Abstract
With a rich abundance of natural polyphenols, green tea has become one of the most popular and healthiest nonalcoholic beverages being consumed worldwide. Epigallocatechin-3-gallate (EGCG) is the predominant catechin found in green tea, which has been shown to promote numerous health benefits, including metabolic regulation, antioxidant, anti-inflammatory, and anticancer. Clinical studies have also shown the inhibitory effects of EGCG on cancers of the male and female reproductive system, including ovarian, cervical, endometrial, breast, testicular, and prostate cancers. Autophagy is a natural, self-degradation process that serves important functions in both tumor suppression and tumor cell survival. Naturally derived products have the potential to be an effective and safe alternative in balancing autophagy and maintaining homeostasis during tumor development. Although EGCG has been shown to play a critical role in the suppression of multiple cancers, its role as autophagy modulator in cancers of the male and female reproductive system remains to be fully discussed. Herein, we aim to provide an overview of the current knowledge of EGCG in targeting autophagy and its related signaling mechanism in reproductive cancers. Effects of EGCG on regulating autophagy toward reproductive cancers as a single therapy or cotreatment with other chemotherapies will be reviewed and compared. Additionally, the underlying mechanisms and crosstalk of EGCG between autophagy and other cellular processes, such as reactive oxidative stress, ER stress, angiogenesis, and apoptosis, will be summarized. The present review will help to shed light on the significance of green tea as a potential therapeutic treatment for reproductive cancers through regulating autophagy.
Keywords: green tea, EGCG, autophagy, reproductive cancers, anticancer
Introduction
Male and female reproductive cancers remain one of the biggest health concerns worldwide due to the high prevalence, yet therapeutic management remains a great challenge. A low survival rate reflects the lack of efficient treatments and risks of recurrence (Garcia-Aranda and Redondo, 2019; Hanna et al., 2020; Hawsawi et al., 2020). Current treatments for reproductive cancers bring fertility-related side effects. The risk of infertility associated with gynecologic malignancies is high, with a successful pregnancy rate of only above 40% (Poorvu et al., 2019). Hysterectomy is one of the most common management of ovarian and endometrial cancers, removing the critical reproductive organs. In breast cancer, a negative impact on the reproductive system has been reported after chemotherapy (Hickey et al., 2009). Radiation therapy that is directed to the reproductive organs disturbs hormone production and imposes fertility risk. Adjuvant therapy for testicular cancer affects fecundity (Kim et al., 2010; Poorvu et al., 2019). Recently, modulation of autophagy is proposed as a novel approach to tackle cancer. In this review article, we will report the therapeutic effects of green tea, particularly EGCG, on female and male reproductive cancers and its possible mechanisms behind it. In addition, this review will emphasize the crosstalk of EGCG between its regulated anti-carcinogenic properties with autophagy on highlighting its role as an autophagy modulator toward reproductive cancer. Importantly, we hope to provide a perspective on green tea catechins and EGCG as potential autophagy modulator for the treatment of reproductive cancer.
Incidence, Mortality, and Prevalence of Reproductive Cancer
Cancer is a major leading burden of disease worldwide, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths. In 2050, an estimated 6.9 million new cancers will be diagnosed in adults aged 80 years or older worldwide (20.5% of all cancer cases) (Pilleron et al., 2021). Among both male and female, reproductive cancer contributes to a high incidence and mortality on the causes of death worldwide (Garcia-Aranda and Redondo, 2019; Hawsawi et al., 2020). The following cancers are exclusive to the female reproductive system including cancer originating at the ovary (ovarian cancer), cervix uteri (cervical cancer), and corpus uteri (endometrial cancer). While these cancers are exclusive to the male reproductive system, these include cancer originating at the prostate gland (prostate cancer) and the testis (testicular cancer). As for breast cancer, this can affect both males and females.
Ovarian cancer cells arise from epithelial, stromal, or germ cells of the ovary (Horowitz et al., 2020). The cells disseminate through the perineal cavity and metastasize to the omentum (Yeung et al., 2015). On the first hand, cancer cells from primary tumor cells can passively be disseminated to a newly established site, with enhanced cancer cells adhesion, invasiveness, motility, and a supportive microenvironment. On the other hand, it can undergo a hematogenous metastatic mechanism via epithelial-mesenchymal transition (Yeung et al., 2015). In 2020, ovarian cancer accounts for 313,959 new cases, and 207,252 deaths. The crude death rate of ovarian cancer was 5.4 per 100,000 female population. The age-standardized death rate of ovarian cancer was 4.2 per 100,000 standard population (WHO, 2022). Diagnosis of ovarian cancer is often delayed; it could be more treatable if it is diagnosed earlier (Evans et al., 2007).
For cervical cancer, it has been widely accepted that Human papillomavirus (HPV) infection is an attributable factor (Walboomers et al., 1999). HPV oncoproteins E6 and E7 block p53 and retinoblastoma protein, which function as tumor suppressors, as well as engage in multi-step carcinogenesis such as viral DNA replication and proliferation, leading to invasive cervical cancer development (Walboomers et al., 1999; Yugawa and Kiyono, 2009; Den Boon et al., 2015; Gupta and Mania-Pramanik, 2019). Worldwide, cervical cancer is reportedly the fourth most incident cancer and the fourth leading cause of cancer-related death, with 604,127 new cases and 341,831 deaths in women in 2020. Early on, typically no symptoms are seen. Later, symptoms may include abnormal vaginal bleeding, pelvic pain, or pain during sexual intercourse (WHO, 2022).
Endometrial cancer can be endometrioid (type 1), arising from excess stimulation of estrogen and atypical glandular hyperplasia; or non-endometrioid (type 2) (Lu and Broaddus, 2020; Terzic et al., 2021). Type 1 tumors are associated with significantly enhanced oncogene mutations and tumor suppressor genes; hence, it resembles proliferative endometrium (Hecht and Mutter, 2006; Morice et al., 2016). Type 2 tumors are mainly associated with enhanced TP53 and HER2/neu mutations (Morice et al., 2016; Terzic et al., 2021). In 2020, endometrial cancers newly occurred in 417,367 women and caused 97,370 deaths. This makes it the third most common cause of death in cancers which only affect women, behind cervical and ovarian cancer. Endometrial cancer arises from the endometrium (the lining of the uterus or womb) and is associated with excessive estrogen exposure, high blood pressure, and diabetes. And often, regular screening in those at normal risk is not common.
Breast cancer originates within the inner lining of ducts or the lobules, then disseminates to lymph nodes or other nearby sites. The major cause of mortalities includes metastasis with enhanced cells, or with dysregulated immune responses (Zheng et al., 2017). In 2020, breast cancer is the most diagnosed cancer, with 2.3 million cases and 685,000 deaths globally (Sung et al., 2021). It affects 1 in 7 (14%) women worldwide, making it the world’s most prevalent cancer (Balasubramanian et al., 2019). Breast cancer occurs in every country of the world in women at any age after puberty but with increasing rates in later life (WHO, 2021).
Genetic and environmental factors contribute to the development of testicular germ cell tumors, supported by ethical and geographic evidence (Al-Obaidy et al., 2020). It is also reported that isochromosome 12p alternation is especially associated with the progression and invasiveness of the tumors; and it can be related to abnormalities in malignant transformation, oxidative damage, and DNA repairing (Reuter, 2005; Cheng et al., 2017; Al-Obaidy et al., 2020). Globally, testicular cancer affected about 74,458 new cases in 2020, which resulted in 9,334 deaths in 2020. As found, rates are lower in the developing than the developed world. Onset most commonly occurs in males 20–34 years old, rarely before 15 years old (Hayes-Lattin and Nichols, 2009). Most often, treatment outcomes are better when the disease remains localized.
Prostate cancer development can be via intraepithelial neoplasia which is the hyperplasia of luminal cells and progressive loss of basal cells. On the other hand, it is also reported as an androgen-dependent adenocarcinoma, that is characterized by the strong phenotype of lumina cells but without basal cells. In contrast, it can also be castration-resistant and independent of androgens (Testa et al., 2019; Tong, 2021). Prostate cancer is the fourth most incident cancer worldwide, with 1,414,259 new cases and 375,304 deaths in 2020. In males, it was the second most common cancer and had the highest prevalence in 2020. Factors that increase the risk of prostate cancer include older age, family history, and race. About 99% of cases occur after age 50 (Rawla, 2019).
Overall, reproductive cancers comprised 28.11% of incidence, 17.34% of mortality, and 38.05% of prevalence (5 years) of all cancers in the world in 2020, demonstrating its relevance as a public health problem (Table 1). The molecular mechanism of reproductive related cancers had been extensively reviewed, which is a complex carcinogenesis process accompanied by related pathophysiology (Zheng et al., 2017; Gupta and Mania-Pramanik, 2019; Testa et al., 2019; Al-Obaidy et al., 2020; Horowitz et al., 2020; Terzic et al., 2021). These include enhanced growth factors and oncogenes but resisting apoptotic factors and tumor suppressors, as a result of enhanced invasiveness and metastasis. These processes are regulated by different signal transduction systems such as the cell cycle and DNA repair system (Torgovnick and Schumacher, 2015; Li et al., 2020). Noteworthily, there is a rapid growth of research that focuses on the autophagy roles in cancer pathophysiology. Our team previously conducted a comprehensive and systematic analysis on the roles of autophagy in cervical, endometrial, and ovarian cancer progression and chemoresistance (Tam et al., 2021). We agreed with the argumentative roles of autophagy, which are inconsistent with those proposed in previous cancer studies (Janku et al., 2011; Mowers et al., 2017; Lim and Murthy, 2020). In this review, we extend the research to explore autophagy modulators in reproductive cancer.
TABLE 1.
Incidence, mortality, and prevalence of male and female reproductive cancers worldwide in 2020.
| Cancer | Prevalence, 5 years (total cases of all cancers: 44,091,402) | Mortality (total deaths of all cancers: 9,894,402) | Incidence (total new cases of all cancers: 18, 094,716) | Proportion mortality/incidence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence in cancer occurrence | Number of cases | % | Cause of cancer-related death | Number of cases | % | Cancer most frequently diagnosed | Number of cases | % | ||
| Breast | 1st | 7,790,717 | 17.67 | 5th | 684,996 | 6.92 | 1st | 2,261,419 | 12.50 | 0.30 |
| Cervical | 9th | 1,495,211 | 3.39 | 9th | 341,831 | 3.45 | 7th | 604,127 | 3.34 | 0.57 |
| Endometrial | 10th | 1,415,213 | 3.21 | 19th | 97,370 | 0.98 | 15th | 417,367 | 2.31 | 0.23 |
| Ovarian | 17th | 823,315 | 1.87 | 14th | 207,252 | 2.09 | 18th | 313,959 | 1.74 | 0.66 |
| Prostate | 3rd | 4,956,901 | 11.24 | 8th | 375,304 | 3.79 | 4th | 1,414,259 | 7.82 | 0.27 |
| Testicular | 23rd | 296,686 | 0.67 | 32nd | 9,334 | 0.09 | 27th | 74,458 | 0.41 | 0.13 |
| Total | — | 16,778,043 | 38.05 | — | 1,716,087 | 17.34 | — | 5,085,589 | 28.11 | — |
Information collected from data published at https://gco.iarc.fr/today, accessed on 21st March 2022
The Role of Autophagy in Reproductive Cancer
In normal physiological conditions, autophagy typically would be maintained at a minimal level of activity. Mainly, cells utilize basal levels of autophagy to aid in the maintenance of biological function, homeostasis, quality-control of cell contents, and elimination of old proteins and damaged organelles for cell survival (Levine and Yuan, 2005; Mizushima et al., 2008). Autophagy happens in lysosome which has a membrane to limit the leakage of the degradative enzyme. There are micro- and macro-autophagy. The former engulfs the cytosolic compound directly by lysosomes, and the latter involves the sequestration of cargoes within an autophagosome, which is a double membrane cytosolic vesicle formed from a phagophore. Macro-autophagy can be specific and targets organelles or invasive microbes, as well as can be nonspecific. The degradation process of autophagosome happens in autolysosome and macromolecules will be released back into the cytosol (Levine and Kroemer, 2008; Mizushima et al., 2008; Mizushima and Levine, 2020). Additionally, the regulation of autophagy can modulate cancer cells’ growth or death.
During the initiation stage of autophagy, phagophore assembly site (PAS) is formed, along with UNC51-like kinase (ULK)1/2 and autophagy-related protein (ATG)13 which translocate to the endoplasmic reticulum to facilitate the formation of class III PI3K-Beclin1-VPS34 complex as phagophore. Phagophore undergoes elongation to form autophagosome, which requires two ubiquitin-like conjugate systems: ATG12-ATG5-ATG16 multimeric system and Phosphatidylethanolamine (PE) to microtubule-associated protein 1 light chain (LC)3 conjugate system. ATG7 and ATG4 facilitate the binding of LC3-PE, which are essential for the expansion of the autophagosome membrane to recognize autophagic cargos. During the maturation stage, the cargo sequestration is completed. The resulting autophagosome fuses with a lysosome to form an autolysosome, meanwhile, lysosomal enzyme degrades the autophagosome contents. At last, the cargo is broken down and released into the cytosol. The lysosome is recycled during the degradation stage (Levine and Kroemer, 2008; Mizushima et al., 2008; Feng et al., 2014; Kaur and Debnath, 2015; Mizushima and Levine, 2020). Based on our previous findings, we reported that several autophagy regulators, consisting of ULK1, Beclin1, ATG5, p62, and LC-3BII, were related to the growth of cervical, endometrial, and ovarian cancer (Tam et al., 2021). Similarly, these autophagy regulators, including Beclin1 and LC-3B, were also shown to modulate prostate adenocarcinoma and breast cancer (Naponelli et al., 2015; Tyutyunyk-Massey and Gewirtz, 2020) while p62, LC3B, and Beclin1 played critical rolesin the survival of testicular cancer (Nakkas et al., 2021). Therefore, by targeting these autophagy regulatory factors directly or indirectly, it could potentially be used to inhibit the progression of reproductive cancer tumors.
Promotion of Cancer Growth
Autophagy is a physiological cellular process for the degradation and elimination of misfolded proteins and damaged organelles that function in adaptation to starvation, development, cell death, and tumor suppression (Klionsky et al., 2021). This process allows unneeded proteins to be degraded and the amino acids recycled for the synthesis of proteins that are essential for survival. Toward cancer survival, autophagy plays dual roles in contributing to the growth of cancer as well as tumor suppression (Klionsky et al., 2021). Such that, the growth of cancer can be promoted by maintaining the survival of tumor cells that have been starved, or to degrade apoptotic mediators, through autophagy (Su et al., 2013). This can be induced by cytotoxic and metabolic stresses, including hypoxia and nutrient deprivation, to activate autophagy for recycling of ATP and to maintain cellular biosynthesis and survival, which promotes HIF-1α-dependent and -independent activation (Semenza, 2010). This activation would increase the expression of angiogenic factors, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor, and nitric oxide synthase. Also, the induction of autophagy by miRNA-4673 has been found to improve the resistance of cancer cells to radiation (Dokumcu et al., 2018). In addition, suppression of autophagy by deletion of Beclin 1 enhances cell death (Degenhardt and White, 2006; White and Dipaola, 2009). Autophagy presents the cytoprotective ability to cancer cells and favors cell survival by coping with the intracellular and environmental stress. Therefore, potent inhibitors to limit the progression of autophagy can be an alternative strategy (Amaravadi et al., 2019; Mizushima and Levine, 2020), such as Metformin in prostate cancer (Farrow et al., 2014); Chloroquine in prostate, breast, and endometrial cancer (Solomon and Lee, 2009; Farrow et al., 2014; Nunez-Olvera et al., 2019); Bortezomib and HDAC6 inhibitor Panobinostat in breast cancer (Maycotte and Thorburn, 2014); Elaiophyl in ovarian cancer (Zhao et al., 2015) etc. Autophagy inhibitors should be used only if cancer cells are more reliant on autophagy than normal cells (Mizushima and Levine, 2020).
Promotion of Cancer Cell Death
Various studies have shown the regulation of autophagy would contribute to the expression of tumor suppressor proteins or oncogenes (Avalos et al., 2014). The previous study has shown xenografts with a lowered expression of Beclin1, a protein that regulates autophagy, to be tumor-prone (Qu et al., 2003). However, another study demonstrated when Beclin1 was overexpressed, the tumor development was inhibited (Liang et al., 1999). Likewise, autophagy can indirectly prevent the release of proinflammatory HMGB1 by limiting necrosis and chronic inflammation to protect against tumorigenesis (Tang et al., 2010). Autophagy brings cytotoxicity effects to promote tumor cell death, which can be completed through degradation of oncogenic proteins (Janku et al., 2011), or via reducing metabolic stress, regulation of oxidative stress, or metastasis during tumorigenesis. DNA would be damaged and lead to genomic instability (Mathew et al., 2009; Mizushima and Levine, 2020). It responds to a range of cellular stresses, including nutrient deprivation, organelle damage, and abnormal protein accumulation. Autophagy inducers may be beneficial when there are excess abnormal macromolecules and organelles (Mizushima and Levine, 2020), for example, Rapamycin in breast cancer (Danko et al., 2021); Trastuzumab in HER2 positive breast cancer (Maycotte and Thorburn, 2014); Atorvastatin in prostate cancer (Farrow et al., 2014); Metformin in cervical, breast and endometrial cancer (Lu G. et al., 2021), etc.
As mentioned, some anticancer drugs have been shown to regulate autophagy (Cuomo et al., 2019). However, targeting these autophagy modulators as a therapeutical strategy remains a huge obstacle (Levy et al., 2017; Amaravadi et al., 2019). Different side effects were reported, ranging from diarrhea, and sensitization to chemotherapy and anemia (Buzun et al., 2021). Autophagy inhibitors had also been reported to sensitize immune response in therapy, as well as influence tumor stroma and normal cell function (Gewirtz, 2016; Noguchi et al., 2020). Hence, natural product as autophagy modulator has been proposed, owing to their safe profiles whilst showing high effectiveness to diminish tumor cells (Zhang Q. Y. et al., 2018; Rahman et al., 2020; Al-Bari et al., 2021). Natural product to regulate autophagy is also a novel therapeutic approach in improving fertility (Zhu et al., 2019; Park et al., 2021). In addition, natural products can be used to target multiple pathways, thus providing greater flexibility and potential for a variety of cancer types and stages.
Green Tea
Tea is brewed from the leaves and buds of the plant Camellia sinensis. With extreme popularity particularly in Asia, it is ranked the second most common beverage consumed worldwide (behind water) (Graham, 1992). In general, teas from this plant can be grouped into green, black, or oolong tea. Among the tea consumption, approximately 20% is in the form of green tea. As green tea has not undergone the same withering and oxidation process as black or oolong tea, it contains the highest amounts of natural polyphenols (catechins) (around 5–7 mg/g in green tea; while that in black tea is only around 0–3 mg/g). These catechins account for approximately 30–40% of the extractable solids of dried green tea leaves, alkaloids (such as caffeine and theobromine), carbohydrates, tannins, and minerals (such as fluoride and aluminum) (Kochman et al., 2020). These catechins have been shown to be more powerful antioxidants, both in vitro and in vivo, when compared to vitamins C and vitamins E (Cabrera et al., 2006; Khan and Mukhtar, 2008; Chacko et al., 2010). The four-major green tea catechins include (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), and (−)-epigallocatechin-3-gallate (EGCG), which EGCG is the most abundant and biologically active (Schramm, 2013) (Supplementary Figure S1). Green tea has been shown to regulate a range of health issues such as improves digestive and brain function, promotes metabolism, as well as fights and protects against cardiovascular disease, diabetes, cancer and others (Cabrera et al., 2006; Khan and Mukhtar, 2008; Chacko et al., 2010; Kochman et al., 2020). Based on previous studies, the consumption of green tea daily was found to be correlated with a slightly lower risk of death from cardiovascular causes (Xi et al., 2015) and a reduced risk of stroke (Zhang et al., 2015). As EGCG is a powerful antioxidant, it is believed to be an important determinant in the therapeutic qualities of green tea. As the structure of EGCG possessed a gallic acid, the presence of the gallate moiety and the extra phenol ring on EGCG enhance its anticancer property, which traps electrons, as a scavenger of free radicals and reduces oxidative stress (Du et al., 2012; Chu et al., 2017). This powerful antioxidant property of EGCG can suppress neovascularization and regulate vessel permeability, thereby cutting off the blood supply to cancerous cells (Maiti et al., 2003).
Potential Anticancer Effect of Green Tea
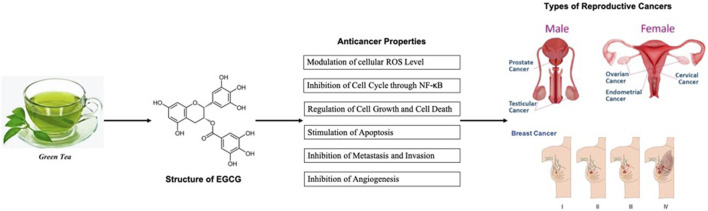
Green tea catechin was shown to target cell cycle regulatory proteins, inhibit cell division, stimulate cell cycle arrest mechanism and promote apoptosis, thus is effective against the progression of the tumor. As green tea catechins, in particular, EGCG, are powerful antioxidants, it is believed they are important determinants in the therapeutic qualities of green tea. As the structure of EGCG possessed a gallic acid, the presence of the gallate moiety and the extra phenol ring on EGCG enhance its anticancer property, which traps electrons, as a scavenger of free radicals and reduces oxidative stress (Du et al., 2012; Chu et al., 2017). This powerful antioxidant property of EGCG can suppress neovascularization and regulating vessel permeability, thereby cutting off the blood supply to cancerous cells (Maiti et al., 2003). EGCG also regulated various transcription factors, protein kinases, and inflammatory cytokines to mediate the invasion, metastasis, and progression of cancerous cells (Shirakami and Shimizu, 2018; Kochman et al., 2020). The summary of the anticancer properties of green tea is shown in Figure 1.
FIGURE 1.
Role of EGCG and green tea catechins on reproductive cancers.
Experimental Studies
Toward ovarian cancer, experimental studies uncovered the mechanisms of green tea, which are mainly involved in regulating cell proliferation and apoptosis in cells or xenograft models. Green tea targeted CD44 as a safe nanomedicine treatment, thus inhibiting tumor progression and recurrence (Bae et al., 2017). It had been demonstrated that EGCG efficacies via targeting a number of genes including proliferative related genes JUN, FADD, NFKB1, HIF1α, and matrix metalloproteinases (MMPs) (Xinqiang et al., 2017), apoptotic related genes Bcl-2, Bax, caspases (Panji et al., 2021a) as well as downregulated PTEN/AKT/mTOR pathway to inhibit proliferation in vitro and in vivo (Qin et al., 2020). EGCG promoted DNA damage response, reduced DNA synthesis, and enhanced oxidative stress to induce apoptosis in cells (Mazumder et al., 2012) (Rao and Pagidas, 2010) (Chan et al., 2006).
Towards cervical cancer, green tea extract was reported to suppress TGF-β induced epithelial–mesenchymal transition via reactive oxygen species (ROS) generation, as well as inhibited proliferation and induced apoptosis via VEGF, NF-KB, and Akt pathways in cell lines or mice with HeLa xenografts (Singh et al., 2011; Panji et al., 2021b; Khojaste and Ahmadizadeh, 2021). EGCG reduced DNMT to regulate DNA hypomethylation (Khan et al., 2015), induced cell cycle arrest (Ahn et al., 2003), and downregulated p53 expression to regulate proliferation and apoptosis (Muthusami et al., 2013). EGCG inhibited hypoxia-induced HIF-1α to inhibit VEGF, thus regulating angiogenesis (Zhang et al., 2006). Toward endometrial cancer, the effect of the prodrug of EGCG (ProEGCG) exhibited anti-proliferative, pro-apoptotic, and anti-tumor actions via ERK/JNK, Akt and PI3K/AKT/mTOR/HIF1α signaling pathway (Wang Q. et al., 2018; Man et al., 2020).
Toward breast cancer, the anti-proliferative and pro-apoptotic effects of green tea catechins were achieved via inhibiting VEGF transcription (Sartippour et al., 2002), increasing Bax/Bcl-2 ratio (Li W. et al., 2016) or inducing p27 (kip1) CK1(Kavanagh et al., 2001), cell cycle arrest (Liu et al., 2017), caspases (Mbuthia et al., 2017) and p53 (Santos et al., 2021). In particular, EGCG mediated multiple signaling pathways to inhibit cell proliferation, migration, and invasion, thus limiting tumor progression, which included PI3K, Akt, NF-κB, MAPK, Wnt (Pianetti et al., 2002; Pan et al., 2007) and estrogen receptor (Belguise et al., 2007; Li et al., 2010) signaling pathways. Additionally, EGCG served as an antiangiogenic inhibitor that downregulated the VEGF pathway (Sartippour et al., 2002; Braicu et al., 2013; Crew et al., 2015), as well as an immune modulator that targeted IL-6 and TNF-α (Jang et al., 2013). Several studies also revealed the anti-invasive ability of catechins through mediating MMPs(Roomi et al., 2005a), VASP(Zhang et al., 2009), FOXO3a (Belguise et al., 2007), or p53 (Santos et al., 2021) expressions. In addition to acting as a chemo-preventive agent, green tea catechins also reversed chemotherapy resistance (Esmaeili, 2016; Tuntiteerawit et al., 2020).
While in testicular cancer, in vitro study revealed the potential of green tea extract to inhibit MMP and regulated invasion (Roomi et al., 2007). Similarly, studies on prostate cancer showed that green tea catechins suppressed cell proliferation and induced apoptosis by an increased Bax/Bcl-2 ratio (Yang et al., 2019) and via regulating the cell cycle (Wang et al., 2012), PI3K, Akt and Erk pathways (Siddiqui et al., 2004). Furthermore, green tea catechins suppressed invasion and migration by TIMPs and MMPs(Deb et al., 2019; Fan et al., 2021); inhibited tumor angiogenesis by downregulating IGF-1 (Harper et al., 2007), HIF1α (Thomas and Kim, 2005) and VEGF (Roomi et al., 2015b); and targets NK-κB (Saedmocheshi et al., 2019), COX-2 (Adhami et al., 2007), interleukin family (Mukherjee et al., 2014), androgen receptor (Stanislawska et al., 2019) to suppress prostate cancer cell growth. A summary of studies based on animal models about the anti-cancer effects of EGCG and green tea catechins on reproductive cancers can be found in Table 2.
TABLE 2.
Effects of Green tea on reproductive cancers in animal models.
| Types | Animal models | Type of green tea | Duration | Positive control | Negative control | Dosage of green tea type/treatment | Outcome measures | Results | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Ovarian Cancer | Subcutaneous human ovarian cancer cells SKOV-3 xenograft in BALB/c nude mice (4–5 weeks old) | EGCG (n = 7) | 21 days | Paclitaxel (n = 7) | Saline (n = 7) | 10, 30 or 50 mg/kg | Tumor weight: EGCG (reduced by 71.25%, compared to control) and paclitaxel (reduced by 39.62%, compared to control) | EGCG significantly inhibited tumor growth | Qin et al. (2020) |
| Subcutaneous human ovarian cancer cells SKOV-3 xenograft in female NCR mice | HA-EGCG (n = 10) (intravenous injection) | 2 weeks (Twice/week) | Free cisplatin (n = 10) and Micellar nanocomplexes (n = 10) | An isotonic dextrose solution (n = 10) | 19.6 mg/kg | Tumor weight: control mice (405.2 ± 141.6 mm3); HA-EGCG (No measurement), MNC-treated mice (181.2 ± 75.1 mm3) | No significant difference between Hthe A-EGCG and control group | Bae et al. (2017) | |
| Cervical Cancer | Subcutaneous HeLa cells in female athymic nude mice (5/6-week-old) | Nutrient mixture (NM) containing green tea extract (n = 12) (diet) | 4 weeks | NA | Regular purina mouse chow (n = 12) | Nutrient formulation in 20 mg NM | Tumor weight: NM (reduced by 59%; p = 0.001, compared to control) | NM group significantly inhibited tumor growth | Roomi et al. (2015b) |
| Subcutaneous HeLa cells in female athymic nude mice (5/6-week-old) | Nutrient mixture (NM) containing green tea extract (n = 12) (diet) | 4 weeks | NA | Regular purina mouse chow (n = 12) | Nutrient formulation in 20 mg NM | Tumor weight: NM (reduced by 59%; p = 0.001, compared to control) | NM group significantly inhibited tumor growth | Roomi et al. (2015a) | |
| Subcutaneous CaSki cells xenograft in nude mice | EGCG (n = 4) (oral) | 30 days | NA | Water (n = 4) | 35 mM | Tumor volume: Control (22.5 mm3) and EGCG (7.5 mm3) | EGCG significantly reduced tumor growth and delayed tumor formation | Ahn et al. (2003) | |
| Endometrial Cancer | Subcutaneous human EC cell lines (RL95–2 and AN3 CA) xenografts in nude mice (5–6 weeks old) | ProEGCG (n = 5) (oral) | RL95-2 xenograft model: 5 weeks; AN3-CA xenograft model: 3 weeks | NA | Olive oil (n = 5) | 50 mg/kg | Tumor weight: 1. RL95–2 xenograft model: control (0.55 ± 0.11 g), ProEGCG (0.33 ± 0.16 g); 2. AN3 CA xenograft model: control (1.45 ± 0.59), ProEGCG (0.60 ± 0.32) | ProEGCG significantly inhibited tumor growth and promoted the apoptotic activity of tumors | Man et al. (2020) |
| Subcutaneous endometrial cancer cells xenograft in nude mouse | ProEGCG (n = 5) (intragastric) | 35 days | NA | Olive oil (n = 5) | 50 mg/kg | No tumor size measurement | ProEGCG significantly inhibited tumor growth and angiogenesis in xenografts | Wang et al. (2018b) | |
| Breast Cancer | Female Sprague–Dawley rat breast cancer carcinogen model (4-week-old) | Green tea powder (n = 15) (oral) | 9 weeks | NA | Water (n = 15) | 0.3% (w/v) | Tumor weight: control (8.3 ± 6.9 g), green tea (2.5 ± 4.5 g) | Green tea significantly inhibited tumor growth | Kavanagh et al. (2001) |
| Subcutaneous human breast cancer cells MDA-MB231 xenograft in female scid mice (8–10 weeks old) | Green tea extracts (n = 12) (oral) | 35 days | NA | Water (n = 12) | 2.5 g/L | No tumor size measurement | GTE significantly inhibited tumor growth and vessel density | Sartippour et al. (2001) | |
| Breast cancer MDA-MB-231 cells xenograft in female athymic nude mice (6-week-old) | 0.5% Nutrient mixture (NM) containing epigallocatechin gallate in regular diet (n = 6) (diet) | 4 weeks | NA | Regular diet (n = 6) | Nutrient formulation | Tumor size: NM (reduced by 27%, p = 0.0082, compared to control) | NM significantly inhibited tumor growth | Roomi et al. (2005a) | |
| Subcutaneous human breast cancer cells MCF-7 xenograft in nude mice | Green tea extracts (oral) | 64 days | Tamoxifen | Water | 2.5 g/L | Tumor volume: Green tea (341.1 ± 48.8 mm3), tamoxifen (177.8 ± 37.6 mm3), control (622.2 ± 163.3 mm3), dual therapy with green tea and tamoxifen (116.5 ± 31.9 mm3) | Green tea and tamoxifen-treated together significantly inhibited growth and promoted apoptosis of tumor | Sartippour et al. (2006) | |
| Breast cancer MDA-MB-231 cells xenograft in female athymic nude mice (5-week-old) | Green tea polyphenols or EGCG (n = 5) (oral) | 10 weeks | NA | Water (n = 15) | 3 mg GTP/1 mg of EGCG | Tumor volume: EGCG (reduced by 45%, compared to control), GTP (reduced by 61%, compared to control) | GTP and EGCG significantly inhibited tumor growth, and proliferation and induced apoptosis of tumors | Thangapazham et al. (2007) | |
| Heterozygous C3 (1) SV40 T,t antigen (TAg) transgenic female mice (4-week-old) | Green tea catechins (n = 16) (oral) | 15 weeks | NA | Water (n = 16) | 0.01 or 0.05% (w/v) | Tumor weights: control (1.93 g), GTC (1.85 g) | GTC significantly inhibited tumor growth | Kaur et al. (2007) | |
| Subconfluent murine breast cancer cells 4T1 in BALB/c nude mice (6–7 weeks old) | EGCG (n = 8) (i.p.) | 24 days | Taxol (n = 8) | Saline (n = 8) | 30 mg/kg | Tumor volume: control (800 mm3), EGCG (700 mm3), Taxol (650 mm3), Combo of EGCG and Taxol (250 mm3); Tumor weight: control (1.25 g), EGCG (1.15 g), Taxol (1 g), Combo of EGCG and Taxol (0.65 g) | EGCG alone had little effect on tumor growth but the combination of EGCG and taxol significantly inhibited tumor growth | Luo et al. (2010) | |
| Breast cancer cells SUM-149 xenograft in female NOD/SCID mice (6-week-old) | EGCG (n = 6) (i.p.) | 42 days | NA | PBS (n = 6) | 16.5 mg/kg | Tumor volume: EGCG (reduced by 37.7 ± 4.4%, compared to control), tumor weight EGCG (reduced by 28.6 ± 6.5%, compared to control) | EGCG significantly inhibited tumor growth | Mineva et al. (2013) | |
| Subcutaneous murine breast cancer cells 4T1 in BALB/c nude mice | EGCG (i.p.) | 3 days | NA | PBS | 10 mg/kg | Tumor volume: Control (8000 mm3), EGCG (3700 mm3); tumor weight: Control (6 g), EGCG (3.8 g) | EGCG significantly inhibited tumor growth, macrophages infiltration, and M2 polarization of tumors | Jang et al. (2013) | |
| Subconfluent murine breast cancer cells 4T1 in BALB/c nude mice (5-week-old) | 0.5% Nutrient mixture (NM) containing epigallocatechin gallate in regular diet (n = 7) (diet) | 4 weeks | NA | Regular Purina mouse chow (n = 7) | 20 mg NM | Tumor weight: NM(0.91 ± 0.43 g), Control (1.83 ± 0.81 g) | NM group significantly inhibited tumor growth | Roomi et al. (2014) | |
| An orthotopic xenograft mouse model by injecting with early transformed breast cancer SHR cells in nude mice (4–6-week-old) | Green tea polyphenols (Sunphenon 90D) (n = 5) (oral) | 9 weeks | NA | Regular diet | 5 mg/ml | Tumor volume: Control (1200 mm3), GTP (300 mm3); Tumor weight: Control (1100 mg), GTP (300 mg) | GTPs significantly inhibited tumor growth and the proliferation rate of tumor growth | Li et al. (2016c) | |
| An orthotopic xenograft mouse model by injecting with MDA-MB-231 cell in nude mice (4–6-week-old) | Green tea polyphenols diet (n = 5) (oral) | 8 weeks | Tamoxifen (n = 5) | Regular diet | 3 mg/ml | Tumor volume: Control (1200 mm3), GTP (1000 mm3), TAM (1200 mm3); Tumor weight: Control (1.4 g), GTP (1 g), TAM (1.2 g) | GTPs alone significantly suppressed tumor growth | Li et al. (2017) | |
| Subcutaneous murine breast cancer cells 4T1 in male BALB/c mice (6-week-old) | Pre-treatment of EGCG (n = 5) (oral) | Pre-treatment for 1 month | NA | Water (n = 5) | 250, 500, 1000 and 2000 μg/ml | Tumor volume: Control (800 mm3), EGCG250ug/ml (600 mm3), EGCG500ug/ml (400 mm3), EGCG 1000 µg/ml (350 mm3) EGCG 2000 µg/ml (400 mm3) | EGCG in a low concentratioof n 250 μg/ml can significantly inhibit tumor growth | Xu et al. (2020) | |
| Prostate Cancer | Male TRAMP mice (8-week-old) | Green tea polyphenols (oral) | 24 weeks | NA | Water | 0.1% (v/v) | No tumor size measurement | GTP significantly inhibited tumor growth, inhibited angiogenesis and metastasis in tumor | Adhami et al. (2004) |
| Male TRAMP mice (8-week-old) | Green tea polyphenols (oral) | 24 weeks (thrice a week) | NA | Water | 0.1% (v/v) | No tumor size measurement | GTP significantly inhibited tumor growth | Saleem et al. (2005) | |
| Inoculation of human prostate cancer cells PC-3 xenograft in male nude mice | Nutrient mixture (NM) containing epigallocatechin gallate in regular diet (diet) | 4 weeks | NA | Regular diet | Nutrient formulation | Tumor weight: NM (reduced by 47%, compared to control); Mean tumor value: NM (reduced by 53%, compared to control) | NM significantly inhibited tumor growth, MMP-9 and VEGF secretion, and mitosis in tumor | Roomi et al. (2005b) | |
| Male TRAMP mice (8-week-old) | Green tea catechins (n = 9) (oral) | 16 weeks | NA | Water (n = 9) | 0.3% (w/v) | No tumor size measurement | GTC significantly inhibited tumor growth | Scaltriti et al. (2006) | |
| Subcutaneous human prostate cancer cells CWR22Rν1 xenografts in male athymic nu/nu mice (6–8-week-old) | Green tea polyphenols (n = 10) (oral) | 28 days | Celecoxib (n = 10) | Water (n = 10) | 0.1% (v/v) | Tumor growth: GTP (reduced by 42%, compared to control), celexocib (reduced by 57%, compared to control), combination (reduced by 81%, compared to control) | EGCG significantly inhibited the tumor growth, but the combination of EGCG and celexocib had greater effects | Adhami et al. (2007) | |
| Male TRAMP mice (8-week-old) | Green tea catechins (n = 9) (oral) | 16 weeks (thrice a week) | NA | Water (n = 9) | 0.3% (v/v) | No tumor size measurement | GTC significantly inhibited tumor growth | Mccarthy et al. (2007) | |
| Male TRAMP mice (8-week-old) | EGCG (oral) | 23 weeks | NA | Water | 0.06% (w/v) | No tumor size measurement | EGCG significantly reduced cell proliferation and induced apoptosis in tumor | Harper et al. (2007) | |
| subcutaneous CWR22R xenografts in male nude mice (4–5-week-old) | EGCG (n = 8), peracetate of EGCG (n = 8) (i.p.) | 20 days | NA | DMSO (n = 8) | EGCG: 50 mg/kg, EGCG-P: 86.7 mg/kg | Tumor size: EGCG (reduced by 36.3%, compared to control) EGCG-P (reduced by 62.9%, compared to control) | EGCG-P had a significantly better therapeutic effect than EGCG and control on inhibiting tumor growth and angiogenesis while promoting apoptosis of tumors | Lee et al. (2008) | |
| Male TRAMP mice (4-week-old) | Green tea polyphenols (n = 20) (oral) | 24 weeks (thrice a week) | NA | Water (n = 20) | 0.1% (v/v) | No tumor size measurement | Green tea polyphenols inducted apoptosis by inhibiting osteopontin and NFκB signaling in TRAMP mice | Siddiqui et al. (2008) | |
| Subcutaneous human prostate carcinoma cell line LNCaP 104-R1 xenografts in castration male BALB/c nu/nu mice | EGCG (i.p.) | 11 weeks | NA | Water | 1 mg | Tumor volume: EGCG (reduced by 40%, compared to control) | EGCG significantly inhibited tumor growth and suppressed cell proliferation of tumors | Chuu et al. (2009) | |
| Male TRAMP mice (8-week-old) | Green tea polyphenols (n = 18) (oral) | 4 weeks (thrice a week) | NA | Water (n = 18) | 0.1% (v/v) | Tumor volume and weight: GTP significantly the parameters compared with the control | GTP significantly inhibited cancer development | Adhami et al. (2009) | |
| Subcutaneous 1 × 106 PC-3 cells in nude mice (6–8 weeks old) | TBS-101 (n = 6) (oral) | 29 days (once every other day) | Etoposide | 5% DMSO in PBS | 80 mg/kg | Tumor growth: TBS-101 significantly inhibited | TBS-101 containing green tea significantly inhibited tumor growth and invasion | Evans et al. (2009) | |
| C57BL/6J and C57BL/6J/Nrf2(−/−) mice | EGCG (n = 4) (i.g.) | 12 h | NA | PEG 400 aqueous solution | 100 mg/kg | No tumor size measurement | EGCG significantly downregulated Nrf2 in tumors | Nair et al. (2010) | |
| Male TRAMP mice after weaning | Green tea polyphenols (oral) | 25 weeks (thrice a week) | NA | Water | 0.05% (v/v) | No tumor size measurement | No significant differences between GTP and control | Teichert et al. (2010) | |
| Human prostate cancer cells 22Rν1 xenografts in male nude mice (6–8-week-old) | EGCG (i.p.) | 6 weeks | NA | Water | 1 mg | No tumor size measurement | EGCG significantly inhibited tumor growth | Siddiqui et al. (2011) | |
| Male rat (10-week-old) | Green tea (oral) | 4 weeks (over 39 weeks long duration) | Soy diet | Water | 2% (v/v) | No tumor size measurement of green tea | Green tea and soy in combination significantly inhibited tumor growth and inflammation | Hsu et al. (2011) | |
| Subcutaneous 5 × 105 LAPC-4 cells in male SCID mice (fed AIN-93G diet) | Green tea bag (n = 12) (oral) | 6 weeks (thrice a week) | Q (quercetin) | Water (n = 12) | One tea bag in 240 ml of water for 5 min | Tumor size: 0.2% Q (reduced by 3%), 0.4% Q (reduced by 15%), GT (reduced by 21%), GT + 0.2% Q (reduced by 28%) and GT + 0.4% Q (reduced by 45%), all compared to control | Green tea and quercetin in combination significantly improved chemoprevention and suppressed proliferation | Hsu et al. (2011) | |
| Subcutaneous 1 × 106 22Rv1 cells in 4 weeks old male athymic nude mice | Chit-nanoEGCG (n = 12), EGCG (n = 12) (oral) | Five times a week, until tumors reached a targeted volume of 1200 mm3 | NA | Void chitosan nanoparticles (n = 12) | Chit-nanoEGCG (3 or 6 mg/kg), EGCG (40 mg/kg) | Times taken to achieve an average tumor volume of 1200 mm3: control (32 days), EGCG (46 days), Chit-nanoEGCG (3 mg/kg) (53 days), and Chit-nanoEGCG (6 mg/kg) (60 days) | EGCG significantly inhibited tumor growth, but Chit-nanoEGCG had significantly greater effects | Khan et al. (2014) | |
| Male TRAMP mice (5-week-old) | Polyphenon E (n = 11–16) (oral) | 3 weeks (over 25 weeks long duration) | NA | Water (n = 11–16) | 200, 500, and 1,000 mg/kg | Tumor volume: Control (107.5 ± 60.1 mm3), Polyphenono E at 200 mg/kg (90.0 ± 76.9 mm3), 500 mg/kg (92.5 ± 53.6 mm3), 1000 mg/kg (80.2 ± 36.3 mm3) | Polyphenon E significantly inhibited tumor growth and metastasis with no evidence of toxicity | Kim et al. (2014) | |
| Male Wistar rats (received an intake of cyproterone acetate, testosterone propionate, and N-Nitroso-N-methylurea) (2 months old) | Green tea extract (n = 10) (i.g.) | Eight weeks (three times per week) | Exercise training | Water (n = 10) | 0.1% | Tumor weight: Control (1.84 ± 0.22 g), training (1.38 ± 0.25 g), GTE (0.95 ± 0.08 g), GTE + training (1.03 ± 0.11 g) | GTE significantly inhibited tumor growth | Saedmocheshi et al. (2019) | |
| Adult male Wistar rats (received an intake of cyproterone acetate, testosterone propionate, and N-Nitroso-N-methylurea) (45 days old) | Green tea extract (n = 8) (oral) | 8 weeks | Exercise training | Water (n = 8) | 0.1% | Tumor weight: Control (2.05 ± 0.08 g), training (0.96 ± 0.08 g), GTE (1.38 ± 0.25 g), training+GTE (1.06 ± 0.10 g) | GTE significantly inhibited tumor growth | Vahabzadeh et al. (2020) | |
| Injection of human prostate cancer cells PC3 cells xenografts through the tail vein in Male NOD-SCID mice (6–8 weeks old) | L-theanine (isolated from green tea leaves) (i.p.) | 10 weeks | NA | Saline | 80 mg/kg | No tumor size measurement | L-theanine significantly suppressed invasion, migration, and adhesion in tumors | Fan et al. (2021) |
EGCG, Epigallocatechin gallate; GTE, green tea extracts; GTP, green tea polyphenols; i.p, intraperitoneal injection; o.g., oral galvage; s.c., subcutaneous injection
These experimental studies demonstrated the ability of EGCG to regulate multi-targets and multi-signaling pathways. Some of these targeted pathways presented potential mechanism of actions of EGCG to regulate autophagy in reproductive cancers. In particular, studies showed that the inhibition of autophagy was conducted via inhibiting the MAPK pathway mechanistically (Han et al., 2021; Tam et al., 2021). The Akt/mTor signaling pathway act as the main autophagy regulator for the survival of reproductive cancers (Amaral et al., 2018; Nunez-Olvera et al., 2019; Tam et al., 2021; Wang et al., 2021). Moreover, the NF- B pathway not only regulated the cell cycle and apoptosis but also controlled autophagy (Verzella et al., 2020). These will be discussed further in section 4.
Clinical Trials
Currently, there are very few clinical trials performed to validate whether green tea possesses chemo-preventive or curative activity in significantly reducing cancer development. In clinical trials on prostate cancer, the majority of studies showed significant beneficial effects of green tea extracts on reducing cancer progression than placebo (Bettuzzi et al., 2006; Brausi et al., 2008; Mclarty et al., 2009; Ram Kumar and Schor, 2018). While in breast cancer, the studies by Samavat et al. demonstrated the consumption of green tea extracts significantly reduced breast tumors in female patients ((Samavat et al., 2017; Samavat et al., 2019). However, it showed no significant effects when conducted by another study group (Crew et al., 2012; Crew et al., 2015)). These results remained inconsistent owing to the presence of numerous confounding variables in these study designs, such as diet and population differences. In addition, prospective and case-control studies had demonstrated the potential of green tea in reducing the risk of ovarian cancer (Zhang et al., 2002; Yoon et al., 2008; Lee et al., 2013), cervical cancer (Jia et al., 2012; Paul et al., 2019), endometrial cancer (Bandera et al., 2010), breast cancer (Dai et al., 2010; Iwasaki et al., 2010; Iwasaki et al., 2014; Li M. et al., 2016; Yuan et al., 2017), and prostate cancer (Kurahashi et al., 2008; Erdrich et al., 2015; Lassed et al., 2016; Lee et al., 2017; Sawada, 2017; Beynon et al., 2019). A summary of studies based on clinical trials about the anti-cancer effects of EGCG and green tea catechins on reproductive cancers can be found in Table 3.
TABLE 3.
Effects of Green tea on reproductive cancers in clinical trials.
| Type | Study design | Subject | Intervention group | Control group | Length of intervention | Time points | Outcome measures | Results | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Ovarian Cancer | Phase II clinical trial | 16 | Double-brewed green tea (DBGT; 500 mg epigallocatechin gallate (EGCG)-enriched tea drink) | NA | Minimum <100 days; Maximum 1.5 years | Every 3 months; Endpoint: recurrence starts | Time of recurrence: 5 ppl have an absence of recurrence at 18 months | No significant maintenance in women with advanced-stage ovarian cancer after standard treatment | Trudel et al. (2013) |
| Cohort study | 244 (Green tea (104), Control (140)) | Green tea (2.1 g ± 1.75 g) | Non-tea drinking | Minimum <1 year; Maximum >3 years | Endpoint: death of participants | The survival rate after 3 years: GT 77.9% vs control 47.9% | Green tea enhanced epithelial ovarian cancer survival dose-response relationships | Zhang et al. (2004) | |
| Breast Cancer | Cohort study | 390 (≤4 cups/day (181), 5–7 cups (131), and ≥8 cups/day (78)) | Green tea (consumption levels of ≤4 cups, 5–7 cups, and ≥8 cups; 30–40 mg EGCG per cup) | NA | 6.5 ± 3 years | Endpoint: recurrence starts | Mean expression of ER (fmol/mg protein): ≤4 cups/day (72.8 ± 9), 5–7 cups (93.4 ± 13.8), and ≥8 cups/day (109.7 ± 21.8) (p = 0.05); Recurrence Rates: ≤4 cups/day (28.8%), ≥5 cups/day (23.6%) (p = 0.05) | Increased consumption of green tea was correlated with decreased recurrence of stage I and II breast cancer | Nakachi et al. (1998) |
| Randomized Phase IB Dose Escalation Study | 34 (control (8), 800 mg EGCG (14), 1200 mg EGCG (11), 1600 mg EGCG (1)) | EGCG (800, 1200, or 1600 mg) | Placebo | 6 months | Baseline, 2, 4, and 6 months | No significant changes in serum hormone levels including oestradiol, testosterone, IGF-1, IGFBP-3, and SHBG; target tissue effects including Ki-67 proliferation index or mammographic density; and quality of life | No significant difference in outcome measures between the intervention and placebo group, but this study obtained preliminary data on the biological effects of Poly E to benefit other ongoing trials | Crew et al. (2012) | |
| Randomized Phase IB Dose Escalation Study | 34 (control (8), 800 mg EGCG (14), 1200 mg EGCG (11), 1600 mg EGCG (1)) | EGCG (800, 1200, or 1600 mg) | Placebo | 6 months | Baseline, 2, 4, and 6 months | Serum HGF levels: Poly E: decreased by 12.7% vs placebo increase of 6.3% (p = 0.04), at 2 months, but no statistically significant at 4 and 6 months; serum VEGF levels: Poly E: decreased by 11.5% at 2 months (p = 0.02) and 13.9% at 4 months (p = 0.05), but no significant difference compared to placebo. Biomarkers of oxidative damage or inflammation, total cholesterol: Poly E treatment: no statistical significance decreased, compared to placebo | No significant difference in outcome measures between intervention and placebo, but this study suggested potential mechanisms of action of tea polyphenols in HGF signaling, angiogenesis, and lipid metabolism | Crew et al. (2015) | |
| Randomized phase II clinical trial | 932 (control (470); GTF (462)) | GTE capsule with 328.8 mg total catechins and 210.7 mg EGCG | Placebo | 1 year | Baseline and 12 months | Percentage mammographic density of women aged 50–55 years: GTE: reduced by 4.40% vs placebo increase of 1.02% (p = 0.05). Overall PMD and modifying effect of COMT genotype: no significant difference in overall | EGCG significantly reduced PMD for women 50–55 years old | Samavat et al. (2017) | |
| Randomized phase II clinical trial | 1075(Placebo (537); GTE-COMT (538)) | Green Tea extract-catechol-O-methyltransferase (GTE-COMT) | Placebo | 1 year | Baseline, 6 and 12 months | Blood total estradiol and bioavailable estradiol: GTE: increase of 16% (p = 0.02) and 21% (p = 0.03), compared to placebo at month 12 | GTE significantly increased circulating estradiol concentrations and prevented breast cancer | Samavat et al. (2019) | |
| Prostate Cancer | Prospective single-arm clinical trial | 19 | Green tea extract (500 mg) | NA | Minimum 2 months; Maximum 6 months | Endpoint: A rise of either a relative prostate-specific antigen, or two largest dimensions of measurable disease on CT scan, or intensity of abnormal bone activity of greater than 25% over baseline over a 2-month period | Prostate-specific antigen percentage change: GTE: slow down disease progression in six patients | GTE produced no discernible clinical activity against hormone-refractory prostate cancer | Choan et al. (2005) |
| Placebo-controlled study | 60 (Placebo (30), GTC (30) | GTC (600 mg) | Placebo | 1 year | Baseline, 3, 6, and 12 months | Prevalence of prostate cancer: GTCs: 90% chemoprevention efficacy in men subjected to high risk for developing CaP (p < 0.01); Total serum PSA values: GTCs: lower, at any time point, compared to control; Changes in LUTS as assessed by IPSS and QoL scores: GTCs: decrease for 3 months, compared with control (p < 0.05)) | GTCs bring significant potent chemoprevention activity to cancer patients | Bettuzzi et al. (2006) | |
| Placebo-controlled study | 60 (Placebo (30), GTC (30)) | GTC (600 mg) | Placebo | 1 year | 2 years later following the GTC administration | Incidence of tumors: GTC (3%), placebo (30%); cancer progression onset time: GTC (23.3 months), placebo (19.1 months) | GTC significantly reduced Prostate cancer diagnosis | Brausi et al. (2008) | |
| single-armed Phase II clinical trial | 25 | Polyphenon E (EGCG 800 mg) | NA | 6 weeks | 4, 8 weeks | Serum levels of HGF, VEGF, PSA, IGF-I, and IGFBP-3: EGCG: reduced the biomarkers significantly (all p < 0.03)) | Polyphenon E played potential roles in prostate cancer treatment or prevention | Mclarty et al. (2009) | |
| Randomized controlled trial | 50 (Placebo (25), GTC (25)) | Polyphenon E of 85–95% total catechins, with 56–72% as EGCG | Placebo | 3–6 weeks | Baseline, 3 days before surgery, and during surgery | Systemic biomarker 8OHdG/dG, IGF-1, IGFBP-3, Ki67, Caspase 3, microvessel density, PSA value: Polyphenon E group: no significant decrease | Polyphenon E brings no significant differences, suggested longer-term interventions should be used for future studies | Nguyen et al. (2012) | |
| Randomized controlled trial | 97 (Placebo (48), Poly E (49)) | Polyphenon E (EGCG 400 mg) | Placebo | 1 year | Baseline, 6 and 12 months | Toxicity symptoms, Lower Urinary Tract Symptoms, and Quality of Life scores: Poly E brings significant symptoms unrelated to the study agent, and no other significant difference compared to control | EGCG was safe to be administrated for prostate cancer prevention or other indications | Kumar et al. (2016) | |
| Single-armed Pre-clinical trial | 90 | Green tea (10 g w/v)) | NA | 6 months | Baseline, 3 and 6 months | lipid peroxidation and antioxidants status: GSH (mg/dl): before (20.79 ± 4.32), after 6 months (34.36 ± 3.64), p < 0.0001; MDA (nmol/gHb): before (99.52 ± 12.49), after 6 months (45.16 ± 7.45), p < 0.0001; CAT (UI/gHb): before (15.29 ± 1.75), after 6 months (22.19 ± 1.78), p < 0.0001 | Green tea significantly improved overall the antioxidative status in patients | Lassed et al. (2017) |
EGCG, Epigallocatechin gallate, GTCs, green tea catechins
Regulation of Autophagy by EGCG and Green Tea Catechins in Reproductive Cancer
As autophagy is regulated by different autophagy-related proteins that could be potential molecular targets in reproductive cancers. Likewise, EGCG has demonstrated its potential to modulate these factors in current literatures. EGCG restored autophagic flux via downregulating Beclin1, Atg5, and p62 in myocardial ischemia/reperfusion injury rats (Xuan and Jian, 2016). Also, EGCG was reported to inhibit dimerization of LC-3BI, promoted LC3B-II production, and activated autophagy in hepatocellular carcinoma cells (Zhao et al., 2017). By promoting the upregulation of Atg5, Beclin-1, and LC3B-II protein levels, EGCG induced apoptosis and autophagy in cisplatin-resistant oral cancer (Yuan et al., 2017). Also, other studies showed that EGCG enhanced LC3 transition and induced Beclin-1in lymphoma (Tsai et al., 2017). Whereas, it regulated Atg5 and Beclin-1 to induce autophagy in subarachnoid hemorrhage (Chen et al., 2018). This regulation of EGCG on the protein level of Atg5 was essentially critical in governing the autophagy and apoptosis switch, as lysosomes would not be able to fuse with phagosome when it was absent (Yousefi et al., 2006). However, the concrete evidence on the modulating effect of EGCG and green tea catechin on autophagy regulation remained controversial. Their role in determining the onset of autophagy might also be dependent on the integration of signaling networks, in which autophagy and cell growth often shared a series of direct or indirect signaling pathways (Neufeld, 2012; Rizzi et al., 2014).
MAPK had been shown to act as a prognostic factor to regulate the incidence of several reproductive cancers ((Eritja et al., 2017; Lim et al., 2019)). EGCG inhibited various transcriptional factors of mitogen-activated protein kinase (MAPK), including c-Jun, and ERK1/2, to inhibit proliferation, and migration and promote apoptosis (Katiyar et al., 2001; Maeda-Yamamoto et al., 2003). As matrix metalloproteinases (MMPs) were correlated with tumor invasion via MAPK, MMPs were reduced upon the treatment with EGCG to revoke the activation of ERK and JNK, which prevented fibrosis, invasion, and migration of cancer cells (Kim et al., 2004; Zhen et al., 2006; Ho et al., 2007; Zeng and Harris, 2014). Autophagy also acts as an adaptive resistance mechanism upon the inhibition of MAPK (Lee et al., 2021). MAPK signaling pathway did not solely regulate autophagy (Raufi et al., 2021). The significance of autophagy against MAPK inhibition was also dependent to the cell’s ability to produce autolysosomes (Kochetkova et al., 2017). Autophagic mechanism was activated dependent on MAPK/JNK pathway after therapeutic stress in endometrial cancer (Eritja et al., 2017). In ovarian cancer, the stimulation of tumor cell proliferation via the MAPK/ERK1/2/mTor pathway induced autophagy (Lim et al., 2019). Likewise, the promotion of autophagy was found to inhibit tumor cell growth in a breast cancer study via the inhibition of MAPK-regulated mTOR activity, and the phosphorylation of ERK was hindered (Sun et al., 2013). Moreover, the downregulation of p-ERK1/2 protein via the MAPK/ERK pathway induced early autophagy and apoptosis for anti-tumor activities in breast and cervical cancer (Hanyu et al., 2020; Liu et al., 2020). On the contrary, the promotion of MAPK/ERK pathway was shown to inhibit autophagy on promoting metastasis (Li et al., 2021).
In another aspect, EGCG targeted PI3K/AKT/mTOR pathway via inhibiting phosphorylation of AKT and mTOR to exert anti-proliferation and pro-apoptosis effects (Liu et al., 2013; Liu et al., 2016). EGCG also inhibited the binding of EGF to EGFR by reducing receptor dimerization and inhibiting EGFR phosphorylation (Adachi et al., 2007). These regulations indirectly inhibited AKT activities to induce cell cycle arrest (Sah et al., 2004; Hou et al., 2005; Shimizu et al., 2005) As a result, EGCG was shown to increase caspases and anti-apoptotic proteins level, and decrease AKT and pro-apoptotic levels (Moradzadeh et al., 2017). The inhibition of the PI3K/AKT/mTOR pathway promoted autophagy for the anti-proliferation activity in endometrial cancer cells (Lin et al., 2019). The blockade of the AKT/mTOR pathway activated the compensatory MEK/ERK pathway to promote autophagy and apoptosis induced cell death and cell cycle arrest in prostate, cervical and ovarian cancers (Zhou et al., 2015; Xu et al., 2016; Butler et al., 2017; Qin et al., 2018). Another study also showed that the downregulation of p-AKT inhibited cancer cell survival, along with the upregulation of p-JNK1/2-induced apoptosis, synergistically lead to autophagy-induced cell death in the breast cancer cells (Innajak et al., 2016). The promotion of apoptosis and autophagic cell death via inhibiting the PI3K/AKT/mTOR signaling pathway could have induced stress to attenuate MAPK/ERK activity in autophagy inhibition (Reddy et al., 2020).
In addition, EGCG also suppressed anti-apoptotic related protein through the inhibition of the NF- B pathway (Hastak et al., 2003). EGCG mediated the cleavage of NF- B/p65 subunit and deregulated the cell cycle. This further inhibited NF- B nuclear translocation and activated caspases for pro-apoptotic activities (Ahmad et al., 2000; Gupta et al., 2004; Kitamura et al., 2012). Typically, It was shown that NF-κB induced autophagy-related genes, including Beclin1 and ATG5, directly trigger autophagy (Salminen et al., 2012). On the other hand, it inhibited autophagocytotic via limiting autophagy inducers (including p53 and ROS) or enhancing autophagy repressors (e.g., Bcl-2) (Djavaheri-Mergny et al., 2006; Salminen et al., 2012; Verzella et al., 2020). Hence, the crosstalk between the NF-κB pathway and autophagy in the canner was proposed. Given the mutual upstream regulators of the interplay, therefore, they could control each other via positive and negative feedback loops (Trocoli and Djavaheri-Mergny, 2011). The complex interconnections of these pathways were demonstrated in breast cancer as both NF-κB and autophagy regulated cell death. Inhibition of the NF-κB pathway downregulated autophagy-induced DNA damage (Vequaud et al., 2015). However, the inhibition of the NF-κB pathway induced ER stress and autophagy, which showed the potential to regulate both cancer cells and cancer stem cells to avoid tumor recurrence in breast and prostate cancers (Lu C. et al., 2021). Although an enhanced interaction between NF-κB p50 and annexin A4 fucosylation promoted autophagy and progressed ovarian clear cell carcinoma malignancy, the interaction and progression were mainly to be associated with clinical stage and chemotherapy resistance (Wang et al., 2017).
EGCG can work like various chemotherapeutic drugs, which enhanced autophagy-induced cancer cell death (Denton et al., 2015). Currently, EGCG as an autophagy inducer was only studied in breast, cervical, and prostate cancers. The summary on the finding of EGCG and green tea catechins on autophagy is shown in Table 4. In breast cancer, EGCG inhibited tumor growth in vitro and in vivo via activating autophagy directly, thus regulating glucose metabolism (Wei et al., 2018). Another study in the breast cancer cell line showed that EGCG induced autophagy via enhancing autophagosome formation, hence inhibiting proinflammatory mediators HMGB1 (Li et al., 2011). The regulated stages of autophagy in breast cancer were shown to be dependent on the EGCG concentration. EGCG in low concentration upregulated Beclin1, Atg5, and LC3B-II/LC3B-I levels, promoting autophagosomes; However, when EGCG is under high concentration, it would only upregulate Beclin1 and Atg5 to enhance the fusion of the autophagosome with lysosomes to form autolysomes, which resulted in a downregulated LC3B-II/LC3B-I levels. Whereas, high concentrations of EGCG were shown to activate later stages of autophagy in breast cancer cells (Wei et al., 2018).
TABLE 4.
Green tea polyphenol (EGCG) as autophagy modulator in cancer study from 2011 to 2022 January.
| Type of cancer | Compound | Study models | Autophagy modulation | Combination strategy | Ref |
|---|---|---|---|---|---|
| GTP as Autophagy Inducer | |||||
| Breast cancer | EGCG | Cell lines: 4T1 and 4T1 | Modulating the levels of the autophagy-related proteins Beclin1, ATG5, and LC3B to induce apoptosis and suppresses glucose metabolism | NA | Wei et al. (2018) |
| Mouse xenograft Model | |||||
| EGCG | Cell lines: MDA-MB-361 and MCF-7 | Stimulated LC3-II production and autophagosome formation, and inhibited LPS-induced HMGB1 up-regulation and extracellular release | NA | Li et al. (2011) | |
| Cervical cancer | EGCG | Mouse xenograft model | Down-regulated p62 and up-regulated Beclin 1 and LC3 to induce apoptosis | Combined with Doxorubicin in chemo-photothermal synergistic therapy | Chen et al. (2020) |
| EGCG palmitate | Cell line: HeLa | Induces autophagosome-lysosome formation for cell death | Encapsulated in ZIF-8 nanoparticles with functionalization of folic acid | Chen et al. (2020) | |
| Prostate cancer | Polyphenon E® | Cell lines: PNT1a and PC3 | Induces ER stress and unfolded protein response | NA | Rizzi et al. (2014) |
| EGCG | Cell line: PC3 cell | Inhibits proteasome activity and induces ER stress | Combined with Bortezomib | Modernelli et al. (2015) | |
| GTP as Autophagy Inhibitor | |||||
| Cervical cancer | EGCG | Cell lines: HeLa | Induce ROS-mediated lysosomal membrane permeabilization to mediate cell death | NA | Zhang et al. (2012) |
| Prostate cancer | Tea polyphenols | Cell lines: PC3 and DU145 | Activation of the mTOR pathway to induce apoptosis | Combined with Docetaxel | Wang et al. (2018b) |
EGCG, epigallocatechin gallate; ER stress, endoplasmic reticulum stress; ROS, reactive oxygen species; VEGF, vascular endothelial growth factors
In cervical cancer, the combination of EGCG and Doxorubicin, which is chemotherapy, was investigated as an autophagy modulator that further improved the therapeutic efficacy of photothermal therapy (Chen et al., 2020). Photothermal therapy solely induced hyperthermia damage to intracellular components and activated autophagy which inhibited apoptotic-induced cancer cell death (Zhang Y. et al., 2018; Li et al., 2019; Chen et al., 2020). However, an autophagy enhancer could suppress inflammation and cancer progression, and therefore promote autophagic-induced cell death. When autophagic flux and the formation of autophagosomes were activated, this would downregulate p62 while upregulating both Beclin1 and LC3 in tumors. Additionally, EGCG also played roles to inhibit the toxins generated from Doxorubicin in vitro and in vivo, and limited chemo-resistance, resulting in greater efficiency (Shan et al., 2019; Chen et al., 2020)
In prostate cancer, Bortezomib is an effective proteasome inhibitor against tumor growth via induction of apoptosis in vitro and in vivo (Papandreou and Logothetis, 2004), followed by activation of unfolded protein regulator, ER stress, and autophagy (Modernelli et al., 2015; Takenokuchi et al., 2015). The proteasome is mechanistically related to autophagy as both pathways act as protein degradation systems and maintain intracellular proteostasis (Zhu et al., 2010). EGCG potently suppressed proteasome activity in prostate cancer (Kazi et al., 2004), and could potentially induce autophagy as Bortezomib. A study hence showed that co-treatment of Bortezomib with EGCG exacerbated autophagy via induction of LC3B transition and an autophagic flux in prostate cancer cells (Modernelli et al., 2015). The therapeutic efficacy of green tea as an autophagy inducer depended on the stage of cancer. Green tea polyphenol activated autophagy in the initial stage of prostate cancer in vitro, demonstrated by an increased interconversion of LC3B-I to LC3B-II, which indicated the accumulation of inactivated autophagosomes (Rizzi et al., 2014). However, in advanced stages of prostate cancer in vitro, although green tea increased the level of LC3B, there was no change in the interconversion level of LC3B-I to LC3B-II to indicate a net autophagic flux (Mizushima and Yoshimori, 2007; Rizzi et al., 2014).
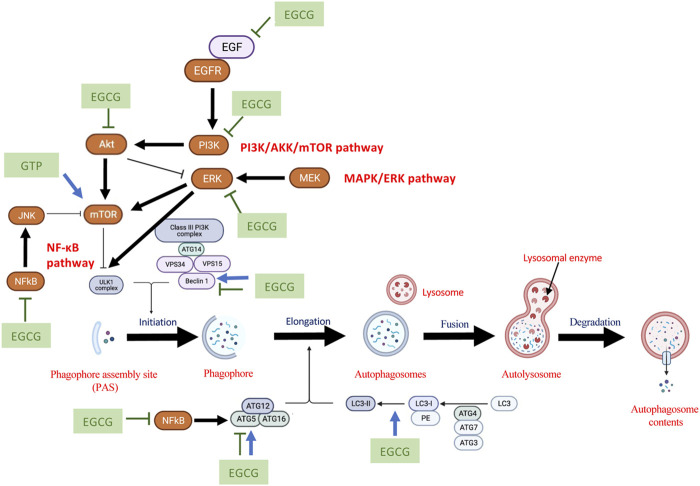
Autophagy degrades unusual macromolecules and organelles that are used to induce apoptosis. An upregulated level of autophagy was shown to limit drug-induced apoptosis in prostate cancer (Herman-Antosiewicz et al., 2006). Induction of autophagy favored cancer cells’ survival under metabolic stress conditions stimulated by chemotherapy or radiation therapy (Ozpolat and Benbrook, 2015). Therefore, a downregulated level of autophagy could enhance the pro-apoptotic properties of chemotherapy. In prostate cancer, patients developed resistance against first-line therapy such as docetaxel (Armstrong and Gao, 2015), which could be due to the induction of autophagy (Wang Q. et al., 2018). Green tea polyphenol inhibited the induced-autophagy events and promoted apoptosis via activation of the mTOR signaling pathway. LC3-II was reduced to enhance the p-mTOR expression level (Wang Q. et al., 2018). The schematic diagram of the chemo-preventive mechanisms of EGCG and green tea catechins is shown in Figure 2.
FIGURE 2.
The schematic diagram of potential chemopreventive mechanisms of EGCG and green tea catechins to regulate autophagy. The schematic diagram was created using BioRender.com.
Potential of EGCG as an Autophagy Modulator in Reproductive Cancer
Pharmacological actions of autophagy modulator are not tumor-selective, which could impose a risk of healthy tissue being transformed into malignant tissue (Gewirtz, 2016; Noguchi et al., 2020). Although autophagy was shown to induce cancer cell death, the anti-oxidative properties of green tea polyphenols inhibited stress-induced autophagy cell death in healthy ovarian granulosa cells. Activities and concentrations of antioxidant SOD and GSH-PX were upregulated. MDA levels, Beclin1, as well as the autophagic vacuoles in cytoplasmic were reduced (Yang et al., 2020). EGCG reduced ROS to limit the enzyme that cleaved LC3-I to form LC3-II, and inhibited LC3-II formation, indicating a reduced level of autophagosome formation and autophagy in healthy ovarian cells (Zhang S. et al., 2020; Yang et al., 2020). Autophagy was induced by environmental stress such as chemicals and toxins (Lee et al., 2011). EGCG shows the potential to regulate autophagy and protect healthy cells against death from chemotherapy.
Reproductive cancer treatments could interfere with the reproductive processes and fertility. EGCG was shown to improve fertility and protect it from reproductive cancers. Its molecular mechanism against infertility is based on regulating ROS, enhancing the antioxidant enzymes’ expression and activity (Roychoudhury et al., 2017; Zhang Y. et al., 2020). From the male fertility aspect, EGCG was able to protect germ cell loss, as well as diminish testes lesions (Ge et al., 2015), sperm deformity (Spinaci et al., 2008), and spermatogenic cell apoptosis (Ge et al., 2015). EGCG in a low concentration improved motility and capacitation of sperm. The addition of EGCG in thawing sperm for in-vitro fertilization after chemotherapy significantly increased penetration rate and fertilization efficiency (Rahman et al., 2018). DNA damage on semen from ROS was protected, and the fertility rate was significantly increased. While on the other hand, from the female fertility aspect, EGCG improved developmental capacity and the porcine oocytes’ maturation quality in vitro. EGCG in premature cultures of oocytes delayed oxidation and reduced ROS in vitro (Roth et al., 2008; Balazi et al., 2019). EGCG inhibitory actions of AMPK, cAMP, calciumion, and ferric iron prevented chromosome impairment, low-density lipoprotein oxidation, and spontaneous mutation. This reduced ROS and regulated gene expression to improve fertility (Rahman et al., 2018).
Current Limitation and Future Perspectives of EGCG Development
Autophagy modulators can act as tumor activators or suppressors, which complicates the discovery stage in terms of pharmacological, technical, and experimental natures. It must be solved before implement as a chemotherapeutic modulator in the clinic. The autophagy strategy should be customizable and used depending on cancer cell types and tumorigenesis stages. Natural products such as cancer therapies attract growing attention due to their high effectiveness to diminish tumor cells and low toxicity to normal cells (Zhang Q. Y. et al., 2018). Their autophagy roles have been put forward (Rahman et al., 2020; Al-Bari et al., 2021). We reviewed and proposed the use of green tea EGCG as an autophagy modulator in reproductive cancer studies. EGCG exerts extraordinary anti-carcinogenic properties, attributes to its multi-targets and multi-pathways functional properties from anti-proliferative, proapoptotic, anti-invasive, anti-angiogenesis, and anti-oxidative effects (Chacko et al., 2010; Gan et al., 2018; Kochman et al., 2020). It was tested with a safe profile and benefited the preservation of fertility (Rahman et al., 2018; Opuwari and Monsees, 2020), yet it still requires more validation using animal studies and clinical trials to prove the efficacies of EGCG. Fertility-related side effects of EGCG for reproductive cancers in clinical use also needs to be investigated further.
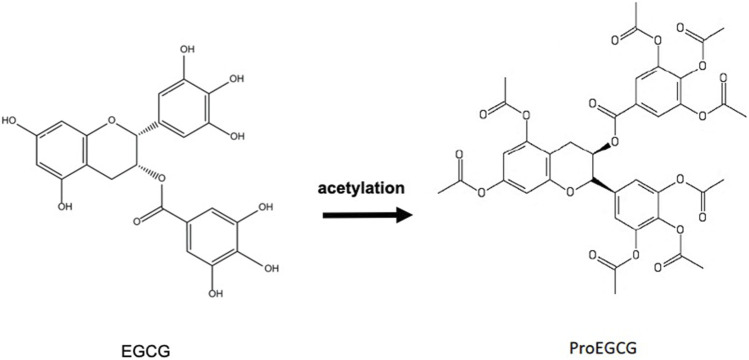
However, the hydroxyl group on EGCG brings low bioavailability in the human body (Du et al., 2012; Chu et al., 2017). EGCG is not stable and is readily oxidized in a neutral or alkaline environment. A basic environment disfavors the protons on phenol groups, causing it to generate phenoxide anion which acts on electrophilic agents such as free radicals in the body (Lam et al., 2004). With innovations and technologies, this could be overcome via different delivery methods including prodrug and encapsulation approaches. Lam et al., team’s derived a prodrug of EGCG (ProEGCG) with enhanced EGCG stability by replacing the -OH hydroxyl groups of EGCG into -COOCH3 acetyl acetoxy groups of ProEGCG (Lam et al., 2004) (Figure 3). ProEGCG showed greater bioavailability and stability than EGCG on breast cancer in vitro and in vivo (Landis-Piwowar et al., 2005; Landis-Piwowar et al., 2007). Its potency to inhibit tumor growth was to a greater extent than EGCG, with an enhanced inhibitory effect on proteasome and induction abilities of caspase3/7 and PARP proteins, thus to inducing apoptosis. In prostate cancer, ProEGCG limited tumor progression and angiogenesis, as well as induced apoptosis in vivo (Lee et al., 2008). Besides these, ProEGCG was shown to enhance the sensitization of leukemia to chemotherapy via caspase 3 activity and cleavage of PARP (Davenport et al., 2010). More recently, our team reported that ProEGCG acts as an angiogenesis inhibitor in endometrial cancer models in vitro and in vivo, and downregulated HIF-1α and VEGFA via PI3K/AKT/mTOR pathway (Wang J. et al., 2018). ProEGCG in low doses could inhibit cancer activity in endometrial cancer cells. Based on our finding, it was shown to upregulate the phosphorylation of JNK and p38 MAPK and downregulate that of AKT and ERK to ultimately inhibit tumor cell proliferation, micro-vessels formation and promote apoptotic activity in vitro and in vivo (Man et al., 2020). Importantly, this anticancer effect was significantlysuperior when compared with EGCG, with no toxicity being found.
FIGURE 3.
Chemical synthesis of ProEGCG from EGCG.
On the other hand, a nanostructure-based drug delivery system improves the bioavailability of even low doses of EGCG. Chitosan nanoparticles (Dube et al., 2010; Li et al., 2014), liposomes (Fang et al., 2006; Dag and Oztop, 2017), nanoliposomes (De Pace et al., 2013) encapsulation material were used as the carriers of EGCG in different studies. Improved chemical structure stability via sustainable release of bioactive and the enhanced permeability of chemicals through the cellular membrane were resulted (Cai et al., 2018). In breast cancer, EGCG nanoparticles FA-NPS-PEG and FA-PEG-NPS were synthesized. It showed improved cellular uptake and anti-proliferation effect in vitro (Zeng et al., 2017). In cervical cancer, EGCG was delivered in a photothermal responsive zeolitic imidazolate framework using polydopamine-PEG nanoparticles (ZIF-PDA-PEG). Infrared laser irradiation assisted the releases of drugs, solving co-loading and multiple drug resistance problems, as well as showed higher efficiency in inducing apoptosis and autophagy (Chen et al., 2020).
Future studies can also focus on combining EGCG with other anticancer drugs to achieve a synergistic enhancement of the anti-cancer effects (Suganuma et al., 2011; Fujiki et al., 2015). In ovarian cancer, green tea with Paclitaxel or other nutrients as combination strategy synergistically inhibited cancer cells (Xinqiang et al., 2017; Panji et al., 2021a). Meanwhile, EGCG in combination with Cruciferous vegetables or Sulforaphane were shown to overcome Cisplatin or Paclitaxel chemotherapy resistance in ovarian cancer (Mazumder et al., 2012; Chen et al., 2013b; a). In cervical cancer, EGCG was treated synergistically with Enoacin or Eugenol-Amarogentin against cancer tumor growth (Pal et al., 2018; Mcdonnell et al., 2019). It was also treated to enhance Cisplatin effects in chemotherapy (Kilic et al., 2015). In breast cancer, EGCG demonstrated synergistic effects with Dexamethasone, U021, Tamoxifen, Quercetin or Paclitaxel (Guo et al., 2006; Sartippour et al., 2006; Li et al., 2009; Luo et al., 2010), thus enhanced cancer cell death. As discussed, tea polyphenol was proposed to be used with other chemotherapies to synergistically advance the therapeutic efficiency of autophagy modulating effect, such as with Docetaxel or Bortezomib in prostate cancer (Modernelli et al., 2015; Wang Q. et al., 2018), with Paclitaxel in ovarian cancer (Panji et al., 2021a), or with Doxorubicin in cervical cancer (Chen et al., 2020).
Conclusion
The role of autophagy has been shown to play a dual role in cancer. On one hand, autophagy may help to protect against cancer, while on the other hand, it may promote the growth of cancer. As autophagy plays an important role in cell death, these autophagy modulators may be a potential target to induce tumor cell death in a manner independent of necrosis or apoptosis. However, targeting these autophagy modulators as therapeutical strategy remained a huge obstacle. Thus, natural products can be used to target multiple pathways, which provide greater flexibility and potential for a variety of cancer types and stages. Green tea catechins and EGCG have been shown to play an important role in modulating autophagy in reproductive cancer cells. A number of in vitro and in vivo studies have confirmed the anti-inflammatory and antioxidant properties of polyphenolic compounds to induce autophagic death in reproductive cancer cells. However, it still required more validation using animal studies and clinical trials. Nevertheless, the current evidence strongly suggests the biologically active ingredients of green tea, namely EGCG, to be a valuable and promising agent in reproductive cancer chemoprevention.
Author Contributions
SH, YL, GC-WM, and CW wrote the first draft of the manuscript. XC, KC, YZ, YL, and XG contributed sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.906746/full#supplementary-material
Chemical structures of the four-major green tea catechins include (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), and (−)-epigallocatechin-3- gallate (EGCG).
References
- Adachi S., Nagao T., Ingolfsson H. I., Maxfield F. R., Andersen O. S., Kopelovich L., et al. (2007). The Inhibitory Effect of (-)-epigallocatechin Gallate on Activation of the Epidermal Growth Factor Receptor Is Associated with Altered Lipid Order in HT29 Colon Cancer Cells. Cancer Res. 67, 6493–6501. 10.1158/0008-5472.CAN-07-0411 [DOI] [PubMed] [Google Scholar]
- Adhami V. M., Malik A., Zaman N., Sarfaraz S., Siddiqui I. A., Syed D. N., et al. (2007). Combined Inhibitory Effects of Green Tea Polyphenols and Selective Cyclooxygenase-2 Inhibitors on the Growth of Human Prostate Cancer Cells Both In Vitro and In Vivo. Clin. Cancer Res. 13, 1611–1619. 10.1158/1078-0432.CCR-06-2269 [DOI] [PubMed] [Google Scholar]
- Adhami V. M., Siddiqui I. A., Ahmad N., Gupta S., Mukhtar H. (2004). Oral Consumption of Green Tea Polyphenols Inhibits Insulin-like Growth Factor-I-Induced Signaling in an Autochthonous Mouse Model of Prostate Cancer. Cancer Res. 64, 8715–8722. 10.1158/0008-5472.CAN-04-2840 [DOI] [PubMed] [Google Scholar]
- Adhami V. M., Siddiqui I. A., Sarfaraz S., Khwaja S. I., Hafeez B. B., Ahmad N., et al. (2009). Effective Prostate Cancer Chemopreventive Intervention with Green Tea Polyphenols in the TRAMP Model Depends on the Stage of the Disease. Clin. Cancer Res. 15, 1947–1953. 10.1158/1078-0432.CCR-08-2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N., Gupta S., Mukhtar H. (2000). Green Tea Polyphenol Epigallocatechin-3-Gallate Differentially Modulates Nuclear Factor kappaB in Cancer Cells versus Normal Cells. Arch. Biochem. Biophys. 376, 338–346. 10.1006/abbi.2000.1742 [DOI] [PubMed] [Google Scholar]
- Ahn W. S., Huh S. W., Bae S. M., Lee I. P., Lee J. M., Namkoong S. E., et al. (2003). A Major Constituent of Green Tea, EGCG, Inhibits the Growth of a Human Cervical Cancer Cell Line, CaSki Cells, through Apoptosis, G(1) Arrest, and Regulation of Gene Expression. DNA Cell Biol. 22, 217–224. 10.1089/104454903321655846 [DOI] [PubMed] [Google Scholar]
- Al-Bari M. A. A., Ito Y., Ahmed S., Radwan N., Ahmed H. S., Eid N. (2021). Targeting Autophagy with Natural Products as a Potential Therapeutic Approach for Cancer. Int. J. Mol. Sci. 22, 9807. 10.3390/ijms22189807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Obaidy K. I., Chovanec M., Cheng L. (2020). Molecular Characteristics of Testicular Germ Cell Tumors: Pathogenesis and Mechanisms of Therapy Resistance. Expert Rev. Anticancer Ther. 20, 75–79. 10.1080/14737140.2020.1717337 [DOI] [PubMed] [Google Scholar]
- Amaral C., Augusto T. V., Tavares-Da-Silva E., Roleira F. M. F., Correia-Da-Silva G., Teixeira N. (2018). Hormone-dependent Breast Cancer: Targeting Autophagy and PI3K Overcomes Exemestane-Acquired Resistance. J. Steroid Biochem. Mol. Biol. 183, 51–61. 10.1016/j.jsbmb.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Amaravadi R. K., Kimmelman A. C., Debnath J. (2019). Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 9, 1167–1181. 10.1158/2159-8290.CD-19-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Gao A. C. (2015). Drug Resistance in Castration Resistant Prostate Cancer: Resistance Mechanisms and Emerging Treatment Strategies. Am. J. Clin. Exp. Urol. 3, 64–76. [PMC free article] [PubMed] [Google Scholar]
- Ávalos Y., Canales J., Bravo-Sagua R., Criollo A., Lavandero S., Quest A. F. (2014). Tumor Suppression and Promotion by Autophagy. Biomed. Res. Int. 2014, 603980. 10.1155/2014/603980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae K. H., Tan S., Yamashita A., Ang W. X., Gao S. J., Wang S., et al. (2017). Hyaluronic Acid-Green Tea Catechin Micellar Nanocomplexes: Fail-Safe Cisplatin Nanomedicine for the Treatment of Ovarian Cancer without Off-Target Toxicity. Biomaterials 148, 41–53. 10.1016/j.biomaterials.2017.09.027 [DOI] [PubMed] [Google Scholar]
- Balasubramanian R., Rolph R., Morgan C., Hamed H. (2019). Genetics of Breast Cancer: Management Strategies and Risk-Reducing Surgery. Br. J. Hosp. Med. (Lond) 80, 720–725. 10.12968/hmed.2019.80.12.720 [DOI] [PubMed] [Google Scholar]
- Balazi A., Sirotkin A. V., Foldesiova M., Makovicky P., Chrastinova L., Makovicky P., et al. (2019). Green Tea Can Supress Rabbit Ovarian Functions In Vitro and In Vivo. Theriogenology 127, 72–79. 10.1016/j.theriogenology.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Bandera E. V., Williams-King M. G., Sima C., Bayuga-Miller S., Pulick K., Wilcox H., et al. (2010). Coffee and Tea Consumption and Endometrial Cancer Risk in a Population-Based Study in New Jersey. Cancer Causes Control 21, 1467–1473. 10.1007/s10552-010-9575-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belguise K., Guo S., Sonenshein G. E. (2007). Activation of FOXO3a by the Green Tea Polyphenol Epigallocatechin-3-Gallate Induces Estrogen Receptor Alpha Expression Reversing Invasive Phenotype of Breast Cancer Cells. Cancer Res. 67, 5763–5770. 10.1158/0008-5472.CAN-06-4327 [DOI] [PubMed] [Google Scholar]
- Bettuzzi S., Brausi M., Rizzi F., Castagnetti G., Peracchia G., Corti A. (2006). Chemoprevention of Human Prostate Cancer by Oral Administration of Green Tea Catechins in Volunteers with High-Grade Prostate Intraepithelial Neoplasia: A Preliminary Report from a One-Year Proof-Of-Principle Study. Cancer Res. 66, 1234–1240. 10.1158/0008-5472.CAN-05-1145 [DOI] [PubMed] [Google Scholar]
- Beynon R. A., Richmond R. C., Santos Ferreira D. L., Ness A. R., May M., Smith G. D., et al. (2019). Investigating the Effects of Lycopene and Green Tea on the Metabolome of Men at Risk of Prostate Cancer: The ProDiet Randomised Controlled Trial. Int. J. Cancer 144, 1918–1928. 10.1002/ijc.31929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braicu C., Gherman C. D., Irimie A., Berindan-Neagoe I. (2013). Epigallocatechin-3-gallate (EGCG) Inhibits Cell Proliferation and Migratory Behaviour of Triple Negative Breast Cancer Cells. J. Nanosci. Nanotechnol. 13, 632–637. 10.1166/jnn.2013.6882 [DOI] [PubMed] [Google Scholar]
- Brausi M., Rizzi F., Bettuzzi S. (2008). Chemoprevention of Human Prostate Cancer by Green Tea Catechins: Two Years Later. A Follow-Up Update. Eur. Urol. 54, 472–473. 10.1016/j.eururo.2008.03.100 [DOI] [PubMed] [Google Scholar]
- Butler D. E., Marlein C., Walker H. F., Frame F. M., Mann V. M., Simms M. S., et al. (2017). Inhibition of the PI3K/AKT/mTOR Pathway Activates Autophagy and Compensatory Ras/Raf/MEK/ERK Signalling in Prostate Cancer. Oncotarget 8, 56698–56713. 10.18632/oncotarget.18082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzun K., Gornowicz A., Lesyk R., Bielawski K., Bielawska A. (2021). Autophagy Modulators in Cancer Therapy. Int. J. Mol. Sci. 22, 5804. 10.3390/ijms22115804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera C., Artacho R., Giménez R. (2006). Beneficial Effects of Green Tea-Aa Review. J. Am. Coll. Nutr. 25, 79–99. 10.1080/07315724.2006.10719518 [DOI] [PubMed] [Google Scholar]
- Cai Z. Y., Li X. M., Liang J. P., Xiang L. P., Wang K. R., Shi Y. L., et al. (2018). Bioavailability of Tea Catechins and its Improvement. Molecules 23, 2346. 10.3390/molecules23092346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko S. M., Thambi P. T., Kuttan R., Nishigaki I. (2010). Beneficial Effects of Green Tea: a Literature Review. Chin. Med. 5, 13. 10.1186/1749-8546-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M. M., Soprano K. J., Weinstein K., Fong D. (2006). Epigallocatechin-3-gallate Delivers Hydrogen Peroxide to Induce Death of Ovarian Cancer Cells and Enhances Their Cisplatin Susceptibility. J. Cell Physiol. 207, 389–396. 10.1002/jcp.20569 [DOI] [PubMed] [Google Scholar]
- Chen H., Landen C. N., Li Y., Alvarez R. D., Tollefsbol T. O. (2013b). Epigallocatechin Gallate and Sulforaphane Combination Treatment Induce Apoptosis in Paclitaxel-Resistant Ovarian Cancer Cells through hTERT and Bcl-2 Down-Regulation. Exp. Cell Res. 319, 697–706. 10.1016/j.yexcr.2012.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Landen C. N., Li Y., Alvarez R. D., Tollefsbol T. O. (2013a). Enhancement of Cisplatin-Mediated Apoptosis in Ovarian Cancer Cells through Potentiating G2/M Arrest and P21 Upregulation by Combinatorial Epigallocatechin Gallate and Sulforaphane. J. Oncol. 2013, 872957. 10.1155/2013/872957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Tong R., Liu B., Liu H., Feng X., Ding S., et al. (2020). Duo of (-)-Epigallocatechin-3-Gallate and Doxorubicin Loaded by Polydopamine Coating ZIF-8 in the Regulation of Autophagy for Chemo-Photothermal Synergistic Therapy. Biomater. Sci. 8, 1380–1393. 10.1039/c9bm01614g [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen J., Sun X., Shi X., Wang L., Huang L., et al. (2018). Evaluation of the Neuroprotective Effect of EGCG: a Potential Mechanism of Mitochondrial Dysfunction and Mitochondrial Dynamics after Subarachnoid Hemorrhage. Food Funct. 9, 6349–6359. 10.1039/c8fo01497c [DOI] [PubMed] [Google Scholar]
- Cheng L., Lyu B., Roth L. M. (2017). Perspectives on Testicular Germ Cell Neoplasms. Hum. Pathol. 59, 10–25. 10.1016/j.humpath.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Choan E., Segal R., Jonker D., Malone S., Reaume N., Eapen L., et al. (2005). A Prospective Clinical Trial of Green Tea for Hormone Refractory Prostate Cancer: An Evaluation of the Complementary/alternative Therapy Approach. Urol. Oncol. 23, 108–113. 10.1016/j.urolonc.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Chu C., Deng J., Man Y., Qu Y. (2017). Green Tea Extracts Epigallocatechin-3-Gallate for Different Treatments. Biomed. Res. Int. 2017, 5615647. 10.1155/2017/5615647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuu C. P., Chen R. Y., Kokontis J. M., Hiipakka R. A., Liao S. (2009). Suppression of Androgen Receptor Signaling and Prostate Specific Antigen Expression by (-)-Epigallocatechin-3-Gallate in Different Progression Stages of LNCaP Prostate Cancer Cells. Cancer Lett. 275, 86–92. 10.1016/j.canlet.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew K. D., Brown P., Greenlee H., Bevers T. B., Arun B., Hudis C., et al. (2012). Phase IB Randomized, Double-Blinded, Placebo-Controlled, Dose Escalation Study of Polyphenon E in Women with Hormone Receptor-Negative Breast Cancer. Cancer Prev. Res. (Phila) 5, 1144–1154. 10.1158/1940-6207.CAPR-12-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew K. D., Ho K. A., Brown P., Greenlee H., Bevers T. B., Arun B., et al. (2015). Effects of a Green Tea Extract, Polyphenon E, on Systemic Biomarkers of Growth Factor Signalling in Women with Hormone Receptor-Negative Breast Cancer. J. Hum. Nutr. Diet. 28, 272–282. 10.1111/jhn.12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo F., Altucci L., Cobellis G. (2019). Autophagy Function and Dysfunction: Potential Drugs as Anti-cancer Therapy. Cancers (Basel) 11, 1465. 10.3390/cancers11101465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dag D., Oztop M. H. (2017). Formation and Characterization of Green Tea Extract Loaded Liposomes. J. Food Sci. 82, 463–470. 10.1111/1750-3841.13615 [DOI] [PubMed] [Google Scholar]
- Dai Q., Shu X. O., Li H., Yang G., Shrubsole M. J., Cai H., et al. (2010). Is Green Tea Drinking Associated with a Later Onset of Breast Cancer? Ann. Epidemiol. 20, 74–81. 10.1016/j.annepidem.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankó T., Petővári G., Sztankovics D., Moldvai D., Raffay R., Lőrincz P., et al. (2021). Rapamycin Plus Doxycycline Combination Affects Growth Arrest and Selective Autophagy-dependent Cell Death in Breast Cancer Cells. Int. J. Mol. Sci. 22, 8019. 10.3390/ijms22158019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport A., Frezza M., Shen M., Ge Y., Huo C., Chan T. H., et al. (2010). Celastrol and an EGCG Pro-drug Exhibit Potent Chemosensitizing Activity in Human Leukemia Cells. Int. J. Mol. Med. 25, 465–470. 10.3892/ijmm_00000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pace R. C., Liu X., Sun M., Nie S., Zhang J., Cai Q., et al. (2013). Anticancer Activities of (-)-Epigallocatechin-3-Gallate Encapsulated Nanoliposomes in MCF7 Breast Cancer Cells. J. Liposome Res. 23, 187–196. 10.3109/08982104.2013.788023 [DOI] [PubMed] [Google Scholar]
- Deb G., Shankar E., Thakur V. S., Ponsky L. E., Bodner D. R., Fu P., et al. (2019). Green Tea-Induced Epigenetic Reactivation of Tissue Inhibitor of Matrix Metalloproteinase-3 Suppresses Prostate Cancer Progression through Histone-Modifying Enzymes. Mol. Carcinog. 58, 1194–1207. 10.1002/mc.23003 [DOI] [PubMed] [Google Scholar]
- Degenhardt K., White E. (2006). A Mouse Model System to Genetically Dissect the Molecular Mechanisms Regulating Tumorigenesis. Clin. Cancer Res. 12, 5298–5304. 10.1158/1078-0432.CCR-06-0439 [DOI] [PubMed] [Google Scholar]
- Den Boon J. A., Pyeon D., Wang S. S., Horswill M., Schiffman M., Sherman M., et al. (2015). Molecular Transitions from Papillomavirus Infection to Cervical Precancer and Cancer: Role of Stromal Estrogen Receptor Signaling. Proc. Natl. Acad. Sci. U. S. A. 112, E3255–E3264. 10.1073/pnas.1509322112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D., Xu T., Kumar S. (2015). Autophagy as a Pro-death Pathway. Immunol. Cell Biol. 93, 35–42. 10.1038/icb.2014.85 [DOI] [PubMed] [Google Scholar]
- Djavaheri-Mergny M., Amelotti M., Mathieu J., Besançon F., Bauvy C., Souquère S., et al. (2006). NF-kappaB Activation Represses Tumor Necrosis Factor-Alpha-Induced Autophagy. J. Biol. Chem. 281, 30373–30382. 10.1074/jbc.M602097200 [DOI] [PubMed] [Google Scholar]
- Dökümcü K., Simonian M., Farahani R. M. (2018). miR4673 Improves Fitness Profile of Neoplastic Cells by Induction of Autophagy. Cell Death Dis. 9, 1068. 10.1038/s41419-018-1088-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G. J., Zhang Z., Wen X. D., Yu C., Calway T., Yuan C. S., et al. (2012). Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea. Nutrients 4, 1679–1691. 10.3390/nu4111679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube A., Nicolazzo J. A., Larson I. (2010). Chitosan Nanoparticles Enhance the Intestinal Absorption of the Green Tea Catechins (+)-catechin and (-)-epigallocatechin Gallate. Eur. J. Pharm. Sci. 41, 219–225. 10.1016/j.ejps.2010.06.010 [DOI] [PubMed] [Google Scholar]
- Erdrich S., Bishop K. S., Karunasinghe N., Han D. Y., Ferguson L. R. (2015). A Pilot Study to Investigate if New Zealand Men with Prostate Cancer Benefit from a Mediterranean-Style Diet. PeerJ 3, e1080. 10.7717/peerj.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eritja N., Chen B. J., Rodríguez-Barrueco R., Santacana M., Gatius S., Vidal A., et al. (2017). Autophagy Orchestrates Adaptive Responses to Targeted Therapy in Endometrial Cancer. Autophagy 13, 608–624. 10.1080/15548627.2016.1271512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili M. A. (2016). Combination of siRNA-Directed Gene Silencing with Epigallocatechin-3-Gallate (EGCG) Reverses Drug Resistance in Human Breast Cancer Cells. J. Chem. Biol. 9, 41–52. 10.1007/s12154-015-0144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J., Ziebland S., Mcpherson A. (2007). Minimizing Delays in Ovarian Cancer Diagnosis: an Expansion of Andersen's Model of 'total Patient Delay'. Fam. Pract. 24, 48–55. 10.1093/fampra/cml063 [DOI] [PubMed] [Google Scholar]
- Evans S., Dizeyi N., Abrahamsson P. A., Persson J. (2009). The Effect of a Novel Botanical Agent TBS-101 on Invasive Prostate Cancer in Animal Models. Anticancer Res. 29, 3917–3924. [PubMed] [Google Scholar]
- Fan X., Zhou J., Bi X., Liang J., Lu S., Yan X., et al. (2021). L-theanine Suppresses the Metastasis of Prostate Cancer by Downregulating MMP9 and Snail. J. Nutr. Biochem. 89, 108556. 10.1016/j.jnutbio.2020.108556 [DOI] [PubMed] [Google Scholar]
- Fang J. Y., Lee W. R., Shen S. C., Huang Y. L. (2006). Effect of Liposome Encapsulation of Tea Catechins on Their Accumulation in Basal Cell Carcinomas. J. Dermatol Sci. 42, 101–109. 10.1016/j.jdermsci.2005.12.010 [DOI] [PubMed] [Google Scholar]
- Farrow J. M., Yang J. C., Evans C. P. (2014). Autophagy as a Modulator and Target in Prostate Cancer. Nat. Rev. Urol. 11, 508–516. 10.1038/nrurol.2014.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., He D., Yao Z., Klionsky D. J. (2014). The Machinery of Macroautophagy. Cell Res. 24, 24–41. 10.1038/cr.2013.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H., Sueoka E., Watanabe T., Suganuma M. (2015). Synergistic Enhancement of Anticancer Effects on Numerous Human Cancer Cell Lines Treated with the Combination of EGCG, Other Green Tea Catechins, and Anticancer Compounds. J. Cancer Res. Clin. Oncol. 141, 1511–1522. 10.1007/s00432-014-1899-5 [DOI] [PubMed] [Google Scholar]
- Gan R. Y., Li H. B., Sui Z. Q., Corke H. (2018). Absorption, Metabolism, Anti-cancer Effect and Molecular Targets of Epigallocatechin Gallate (EGCG): An Updated Review. Crit. Rev. Food Sci. Nutr. 58, 924–941. 10.1080/10408398.2016.1231168 [DOI] [PubMed] [Google Scholar]
- García-Aranda M., Redondo M. (2019). Immunotherapy: A Challenge of Breast Cancer Treatment. Cancers (Basel) 11, 1822. 10.3390/cancers11121822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J., Han B., Hu H., Liu J., Liu Y. (2015). Epigallocatechin-3-O-Gallate Protects against Hepatic Damage and Testicular Toxicity in Male Mice Exposed to Di-(2-ethylhexyl) Phthalate. J. Med. Food 18, 753–761. 10.1089/jmf.2014.3247 [DOI] [PubMed] [Google Scholar]
- Gewirtz D. A. (2016). The Challenge of Developing Autophagy Inhibition as a Therapeutic Strategy. Cancer Res. 76, 5610–5614. 10.1158/0008-5472.CAN-16-0722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham H. N. (1992). Green Tea Composition, Consumption, and Polyphenol Chemistry. Prev. Med. 21, 334–350. 10.1016/0091-7435(92)90041-f [DOI] [PubMed] [Google Scholar]
- Guo S., Lu J., Subramanian A., Sonenshein G. E. (2006). Microarray-assisted Pathway Analysis Identifies Mitogen-Activated Protein Kinase Signaling as a Mediator of Resistance to the Green Tea Polyphenol Epigallocatechin 3-gallate in Her-2/neu-Overexpressing Breast Cancer Cells. Cancer Res. 66, 5322–5329. 10.1158/0008-5472.CAN-05-4287 [DOI] [PubMed] [Google Scholar]
- Gupta S., Hastak K., Afaq F., Ahmad N., Mukhtar H. (2004). Essential Role of Caspases in Epigallocatechin-3-Gallate-Mediated Inhibition of Nuclear Factor Kappa B and Induction of Apoptosis. Oncogene 23, 2507–2522. 10.1038/sj.onc.1207353 [DOI] [PubMed] [Google Scholar]
- Gupta S. M., Mania-Pramanik J. (2019). Molecular Mechanisms in Progression of HPV-Associated Cervical Carcinogenesis. J. Biomed. Sci. 26, 28. 10.1186/s12929-019-0520-2 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Han Z., Liu D., Chen L., He Y., Tian X., Qi L., et al. (2021). PNO1 Regulates Autophagy and Apoptosis of Hepatocellular Carcinoma via the MAPK Signaling Pathway. Cell Death Dis. 12, 552. 10.1038/s41419-021-03837-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna T. P., King W. D., Thibodeau S., Jalink M., Paulin G. A., Harvey-Jones E., et al. (2020). Mortality Due to Cancer Treatment Delay: Systematic Review and Meta-Analysis. BMJ 371, m4087. 10.1136/bmj.m4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu X., Lanyue L., Miao D., Wentao F., Cangran C., Hui S. (2020). Effect of Ganoderma Applanatum Polysaccharides on MAPK/ERK Pathway Affecting Autophagy in Breast Cancer MCF-7 Cells. Int. J. Biol. Macromol. 146, 353–362. 10.1016/j.ijbiomac.2020.01.010 [DOI] [PubMed] [Google Scholar]
- Harper C. E., Patel B. B., Wang J., Eltoum I. A., Lamartiniere C. A. (2007). Epigallocatechin-3-Gallate Suppresses Early Stage, but Not Late Stage Prostate Cancer in TRAMP Mice: Mechanisms of Action. Prostate 67, 1576–1589. 10.1002/pros.20643 [DOI] [PubMed] [Google Scholar]
- Hastak K., Gupta S., Ahmad N., Agarwal M. K., Agarwal M. L., Mukhtar H. (2003). Role of P53 and NF-kappaB in Epigallocatechin-3-Gallate-Induced Apoptosis of LNCaP Cells. Oncogene 22, 4851–4859. 10.1038/sj.onc.1206708 [DOI] [PubMed] [Google Scholar]
- Hawsawi Y. M., Zailaie S. A., Oyouni A. A. A., Alzahrani O. R., Alamer O. M., Aljohani S. A. S. (2020). Prostate Cancer and Therapeutic Challenges. J. Biol. Res. Thessal. 27, 20. 10.1186/s40709-020-00128-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes-Lattin B., Nichols C. R. (2009). Testicular Cancer: a Prototypic Tumor of Young Adults. Semin. Oncol. 36, 432–438. 10.1053/j.seminoncol.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht J. L., Mutter G. L. (2006). Molecular and Pathologic Aspects of Endometrial Carcinogenesis. J. Clin. Oncol. 24, 4783–4791. 10.1200/JCO.2006.06.7173 [DOI] [PubMed] [Google Scholar]
- Herman-Antosiewicz A., Johnson D. E., Singh S. V. (2006). Sulforaphane Causes Autophagy to Inhibit Release of Cytochrome C and Apoptosis in Human Prostate Cancer Cells. Cancer Res. 66, 5828–5835. 10.1158/0008-5472.CAN-06-0139 [DOI] [PubMed] [Google Scholar]
- Hickey M., Peate M., Saunders C. M., Friedlander M. (2009). Breast Cancer in Young Women and its Impact on Reproductive Function. Hum. Reprod. Update 15, 323–339. 10.1093/humupd/dmn064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. C., Yang S. F., Peng C. Y., Chou M. Y., Chang Y. C. (2007). Epigallocatechin-3-gallate Inhibits the Invasion of Human Oral Cancer Cells and Decreases the Productions of Matrix Metalloproteinases and Urokinase-Plasminogen Activator. J. Oral Pathol. Med. 36, 588–593. 10.1111/j.1600-0714.2007.00588.x [DOI] [PubMed] [Google Scholar]
- Horowitz M., Esakov E., Rose P., Reizes O. (2020). Signaling within the Epithelial Ovarian Cancer Tumor Microenvironment: the Challenge of Tumor Heterogeneity. Ann. Transl. Med. 8, 905. 10.21037/atm-2019-cm-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z., Sang S., You H., Lee M. J., Hong J., Chin K. V., et al. (2005). Mechanism of Action of (-)-Epigallocatechin-3-Gallate: Auto-oxidation-dependent Inactivation of Epidermal Growth Factor Receptor and Direct Effects on Growth Inhibition in Human Esophageal Cancer KYSE 150 Cells. Cancer Res. 65, 8049–8056. 10.1158/0008-5472.CAN-05-0480 [DOI] [PubMed] [Google Scholar]
- Hsu A., Bruno R. S., Löhr C. V., Taylor A. W., Dashwood R. H., Bray T. M., et al. (2011). Dietary Soy and Tea Mitigate Chronic Inflammation and Prostate Cancer via NFκB Pathway in the Noble Rat Model. J. Nutr. Biochem. 22, 502–510. 10.1016/j.jnutbio.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innajak S., Mahabusrakum W., Watanapokasin R. (2016). Goniothalamin Induces Apoptosis Associated with Autophagy Activation through MAPK Signaling in SK-BR-3 Cells. Oncol. Rep. 35, 2851–2858. 10.3892/or.2016.4655 [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Inoue M., Sasazuki S., Miura T., Sawada N., Yamaji T., et al. (2010). Plasma Tea Polyphenol Levels and Subsequent Risk of Breast Cancer Among Japanese Women: A Nested Case-Control Study. Breast Cancer Res. Treat. 124, 827–834. 10.1007/s10549-010-0916-x [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Mizusawa J., Kasuga Y., Yokoyama S., Onuma H., Nishimura H., et al. (2014). Green Tea Consumption and Breast Cancer Risk in Japanese Women: A Case-Control Study. Nutr. Cancer 66, 57–67. 10.1080/01635581.2014.847963 [DOI] [PubMed] [Google Scholar]
- Jang J. Y., Lee J. K., Jeon Y. K., Kim C. W. (2013). Exosome Derived from Epigallocatechin Gallate Treated Breast Cancer Cells Suppresses Tumor Growth by Inhibiting Tumor-Associated Macrophage Infiltration and M2 Polarization. BMC Cancer 13, 421. 10.1186/1471-2407-13-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F., Mcconkey D. J., Hong D. S., Kurzrock R. (2011). Autophagy as a Target for Anticancer Therapy. Nat. Rev. Clin. Oncol. 8, 528–539. 10.1038/nrclinonc.2011.71 [DOI] [PubMed] [Google Scholar]
- Jia Y., Hu T., Hang C. Y., Yang R., Li X., Chen Z. L., et al. (2012). Case-control Study of Diet in Patients with Cervical Cancer or Precancerosis in Wufeng, a High Incidence Region in China. Asian Pac J. Cancer Prev. 13, 5299–5302. 10.7314/apjcp.2012.13.10.5299 [DOI] [PubMed] [Google Scholar]
- Katiyar S. K., Afaq F., Azizuddin K., Mukhtar H. (2001). Inhibition of UVB-Induced Oxidative Stress-Mediated Phosphorylation of Mitogen-Activated Protein Kinase Signaling Pathways in Cultured Human Epidermal Keratinocytes by Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate. Toxicol. Appl. Pharmacol. 176, 110–117. 10.1006/taap.2001.9276 [DOI] [PubMed] [Google Scholar]
- Kaur J., Debnath J. (2015). Autophagy at the Crossroads of Catabolism and Anabolism. Nat. Rev. Mol. Cell Biol. 16, 461–472. 10.1038/nrm4024 [DOI] [PubMed] [Google Scholar]
- Kaur S., Greaves P., Cooke D. N., Edwards R., Steward W. P., Gescher A. J., et al. (2007). Breast Cancer Prevention by Green Tea Catechins and Black Tea Theaflavins in the C3(1) SV40 T,t Antigen Transgenic Mouse Model Is Accompanied by Increased Apoptosis and a Decrease in Oxidative DNA Adducts. J. Agric. Food Chem. 55, 3378–3385. 10.1021/jf0633342 [DOI] [PubMed] [Google Scholar]
- Kavanagh K. T., Hafer L. J., Kim D. W., Mann K. K., Sherr D. H., Rogers A. E., et al. (2001). Green Tea Extracts Decrease Carcinogen-Induced Mammary Tumor Burden in Rats and Rate of Breast Cancer Cell Proliferation in Culture. J. Cell Biochem. 82, 387–398. 10.1002/jcb.1164 [DOI] [PubMed] [Google Scholar]
- Kazi A., Wang Z., Kumar N., Falsetti S. C., Chan T. H., Dou Q. P. (2004). Structure-activity Relationships of Synthetic Analogs of (-)-Epigallocatechin-3-Gallate as Proteasome Inhibitors. Anticancer Res. 24, 943–954. [PubMed] [Google Scholar]
- Khan M. A., Hussain A., Sundaram M. K., Alalami U., Gunasekera D., Ramesh L., et al. (2015). (-)-Epigallocatechin-3-gallate Reverses the Expression of Various Tumor-Suppressor Genes by Inhibiting DNA Methyltransferases and Histone Deacetylases in Human Cervical Cancer Cells. Oncol. Rep. 33, 1976–1984. 10.3892/or.2015.3802 [DOI] [PubMed] [Google Scholar]
- Khan N., Bharali D. J., Adhami V. M., Siddiqui I. A., Cui H., Shabana S. M., et al. (2014). Oral Administration of Naturally Occurring Chitosan-Based Nanoformulated Green Tea Polyphenol EGCG Effectively Inhibits Prostate Cancer Cell Growth in a Xenograft Model. Carcinogenesis 35, 415–423. 10.1093/carcin/bgt321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Mukhtar H. (2008). Multitargeted Therapy of Cancer by Green Tea Polyphenols. Cancer Lett. 269, 269–280. 10.1016/j.canlet.2008.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khojaste E., Ahmadizadeh C. (2021). Catechin Metabolites along with Curcumin Inhibit Proliferation and Induce Apoptosis in Cervical Cancer Cells by Regulating VEGF Expression In-Vitro. Nutr. Cancer 74 (3), 1048–1057. 10.1080/01635581.2021.1936082 [DOI] [PubMed] [Google Scholar]
- Kilic U., Sahin K., Tuzcu M., Basak N., Orhan C., Elibol-Can B., et al. (2015). Enhancement of Cisplatin Sensitivity in Human Cervical Cancer: Epigallocatechin-3-Gallate. Front. Nutr. 1, 28. 10.3389/fnut.2014.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Mcglynn K. A., Mccorkle R., Zheng T., Erickson R. L., Niebuhr D. W., et al. (2010). Fertility Among Testicular Cancer Survivors: a Case-Control Study in the U.S. J. Cancer Surviv 4, 266–273. 10.1007/s11764-010-0134-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S., Kim M. H., Jeong M., Hwang Y. S., Lim S. H., Shin B. A., et al. (2004). EGCG Blocks Tumor Promoter-Induced MMP-9 Expression via Suppression of MAPK and AP-1 Activation in Human Gastric AGS Cells. Anticancer Res. 24, 747–753. [PubMed] [Google Scholar]
- Kim S. J., Amankwah E., Connors S., Park H. Y., Rincon M., Cornnell H., et al. (2014). Safety and Chemopreventive Effect of Polyphenon E in Preventing Early and Metastatic Progression of Prostate Cancer in TRAMP Mice. Cancer Prev. Res. (Phila) 7, 435–444. 10.1158/1940-6207.CAPR-13-0427-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M., Nishino T., Obata Y., Furusu A., Hishikawa Y., Koji T., et al. (2012). Epigallocatechin Gallate Suppresses Peritoneal Fibrosis in Mice. Chem. Biol. Interact. 195, 95–104. 10.1016/j.cbi.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Abdelmohsen K., Abe A., Abedin M. J., Abeliovich H., Acevedo Arozena A., et al. (2021). Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy. Autophagy 17, 1–382. 10.1080/15548627.2015.1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochetkova E. Y., Blinova G. I., Bystrova O. A., Martynova M. G., Pospelov V. A., Pospelova T. V. (2017). Targeted Elimination of Senescent Ras-Transformed Cells by Suppression of MEK/ERK Pathway. Aging (Albany NY) 9, 2352–2375. 10.18632/aging.101325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochman J., Jakubczyk K., Antoniewicz J., Mruk H., Janda K. (2020). Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 26, 85. 10.3390/molecules26010085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. B., Pow-Sang J., Spiess P. E., Park J., Salup R., Williams C. R., et al. (2016). Randomized, Placebo-Controlled Trial Evaluating the Safety of One-Year Administration of Green Tea Catechins. Oncotarget 7, 70794–70802. 10.18632/oncotarget.12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi N., Sasazuki S., Iwasaki M., Inoue M. (2008). Green Tea Consumption and Prostate Cancer Risk in Japanese Men: A Prospective Study. Am. J. Epidemiol. 167, 71–77. 10.1093/aje/kwm249 [DOI] [PubMed] [Google Scholar]
- Lam W. H., Kazi A., Kuhn D. J., Chow L. M., Chan A. S., Dou Q. P., et al. (2004). A Potential Prodrug for a Green Tea Polyphenol Proteasome Inhibitor: Evaluation of the Peracetate Ester of (-)-epigallocatechin Gallate [(-)-EGCG]. Bioorg Med. Chem. 12, 5587–5593. 10.1016/j.bmc.2004.08.002 [DOI] [PubMed] [Google Scholar]
- Landis-Piwowar K. R., Huo C., Chen D., Milacic V., Shi G., Chan T. H., et al. (2007). A Novel Prodrug of the Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate as a Potential Anticancer Agent. Cancer Res. 67, 4303–4310. 10.1158/0008-5472.CAN-06-4699 [DOI] [PubMed] [Google Scholar]
- Landis-Piwowar K. R., Kuhn D. J., Wan S. B., Chen D., Chan T. H., Dou Q. P. (2005). Evaluation of Proteasome-Inhibitory and Apoptosis-Inducing Potencies of Novel (-)-EGCG Analogs and Their Prodrugs. Int. J. Mol. Med. 15, 735–742. 10.3892/ijmm.15.4.735 [DOI] [PubMed] [Google Scholar]
- Lassed S., Deus C. M., Djebbari R., Zama D., Oliveira P. J., Rizvanov A. A., et al. (2017). Protective Effect of Green Tea (Camellia Sinensis (L.) Kuntze) against Prostate Cancer: From In Vitro Data to Algerian Patients. Evid. Based Complement. Altern. Med. 2017, 1691568. 10.1155/2017/1691568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassed S., Deus C. M., Lourenco N., Dahdouh A., Rizvanov A. A., Oliveira P. J., et al. (2016). Diet, Lifestyles, Family History, and Prostate Cancer Incidence in an East Algerian Patient Group. Biomed. Res. Int. 2016, 5730569. 10.1155/2016/5730569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. H., Su D., Pasalich M., Binns C. W. (2013). Tea Consumption Reduces Ovarian Cancer Risk. Cancer Epidemiol. 37, 54–59. 10.1016/j.canep.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Lee J. J., Jain V., Amaravadi R. K. (2021). Clinical Translation of Combined MAPK and Autophagy Inhibition in RAS Mutant Cancer. Int. J. Mol. Sci. 22, 12402. 10.3390/ijms222212402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. S., Cherla R. P., Jenson M. H., Leyva-Illades D., Martinez-Moczygemba M., Tesh V. L. (2011). Shiga Toxins Induce Autophagy Leading to Differential Signalling Pathways in Toxin-Sensitive and Toxin-Resistant Human Cells. Cell Microbiol. 13, 1479–1496. 10.1111/j.1462-5822.2011.01634.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. M. Y., Ng C. F., Liu Z. M., Ho W. M., Lee M. K., Wang F., et al. (2017). Reduced Prostate Cancer Risk with Green Tea and Epigallocatechin 3-gallate Intake Among Hong Kong Chinese Men. Prostate Cancer Prostatic Dis. 20, 318–322. 10.1038/pcan.2017.18 [DOI] [PubMed] [Google Scholar]
- Lee S. C., Chan W. K., Lee T. W., Lam W. H., Wang X., Chan T. H., et al. (2008). Effect of a Prodrug of the Green Tea Polyphenol (-)-epigallocatechin-3- Gallate on the Growth of Androgen-independent Prostate Cancer In Vivo. Nutr. Cancer 60, 483–491. 10.1080/01635580801947674 [DOI] [PubMed] [Google Scholar]
- Levine B., Kroemer G. (2008). Autophagy in the Pathogenesis of Disease. Cell 132, 27–42. 10.1016/j.cell.2007.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Yuan J. (2005). Autophagy in Cell Death: an Innocent Convict? J. Clin. Invest 115, 2679–2688. 10.1172/JCI26390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. M. M., Towers C. G., Thorburn A. (2017). Targeting Autophagy in Cancer. Nat. Rev. Cancer 17, 528–542. 10.1038/nrc.2017.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. Y., Guan Y. D., Chen X. S., Yang J. M., Cheng Y. (2020). DNA Repair Pathways in Cancer Therapy and Resistance. Front. Pharmacol. 11, 629266. 10.3389/fphar.2020.629266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Tse L. A., Chan W. C., Kwok C. H., Leung S. L., Wu C., et al. (2016a). Evaluation of Breast Cancer Risk Associated with Tea Consumption by Menopausal and Estrogen Receptor Status Among Chinese Women in Hong Kong. Cancer Epidemiol. 40, 73–78. 10.1016/j.canep.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Wang J., Jing J., Hua H., Luo T., Xu L., et al. (2009). Synergistic Promotion of Breast Cancer Cells Death by Targeting Molecular Chaperone GRP78 and Heat Shock Protein 70. J. Cell. Mol. Med. 13, 4540–4550. 10.1111/j.1582-4934.2008.00575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhao X., Yong H., Shang B., Lou W., Wang Y., et al. (2021). FBXO22 Promotes Growth and Metastasis and Inhibits Autophagy in Epithelial Ovarian Cancers via the MAPK/ERK Pathway. Front. Pharmacol. 12, 778698. 10.3389/fphar.2021.778698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., He N., Tian L., Shi X., Yang X. (2016b). Inhibitory Effects of Polyphenol-Enriched Extract from Ziyang Tea against Human Breast Cancer MCF-7 Cells through Reactive Oxygen Species-dependent Mitochondria Molecular Mechanism. J. Food Drug Analysis 24, 527–538. 10.1016/j.jfda.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Yang J., Luo L., Jiang M., Qin B., Yin H., et al. (2019). Targeting Photodynamic and Photothermal Therapy to the Endoplasmic Reticulum Enhances Immunogenic Cancer Cell Death. Nat. Commun. 10, 3349. 10.1038/s41467-019-11269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhu S., Li J., Assa A., Jundoria A., Xu J., et al. (2011). EGCG Stimulates Autophagy and Reduces Cytoplasmic HMGB1 Levels in Endotoxin-Stimulated Macrophages. Biochem. Pharmacol. 81, 1152–1163. 10.1016/j.bcp.2011.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Buckhaults P., Cui X., Tollefsbol T. O. (2016c). Combinatorial Epigenetic Mechanisms and Efficacy of Early Breast Cancer Inhibition by Nutritive Botanicals. Epigenomics 8, 1019–1037. 10.2217/epi-2016-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Meeran S. M., Tollefsbol T. O. (2017). Combinatorial Bioactive Botanicals Re-sensitize Tamoxifen Treatment in ER-Negative Breast Cancer via Epigenetic Reactivation of ERα Expression. Sci. Rep. 7, 1. 10.1038/s41598-017-09764-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Yuan Y. Y., Meeran S. M., Tollefsbol T. O. (2010). Synergistic Epigenetic Reactivation of Estrogen Receptor-α (ERα) by Combined Green Tea Polyphenol and Histone Deacetylase Inhibitor in ERα-Negative Breast Cancer Cells. Mol. Cancer 9, 1. 10.1186/1476-4598-9-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Ha J., Zou T., Gu L. (2014). Fabrication of Coated Bovine Serum Albumin (BSA)-epigallocatechin Gallate (EGCG) Nanoparticles and Their Transport across Monolayers of Human Intestinal Epithelial Caco-2 Cells. Food Funct. 5, 1278–1285. 10.1039/c3fo60500k [DOI] [PubMed] [Google Scholar]
- Liang X. H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., et al. (1999). Induction of Autophagy and Inhibition of Tumorigenesis by Beclin 1. Nature 402, 672–676. 10.1038/45257 [DOI] [PubMed] [Google Scholar]
- Lim J., Murthy A. (2020). Targeting Autophagy to Treat Cancer: Challenges and Opportunities. Front. Pharmacol. 11, 590344. 10.3389/fphar.2020.590344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim V., Zhu H., Diao S., Hu L., Hu J. (2019). PKP3 Interactions with MAPK-JNK-Erk1/2-mTOR Pathway Regulates Autophagy and Invasion in Ovarian Cancer. Biochem. Biophys. Res. Commun. 508, 646–653. 10.1016/j.bbrc.2018.11.163 [DOI] [PubMed] [Google Scholar]
- Lin Q., Chen H., Zhang M., Xiong H., Jiang Q. (2019). Knocking Down FAM83B Inhibits Endometrial Cancer Cell Proliferation and Metastasis by Silencing the PI3K/AKT/mTOR Pathway. Biomed. Pharmacother. 115, 108939. 10.1016/j.biopha.2019.108939 [DOI] [PubMed] [Google Scholar]
- Liu F., Chang L., Hu J. (2020). Activating Transcription Factor 6 Regulated Cell Growth, Migration and Inhibiteds Cell Apoptosis and Autophagy via MAPK Pathway in Cervical Cancer. J. Reprod. Immunol. 139, 103120. 10.1016/j.jri.2020.103120 [DOI] [PubMed] [Google Scholar]
- Liu S. M., Ou S. Y., Huang H. H. (2017). Green Tea Polyphenols Induce Cell Death in Breast Cancer MCF-7 Cells through Induction of Cell Cycle Arrest and Mitochondrial-Mediated Apoptosis. J. Zhejiang Univ. Sci. B 18, 89–98. 10.1631/jzus.B1600022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Wang X. J., Liu Y., Cui Y. F. (2013). PI3K/AKT/mTOR Signaling Is Involved in (-)-Epigallocatechin-3-Gallate-Induced Apoptosis of Human Pancreatic Carcinoma Cells. Am. J. Chin. Med. 41, 629–642. 10.1142/S0192415X13500444 [DOI] [PubMed] [Google Scholar]
- Liu S., Xu Z. L., Sun L., Liu Y., Li C. C., Li H. M., et al. (2016). Epigallocatechin3gallate Induces Apoptosis in Human Pancreatic Cancer Cells via PTEN. Mol. Med. Rep. 14, 599–605. 10.3892/mmr.2016.5277 [DOI] [PubMed] [Google Scholar]
- Lu C., Li X., Ren Y., Zhang X. (2021a). Disulfiram: a Novel Repurposed Drug for Cancer Therapy. Cancer Chemother. Pharmacol. 87, 159–172. 10.1007/s00280-020-04216-8 [DOI] [PubMed] [Google Scholar]
- Lu G., Wu Z., Shang J., Xie Z., Chen C., Zhang C. (2021b). The Effects of Metformin on Autophagy. Biomed. Pharmacother. 137, 111286. 10.1016/j.biopha.2021.111286 [DOI] [PubMed] [Google Scholar]
- Lu K. H., Broaddus R. R. (2020). Endometrial Cancer. N. Engl. J. Med. 383, 2053–2064. 10.1056/NEJMra1514010 [DOI] [PubMed] [Google Scholar]
- Luo T., Wang J., Yin Y., Hua H., Jing J., Sun X., et al. (2010). (-)-Epigallocatechin Gallate Sensitizes Breast Cancer Cells to Paclitaxel in a Murine Model of Breast Carcinoma. Breast Cancer Res. 12, R8. 10.1186/bcr2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda-Yamamoto M., Suzuki N., Sawai Y., Miyase T., Sano M., Hashimoto-Ohta A., et al. (2003). Association of Suppression of Extracellular Signal-Regulated Kinase Phosphorylation by Epigallocatechin Gallate with the Reduction of Matrix Metalloproteinase Activities in Human Fibrosarcoma HT1080 Cells. J. Agric. Food Chem. 51, 1858–1863. 10.1021/jf021039l [DOI] [PubMed] [Google Scholar]
- Maiti T. K., Chatterjee J., Dasgupta S. (2003). Effect of Green Tea Polyphenols on Angiogenesis Induced by an Angiogenin-like Protein. Biochem. Biophys. Res. Commun. 308, 64–67. 10.1016/s0006-291x(03)01338-x [DOI] [PubMed] [Google Scholar]
- Man G. C. W., Wang J., Song Y., Wong J. H., Zhao Y., Lau T. S., et al. (2020). Therapeutic Potential of a Novel Prodrug of Green Tea Extract in Induction of Apoptosis via ERK/JNK and Akt Signaling Pathway in Human Endometrial Cancer. BMC Cancer 20, 964. 10.1186/s12885-020-07455-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R., Karp C. M., Beaudoin B., Vuong N., Chen G., Chen H. Y., et al. (2009). Autophagy Suppresses Tumorigenesis through Elimination of P62. Cell 137, 1062–1075. 10.1016/j.cell.2009.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycotte P., Thorburn A. (2014). Targeting Autophagy in Breast Cancer. World J. Clin. Oncol. 5, 224–240. 10.5306/wjco.v5.i3.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder M. E. H., Beale P., Chan C., Yu J. Q., Huq F. (2012). Epigallocatechin Gallate Acts Synergistically in Combination with Cisplatin and Designed Trans -palladiums in Ovarian Cancer Cells. Anticancer Res. 32, 4851–4860. [PubMed] [Google Scholar]
- Mbuthia K. S., Mireji P. O., Ngure R. M., Stomeo F., Kyallo M., Muoki C., et al. (2017). Tea (Camellia Sinensis) Infusions Ameliorate Cancer in 4TI Metastatic Breast Cancer Model. BMC Complementary Altern. Med. 17, 202. 10.1186/s12906-017-1683-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccarthy S., Caporali A., Enkemann S., Scaltriti M., Eschrich S., Davalli P., et al. (2007). Green Tea Catechins Suppress the DNA Synthesis Marker MCM7 in the TRAMP Model of Prostate Cancer. Mol. Oncol. 1, 196–204. 10.1016/j.molonc.2007.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonnell A. M., Pyles H. M., Diaz-Cruz E. S., Barton C. E. (2019). Enoxacin and Epigallocatechin Gallate (EGCG) Act Synergistically to Inhibit the Growth of Cervical Cancer Cells in Culture. Molecules 24, 1580. 10.3390/molecules24081580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclarty J., Bigelow R. L. H., Smith M., Elmajian D., Ankem M., Cardelli J. A. (2009). Tea Polyphenols Decrease Serum Levels of Prostate-specific Antigen, Hepatocyte Growth Factor, and Vascular Endothelial Growth Factor in Prostate Cancer Patients and Inhibit Production of Hepatocyte Growth Factor and Vascular Endothelial Growth Factor in V. Cancer Prev. Res. 2, 673–682. 10.1158/1940-6207.CAPR-08-0167 [DOI] [PubMed] [Google Scholar]
- Mineva N. D., Paulson K. E., Naber S. P., Yee A. S., Sonenshein G. E. (2013). Epigallocatechin-3-gallate Inhibits Stem-like Inflammatory Breast Cancer Cells. PloS one 8, e73464. 10.1371/journal.pone.0073464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Levine B. (2020). Autophagy in Human Diseases. N. Engl. J. Med. 383, 1564–1576. 10.1056/NEJMra2022774 [DOI] [PubMed] [Google Scholar]
- Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008). Autophagy Fights Disease through Cellular Self-Digestion. Nature 451, 1069–1075. 10.1038/nature06639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Yoshimori T. (2007). How to Interpret LC3 Immunoblotting. Autophagy 3, 542–545. 10.4161/auto.4600 [DOI] [PubMed] [Google Scholar]
- Modernelli A., Naponelli V., Giovanna Troglio M., Bonacini M., Ramazzina I., Bettuzzi S., et al. (2015). EGCG Antagonizes Bortezomib Cytotoxicity in Prostate Cancer Cells by an Autophagic Mechanism. Sci. Rep. 5, 15270. 10.1038/srep15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradzadeh M., Hosseini A., Erfanian S., Rezaei H. (2017). Epigallocatechin-3-gallate Promotes Apoptosis in Human Breast Cancer T47D Cells through Down-Regulation of PI3K/AKT and Telomerase. Pharmacol. Rep. 69, 924–928. 10.1016/j.pharep.2017.04.008 [DOI] [PubMed] [Google Scholar]
- Morice P., Leary A., Creutzberg C., Abu-Rustum N., Darai E. (2016). Endometrial Cancer. Lancet 387, 1094–1108. 10.1016/S0140-6736(15)00130-0 [DOI] [PubMed] [Google Scholar]
- Mowers E. E., Sharifi M. N., Macleod K. F. (2017). Autophagy in Cancer Metastasis. Oncogene 36, 1619–1630. 10.1038/onc.2016.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Siddiqui M. A., Dayal S., Ayoub Y. Z., Malathi K. (2014). Epigallocatechin-3-gallate Suppresses Proinflammatory Cytokines and Chemokines Induced by Toll-like Receptor 9 Agonists in Prostate Cancer Cells. J. Inflamm. Res. 7, 89–101. 10.2147/JIR.S61365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusami S., Prabakaran D. S., An Z., Yu J. R., Park W. Y. (2013). EGCG Suppresses Fused Toes Homolog Protein through P53 in Cervical Cancer Cells. Mol. Biol. Rep. 40, 5587–5596. 10.1007/s11033-013-2660-x [DOI] [PubMed] [Google Scholar]
- Nair S., Barve A., Khor T. O., Shen G. X., Lin W., Chan J. Y., et al. (2010). Regulation of Nrf2-And AP-1-Mediated Gene Expression by Epigallocatechin-3-Gallate and Sulforaphane in Prostate of Nrf2-Knockout or C57BL/6J Mice and PC-3 AP-1 Human Prostate Cancer Cells. Acta Pharmacol. Sin. 31, 1223–1240. 10.1038/aps.2010.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakachi K., Suemasu K., Suga K., Takeo T., Imai K., Higashi Y. (1998). Influence of Drinking Green Tea on Breast Cancer Malignancy Among Japanese Patients. Jpn. J. Cancer Res. 89, 254–261. 10.1111/j.1349-7006.1998.tb00556.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakkas H., Ocal B. G., Kipel S., Akcan G., Sahin C., Ardicoglu A., et al. (2021). Ubiquitin Proteasome System and Autophagy Associated Proteins in Human Testicular Tumors. Tissue Cell 71, 101513. 10.1016/j.tice.2021.101513 [DOI] [PubMed] [Google Scholar]
- Naponelli V., Modernelli A., Bettuzzi S., Rizzi F. (2015). Roles of Autophagy Induced by Natural Compounds in Prostate Cancer. Biomed. Res. Int. 2015, 121826. 10.1155/2015/121826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld T. P. (2012). Autophagy and Cell Growth-Tthe Yin and Yang of Nutrient Responses. J. Cell Sci. 125, 2359–2368. 10.1242/jcs.103333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. M., Ahmann F. R., Nagle R. B., Hsu C. H., Tangrea J. A., Parnes H. L., et al. (2012). Randomized, Double-Blind, Placebo-Controlled Trial of Polyphenon E in Prostate Cancer Patients before Prostatectomy: Evaluation of Potential Chemopreventive Activities. Cancer Prev. Res. (Phila) 5, 290–298. 10.1158/1940-6207.CAPR-11-0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M., Hirata N., Tanaka T., Suizu F., Nakajima H., Chiorini J. A. (2020). Autophagy as a Modulator of Cell Death Machinery. Cell Death Dis. 11, 517. 10.1038/s41419-020-2724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Olvera S. I., Gallardo-Rincon D., Puente-Rivera J., Salinas-Vera Y. M., Marchat L. A., Morales-Villegas R., et al. (2019). Autophagy Machinery as a Promising Therapeutic Target in Endometrial Cancer. Front. Oncol. 9, 1326. 10.3389/fonc.2019.01326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opuwari C., Monsees T. (2020). Green Tea Consumption Increases Sperm Concentration and Viability in Male Rats and Is Safe for Reproductive, Liver and Kidney Health. Sci. Rep. 10, 15269. 10.1038/s41598-020-72319-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozpolat B., Benbrook D. M. (2015). Targeting Autophagy in Cancer Management - Strategies and Developments. Cancer Manag. Res. 7, 291–299. 10.2147/CMAR.S34859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D., Sur S., Roy R., Mandal S., Kumar Panda C. (2018). Epigallocatechin Gallate in Combination with Eugenol or Amarogentin Shows Synergistic Chemotherapeutic Potential in Cervical Cancer Cell Line. J. Cell. Physiology 234, 825–836. 10.1002/jcp.26900 [DOI] [PubMed] [Google Scholar]
- Pan M. H., Lin C. C., Lin J. K., Chen W. J. (2007). Tea Polyphenol (-)-epigallocatechin 3-gallate Suppresses Heregulin-Β1-Induced Fatty Acid Synthase Expression in Human Breast Cancer Cells by Inhibiting Phosphatidylinositol 3-kinase/Akt and Mitogen-Activated Protein Kinase Cascade Signaling. J. Agric. Food Chem. 55, 5030–5037. 10.1021/jf070316r [DOI] [PubMed] [Google Scholar]
- Panji M., Behmard V., Zare Z., Malekpour M., Nejadbiglari H., Yavari S., et al. (2021a). Synergistic Effects of Green Tea Extract and Paclitaxel in the Induction of Mitochondrial Apoptosis in Ovarian Cancer Cell Lines. Gene 787, 145638. 10.1016/j.gene.2021.145638 [DOI] [PubMed] [Google Scholar]
- Panji M., Behmard V., Zare Z., Malekpour M., Nejadbiglari H., Yavari S., et al. (2021b). Suppressing Effects of Green Tea Extract and Epigallocatechin-3-Gallate (EGCG) on TGF-β- Induced Epithelial-To-Mesenchymal Transition via ROS/Smad Signaling in Human Cervical Cancer Cells. Gene 794, 145774. 10.1016/j.gene.2021.145774 [DOI] [PubMed] [Google Scholar]
- Papandreou C. N., Logothetis C. J. (2004). Bortezomib as a Potential Treatment for Prostate Cancer. Cancer Res. 64, 5036–5043. 10.1158/0008-5472.CAN-03-2707 [DOI] [PubMed] [Google Scholar]
- Park H., Cho M., Do Y., Park J. K., Bae S. J., Joo J., et al. (2021). Autophagy as a Therapeutic Target of Natural Products Enhancing Embryo Implantation. Pharm. (Basel) 15, 53. 10.3390/ph15010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul P., Koh W. P., Jin A., Michel A., Waterboer T., Pawlita M., et al. (2019). Soy and Tea Intake on Cervical Cancer Risk: the Singapore Chinese Health Study. Cancer Causes Control 30, 847–857. 10.1007/s10552-019-01173-3 [DOI] [PubMed] [Google Scholar]
- Pianetti S., Guo S., Kavanagh K. T., Sonenshein G. E. (2002). Green Tea Polyphenol Epigallocatechin-3 Gallate Inhibits Her-2/neu Signaling, Proliferation, and Transformed Phenotype of Breast Cancer Cells. Cancer Res. 62, 652–655. [PubMed] [Google Scholar]
- Pilleron S., Soto-Perez-De-Celis E., Vignat J., Ferlay J., Soerjomataram I., Bray F., et al. (2021). Estimated Global Cancer Incidence in the Oldest Adults in 2018 and Projections to 2050. Int. J. Cancer 148, 601–608. 10.1002/ijc.33232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorvu P. D., Frazier A. L., Feraco A. M., Manley P. E., Ginsburg E. S., Laufer M. R., et al. (2019). Cancer Treatment-Related Infertility: A Critical Review of the Evidence. JNCI Cancer Spectr. 3, pkz008. 10.1093/jncics/pkz008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G., Li P., Xue Z. (2018). Triptolide Induces Protective Autophagy and Apoptosis in Human Cervical Cancer Cells by Downregulating Akt/mTOR Activation. Oncol. Lett. 16, 3929–3934. 10.3892/ol.2018.9074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Fu M., Wang J., Huang F., Liu H., Huangfu M., et al. (2020). PTEN/AKT/mTOR Signaling Mediates Anticancer Effects of Epigallocatechin-3-Gallate in Ovarian Cancer. Oncol. Rep. 43, 1885–1896. 10.3892/or.2020.7571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Yu J., Bhagat G., Furuya N., Hibshoosh H., Troxel A., et al. (2003). Promotion of Tumorigenesis by Heterozygous Disruption of the Beclin 1 Autophagy Gene. J. Clin. Invest 112, 1809–1820. 10.1172/JCI20039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. A., Saha S. K., Rahman M. S., Uddin M. J., Uddin M. S., Pang M. G., et al. (2020). Molecular Insights into Therapeutic Potential of Autophagy Modulation by Natural Products for Cancer Stem Cells. Front. Cell Dev. Biol. 8, 283. 10.3389/fcell.2020.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S. U., Huang Y., Zhu L., Feng S., Khan I. M., Wu J., et al. (2018). Therapeutic Role of Green Tea Polyphenols in Improving Fertility: A Review. Nutrients 10, 834. 10.3390/nu10070834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram Kumar R. M., Schor N. F. (2018). Methylation of DNA and Chromatin as a Mechanism of Oncogenesis and Therapeutic Target in Neuroblastoma. Oncotarget 9, 22184–22193. 10.18632/oncotarget.25084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S. D., Pagidas K. (2010). Epigallocatechin-3-gallate, a Natural Polyphenol, Inhibits Cell Proliferation and Induces Apoptosis in Human Ovarian Cancer Cells. Anticancer Res. 30, 2519–2523. [PubMed] [Google Scholar]
- Raufi A. G., Liguori N. R., Carlsen L., Parker C., Hernandez Borrero L., Zhang S., et al. (2021). Therapeutic Targeting of Autophagy in Pancreatic Ductal Adenocarcinoma. Front. Pharmacol. 12, 751568. 10.3389/fphar.2021.751568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawla P. (2019). Epidemiology of Prostate Cancer. World J. Oncol. 10, 63–89. 10.14740/wjon1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D., Kumavath R., Tan T. Z., Ampasala D. R., Kumar A. P. (2020). Peruvoside Targets Apoptosis and Autophagy through MAPK Wnt/beta-Catenin and PI3K/AKT/mTOR Signaling Pathways in Human Cancers. Life Sci. 241, 117147. 10.1016/j.lfs.2019.117147 [DOI] [PubMed] [Google Scholar]
- Reuter V. E. (2005). Origins and Molecular Biology of Testicular Germ Cell Tumors. Mod. Pathol. 18 (Suppl. 2), S51–S60. 10.1038/modpathol.3800309 [DOI] [PubMed] [Google Scholar]
- Rizzi F., Naponelli V., Silva A., Modernelli A., Ramazzina I., Bonacini M., et al. (2014). Polyphenon E(R), a Standardized Green Tea Extract, Induces Endoplasmic Reticulum Stress, Leading to Death of Immortalized PNT1a Cells by Anoikis and Tumorigenic PC3 by Necroptosis. Carcinogenesis 35, 828–839. 10.1093/carcin/bgt481 [DOI] [PubMed] [Google Scholar]
- Roomi M. W., Cha J., Kalinovsky T., Roomi N., Niedzwiecki A., Rath M. (2015a). Effect of a Nutrient Mixture on the Localization of Extracellular Matrix Proteins in HeLa Human Cervical Cancer Xenografts in Female Nude Mice. Exp. Ther. Med. 10, 901–906. 10.3892/etm.2015.2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomi M. W., Ivanov V., Kalinovsky T., Niedzwiecki A., Rath M. (2005a). In Vitro and In Vivo Antitumorigenic Activity of a Mixture of Lysine, Proline, Ascorbic Acid, and Green Tea Extract on Human Breast Cancer Lines MDA-MB-231 and MCF-7. Med. Oncol. 22, 129–138. 10.1385/MO:22:2:129 [DOI] [PubMed] [Google Scholar]
- Roomi M. W., Ivanov V., Kalinovsky T., Niedzwiecki A., Rath M. (2005b). In Vivo antitumor Effect of Ascorbic Acid, Lysine, Proline and Green Tea Extract on Human Prostate Cancer PC-3 Xenografts in Nude Mice: Evaluation of Tumor Growth and Immunohistochemistry. Vivo 19, 179–184. [PubMed] [Google Scholar]
- Roomi M. W., Ivanov V., Kalinovsky T., Niedzwiecki A., Rath M. (2007). Inhibitory Effects of a Nutrient Mixture on Human Testicular Cancer Cell Line NT 2/DT Matrigel Invasion and MMP Activity. Med. Oncol. 24, 183–188. 10.1007/BF02698038 [DOI] [PubMed] [Google Scholar]
- Roomi M. W., Kalinovsky T., Cha J., Roomi N. W., Niedzwiecki A., Rath M. (2015b). Effects of a Nutrient Mixture on Immunohistochemical Localization of Cancer Markers in Human Cervical Cancer Hela Cell Tumor Xenografts in Female Nude Mice. Exp. Ther. Med. 9, 294–302. 10.3892/etm.2014.2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomi M. W., Kalinovsky T., Roomi N. M., Cha J., Rath M., Niedzwiecki A. (2014). In Vitro and In Vivo Effects of a Nutrient Mixture on Breast Cancer Progression. Int. J. Oncol. 45, 1933–1944. 10.3892/ijo.2014.2379 [DOI] [PubMed] [Google Scholar]
- Roth Z., Aroyo A., Yavin S., Arav A. (2008). The Antioxidant Epigallocatechin Gallate (EGCG) Moderates the Deleterious Effects of Maternal Hyperthermia on Follicle-Enclosed Oocytes in Mice. Theriogenology 70, 887–897. 10.1016/j.theriogenology.2008.05.053 [DOI] [PubMed] [Google Scholar]
- Roychoudhury S., Agarwal A., Virk G., Cho C. L. (2017). Potential Role of Green Tea Catechins in the Management of Oxidative Stress-Associated Infertility. Reprod. Biomed. Online 34, 487–498. 10.1016/j.rbmo.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Saedmocheshi S., Saghebjoo M., Vahabzadeh Z., Sheikholeslami-Vatani D. (2019). Aerobic Training and Green Tea Extract Protect against N-Methyl-N-Nitrosourea-Induced Prostate Cancer. Med. Sci. Sports Exerc 51, 2210–2216. 10.1249/MSS.0000000000002054 [DOI] [PubMed] [Google Scholar]
- Sah J. F., Balasubramanian S., Eckert R. L., Rorke E. A. (2004). Epigallocatechin-3-gallate Inhibits Epidermal Growth Factor Receptor Signaling Pathway. Evidence for Direct Inhibition of ERK1/2 and AKT Kinases. J. Biol. Chem. 279, 12755–12762. 10.1074/jbc.M312333200 [DOI] [PubMed] [Google Scholar]
- Saleem M., Adhami V. M., Ahmad N., Gupta S., Mukhtar H. (2005). Prognostic Significance of Metastasis-Associated Protein S100A4 (Mts1) in Prostate Cancer Progression and Chemoprevention Regimens in an Autochthonous Mouse Model. Clin. Cancer Res. 11, 147–153. [PubMed] [Google Scholar]
- Salminen A., Hyttinen J. M., Kauppinen A., Kaarniranta K. (2012). Context-Dependent Regulation of Autophagy by IKK-NF-kappaB Signaling: Impact on the Aging Process. Int. J. Cell Biol. 2012, 849541. 10.1155/2012/849541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavat H., Ursin G., Emory T. H., Lee E., Wang R., Torkelson C. J., et al. (2017). A Randomized Controlled Trial of Green Tea Extract Supplementation and Mammographic Density in Postmenopausal Women at Increased Risk of Breast Cancer. Cancer Prev. Res. 10, 710–718. 10.1158/1940-6207.CAPR-17-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavat H., Wu A. H., Ursin G., Torkelson C. J., Wang R., Yu M. C., et al. (2019). Green Tea Catechin Extract Supplementation Does Not Influence Circulating Sex Hormones and Insulin-like Growth Factor axis Proteins in a Randomized Controlled Trial of Postmenopausal Women at High Risk of Breast Cancer. J. Nutr. 149, 619–627. 10.1093/jn/nxy316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R. A., Andrade E. D. S., Monteiro M., Fialho E., Silva J. L., Daleprane J. B., et al. (2021). Green Tea (Camellia Sinensis) Extract Induces P53-Mediated Cytotoxicity and Inhibits Migration of Breast Cancer Cells. Foods 10, 3154. 10.3390/foods10123154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartippour M. R., Heber D., Ma J., Lu Q., Go V. L., Nguyen M. (2001). Green Tea and its Catechins Inhibit Breast Cancer Xenografts. Nutr. Cancer 40, 149–156. 10.1207/S15327914NC402_11 [DOI] [PubMed] [Google Scholar]
- Sartippour M. R., Pietras R., Marquez-Garban D. C., Chen H. W., Heber D., Henning S. M., et al. (2006). The Combination of Green Tea and Tamoxifen Is Effective against Breast Cancer. Carcinogenesis 27, 2424–2433. 10.1093/carcin/bgl066 [DOI] [PubMed] [Google Scholar]
- Sartippour M. R., Shao Z. M., Heber D., Beatty P., Zhang L., Liu C., et al. (2002). Green Tea Inhibits Vascular Endothelial Growth Factor (VEGF) Induction in Human Breast Cancer Cells. J. Nutr. 132, 2307–2311. 10.1093/jn/132.8.2307 [DOI] [PubMed] [Google Scholar]
- Sawada N. (2017). Risk and Preventive Factors for Prostate Cancer in Japan: The Japan Public Health Center-based Prospective (JPHC) Study. J. Epidemiol. 27, 2–7. 10.1016/j.je.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaltriti M., Belloni L., Caporali A., Davalli P., Remondini D., Rizzi F., et al. (2006). Molecular Classification of Green Tea Catechin-Sensitive and Green Tea Catechin-Resistant Prostate Cancer in the TRAMP Mice Model by Quantitative Real-Time PCR Gene Profiling. Carcinogenesis 27, 1047–1053. 10.1093/carcin/bgi287 [DOI] [PubMed] [Google Scholar]
- Schramm L. (2013). Going Green: The Role of the Green Tea Component EGCG in Chemoprevention. J. Carcinog. Mutagen 4, 1000142. 10.4172/2157-2518.1000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L. (2010). HIF-1: Upstream and Downstream of Cancer Metabolism. Curr. Opin. Genet. Dev. 20, 51–56. 10.1016/j.gde.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L., Gao G., Wang W., Tang W., Wang Z., Yang Z., et al. (2019). Self-assembled Green Tea Polyphenol-Based Coordination Nanomaterials to Improve Chemotherapy Efficacy by Inhibition of Carbonyl Reductase 1. Biomaterials 210, 62–69. 10.1016/j.biomaterials.2019.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Deguchi A., Lim J. T., Moriwaki H., Kopelovich L., Weinstein I. B. (2005). (-)-Epigallocatechin Gallate and Polyphenon E Inhibit Growth and Activation of the Epidermal Growth Factor Receptor and Human Epidermal Growth Factor Receptor-2 Signaling Pathways in Human Colon Cancer Cells. Clin. Cancer Res. 11, 2735–2746. 10.1158/1078-0432.CCR-04-2014 [DOI] [PubMed] [Google Scholar]
- Shirakami Y., Shimizu M. (2018). Possible Mechanisms of Green Tea and its Constituents against Cancer. Molecules 23, 2284. 10.3390/molecules23092284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui I. A., Adhami V. M., Afaq F., Ahmad N., Mukhtar H. (2004). Modulation of Phosphatidylinositol-3-Kinase/protein Kinase B- and Mitogen-Activated Protein Kinase-Pathways by Tea Polyphenols in Human Prostate Cancer Cells. J. Cell. Biochem. 91, 232–242. 10.1002/jcb.10737 [DOI] [PubMed] [Google Scholar]
- Siddiqui I. A., Asim M., Hafeez B. B., Adhami V. M., Tarapore R. S., Mukhtar H. (2011). Green Tea Polyphenol EGCG Blunts Androgen Receptor Function in Prostate Cancer. FASEB J. 25, 1198–1207. 10.1096/fj.10-167924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui I. A., Shukla Y., Adhami V. M., Sarfaraz S., Asim M., Hafeez B. B., et al. (2008). Suppression of NFκB and its Regulated Gene Products by Oral Administration of Green Tea Polyphenols in an Autochthonous Mouse Prostate Cancer Model. Pharm. Res. 25, 2135–2142. 10.1007/s11095-008-9553-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Singh R., Bhui K., Tyagi S., Mahmood Z., Shukla Y. (2011). Tea Polyphenols Induce Apoptosis through Mitochondrial Pathway and by Inhibiting Nuclear Factor-Κb and Akt Activation in Human Cervical Cancer Cells. Oncol. Res. 19, 245–257. 10.3727/096504011x13021877989711 [DOI] [PubMed] [Google Scholar]
- Solomon V. R., Lee H. (2009). Chloroquine and its Analogs: a New Promise of an Old Drug for Effective and Safe Cancer Therapies. Eur. J. Pharmacol. 625, 220–233. 10.1016/j.ejphar.2009.06.063 [DOI] [PubMed] [Google Scholar]
- Spinaci M., Volpe S., De Ambrogi M., Tamanini C., Galeati G. (2008). Effects of Epigallocatechin-3-Gallate (EGCG) on In Vitro Maturation and Fertilization of Porcine Oocytes. Theriogenology 69, 877–885. 10.1016/j.theriogenology.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Stanislawska I. J., Granica S., Piwowarski J. P., Szawkalo J., Wiazecki K., Czarnocki Z., et al. (2019). The Activity of Urolithin A and M4 Valerolactone, Colonic Microbiota Metabolites of Polyphenols, in a Prostate Cancer In Vitro Model. Planta Med. 85, 118–125. 10.1055/a-0755-7715 [DOI] [PubMed] [Google Scholar]
- Su M., Mei Y., Sinha S. (2013). Role of the Crosstalk between Autophagy and Apoptosis in Cancer. J. Oncol. 2013, 102735. 10.1155/2013/102735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma M., Saha A., Fujiki H. (2011). New Cancer Treatment Strategy Using Combination of Green Tea Catechins and Anticancer Drugs. Cancer Sci. 102, 317–323. 10.1111/j.1349-7006.2010.01805.x [DOI] [PubMed] [Google Scholar]
- Sun Y., Zou M., Hu C., Qin Y., Song X., Lu N., et al. (2013). Wogonoside Induces Autophagy in MDA-MB-231 Cells by Regulating MAPK-mTOR Pathway. Food Chem. Toxicol. 51, 53–60. 10.1016/j.fct.2012.09.012 [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Takenokuchi M., Miyamoto K., Saigo K., Taniguchi T. (2015). Bortezomib Causes ER Stress-Related Death of Acute Promyelocytic Leukemia Cells through Excessive Accumulation of PML-RARA. Anticancer Res. 35, 3307–3316. [PubMed] [Google Scholar]
- Tam C., Rao S., Waye M. M. Y., Ng T. B., Wang C. C. (2021). Autophagy Signals Orchestrate Chemoresistance of Gynecological Cancers. Biochim. Biophys. Acta Rev. Cancer 1875, 188525. 10.1016/j.bbcan.2021.188525 [DOI] [PubMed] [Google Scholar]
- Tang D., Kang R., Livesey K. M., Cheh C. W., Farkas A., Loughran P., et al. (2010). Endogenous HMGB1 Regulates Autophagy. J. Cell Biol. 190, 881–892. 10.1083/jcb.200911078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert F., Verschoyle R. D., Greaves P., Jones D. J. L., Wilson I. D., Farmer P. B., et al. (2010). Plasma Metabolic Profiling Reveals Age-Dependency of Systemic Effects of Green Tea Polyphenols in Mice with and without Prostate Cancer. Mol. Biosyst. 6, 1911–1916. 10.1039/c004702c [DOI] [PubMed] [Google Scholar]
- Terzic M., Aimagambetova G., Kunz J., Bapayeva G., Aitbayeva B., Terzic S., et al. (2021). Molecular Basis of Endometriosis and Endometrial Cancer: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 22, 9274. 10.3390/ijms22179274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa U., Castelli G., Pelosi E. (2019). Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications. Med. (Basel) 6, 82. 10.3390/medicines6030082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangapazham R. L., Singh A. K., Sharma A., Warren J., Gaddipati J. P., Maheshwari R. K. (2007). Green Tea Polyphenols and its Constituent Epigallocatechin Gallate Inhibits Proliferation of Human Breast Cancer Cells In Vitro and In Vivo. Cancer Lett. 245, 232–241. 10.1016/j.canlet.2006.01.027 [DOI] [PubMed] [Google Scholar]
- Thomas R., Kim M. H. (2005). Epigallocatechin Gallate Inhibits HIF-1α Degradation in Prostate Cancer Cells. Biochem. Biophysical Res. Commun. 334, 543–548. 10.1016/j.bbrc.2005.06.114 [DOI] [PubMed] [Google Scholar]
- Tong D. (2021). Unravelling the Molecular Mechanisms of Prostate Cancer Evolution from Genotype to Phenotype. Crit. Rev. Oncol. Hematol. 163, 103370. 10.1016/j.critrevonc.2021.103370 [DOI] [PubMed] [Google Scholar]
- Torgovnick A., Schumacher B. (2015). DNA Repair Mechanisms in Cancer Development and Therapy. Front. Genet. 6, 157. 10.3389/fgene.2015.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trocoli A., Djavaheri-Mergny M. (2011). The Complex Interplay between Autophagy and NF-kappaB Signaling Pathways in Cancer Cells. Am. J. Cancer Res. 1, 629–649. [PMC free article] [PubMed] [Google Scholar]
- Trudel D., Labbé D. P., Araya-Farias M., Doyen A., Bazinet L., Duchesne T., et al. (2013). A Two-Stage, Single-Arm, Phase II Study of EGCG-Enriched Green Tea Drink as a Maintenance Therapy in Women with Advanced Stage Ovarian Cancer. Gynecol. Oncol. 131, 357–361. 10.1016/j.ygyno.2013.08.019 [DOI] [PubMed] [Google Scholar]
- Tsai C. Y., Chen C. Y., Chiou Y. H., Shyu H. W., Lin K. H., Chou M. C., et al. (2017). Epigallocatechin-3-Gallate Suppresses Human Herpesvirus 8 Replication and Induces ROS Leading to Apoptosis and Autophagy in Primary Effusion Lymphoma Cells. Int. J. Mol. Sci. 19, 16. 10.3390/ijms19010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuntiteerawit P., Jarukamjorn K., Porasuphatana S. (2020). The Effect of Green Tea Catechins on Breast Cancer Resistance Protein Activity and Intestinal Efflux of Aflatoxin B1 via Breast Cancer Resistance Protein in Caco-2 Cells. Toxicol. Res. 36, 293–300. 10.1007/s43188-019-00032-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyutyunyk-Massey L., Gewirtz D. A. (2020). Roles of Autophagy in Breast Cancer Treatment: Target, Bystander or Benefactor. Semin. Cancer Biol. 66, 155–162. 10.1016/j.semcancer.2019.11.008 [DOI] [PubMed] [Google Scholar]
- Vahabzadeh Z., Molodi M., Nikkho B., Saghebjoo M., Saedmocheshi S., Zamani F., et al. (2020). Aerobic Training and Hydroalcoholic Extracts of Green Tea Improve Pro-oxidant-antioxidant Balance and Histopathological Score in the N-Methyl-N-Nitrosourea-Induced Prostate Cancer Model of Rat. EXCLI J. 19, 762–772. 10.17179/excli2019-2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vequaud E., Seveno C., Loussouarn D., Engelhart L., Campone M., Juin P., et al. (2015). YM155 Potently Triggers Cell Death in Breast Cancer Cells through an Autophagy-NF-kB Network. Oncotarget 6, 13476–13486. 10.18632/oncotarget.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzella D., Pescatore A., Capece D., Vecchiotti D., Ursini M. V., Franzoso G., et al. (2020). Life, Death, and Autophagy in Cancer: NF-kappaB Turns up Everywhere. Cell Death Dis. 11, 210. 10.1038/s41419-020-2399-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers J. M., Jacobs M. V., Manos M. M., Bosch F. X., Kummer J. A., Shah K. V., et al. (1999). Human Papillomavirus Is a Necessary Cause of Invasive Cervical Cancer Worldwide. J. Pathol. 189, 12–19. [DOI] [PubMed] [Google Scholar]
- Wang H., Deng L., Cai M., Zhuang H., Zhu L., Hao Y., et al. (2017). Annexin A4 Fucosylation Enhances its Interaction with the NF-kB P50 and Promotes Tumor Progression of Ovarian Clear Cell Carcinoma. Oncotarget 8, 108093–108107. 10.18632/oncotarget.10226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Man G. C. W., Chan T. H., Kwong J., Wang C. C. (2018a). A Prodrug of Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (Pro-EGCG) Serves as a Novel Angiogenesis Inhibitor in Endometrial Cancer. Cancer Lett. 412, 10–20. 10.1016/j.canlet.2017.09.054 [DOI] [PubMed] [Google Scholar]
- Wang P., Heber D., Henning S. M. (2012). Quercetin Increased the Antiproliferative Activity of Green Tea Polyphenol (-)-epigallocatechin Gallate in Prostate Cancer Cells. Nutr. Cancer 64, 580–587. 10.1080/01635581.2012.661514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., He W. Y., Zeng Y. Z., Hossain A., Gou X. (2018b). Inhibiting Autophagy Overcomes Docetaxel Resistance in Castration-Resistant Prostate Cancer Cells. Int. Urol. Nephrol. 50, 675–686. 10.1007/s11255-018-1801-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wu N., Jiang N. (2021). Autophagy Provides a Conceptual Therapeutic Framework for Bone Metastasis from Prostate Cancer. Cell Death Dis. 12, 909. 10.1038/s41419-021-04181-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R., Mao L., Xu P., Zheng X., Hackman R. M., Mackenzie G. G., et al. (2018). Suppressing Glucose Metabolism with Epigallocatechin-3-Gallate (EGCG) Reduces Breast Cancer Cell Growth in Preclinical Models. Food Funct. 9, 5682–5696. 10.1039/c8fo01397g [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Dipaola R. S. (2009). The Double-Edged Sword of Autophagy Modulation in Cancer. Clin. Cancer Res. 15, 5308–5316. 10.1158/1078-0432.CCR-07-5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2021). Breast Cancer [Online]. Geneva: World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (Accessed 03, 2022). [Google Scholar]
- WHO (2022). Cervical Cancer [Online]. Geneva: World Health Organization. Available: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (Accessed 03, 2022). [Google Scholar]
- Xi B., Huang Y., Reilly K. H., Li S., Zheng R., Barrio-Lopez M. T., et al. (2015). Sugar-sweetened Beverages and Risk of Hypertension and CVD: a Dose-Response Meta-Analysis. Br. J. Nutr. 113, 709–717. 10.1017/S0007114514004383 [DOI] [PubMed] [Google Scholar]
- Xinqiang S., Mu Z., Lei C., Mun L. Y. (2017). Bioinformatics Analysis on Molecular Mechanism of Green Tea Compound Epigallocatechin-3-Gallate against Ovarian Cancer. Clin. Transl. Sci. 10, 302–307. 10.1111/cts.12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Zhang X., Li Y., Lu S., Lu S., Li J., et al. (2016). Neferine Induces Autophagy of Human Ovarian Cancer Cells via P38 MAPK/JNK Activation. Tumour Biol. 37, 8721–8729. 10.1007/s13277-015-4737-8 [DOI] [PubMed] [Google Scholar]
- Xu P., Yan F., Zhao Y., Chen X., Sun S., Wang Y., et al. (2020). Green Tea Polyphenol EGCG Attenuates MDSCs-Mediated Immunosuppression through Canonical and Non-canonical Pathways in a 4T1 Murine Breast Cancer Model. Nutrients 12, 1042. 10.3390/nu12041042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan F., Jian J. (2016). Epigallocatechin Gallate Exerts Protective Effects against Myocardial Ischemia/reperfusion Injury through the PI3K/Akt Pathway-Mediated Inhibition of Apoptosis and the Restoration of the Autophagic Flux. Int. J. Mol. Med. 38, 328–336. 10.3892/ijmm.2016.2615 [DOI] [PubMed] [Google Scholar]
- Yang K., Gao Z. Y., Li T. Q., Song W., Xiao W., Zheng J., et al. (2019). Anti-tumor Activity and the Mechanism of a Green Tea (Camellia Sinensis) Polysaccharide on Prostate Cancer. Int. J. Biol. Macromol. 122, 95–103. 10.1016/j.ijbiomac.2018.10.101 [DOI] [PubMed] [Google Scholar]
- Yang S., Shao S., Huang B., Yang D., Zeng L., Gan Y., et al. (2020). Tea Polyphenols Alleviate Tri-ortho-cresyl Phosphate-Induced Autophagy of Mouse Ovarian Granulosa Cells. Environ. Toxicol. 35, 478–486. 10.1002/tox.22883 [DOI] [PubMed] [Google Scholar]
- Yeung T. L., Leung C. S., Yip K. P., Au Yeung C. L., Wong S. T., Mok S. C. (2015). Cellular and Molecular Processes in Ovarian Cancer Metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am. J. Physiol. Cell Physiol. 309, C444–C456. 10.1152/ajpcell.00188.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. S., Kristal A. R., Wicklund K. G., Cushing-Haugen K. L., Rossing M. A. (2008). Coffee, Tea, Colas, and Risk of Epithelial Ovarian Cancer. Cancer Epidemiol. Biomarkers Prev. 17, 712–716. 10.1158/1055-9965.EPI-07-2511 [DOI] [PubMed] [Google Scholar]
- Yousefi S., Perozzo R., Schmid I., Ziemiecki A., Schaffner T., Scapozza L., et al. (2006). Calpain-mediated Cleavage of Atg5 Switches Autophagy to Apoptosis. Nat. Cell Biol. 8, 1124–1132. 10.1038/ncb1482 [DOI] [PubMed] [Google Scholar]
- Yuan C. H., Horng C. T., Lee C. F., Chiang N. N., Tsai F. J., Lu C. C., et al. (2017). Epigallocatechin Gallate Sensitizes Cisplatin-Resistant Oral Cancer CAR Cell Apoptosis and Autophagy through Stimulating AKT/STAT3 Pathway and Suppressing Multidrug Resistance 1 Signaling. Environ. Toxicol. 32, 845–855. 10.1002/tox.22284 [DOI] [PubMed] [Google Scholar]
- Yugawa T., Kiyono T. (2009). Molecular Mechanisms of Cervical Carcinogenesis by High-Risk Human Papillomaviruses: Novel Functions of E6 and E7 Oncoproteins. Rev. Med. Virol. 19, 97–113. 10.1002/rmv.605 [DOI] [PubMed] [Google Scholar]
- Zeng F., Harris R. C. (2014). Epidermal Growth Factor, from Gene Organization to Bedside. Semin. Cell Dev. Biol. 28, 2–11. 10.1016/j.semcdb.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Yan J., Luo L., Ma M., Zhu H. (2017). Preparation and Characterization of (-)-Epigallocatechin-3-Gallate (EGCG)-loaded Nanoparticles and Their Inhibitory Effects on Human Breast Cancer MCF-7 Cells. Sci. Rep. 7, 45521. 10.1038/srep45521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Qin Y. Y., Wei X., Yu F. F., Zhou Y. H., He J. (2015). Tea Consumption and Risk of Cardiovascular Outcomes and Total Mortality: a Systematic Review and Meta-Analysis of Prospective Observational Studies. Eur. J. Epidemiol. 30, 103–113. 10.1007/s10654-014-9960-x [DOI] [PubMed] [Google Scholar]
- Zhang M., Binns C. W., Lee A. H. (2002). Tea Consumption and Ovarian Cancer Risk: A Case-Control Study in China. Cancer Epidemiol. Biomarkers Prev. 11, 713–718. [PubMed] [Google Scholar]
- Zhang M., Lee A. H., Binns C. W., Xie X. (2004). Green Tea Consumption Enhances Survival of Epithelial Ovarian Cancer. Int. J. Cancer 112, 465–469. 10.1002/ijc.20456 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Tang X., Lu Q., Zhang Z., Rao J., Le A. D. (2006). Green Tea Extract and (-)-Epigallocatechin-3-Gallate Inhibit Hypoxia- and Serum-Induced HIF-1alpha Protein Accumulation and VEGF Expression in Human Cervical Carcinoma and Hepatoma Cells. Mol. Cancer Ther. 5, 1227–1238. 10.1158/1535-7163.MCT-05-0490 [DOI] [PubMed] [Google Scholar]
- Zhang Q. Y., Wang F. X., Jia K. K., Kong L. D. (2018a). Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 9, 1253. 10.3389/fphar.2018.01253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Cao M., Fang F. (2020a). The Role of Epigallocatechin-3-Gallate in Autophagy and Endoplasmic Reticulum Stress (ERS)-Induced Apoptosis of Human Diseases. Med. Sci. Monit. 26, e924558. 10.12659/MSM.924558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Han G., Fan B., Zhou Y., Zhou X., Wei L., et al. (2009). Green Tea (-)-Epigallocatechin-3-Gallate Down-Regulates VASP Expression and Inhibits Breast Cancer Cell Migration and Invasion by Attenuating Rac1 Activity. Eur. J. Pharmacol. 606, 172–179. 10.1016/j.ejphar.2008.12.033 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lin H., Liu C., Huang J., Liu Z. (2020b). A Review for Physiological Activities of EGCG and the Role in Improving Fertility in Humans/mammals. Biomed. Pharmacother. 127, 110186. 10.1016/j.biopha.2020.110186 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Sha R., Zhang L., Zhang W., Jin P., Xu W., et al. (2018b). Harnessing Copper-Palladium Alloy Tetrapod Nanoparticle-Induced Pro-survival Autophagy for Optimized Photothermal Therapy of Drug-Resistant Cancer. Nat. Commun. 9, 4236. 10.1038/s41467-018-06529-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yang N. D., Zhou F., Shen T., Duan T., Zhou J., et al. (2012). (-)-Epigallocatechin-3-gallate Induces Non-apoptotic Cell Death in Human Cancer Cells via ROS-Mediated Lysosomal Membrane Permeabilization. PLoS One 7, e46749. 10.1371/journal.pone.0046749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Liu S., Xu J., Li W., Duan G., Wang H., et al. (2017). A New Molecular Mechanism Underlying the EGCG-Mediated Autophagic Modulation of AFP in HepG2 Cells. Cell Death Dis. 8, e3160. 10.1038/cddis.2017.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Fang Y., Yang Y., Qin Y., Wu P., Wang T., et al. (2015). Elaiophylin, a Novel Autophagy Inhibitor, Exerts Antitumor Activity as a Single Agent in Ovarian Cancer Cells. Autophagy 11, 1849–1863. 10.1080/15548627.2015.1017185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M. C., Huang X. H., Wang Q., Sun K., Liu Y. J., Li W., et al. (2006). Green Tea Polyphenol Epigallocatechin-3-Gallate Suppresses Rat Hepatic Stellate Cell Invasion by Inhibition of MMP-2 Expression and its Activation. Acta Pharmacol. Sin. 27, 1600–1607. 10.1111/j.1745-7254.2006.00439.x [DOI] [PubMed] [Google Scholar]
- Zheng T., Wang A., Hu D., Wang Y. (2017). Molecular Mechanisms of Breast Cancer Metastasis by Gene Expression Profile Analysis. Mol. Med. Rep. 16, 4671–4677. 10.3892/mmr.2017.7157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. W., Li X. X., He Z. X., Pan S. T., Yang Y., Zhang X., et al. (2015). Induction of Apoptosis and Autophagy via Sirtuin1- and PI3K/Akt/mTOR-Mediated Pathways by Plumbagin in Human Prostate Cancer Cells. Drug Des. Devel Ther. 9, 1511–1554. 10.2147/DDDT.S75976 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhu K., Dunner K., Jr., Mcconkey D. J. (2010). Proteasome Inhibitors Activate Autophagy as a Cytoprotective Response in Human Prostate Cancer Cells. Oncogene 29, 451–462. 10.1038/onc.2009.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Yin Q., Wei D., Yang Z., Du Y., Ma Y. (2019). Autophagy in Male Reproduction. Syst. Biol. Reprod. Med. 65, 265–272. 10.1080/19396368.2019.1606361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemical structures of the four-major green tea catechins include (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), and (−)-epigallocatechin-3- gallate (EGCG).