Abstract
Introduction
Sickle cell disease (SCD) is a heritable blood disorder resulting in deformed, rigid red blood cells, rendering them more prone to vaso-occlusion. Ocular complications are known to affect multiple organs through the body's vasculature. Here, we evaluated the incidence of eye complications in patients with SCD at King Abdulaziz Medical City in Jeddah, Saudi Arabia.
Materials and methods
We used a cross-sectional approach and collected relevant medical data from nine patients with SCD. Ophthalmic assessment included visual acuity and an in-clinic dilated fundus examination. All patients were asked to attend the ophthalmology clinic to undergo optical coherence tomography (OCT) angiography, macular OCT scan, and fundus photography. The results of the imaging tests were interpreted by a certified ophthalmology consultant specializing in retinal diseases. Descriptive analyses of the results were also performed.
Results
The mean age of the nine patients was 24.78 ± 10.9 years. All patients were non-hypertensive, one had type 1 diabetes, and another had type 2 diabetes. Five patients had normal visual acuity, two had slight impairments in at least one eye, and two had moderate impairment in at least one eye. One of the nine patients exhibited retinal ischemia in the right eye despite normal macular thickness (visual acuity, OD, 6/30; OS, 6/21). The remaining eight patients showed no ocular abnormalities.
Conclusion
Of the nine patients with SCD, five showed no symptoms of ocular complications. One patient showed retinal ischemia in the right eye, despite a normal macular thickness. This study's results suggest routine ophthalmologic examination may not be able to detect or monitor macular or retinal abnormalities unless augmented with detailed imaging techniques.
Keywords: Eye complications, Retinopathy, Saudi Arabia, Sickle cell disease, Ophthalmology screening
Highlights
-
•
Out of nine patients with SCD, five patients exhibited normal visual acuity.
-
•
One patient presented perifoveal retinal ischemia in the right eye.
-
•
Results suggest augmenting routine ophthalmology screening with imaging techniques.
Abbreviations
- SCD
sickle cell disease
- OCT
optical coherence tomography
- PSR
proliferative sickle cell retinopathy
- NPSR
non-proliferative sickle cell retinopathy
1. Introduction
Sickle cell disease (SCD) is a well-known inherited disorder of red blood cells and one of the most prevalent monogenic disorders globally [1]. It presents itself in different forms, the most common types being the homozygous beta-globin gene mutation (HbSS), heterozygous SC, and heterozygous S beta-thalassemia [2]. HbSS is the most severe form of SCD, with each sickle cell gene “S” inherited from both parents. SCD is multisystemic as its pathophysiology primarily involves sickle hemoglobin polymerization in erythrocytes, which are responsible for supplying oxygen to different organs in the body [1]. Patients with SCD tend to have sickle-shaped red blood cells, which contribute to the rigidity of erythrocytes, resulting in a higher chance of vaso-occlusion, chronic anemia, hemolysis, and vasculopathy [1]. Globally, SCD is estimated to affect 305,800 newborns in 2010 and is predicted to affect 404,200 newborns in 2050 [3]. Most patients with SCD can be found across sub-Saharan Africa, where 230,000 newborns are affected every year, representing as much as 80% of the global total of SCD cases [1].
Given the chronic and multisystemic nature of SCD, its complications can manifest in multiple ways. These have been reported to include ocular problems resulting from vaso-occlusion of the conjunctiva, retina, choroid, and/or iris. The most prevalent among these is sickle cell retinopathy, wherein sickle vasculopathy affects the retinal vasculature [4]. It can be either proliferative sickle cell retinopathy (PSR) or non-proliferative sickle cell retinopathy (NPSR) [5]. The clinical presentation of sickle cell retinopathy is usually asymptomatic until complications, such as retinal detachment or vitreous hemorrhage, emerge [6]. For this reason, routine ophthalmology screening is carried out among children with SCD to detect proliferative changes and address their possible long-term consequences [4].
In Saudi Arabia, a study published in 2007 estimated a prevalence of 0.26% of SCD and 4.20% for SCD traits among 488,315 individuals who took part in the National Premarital Screening Program [7]. Another study employed retinal examination with fluorescein angiography to investigate the prevalence of eye complications in Saudi Arabia in relation to sickle cell disease [8]. Their results revealed that retinal vascular changes are a common occurrence among Saudi patients, independent of their haplotype. Interestingly, the risk associated with PSR was observed to be significantly higher among patients from the southwestern region as an implication of an abnormal vascular border [8]. In Jeddah, a populated city in the western region of Saudi Arabia, the prevalence of SCD was identified to be 0.04% and 3.46% for SCD trait [7]. This study aimed to determine the prevalence of eye complications among patients with SCD in Jeddah, Saudi Arabia. To the author's knowledge, this is the first study to look at the incidence of SCD-related eye complications at an administrative regional level, that is, Jeddah.
2. Methods
2.1. Study design
This study was carried out according to a cross-sectional approach in accordance with the STROCSS 2021 guidelines [9] to assess the incidence of ocular complications among patients with SCD in a particular hospital King Abdulaziz Medical City in Jeddah, Saudi Arabia. The inclusion criteria were as follows: all patients with major sickle cell hemoglobinopathies as diagnosed by a certified hematologist, from all age groups over the period 2018–2020 were enrolled in this study. Patients who were not able to undergo imaging examinations for various reasons were excluded. A total of 9 patients were eligible for inclusion in the study. To maintain patient privacy, each study participant was assigned a serial number instead of a medical record number.
2.2. Data collection
Most of the patients’ data were sourced from their electronic medical files through BESTCare using a data collection sheet. The variables included in the sheet were devised from related published literature, as well as from discussions among experts. These included patient serial number, sex, age, diagnosis, ophthalmic assessment results, and presence of comorbidities. All patients were asked to attend the ophthalmology clinic to undergo imaging tests.
2.3. Ophthalmic assessment
Ophthalmic assessments focused on visual acuity, in-clinic dilated fundus examination, optical coherence tomography (OCT) angiography, macular OCT scan, and fundus photography. Visual acuity was assessed using the tumbling E chart, while the in-clinic dilated fundus examination involved retinal examination with the aid of mydriatic eye drops. Imaging results were interpreted by a certified ophthalmology consultant specializing in retinal diseases with thirteen years of clinical experience.
2.4. Ethical standards
Informed consent was obtained from the patients in accordance with the basic principles of the Declaration of Helsinki. Access to patient data was limited by the authors of this study. Approval from the Institutional Review Board of King Abdullah International Medical Research Center, Riyadh, Saudi Arabia was accomplished prior to the conduct of the study, with Memorandum Reference No. IRB/0453/22.
2.5. Data management and analysis
Data were analyzed using the Statistical Package for the Social Sciences version 23 (IBM Corp., Armonk, NY). Descriptive statistics were used, and normally distributed variables were presented as means and standard deviations.
3. Results
The study variables and their counts are summarized in Table 1.
Table 1.
Characteristics of the patients.
| Variable | N | Min | Max | Mean | SD | |
|---|---|---|---|---|---|---|
| Age | 9 | 17 | 50 | 24.78 | 10.9 | |
| Count | % | |||||
| Sex | Male | 4 | 44.4 | |||
| Female | 5 | 55.6 | ||||
| Diagnosis | Sickle cell disease | 8 | 88.9 | |||
| Sickle cell trait | 1 | 11.1 | ||||
| In-clinic dilated fundus exam | Normal Findings | 8 | 88.9 | |||
| Patient did not undergo examination | 1 | 11.1 | ||||
| OCT angiography | Normal Findings | 8 | 88.9 | |||
| Abnormal Findings | 1 | 11.1 | ||||
| OCT macula | Normal Findings | 9 | 100.0 | |||
| Fundus photography | Normal Findings | 9 | 100.0 | |||
| Hypertension | No | 29 | 100.0 | |||
| Diabetes mellitus | Type 2 | 1 | 11.1 | |||
| Type 1 | 1 | 11.1 | ||||
| No | 7 | 77.8 | ||||
OCT, optical coherence tomography; Min, minimum; Max, maximum; SD, standard deviation.
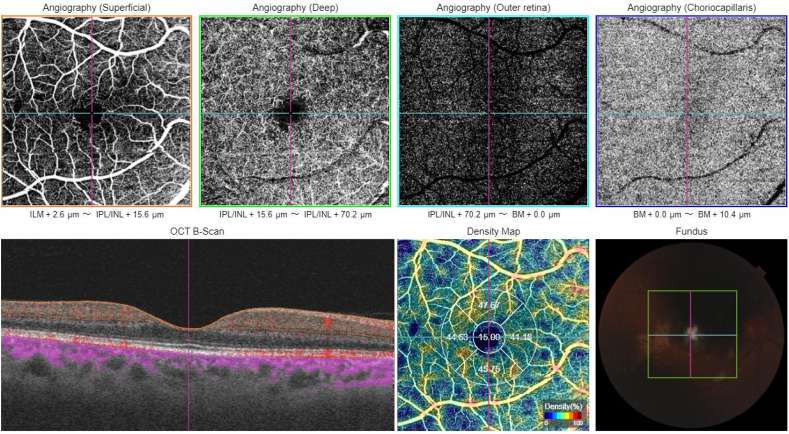
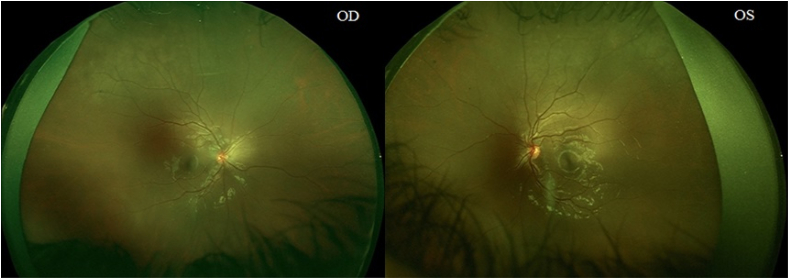
One of the patients exhibited abnormal findings on OCT angiography. Retinal perifoveal ischemia was observed in the patient's right eye, despite having a normal macular thickness. No abnormalities were observed in the patient's left eye. Visual acuity was 6/30 for the right eye and 6/21 for the left eye. Fig. 1, Fig. 2 show the imaging results.
Fig. 1.
OCT angiography report of the right eye for the patient with retinal abnormalities. Abbreviations: OCT, optical coherence tomography.
Fig. 2.
Wide-angle retinal photography of the patient with retinal abnormalities. Abbreviations: OD, right eye; OS, left eye.
4. Discussion
In this study, we acquired data from nine patients with SCD to assess the incidence of eye complications. Five patients revealed no signs of ocular impairment based on their visual acuity. One of the patients demonstrated signs of retinal perifoveal ischemia in the right eye even when it had normal macular thickness at the time of observation.
The pathophysiology of SCD has been well studied and dissected. The organs most commonly involved are kidneys, lungs, liver, bones, and skin [10]. However, ocular complications attributed to vaso-occlusions may also occur during disease progression [10]. In a 2019 study conducted on the general population in the Eastern region of Saudi Arabia, the majority (57.3%) of participants were not aware of ocular complications arising from SCD [11]. This is important as the Eastern region exhibited a significantly higher prevalence of SCD (0.87%) and SCD traits (10.74%) than the other regions [7]. Meanwhile, a trial in Jeddah reported a 0.04% prevalence of SCD and a 3.46% prevalence of SCD traits among the participants [7]. This study sought to estimate the incidence of eye complications among patients with SCD in Jeddah, Saudi Arabia.
A study carried out on approximately 1000 patients with SS observed a higher incidence of PSR among patients from the older age group (30 years old and above), although the difference was not statistically significant [12]. Another study observed the same phenomenon; most SS cases were seen in the group of individuals aged 25–39 years of either sex, and the majority of SC incidences were seen in men aged 15–24 years and women aged 20–39 years, although this did not reach statistical significance [13]. The probable reason for this is that age may be reflective of the duration of vaso-occlusion and the consequential accumulation of organ damage [12].
The sex ratio observed in our study was 1.25:1 (female-to-male). This is consistent with a study conducted in Jordan in which the sample population had a female-to-male ratio of 1.5:1 [14] and another study that had a female-to-male ratio of 2.38:1 [15]. However, it is known that no sex bias exists for SCD or SCD traits and that these observations of sex ratio biases may be attributed to probable sampling biases and small sample sizes.
The majority (88.9%) of the sample population was diagnosed with sickle cell disease. In terms of comorbidities, all patients were non-hypertensive; one had type 1 diabetes, another had type 2 diabetes, and the rest were non-diabetic. A study carried out in 2007 identified an association between pulmonary hypertension and retinopathy [16]. Lim et al. (2021) also found that hypertension and diabetes are risk factors associated with retinal thinning [17].
Five had normal visual acuity (6/6), two had slight impairments of up to 6/12 in at least one eye, and the remaining two patients had moderate visual acuity impairment in at least one eye (6/30) (Supplementary Table 1). Loss of vision has been shown to have a strong association with PSR in a study conducted in Jamaica, with a higher incidence of loss of vision among individuals with PSR than those with NPSR [18]. In Saudi Arabia, it was found that peripheral vascular changes were frequently detected by fluorescent angiography among 61 subjects with SS disease and 10 subjects with heterozygous S beta-thalassemia [19]. Changes occurred regardless of the halotype, and were similar to what had been reported in Jamaica [19].
Among the sample population, only one patient exhibited abnormal findings on OCT angiography. Retinal ischemia occurs in patients with SCD as a result of the sickling of red blood cells and eventual vaso-occlusion of retinal blood vessels [20]. Retinal ischemia can progress to vitreous hemorrhage and retinal detachment [20]. A review by Do and Rodger (2017) stated that retinal ischemia can occur in patients without any visual symptoms [21]. This was also reported by Murthy in 2011 [20], thus suggesting that ischemia is easily overseen during a routine clinical examination, thereby implying the need for more detailed imaging techniques, such as wide-field angiography, to assess such changes among asymptomatic patients [20]. Overall, this study's results suggest that routine ophthalmological screening may not be able to detect and/or monitor the progression of ocular complications associated with SCD, and that supplementary investigation through detailed imaging techniques such as OCT angiography may be necessary.
This study has several limitations. First, the sample size for this study was small. Initially, patient data were collected from 29 patients, but since only nine underwent imaging tests due to inability of other patients to do follow ups (e.g., busy schedule, living in a different city), only those nine were considered as the sample population. Second, fluorescent angiography, an imaging technique known to be more sensitive in detecting vascular changes in the eye, was not carried out. This could have resulted in a probable underestimation of the incidence of eye complications in this study. Finally, although this study used wide-angle retinal photography, it was limited to patients with retinal abnormalities. A previously published study demonstrated that wide-field images can detect vascular changes that can be overseen using standard-field photography [22]. Despite these limitations, the authors believe that the strength of this study lies in the fact that the data on the association between ocular complications and SCD remains relatively scarce, particularly in the Kingdom of Saudi Arabia. The findings of this study suggest that routine ophthalmological screening may not be able to distinguish macular or retinal abnormalities unless supplemented by detailed imaging techniques such as OCT angiography. Additionally, this study may inspire further discussions on the practice of regular ophthalmology screening to monitor visual symptoms in patients with progressive SCD.
5. Conclusion
SCD-associated ocular complications can easily be overseen during routine clinical examination and in asymptomatic patients, highlighting the utility of more intensive ophthalmology checkups and more advanced imaging techniques to monitor the progression of such complications. Further work needs to be done to ascertain the cost-effectiveness of routine ophthalmological screening in the detection and monitoring of SCD-associated ocular complications. Recommendations for future research include focusing work on a larger sample size, taking a deeper look into the risk factors of eye complications in relation to SCD, and using advanced imaging techniques, such as OCT angiography, to detect meaningful vascular changes in the eye.
Ethical approval
Approval from the Institutional Review Board of King Abdullah International Medical Research Center, Riyadh, Saudi Arabia was accomplished prior to the conduct of the study, with Memorandum Reference No. IRB/0453/22.
Sources of funding
There are no sources of funding to declare.
Author contribution
Eid Ayed Almasoudi and Sultan Fahad Magliah contributed in all stages of the study. Abubakr Salem Alzwaihri participated in proposal and manuscript writing in addition to data collection. Abdullah Omar Aljuwaybiri contributed in proposal and manuscript writing. Abdullah Saleh Alqahtani contributed in interpretation of the data, revising the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Consent
Informed consent was obtained from the patients who presented to the clinic for imaging tests in accordance with the basic principles of the Declaration of Helsinki.
Registration of research studies
1.Name of the registry: Research Registry http://www.researchregistry.com
2.Unique Identifying number or registration ID: researchregistry7845
3.Hyperlink to your specific registration (must be publicly accessible and will be checked): https://researchregistry.knack.com/research-registry#home/registrationdetails/626809b1cadd9e001ec91e62/
Guarantor
Abdullah Saleh Alqahtani.
Department of Surgery, Division of Ophthalmology, Ministry of the National Guard Health Affairs, King Abdulaziz Medical City, King Saud bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Jeddah, Saudi Arabia 22384 Email: dr-a-saleh@hotmail.com.
Provenance and peer review
Not commissioned, externally peer reviewed.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgments
This research has received no external funding.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103999.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rees D.C., Williams T.N., Gladwin M.T. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 2.Galadanci A.A., DeBaun M.R., Galadanci N.A. Neurologic complications in children under five years with sickle cell disease. Neurosci. Lett. 2019;706:201–206. doi: 10.1016/j.neulet.2019.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piel F.B., Hay S.I., Gupta S., Weatherall D.J., Williams T.N. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10(7) doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pahl D.A., Green N.S., Bhatia M., Lee M.T., Chang J.S., Licursi M., Briamonte C., Smilow E., Chen R.W.S. Optical coherence tomography angiography and ultra-widefield fluorescein angiography for early detection of adolescent sickle retinopathy. Am. J. Ophthalmol. 2017;183:91–98. doi: 10.1016/j.ajo.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan T., Badr M., Hanna D., Arafa M., Elhewala A., Dabour S., Shehata S., Rahman D.A. Retinopathy in Egyptian patients with sickle cell disease: a cross-sectional study. Medicine (Baltim.) 2021;100(51) doi: 10.1097/md.0000000000028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Downes S.M., Hambleton I.R., Chuang E.L., Lois N., Serjeant G.R., Bird A.C. Incidence and natural history of proliferative sickle cell retinopathy: observations from a cohort study. Ophthalmology. 2005;112(11):1869–1875. doi: 10.1016/j.ophtha.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Alhamdan N.A., Almazrou Y.Y., Alswaidi F.M., Choudhry A.J. Premarital screening for thalassemia and sickle cell disease in Saudi Arabia. Genet. Med. 2007;9(6):372–377. doi: 10.1097/gim.0b013e318065a9e8. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hazzaa S., Bird A.C., Kulozik A., Serjeant B.E., Serjeant G.R., Thomas P., Padmos A. Ocular findings in Saudi Arabian patients with sickle cell disease. Br. J. Ophthalmol. 1995;79(5):457–461. doi: 10.1136/bjo.79.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew G., Agha R. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int. J. Surg. 2021;96 doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 10.Abdalla Elsayed M.E.A., Mura M., Al Dhibi H., Schellini S., Malik R., Kozak I., Schatz P. Sickle cell retinopathy. A focused review. Graefes Arch. Clin. Exp. Ophthalmol. 2019;257(7):1353–1364. doi: 10.1007/s00417-019-04294-2. [DOI] [PubMed] [Google Scholar]
- 11.Alshehri A.M., Feroze K.B., Amir M.K. Awareness of ocular manifestations, complications, and treatment of sickle cell disease in the eastern province of Saudi Arabia: a cross-sectional study. Middle East Afr. J. Ophthalmol. 2019;26(2):89–94. doi: 10.4103/meajo.MEAJO_200_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes R.J., Condon P.I., Serjeant G.R. Haematological factors associated with proliferative retinopathy in homozygous sickle cell disease. Br. J. Ophthalmol. 1981;65(1):29–35. doi: 10.1136/bjo.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox P.D., Dunn D.T., Morris J.S., Serjeant G.R. Risk factors for proliferative sickle retinopathy. Br. J. Ophthalmol. 1990;74(3):172–176. doi: 10.1136/bjo.74.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Yaghi N.E., AlNawaiseh A.M., Khourshid I.M., AlRawashdeh T.J., Rawashdeh M.M.A., Zghoul A.M., Shafagoj A.N., Alomairi Y.A., Muhsen S.M., AlRyalat S.S. Central macular thickness in patients with sickle cell disease and no signs of retinopathy: a cross-sectional study of Jordanian patients. J. Int. Med. Res. 2021;49(4) doi: 10.1177/0300060520977387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oltra E.Z., Chow C.C., Wubben T., Lim J.I., Chau F.Y., Moss H.E. Cross-sectional analysis of neurocognitive function, retinopathy, and retinal thinning by spectral-domain optical coherence tomography in sickle cell patients. Middle East Afr. J. Ophthalmol. 2016;23(1):79–83. doi: 10.4103/0974-9233.150632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akgül F., Yalçin F., Seyfeli E., Uçar E., Karazincir S., Balci A., Gali E. Pulmonary hypertension in sickle-cell disease: comorbidities and echocardiographic findings. Acta Haematol. 2007;118(1):53–60. doi: 10.1159/000102588. [DOI] [PubMed] [Google Scholar]
- 17.Lim J.I., Niec M., Sun J., Cao D. Longitudinal assessment of retinal thinning in adults with and without sickle cell retinopathy using spectral-domain optical coherence tomography. JAMA Ophthalmol. 2021;139(3):330–337. doi: 10.1001/jamaophthalmol.2020.6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriarty B.J., Acheson R.W., Condon P.I., Serjeant G.R. Patterns of visual loss in untreated sickle cell retinopathy. Eye. 1988;2(Pt 3):330–335. doi: 10.1038/eye.1988.62. [DOI] [PubMed] [Google Scholar]
- 19.al-Hazzaa S., Bird A.C., Kulozik A., Serjeant B.E., Serjeant G.R., Thomas P., et al. Ocular findings in Saudi Arabian patients with sickle cell disease. Br. J. Ophthalmol. 1995;79:457–461. doi: 10.1136/bjo.79.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy R.K., Grover S., Chalam K.V. Temporal macular thinning on spectral-domain optical coherence tomography in proliferative sickle cell retinopathy. Arch. Ophthalmol. 2011;129(2):247–249. doi: 10.1001/archophthalmol.2010.357. [DOI] [PubMed] [Google Scholar]
- 21.Do B.K., Rodger D.C. Sickle cell disease and the eye. Curr. Opin. Ophthalmol. 2017;28(6):623–628. doi: 10.1097/icu.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 22.Cho M., Kiss S. Detection and monitoring of sickle cell retinopathy using ultra wide-field color photography and fluorescein angiography. Retina. 2011;31(4):738–747. doi: 10.1097/IAE.0b013e3181f049ec. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.