Abstract
Purpose
We report a case of corneal verticillata in a patient who had been taking raloxifene for a prolonged period. To our knowledge, this is the first case report of an ocular side effect of raloxifene.
Observations
A 69-year-old female patient presented to our clinic for her routine eye check-up. On slit-lamp examination, whorl-like subepithelial deposits were observed in the bilateral corneas. She was diagnosed with corneal verticillata (vortex keratopathy) caused by raloxifene. A follow-up evaluation was conducted after discontinuation of the drug; however, the corneal opacity did not improve.
Conclusions and importance
Patients with corneal verticillata should be asked regarding any intake of raloxifene for osteoporosis, as it may cause corneal verticillata.
Keywords: Drug-induced keratopathy, Keratopathy, Raloxifene, Selective estrogen receptor modulator
1. Introduction
Raloxifene is a selective estrogen receptor modulator (SERM), which acts as either an estrogen agonist or antagonist depending on the tissue cell type and surrounding environment.1 It was approved by the Food and Drug Administration in 1997 for the prevention of postmenopausal bone loss, and reportedly prevents breast cancer among postmenopausal women.2 Although it was initially developed as a treatment for breast cancer, it was shown to be less effective than tamoxifen and other SERMs. Thus, it is currently administered for osteoporosis prevention.
Another SERM, tamoxifen, is reportedly associated with certain ophthalmic complications.3,4 In contrast, raloxifene has no known ophthalmic complications. We herein present the first reported case of corneal verticillata in a patient who had been taking raloxifene for a significant period.
2. Case report
A 69-year-old female patient presented to our clinic for her routine eye check-up. She had no significant medical history aside from osteoporosis, for which she had been taking raloxifene (Evista) tablets and calcium carbonate/cholecalciferol (Cavid) chewable tablets for the past 2 years. Besides the medications for osteoporosis, she was not taking any other medications for other diseases. She had no specific ophthalmic complaints. During her last visit 18 months prior, she had no abnormal findings other than a mild cortical cataract and dry eye. She said that she had undergone a regular health checkup every year, and that there had been no specific findings in the blood test and urine tests. She also denied any family history of genetic disorders.
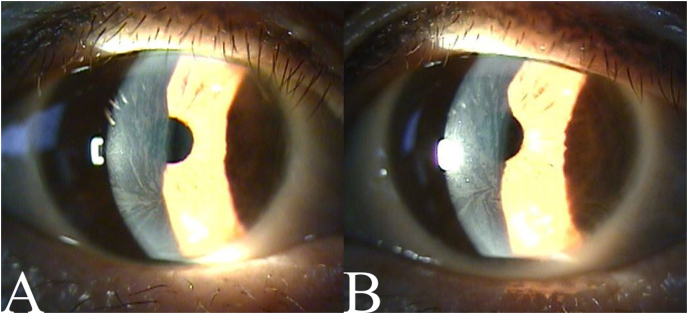
On visual acuity testing, her best corrected visual acuity (BCVA) was 20/25 for the right eye and 20/32 for the left eye. The manifest refraction was −0.25 Dsph −0.50 Dcyl 80° for the right eye and −1.50 Dcyl 105° for the left eye. Her light reflex was intact, and the color blindness test results were normal. On slit-lamp examination, whitish, whorl-like subepithelial deposits were observed in the bilateral corneas, with no significant findings in the anterior chamber (Fig. 1). The patterns and opacities of the deposits were similar in both eyes, spanning a wide area, including the center of the cornea. The center of the whorl-like pattern was located below the center of the cornea. Drusen were observed in both eyes, and both optic discs and maculae were normal on fundoscopic examination and optical coherence tomography.
Fig. 1.
Initial anterior segment photographs
Anterior segment photographs at the patient's first visit are shown. Bilateral whorl-like subepithelial deposits are observed. (A: right eye, B: left eye).
The patient was informed about the possibility of raloxifene-induced corneal verticillata and was asked whether it was possible for her to discontinue the drug. She decided to discontinue raloxifene after consulting with her orthopedic surgeon.
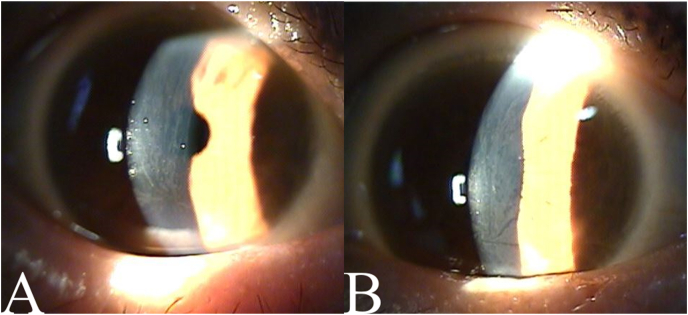
When she returned to the hospital 3 months later, her BCVA was 20/25 for both eyes, and her manifest refraction was unchanged. On slit-lamp examination, corneal verticillata was still observed (Fig. 2). The patient noted no differences in her condition. Since raloxifene discontinuation did not appear to worsen her osteoporosis, she subsequently decided to stop taking raloxifene while continuing the follow-up evaluations.
Fig. 2.
Anterior segment photographs on follow-up
The patient's anterior segment photographs 3 months later. No significant changes are observed. (A: right eye, B: left eye).
3. Discussion
Corneal verticillata (vortex keratopathy) is a disease characterized by the formation of a whorl-like pattern of deposits in the inferior interpalpebral portion of the cornea. It is reportedly caused by various drugs, such as amiodarone and chloroquine.
The first SERM agent, tamoxifen, has an antagonistic effect on breast cancer cell proliferation and has been used as an adjuvant therapy.5 However, several ocular toxic reactions have been reported with its use. Tamoxifen may induce vortex keratopathy.3 This was first reported in 1978, and its prevalence ranged from 1 to 10%.4 Corneal opacity can also occur, causing vision loss, which may be reversed upon discontinuing the drug.6 However, the specific pathogenesis of this condition remain unknown. It may also lead to formation of yellow crystalline deposits in the inner retina of both eyes and punctate gray lesions in the outer retina and retinal pigment epithelium. It may likewise lead to macular edema, which can reduce vision.4 Cases of optic neuritis have also been reported.4
Raloxifene is a second-generation SERM agent and is structurally different from tamoxifen. Although classified as an SERM, raloxifene is a benzothiophene derivative, while tamoxifen is a triphenylethylene derivative.7 The reported side effects of raloxifene include hot flashes, influenza-like syndrome, peripheral edema, and increased risk of venous thromboembolic disease.8 There have been 5 cases of raloxifene-related eye disorders reported to the FDA Adverse Events Reporting System: 3 cases of blurred vision, 1 case of glaucoma, and 1 case of mydriasis, but no cases of corneal complications. However, in a literature review using PubMed, no reports of ophthalmic side effects of raloxifene were found.
Drugs such as tamoxifen, chloroquine, chlorpromazine, thioridazine, and amiodarone have reportedly caused keratopathy and retinopathy. They share one similarity, in that they are all cationic amphiphilic drugs.9 They cause accumulation of phospholipids, thereby creating vortex pattern deposits. Their chemical properties lead to an intralysosomal accumulation of lipids.10 One possible mechanism is that these drugs inhibits lysosomal phospholipase, which is involved in lipid metabolism. Another possible mechanism is that the drug binds to the lipid to form a drug-lipid complex that does not pass through the lysosome or is not degraded.10 Since raloxifene is also a cationic amphiphilic drug,11 it may potentially cause keratopathy. However, further studies are required to elucidate its specific pathogenesis. Unlike tamoxifen, another SERM agent, retinal deposits, macular edema, or optic neuritis was not observed in this patient, and only corneal verticillata was present.
Genetic disorders such as Fabry disease can also cause corneal verticillata. Fabry disease is a lysosomal storage disorder secondary to alpha-galactosidase A enzyme deficiency that causes deposits due to a buildup of complex lipid in the corneal epithelium. This disease can cause systemic problems, including hypertrophic cardiomyopathy, stroke, and chronic kidney disease with proteinuria.12 Although it can be detected late in women, this patient had no family history of genetic diseases, no specific findings on regular health checkup, and no skin lesions such as angiokeratoma; thus, Fabry disease could be excluded.
4. Conclusions
To our knowledge, this was the first case report on an ocular side effect of raloxifene. In this case, keratopathy was observed. However, the retinopathy that reportedly occurs as a side effect of tamoxifen was not observed with raloxifene in our case. The keratopathy did not improve even after discontinuation of the drug for 3 months. Thus, prompt follow-up is necessary to evaluate the clinical improvement of keratopathy or development of retinopathy. In conclusion, patients with vortex keratopathy should be asked regarding any intake of raloxifene for osteoporosis, as this may be a possible cause of their condition.
Patient consent
We have obtained written informed consent from the patient in this case report.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Smith C.L., O'Malley B.W. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25(1):45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 2.Muchmore D.B. Raloxifene: a selective estrogen receptor modulator (SERM) with multiple target system effects. Oncol. 2000;5(5):388–392. doi: 10.1634/theoncologist.5-5-388. [DOI] [PubMed] [Google Scholar]
- 3.Martinkovich S., Shah D., Planey S.L., Arnott J.A. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Interv Aging. 2014;9:1437–1452. doi: 10.2147/CIA.S66690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinchuk O., Watanabe M., Hayashi N., Fukushima A., Ueno H. A case of tamoxifen keratopathy. Arch Ophthalmol. 2006;124(7):1046–1048. doi: 10.1001/archopht.124.7.1046. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser-Kupfer M.I., Lippman M.E. Tamoxifen retinopathy. Cancer Treat Rep. 1978;62(3):315–320. doi: 10.1016/S0161-6420(81)35071-4. [DOI] [PubMed] [Google Scholar]
- 6.Pavlidis N.A., Petris C., Briassoulis E., et al. Clear evidence that long-term, low-dose tamoxifen treatment can induce ocular toxicity. A prospective study of 63 patients. Cancer. 1992;69(12):2961–2964. doi: 10.1002/1097-0142(19920615)69:12<2961::aid-cncr2820691215>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Patel H.K., Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther. 2018;186:1–24. doi: 10.1016/j.pharmthera.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Cummings S.R., Eckert S., Krueger K.A., et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of raloxifene Evaluation. JAMA. 1999;281(23):2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 9.Imperia P.S., Lazarus H.M., Lass J.H. Ocular complications of systemic cancer chemotherapy. Surv Ophthalmol. 1989;34(3):209–230. doi: 10.1016/0039-6257(89)90105-7. [DOI] [PubMed] [Google Scholar]
- 10.Raizman M.B., Hamrah P., Holland E.J., et al. Drug-induced corneal epithelial changes. Surv Ophthalmol. 2017;62(3):286–301. doi: 10.1016/j.survophthal.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Muehlbacher M., Tripal P., Roas F., Kornhuber J. Identification of drugs inducing phospholipidosis by novel in vitro data. ChemMedChem. 2012;7(11):1925–1934. doi: 10.1002/cmdc.201200306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaud M., Mauhin W., Belmatoug N., et al. When and how to diagnose Fabry disease in clinical pratice. Am J Med Sci. 2020;360(6):641–649. doi: 10.1016/j.amjms.2020.07.011. [DOI] [PubMed] [Google Scholar]