Abstract
Introduction and importance
Ectopic Pancreas (EP) is a rare condition that is mostly found in the Gastrointestinal tract and especially in the stomach. Although the lesion is mainly asymptomatic, non-specific symptoms can be present, making the diagnosis even more challenging.
Case presentation
In our case a 52-year-old woman, with heartburn as the only symptom, was undergone successive examinations, indicating a subepithelial lesion in the antrum of the stomach, from which only Magnetic Resonance Imaging (MRI) indicated the presence of ectopic pancreas, while Computed Tomography results considered the mass as Gastrointestinal Stromal Tumor. Wedge gastrectomy was performed in order to extract the lesion and the histopathological examination confirmed the findings of the MRI. The patient fully recovered with no complications.
Clinical discussion
In most cases, EP is described in endoscopy as a subepithelial mass with normal mucosa. As EP can mimic other subepithelial masses, even adenocarcinoma, it is of utmost importance not to omit the performance of surgical removal and histopathological examination. Consequently, resection is essential not only for the diagnosis but also for the treatment of the patient.
Conclusion
EP is not a usually detected clinical pathology. There is no specific algorithm, which physicians should follow in order to reach the diagnosis without the surgical intervention. For this reason, clinicians should be conscious of the existence of EP in the stomach.
Κeywords: Ectopic pancreas, Stomach, GIST, Histopathological examination, Surgical resection
Highlights
-
•
Ectopic pancreas (EP) is mostly found in the gastrointestinal tract.
-
•
EP in stomach first considered as a Gastrointestinal Stromal Tumor (GIST).
-
•
Resection is essential for the diagnosis and for the treatment of the patient.
1. Introduction
Ectopic pancreas (EP), also recognized as heterotopic, aberrant, accessory, or pancreatic rest [1,2], is a rare congenital condition in which pancreatic tissue is detected in areas where is normally absent and it has no vascular, anatomical, or ductal association with the orthotopic pancreas. The prevalence of EP at autopsies fluctuates between 0,6%–13% and is found in 1 out of 500 surgical operations implicating the upper abdomen [2]. It is notable that ectopic pancreatic lesions can occur not only in the gastrointestinal tract but also in the thorax and pelvis [3]. However, EP mainly develops in the stomach (25%–38%), duodenum (17%–36%) or jejunum (15%-21,7%) [4]. Although EP is foremost asymptomatic and the lesion is found incidentally during surgical investigations or gastrointestinal endoscopies, it may manifest as epigastric pain (27%), nausea, and vomiting (27%), ulceration (27%), weight loss (18%) and dyspepsia [5,6,13]. Furthermore, pathologic conditions found in the pancreas, can infrequently occur in the ectopic lesion [7]. Due to the fact that EP is a rare entity, there are no gold standards for the diagnosis. Nevertheless, resection and histopathologic examination are still considered the optimal diagnostic tools.
We present the case of a 52-year-old woman with an ectopic pancreatic lesion in the posterior wall of the stomach. This case report has been reported in line with the SCARE Criteria [17].
2. Case report
Α 52-year-old Caucasian woman presented in Emergency Department, with a 1-year history of a burning feeling behind her sternum (heartburn). Her medical history included Hashimoto disease and dyslipidemia, as well as gastroesophageal reflux disease with symptoms of epigastric pain and preprandial heartburn 20 years ago. For these conditions, she was taking levothyroxine, rosuvastatin, and omeprazole, while she was not a smoker and she was not drinking alcohol. No allergies were reported by the patient.
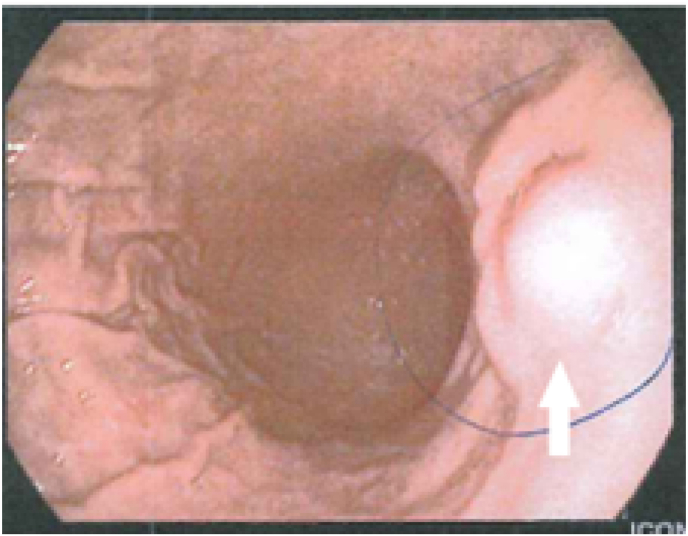
When the clinical examination was conducted, her vital signs were normal (Blood pressure: 125/78 mmHg; Heart rate: 68 pulses/min.; Respiration rate: 14 breaths/min.; Temperature: 36,2 °C; Oxygen Saturation: 98%). Physical examination revealed a soft abdomen and no significant findings were detected. Also, routine blood and biochemical laboratory examinations were performed with no significant evidence. In the past year, she has undergone several diagnostic procedures which were contradictory. The patient underwent gastroscopy, which revealed a subepithelial nodule, located in the middle of the perpendicular region of the stomach's body (Fig. 1).
Fig. 1.
Gastroscopy of the lesion with normal mucosa.
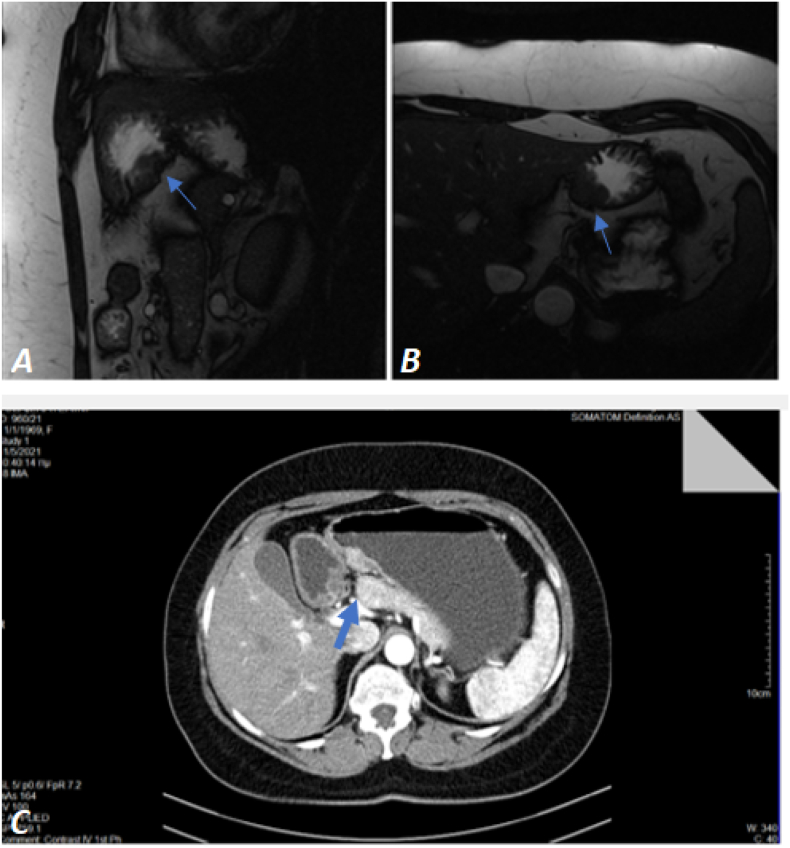
The histopathologic examination which followed showed mild gastritis. Computed Tomography (CT) was conducted with no diagnostic value and Magnetic Resonance Imaging (MRI) revealed ectopic pancreatic tissue in the perpendicular wall of the stomach. Other abdominal organs, including the pancreas, were normal. Also, an Endoscopic Ultrasound (EUS) was performed indicating a hypoechoic mass in the margin of the body-antrum of the stomach's posterior wall and the findings were consistent with Gastrointestinal Stromal Tumor (GIST). A second gastroscopy and histopathologic examination were performed, confirming the findings of the previous gastroscopy and biopsy, revealing redness located in the antrum with no signs of malignancy. Due to the absence of specific findings, a triphasic CT scan was conducted indicating GIST as the most probable diagnosis (Fig. 2).
Fig. 2.
(A), (B) Coronal and axial MRI accordingly, indicating ectopic pancreatic tissue in the perpendicular wall of the stomach. (C) Triphasic CT (axial) indicating GIST as the most probable diagnosis.
Ultimately, the third gastroscopy confirmed the presence of a 2 cm subepithelial nodule upper the gastric angle of the lesser curvature, with normal mucosa and ulceration on its top, reinforcing the previous theories about GIST.
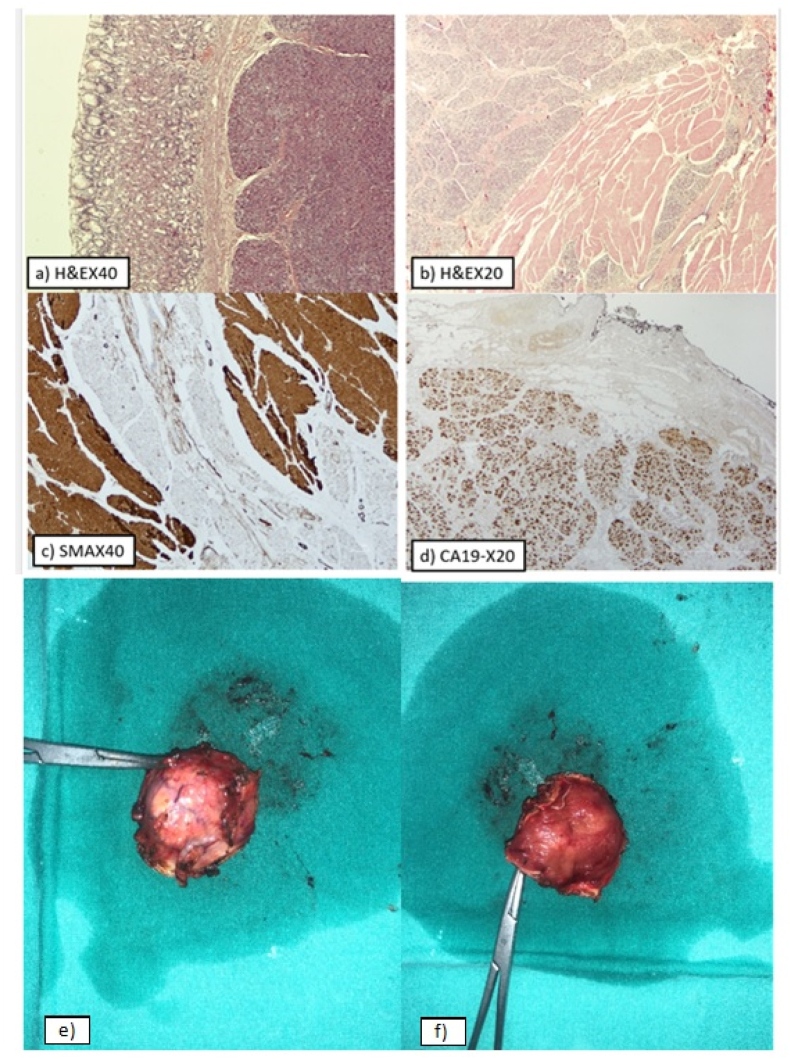
In order to remove the lesion, a wedge gastrectomy was conducted after 5 days of the patient's admission by the professor of surgery of our department with the help of two junior trainees with approximately 3 years of surgical specialty training. For practical reasons, a laparotomy was held, as there were difficulties in the surgical approach. Then a histopathologic examination was performed in the excised tissue. Macroscopically, a circumscribed, fibroelastic, round tissue specimen measuring 4 × 3.5 × 1.5 cm was received. A yellow tan nodule of diameter 1.8 cm was identified. An intraoperative consultation was requested and a routine histopathology examination followed. Microscopically, gastric mucosa with pancreatic parenchyma located beneath the muscularis mucosa was found. The pancreatic parenchyma consisted of lobules with acini, dispersed islets of Langerhans, and ducts of various sizes, with some of them dilated. Periductal fibroconnective tissue was occasionally observed. Also, part of the muscularis propria with dispersed pancreatic ducts and acini was found. The gastric mucosa was unremarkable. Neither inflammation, intestinal metaplasia nor microorganisms Helicobacter pylori were identified. Immunohistochemically, CD117 and CD34 were negative, with the last marker highlighting the vascular background. A diagnosis of heterotopic pancreatic tissue in the stomach, with no signs of malignancy, was reached (Fig. 3).
Fig. 3.
Histopathological findings. (a) Hematoxylin and eosin stain, (40× magnification). Pancreatic parenchyma is seen beneath the gastric mucosa. (b) Hematoxylin and eosin stain, (20× magnification), showing the gastric muscularis propria with dispersed pancreatic ducts and acini. (c) Immunostaining for Smooth Muscle Actin, (40× magnification), highlighting the muscularis propria containing dispersed pancreatic ducts and acini. (d) Immunostaining for CA19-9, (20× magnification), highlighting the pancreatic parenchyma. Specimen of the excised pancreatic mass. (e) Ectopic pancreatic tissue. (f) Gastric mucosa.
After the surgery, a specific diet, which is followed by patients suffering from a stomach ulcer, and reception of esomeprazole were given to the patient as post-surgery instructions. The recuperation of the patient was uneventful, she affirmed that the main symptom of preprandial heartburn had subsided, as it was expected, and she was discharged from the hospital on the 6th post-operative day.
3. Discussion
Jean Schulz was the first who described EP in 1727 when noted in an ileal diverticulum [8]. Later in 1859, the histological characteristics were observed by Klob [8]. Although the pathogenesis remains still unresolved, two are the theories that are mainly related to the EP development: the misplacement theory and the metaplasia theory [8]. According to the first one, during the embryological foregut rotation, fragments originating from the wall of primitive foregut, instead of forming the normal pancreas, remain in the wall of the bowel and eventually develop as EP [9]. The second one is based on the migration of endoderm tissue to the submucosa during embryogenesis, in which metaplasia occurs and finally evolves into pancreatic tissue [9].
It is notable that more than 95% of the lesions of the stomach are found in the antrum and especially in the greater curvature [10]. However, it has been observed in most places of the gastrointestinal tract, including the esophagus, common bile duct, gallbladder, papilla of Vater, Meckel's diverticulum, and mesocolon [11]. Moreover, it is infrequently detected in the liver, spleen, omentum, lungs, mediastinum, fallopian tubes, or umbilicus [12]. As far as the incidence in the stomach's wall is concerned, 73% is found in the submucosal, 17% in the muscularispropria, and 10% in the subserosal layer [11].
Despite the fact that EP is usually asymptomatic, it may present with a variety of symptoms, usually due to the irritation that secreted pancreatic hormones or enzymes are responsible for [2]. Heartburn, as in our case, is a common symptom. Notable is that clinical symptoms depend on the location and size of the lesion and especially if the lesion's size is more than 1,5 cm, clinical manifestations are more probable [13]. Rare conditions such as pancreatitis, pseudocyst, bleeding, or malignant transformation (adenocarcinoma) can occur in the lesion, like in the orthotopic pancreas, with malignancy emerging in only 0,7–1,9% of the cases [5,13,14]. Furthermore, owing to the anatomical position, gastric outlet obstruction, intussusceptions, obstructive jaundice, intestinal obstruction, or thoracic pain may arise [2,14].
The preoperative diagnosis of the disease is demanding. Due to the infrequency of EP, there are no guidelines for the diagnosis of this congenital lesion. A large variety of diagnostic tools have been used, for example, CT, MRI, and EUS, but their results are predominantly inconclusive as EP cannot be easily differentiated from subepithelial lesions (SEL) [8]. Abnormalities that should be taken into consideration as a differential diagnosis are lymphoma, GIST, neuroendocrine tumors (NET) [8,11]. Typically, the finding during endoscopy is characterized by a firm round or oval subepithelial mass with normal mucosa and a central dimpling (35%–90%) with the outlet of a duct on its top, which, however, can be present in GIST and NET too [2,14]. Moreover, in EUS the lesion appears with heterogeneous echogenicity, mainly hypoechoic, and can be classified as M-type or S-type if the lesion occupies the muscularis propria or the submucosal and the mucosal layer [2,8]. It has been reported that the size of the lesion ranges between 0,2–4 cm and pancreatic acini ducts and islets of Langerhans can be observed histopathologically [13]. It was first categorized by Heinrich in 1909, but later in 1973 Gaspar Fuentes modified the classification as follows: Type I includes acini, ducts, and islets cells similar to the normal pancreas, Type II contains only ducts and is referred as canalicular variety, Type III comprises only acinar tissue (exocrine pancreas) and Type IV consists only of islet cells (endocrine pancreas) [8]. Generally, the differentiation of ectopic pancreas in the stomach from other submucosal tumors, such as GIST, as happened in our patient, still remains a challenge [15]. Consequently, the final diagnosis results from the histological examination after surgery or endoscopic resection of the lesion [11.].
Local resection is completely essential when symptoms are present, and it is recommended if EP is found incidentally during other surgery and is asymptomatic. Local resection can be conducted laparoscopically or endoscopically [16].
The contribution of this case report to the existing literature is the confirmation that EP in the stomach cannot be easily diagnosed preoperatively, as imaging techniques are in most cases contradictory and not in accordance with the final histopathological results. However, the basic barrier that deters this case report from being innovative is the absence of specific symptoms and pathognomonic signs, which could lead the physicians to the final diagnosis in advance. Nevertheless, this study provides a piece of information about this rare entity, which may be essential and useful during the effort of clinicians to approach effectively the cases of EP in the future.
4. Conclusion
We experienced a case of subepithelial mass in the stomach's lesser curvature, which was first considered as a GIST. However, the histopathological examination revealed ectopic pancreatic tissue. Consequently, it is crucial during differential diagnosis of such a lesion, the presence of ectopic pancreas to be taken into consideration.
Ethical approval
Our case report obtained ethics approval from the ethics committee of our hospital and the patient gave her informed consent to participate.
Source of funding
This research received no external funding.
Author contribution
D.P., management and follow up, conducting the clinical examination, supervising, conducting the surgery of the patient, correspondence; A.S.K., writing manuscript; T.S., writing manuscript; E.K, writing manuscript, conducting the clinical examination; I.A.D., pathological analysis; G.P, pathological analysis; T.D, conducting the clinical examination, management and follow up; A.M., supervising, management and follow up. All authors have read and agreed to the published version of the manuscript
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Trail registry number
None declared.
Grantor
D.P. and A.S.K. accept full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgement
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.104005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Matsumoto T., Tanaka N., Nagai M., Koike D., Sakuraoka Y., Kubota K. A case of gastric heterotopic pancreatitis resected by laparoscopic surgery. Int. Surg. 2015;100:678–682. doi: 10.9738/INTSURG-D-14-00182.1. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryu D.Y., Kim G.H., Park D.Y., et al. Endoscopic removal of gastric ectopic pancreas: an initial experience with endoscopic submucosal dissection. World J. Gastroenterol. 2010;16:4589–4593. doi: 10.3748/wjg.v16.i36.4589. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurocak B., Gokturk H.S., Kayacetin S., Bakdik S. A rare case of heterotopic pancreas in the stomach which caused closed perforation. Neth. J. Med. 2009;67:285–287. [Google Scholar] [PubMed] [Google Scholar]
- 4.Christodoulidis G., Zacharoulis D., Barbanis S., Katsogridakis E., Hatzitheofilou K. Heterotopic pancreas in the stomach: a case report and literature review. World J. Gastroenterol. 2007;13:6098–6100. doi: 10.3748/wjg.v13.45.6098. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou J.W., Cheng K.S., Ting C.F., Feng C.L., Lin Y.T., Huang W.H. Endosonographic features of histologically proven gastric ectopic pancreas. Gastroenterol Res Pract. 2014;2014 doi: 10.1155/2014/160601. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiriatti E., Kuczma P., Galasso D., Koliakos E., Pezzetta E., Martinet O. Intramural ectopic pancreatic tissue of the stomach: a case report of an uncommon origin of a non-cancerous gastric tumour. Int J Surg Case Rep. 2020;73:48–51. doi: 10.1016/j.ijscr.2020.06.081. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papaziogas B., Koutelidakis I., Tsiaousis P., et al. Carcinoma developing in ectopic pancreatic tissue in the stomach: a case report. Cases J. 2008;1:249. doi: 10.1186/1757-1626-1-249. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottschalk U., Dietrich C.F., Jenssen C. Ectopic pancreas in the upper gastrointestinal tract: is endosonographic diagnosis reliable? Data from the German Endoscopic Ultrasound Registry and review of the literature. Endosc Ultrasound. 2018;7:270–278. doi: 10.4103/eus.eus_18_17. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandan Vishal S., Wang Weichen. Pancreatic heterotopia in the gastric antrum. Arch Pathol Lab Med 1. 2004;128:111–112. doi: 10.5858/2004-128-111-PHITGA. ([PubMed]) [DOI] [PubMed] [Google Scholar]
- 10.Seneviratne S.A., Ramanayaka I.T., Samarasekera D.N. Heterotopic pancreas in the body of the stomach. Ceylon Med. J. 2009;54:57–58. doi: 10.4038/cmj.v54i2.869. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Filip R., Walczak E., Huk J., Radzki R.P., Bieńko M. Heterotopic pancreatic tissue in the gastric cardia: a case report and literature review. World J. Gastroenterol. 2014;20:16779–16781. doi: 10.3748/wjg.v20.i44.16779. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persano G., Cantone N., Pani E., Ciardini E., Noccioli B. Heterotopic pancreas in the gastrointestinal tract in children: a single-center experience and a review of the literature. Ital. J. Pediatr. 2019;45:142. doi: 10.1186/s13052-019-0738-3. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang L.X., Xu J., Wang X.W., et al. Gastric outlet obstruction caused by heterotopic pancreas: a case report and a quick review. World J. Gastroenterol. 2008;14:6757–6759. doi: 10.3748/wjg.14.6757. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calcagno P., Lotti M., Campanati L., et al. Emergency presentation of gastric ectopic pancreas. Cureus. 2018;10:e3565. doi: 10.7759/cureus.3565. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subasinghe D., Sivaganesh S., Perera N., Samarasekera D.N. Gastric fundal heterotopic pancreas mimicking a gastrointestinal stromal tumour (GIST): a case report and a brief review. BMC Res. Notes. 2016;9:185. doi: 10.1186/s13104-016-1995-5. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alqahtani A., Aljohani E., Almadi F., Billa S., Alqahtani M., Alkhaldi H. Heterotopic pancreatic tissue in the gastric antrum an incidental finding during bariatric surgery: a case report and literature review. Int J Surg Case Rep. 2020;67:39–41. doi: 10.1016/j.ijscr.2019.12.040. [PubMed] [Google Scholar] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. ([PubMed] [Google Scholar]) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.