 3A; Perkin-Elmer). One unit of enzyme activity was defined as the amount of protein that resulted in an increase in absorbance at 420 nm of 0.01 in 2 h under the assay conditions used.
The amount of total protein was estimated by a standard method, using bovine serum albumin (37).
Protease purification.
Five-milliliter portions of a stationary-phase culture of F. psychrophilum were used to inoculate 2-liter Erlenmeyer flasks containing 500 ml of NBF medium. After 96 h of incubation at 12°C and 250 rpm, cells were harvested by centrifugation (23,500 × g for 15 min at 4°C), and the supernatant was used as the starting point for purification. All steps were carried out at 4°C.
(i) Ammonium sulfate precipitation.
First, 741 g of ammonium sulfate was slowly added to 1,900 ml of supernatant in order to obtain 60% saturation. After 2 h, the solution was centrifuged (30,000 × g for 45 min), and the pellet was dissolved in 20 ml of 25 mM Tris-HCl (pH 7.6) containing 10 mM CaCl2 (Tris-calcium buffer) and dialyzed twice (for 18 h the first time and for 4 h the second time) against the same buffer. After dialysis the total volume was adjusted to 95 ml by adding Tris-calcium buffer.
(ii) Ion-exchange chromatography.
Dialyzed material (95 ml) was added to a beaker containing 25 ml of DEAE-Sephacel (Pharmacia, Uppsala, Sweden) previously equilibrated with Tris-calcium buffer. After 2 h of shaking at 4°C, the resin was separated from the buffer by centrifugation (30,000 × g for 5 min) and washed with the same buffer, and the supernatant was used for the next chromatography step.
(iii) Hydrophobic chromatography.
Next, 42 ml of a 4 M ammonium sulfate solution (final concentration 1.2 M) was slowly added to 100 ml of the unbound protein suspension obtained after the anion-exchange step. Then the protein solution was loaded at a flow rate of 1.1 ml/min onto a 25-ml Phenyl-Sepharose 6 fast flow column (2.5 by 10 cm; Pharmacia) previously equilibrated with Tris-calcium buffer containing 1.2 M ammonium sulfate. The column was washed with 100 ml of loading buffer, and bound proteins were eluted with a 200-ml linear gradient of ammonium sulfate ranging from 1.2 to 0 M at a flow rate of 1 ml/min. Fractions (2.3 ml) were collected, and 75-μl aliquots were assayed for protease activity as described above. Two peaks of protease activity were obtained. The most active fractions from the larger peak (fractions 48 to 85) were pooled (total volume, 83 ml), and ammonium sulfate was added to the sample to obtain a final concentration of 1.2 M. The sample was then rechromatographed on a 10-ml Phenyl-Sepharose 6 Fast Flow column (1.5 by 10 cm) equilibrated with Tris-calcium buffer containing 1.2 M ammonium sulfate. The proteolytic activity was eluted stepwise by using a solution of 0.6 M ammonium sulfate in Tris-calcium buffer at a flow rate of 0.4 ml/min. Twenty-five milliliters of a protein solution was recovered, concentrated, and washed with Tris-calcium buffer containing 5% dimethyl sulfoxide by filtering it through a Centricon 4M-30 filter (Amicon). The concentrated solution (0.5 ml) was used for size exclusion chromatography.
(iv) FPLC gel filtration chromatography.
For fast protein liquid chromatography (FPLC) (Superdex 75); (Pharmacia) a column was equilibrated with Tris-calcium buffer containing 150 mM NaCl, and a 100-μl sample was loaded at a flow rate of 0.2 ml/min. Fractions (0.5 ml) were collected and analyzed for proteolytic activity. Protein standards were subsequently injected into the column in order to estimate the molecular mass of the protease. Aliquots of the purified protein were stored at −20°C. For some experiments, fractions of the purified Fpp1 protease were filtered extensively through a Centricon 4M-30 filter with 25 mM Tris-HCl (pH 7.6) in order to completely eliminate the calcium in the sample. The enzyme obtained in this way was designated calcium-free Fpp1.
Characterization of the enzyme activity.
The caseinolytic activity of pure Fpp1 was assayed at pH values ranging from 4.7 to 11.2 at 37°C in the presence of 5 mM CaCl2 by using azocasein as the substrate. The following buffers were used: for pH 4.7, 25 mM acetate; for pH 5.6 and 6.3, 25 mM MES (2-N-morpholinoethanesulfonic acid); for pH 6.5, 6.8, and 7.3, 25 mM PIPES; for pH 7.6, 25 mM MOPS (3-N-morpholinopropanesulfonic acid); for pH 7.9, 8.7, and 9.1, 25 mM Tris-HCl; and for pH 9.9, 10.4, and 11.2, 25 mM CAPS [3-(cyclo-hexylamino)1-propanesulfonic acid)].
To test the effect of temperature on the activity of the enzyme, purified calcium-free Fpp1 was incubated at 4, 12, 15, 18, 25, 30, 35, 40, 45, and 50°C for 2 h in 25 mM PIPES (pH 6.5) by using 1% azocasein as the substrate. The enzyme was tested in the presence of 5 mM CaCl2. The activation energy (Ea) was determined from the slope (−Ea/R) of Arrhenius plots of ln k (k = 100 × enzyme units [EU]) versus the reciprocal of the temperature (in Kelvins). Thermal stability was assayed by incubating Fpp1 at 40°C in the presence or absence of 5 mM CaCl2 for different periods of time and then measuring the residual activity under the standard conditions.
For inhibition studies, Fpp1 was incubated with different inhibitors for 10 min on ice in 25 mM PIPES buffer (pH 6.5) before the caseinolytic activity was measured under the standard conditions.
Electrophoresis and zymograms.
The method used for SDS-PAGE was essentially the method described by Laemmli (30). For enzymatic activity assays, Fpp1 (0.8 μg) was incubated with various protein substrates (12 μg), including type I, type II, and type IV collagens, type I gelatin, type I laminin, fibronectin, fibrinogen, actin, and myosin, at 14°C for 24 h in 25 mM PIPES (pH 6.5) containing 5 mM CaCl2 and 0.05% (vol/vol) Brij 35. The reactions were terminated by adding 10 mM EGTA, and the products were analyzed by SDS–10% PAGE.
For zymogram analysis, 0.1% sodium caseinate or 0.1% gelatin copolymerized with gels was used. Heating was avoided prior to and during electrophoresis, which was performed at 4°C at a constant current of 30 mA. Following electrophoresis the gels were washed twice with deionized water and twice with 25 mM PIPES buffer (pH 6.5), both containing 2.5% (vol/vol) Triton X-100. Each wash was performed for 30 min at 4°C. The gels were then incubated overnight in the same buffer containing 5 mM CaCl2 at room temperature. Finally, the gels were stained with 0.1% Coomassie brilliant blue R250 in methanol-acetic-water (4:1:5, vol/vol/vol) and destained in the same solution without the dye, which revealed zones of substrate hydrolysis. Silver staining was carried out by the method of Sammons et al. (49).
Antiserum preparation and protein immunodetection.
Forty micrograms of the purified protease was used to immunize a New Zealand White rabbit in order to raise antibodies. Twenty-seven days after immunization the rabbit was exsanguinated, and the serum was separated and stored in aliquots. For immunodetection experiments, culture supernatants were electrophoresed on SDS-PAGE gels and transferred to nitrocellulose membranes by standard techniques. The membranes were then blocked by incubation at room temperature in 1% skim dry milk in phosphate-buffered saline containing 0.1% Tween 20 for 1 h. After washing, the blots were successively incubated with the antiserum (1:500) raised against Fpp1 (see above), anti-rabbit immunoglobulin-alkaline phosphatase antibodies (1:10,000; Sigma), and 5-bromo-4-chloro-3-indolylphosphate (BCIP)–Nitro Blue Tetrazolium substrate in alkaline phosphatase buffer (4 mM MgCl2, 50 mM Tris-HCl; pH 9.6). Three washes with phosphate-buffered saline containing 0.1% Tween 20 were performed between all incubations except the last one, for which we used alkaline phosphate buffer just before the substrate solution was added. Color development was stopped by washing with 0.1 M EDTA in distilled water.
Effect of culture conditions on Fpp1 production.
For all experiments, 250-ml flasks containing 50 ml of culture medium were inoculated with 0.5 ml of stationary-phase cultures. The effect of temperature on protease production was assayed by growing the microorganism in NB or NBF medium at 12 or 18°C. Calcium concentration effects were studied by adding CaCl2 at concentrations ranging from 0 to 20 mM to NB at pH 6. Finally, for pH studies, the pH of NBF medium was adjusted to 6, 6.5, 7, or 7.5 with HCl or NaOH. In all cases, after 4 days of incubation at 12°C 5-ml aliquots were removed and 4 ml of each culture supernatant was precipitated with 10% (wt/vol) trichloroacetic acid and left on ice for 60 min. After centrifugation at 30,000 × g and 4°C for 15 min, samples were resuspended in Laemmli sample buffer (30) and used for Western blot analysis.
Time course experiments were carried out in NBF medium at 12°C, and at different times samples were withdrawn and processed for immunoblot analysis as described above. Induction experiments were performed by incubating the microorganism in NB until the mid-exponential or stationary growth phase was reached, and then 10 mM (final concentration) CaCl2 was added. The bacteria were grown for two additional days, and then samples were withdrawn and processed for immunoblot analysis. Repression experiments were carried out by incubating the microorganism in NBF medium for 2 days; then after the cells were washed twice with distilled water, the bacteria were resuspended in the same volume of NB and the preparation was split into two parts. CaCl2 (final concentration, 10 mM) was added to one of the parts, and both parts were incubated for two additional days under the same conditions. Culture supernatants were obtained and used for Western blot analysis as described above.
RESULTS
Purification of Fpp1 from F. psychrophilum.
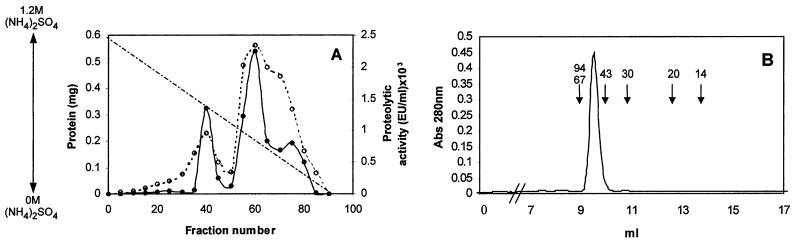
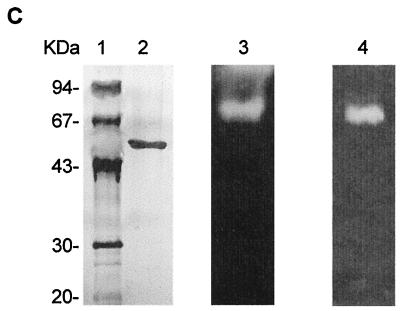
Different culture conditions were used to obtain the maximum levels of proteolytic activity of F. psychrophilum 1947 (data not shown). The optimum level (235 EU/ml), as indicated by azocasein degradation, was observed in NBF medium (see Materials and Methods) after 4 days of incubation at 12°C and 250 rpm. After the microorganism was grown under these conditions, a procedure for purifying a major protease was developed (Table 1). Ammonium sulfate precipitation of the culture supernatant followed by dialysis resulted in a 25.9-fold increase in the specific activity. The protein solution obtained in this way was then adsorbed onto DEAE-Sephacel, and nonadsorbed proteins were recovered. During this step, proteolytic activity was detected in the DEAE-Sephacel-bound fraction, although most proteolytic activity remained in the unbound fraction. This material was then subjected to hydrophobic chromatography. First, the protein was eluted from a Phenyl-Sepharose column (see Materials and Methods), which resulted in two separate peaks of proteolytic activity, one eluting at 0.67 M ammonium sulfate and the larger one eluting at 0.41 M ammonium sulfate (Fig. 1A). Fractions containing the major peak were pooled, and after ammonium sulfate was added to bring the concentration to 1.2 M, the solution was loaded onto a smaller Phenyl-Sepharose column. The proteolytic activity was then eluted in a stepwise fashion (see Materials and Methods). At this stage, SDS-PAGE of the eluted protein showed a high level of purity, although the yield was low (approximately 3.8%). After the FPLC gel filtration step 231-fold purification was obtained, and only one protein, a 55-kDa protein, was observed when the protein in the SDS-PAGE gel was silver stained (Fig. 1C, lane 2). The proteolytic activity of this protein was confirmed by zymogram analysis by using casein or gelatin as the substrate (Fig. 1C, lanes 3 and 4). The molecular mass of the native enzyme was estimated to be 55 kDa by FPLC gel filtration on a Superdex 75 column (Fig. 1B). On the SDS-PAGE gel, the apparent molecular mass was also 55 kDa, indicating that the protein is active as a monomer (Fig. 1C, lane 2).
TABLE 1.
Purification of F. psychrophilum 55-kDa metalloprotease
| Purification step | Total protein (mg) | Total activity (EU) | Sp act. (EU/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Culture supernatant | 4,325 | 478,800 | 110.7 | 1 | 100 |
| Ammonium sulfate | 88.3 | 253,650 | 2,870 | 25.9 | 52.9 |
| DEAE-Sephacel (batch) | 41 | 195,300 | 4,763 | 43 | 40.8 |
| Phenyl-Sepharose (gradient) | 8.9 | 126,072 | 14,133 | 127.6 | 26.3 |
| Phenyl-Sepharose (stepwise) | 0.8 | 18,170 | 22,712 | 205 | 3.8 |
| Superdex 75 (FPLC gel filtration) | 0.7 | 17,950 | 25,642 | 231.5 | 3.7 |
FIG. 1.
Chromatography steps and SDS-PAGE used for purification of calcium-dependent protease Fpp1 from F. psychrophilum. (A) Phenyl-Sepharose chromatography profile. The active unbound fraction from DEAE-Sephacel chromatography was applied to a Phenyl-Sepharose column in 1.2 M ammonium sulfate. The samples were eluted with a linear 1.2 to 0 M ammonium sulfate gradient (dashed line). Fractions were collected and assayed to determine caseinolytic activity (dotted line) and protein content (solid line) as described in Materials and Methods. (B) Elution pattern for Fpp1 protease with FPLC Superdex 75 gel filtration chromatography. A concentrated solution containing the proteolytic activity recovered during Phenyl-Sepharose column stepwise elution was loaded onto a Superdex 75 FPLC gel filtration column and chromatographed as described in Materials and Methods. The positions of molecular mass markers (in kilodaltons) are indicated by arrows. Abs 280 nm, absorbance at 280 nm. (C) SDS–12.5% PAGE of the purified calcium-dependent Fpp1 protease. The positions of molecular mass markers (in kilodaltons) are indicated on the left. Lane 1, molecular mass markers; lane 2, silver-stained purified Fpp1 (0.8 μg); lanes 3 and 4, caseinolytic and gelatinolytic activities of purified Fpp1 as determined by substrate gel electrophoresis with 1% sodium caseinate and gelatin, respectively, as described in Materials and Methods.
Fpp1 is a metalloprotease with an optimum pH for activity of 6.5.
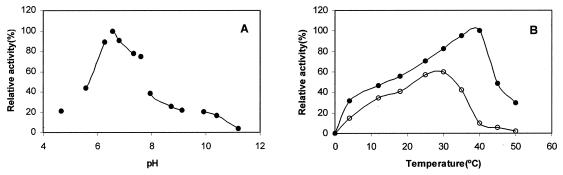
The effect of pH on Fpp1 activity was tested at pH 4.7 to 11.2 (Fig. 2A). Optimal Fpp1 proteolytic activity occurred at a moderately acidic pH (pH 6.5), and there was a sharp dependence profile (Fig. 2A). Levels of activity slightly less than 50% of the maximal level of protease activity occurred at pH 5.6 and 7.9.
FIG. 2.
Effect of pH and temperature on the activity of the Fpp1 protease. (A) The enzyme (0.2 μg) was incubated for 2 h in 600-μl portions of the buffers described in Materials and Methods containing 5 mM CaCl2 and 1% azocasein. The value obtained at pH 6.5 was defined as 100%. (B) The enzyme (0.2 μg) was incubated in 600-μl portions of 25 mM PIPES buffer (pH 6.5) containing 1% azocasein for 2 h at various temperatures, and caseinolytic activity was measured as described in the text. Symbols: (○), calcium-free enzyme; (●) enzyme in the presence of 5 mM CaCl2. The relative activities are averages based on two independent experiments.
A range of protease inhibitors were tested (Table 2). Fpp1 activity was completely inhibited (less than 10% activity) by the chelating agents EDTA and EGTA (10 mM) (Table 2) and also by the metalloprotease inhibitor 1,10-phenanthroline at a concentration of 1 mM. The zinc chelator Zincov (Calbiochem), as well as the serine protease inhibitor phenylmethylsulfonyl fluoride, failed to suppress activity, while 10 mM dithiothreitol caused 80% inhibition. In contrast, when CaCl2 was added to reaction mixtures containing calcium-free Fpp1, Fpp1 proteolytic activity increased as the CaCl2 concentration increased from 10 μM to 5 mM. However, a level of inhibition of about 36% was observed when the calcium concentration was increased from 5 to 10 mM. SrCl2 and BaCl2 had similar effects, whereas other cations, such as Mg2+, Mn2+, and Zn2+, at concentrations less than 1 mM had no significant effect on Fpp1 activity. On the other hand, reconstitution assays aimed at defining the nature of the metal ion(s) required for Fpp1 proteolytic activity showed that when CaCl2, BaCl2, or SrCl2 (final concentration, 5 mM) was added to Fpp1 treated with EGTA, about 75% of the proteolytic activity was recovered.
TABLE 2.
Metal ion and inhibitor effects on the activity of the Fpp1 protease
| Compound(s) (concn [mM]) | Caseinolytic activity (%) |
|---|---|
| None | 100 |
| CaCl2 (0.01) | 100.6 ± 1.2 |
| CaCl2 (0.1) | 100.1 ± 1 |
| CaCl2 (0.5) | 146.8 ± 2.4 |
| CaCl2 (1) | 297.6 ± 5.3 |
| CaCl2 (5) | 301.6 ± 5.2 |
| CaCl2 (10) | 191.5 ± 3.6 |
| MgCl2 (1) | 99.2 ± 0.9 |
| MgCl2 (10) | 88.5 ± 1.8 |
| ZnCl2 (0.005) | 94.6 ± 0.5 |
| ZnCl2 (0.01) | 97.7 ± 2.0 |
| ZnCl2 (0.1) | 94.6 ± 0.7 |
| ZnCl2 (1) | 84 ± 1.5 |
| MnCl2 (1) | 117.7 ± 2.1 |
| MnCl2 (10) | 21.5 ± 3.8 |
| SrCl2 (1) | 250.8 ± 4.9 |
| SrCl2 (5) | 258.5 ± 5.2 |
| BaCl2 (1) | 172.6 ± 3.6 |
| BaCl2 (5) | 264.4 ± 5.8 |
| Phenylmethysulfonyl fluoride (1) | 97.9 ± 2.3 |
| 1,10-Phenanthroline (1) | 4.2 ± 1.5 |
| Dithiothreitol (1) | 78.8 ± 6 |
| Dithiothreitol (10) | 19.4 ± 1.4 |
| EDTA (1) | 18.5 ± 0.5 |
| EDTA (10) | 9.1 ± 1 |
| EGTA (1) | 12.3 ± 3 |
| EGTA (10) | 8.7 ± 1.2 |
| Zincov (1) | 101.1 ± 0.3 |
| EGTA (1) + CaCl2 (5) | 74.4 ± 3.2 |
| EGTA (1) + BaCl2 (5) | 70.5 ± 4.7 |
| EGTA (1) + SrCl2 (5) | 85.1 ± 5.3 |
Protease Fpp1 is thermally stabilized by calcium.
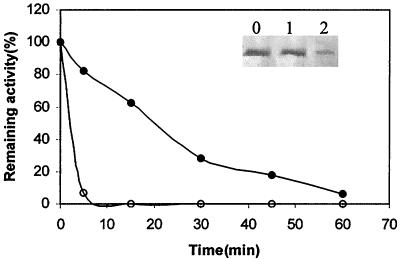
The temperature dependence curve for Fpp1 is shown in Figure 2B. The calcium-free enzyme is a psychrophilic heat-sensitive protease that remains active at temperatures ranging from 4 to 37°C; approximately 60% relative activity occurs at 12°C. However, a different profile was observed when 5 mM CaCl2 was present in the reaction mixtures. Addition of calcium resulted in a shift in the apparent optimal temperature for activity from 25 to 30°C to 37 to 40°C. The enzyme became unstable in the presence of calcium at about 40°C, as deduced from an Arrhenius plot (data not shown), but 30% of the activity remained at 50°C. The Ea for hydrolysis of azocasein at 4 to 40°C was estimated to be 5.58 ± 0.09 kcal/mol from the linear portion of the Arrhenius plot (data not shown). All calcium-free enzyme activity was lost after incubation of the protein for 5 min at 40°C (Fig. 3). This loss was accompanied by degradation of the protein, as shown by Western blot analysis (Fig. 3, lane 2). Under the same incubation conditions in the presence of 5 mM CaCl2, the enzyme was not substantially degraded (Fig. 3, lane 1). Moreover, the Fpp1 protease exhibited 80 and 30% relative activity after 5 and 30 min, respectively, in the presence of 5 mM CaCl2 (Fig. 3).
FIG. 3.
Thermostability of the Fpp1 protease in the presence (●) or absence (○) of 5 mM CaCl2. The enzyme (0.2 μg) was incubated in 100-μl portions of 25 mM PIPES (pH 6.5) at 40°C for different times. Then the remaining activity was measured by adding 500 μl of a 1% azocasein solution to the same buffer. The reactions were carried out as described in Materials and Methods. (Inset) Immunoblot probed with antibodies (1:500) raised against the Fpp1 protein. The calcium-free enzyme (0.4 μg) was incubated in 25 mM PIPES (pH 6.5) in the presence (lane 1) or in the absence (lane 2) of 5 mM CaCl2 at 40°C for 5 min, and its presence was analyzed by immunoblotting. Lane 0, no temperature treatment. The relative activities are averages based on two independent experiments.
As Fig. 4A shows, gelatin was completely degraded by Fpp1, whereas digestion of laminin, fibrinogen, fibronectin, and type IV collagen generated both small and large fragments. In contrast, the levels of hydrolysis of type I and type II collagens were quite low (Fig. 4A, lanes 7 and 5, respectively). Fpp1 cleaved actin and myosin (Fig. 4B), proteins which constitute muscle fibers. Under the same conditions the elastin Congo red was not degraded by Fpp1 (data not shown).
FIG. 4.
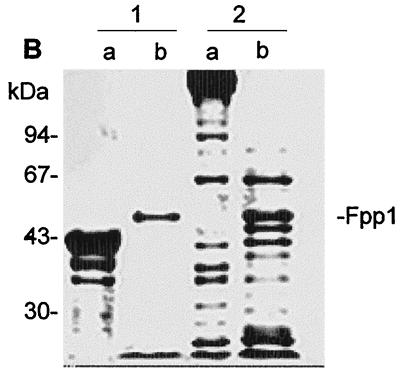
Degradation of different proteins by Fpp1. (A) Extracellular matrix compounds; (B) muscular proteins. The substrates (12 μg) were incubated in the absence (lanes a) or in the presence (lanes b) of Fpp1 (0.8 μg) at 14°C for 24 h. Each reaction was terminated with 10 mM EGTA, and the reaction products were electrophoresed on SDS–10% PAGE gels. (A) Lanes 1, fibrinogen; lanes 2, laminin; lanes 3, gelatin; lanes 4, fibronectin; lanes 5, type II collagen; lanes 6, type IV collagen; lanes 7, type I collagen. (B) Lanes 1, actin; lanes 2, myosin. The positions of molecular mass markets are indicated on the left. The position of Fpp1 in lanes b is indicated on the right.
Fpp1 is induced by calcium and is produced during the exponential growth phase.
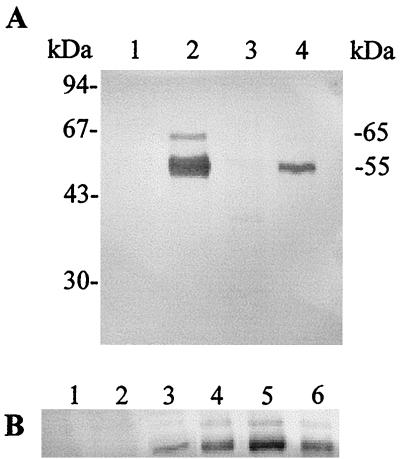
Using the antibodies raised against Fpp1, it was possible to establish a relationship between the presence of Fpp1 protease in the culture supernatant and environmental factors (growth phase, pH, temperature, and calcium). The Fpp1 enzyme was detected in the supernatant when the microorganism was grown in NBF medium at 12°C (Fig. 5A, lane 2) and at 18°C (Fig. 5A, lane 4) but not when it was grown in NB (Fig. 5A, lanes 1 and 3). The lower intensity of the protein band when the cells were grown at 18°C suggests that Fpp1 activity is also regulated by temperature (Fig. 5A, lane 4). Production of the enzyme can, therefore, be considered calcium dependent and temperature regulated. Other cations (Zn2+, Mn2+, Mg2+, Sr2+, Ba2+) were not able to induce Fpp1 protease activity (data not shown). As Fig. 5 shows, a band at about 65 kDa appeared in all the Western experiments along with the corresponding Fpp1 band.
FIG. 5.
Detection of Fpp1 in culture supernatants of F. psychrophilum. Culture supernatants (4 ml) from cultures incubated under different conditions were precipitated with trichloroacetic acid and then loaded onto a SDS–12.5% PAGE gel. Immunoblotting was carried out as described in Materials and Methods. (A) F. psychrophilum was grown until the onset of the stationary phase at 12°C (lanes 1 and 2) and 18°C (lanes 3 and 4) in NB (lanes 1 and 3) or NBF medium (lanes 2 and 4). The positions of the 55-kDa (Fpp1) and 65-kDa immunoreacting bands are indicated on the right. (B) The microorganism was grown until the stationary phase at 12°C in NB containing 0 to 20 mM CaCl2. Lane 1, no CaCl2; lanes 2 to 6, 0.1, 1, 5, 10, and 20 mM CaCl2, respectively. All of the immunoblot experiments were carried out at least three times.
Furthermore, when the microorganism was grown at 12°C in the presence of concentrations of CaCl2 ranging from 0.1 to 20 mM, maximum induction was observed in the presence of 10 mM CaCl2, while no Fpp1 induction was detected when the CaCl2 concentration was 0.1 mM. An almost linear relationship between increasing levels of Fpp1 and increasing calcium concentrations in the culture medium was observed at concentrations from 1 to 10 mM (Fig. 5B).
Additionally, the presence and amount of Fpp1 were pH dependent at pH 6 to 7.5, and a maximum production occurred at pH 6 (data not shown). At pHs below this pH (pH 5.4 and 5.7), low levels of Fpp1 production were detected (data not shown).
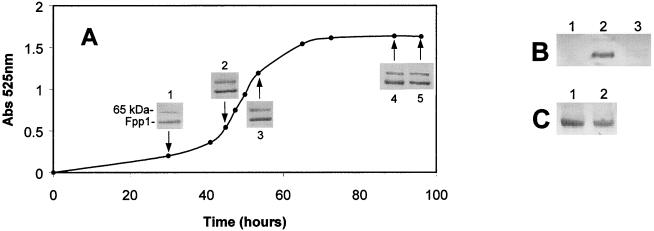
Fpp1 production was initially detected in exponential-phase cells of F. psychrophilum, and maximum production occurred during this phase (Fig. 6A); the amount of enzyme was constant during the stationary phase. Addition of CaCl2 during the exponential growth phase resulted in the presence of the Fpp1 protease in the medium (Fig. 6B, lane 2). However, when 10 mM CaCl2 was added to NB cultures at the early stationary phase, no Fpp1 protease production occurred (Fig. 6B, lane 3). On the other hand, when cells were grown in NBF medium until the early stationary phase and subsequently transferred to NB, only a small decrease in the amount of Fpp1 protease was observed compared with the amount of Fpp1 produced on NBF medium (Fig. 6C, lanes 1 and 2).
FIG. 6.
Fpp1 production by F. psychrophilum during growth. (A) The bacterium was grown at 12°C in NBF medium, and at different times (arrow 1, 28 h; arrow 2, 48 h; arrow 3, 52 h; arrow 4, 90 h; arrow 5, 96 h) Fpp1 was detected by Western blotting. Growth was monitored by determining culture absorbance at 525 nm (Abs 525 nm). (B) The bacterium was grown at 12°C in NB, and in the middle of the exponential phase (lane 2) or in the stationary phase (lane 3) 10 mM CaCl2 was added to the cultures. In both cases, incubation was continued for two additional days, and Fpp1 was detected by Western blotting. Lane 1 contained with an NB culture used as a negative control. (C) The microorganism was grown at 12°C in NBF medium for 2 days (stationary-phase culture), and then cells were washed by centrifugation and resuspended in NB (lane 2) or NBF medium (lane 1). Incubation was continued for two additional days, and samples for immunoblot analysis were obtained as described in Materials and Methods. All of the immunoblot experiments were carried out three times.
DISCUSSION
F. psychrophilum grows at temperatures between 4 and 20°C. Using gel substrate electrophoresis, Bertolini et al. (5) observed that an array of proteolytic enzymes were present and suggested that some of them were related to virulence. In this study we purified a protease and examined the physiological conditions under which this enzyme was produced.
Data presented here shows that a pronounced increase in specific proteolytic activity occurred after ammonium sulfate precipitation. However, during this step there was a marked reduction in yield. This reduction was probably due to elimination of peptides and proteins present in NBF medium, together with some inactivation of the proteolytic activity by ammonium sulfate. Utilization of two sequential hydrophobic chromatography steps with different elution techniques turned out to be a valuable method for purification of the protease. As the Fpp1 proteolytic activity started to elute from the first Phenyl-Sepharose column at 0.6 M ammonium sulfate, we decided to carry out stepwise elution in a second column by suddenly reducing the concentration of the salting-out ions in the buffer from 1.2 to 0.6 M. This resulted in elution of very pure Fpp1 protease, although the yield was greatly decreased. The purification process increased the specific activity of the protease by more than 25,642-fold, whereas the level of recovery of Fpp1 activity was only 3.7%.
Although pure Fpp1 was used to raise antibodies, a 65-kDa cross-reacting band was observed in all of the immunodetection experiments performed with the anti-Fpp1 antibodies. Thus, there was a correlation between Fpp1 and the 65-kDa band in terms of intensity and appearance conditions (Fig. 6A). This suggests that the two proteins are related. The 65-kDa cross-reacting band could be the precursor of the mature 55-kDa Fpp1 protein, as occurs in a subtilisin-like protease produced by a psychrotolerant Vibrio sp. (28). This protease is secreted as a 47-kDa protein and then undergoes C-terminal autolysis which gives rise to 36-kDa enzyme (28). Aqualisin I is also produced as a 51-kDa precursor, although the mature protease has a molecular mass of 29 kDa (29).
Fpp1 protease can be presumptively classified as a metalloprotease since EGTA, EDTA, and 1,10-phenanthroline but not phenylmethylsulfonyl fluoride inhibited the enzyme's activity. Fpp1 activity depends on the presence of Ca2+, and we also observed that related cations such as Sr2+ and Ba2+, are able to increase the activity. Ca2+ was important not only, for Fpp1 activity since it also increased the thermostability of Fpp1. The temperature dependence profile showed that Fpp1 is a thermolabile cold-adapted enzyme. Fpp1 displays high catalytic efficiency at low temperatures (at 18°C it exhibits 50% of its maximum activity). It is also unstable at temperatures over 40°C, and it has a low Ea (5.58 kcal/mol). Some of these characteristics are typical of exocellular enzymes from psychrotrophic bacteria, including amylases from a psychrophilic bacterium isolated from Japan Sea sediments (21), lipases from Pseudomonas sp. (6), a subtilisin-like protease from Bacillus strain TA41 (10), and metalloproteases from Pseudomonas fluorescens (39) and Y. ruckeri (50). The experiments described here indicate that the thermal stability of the Fpp1 protease is reduced when calcium is removed from the buffer. In contrast to calcium-saturated Fpp1, which lost one-half of its activity after approximately 25 min of incubation at 40°C, the calcium-free enzyme underwent complete autolysis after 2 min of incubation at 40°C. Thus, this biochemical behavior of Fpp1 is similar to the behavior of the metalloproteases produced by P. fluorescens (34), which required Ca2+ ions for activity and/or thermal stability, and to the behavior of the subtilisin family of proteases, which are calcium-dependent serine proteases (52, 54). Of these enzymes, subtilisin (52), proteinase K (1), the calcium-stimulated protease from the cyanobacterium Anabaena variabilis (36), aqualisin from Thermus aquaticus (35), and the cell envelope proteinase from Lactococcus lactis (14, 15) are probably the best studied. The thermostability of all of these enzymes depends on the presence of Ca2+. Additionally, the fact that zinc ions and zincov have no effect on Fpp1 activity indicates that this protease is not a zinc metalloprotease. Furthermore, the inhibition of Fpp1 activity by dithiothreitol suggests that disulfide bonds could be important in maintaining the molecular conformation required for activity. On the other hand, depletion of Ca2+ by treatment with EGTA and subsequent filtration incubation of Fpp1 with Ca2+, Sr2+, or Ba2+ restored approximately 75% of the activity. This suggests that as in proteinase K (1) and the cell envelope proteinase from L. lactis (14), removal of Ca2+ triggers a conformational change of the substrate recognition site that impedes complete recovery of activity.
Our studies on degradation of various substrates showed that at 14°C Fpp1 readily hydrolyzes matrix and basement membrane proteins to a greater or lesser extent. In this regard the behavior of Fpp1 is similar to that of the matrix metalloproteases, a family of eucaryotic enzymes which have attracted considerable interest due to their abilities to degrade essentially all protein constituents of connective tissue (46). Matrix metalloproteases are associated with tumor progression in cancer due to proteolytic breakdown of the connective tissue that contributes to metastatic spread (53). The wide range of proteolytic activity of Fpp1 is shown by hydrolysis of the fish muscular proteins actin and myosin. This clearly indicates that Fpp1 could participate in invasion of different tissues during progression of an infection. However, it must be pointed out that although Fpp1 was the protease that was most active against azocasein found in the supernatant of F. psychrophilum cultures, it may not turn out to be the protease that is most active against the proteins of interest in pathogenesis.
Despite the probable importance of proteases in establishment and maintenance of CWD, nothing is known about the environmental signals that regulate biosynthesis of these molecules. Of special interest was the finding that the Fpp1 protease was induced by calcium. The calcium requirement was highly specific since no other ions (Zn2+, Mg2+, and Mn2+, as well as Sr2+ and Ba2+, both of which activated Fpp1) were able to induce enzyme production. In addition, calcium had to be present during the exponential growth phase in order to induce Fpp1, and maximum production occurred before the beginning of the stationary phase. However, addition of calcium during the stationary phase did not induce production of Fpp1, suggesting that induction is growth phase related. This kind of growth phase dependence observed for the Fpp1 protease also occurs with virulence factors of Staphylococcus aureus (25), Yersinia enterocolitica (43), Clostridium difficile (12), and Streptococcus pyogenes (40). Furthermore, a constant presence of calcium was necessary for Fpp1 production, as deduced from the decrease in Fpp1 levels after calcium depletion. Liao et al. found in P. fluorescens CY091 a pectate lyase (33) and a protease (34) which required the presence of Ca2+ or Sr2+ in the medium for production. The calcium ion has a wide variety of biological roles, and specific induction of Fpp1 by calcium can be mediated through calmodulin-like regulatory proteins which have been described previously for some bacteria (45, 51). Moreover, as in P. fluorescens, the level of protease induction in F. psychrophilum was directly related to the amount of calcium in the culture medium, suggesting that there is a tight regulatory system. The calcium concentration dependentce of Fpp1 expression must be a reflection of the environmental conditions present in the host. The characteristic calcium concentrations in rainbow trout and salmon sera range from 5.5 to 6.4 mM (23) and from 3.5 to 12.5 mM (26), respectively. This indicates that the Fpp1 protease would be induced at optimal levels during the fish infection process. Thus, the calcium concentration is one of the environmental signals that could alert the bacteria about a possible interaction with the host. A similar situation has been found in V. anguillarum, in which the gastrointestinal mucus of the Atlantic salmon induces synthesis of a protease and several outer membrane proteins (11, 16).
Incubation of F. psychrophilum at 18°C and in the presence of calcium resulted in significantly lower levels of Fpp1 production than the levels observed at 12°C. This observation suggests that Fpp1 production is also regulated by temperature and is higher at temperatures below the optimum growth temperature. At this point it is necessary to recall that although the bacterium grows better at 18°C, development of the disease (CWD) occurs mainly when the water temperature is between 10 and 15°C (9). Thus, all the data show that Fpp1 production was not necessary for in vitro growth of the bacterium, but (as a putative virulence factor) Fpp1 induction may be an adaptation to the conditions needed for efficient infection. This kind of temperature-dependent regulation of protease production also occurs in Y. ruckeri (50). In this case, there is inhibition of protease production at 28°C, while the growth rate is optimal. Other Yersinia species (Y. enterocolitica and Y. pseudotuberculosis) also express temperature-dependent proteins at 37°C but not at 26°C (7, 8). Although in a different way, temperature- and calcium-regulated virulence occurs with species of Yersinia. In these cases, at 37°C growth is dependent on millimolar levels of calcium, but expression of virulence genes occurs only when calcium is absent (2, 3).
Taken together, the results described above provide evidence that Fpp1 regulation is a new example of enzyme regulation by conditions present in the host environment. It is probable that other genes and proteins are regulated in a similar way by coordinated control that senses environmental signals (i.e., calcium levels) which affect entry of the microbe into the host tissues. The calcium levels needed for optimal in vitro Fpp1 induction are those found in the blood of fish. The enzyme had a wide range of matrix and muscle protein substrates, which strongly suggests that it participates in pathogenensis by contributing to colonization and/or invasion of the fish tissues. Further work is needed to elucidate the role of calcium in induction of proteins in F. psychrophilum and the relationship of calcium to progress of the disease.
ACKNOWLEDGMENTS
This research was supported by grant 1FD97-0426 to J.A.G.
We thank V. Quesada and J. Huergo for the FPLC analysis; J. Uria for the connective tissue protein degradation studies; D. Rodriguez, L. M. Quiros, and L. M. Sanchez for assistance with protein purification and biochemical studies; J.-F. Bernardet for help with the world of Flavobacterium; and A. Obaya and J. F. Aparicio for critical reading of the manuscript. In particular, we thank S. Cal for his comments, ideas, and constant help and generosity.
REFERENCES
- 1.Bajorath J, Hinrichs W, Saenger W. The enzymatic activity of proteinase K is controlled by calcium. Eur J Biochem. 1988;176:441–447. doi: 10.1111/j.1432-1033.1988.tb14301.x. [DOI] [PubMed] [Google Scholar]
- 2.Barve S S, Straley S C. lcrR, a low-Ca2+-response locus with dual Ca2+-dependent functions in Yersinia pestis. J Bacteriol. 1990;172:4661–4671. doi: 10.1128/jb.172.8.4661-4671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman T, Hakansson S, Forsberg A, Norlander L, Macellara A, Backman A, Bolin I, Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardet J F, Sergers P, Vancanneyt M, Berthe F, Kersters K, Vandamme P. Cutting a Gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. Int J Syst Bacteriol. 1996;46:128–148. [Google Scholar]
- 5.Bertolini J M, Wakabayashi H, Watral V G, Whipple M J, Rohovec J S. Electrophorectic detection of proteases from selected strains of Flexibacter psychrophilus and assessment of their variability. J Aquat Anim Health. 1994;6:224–233. [Google Scholar]
- 6.Choo D-W, Kurihara T, Suzuki T, Soda K, Esaki N. A cold-adapted lipase of an Alaskan psychrotroph, Pseudomonas sp. strain B11–1: gene cloning and enzyme purification and characterization. Appl Environ Microbiol. 1998;64:486–491. doi: 10.1128/aem.64.2.486-491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelis G R. The Yersinia deadly kiss. J Bacteriol. 1998;180:5495–5504. doi: 10.1128/jb.180.21.5495-5504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis G R, Boland A, Boyd A P, Genijen C, Iriarte M, Neyt C, Sory M-P, Stanier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalsgaard I. Virulence mechanisms in Cytophaga psychrophila and other Cytophaga-like bacteria pathogenic for fish. Annu Rev Fish Dis. 1993;3:127–144. [Google Scholar]
- 10.Davail S, Feller G, Narix E, Gerday C. Cold adaptation of proteins. J Biol Chem. 1994;269:17448–17453. [PubMed] [Google Scholar]
- 11.Denkin S M, Nelson D R. Induction of protease activity in Vibrio anguillarum by gastrointestinal mucus. Appl Environ Microbiol. 1999;65:3555–3560. doi: 10.1128/aem.65.8.3555-3560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupuy B, Sonenshein A L. Regulated transcription of Clostridium difficile toxin genes. Mol Microbiol. 1998;27:107–120. doi: 10.1046/j.1365-2958.1998.00663.x. [DOI] [PubMed] [Google Scholar]
- 13.Ekman E, Borjeson H, Johansson N. Flavobacterium psychrophilum in Baltic salmon Salmo salar brood fish and their offspring. Dis Aquat Org. 1999;37:159–163. doi: 10.3354/dao037159. [DOI] [PubMed] [Google Scholar]
- 14.Exterkate F A. Structural changes and interactions involved in the Ca2+-triggered stabilization of the cell-bound cell envelope proteinase in Lactococcus lactis subsp. cremoris SK11. Appl Environ Microbiol. 2000;66:2021–2028. doi: 10.1128/aem.66.5.2021-2028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exterkate F A, Alting A C. Role of calcium in activity and stability of the Lactococcus lactis cell envelope proteinase. Appl Environ Microbiol. 1999;65:1390–1396. doi: 10.1128/aem.65.4.1390-1396.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia T, Otto K, Kjellberg S, Nelson D R. Growth of Vibrio anguillarum in salmon intestinal mucus. Appl Environ Microbiol. 1997;63:1034–1039. doi: 10.1128/aem.63.3.1034-1039.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin B R. Columnaris disease: recent advances in research. Aquaculture. 1987;13:48–50. [Google Scholar]
- 18.Griffiths E. Environmental regulation of bacterial virulence-implication for vaccine design and production. Trends Biotechnol. 1991;9:309–315. doi: 10.1016/0167-7799(91)90101-m. [DOI] [PubMed] [Google Scholar]
- 19.Gunnlaugsdottir B, Gudmundsdottir B K. Pathogenicity of atypical Aeromonas salmonicida in Atlantic salmon compared with protease production. J Appl Microbiol. 1997;83:543–541. doi: 10.1046/j.1365-2672.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- 20.Hale T L. Genetic basis for virulence in Shigella species. Microbiol Rev. 1991;55:206–224. doi: 10.1128/mr.55.2.206-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamamoto T, Russel N J. Psychrophiles. In: Horikoshi K, Grant W D, editors. Extremophiles. New York, N.Y: Wiley; 1988. pp. 1–21. [Google Scholar]
- 22.Haulon G H, Hodges N A, Russell A D. The influence of glucose, ammonium and magnesium availability on the production of protease and bacitracine by Bacillus licheniformis. J Gen Microbiol. 1982;128:845–851. doi: 10.1099/00221287-128-4-845. [DOI] [PubMed] [Google Scholar]
- 23.Hille S. A literature review of the blood chemistry of rainbow trout Salmo gairdneri. J Fish Biol. 1982;20:535–569. [Google Scholar]
- 24.Holt R A. Cytophaga psychrophila, the causative agent of bacterial coldwater disease in salmonid fish. Ph. D. thesis. Corvallis: Oregon State University; 1987. [Google Scholar]
- 25.Janzon L, Arvidson S. The role of the delta-lysin gene (hdl) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 1990;9:1391–1399. doi: 10.1002/j.1460-2075.1990.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson C E, Gray R W, McLennan A, Paterson A. Effects of photoperiod, temperature and diet on the reconditioning response, blood chemistry and gonad maturation of Atlantic salmon kelts Salmo salar held in freshwater. Can J Fish Aquat Sci. 1987;44:702–711. [Google Scholar]
- 27.Kothary M H, Kreger A S. Production and partial characterization of an elastolytic protease of Vibrio vulnificus. Infect Immun. 1985;50:534–540. doi: 10.1128/iai.50.2.534-540.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristjansson M M, Magnusson O T, Gudmundsson H M, Alfredsson G A, Matsuzawa H. Properties of a subtilisin-like proteinase from a psychrotropic Vibrio species. Eur J Biochem. 1999;260:752–760. doi: 10.1046/j.1432-1327.1999.00205.x. [DOI] [PubMed] [Google Scholar]
- 29.Kurosaka K, Ohta T, Matsuzawa H. A 38 kDa precursor protein of aqualisin I (a thermophilic subtilisin-type protease) with a C-terminal extended sequence: its precipitation and in vivo processing. Mol Microbiol. 1995;20:385–389. doi: 10.1111/j.1365-2958.1996.tb02625.x. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Leung K Y, Stevenson R M W. Tn5-induced protease-deficient strains of Aeromonas hydrophila with reduced virulence for fish. Infect Immun. 1988;56:2639–2644. doi: 10.1128/iai.56.10.2639-2644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levishon S, Aronson A I. Regulation of extracellular protease production in Bacillus cereus. J Bacteriol. 1967;93:1023–1030. doi: 10.1128/jb.93.3.1023-1030.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao C-H, McCallus D E, Wells J M. Calcium-dependent pectate lyase production in the soft-rotting bacterium Pseudomonas fluorescens. Phytopathology. 1993;83:813–818. [Google Scholar]
- 34.Liao C-H, McCallus D E. Biochemical and genetic characterization of an extracellular protease from Pseudomonas fluorescens CY091. Appl Environ Microbiol. 1998;64:914–921. doi: 10.1128/aem.64.3.914-921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin S J, Yoshimura E, Sakai H, Wakagi T, Matsuzawa H. Weakly bound calcium ions involved in the thermostability of aqualisin I, a heat-stable subtilisin-type protease of Thermus aquaticus YT-1. Biochim Biophys Acta. 1999;1433:132–138. doi: 10.1016/s0167-4838(99)00140-5. [DOI] [PubMed] [Google Scholar]
- 36.Lockau W, Massalsky B, Dirmeier A. Purification and partial characterization of a calcium-stimulated protease from the cyanobacterium Anabaena variabilis. Eur J Biochem. 1988;172:433–438. doi: 10.1111/j.1432-1033.1988.tb13906.x. [DOI] [PubMed] [Google Scholar]
- 37.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 38.Madsen L, Dalsgaard I. Characterization of Flavobacterium psychrophilum; comparison of proteolytic activity and virulence of strains isolated from trout (Oncorhynchus mykiss) In: Barnes A C, Davidson G A, Hiney M P, McIntosh D, editors. Methodology in fish disease research. Aberdeen, Scotland: Fisheries Research Service; 1998. pp. 45–52. [Google Scholar]
- 39.Margesin R, Schinner F. Production and properties of an extracellular metalloprotease from a psychrophilic Pseudomonas fluorescens. J Biotechnol. 1992;24:207–210. [Google Scholar]
- 40.McIver K S, Scott J R. Role of mga in growth phase regulation of virulence genes of the group A streptococcus. J Bacteriol. 1997;179:5178–5187. doi: 10.1128/jb.179.16.5178-5187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michel C, Antonio D, Hedrick R P. Production of viable cultures of Flavobacterium psychrophilum: approach and control. Res Microbiol. 1999;150:351–358. doi: 10.1016/s0923-2508(99)80061-8. [DOI] [PubMed] [Google Scholar]
- 43.Mikulskis A V, Delor I, Thi V H, Cornelis G R. Regulation of the Yersinia enterocolitica enterotoxin Yst gene: influence of growth phase, temperature, osmolarity, pH and bacterial host factors. Mol Microbiol. 1994;14:905–915. doi: 10.1111/j.1365-2958.1994.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 44.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP, phoQ) controls Salmonella typhymurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mock M, Ullmann A. Calmodulin-activated bacterial adenylate cyclases as virulence factors. Trends Microbiol. 1993;1:187–192. doi: 10.1016/0966-842x(93)90089-a. [DOI] [PubMed] [Google Scholar]
- 46.Nagase H, Woessner J F. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 47.Norqvist A, Norrman B, Wolf-Watz H. Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J Bacteriol. 1992;174:111–118. doi: 10.1128/jb.174.22.7235-7244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacha R E, Porter S. Characteristics of myxobacteria isolated from the surface of freshwater fish. Appl Microbiol. 1968;16:1901–1906. doi: 10.1128/am.16.12.1901-1906.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sammons D W, Adams L D, Nishizawa E E. Ultrasensitive silver-based colour staining of polypeptides in polyacrylamide gels. Electrophoresis. 1981;2:135–140. [Google Scholar]
- 50.Secades P, Guijarro J A. Purification and characterization of an extracellular protease from the fish pathogen Yersinia ruckeri and effect of culture conditions on production. Appl Environ Microbiol. 1999;65:3969–3975. doi: 10.1128/aem.65.9.3969-3975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma S, Giri S, Khuller G K. Ca2+/calmodulin dependent protein kinase from Mycobacterium smegmatis ATCC 607. Mol Cell Biol. 1998;183:183–191. doi: 10.1023/a:1006883006402. [DOI] [PubMed] [Google Scholar]
- 52.Siezen R J, de Vos W M, Levnissen J A M, Dijkstra W. Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteinases. Protein Eng. 1991;4:719–737. doi: 10.1093/protein/4.7.719. [DOI] [PubMed] [Google Scholar]
- 53.Stetler-Stevenson W G, Aznavoorian S, Liotta L A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 54.Voordouw G, Milo C, Roche R S. Role of bound calcium ions in thermostable, proteolytic enzymes. Separation of intrinsic and calcium ion contributions to the kinetic thermal stability. Biochemistry. 1976;15:3716–3723. doi: 10.1021/bi00662a012. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein D L, Holmes R K, O'Brien A D. Effects of iron and temperature on Shiga-like toxin I production by Escherichia coli. Infect Immun. 1988;56:106–111. doi: 10.1128/iai.56.1.106-111.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]