Abstract
Nervous system disorders are one of the common problems that affect many people around the world every year. Regarding the beneficial effects of the probiotics on the gut and the gut-brain axis, their application along with current medications has been the subject of intense interest. Psychobiotics are a probiotic strain capable to affect the gut-brain axis. The effective role of Psychobiotics in several neurological disorders is documented. Consumption of the Psychobiotics containing nutrients has positive effects on the improvement of microbiota as well as alleviation of some symptoms of central nervous system (CNS) disorders. In the present study, the effects of probiotic strains on some CNS disorders in terms of controlling the disease symptoms were reviewed. Finding suggests that Psychobiotics can efficiently alleviate the symptoms of several CNS disorders such as autism spectrum disorders, Parkinson’s disease, multiple sclerosis, insomnia, depression, diabetic neuropathy, and anorexia nervosa. It can be concluded that functional foods containing psychotropic strains can help to improve mental health.
Graphical Abstract
Keywords: Probiotic, Psychobiotics, Gut-brain axis, Central nervous system disorders
Introduction
According to the National Academy of Sciences’ Food and Nutrition Board (FNB) definition in 1994, functional foods are “any modified food or nutrient that may be beneficial to health beyond its contained traditional nutrients” (Chauhan and Kaur 2018). Functional food has various components with health-promoting properties. These compounds include bioactive peptides, unsaturated fatty acids, minerals and vitamins, prebiotics, and probiotics (Valero-Cases et al. 2020; da Silva et al. 2016). The term “probiotic” is derived from the Greek word and means “pro-life.” According to the World Health Organization (WHO) definition, probiotics are defined as living microorganisms that confer benefits to host health when consumed in adequate amounts (Wasilewski et al. 2015). Various species of lactic acid bacteria are classified as probiotics that are isolated from diverse sources such as traditional dairy products, human samples, and plants (Nami et al. 2018). The health effects of probiotics are species-special and are majorly used to prevent or treat different diseases. Induction of inflammatory response by Bifidobacterium animalis subspecies lactis Bl-04 (Turner et al. 2017), anti-diabetic/obese potential by Lactobacillus johnsonni 3121, Lactobacillus rhamnosus 86, and Lactobacillus casei YRL577 (Lee et al. 2021; Zhang et al. 2021), treatment of arthritis, pouchitis, ulcerative colitis, Crohn disease by Lactobacillus casei, Lactococcus lactis NCDO 2118 (Ferro et al. 2021; Cordeiro et al. 2021), anti-cancer effects of Saccharomyces cerevisiae and engineered Escherichia coli Nissle 1917 (EcN) (Chiang and Hong 2021), management of COVID-19 by Bifidobacteria (Bozkurt and Quigley 2020), and fertility improving properties of Lactobacillus plantarum 2621 (Bhandari and Prabha 2015) are some examples of probiotics health effects. In addition to these positive properties, it has recently been well known that the gut has a major impact on human gut-brain axis which is of great importance in health. The gut microbiota effect on improving the physiology and behavior of the host is well known, but the main focus recently has been on the microbiome effect on the brain function (Tengeler 2020). In recent years, researchers have identified the bidirectional correlation between the central nervous system (CNS) and gut microbiota (Bauer et al. 2019). The brain-gut-microbiome (BGM) system comprises the enteric nervous systems (ENS), CNS, and endocrine system, as well as metabolic, neural, and immune mediators (Cowan et al. 2020). Psychobiotics are probiotics strains with potential benefits for host physical health and is widely used for mental disease therapy. Several molecular byproducts of the microbiota such as neuroactive metabolites and associated inflammatory mediators could pass the gut and blood–brain barriers that allow transport through the BGM system (Logsdon et al. 2018). Growing evidence has shown that microbial dysbiosis plays a pivotal role in the pathology of common neurological disorders like autism, depression, Alzheimer’s, and Parkinson’s diseases (Sherwin et al. 2018). Given the gut microbiome effect on brain communications especially for improving mental health, it seems that a proper diet consisting of probiotics could maintain the gut-brain conjunction. The aim of this review is to emphasize on the effects of Psychobiotics on the gut-brain axis function and related neurological diseases.
Microbiota-Gut-Brain Axis
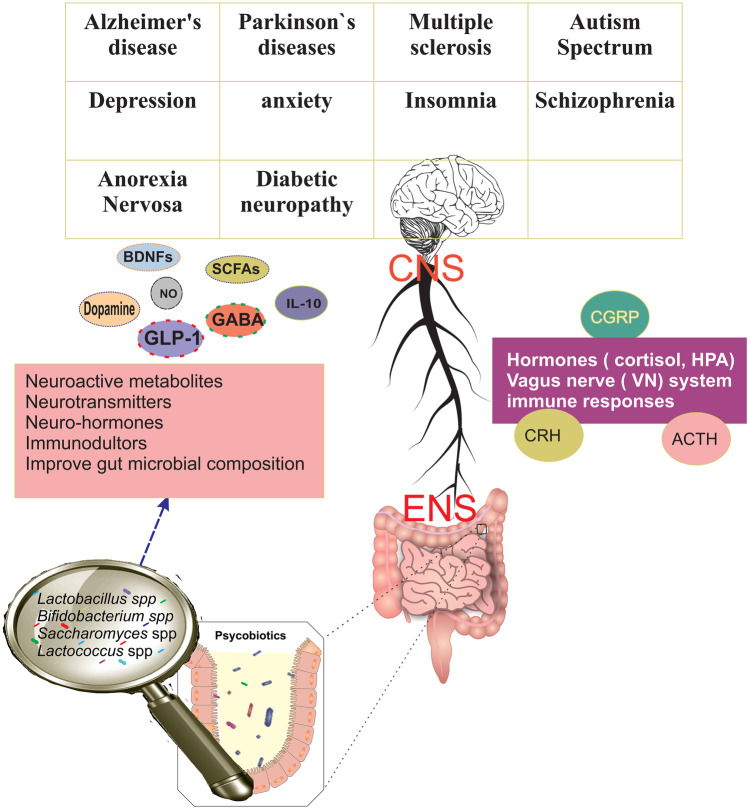
The gut-brain axis is bidirectional association between the gastrointestinal system and the CNS that coordinates the functions of the ENS and CNS (Fichna and Storr 2012; Quigley 2018). This association is regulated by hormones such as cortisol and the hypothalamic–pituitary–adrenal (HPA) hormones, the vagus nerve (VN) system, and immune responses. VN emissions are a major component of the parasympathetic system that transmits intestinal signals to the CNS and stimulates the response (Bonaz et al. 2018). The gut-brain axis role has been identified in several physiological processes such as satiety, food intake, as well as fat and bone metabolism, glucose regulation, and insulin secretion (Romijn et al. 2008). In addition, the host behavior is affected by the correlation of ENS and CNS with gut-brain axis. Reduced activity of these systems is associated with increased stress or anxiety (Vaiserman et al. 2017). It has been determined that the gut-brain axis activity is regulated by calcitonin gene-related peptide (CGRP). Upon disruption of gut microbiota by increased pathogenic microorganisms, neurons can produce CGRP and release it into the gut. The infection could induce the secretion of CGRP which in turn activates the host defense and the corresponding immune response by the calcitonin receptor (CRLR). It has been documented that the intestinal microbiota is balanced by probiotic microorganisms and the gut-brain axis is regulated by CGRP. Corticotropin-releasing hormone (CRH) and adrenocorticotrophic hormone (ACTH) are released on the HPA axis and respond to inflammation and are able to induce the immune responses against pathogens such as bacteria, viruses, and fungi (Wei et al. 2018). Previous studies have demonstrated that during neurological stress, CRH enhances intestinal permeability and plasma levels of ACTH (Vanuytsel et al. 2014). Followed by pathogenic infections, the HPA axis is activated by CRH and ACTH. It has been found that gut microbiota is imbalanced during anxiety and stress which is referred to the interference with the HPA. Due to the importance of the gut-brain axis, modification of the gut microbiota could help the treatment and improvement of psychiatric disorders. Current research has demonstrated that the composition of gut microbiota in autistic patients (Parracho et al. 2005; Bercik et al. 2012) and psychotic patients has changed after administration of various antibiotics (Mehdi 2010). The Microbiota-gut-brain axis could be modified by the administration of prebiotics, probiotics, synbiotics, and postbiotics (Zmora et al. 2019). Altogether, evidence has shown the role of Psychobiotics in the improvement of mental health by modification of microbiota properties (Sarkar et al. 2016).
Psychobiotics
The term “Psychobiotics” refers to probiotics, prebiotics, and all microbiota-targeted interventions that can manipulate microbiota-gut-brain signals and have positive effects on neurological functions such as mood, cognition, and anxiety (Dinan et al. 2013; Evrensel et al. 2019). The potential of Psychobiotics for treating psychiatric disorders are listed in Table 1.
Table 1.
The list of Psychobiotics and their positive psycho effects
| Potential Psychobiotics | Dosage of Psychobiotics | Observation psycho effect | Study model | Ref |
|---|---|---|---|---|
| B. longum 1714 | 1 × 109 CFU/day by the stick with probiotic strains mixed into milk and drunk each morning for 4 weeks | Decreased stress and enhanced memory | Clinical/N = 22 healthy male volunteers | (Allen et al. 2016) |
| L. rhamnosus (JB-1) | 1 × 109 CFU/day as capsule for 4 weeks | Decreased stress-related behaviors, corticosterone release, and altered expression of central GABA* receptors | Clinical N = 29 healthy male volunteers | (Kelly et al. 2017) |
| L. reuteri ATG-F4 | 1 × 107 CFU/day as drinking water for 4 weeks | Anti-inflammatory effects interleukin (IL)-10 and serum dopamine level significantly increased | Animal model N = 10 male mice | (Beck et al. 2019) |
| Pedicoccus pentosaceus WS11, L. plantarum SK321, L. fermentum SK324, L. brevis TRBC 3003, B. adolescentis TBRC 7154, Lactococcus lactis subsp. lactis TBRC 375 | 6 × 109 CFU/day as cell pellets were administered daily via oral gavage for 14 days | Reduced anxiety level, increased locomotor function, improved short-term memory | Animal model N = 7 male Wistar rats | (Luang-In et al. 2020) |
| L.gasseri CP2305 | 1 × 1010 CFU/day mixed with acid milk beverages for 5 weeks | Improved the sleep quality, effect on the growth of fecal Bacteroides spp. involved in the intestinal inflammation | Clinical N = 21 male and N = 11 female healthy students | (Nishida et al. 2017) |
| L. plantarum PS128 | 1 × 1010 CFU/day as pellet for 2 weeks by oral administration | Reduced tic-like behaviors | Animal model N = 10 male Wistar rat | (Liao et al. 2019) |
| L. plantarum PS128 | 3 × 1010 CFU/day as capsule for 4 weeks | Improve opposition/defiance behaviors in ASD children | Clinical N = 80 children (7–15 age) with ASD | (Liu et al. 2019) |
| L. plantarum PS128 | 1 × 1010 CFU/day as pellet by oral gavage for 4 weeks | Reduced motor deficits, elevated corticosterone, and prevention of Parkinson’s disease | Animal model N = 18 male mice | (Liao et al. 2020) |
| Multi-strain probiotic (Bacillus coagulans Unique IS2, L. rhamnosus UBLR58, B. lactis UBBLa70, L. plantarum UBLP40, B. breve UBBr01, B. infantis UBBI01) | 1 × 109 CFU/capsule 2 times a day for 28 days | Reduction in depression anxiety stress scale and state-trait anxiety inventory | Clinical N = 80 student (63 female and 17 male) | (Venkataraman et al. 2021) |
| L. plantarum 90sk and B. adolescentis 150 | 0.5 mL/day of the mixture includes 1 × 107 CFU of B. adolescentis and 1 × 108 CFU of L. plantarum by oral gavage for 14 days | Reduced depressive-like behavior | Animal model N = 48 male mice with anxiety-like behavior and measures of despair | (Yunes et al. 2020) |
| L. rhamnosus JB-1 | 1 × 109 CFU/day by oral treatment for 14 days | Antidepressant effects | Animal model N = 46 male mice with anxiety-like behavior and measures of despair | (McVey Neufeld et al. 2018) |
| L. casei W56, L. acidophilus W22, L. paracasei W20, B. lactis W51, L. salivarius W24, L. lactis W19, B. lactis W52, L. plantarum W62, and B. bifidum W23 | 3000 mg daily oral treatment for 6 months | Normalized the gut-microbiome composition, reduced inflammation and gastrointestinal discomfort, and increased body weight | Clinical N = 60 patients with anorexia nervosa (13–19 years) | (Gröbner et al. 2022) |
*GABA: γ-aminobutyric acid, L: Lactobacillus, B: Bifidobacterium
Functional Foods Rich with Psychobiotics
Fermented foods including yogurt, kefir, tempeh, and kimchi are rich sources of probiotics (Dimidi et al. 2019). Generally, a probiotic product should contain at least 107 CFU/g or 107 CFU/dried viable probiotic cells (Homayouni-Rad et al. 2020). Some probiotic bacteria produce active neuronal compounds or act as carriers (Barros et al. 2020). Some strains of Bifidobacterium and Lactobacillus secrete gamma-aminobutyric acid (GABA). In addition analysis of fecal samples from healthy people through transcriptome methods presented that Bacteroides, Parabacteroides, and Escherichia species are able to regulate GABA-producing pathways (Strandwitz et al. 2019).
This neurotransmitter is one of the key inhibitors in the brain that regulate many physiological and psychological processes, and its dysfunction is involved in anxiety and depression. GABA is an amino acid with a non-protein conformation and one of the secretory metabolites of probiotics, especially lactic acid bacteria (LAB). It is the main mediator of inhibitory transmission in the mammalian CNS (Diez-Gutiérrez et al. 2020). It acts through inotropic A and metabotropic type B (Kalueff and Nutt 2007). GABA-A receptors are ion channels with heteropentamer ligands that cross the nerve membrane. GABA-B receptors are considered to modulate the generation of excitatory postsynaptic potentials and long-term potentiation. Both of these receptors bind to positive modulators such as barbiturates, benzodiazepines, steroids, and ethanol. GABA plays a major role in preventing neurological diseases, cancer, type 1 diabetes, and immune disorders (Diez-Gutiérrez et al. 2020). Medications that are used to treat depression and anxiety can increase GABA-positive modulators. Previous reports have shown that the GABA-A receptor is the active site of anti-anxiety drugs (Diez-Gutiérrez et al. 2020). Better effects and less dependence have also been reported in treatment with GABA (Soussan and Kjellgren 2016). Remarkably, the food industry has a critical role in reducing depression and anxiety in the society by producing functional foods containing probiotic species with the ability to produce GABA. It is defined that Psychobiotics are a subclass of probiotics that bring mental health benefits to the host by interacting with gut bacteria as well as oncobiotics, pharmabiotics, and metabiotics (Nataraj et al. 2020; Toro-Barbosa et al. 2020). In addition to their positive psychological effects, Psychobiotics could induce the production of neuro-transmitters and neuro-hormones that exhibit psychotropic effects in the studied models. Several gut microbes with the potential of producing neuro-transmitters or neuro-hormones are listed in Table 2. Regarding the importance of Psychobiotics, these strains could be used in functional foods. Luang-In et al. (2020), isolated eighteen microbes from fermented Thai food sources and investigated their probiotic attributes, production capacity capacities, and cytotoxic effects. Accordingly, Enterobacter xiangfangensis 4A-2A3.1 and Bacillus spp. PS15 were introduced as GABA-producing bacteria (Luang-In et al. 2020). Ton et al. (2020), confirmed that kefir grains contain species such as Acetobacter aceti, L. fructivorans, Acetobacter sp., Enterococcus faecium, Leuconostoc spp., L. delbrueckii delbrueckii, L. fermentum, L. kefiranofaciens, Candida famata, and Candida krusei. In addition, they reported positive effects of these strains in improving memory, visual-spatial and abstraction properties, as well as executive and language functions in Alzheimer (Ton et al. 2020). Likewise, the probiotic strain L. helveticus was isolated from fermented milk which can improve cognitive function in elderly and middle-aged adults (Chung et al. 2014; Ohsawa et al. 2018). Ko et al. (2013), enriched fermented black soybean milk by GABA producing L. brevis FPA 3709 and found antidepressant effects in rat models without side effects (Ko et al. 2013). Reid et al. (2018), enriched fermented Laminaria japonica with L. brevis BJ20 to determine its effects on physical fitness and short-time working memory in the elderly. They reported that consumption of this fermented food could provide a protective mechanism against dementia in the elderly (Reid et al. 2018). Kim et al. (2002) determined the anti-fatigue and anti-stress effects of rice bran extract which was fermented with Saccharomyces cerevisiae IFO 2346 on rat and mice models (Kim et al. 2002). Due to the widespread use of fermented products including milk, dairy, and soybean products, the probiotic strains in these fermented products have been comprehensively studied for their psychotropic effects. Other studies have identified that fermented milk with L. casei Shirota can improve mood, increase fecal serotonin, and reduce stress level in the investigated students (Benton et al. 2007; Kato-Kataoka et al. 2016), and L. helveticus impact cognitive function (Selhub et al. 2014). Fermented cow milk containing a mixture of L. fermentum LAB9, L. casei LABPC decreased nitrosative stress parameters in mice (Musa et al. 2017). B. lactis BB12 and L. acidophilus LA5 probiotics containing yogurt significantly enhance the general health and alleviate anxiety, depression, and stress scale scores as well (Mohammadi et al. 2016). Moreover, the fermented milk kefir containing L. reuteri could increase GABA biosynthesis in mice (van de Wouw et al. 2020). Similarly, the use of fermented black soy milk containing L. brevis FPA 3709 capable of producing GABA showed antidepressant activity in mice (Ko et al. 2013). On the other hands, administration of fermented soybean containing L. plantarum C29 can improve cognitive function in patients with mild cognitive abnormality (Hwang et al. 2019).
Table 2.
The list of some gut microbes with the production potential of neuro-transmitters or neuro-hormones
| Gut microbe | Neurotransmitters/neurohormones | References |
|---|---|---|
| Proteus vulgaris, B. subtilis, Bacillus mycoides, Serratia marcescens | Dopamine and norepinephrine | (Tsavkelova et al. 2000) |
| Bifidobacterium infantis | Serotonin precursor, tryptophan | (Desbonnet et al. 2008) |
| L. plantarum DSM 19,463 | GABA | (Di Cagno et al. 2010) |
| L. plantarum | Acetylcholine | (Marquardt and Falk 1957) |
| B. amyloliquefaciens SB-9 | Melatonin, serotonin, 5-hydroxytryptophan, and N-acetylserotonin | (Jiao et al. 2016) |
| H. alvei, K. pneumoniae, and M. morganii | Serotonin, histamine, and dopamine | (Özoğul 2004) |
| E. coli | Serotonin, dopamine, and norepinephrine | (Roshchina 2016; Shishov et al. 2009) |
| Enterococcus faecium BS5 | GABA | (Sabna et al. 2021) |
| Enterococcus fecalis (EC-12) | Adrb3, Avpr1a, and Drd5* | (Kambe et al. 2020) |
| L. plantarum 8P-A3, L. fermentum, L. farciminis | Nitric oxide ( NO) | (Morita et al. 1997; Yarullina et al. 2016) |
| Clostridium species, E. coli strain | Catecholamines (CA) | (Asano et al. 2012) |
*The β-3 adrenergic receptor, arginine vasopressin 1a receptor, dopamine D5 receptor
Nitric oxide (NO) is another neurotransmitter involved in various GI functions such as preserving the vascular tone, interfering with immune responses, transmitting nerve impulses, and aiding GI motility by relaxation of the intestinal smooth muscle (Toda and Herman 2005). NO could be produced enzymatically (NO syntheses) or by enteric microflora (e.g., Bacillus subtilis) (Gusarov et al. 2013). In addition, Lactobacillus spp. as the main probiotic strains and normal enteric flora have gained great amount of attention based on the potential of NO production. Korhonen et al. investigated the potential of probiotic L. rhamnosus GG in inducing and regulating the NO production using J774 murine macrophages and T84 colon epithelial cells. It was reported that L. rhamnosus GG could induce NO production from Lipoteichoic acid as an active component in the presence of IFN-γ (Korhonen et al. 2001).
The Effect of Probiotics on CNS Function and Neurological Disorders
Although the exact cause of neurodegenerative disorders is still unknown, some factors including lifestyle, diet, aging, and genetic are contributed to the initiation and development of these diseases. The probiotics effects on the gut-brain axis can be through in CNS biochemistry. For instance, these microorganisms could affect the levels of serotonin (5 hydroxytryptamine; 5 HT), brain-derived neurotrophic factor (BDNF), dopamine (DA), and GABA. The vagus nerve and ENS have a critical role in this connection. Probiotics also indirectly alter CNS function by producing metabolites including tryptophan and short-chain fatty acids (SCFAs) (Ansari et al. 2020). Furthermore, prebiotics have a positive effect on mental health by modulating the composition of gut microbiota (Tabrizi et al. 2019). For example, the effects of SCFAs on the cellular system are mediated through immune system and endocrine pathways as well as neural and humoral routes. SCFAs activate free fatty acid receptors and interact with immune and intestinal epithelial cells, which can affect the safety and function of the intestinal mucosa. Environmentally, it also affects systemic inflammation and neuroinflammation by secreting interleukin and controlling the morphology and function of microglia cells, respectively. It also induces the secretion of intestinal hormones such as glucagon-like peptide 1 (GLP-1), which transfer indirect signals to the brain through vagus nerve and systemic pathways. All of these pathways ultimately affect learning, mood, and memory (Dalile et al. 2019). Alzheimer’s disease (AD) is a major reason for dementia among diseases of the nervous system. The accurate cause of AD is not comprehensively understood. Dementia usually occurs in people over 60 years of age. There is no definitive and effective treatment for this disease. Researches show that the primary manifestations of AD are associated with the production of intracellular Tau neurofibrillary tangles (NFTs) and extracellular amyloid plaques (Yiannopoulou and Papageorgiou 2020). A better understanding of the physiological mechanisms involved in this disease could help identify effective treatments. Given that damage to the gut microbiota can be linked to neurodegenerative diseases such as Alzheimer’s, support for gut microbiota is a possible treatment for AD. Inflammation and oxidative stress destroy nerve cells in the CNS, which could subsequently lead to Alzheimer’s. Prevention of cholinergic neurons destruction along with increasing acetylcholine levels in the brain is desired in successful treatment of AD. It has been documented that continuous application of D-galactose could lead to cognitive abnormalities and memory impairment through increasing oxidative stress and reactive oxygen species. Moreover, reduced expression of nerve growth factors and associated proteins could result from excessive and continuous consumption of D-galactose which consequently results in the nerve cells’ decline (Ansari et al. 2020). Some probiotic bacteria, including L. plantarum, are capable of producing acetylcholine and could protect memory deficiency caused by D-galactose consumption (Nimgampalle and Kuna 2017). Mehrabadi and Sadr (2020) demonstrated that probiotic strains L. reuteri, L. rhamnosus, and B. infantis (1010 CFU/day) treatment in rat models of AD for 10 weeks is helpful in attenuating inflammation and oxidative stress (Mehrabadi and Sadr 2020). Nimgampalle and Kuna (2017) evaluated the anti-Alzheimer properties of L. plantarum MTCC1325 in AD rat models and reported its effects against D-galactose induced AD (Nimgampalle and Kuna 2017). In the clinical studies, Tamtaji et al. (2019a, b) determined the effects of probiotic strains containing L. acidophilus, B. longumB. bifidum (2 × 109 CFU/day), and selenium co-supplementation on the metabolic status and cognitive function of AD patients for 12 weeks. The results confirmed the improved metabolic profiles and cognitive function in patients with AD (Tamtaji et al. 2019a). Akbari et al. (2016) used a mixture of probiotics containing L. acidophilus, L. casei, B. bifidum, and L. fermentum (both 2 × 109 CFU/g) for 12 weeks demonstrating their positive effects on metabolic and status cognitive function in AD patients (Akbari et al. 2016). An important risk factor for AD is type 2 diabetes mellitus (T2D). The peptide hormone GLP-1 is produced in the intestine and CNS (especially in the brainstem) and is associated with neurological protection as well as cognitive functions and glucose metabolism as well. The receptors of GLP-1 are expressed in different tissues (kidney, lung, heart, CNS, etc.) and could up-regulate the expression of various genes that are involved in brain cell repair and differentiation. GLP-1 could also promote the insulin secretion in the hyperglycemic state (Athauda and Foltynie 2016). Previous studies have indicated that GLP-1 act as neuroprotective factor in the CNS followed by inducing the proliferation/apoptosis of neural cells, improvement of learning and memory, lowering the Aβ plaque deposition, reserving dopaminergic neurons, and stimulating nerve regeneration (Kim et al. 2017). Although the benefits of GLP-1 drugs in AD have been approved, the identification is still an important challenge due to the high degradation rate of GLP-1 by dipeptidyl peptidase IV (DPP-IV) in the body with a half-life time of less than 2 min (Gejl et al. 2016). Fang et al. investigated the effects of GLP-1 engineered probiotic (Lactococcus lactis MG1363) on AD mouse model after oral administration. Memory impairment and motor dysfunction were induced by LPS and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), respectively. The results indicated that engineered strain is able to reduce memory impairment and motor dysfunction via two signaling pathways including TLR4/NFκB and AKT/glycogen synthase kinase-3β (GSK3β). In addition, the engineered probiotic reduced the frequency of pathogens (Enterococcus, Proteus) and increase the frequency of Akkermansia muciniphila (Fang et al. 2020). Similarly, Chan et al. constructed two engineering strains to overcome GLP-1 degradation by continuous expression. Administration of MG1363-pMG36e-GLP-1 and VNP20009-pLIVE-GLP-1 could improve the LPS-induced learning and memory impairment, inhibit the glial cell activation, and aggregation of Aβ. Moreover, downregulation of inflammatory response (COX-2, TLR-4, TNF-a, and IL-1β), blocking NF-κB signals, and MAPKs/PI3K/AKT were observed (Chen et al. 2018). The effectiveness of GLP-1 and GIP (glucose-dependent insulinotropic polypeptide) as neuroprotective factors has been determined for T2D treatment. Li et al. explored the impacts of GLP-1/GIP/glucagon triagonist administration (for 30 days) on the cognitive behaviors of AD mice. The results showed the beneficial effects of triagonist on AD by decreasing cognitive deficits and pathological changes (Li et al. 2018a). Bonfili et al. reported that oral administration of SLAB51 (formulated with LAB and bifidobacteria) in AD mice could restore the glucose transporters in brain (GLUT3 and GLUT1), ameliorate glucose homeostasis in mice brain, and decrease the hyperphosphorylation of Tau by pAMPK/pAkt signaling pathways. In addition, SLAB51 could increase the expression level of insulin-like growth factor receptor β and counteract the increasing of HbA1c and advanced glycation end products (AGEs) in AD mice (Bonfili et al. 2020). Collectively, these information elucidated that manipulating gut microbiota with probiotics in AD could ameliorate impaired glucose metabolism, prevent AD progression, and reduce neuroinflammation benefitting the GLP-1 effects.
Parkinson’s disease (PD) is the second common and leading neurodegenerative disorders (Poewe et al. 2017). The disease is associated with synuclein accumulation and progressive loss of dopaminergic neurons (Balestrino and Schapira 2020) and is characterized by motor and non-motor symptoms (Castelli et al. 2020). Non-motor symptoms of abnormalities in the gut function are mainly weight loss, gastroparesis, constipation, and defecation dysfunction (Cloud and Greene 2011; Kim and Sung 2015). Psychobiotics consumption is currently of great attention in the treatment of PD. Hsieh et al. (2020) reported that consumption of a probiotic mixture (1010 CFU/day) containing B. bifidum, B. longum, L. rhamnosis, L. rhamnosus GG, Lactococcus lactis subsp. Lactis, and L. plantarum LP28 for 16 weeks provide effective protection on dopamine releasing neurons and subsequently reduce the motor dysfunctions deterioration in MitoPark PD mice (Hsieh et al. 2020). Khandestan et al. (2020) determined the effect of probiotic L. paracasei on motor disorders in PD rats and presented less apomorphine rotation test than the saline receiving group (Khandestan et al. 2020).
In a clinical study, Cassani et al. (2011) assessed the fermented milk effects that contain 6.5 × 109 of L. casei Shirota in PD patients for 5 weeks and reported reduced bloating, decreased constipation, and less abdominal pain (Cassani et al. 2011). Barichella et al. (2016) administrated the fermented milk with multiple probiotic strains and prebiotics once a day for 4 weeks and reported improved constipation in PD patients (Barichella et al. 2016). Georgescu et al. (2016) used 60 mg per tablet of two LAB: L. acidophilus and B. infantis, twice daily for 3 months in 40 PD patients and reported alleviation of abdominal pain and bloating (Georgescu et al. 2016). Tamtaji et al. (2019a, b) reported a decreased hypersensitivity and reduced malondialdehyde levels along with increased glutathione levels in PD patients receiving 8 × 109 CFU/day probiotic for 12 weeks (Tamtaji et al. 2019b). Tan et al. (2021) stated that multi-strain probiotics treatment for 4 weeks was effective for constipation in PD (Tan et al. 2021).
Multiple sclerosis (MS) is a well-known autoimmune disease involving CNS in which myelin covering axons are destroyed. Genetics and environmental factors, as well as viral infections, are considered as main risk factors for MS development. However, the accurate cause of this disorder is not clear (Goldenberg 2012). Accumulating evidence has proposed that probiotics can improve the immune system of MS patients by altering the gut microbiome, suppressing inflammatory pathways, and regulating the immune system (Morshedi et al. 2019). Kouchaki et al. (2017) evaluated the effects of probiotic strains containing L. fermentum, L. casei, L. acidophilus, and B. bifidum (each 2 × 109 CFU/g) on mental health, disability, and metabolic condition in MS patients receiving the probiotic mixture for 12 weeks. Based on the results, favorable effects of probiotics on the expanded disability status, mental health, inflammatory factors, insulin resistance markers, HDL, total HDL-cholesterol, and malondialdehyde levels of MS patients are documented (Kouchaki et al. 2017). Rahimlou et al. (2020) reported a considerable increase in BDNF along with a reduction in IL-6 levels in MS patients receiving probiotic strains (including Bacillus subtilis, B. bifidum, B. breve, B. infantis, B. longum, L. acidophilus, L. delbrueckii ssp. bulgaricus, L. casei, L. plantarum, L. rhamnosus, L. helveticus, L. salivarius, Lactococcus lactis ssp. lactis, Streptococcus thermophilus) for 6 months (Rahimlou et al. 2020). Dargahi et al. (2020) demonstrated that probiotic strain S. thermophiles increase the expression of anti-inflammatory cytokines including IL-4, IL-5, IL-10 and decrease the secretion of pro-inflammatory IFN-γ and IL-1β in MS mice model (Dargahi et al. 2020).
Autism Spectrum Disorders (ASD) are a range of deficits in social communication, sensory-motor behaviors, and limited interests that are considered to be associated with genetic or other factors (Lord et al. 2018). ASD manifestation begins in early childhood (Lord et al. 2020). Autistic patients show gastrointestinal (GI) symptoms. GI dysfunction in autistic children is usually associated with aggressive behaviors, increased irritability, and sleep disturbances (Critchfield et al. 2011). Shaaban et al. (2018) reported the beneficial effects of probiotics on behavioral and GI manifestations of ASD. Treatment of autistic children with probiotic strains containing L. acidophilus, L. rhamnosus, and B. longum for 3 months showed an increase in the population of Bifidobacteria and Lactobacilli levels along with weight reduction and improvement in GI symptoms (Shaaban et al. 2018). Moreover, Grossi et al. (2016) reported an improvement in the autistic core symptoms and reduced abdominal symptoms in autistic children receiving probiotic strains for 1 month (Grossi et al. 2016).
Depression and anxiety are among the common illnesses in the world. Both disorders mostly occur together (Tiller 2013). Antidepressants, anxiolytics, and hypnotics are commonly used for treatment (Miller and Massie 2006). Clinically, there is an association between periods of depression and HPA dysregulation (Foster and Neufeld 2013). Studies have demonstrated that the gut microbiota of healthy individuals and MDD patients is different. Decreases in Bifidobacterium and Lactobacillus along with increases in Clostridium, Streptococcus, Klebsiella, Oscillibacter, and Allistipes are among these changes (Yong et al. 2020). Research has shown that gastrointestinal bacteria activate stress circuits through the vagus pathways (Lyte et al. 2006). Chronic exposure to stressors causes long-term secretion of norepinephrine, which alters the gut microbiota and makes the gut more permeable to bacteria and toxins, which is followed by stressful reactions in the HPA axis. Tian et al. (2020) investigated the effect of probiotic strain B. breve CCFM1025 on fecal microbial composition and brain neurological alterations, as well as serum levels of corticosterone, cytokines, and SCFAs. Totally, depression- and anxiety-like behaviors were reduced (Tian et al. 2020).
Allen et al. (2016) reported that the probiotic strain B. longum 1714 is able to reduce stress levels and improve the memory in healthy volunteers (Allen et al. 2016). Slykerman et al. (2017) evaluated the effect of L. rhamnosus HN001 on postpartum manifestations of anxiety and depression in 423 pregnant women and stated considerably lower scores of depression in the probiotic-treated group (Slykerman et al. 2017). The study by Rudzki et al. (2019) showed that the probiotic strain L. plantarum 299v reduced the kynurenine levels and enhanced the cognitive functions of patients with major depression (Rudzki et al. 2019). However, other investigations did not report any significant differences between patients receiving probiotics and the control group in terms of anxiety and well-being scores (Dawe et al. 2020; Romijn et al. 2017). Pinto-sanchez et al. (2017) demonstrated that the probiotic strain B. longum NCC3001 could increase the life quality of patients with irritable bowel syndrome and could reduce depression in these cases (Pinto-Sanchez et al. 2017).
Insomnia and Schizophrenia
As a matter of fact, sufficient sleep is a critical factor for life quality. The gut microbiome affects the mental states and sleep status of the host by the microbiome-gut-brain axis. The circadian rhythm and sleep quality of the host depend on the microbiome profile and metabolism properties (Li et al. 2018b). Probiotics are considered to improve sleep health. GABA is an inhibitory neurotransmitter capable of promoting the relaxation by reducing anxiety. L. brevis DL1-11 is a probiotic strain with high GABA production capacity, and its potential for improved sleep in mice has been confirmed (Yu et al. 2020). Schizophrenia is a chronic debilitating disease that affects less than 1% of the world’s population (Saha et al. 2005). Research shows that the gut microbiota disruption increases systemic inflammation. Therefore, neuroinflammation can cause schizophrenia (Rogers et al. 2016). Schizophrenic patients usually suffer from the compromised nutritional status, high stress responses, increased inflammatory status, and lactose intolerance (Nemani et al. 2015). Probiotics with anti-inflammatory and immunomodulatory properties could be beneficial in reducing symptoms in schizophrenia patients (Frei et al. 2015). Nagamine et al. (2012) reported that a probiotic mix of Clostridium butyricum, Streptococcus faecalis, and Bacillus mesentericus could reduce schizophrenia symptoms (Nagamine et al. 2012). Dickerson et al. (2014) showed that combination of probiotic strains B. animalis subsp. lactis strain Bb12 GG and L. rhamnosus strain could decrease bowel difficulty in schizophrenia patients (Dickerson et al. 2014). Tomasik et al. (2015) examined the possible immunomodulatory effects of L. rhamnosus strain GG and B. animalis subsp. lactis strain Bb12 in chronic schizophrenia. They reported a significant reduction in von Willebrand factor concentration along with increased levels of BDNF, macrophage inflammatory protein-1 beta, monocyte chemotactic protein-1, and RANTES (regulated on activation, normal T-cell expressed and secreted). Consequently, they demonstrated that consumption of probiotic supplements in schizophrenia patients may improve GI leakage (Tomasik et al. 2015). Severance et al. (2017) reported that L. rhamnosus strain GG and B. animalis subsp. lactis Bb12 help to normalize Candida albicans antibody levels and C. albicans-associated gut discomfort in male patients (Severance et al. 2017). Ghaderi et al. (2019) verified the beneficial effects of B. lactis Bb12 and L. rhamnosus on the total positive and negative syndrome scale score as well as patients metabolic profile in schizophrenia (Ghaderi et al. 2019).
Diabetic Neuropathy (DN)
Diabetic neuropathy is a nutritional neurodegenerative disease associated with axonal atrophies, demyelinating disorders, decreased regenerative capacity, neuronal inflammation, and peripheral neuropathy. DN could change glycemia regulation as well as intestinal glucose malabsorption through neuronal gut-brain axis, portal vein (regulation of energy metabolism in CNS), and loss of peripheral neuronal conduction (Zochodne 2007). Type 2 diabetes (T2D) is characterized by a dysregulated metabolism of glucose that lead to fasting and postprandial hyperglycemia. Impaired insulin and glucagon secretion and function are the main causes of this disorder. GLP-1 is an incretin hormone secreted by intestinal L cells in response to glucose and is used for T2D treatment. It activates enteric neurons and modulates the intestinal transit through specific receptors, increases the proliferation of pancreatic islet b cells as well as enhances the glucose-dependent insulin secretion and reduces the secretion of glucagon from pancreatic α-cells. As a result, it decreases blood glucose and food intake in patients with T2D. When GLP-1 is activated, it transmits a neural message to the VN that is involved in glycemia regulation through the gut-brain axis (Perry and Greig 2005). GLP-1 also has neuro-protective and neurogenic potential, and research has shown that it induces axons in the primary culture of neurons from the dorsal root ganglion (Anand et al. 2018). Therefore, it can be effective in the treatment of DN. Wang et al. (2020) isolated 14 species of probiotics, including 10 Lactobacillus strains (1 × 108, 1 × 1010 CFU/mL) and 4 Saccharomyces (1 × 106, 1 × 108 CFU/mL) from camel fermented milk and examined their effect on various parameters in mice model of T2D for 6 months. They found that probiotics enhanced insulin secretion through glucose-triggered GLP-1 secretion by up-regulating G protein-coupled receptor 43/41 (GPR43/41), proglucagon, and proconvertase 1/3 activity (Wang et al. 2020). It has been determined that when the germ-free (GF) mice were colonized with healthy gut microbiota homeostasis, neuronal activities of ENS and VN were restored but not with diabetic mice gut microbiota (Grasset et al. 2017). Kunze et al. found that feeding rats with L. reuteri for 9 days could improve the ENS by targeting the calcium-dependent potassium channels in enteric sensory nerves (Kunze et al. 2009). Some Lactobacillus strains (L. farciminis, plantarum, and fermentum) could produce nitric oxide (NO) as a neurotransmitter, which is able to influence the neuronal response to GLP-1 and glucose metabolism (Grasset et al. 2017). Pegah et al. investigated the resveratrol and probiotics effects of GLP-1 in T2D rats. Rats were fed with various probiotic bacteria, including L. plantarum, L. bulgaricu, L. casei, B. infantis, L. acidophilus, B. longum, B. breve, at a dose of 50 × 109 for 4 weeks. They found that these probiotics and resveratrol could decrease glucose and insulin resistance (significantly, p < 0.001) and increase GLP1 as well as total antioxidant capacity (significantly, p < 0.001) compared to the diabetic group (Pegah et al. 2021). Wei et al. identified that two strains, L. kefiranofaciens M and L. kefiri K (denoted as Strain K), could decrease the T1D progression in mice model by inducing the GLP-1 secretion, inhibiting the cytokine production (proinflammatory and inflammatory factors), raising the IL-10 production, and modifying the gut microbiota towards LAB and Bifidobacterium spp. along with decreasing the Clostridium perfringens and coliform (Wei et al. 2015). These results revealed the role of probiotics in increasing GLP-1 levels that could subsequently alleviate hyperglycemia and could be suggested as a potential candidate for diabetes treatment.
Anorexia Nervosa
According to the great knowledge of the gut-brain interaction and the positive effects of probiotics on this axis, several novel treatment strategies could be provided for anorexia nervosa (AN) treatment. AN is another important mental disorder that is associated with severe weight loss, psychiatric comorbidities, fear of fatness, and dietary restrictions.
Gröbner et al. assayed the efficacy of probiotics administration on AN patients and measured the body mass index (BMI), psycho/neuro psychological parameters by analyzing serum and stool samples. They reported positive regulation of gut microbial community in AN to improve weight gain, gastrointestinal discomfort, and inflammation reduction (Gröbner et al. 2022). Liu et al. evaluated the effects of probiotics on AN model by pre (fructooligosaccharides (FOS), 1.67 g/daily) and probiotic (Saccharomyces boulardii (5 × 108 CFU)) intervention. After dietary restrictions, total microbiota and metabolites were reduced compared to healthy status, but supplementation with FOS and S. boulardii restored the microbial community by modifying Bifidobacterium, Bacteroides spp. Roseburia Clostridium coccoides-Eubacterium rectale group, Clostridium histolyticum, and Phascolartobacterium faecium (Liu et al. 2021).
Solis et al. showed the positive effect of two diets (yoghurt or milk) including L. bulgaricus and S. thermphilus which is able to induce IFN-γ production against infections on children with diarrhea and AN patients (Solis et al. 2002). It is considerable that antibiotics and 25% of drugs could impact the microbial community, and feeding therapy based on the pre/probiotics is required for AN patients to obtain main goals such as energy harvesting, weight gain, lower gut permeability, and inflammation process as well as modification of the gut microbiome.
Conclusions
Ultimately, it can be concluded that probiotics have effective features on controlling the symptoms of CNS disorders. The probiotics effect mainly enhance gut health. Consumption of healthy foods containing probiotics has an important role in the prevention of CNS disorders and controlling related symptoms by gut microbiota modulation. This effect occurs through the gut-brain axis and can be integrated into clinical trials. Therefore, healthy food diets are of great importance. Consumption of fermented foods along with designing new functional foods containing probiotic species is an important step for enhancement of mental health. It is suggested that the prevalence of CNS disorders in probiotics and fermented foods consuming patients is significantly lesser compared to the patients with no probiotics regimen, indicating the probiotics effects in improving the symptoms of these disorders. Overall, probiotics/prebiotics can be administered as an add-on adjuvant treatment for various diseases. Hence, their positive effects on neurological disorders still require further investigations in the future studies.
Author Contribution
HL had the idea for the article and critically revised the manuscript. PO and SYB performed the literature search and wrote the first draft of the manuscript.
Availability of Data and Materials
Data will be requested from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
This review paper has not obtained any ethical approval and does not contain any studies with human or animal subjects.
Competing Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akbari E, Asemi Z, Daneshvar Kakhaki R, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AP, Hutch W, Borre YE, et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry. 2016;6:e939–e939. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U, Yiangou Y, Akbar A, et al. Glucagon-like peptide 1 receptor (GLP-1R) expression by nerve fibres in inflammatory bowel disease and functional effects in cultured neurons. PLoS ONE. 2018;13:e0198024. doi: 10.1371/journal.pone.0198024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari F, Pourjafar H, Tabrizi A, Homayouni A. The effects of probiotics and prebiotics on mental disorders: a review on depression, anxiety, Alzheimer, and autism spectrum disorders. Curr Pharm Biotechnol. 2020;21:555–565. doi: 10.2174/1389201021666200107113812. [DOI] [PubMed] [Google Scholar]
- Asano Y, Hiramoto T, Nishino R, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today. 2016;21:802–818. doi: 10.1016/j.drudis.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27:27–42. doi: 10.1111/ene.14108. [DOI] [PubMed] [Google Scholar]
- Barichella M, Pacchetti C, Bolliri C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology. 2016;87:1274–1280. doi: 10.1212/WNL.0000000000003127. [DOI] [PubMed] [Google Scholar]
- Barros CP, Guimarães JT, Esmerino EA, et al. Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr Opin Food Sci. 2020;32:1–8. doi: 10.1016/j.cofs.2019.12.003. [DOI] [Google Scholar]
- Bauer KC, Rees T, Finlay BB. The gut microbiota–brain axis expands neurologic function: a nervous rapport. BioEssays. 2019;41:1800268. doi: 10.1002/bies.201800268. [DOI] [PubMed] [Google Scholar]
- Beck BR, Park G-S, Jeong DY, et al. Multidisciplinary and comparative investigations of potential psychobiotic effects of Lactobacillus strains isolated from newborns and their impact on gut microbiota and ileal transcriptome in a healthy murine model. Front Cell Infect Microbiol. 2019;9:269. doi: 10.3389/fcimb.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61:355–361. doi: 10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24:405–413. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- Bhandari P, Prabha V. Evaluation of profertility effect of probiotic Lactobacillus plantarum 2621 in a murine model. Indian J Med Res. 2015;142:79–84. doi: 10.4103/0971-5916.162127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfili L, Cecarini V, Gogoi O et al (2020) Gut microbiota manipulation through probiotics oral administration restores glucose homeostasis in a mouse model of Alzheimer’s disease. Neurobiol Aging 87:35–43. 10.1016/j.neurobiolaging.2019.11.004 [DOI] [PubMed]
- Bozkurt HS, Quigley EMM. The probiotic Bifidobacterium in the management of Coronavirus: a theoretical basis. Int J Immunopathol Pharmacol. 2020;34:2058738420961304. doi: 10.1177/2058738420961304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani E, Privitera G, Pezzoli G, et al. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol Dietol. 2011;57:117–121. [PubMed] [Google Scholar]
- Castelli V, d’Angelo M, Lombardi F, et al. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models. Aging (albany NY) 2020;12:4641. doi: 10.18632/aging.102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan V, Kaur S. Functional foods—urgent need for healthy mind and body. Int J Food Sci Nutr. 2018;3:180–183. [Google Scholar]
- Chen T, Tian P, Huang Z, et al. Engineered commensal bacteria prevent systemic inflammation-induced memory impairment and amyloidogenesis via producing GLP-1. Appl Microbiol Biotechnol. 2018;102:7565–7575. doi: 10.1007/s00253-018-9155-6. [DOI] [PubMed] [Google Scholar]
- Chiang C-J, Hong Y-H. In situ delivery of biobutyrate by probiotic Escherichia coli for cancer therapy. Sci Rep. 2021;11:1–17. doi: 10.1038/s41598-021-97457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y-C, Jin H-M, Cui Y, et al. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J Funct Foods. 2014;10:465–474. doi: 10.1016/j.jff.2014.07.007. [DOI] [Google Scholar]
- Cloud LJ, Greene JG. Gastrointestinal features of Parkinson’s disease. Curr Neurol Neurosci Rep. 2011;11:379–384. doi: 10.1007/s11910-011-0204-0. [DOI] [PubMed] [Google Scholar]
- Cordeiro BF, Alves JL, Belo GA, et al. Therapeutic effects of probiotic Minas Frescal cheese on the attenuation of ulcerative colitis in a murine model. Front Microbiol. 2021 doi: 10.3389/fmicb.2021.623920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CSM, Dinan TG, Cryan JF. Annual research review: critical windows—the microbiota–gut–brain axis in neurocognitive development. J Child Psychol Psychiatry. 2020;61:353–371. doi: 10.1111/jcpp.13156. [DOI] [PubMed] [Google Scholar]
- Critchfield JW, Van Hemert S, Ash M et al (2011) The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract [DOI] [PMC free article] [PubMed]
- da Silva BV, Barreira JCM, Oliveira MBPP. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: extraction, biochemistry and protected-delivery technologies. Trends Food Sci Technol. 2016;50:144–158. doi: 10.1016/j.tifs.2015.12.007. [DOI] [Google Scholar]
- Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- Dargahi N, Matsoukas J, Apostolopoulos V. Streptococcus thermophilus ST285 alters pro-inflammatory to anti-inflammatory cytokine secretion against multiple sclerosis peptide in mice. Brain Sci. 2020;10:126. doi: 10.3390/brainsci10020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe JP, McCowan LME, Wilson J, et al. Probiotics and maternal mental health: a randomised controlled trial among pregnant Women with obesity. Sci Rep. 2020;10:1–11. doi: 10.1038/s41598-020-58129-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, et al. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Di Cagno R, Mazzacane F, Rizzello CG, et al. Synthesis of γ-aminobutyric acid (GABA) by Lactobacillus plantarum DSM19463: functional grape must beverage and dermatological applications. Appl Microbiol Biotechnol. 2010;86:731–741. doi: 10.1007/s00253-009-2370-4. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Stallings C, Origoni A et al (2014) Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim care companion CNS Disord 16 [DOI] [PMC free article] [PubMed]
- Diez-Gutiérrez L, San Vicente L, Barron LJR, et al. Gamma-aminobutyric acid and probiotics: multiple health benefits and their future in the global functional food and nutraceuticals market. J Funct Foods. 2020;64:103669. doi: 10.1016/j.jff.2019.103669. [DOI] [Google Scholar]
- Dimidi E, Cox SR, Rossi M, Whelan K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. 2019;11:1806. doi: 10.3390/nu11081806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Evrensel A, Ünsalver BÖ, Ceylan ME (2019) Psychobiotics. In: Frontiers in psychiatry. Springer, 565–581 [DOI] [PubMed]
- Fang X, Zhou X, Miao Y, et al. Therapeutic effect of GLP-1 engineered strain on mice model of Alzheimer’s disease and Parkinson’s disease. AMB Express. 2020;10:80. doi: 10.1186/s13568-020-01014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro M, Charneca S, Dourado E, et al. Probiotic supplementation for rheumatoid arthritis: a promising adjuvant therapy in the gut microbiome era. Front Pharmacol. 2021;12:711788. doi: 10.3389/fphar.2021.711788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichna J, Storr M. Brain-Gut Interactions in IBS Front Pharmacol. 2012;3:127. doi: 10.3389/fphar.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, Neufeld K-AM. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Frei R, Akdis M, O’Mahony L. Prebiotics, probiotics, synbiotics, and the immune system: experimental data and clinical evidence. Curr Opin Gastroenterol. 2015;31:153–158. doi: 10.1097/MOG.0000000000000151. [DOI] [PubMed] [Google Scholar]
- Gejl M, Gjedde A, Egefjord L, et al. In Alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci. 2016;8:108. doi: 10.3389/fnagi.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Ancusa OE, Georgescu LA, et al. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: is there hope? Clin Interv Aging. 2016;11:1601. doi: 10.2147/CIA.S106284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi A, Banafshe HR, Mirhosseini N, et al. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry. 2019;19:1–10. doi: 10.1186/s12888-019-2059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg MM. Multiple Sclerosis Review Pharm Ther. 2012;37:175. [PMC free article] [PubMed] [Google Scholar]
- Grasset E, Puel A, Charpentier J et al (2017) A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metab 25:1075–1090.e5. 10.1016/j.cmet.2017.04.013 [DOI] [PubMed]
- Gröbner EM, Zeiler M, Fischmeister FPS, et al. The effects of probiotics administration on the gut microbiome in adolescents with anorexia nervosa—a study protocol for a longitudinal, double-blind, randomized, placebo-controlled trial. Eur Eat Disord Rev. 2022;30:61–74. doi: 10.1002/erv.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi E, Melli S, Dunca D, Terruzzi V (2016) Unexpected improvement in core autism spectrum disorder symptoms after long-term treatment with probiotics. SAGE Open Med Case Reports 4:2050313X16666231 [DOI] [PMC free article] [PubMed]
- Gusarov I, Gautier L, Smolentseva O, et al. Bacterial nitric oxide extends the lifespan of C. elegans. Cell. 2013;152:818–830. doi: 10.1016/j.cell.2012.12.043. [DOI] [PubMed] [Google Scholar]
- Homayouni-Rad A, Azizi A, Oroojzadeh P, Pourjafar H. Kluyveromyces marxianus as a probiotic yeast: a mini-review. Curr Nutr Food Sci. 2020;16:1163–1169. doi: 10.2174/1573401316666200217113230. [DOI] [Google Scholar]
- Hsieh T-H, Kuo C-W, Hsieh K-H, et al. Probiotics alleviate the progressive deterioration of motor functions in a mouse model of Parkinson’s disease. Brain Sci. 2020;10:206. doi: 10.3390/brainsci10040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y-H, Park S, Paik J-W, et al. Efficacy and safety of Lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: a 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2019;11:305. doi: 10.3390/nu11020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Ma Y, Chen S, et al. Melatonin-producing endophytic bacteria from grapevine roots promote the abiotic stress-induced production of endogenous melatonin in their hosts. Front Plant Sci. 2016;7:1387. doi: 10.3389/fpls.2016.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- Kambe J, Watcharin S, Makioka-Itaya Y, et al. Heat-killed Enterococcus fecalis (EC-12) supplement alters the expression of neurotransmitter receptor genes in the prefrontal cortex and alleviates anxiety-like behavior in mice. Neurosci Lett. 2020;720:134753. doi: 10.1016/j.neulet.2020.134753. [DOI] [PubMed] [Google Scholar]
- Kato-Kataoka A, Nishida K, Takada M, et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef Microbes. 2016;7:153–156. doi: 10.3920/BM2015.0100. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Allen AP, Temko A, et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun. 2017;61:50–59. doi: 10.1016/j.bbi.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Khandestan K, Pakpour B, Tajabadi Ebrahimi M. Effect of Lactobacillus paracasei probiotic in Parkinson’s male rats. J Gorgan Univ Med Sci. 2020;22:65–72. [Google Scholar]
- Kim DS, Choi H-I, Wang Y, et al. A new treatment strategy for Parkinson’s disease through the gut–brain axis: the glucagon-like peptide-1 receptor pathway. Cell Transplant. 2017;26:1560–1571. doi: 10.1177/0963689717721234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-S, Sung H-Y. Gastrointestinal autonomic dysfunction in patients with Parkinson’s disease. J Mov Disord. 2015;8:76. doi: 10.14802/jmd.15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Yu KW, Kang DH, Suh HJ. Anti-stress and anti-fatigue effect of fermented rice bran. Phyther Res an Int J Devoted to Pharmacol Toxicol Eval Nat Prod Deriv. 2002;16:700–702. doi: 10.1002/ptr.1019. [DOI] [PubMed] [Google Scholar]
- Ko CY, Lin H-TV, Tsai GJ. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochem. 2013;48:559–568. doi: 10.1016/j.procbio.2013.02.021. [DOI] [Google Scholar]
- Korhonen R, Korpela R, Saxelin M, et al. Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation. 2001;25:223–232. doi: 10.1023/a:1010971703271. [DOI] [PubMed] [Google Scholar]
- Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36:1245–1249. doi: 10.1016/j.clnu.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Mao YK, Wang B, et al. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med. 2009;13:2261–2270. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Park MH, Kim BK, Kim SH. Antiobesity effect of novel probiotic strains in a mouse model of high-fat diet–induced obesity. Probiotics Antimicrob Proteins. 2021;13:1054–1067. doi: 10.1007/s12602-021-09752-0. [DOI] [PubMed] [Google Scholar]
- Li T, Jiao JJ, Hölscher C, et al. A novel GLP-1/GIP/Gcg triagonist reduces cognitive deficits and pathology in the 3xTg mouse model of Alzheimer’s disease. Hippocampus. 2018;28:358–372. doi: 10.1002/hipo.22837. [DOI] [PubMed] [Google Scholar]
- Li Y, Hao Y, Fan F, Zhang B. The role of microbiome in insomnia, circadian disturbance and depression. Front Psychiatry. 2018;9:669. doi: 10.3389/fpsyt.2018.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J-F, Cheng Y-F, Li S-W, et al. Lactobacillus plantarum PS128 ameliorates 2, 5-dimethoxy-4-iodoamphetamine-induced tic-like behaviors via its influences on the microbiota–gut-brain-axis. Brain Res Bull. 2019;153:59–73. doi: 10.1016/j.brainresbull.2019.07.027. [DOI] [PubMed] [Google Scholar]
- Liao J-F, Cheng Y-F, You S-T, et al. Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced mouse models of Parkinson’s disease. Brain Behav Immun. 2020;90:26–46. doi: 10.1016/j.bbi.2020.07.036. [DOI] [PubMed] [Google Scholar]
- Liu L, Poveda C, Jenkins PE, Walton GE. An in vitro approach to studying the microbial community and impact of pre and probiotics under anorexia nervosa related dietary restrictions. Nutrients. 2021;13:4447. doi: 10.3390/nu13124447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-W, Liong MT, Chung Y-CE, et al. Effects of Lactobacillus plantarum PS128 on children with autism spectrum disorder in Taiwan: a randomized, double-blind, placebo-controlled trial. Nutrients. 2019;11:820. doi: 10.3390/nu11040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon AF, Erickson MA, Rhea EM, et al. Gut reactions: how the blood–brain barrier connects the microbiome and the brain. Exp Biol Med. 2018;243:159–165. doi: 10.1177/1535370217743766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Brugha TS, Charman T, et al. Autism spectrum disorder. Nat Rev Dis Prim. 2020;6:1–23. doi: 10.1038/s41572-019-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. 2018;392:508–520. doi: 10.1016/S0140-6736(18)31129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luang-In V, Katisart T, Konsue A, et al. Psychobiotic effects of multi-strain probiotics originated from thai fermented foods in a rat model. Food Sci Anim Resour. 2020;40:1014. doi: 10.5851/kosfa.2020.e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M, Li W, Opitz N, et al. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89:350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Marquardt P, FALK H, Incidence and synthesis of acetylcholine in plants and bacteria. Arzneimittelforschung. 1957;7:203–211. [PubMed] [Google Scholar]
- McVey Neufeld K-A, Kay S, Bienenstock J. Mouse strain affects behavioral and neuroendocrine stress responses following administration of probiotic Lactobacillus rhamnosus JB-1 or traditional antidepressant fluoxetine. Front Neurosci. 2018;12:294. doi: 10.3389/fnins.2018.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi S. Antibiotic-induced psychosis: a link to D-alanine? Med Hypotheses. 2010;6:676–677. doi: 10.1016/j.mehy.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Mehrabadi S, Sadr SS. Assessment of probiotics mixture on memory function, inflammation markers, and oxidative stress in an Alzheimer’s disease model of rats. Iran Biomed J. 2020;24:220. doi: 10.29252/ibj.24.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Massie MJ. Depression and anxiety. Cancer J. 2006;12:388–397. doi: 10.1097/00130404-200609000-00008. [DOI] [PubMed] [Google Scholar]
- Mohammadi AA, Jazayeri S, Khosravi-Darani K, et al. The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr Neurosci. 2016;19:387–395. doi: 10.1179/1476830515Y.0000000023. [DOI] [PubMed] [Google Scholar]
- Morita H, Yoshikawa H, Sakata R, et al. Synthesis of nitric oxide from the two equivalent guanidino nitrogens of L-arginine by Lactobacillus fermentum. J Bacteriol. 1997;179:7812–7815. doi: 10.1128/jb.179.24.7812-7815.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedi M, Hashemi R, Moazzen S, et al. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: a systematic review. J Neuroinflammation. 2019;16:1–11. doi: 10.1186/s12974-019-1611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa NH, Mani V, Lim SM, et al. Lactobacilli-fermented cow’s milk attenuated lipopolysaccharide-induced neuroinflammation and memory impairment in vitro and in vivo. J Dairy Res. 2017;84:488–495. doi: 10.1017/S0022029917000620. [DOI] [PubMed] [Google Scholar]
- Nagamine T, Sato N, Seo G. Probiotics reduce negative symptoms of schizophrenia: a case report. Int Med J. 2012;19:72–73. [Google Scholar]
- Nami Y, Haghshenas B, Vaseghi Bakhshayesh R, et al. Novel autochthonous lactobacilli with probiotic aptitudes as a main starter culture for probiotic fermented milk. LWT. 2018 doi: 10.1016/j.lwt.2018.08.035. [DOI] [Google Scholar]
- Nataraj BH, Shivanna SK, Rao P et al (2020) Evolutionary concepts in the functional biotics arena: a mini-review. Food Sci Biotechnol 1–10 [DOI] [PMC free article] [PubMed]
- Nemani K, Ghomi RH, McCormick B, Fan X. Schizophrenia and the gut–brain axis. Prog Neuro-Psychopharmacology Biol Psychiatry. 2015;56:155–160. doi: 10.1016/j.pnpbp.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Nimgampalle M, Kuna Y (2017) Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s disease induced albino rats. J Clin diagnostic Res JCDR 11:KC01 [DOI] [PMC free article] [PubMed]
- Nishida K, Sawada D, Kawai T, et al. Para-psychobiotic Lactobacillus gasseri CP 2305 ameliorates stress-related symptoms and sleep quality. J Appl Microbiol. 2017;123:1561–1570. doi: 10.1111/jam.13594. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Nakamura F, Uchida N, et al. Lactobacillus helveticus-fermented milk containing lactononadecapeptide (NIPPLTQTPVVVPPFLQPE) improves cognitive function in healthy middle-aged adults: a randomised, double-blind, placebo-controlled trial. Int J Food Sci Nutr. 2018;69:369–376. doi: 10.1080/09637486.2017.1365824. [DOI] [PubMed] [Google Scholar]
- Özoğul F. Production of biogenic amines by Morganella morganii, Klebsiella pneumoniae and Hafnia alvei using a rapid HPLC method. Eur Food Res Technol. 2004;219:465–469. doi: 10.1007/s00217-004-0988-0. [DOI] [Google Scholar]
- Parracho HMRT, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54:987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- Pegah A, Abbasi-Oshaghi E, Khodadadi I et al (2021) Probiotic and resveratrol normalize GLP-1 levels and oxidative stress in the intestine of diabetic rats. Metab Open 10:100093. 10.1016/j.metop.2021.100093 [DOI] [PMC free article] [PubMed]
- Perry T, Greig NH. Enhancing central nervous system endogenous GLP-1 receptor pathways for intervention in Alzheimer’s disease. Curr Alzheimer Res. 2005;2:377–385. doi: 10.2174/1567205054367892. [DOI] [PubMed] [Google Scholar]
- Pinto-Sanchez MI, Hall GB, Ghajar K, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(448–459):e8. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Prim. 2017;3:1–21. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- Quigley EMM. The gut-brain axis and the microbiome: clues to pathophysiology and opportunities for novel management strategies in irritable bowel syndrome (IBS) J Clin Med. 2018;7:6. doi: 10.3390/jcm7010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimlou M, Hosseini SA, Majdinasab N et al (2020) Effects of long-term administration of Multi-Strain Probiotic on circulating levels of BDNF, NGF, IL-6 and mental health in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Nutr Neurosci 1–12 [DOI] [PubMed]
- Reid SNS, Ryu J, Kim Y, Jeon BH (2018) The effects of fermented Laminaria japonica on short-term working memory and physical fitness in the elderly. Evidence-Based Complement Altern Med [DOI] [PMC free article] [PubMed]
- Rogers GB, Keating DJ, Young RL, et al. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust New Zeal J Psychiatry. 2017;51:810–821. doi: 10.1177/0004867416686694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn JA, Corssmit EP, Havekes LM, Pijl H. Gut–brain axis. Curr Opin Clin Nutr Metab Care. 2008;11:518–521. doi: 10.1097/MCO.0b013e328302c9b0. [DOI] [PubMed] [Google Scholar]
- Roshchina VV (2016) New trends and perspectives in the evolution of neurotransmitters in microbial, plant, and animal cells. Microb Endocrinol Interkingdom Signal Infect Dis Heal 25–77 [DOI] [PubMed]
- Rudzki L, Ostrowska L, Pawlak D, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–222. doi: 10.1016/j.psyneuen.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Sabna BS, Thankappan B, Mahendran R et al (2021) Evaluation of GABA production and probiotic activities of Enterococcus faecium BS5. Probiotics Antimicrob Proteins 1–12 [DOI] [PubMed]
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Lehto SM, Harty S, et al. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub EM, Logan AC, Bested AC. Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. J Physiol Anthropol. 2014;33:1–12. doi: 10.1186/1880-6805-33-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, et al. Probiotic normalization of Candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain Behav Immun. 2017;62:41–45. doi: 10.1016/j.bbi.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban SY, El Gendy YG, Mehanna NS, et al. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr Neurosci. 2018;21:676–681. doi: 10.1080/1028415X.2017.1347746. [DOI] [PubMed] [Google Scholar]
- Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2018;1420:5–25. doi: 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- Shishov VA, Kirovskaya TA, Kudrin VS, Oleskin AV. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Appl Biochem Microbiol. 2009;45:494–497. doi: 10.1134/S0003683809050068. [DOI] [PubMed] [Google Scholar]
- Slykerman RF, Hood F, Wickens K, et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. 2017;24:159–165. doi: 10.1016/j.ebiom.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis B, Nova E, Gómez S, et al. The effect of fermented milk on interferon production in malnourished children and in anorexia nervosa patients undergoing nutritional care. Eur J Clin Nutr. 2002;56:S27–S33. doi: 10.1038/sj.ejcn.1601659. [DOI] [PubMed] [Google Scholar]
- Soussan C, Kjellgren A. The users of novel psychoactive substances: online survey about their characteristics, attitudes and motivations. Int J Drug Policy. 2016;32:77–84. doi: 10.1016/j.drugpo.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Strandwitz P, Kim KH, Terekhova D, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4:396–403. doi: 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi A, Khalili L, Homayouni-Rad A et al (2019) Prebiotics, as promising functional food to patients with psychological disorders: a review on mood disorders, sleep, and cognition. NeuroQuantology 17
- Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr. 2019;38:2569–2575. doi: 10.1016/j.clnu.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Tamtaji OR, Taghizadeh M, Kakhaki RD, et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- Tan AH, Lim S-Y, Chong KK, et al. Probiotics for constipation in Parkinson disease: a randomized placebo-controlled study. Neurology. 2021;96:e772–e782. doi: 10.1212/WNL.0000000000010998. [DOI] [PubMed] [Google Scholar]
- Tengeler AC (2020) Mind the microbes. The impact of the gut microbiota on brain structure and function in mice. [Sl: sn]
- Tian P, O’Riordan KJ, Lee Y, et al. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol Stress. 2020;12:100216. doi: 10.1016/j.ynstr.2020.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller JWG. Depression and anxiety. Med J Aust. 2013;199:S28–S31. doi: 10.5694/mja12.10628. [DOI] [PubMed] [Google Scholar]
- Toda N, Herman AG. Gastrointestinal function regulation by nitrergic efferent nerves. Pharmacol Rev. 2005;57:315–338. doi: 10.1124/pr.57.3.4. [DOI] [PubMed] [Google Scholar]
- Tomasik J, Yolken RH, Bahn S, Dickerson FB (2015) Immunomodulatory effects of probiotic supplementation in schizophrenia patients: a randomized, placebo-controlled trial. Biomark Insights 10:BMI. S22007 [DOI] [PMC free article] [PubMed]
- Ton AMM, Campagnaro BP, Alves GA et al (2020) Oxidative stress and dementia in Alzheimer’s patients: effects of synbiotic supplementation. Oxid Med Cell Longev 2020: [DOI] [PMC free article] [PubMed]
- Toro-Barbosa D, Hurtado-Romero A, Garcia-Amezquita LE, García-Cayuela T. Psychobiotics: mechanisms of action, evaluation methods and effectiveness in applications with food products. Nutrients. 2020;12:3896. doi: 10.3390/nu12123896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavkelova EA, Botvinko IV, Kudrin VS, Oleskin AV (2000) Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl Biochem 372:115–117 [PubMed]
- Turner RB, Woodfolk JA, Borish L, et al. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection—a randomised controlled trial. Benef Microbes. 2017;8:207–215. doi: 10.3920/BM2016.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiserman AM, Koliada AK, Marotta F. Gut microbiota: a player in aging and a target for anti-aging intervention. Ageing Res Rev. 2017;35:36–45. doi: 10.1016/j.arr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Valero-Cases E, Cerdá-Bernad D, Pastor J-J, Frutos M-J. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients. 2020;12:1666. doi: 10.3390/nu12061666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wouw M, Walsh AM, Crispie F, et al. Distinct actions of the fermented beverage kefir on host behaviour, immunity and microbiome gut-brain modules in the mouse. Microbiome. 2020;8:1–20. doi: 10.1186/s40168-020-00846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63:1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- Venkataraman R, Madempudi RS, Neelamraju J, et al. Effect of multi-strain probiotic formulation on students facing examination stress: a double-blind, placebo-controlled study. Probiotics Antimicrob Proteins. 2021;13:12–18. doi: 10.1007/s12602-020-09681-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dilidaxi D, Wu Y, et al. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed Pharmacother. 2020;125:109914. doi: 10.1016/j.biopha.2020.109914. [DOI] [PubMed] [Google Scholar]
- Wasilewski A, Zielińska M, Storr M, Fichna J. Beneficial effects of probiotics, prebiotics, synbiotics, and psychobiotics in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1674–1682. doi: 10.1097/mib.0000000000000364. [DOI] [PubMed] [Google Scholar]
- Wei S-H, Chen Y-P, Chen M-J (2015) Selecting probiotics with the abilities of enhancing GLP-1 to mitigate the progression of type 1 diabetes in vitro and in vivo. J Funct Foods 18:473–486. 10.1016/j.jff.2015.08.016
- Wei X, Zhao J, Jia X, et al. Abnormal gut microbiota metabolism specific for liver cirrhosis. Front Microbiol. 2018;9:3051. doi: 10.3389/fmicb.2018.03051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarullina DR, Mikheeva RO, Sabirullina GI, et al. Role of nitric oxide produced by lactobacilli in relaxation of intestinal smooth muscles. Bull Exp Biol Med. 2016;160:343–346. doi: 10.1007/s10517-016-3166-z. [DOI] [PubMed] [Google Scholar]
- Yiannopoulou KG, Papageorgiou SG. Current and future treatments in alzheimer disease: an update. J Cent Nerv Syst Dis. 2020;12:1179573520907397. doi: 10.1177/1179573520907397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong SJ, Tong T, Chew J, Lim WL. Antidepressive mechanisms of probiotics and their therapeutic potential. Front Neurosci. 2020;13:1361. doi: 10.3389/fnins.2019.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Han X, Cen S, et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol Res. 2020;233:126409. doi: 10.1016/j.micres.2020.126409. [DOI] [PubMed] [Google Scholar]
- Yunes RA, Poluektova EU, Vasileva EV, et al. A multi-strain potential probiotic formulation of GABA-producing Lactobacillus plantarum 90sk and bifidobacterium adolescentis 150 with antidepressant effects. Probiotics Antimicrob Proteins. 2020;12:973–979. doi: 10.1007/s12602-019-09601-1. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhou H, Guan M, et al. Lactobacillus casei YRL577 combined with plant extracts reduce markers of non-alcoholic fatty liver disease in mice. Br J Nutr. 2021;125:1081–1091. doi: 10.1017/S0007114520003013. [DOI] [PubMed] [Google Scholar]
- Zmora N, Soffer E, Elinav E. Transforming medicine with the microbiome. Sci Transl Med. 2019 doi: 10.1126/scitranslmed.aaw1815. [DOI] [PubMed] [Google Scholar]
- Zochodne DW. Diabetes mellitus and the peripheral nervous system: manifestations and mechanisms. Muscle Nerve off J Am Assoc Electrodiagn Med. 2007;36:144–166. doi: 10.1002/mus.20785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be requested from the corresponding author.