Abstract
Purpose
The incidence of aspergillosis is increasing, and the risk factors for infection include cancer, admission to the intensive care unit, chronic pulmonary diseases, immunocompromised status, and taking immunomodulatory drugs. There are limited data about the incidence of aspergillosis in patients with different types of cancer. The aim of our study was to survey the incidence of aspergillosis in different cancer types from 2006 to 2017.
Patients and Methods
Data were collected from the Taiwan Cancer Registry database and International Classification of Diseases, 9th, 10th Revision, and Clinical Modification codes for diagnosing aspergillosis. Patients with a history of aspergillosis before cancer were excluded, and the secondary outcome was the risk of mortality in cancer patients with and without aspergillosis after 1 year.
Results
Among 951 cancer patients with a diagnosis of aspergillosis, there were 614 hematopoietic and reticuloendothelial system patients, 100 lung cancer patients, and 73 lymphoma cancer patients. The overall incidence rates of aspergillosis tended to increase significantly from 2006 to 2017 (from 3.50 to 13.37 per 10,000 person-years, p value: <0.0001). Regarding sex, the incidence rates of aspergillosis in males and females were 12.52 and 7.53 per 10,000 person-years, respectively. Patients with a diagnosis of aspergillosis had a 2.30-fold (95% CI: 2.14–2.48, p value: <0.0001) higher risk of mortality than those without aspergillosis.
Conclusion
The incidence of aspergillosis was increased in cancer patients, and cancer patients with aspergillosis had a significantly higher risk of mortality than those without aspergillosis.
Keywords: aspergillosis, cancer patients, Taiwan Cancer Registry, incidence rate
Introduction
Aspergillosis is an infectious disease caused by a fungus, Aspergillus, and may lead to a variety of diseases with different symptoms and signs depending on the patient’s immune status and pulmonary structure. Aspergillus spp. can be isolated from our outdoor and indoor environments, including soil and hospitals. Most strains of Aspergillus are harmless, but some can cause severe disease, particularly among immunocompromised patients.1 Invasive pulmonary aspergillosis commonly occurs in hematologic cancer but also in solid cancer. Invasive aspergillosis is commonly associated with hematologic malignancies and solid tumors2,3 and is also encountered in children with cancer.4 The risk of aspergillosis included patients with solid tumors, hematologic cancer, allogeneic hematopoietic stem cell transplantation, solid organ transplantation, and other causes of immunosuppression, including burns, alemtuzumab therapy, and intensive care units.5,6 Among patients with putative or proven invasive aspergillosis, 70% were in an immunocompromised state. Mortality associated with fungal disease was more than 1.5 million, and more than 150 million people had serious fungal diseases, which have a major impact in the world.7 There is an increasing incidence of invasive pulmonary aspergillosis in Taiwan for males from 0.87 to 4.55 per million person-years and females from 0.36 to 2.07 per million person-years between 2002 and 2011.8 Aspergillus can also live as a saprophyte in the human body. Saprophytic colonization of a parenchymal lung cavity by Aspergillus is referred to as aspergilloma. It is important for diagnosis to distinguish between disease and colonization. There are increasing numbers of reports of invasive aspergillosis in immunocompetent patients. The risk groups included patients with severe chronic obstructive pulmonary disease and critically ill patients. There are limited data about the incidence of aspergillosis in cancer patients, including those with solid and hematologic cancer. The aim of our study was to survey the incidence of aspergillosis in different cancer types from 2006 to 2017 using the Taiwan Cancer Registry database.
Materials and Methods
Data Source
In this study, data for malignancy diagnosis were collected from the Taiwan Cancer Registry database (TCR), which captures 97% of cancer cases in Taiwan. The TCR has collected the data of patients with newly diagnosed cancer in Taiwan since 1979, and the TCR central office uses standardized algorithms to validate the received data. The completeness was 98.4%, the proportion of cases with only a death certificate was 0.9%, and the interval between the date of cancer diagnosis and the date of reporting was 14 months in 2016.9,10 The National Health Insurance of Taiwan is a nationwide compulsory health care program and enrolled more than 99.6% of the population. The National Health Insurance contracts with more than 90% of Taiwan’s clinics and hospitals. The database comprises detailed information regarding diagnostic codes, date of diagnosis, payments for consultations, and prescription details. The National Health Insurance Research database (NHIR) provides an encrypted identification number for each patient as well as information on age, sex, admission records, and International Classification of Diseases, 9th Revision, and Clinical Modification (ICD-9-CM) codes for diagnoses and procedures before 2016, and the 10th Revision became effective after 2016. This deidentified, secondary database was released for public research by the Taiwan National Health Insurance Program. This study was granted exemption from full review, with waiver of patient consent in view of the anonymity of the data, by the Institutional Review Board of Chi Mei Medical Centre (IRB-10912-E02).
Study Participants and Outcome
The International Classification of Disease for Oncology, 3rd edition (ICD-O-3) was used to identify patients with new-onset cancer corresponding to the period of 2006 to 2017 in the TCR database. Details of the cancer type are shown in the Supplementary Table S1. All participants were classified into three categories according to age: <50, 50–70, and ≥70 years. Demographic details were also considered in this study. The primary outcome was the incidence rate of aspergillosis, which was defined by the ICD-9-CM code (117.3, 484.6) and ICD-10 codes (B44.0 to B44.2, B44.7, B44.89, and B44.9), including nonpulmonary aspergillosis and pulmonary aspergillosis,11 in the NHIR database 1 year after initial cancer diagnosis. The secondary outcome was defined by the cause of death database in NHIRD to estimate the mortality risk between cancer patients with aspergillosis and those without. Patients with a history of aspergillosis and ABPA, which was defined by the ICD-9 code (518.6) and ICD-10 code (B44.81), were excluded. We also excluded patients who had the same cancer diagnosis date and death date and an incomplete death date (just show year or month) to avoid incorrect follow-down periods. The potential confounder of lung disease, including tuberculosis (TB), chronic obstructive pulmonary disease (COPD), bronchiectasis, and interstitial lung disease (ILD), was defined as 1 year before aspergillosis diagnosed with an inpatient record one time or an outpatient record three times. The corresponding treatments of aspergillosis starting a year after diagnosis were extracted from the NHIR database (Table S2). Cancer treatments, including operation, radiotherapy, chemotherapy, immunotherapy, hormones, and supportive therapy, were classified under the TCR database. Finally, 1,096,276 participants were included in this study and divided into the aspergillosis group (N=961) and the nonaspergillosis group (N=1,095,315). The flow chart is shown in Figure S1.
Statistical Analysis
The demographic information of the patients included sex, age divided into 3 categories, and cancer type. The number of cases of aspergillosis, including nonpulmonary aspergillosis and pulmonary aspergillosis, and the incidence rate per 10,000 person-years at different patient baselines are presented. The linear trend of the overall and subtype of aspergillosis incidence rates was estimated to change by calendar year. The trend of the aspergillosis incidence rate between males and females was also considered. The Cox proportional hazards regression model was used to compare the risk of mortality between the aspergillosis group and the nonaspergillosis group. The subgroup analysis of mortality was also derived from the Cox proportional hazards regression model. Hazard ratios (HRs) and adjusted hazard ratios (aHRs) with 95% CIs were used to estimate the crude and adjusted risk of mortality between the two comparison groups. The type of aspergillosis, potential confounders of lung disease and treatment among death and nondeath cancer patients with aspergillosis were described as frequencies with percentages. The time information, including the interval from cancer diagnosis to death and the time from aspergillosis to death between patient death or not, is shown as the median and interquartile range. Those differences in cancer patients with or without aspergillosis death were compared by using Pearson’s chi-square test for categorical variables and Wilcoxon rank-sum test for continuous variables after checking with normality test. All analyses were conducted using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC, USA). The statistical significance was set at p value < 0.05.
Results
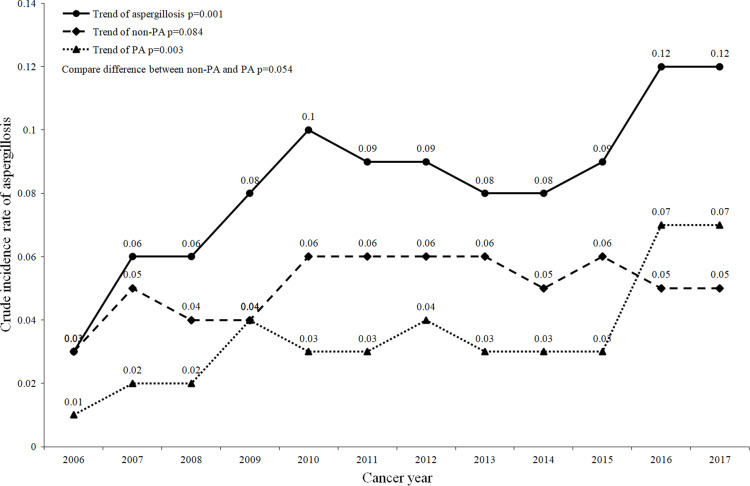
Among the 1,094,896 cancer patients included in this study, 52.39% were males, and the other 47.61% were females. Most cancer patients were older than 50 years old (aged 50–70: 44.61%; age ≥70: 32.71%). There were 951 patients who were diagnosed with aspergillosis within 1 year after cancer diagnosis. Regarding the type of aspergillosis among 951 patients with aspergillosis, 58.89% had nonpulmonary aspergillosis (non-PA), and 41.11% had pulmonary aspergillosis (PA). Overall, the average annual crude incidence rate of aspergillosis was 0.09 per 100 patients. The overall crude incidence rate of aspergillosis tended to increase significantly from 2006 to 2017 (from 0.03 to 0.12, p value: 0.001). By the type of aspergillosis, the overall crude incidence rate of PA also tended to increase significantly (p value: 0.003), but the overall crude incidence rate of non-PA did not (Figure 1).
Figure 1.
Crude incidence rate of aspergillosis in cancer patients from 2006 to 2017.
Abbreviations: PA, pulmonary aspergillosis; non-PA, nonpulmonary aspergillosis.
For sex, the average annual crude incidence rates of aspergillosis in males and females were 0.10 and 0.07 per 100 patients, respectively. An increasing trend in the crude incidence rate of aspergillosis was observed for both males and females from 2006 to 2017 [from 0.03 to 0.15 (p value: 0.001) and 0.03 to 0.09 (p value: 0.001)]. There was not a significant difference in the trend of crude incidence rate between males and females (p value: 0.241) (Figure S2). Table 1 shows that the crude incidence rates of aspergillosis, non-PA, and PA among males were higher than those among females.
Table 1.
The Incidence Rate of Aspergillosis Among Cancer Patients
| Number of Patients | Number of Aspergillosis(%) | Number of Non-PA(%) | Number of PA(%) | PY | Incidence Rate of Aspergillosis* | Incidence Rate of Non-PA * | Incidence Rate of PA * | |
|---|---|---|---|---|---|---|---|---|
| Overall | 1,094,896 | 951(0.09) | 560(0.05) | 391(0.04) | 947,927.64 | 10.03 | 5.91 | 4.12 |
| Cancer year | ||||||||
| 2006 | 74,653 | 25(0.03) | 20(0.03) | 5(0.01) | 71,409.51 | 3.50 | 2.80 | 0.70 |
| 2007 | 77,902 | 50(0.06) | 37(0.05) | 13(0.02) | 65,150.8 | 7.67 | 5.68 | 2.00 |
| 2008 | 79,172 | 44(0.06) | 29(0.04) | 15(0.02) | 66,583.36 | 6.61 | 4.36 | 2.25 |
| 2009 | 85,067 | 71(0.08) | 35(0.04) | 36(0.04) | 72,066.56 | 9.85 | 4.86 | 5.00 |
| 2010 | 88,511 | 86(0.10) | 57(0.06) | 29(0.03) | 75,427.85 | 11.40 | 7.56 | 3.84 |
| 2011 | 89,882 | 80(0.09) | 52(0.06) | 28(0.03) | 76,893.18 | 10.40 | 6.76 | 3.64 |
| 2012 | 93,229 | 88(0.09) | 53(0.06) | 35(0.04) | 80,358.01 | 10.95 | 6.60 | 4.36 |
| 2013 | 96,660 | 82(0.08) | 56(0.06) | 26(0.03) | 83,619.61 | 9.81 | 6.70 | 3.11 |
| 2014 | 99,739 | 82(0.08) | 54(0.05) | 28(0.03) | 86,535.58 | 9.48 | 6.24 | 3.24 |
| 2015 | 101,865 | 95(0.09) | 62(0.06) | 33(0.03) | 88,285.39 | 10.76 | 7.02 | 3.74 |
| 2016 | 102,299 | 124(0.12) | 51(0.05) | 73(0.07) | 88,883.42 | 13.95 | 5.74 | 8.21 |
| 2017 | 105,917 | 124(0.12) | 54(0.05) | 70(0.07) | 92,714.39 | 13.37 | 5.82 | 7.55 |
| Sex | ||||||||
| Male | 573,575 | 595(0.10) | 348(0.06) | 247(0.04) | 475,374.31 | 12.52 | 7.32 | 5.20 |
| Female | 521,321 | 356(0.07) | 212(0.04) | 144(0.03) | 472,553.33 | 7.53 | 4.49 | 3.05 |
| Age | ||||||||
| <50 | 248,348 | 335(0.13) | 222(0.09) | 111(0.04) | 234,335.73 | 14.3 | 9.47 | 4.74 |
| 50–70 | 488,430 | 419(0.09) | 233(0.05) | 186(0.04) | 438,218.98 | 9.56 | 5.32 | 4.24 |
| ≧70 | 358,118 | 197(0.06) | 105(0.03) | 92(0.03) | 275,372.93 | 7.15 | 3.81 | 3.34 |
Note: *Incidence of 10,000 person-years.
Abbreviations: PA, pulmonary aspergillosis; non-PA, nonpulmonary aspergillosis; PY, person-year.
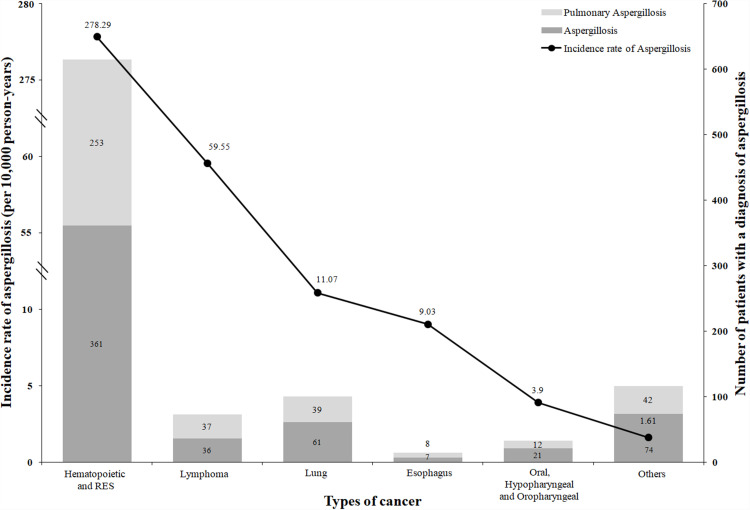
In addition, the crude incidence rates of aspergillosis, non-PA, and PA among patients aged <50 years were also higher than those aged ≥50 years. Among the 951 cancer patients with a diagnosis of aspergillosis, there were 614 hematopoietic and reticuloendothelial system patients, 100 lung cancer patients, and 73 lymphoma cancer patients. (Figure 2). For cancer type, hematopoietic and reticuloendothelial system cancers had the highest crude incidence of aspergillosis and non-PA. Lymphoma and lung cancer were the second-highest and third-highest, respectively. However, the third-highest crude incidence rate of PA was esophagus cancer, which was higher than that of lung cancer (Table S1).
Figure 2.
Incidence rate of aspergillosis and the number of patients with different cancer types.
Table 1 shows that the overall incidence rate of aspergillosis was 10.03 per 10,000 person-years and tended to increase significantly from 2006 to 2017 (from 3.50 to 13.37, p value: 0.001). The overall incidence rates of non-PA and PA were 5.91 and 4.12 per 10,000 person-years, respectively. In addition, the overall incidence rates of PA tended to increase significantly from 2006 to 2017 (from 0.70 to 7.55, p value: 0.003). The overall incidence rate of non-PA increased but did not reach statistical significance from 2006 to 2017 (p value: 0.069). Regarding sex, the incidence rates of aspergillosis in males and females were 12.52 and 7.53 per 10,000 person-years, respectively. For age groups, the incidence rate of aspergillosis among patients at age <50 was 14.30, 50–70 was 9.56, and ≥70 was 7.15 per 10,000 person-years. For cancer type, the incidence rate of aspergillosis was 278.29 per 10,000 person-years in hematopoietic and reticuloendothelial system cancer patients. In lymphoma and lung cancer patients, the incidence rates of aspergillosis were 59.55 and 11.07 per 10,000 person-years, respectively (Figure 2).
As shown in Table 2, patients with a diagnosis of aspergillosis had a 2.30-fold (95% CI: 2.14–2.48, p value: <0.001) higher risk of mortality than those without aspergillosis. Regarding the type of aspergillosis, both non-PA patients and PA patients had a higher risk of death than patients without aspergillosis. In males with aspergillosis, there was a 1.95-fold (95% CI: 1.78–2.14, p value: <0.001) risk of mortality compared with male patients without aspergillosis. In females with aspergillosis, there was a 3.17-fold (95% CI: 2.78–3.61, p value: <0.001) risk of mortality compared with female patients without aspergillosis. Within each age group, the patients with aspergillosis also had a significantly higher risk of mortality than those without aspergillosis.
Table 2.
The Risk of Mortality Between Patients with Aspergillosis and Nonaspergillosis
| Aspergillosis | Non-Aspergillosis | Aspergillosis vs Non-Aspergillosis | ||||||
|---|---|---|---|---|---|---|---|---|
| Total, N | Death, N(%) | Total, N | Death, N (%) | Crude HR | p value | aHR* | p value | |
| Overall | 951 | 669(70.35) | 1,093,945 | 506,145(46.27) | 2.05(1.90–2.21) | <0.0001 | 2.30(2.14–2.48) | <0.001 |
| Non-PA | 560 | 394(70.36) | 1.99(1.80–2.20) | <0.0001 | 2.29(2.08–2.53) | <0.001 | ||
| PA | 391 | 275(70.33) | 2.14(1.90–2.41) | <0.0001 | 2.32(2.06–2.61) | <0.001 | ||
| Stratified | ||||||||
| Gender | ||||||||
| Male | 595 | 448(75.29) | 572,980 | 322,514(56.29) | 1.72(1.56–1.88) | <0.0001 | 1.95(1.78–2.14) | <0.001 |
| Female | 356 | 221(62.08) | 520,965 | 183,631(35.25) | 2.49(2.18–2.84) | <0.0001 | 3.17(2.78–3.61) | <0.001 |
| Age group | ||||||||
| <50 | 335 | 198(59.1) | 248,013 | 66,162(26.68) | 3.06(2.66–3.51) | <0.0001 | 2.50(2.18–2.88) | <0.001 |
| 50–70 | 419 | 298(71.12) | 488,011 | 199,105(40.80) | 2.61(2.33–2.93) | <0.0001 | 2.38(2.13–2.67) | <0.001 |
| ≥70 | 197 | 173(87.82) | 357,921 | 240,878(67.30) | 1.85(1.59–2.15) | <0.0001 | 1.83(1.58–2.12) | <0.001 |
Note: *Adjusted for age group and gender.
Abbreviations: PA, pulmonary aspergillosis; non-PA, nonpulmonary aspergillosis; HR, hazard ratios; aHRs, adjusted hazard ratios.
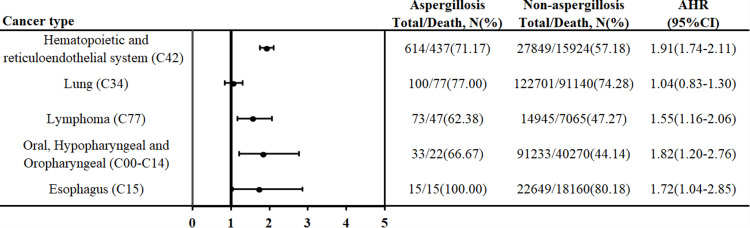
Figure 3 shows the risk of mortality between patients with and without aspergillosis in different cancer types. In hematopoietic and reticuloendothelial system cancer, patients with aspergillosis had a 1.91-fold (95% CI: 1.74–2.11, p<0.001) risk of mortality compared with patients without aspergillosis. In oral, hypopharyngeal, and oropharyngeal cancer, patients with aspergillosis had a 1.82-fold (95% CI: 1.20–2.76, p value: 0.005) risk of mortality compared with patients without aspergillosis. Additionally, there was also a higher risk of mortality among patients with aspergillosis than among those without aspergillosis in lymphoma and esophageal cancer.
Figure 3.
The risk of mortality between patients with aspergillosis and nonaspergillosis with different cancer types.
Abbreviation: AHR, adjusted hazard ratios.
Table 3 shows the crude mortality rate of 951 cancer patients with aspergillosis. Overall, the average follow-up time for these cases was 2.39 years. The crude mortality rates of non-PA and PA were 70.36% and 70.33%, respectively. The crude mortality rate of males was higher than that of females and increased with age. Regarding the treatment of disease, 899 (94.53%) cancer patients with aspergillosis had treatment for cancer, but only 510 (53.63%) patients had treatment for aspergillosis. The crude mortality rates of patients with ILD, COPD, TB, and bronchiectasis were 82.35, 78.53, 77.14, and 67.86%, respectively.
Table 3.
The Characteristics of Cancer Patients Who Died and Did Not Die with Aspergillosis
| Number of Patients, N | Death, N (%) | Non Death, N (%) | p value | |
|---|---|---|---|---|
| Overall | 951 | 669(70.35) | 282(29.65) | |
| Aspergillosis type | ||||
| Non-PA | 560 | 394(70.36) | 166(29.64) | 0.994 |
| PA | 391 | 275(70.33) | 116(29.67) | |
| Cancer to Aspergillosis, (years, median (IQR)) | 0.31(0.11–0.59) | 0.30(0.11–0.60) | 0.32(0.11–0.57) | 0.622 |
| Aspergillosis to death, (years, median (IQR)) | 0.83(0.24–2.66) | 0.42(0.15–1.03) | 3.82(1.86–7.10) | <0.001 |
| Cancer to death, (years, median (IQR)) | 1.23(0.69–3.02) | 0.90(0.51–10.46) | 4.13(2.26–7.33) | <0.001 |
| Sex | ||||
| Male | 595 | 448(75.29) | 147(24.71) | <0.001 |
| Female | 356 | 221(62.08) | 135(37.92) | |
| Age | ||||
| <50 | 335 | 198(59.1) | 137(40.9) | <0.001 |
| 50–70 | 419 | 298(71.12) | 121(28.88) | |
| ≧70 | 197 | 173(87.82) | 24(12.18) | |
| Aspergillosis treatment | ||||
| No | 441 | 276(62.59) | 165(37.41) | <0.001 |
| Yes | 510 | 393(77.06) | 117(22.94) | |
| Cancer treatment | ||||
| No | 52 | 45(86.54) | 7(13.46) | 0.009 |
| Yes | 899 | 624(69.41) | 275(30.59) | |
| Operation | 114 | 70(61.4) | 44(38.6) | 0.006 |
| Radiotherapy | 136 | 86(63.24) | 50(36.76) | 0.050 |
| Chemotherapy | 790 | 552(69.87) | 238(30.13) | 0.479 |
| Immunotherapy | 16 | 11(68.75) | 5(31.25) | 1.000 |
| Hormone | 236 | 145(61.44) | 91(38.56) | 0.001 |
| Supportive therapy | 141 | 119(84.4) | 22(15.6) | <0.001 |
| Others | 270 | 152(56.3) | 118(43.7) | <0.001 |
| Structural lung disease23 | ||||
| TB | 70 | 54(77.14) | 16(22.86) | 0.196 |
| COPD | 177 | 139(78.53) | 38(21.47) | 0.008 |
| Bronchiectasis | 28 | 19(67.86) | 9(32.14) | 0.770 |
| ILD | 17 | 14(82.35) | 3(17.65) | 0.274 |
Abbreviations: PA, pulmonary aspergillosis; non-PA, nonpulmonary aspergillosis; TB, tuberculosis; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; SD, standard deviation.
Discussion
To our knowledge, this is the first study to use a national cancer registry database to survey the incidence of aspergillosis in cancer patients with solid cancer and hematologic cancer. From this study, we found that the incidence of aspergillosis in cancer patients gradually increased from 2006 to 2017. The three highest incidences of aspergillosis were hematopoietic and reticuloendothelial system cancer, lymphoma, and lung cancer.
Incidence of Aspergillosis in Cancer Patients
Huang et al11 estimated the annual incident cases and incidence rates of various fungal diseases in 2013 in Taiwan. Their study cohort consisted of patients randomly selected with a one-in-three sampling ratio from the total population (inpatient and outpatient settings) registered from 1 January 2013 through 31 December 2013 in NHIRD by the ICD-9-CM code. Our study enrolled all new-onset cancers during the period from 2006 to 2017 in the TCR database. The incidence rate of aspergillosis was defined by the ICD-9-CM code and ICD-10 codes. Huang et al estimated an incidence rate of 2.43 cases of aspergillosis per 100,000 population in 2013. In our study, the incidence rates of aspergillosis were calculated in a different way, and we concluded that we found an increase from 3.04 to 13.51 per 10,000 person-years between 2006 and 2017.
There may be several reasons to explain why the incidence of aspergillosis in cancer patients gradually increased from 2006 to 2017. First, the survival rate of different types of cancer has increased, leading to aspergillosis. A study using cancer registry data enrolled 7 countries and observed them over 19 years, finding that cancer survival increased in each country across almost all cancer types.12 Second, prompt diagnosis of aspergillosis is complicated and requires the integration of clinical information of risk factors, symptoms, radiological images and microbiology. With improvements in the rates of Aspergillus detection13 and diagnostic accuracy for aspergillosis in the clinic14 and international groups, guidance and advice on this topic have been published that can assist physicians in their patient management to detect aspergillosis early.15 The increased incidence of aspergillosis might be attributed to the rising awareness of aspergillosis by physicians that could assist the capacity of diagnosis. The diagnosis of aspergillosis and the identification of prognosis factors for survival in patients with aspergillosis are often challenging due to the great variability in the expression of disease.16 Early diagnosis of aspergillosis and prompt treatment have a great impact on morbidity and mortality. After many scientific societies published guidelines17–19 for the diagnosis and management of aspergillosis, physicians were made aware of aspergillosis and its diagnosis.
Solid Cancer and Aspergillosis
In the Dandachi et al study,6 the most common underlying solid cancers diagnosed before aspergillosis were lung cancer (51%), head and neck cancer (19%), and gastrointestinal cancer (13%). In our study, we found similar results, and the most common solid cancers were lung cancer, esophageal cancer and oral cancer, hypopharyngeal cancer and oropharyngeal cancer. Ohmagari et al retrospectively identified 13 patients with invasive aspergillosis with solid tumors who were treated between 1994 and 2003.20 They also found that the majority of patients (57%) had lung cancer. There were differences between our study and these two studies. Not only did we enroll patients with invasive aspergillosis but also those with tonsillar aspergillosis, disseminated aspergillosis, allergic bronchopulmonary aspergillosis, and other forms of aspergillosis. Even though there were differences in the enrollment criteria, lung cancer had the highest incidence of aspergillosis among the solid cancer population.
Although extensive information about aspergillosis in cancer patients is available, aspergillosis and skin cancers occur relatively infrequently. We compared the risk of mortality between patients with and without aspergillosis in different cancer types. We found that skin cancer patients with aspergillosis had a 15.57-fold increased risk of mortality compared with those without aspergillosis. However, it remains poorly understood and described, especially aspergillosis among skin cancers. The major reason was that the present studies mainly focused on the more common and severe clinical scenario of aspergillosis or disseminated infection in frequent cancers.
Risk of Mortality in Patients with Aspergillosis
It is not surprising that patients with aspergillosis had increased mortality, and the overall adjusted HR was 2.32. After subgroup analysis, males had a higher risk of mortality, with an adjusted HR of 1.96, and females had an adjusted HR of 3.15. Sun et al used another nationwide database, the NHIRD, between 2002 and 2012 and enrolled 407 invasive pulmonary aspergillosis patients in Taiwan.21 They found that the overall mortality rate was higher in females than in males (32.67% compared to 28.79%). Furthermore, their study showed that females had a higher case fatality rate than males among most age groups. Their study only used ICD-9 codes and hospitalization periods between 2002 and 2012. Our study focused on cancer patients who used ICD-9-CM and ICD-10 codes between 2006 and 2017. Their study found that mortality risk increased with age, but this was not observed in our study. A possible explanation for this discrepancy was that our mortality risk was only controlled for age group and sex, and we did not control for patients’ comorbidities or underlying cancer stage to determine the survival of cancer patients.
A previous study showed that the one- and 5-year survival rates in chronic pulmonary aspergillosis patients were 86% and 62%, respectively.22 From our study, we found that cancer patients with aspergillosis had a poorer prognosis than those without aspergillosis. In the mortality population, the duration from cancer diagnosis to death was 1.26 years. Among cancer patients with aspergillosis, the duration from aspergillosis diagnosis to death was less than one year. Cancer patients with superimposed aspergillosis infection have a poor prognosis. We also analyzed structural lung disease among cancer patients with aspergillosis and found that the prevalence of COPD was higher in the mortality group, which was similar to the results of a previous study.22 Their study also showed that the prevalence of COPD was higher in the mortality group but was not observed in TB and bronchiectasis.
There were some limitations to this study. First, our study was retrieved from national cancer registry data, and we did not have clinical symptoms or culture results. Second, the lack of imaging and laboratory data also made it difficult for us to account for disease severity. The aim of our study was to estimate the incidence of aspergillosis in different types of cancer and its trend regardless of cancer stage and cancer treatment. Third, we did not survey the treatment of aspergillosis or cause of mortality. Mortality in our population may have contributed to cancer disease, severe infection, other comorbidities or aspergillosis. Fourth, our study was based on the ICD9/10 coding, which does not necessarily reflect current epidemiology. It is not clear what laboratory investigation is performed to establish the diagnosis of aspergillosis. Patients with aspergillosis are most effectively diagnosed according to the EORCT-MSG 2008 criteria, but it could not be found in these claims data. In addition, the clinical definition of invasive aspergillosis comprises possible, probable and proven invasive aspergillosis.24 In clinical practice, invasive pulmonary aspergillosis is often only suspected or may be a possible invasive pulmonary aspergillosis but rarely a proven invasive pulmonary aspergillosis. Colonization with Aspergillus spp. may be detected in lung cancer patients but is rarely invasive pulmonary disease. Most lung cancer patients have chronic underlying pulmonary lung disease. The study by Dandachi et al6 looked at clinical diagnosis and reviewed cases retrospectively according to a positive Aspergillus culture. During a 13-year period, only 51 cases of invasive pulmonary aspergillosis could be identified in lung cancer patients. To ensure medical quality and to avoid unnecessary expenses, the National Health Insurance Administration retrospectively checks the appropriateness of medical claims by random peer review to reduce coding error. In addition, our outcomes were the incidence of nonpulmonary aspergillosis and pulmonary aspergillosis, and we did not investigate invasive pulmonary aspergillosis. Although limitations exist, because all cancer patients had been followed via professional oncology case managers, we believe that all cancer patients with aspergillosis had mostly been covered. A guideline in Taiwan suggests that prophylactic antifungal strategies for patients with hematopoietic stem cell transplantation recipients should be individualized at each hospital or, even for each patient, after considering epidemiological factors: local incidences and risk of invasive fungal diseases, diagnostic tools in the facility, accessibility to the health care setting during the high-risk period, compliance, bioavailability, direct toxicity, drug–drug interactions and cost-effectiveness.25
Conclusion
Our study was based on the ICD9/10 diagnosis codes, which do not provide sufficient clinical information and specificity to describe the severity or complexity of the aspergillosis conditions, but there was a trend of high incidence and mortality of aspergillosis in cancer patients from 2006 to 2017. The overall incidence rate of aspergillosis was 13.57 per 10,000 person-years in cancer patients, and it is important for physicians to be aware of aspergillosis, so that they can make a prompt diagnosis and begin early treatment in cancer patients.
Acknowledgments
The authors are grateful to the Health Data Science Center, National Cheng Kung University Hospital for providing administrative and technical support.
Funding Statement
This study was supported by MOHW110-TDU-B-212-144020 from the health and welfare surcharge of tobacco products.
Data Sharing Statement
The data sources are the Taiwan Nation Health Insurance Database and Taiwan Cancer Registry. The data are available with permission from the Taiwan Health and Welfare Data Science Centre (https://dep.mohw.gov.tw/DOS/np-2497-113.html, accessed on 10 Dec 2021). Restrictions apply to the availability of these data, which were used under license for this study.
Ethics Approval and Informed Consent
We declare compliance with ethical standards as a waiver from the informed consent process approved by the Institutional Review Board of Chi Mei Medical Center (IRB no. 10912-E02). Patient consent was waived because the NHIRD contains anonymized information only.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Douglas AP, Smibert OC, Bajel A, et al. Consensus guidelines for the diagnosis and management of invasive aspergillosis, 2021. Intern Med J. 2021;51(Suppl 7):143–176. doi: 10.1111/imj.15591 [DOI] [PubMed] [Google Scholar]
- 2.Dib RW, Khalil M, Fares J, et al. Invasive pulmonary aspergillosis: comparative analysis in cancer patients with underlying haematologic malignancies versus solid tumours. J Hosp Infect. 2020;104(3):358–364. doi: 10.1016/j.jhin.2019.09.020 [DOI] [PubMed] [Google Scholar]
- 3.Saghrouni F, Youssef YB, Gheith S, et al. Twenty-nine cases of invasive aspergillosis in neutropenic patients. Med Mal Infect. 2011;41(12):657–662. doi: 10.1016/j.medmal.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 4.Abbasi S, Shenep JL, Hughes WT, Flynn PM. Aspergillosis in children with cancer: a 34-year experience. Clin Infect Dis. 1999;29(5):1210–1219. doi: 10.1086/313445 [DOI] [PubMed] [Google Scholar]
- 5.Maschmeyer G, Haas A, Cornely OA. Invasive aspergillosis: epidemiology, diagnosis and management in immunocompromised patients. Drugs. 2007;67(11):1567–1601. doi: 10.2165/00003495-200767110-00004 [DOI] [PubMed] [Google Scholar]
- 6.Dandachi D, Wilson Dib R, Fernández-Cruz A, et al. Invasive pulmonary aspergillosis in patients with solid tumors: risk factors and predictors of clinical outcomes. Ann Med. 2018;50(8):713–720. doi: 10.1080/07853890.2018.1518581 [DOI] [PubMed] [Google Scholar]
- 7.Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi. 2017;3(4):57. doi: 10.3390/jof3040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun K-S, Tsai C-F, Chen S-C-C, Chen -Y-Y, Huang W-C. Galactomannan testing and the incidence of invasive pulmonary aspergillosis: a 10-year Nationwide Population-Based Study in Taiwan. PLoS One. 2016;11(2):e0149964. doi: 10.1371/journal.pone.0149964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang CJ, Wang YW, Lee WC. Taiwan’s nationwide cancer registry system of 40 years: past, present, and future. J Formos Med Assoc. 2019;118(5):856–858. doi: 10.1016/j.jfma.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 10.Chiang CJ, You SL, Chen CJ, Yang YW, Lo WC, Lai MS. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol. 2015;45(3):291–296. doi: 10.1093/jjco/hyu211 [DOI] [PubMed] [Google Scholar]
- 11.Huang YS, Denning DW, Shih SM, et al. Fungal diseases in Taiwan—National insurance data and estimation. J Fungi. 2019;5(3):78. doi: 10.3390/jof5030078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493–1505. doi: 10.1016/S1470-2045(19)30456-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenks JD, Salzer HJF, Hoenigl M. Improving the rates of Aspergillus detection: an update on current diagnostic strategies. Expert Rev Anti Infect Ther. 2019;17(1):39–50. doi: 10.1080/14787210.2018.1558054 [DOI] [PubMed] [Google Scholar]
- 14.Kaziani K, Mitrakou E, Dimopoulos G. Improving diagnostic accuracy for invasive pulmonary aspergillosis in the intensive care unit. Ann Transl Med. 2016;4(18):352. doi: 10.21037/atm.2016.08.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1–e38. doi: 10.1016/j.cmi.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 16.Salzer HJ, Cornely OA. Awareness of predictors of mortality may help improve outcome in chronic pulmonary aspergillosis. Eur Respir J. 2017;49(2):1602520. doi: 10.1183/13993003.02520-2016 [DOI] [PubMed] [Google Scholar]
- 17.Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45–68. doi: 10.1183/13993003.00583-2015 [DOI] [PubMed] [Google Scholar]
- 18.Patterson TF, Thompson IIIGR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salzer HJF, Heyckendorf J, Kalsdorf B, Rolling T, Lange C. Characterization of patients with chronic pulmonary aspergillosis according to the new ESCMID/ERS/ECMM and IDSA guidelines. Mycoses. 2017;60(2):136–142. doi: 10.1111/myc.12589 [DOI] [PubMed] [Google Scholar]
- 20.Ohmagari N, Raad II, Hachem R, Kontoyiannis DP. Invasive aspergillosis in patients with solid tumors. Cancer. 2004;101(10):2300–2302. doi: 10.1002/cncr.20647 [DOI] [PubMed] [Google Scholar]
- 21.Sun KS, Tsai CF, Chen SC, Huang WC. Clinical outcome and prognostic factors associated with invasive pulmonary aspergillosis: an 11-year follow-up report from Taiwan. PLoS One. 2017;12(10):e0186422. doi: 10.1371/journal.pone.0186422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowes D, Al-Shair K, Newton PJ, et al. Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J. 2017;49(2):1601062. doi: 10.1183/13993003.01062-2016 [DOI] [PubMed] [Google Scholar]
- 23.Huang HL, Cheng MH, Lu PL, et al. Epidemiology and predictors of NTM pulmonary infection in Taiwan-a retrospective, five-year multicenter study. Sci Rep. 2017;7(1):16300. doi: 10.1038/s41598-017-16559-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group education and research consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko BS, Chen WT, Kung HC, et al. 2016 guideline strategies for the use of antifungal agents in patients with hematological malignancies or hematopoietic stem cell transplantation recipients in Taiwan. J Microbiol Immunol Infect. 2018;51(3):287–301. doi: 10.1016/j.jmii.2017.07.005 [DOI] [PubMed] [Google Scholar]