Summary
Background
Liver disease is the only major chronic disease and mortality is increasing. Earlier detection of liver fibrosis can reduce progression to cirrhosis and hepatocellular carcinoma. Many studies have reported an increased prevalence in liver fibrosis among adults in urban regions but there are few data in physically active rural populations without attributable metabolic risk factors. This aim of this study is to investigate the prevalence of abnormal liver functions tests (LFTs) and liver fibrosis among adults in a rural population.
Methods
This cross-sectional study included observations from KMCH-NNCD-II (2017) study (n = 907) from a farming village, Nallampatti, located in South India. We assessed lifestyle (occupation, tobacco use and alcohol consumption using AUDIT-C questionnaire), markers for metabolic diseases (obesity, hypertension, diabetes, hypercholesterolemia), LFTs and markers for hepatitis viruses B and C. 901 participants had transient elastography to assess fibrosis. Participants with abnormal LFTs and significant liver fibrosis (F2-F4) underwent additional liver screening (caeruloplasmin, iron studies and autoimmune hepatitis panel). Multiple logistic regression analyses were performed to understand the association of liver fibrosis with lifestyle and metabolic risk factors after adjustment for co-variates.
Findings
Significant liver fibrosis (F2-F4) was observed in 14.4%, and cirrhosis in 0.8%. There was an association of liver fibrosis with abnormal LFTs but no association between alcohol consumption, viral hepatitis, hepatic liver screening and liver fibrosis. Among metabolic risk factors, no association was observed for hypertension and hypercholesterolemia but diabetes [OR – 3.206 (95% CI: 1.792 – 5.736)], obesity [1.987 (1.341 – 2.944)] and metabolic syndrome [2.539 (1.680 – 3.836)] showed association with significant liver fibrosis (F2-F4) after adjustment for confounding factors.
Interpretation
Our results suggest that the prevalence of liver fibrosis in rural population is similar to urban counterparts. The association of metabolic risk factors with liver fibrosis in physically active rural population warrants further investigations in future studies.
Funding
This study is funded by KMCH Research Foundation, India.
Keywords: Liver fibrosis, prevalence, Abnormal liver tests, Aetiological screening for abnormal LFT's, Elastography screening, Metabolic syndrome, Low and middle income country
Research in context.
Evidence before this study
Low-to-middle Income Countries (LMICs), where two thirds of the global population live, are physically active with majority residing in rural areas, have the highest percentage of deaths from liver disease. In developed countries, the prevalence of abnormal liver tests and cirrhosis is around 8% and 1% respectively; in contrast, there are only few studies from LMIC assessing prevalence of abnormal LFT's and fibrosis but documentation of causes of liver disease in community studies in LMICs, prior to this study without language restrictions, were not available in PubMed and Google Scholar with search terms ‘causes’, ‘abnormal LFT's’, ‘prevalence’, ‘fibrosis’ prior to May 15, 2022.
Added value of this study
The main strength of this study is that it addresses 3 issues in the poorly studied population of rural adults in LMIC: the prevalence of abnormal liver tests; the major causes of abnormal liver tests and the prevalence of hepatic fibrosis.
Implications of all the available evidence
Our observation of liver fibrosis (F2-F4) in 14.4%, cirrhosis in 0.8%, its association with metabolic risk factors, would guide service providers on areas to focus on at a population level, to reduce the health-care burden of advanced liver disease given the limited resources in LMIC. The association of fibrosis with the metabolic syndrome among rural population needs further investigation to see whether the pathogenesis differs from that seen in those in urban areas.
Alt-text: Unlabelled box
Introduction
Liver disease worldwide is a major source of morbidity and mortality and is the only major chronic disease with increasing mortality.1 Chronic liver disease often has a long latent course and early identification and intervention may not only prevent the onset of fibrosis/cirrhosis but may even reverse fibrosis.2 The estimated prevalence of cirrhosis3 of 1% makes it an appropriate disease for screening since it meets all 10 principles defined by the WHO.4 Liver function tests (LFTs) such as alanine aminotransferase (ALT), aspartate aminotransferase (AST) and γ-glutamyl transferase (GTT) are relatively inexpensive tests often done to screen for liver diseases and are a good predictor of not only liver disease mortality but also other cause mortality.5 Since LFTs are neither specific nor indicative of any particular disease, further testing is usually required to define the cause and extent of disease. The common causes of liver diseases are viral hepatitis B/C, chronic alcohol use and fatty liver disease,1 but other rarer causes of liver disease, although low in prevalence, need to be excluded. Defining the cause of abnormal LFTs needs extensive screening; however, a specific cause is detected in only 55% [mainly 48% non-alcoholic liver disease (NAFLD) and 46% alcohol-related liver disease (ARLD)]. Since these screening tests are costly,6 it has been suggested that further screening should be undertaken only if the ALT, ALP, GGT are abnormal.7
Chronic liver disease patients often do not come to medical attention until late, and when complications have developed, death or liver transplantation are the outcomes. Detection and treatment of common liver diseases in the earlier stages can contribute to a decrease in disease burden of not only liver diseases but also other diseases.2 Although the cause(s) of liver diseases can usually be established through blood tests/history, liver biopsy remains the gold-standard to diagnose fibrosis/cirrhosis, but its invasive nature and risks makes it unfeasible for screening large populations. Validated non-invasive methods for the assessment of fibrosis, particularly transient elastography (TE), has become a pragmatic and feasible strategy for liver fibrosis/cirrhosis screening3 in the general population.8, 9, 10 These population-based studies are mainly from urban population with attributable behavioral (excess alcohol consumption) and biological risk factors (increased body mass index) in western countries with reported prevalence of significant fibrosis and 1% with liver cirrhosis.8, 9, 10 According to World Bank data, 67% of the global population in Low-to-Middle-Income Country (LMIC) live in rural areas, with a marked disparity existing in health structure between urban and rural in LMIC, but studies on the prevalence of liver fibrosis in physically active rural population is scarce.
The Kovai Medical Center and Hospital (KMCH) – Nallampatti Non-communicable disease (KMCH-NNCD II) study is a longitudinal study on the prevalence of non-communicable diseases (NCDs) in Nallampatti, a rural farming village in South India with 85%, a physically active population, involved in agricultural farming activities. Our 2015 study indicated huge prevalence of diabetes mellitus, systemic hypertension, hypercholesterolemia and atherosclerosis, and the association between heavy metals and organophosphate insecticides with diabetes and atherosclerosis in this farming community11 was a novel finding.
Our primary aims were to look at aetiology of liver disease in those with abnormal LFTs, the relationship between abnormal LFTs and hepatic fibrosis and to estimate the prevalence of liver fibrosis using TE. Secondary aims were to evaluate the association of liver fibrosis with screened aetiologies and to evaluate the association of liver fibrosis with screened non-communicable diseases NCDs [diabetes/hypertension/hypercholesterolaemia/obesity- components of metabolic syndrome (MS)] among the participants of KMCH-NNCD study in rural India.
Methods
Study population
KMCH-NNCD-II was a cross-sectional study performed in rural Nallampatti, a typical farming village in Tamil Nadu, South India (latitude: 11°21′2.39″ N; longitude: 77°32′4.79″ E), that fits all defined rural demographics criteria. Inclusion criteria included all those native to the village and ≥ 20 and ≤ 85 years of age. Pregnant women, people outside the age criteria and those not native to Nallampatti were excluded. All inhabitants of the village were invited through pamphlets and word of mouth via village authorities and volunteers. The KMCH-NNCD-II study was conducted on every Sunday during a period of eight weeks in August and September 2017 with screening done in weekly batches of 100 to 150 participants. The eligibility of the participants was confirmed by government records. The study purpose was explained to participants in local language and informed written consent was obtained from all participants according to the principles of the Declaration of Helsinki, 1975. The study protocol was approved by the clinical research ethics committee of Kovai Medical Center and Hospital (Ref.: EC/AP/556/08/2017).
Data collection
A detailed questionnaire, as described in our previous publication, was administered to document the age, sex, educational status, occupation, source of drinking water, familial disease history, prevalence of known diseases and details of all medications.11 The questions on alcohol intake and cigarette smoking were limited only to males as women are generally self-restricted themselves from these habits due to socio-cultural reasons. Alcohol consumption was assessed with Alcohol Use Disorder Identification – Concise (AUDIT-C)12 questionnaire. Scores were calculated based on the response, alcohol consumers were categorized as low-risk drinkers (AUDIT-C score <5) and hazardous drinkers (AUDIT-C score ≥5) according to standard protocols.
Measurements
Body weight was measured using an electronic weighing scale (SECA 813), height was measured by a stadiometer (SECA 208), and waist circumference was measured using a non-stretchable measuring tape between the costal margins and the iliac crest at the end of expiration. Blood pressure was recorded using the electronic Omron machine in sitting position in the right arm (Model HEM-7130, Omron Healthcare, Singapore) on two occasions 15 minutes apart. The average value was used to determine the hypertension status. Fibrosis screening was done, by two experienced Echosens certified operators using Echosens Fibroscan machine 402. The manufacturer's protocol for validity of liver stiffness measurement (LSM) readings – 10 valid readings, > 50 % success, IQR < 30% was followed.
Blood chemistry
Five ml of non-fasting blood was collected from all participants. Glycated haemoglobin (HbA1c) was measured using an automated high-performance liquid chromatography method (D-10-Bio-Rad). Serum and plasma were prepared from the blood as per standard protocols. The total lipid profile and creatinine in serum were measured using auto-analyzers (Abbott Architech ci8200). Liver function tests were performed by auto analyser (Cobas 6000). Initial viral hepatitis screening was done with WHO approved SD BIOLINE cards for Hepatitis B surface antigen (HBsAg), Hepatitis C antibody (Anti HCV) using whole blood samples.
Definition of outcomes
Obesity and abdominal obesity were defined as body mass index (BMI) ≥25 kg/m2 and waist circumference ≥90 cm for men and ≥80 cm for women, respectively.13 Hypertension was defined as either having a history of hypertension on medications or a systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg. Diabetes was defined as either having a history of diabetes on medications or HbA1c level of ≥ 6.5% in those without a history of diabetes. Prediabetes was defined as HbA1c between or 5.7-6.4% (American Diabetes Association) in those without a history of diabetes. Generalized hypercholesterolemia was defined as a total cholesterol ≥ 200 mg/dL or history of hypercholesterolemia. Dyslipidemia was defined as a Total Cholesterol ≥ 200 mg/ dL, LDL-C ≥ 130 mg/dL, HDL-C < 40 mg/dL in men and < 50 mg/dL in women. Metabolic syndrome (MS) was defined according to the criteria of the International Diabetes Federation14 i.e., those with abdominal obesity and any two of the following risk factors [diabetes, hypertension or reduced c-HDL, (triglycerides not considered as they were non-fasting samples)].
LSM cut-offs used to define fibrosis and cirrhosis (F0/F1 < 6.5, F2 = 6.5- 9.4, F3 = 9.5–11.9, F4/Cirrhosis ≥12Kpa) were extrapolated from published meta-analysis.15 Abnormal LFTs were defined as one of the LFTs (ALT, AST or GTT) above our lab's normal limits.
Aetiology of liver fibrosis
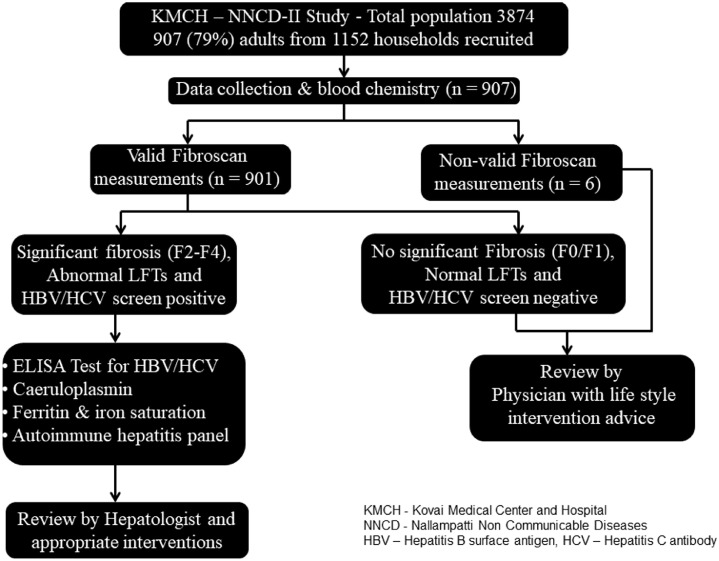
The participants who were found to have either abnormal LFTs or significant liver fibrosis (>F2) or positive for Hepatitis B/C were invited for consultation with the hepatologist during the following week (Figure 1). After clinical review and intervention with informed consent, the participants were subjected for further analyses of etiological factors of liver fibrosis that includes ELISA test for Hepatitis B surface antigen (HBsAg), Hepatitis C antibody (Anti HCV), caeruloplasmin, ferritin and iron saturation and auto-immune hepatitis panel consisting of serum immunoglobulins, anti-nuclear antibody, anti-mitochondrial antibody, anti-smooth muscle antibody, anti-liver kidney microsome antibody were performed by SRL laboratories using immunofluorescence.
Figure 1.
Stepwise methodology followed for assessment of liver fibrosis and its risk factors in KMCH-NNCD-II study.
Statistical analysis
Fisher's exact test was used to examine differences across the ordinal categories of fibrosis stage. Spearman correlation analysis was performed to study the association of liver stiffness with hepatic markers. Multivariate logistic regression was done after dichotomizing the prevalence of fibrosis, following which odds ratios (OR) were calculated for different risk factors previously known to be associated with prevalence of liver fibrosis. The risk factors included age, sex, alcohol consumption, obesity, diabetes, hypercholesterolemia, and hypertension. The control population with no fibrosis was defined as the reference group for logistic regression analyses. Our logistic regression models were fitted with appropriate degrees of adjustment. Statistical significance was determined based on two-sided tests at a 5% significance level with description presented in tables. All statistical analysis were performed using SPSS version 23.0.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of this manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Through purposive sampling techniques, we enrolled 907 adults from 1152 households (79%), out of a population of 3874 (Figure 1); there were no dropouts after enrolment. The characteristics of the study population is given in Table 1. Valid elastography readings were achieved in 99.3% (901/907). Alcohol use recorded only in males, in whom 22.6% (98/433) admitted to drinking and among them based on AUDIT-C score, 74.5% (73/98) of whom were heavy drinkers with high risk (≥ 5). As observed in our previous study, a remarkably high prevalence of metabolic risk factors was observed; 23.6% of the population had the metabolic syndrome according to the IDF criteria. Based on screening for blood chemistry, 8.8% of the population had abnormal LFTs. Liver stiffness measurements showed that 85.6% has no fibrosis (F0 & F1), 14.4% F2-F4 fibrosis and cirrhosis (>F4) was observed in 0.8%. Male sex, generalized obesity, elevated glycated hemoglobulin, high blood pressure, abnormal LFTs and metabolic syndrome showed a statistically significant association with significant fibrosis (Table 1).
Table 1.
Characteristics of the study population.
| KMCH-NNCD-II, 2017 | Whole Population (n = 901a) | No fibrosis F0 & F1(n = 771) | Fibrosis (F2, F3 & F4)(n = 130) | P valueb | |
|---|---|---|---|---|---|
| Sex | Male | 433 (48.1%) | 359 (46.6%) | 74 (56.9%) | 0.029 |
| Female | 468 (51.9%) | 412 (53.4%) | 56 (43.1%) | ||
| Age group | 20-40 | 224 (24.9%) | 200 (25.9%) | 24 (18.5%) | 0.090 |
| 41-60 | 405 (45.0%) | 347 (45.0%) | 58 (44.6%) | ||
| > 60 | 272 (30.2%) | 224 (29.1%) | 48 (36.9%) | ||
| Alcohol intake (only males) | Daily/ weekly | 98 (22.6%) | 76 (21.2%) | 22 (29.7%) | 0.397 |
| AUDIT-C score (only males) | High risk (≥ 5) | 73 (16.9%) | 58 (16.2%) | 15 (20.3%) | 0.541 |
| Low risk (1-4) | 25 (5.8%) | 18 (5.0%) | 7 (9.5%) | ||
| Smoking (only males) | Daily | 92 (21.2%) | 75 (20.9%) | 17 (23.0%) | 0.755 |
| Tobacco chewing | Daily | 127 (14.1%) | 110 (14.3%) | 17 (13.1%) | 0.787 |
| BMI kg/m2 | Generalized obesity (≥ 25) | 360 (40.0%) | 287 (37.2%) | 73 (56.2%) | <0.0001 |
| Abdominal obesity (≥90 for males; ≥80 for females) | 663 (73.6%) | 565 (73.3%) | 98 (75.4%) | 0.668 | |
| HbA1c | Diabetes (≥ 6.5) | 165 (18.3%) | 115 (14.9%) | 50 (38.5%) | <0.0001 |
| Prediabetes (5.6-6.4) | 483 (53.6%) | 426 (55.3%) | 57 (43.8%) | ||
| No diabetes/ prediabetes (≤ 5.6) | 253 (28.1%) | 230 (29.8%) | 23 (17.7%) | ||
| Blood Pressure | Hypertension (≥ 140/90) | 325 (36.1%) | 261 (33.9%) | 64 (49.2%) | 0.001 |
| Lipid Profile mg/dL | Hypercholesterolemia (≥ 200) | 266 (29.5%) | 222 (28.8%) | 44 (33.8%) | 0.254 |
| High LDL-c (≥ 130) | 318 (35.3%) | 271 (35.1%) | 47 (36.2%) | 0.843 | |
| Reduced HDL-c (<40 for males; <50 for females) | 553 (61.4%) | 463 (60.1%) | 90 (69.2%) | 0.051 | |
| LFTs | Abnormal | 79 (8.8%) | 52 (6.7%) | 27 (20.8%) | <0.0001 |
| Liver stiffness | F0 & F1 (< 6.5 kPa) | 771 (85.6%) | 771 (100%) | 0 | - |
| F2 (6.5–9.4) | 110 (12.2%) | 0 | 110 (84.6%) | ||
| F3 (9.4–12.0) | 13 (1.4%) | 0 | 13 (10.0%) | ||
| F4 (> 12.0) | 7 (0.8%) | 0 | 7 (5.4%) | ||
| Metabolic syndrome | Abdominal obesity + two of the following three risk factors (Diabetes, hyper-tension & reduced HDL-c) | 213 (23.6%) | 160 (20.8%) | 53 (40.8 %) | <0.0001 |
Out of 907 participants, only 901 underwent fibroscan screening.
Fisher's exact test.
On multivariate logistic regression analyses, we observed no association between alcohol use, hepatitis virus infection and increased liver stiffness. There was a strong association between abnormal LFTs and increased liver fibrosis (Table 2). Screening for other causes of liver disease (Viral screen/Ferritin/Caeruloplasmin/Auto-immune panel) was done in those with abnormal LFTs and significant liver fibrosis (Figure 1). Apart from a prevalence of Hepatitis B surface antigen (HBsAg) positivity in 0.1%, none of the other liver screening aetiology was positive in this group. Spearman correlation analyses among liver markers indicated a positive association of liver fibrosis with ALT, ALP, GGT and direct bilirubin only, whereas neither AST nor albumin showed any significant correlation with liver fibrosis (Table 3).
Table 2.
Association of liver fibrosis with its etiological factors (age, sex and lifestyle factors, hepatitis virus infection and immune markers).
| Risk factor | Odds Ratio (95% Confidence interval) |
P value | ||
|---|---|---|---|---|
| No significant fibrosis (F0 & F1) | Significant Fibrosis (F2, F3 & F4) | |||
| Sex (Male) | 1 | 1.517 (1.042–2.206) | 0.029 | |
| Age | 41–60 years | 1 | 1.393 (0.839–2.312) | 0.200 |
| > 60 years | 1 | 1.786 (1.056–3.021) | 0.031 | |
| Alcohol consumption (Daily/ weekly) | 1 | 1.723 (0.688–4.318) | 0.245 | |
| AUDIT-C Risk score (≥ 5) | 1 | 1.603 (0.879–2.924) | 0.124 | |
| Smoking (Daily) | 1 | 1.662 (0.694–3.980) | 0.254 | |
| Tobacco chewing (Daily) | 1 | 0.773 (0.202-2.953) | 0.706 | |
| Hepatitis B/C virus infection | Only one positive | - | ||
| Abnormal LFTs | 1 | 3.625 (2.179–6.028) | <0.0001 | |
Table 3.
Correlation and linear regression between median liver stiffness and liver markers.
| Spearman Correlation co-efficient of kPa |
|||
|---|---|---|---|
| Whole population | No fibrosis (F0 & F1) | Fibrosis (F2, F3 & F4) | |
| AST | 0.027 | 0.062 | -0.19* |
| ALT | 0.160** | 0.148** | -0.20* |
| ALP | 0.157** | 0.120** | 0.003 |
| GGT | 0.249** | 0.177** | -0.164 |
| Total bilirubin | 0.041 | 0.05 | 0.056 |
| Direct bilirubin | 0.092** | 0.076* | 0.016 |
| Indirect bilirubin | 0.011 | 0.039 | 0.096 |
| Albumin | -0.024 | 0.013 | 0.021 |
*P < 0.05; **P < 0.01.
We performed multivariate regression analyses to study the association of metabolic risk factors with significant liver fibrosis with varying degrees of adjustment with confounding risk factors. Among the metabolic risk factors, diabetes [OR – 3.206 (95% CI: 1.792 – 5.736)] and generalized obesity [1.987 (1.341 – 2.944)] showed an association with significant liver fibrosis (F2-F4) after adjustment for all confounding factors (Table 4). Though hypertension showed an association with fibrosis in the initial analysis, but lost significance after adjustment. Logistic regression analysis showed a significant association between the metabolic syndrome and liver fibrosis [O.R. 2.539 (1.680-3.836)] (Table 5).
Table 4.
Association of metabolic risk factors (glycaemic status, obesity, hypertension, and hypercholesterolemia) with liver fibrosis.
| Risk factor | Odds Ratio (95% Confidence interval) |
P value | ||
|---|---|---|---|---|
| No significant fibrosis (F0 & F1) | Significant Fibrosis (F2, F3 & F4) | |||
| Diabetes | Unadjusted | 1 | 4.348 (2.528–7.477) | < 0.0001 |
| Model 1 | 1 | 4.026 (2.290–7.080) | < 0.0001 | |
| Model 2 | 1 | 3.991 (2.267–7.026) | < 0.0001 | |
| Model 3 | 1 | 3.206 (1.792–5.736) | < 0.0001 | |
| Pre-diabetes | Unadjusted | 1 | 1.338 (0.803–2.228) | 0.263 |
| Model 1 | 1 | 1.301 (0.775–2.185) | 0.320 | |
| Model 2 | 1 | 1.291 (0.768–2.171) | 0.335 | |
| Model 3 | 1 | 1.154 (0.680–1.956) | 0.596 | |
| Obesity | Unadjusted | 1 | 2.160 (1.483–3.145) | < 0.0001 |
| Model 1 | 1 | 2.329 (1.590–3.412) | < 0.0001 | |
| Model 2 | 1 | 2.321 (1.583–3.404) | < 0.0001 | |
| Model 3 | 1 | 1.987 (1.341–2.944) | 0.001 | |
| Hypertension | Unadjusted | 1 | 1.895 (1.303–2.756) | 0.001 |
| Model 1 | 1 | 1.725 (1.156–2.573) | 0.008 | |
| Model 2 | 1 | 1.774 (1.186–2.652) | 0.005 | |
| Model 3 | 1 | 1.370 (0.901–2.082) | 0.141 | |
| Hypercholesterolemia | Unadjusted | 1 | 1.265 (0.852–1.879) | 0.243 |
| Model 1 | 1 | 1.277 (0.855–1.906) | 0.232 | |
| Model 2 | 1 | 1.273 (0.852–1.901) | 0.238 | |
| Model 3 | 1 | 1.131 (0.747–1.712) | 0.562 | |
Model 1 – adjusted for age & sex; Model 2 – adjusted age, sex and lifestyle factors (alcohol, smoking and tobacco chewing); Model 3 – adjusted for age, sex, lifestyle and metabolic factors (diabetes, blood pressure, waist circumference, BMI status, blood cholesterol profile).
Table 5.
Association of metabolic syndrome with liver fibrosis.
| Risk factor | Odds Ratio (95% Confidence interval) |
P value | ||
|---|---|---|---|---|
| No significant fibrosis (F0 & F1) | Significant Fibrosis (F2, F3 & F4) | |||
| Unadjusted | 1 | 2.628 (1.778–3.885) | < 0.0001 | |
| Metabolic Syndrome | Model 1 | 1 | 2.522 (1.674–3.800) | < 0.0001 |
| Model 2 | 1 | 2.539 (1.680–3.836) | < 0.0001 | |
Model 1 – adjusted for age & sex; Model 2 – adjusted age, sex and lifestyle factors (alcohol, smoking and tobacco chewing).
Discussion
The aim of the study was to determine the prevalence, severity and causes of liver fibrosis in a rural population in Low-to-Middle-Income Countries setting. We enrolled one adult from each household, suggesting this is a representative sampling of majority of the adult population. The prevalence of abnormal LFTs in our study is 8.8% is very much in line with published data16 in those in urban communities. Elastography results in our study are also in line with results obtained in similar community screening.8, 9, 10 Minor differences were due to differences in classification of fibrosis. Our F4 fibrosis rates of 0.8% have been validated and accepted as the prevalence rate of significant fibrosis.3
Our questionnaire did not include women's alcohol consumption because for cultural reasons. alcohol consumption is considered taboo among Indian women. Therefore, in line with the National Survey on Extent, Pattern and Trends of Drug Abuse in India, we did not question women to avoid causing distress and offence Our estimates of alcohol consumption among males are similar to overall prevalence data (20-39.9%) in India.17 Little is known about the association between the risk of fibrosis and the drinking pattern as defined by AUDIT questionnaire; we found no significant difference in fibrosis between those with non-hazardous and hazardous drinking, this association is seen only in those with a diagnosed ARLD.18
Our previous epidemiological11,19 studies indicated the role of insecticides and heavy metals and its association with diabetes among rural farming communities. Non-alcoholic fatty liver disease (NAFLD) has been established as the most common cause of abnormal LFTs both in the western and eastern populations20, 21, 22 and increased liver fibrosis has been reported in those with metabolic syndrome (MS) and in those with diabetes.8,9 Development of NAFLD by gut microbiota alteration via translocated microbial products have been shown in many preclinical studies but different NAFLD subtypes may have different metabolites as a driver for disease progression because not all patients with NAFLD have a disrupted gut barrier.23 Our data suggesting involvement of insecticides and heavy metals19 in development of metabolic syndrome makes us feel that the same factors may contribute to a higher prevalence of fibrosis/NAFLD in Indian sub-continent.22 Since advanced liver fibrosis occurs in non-obese Indians,22 the role of insecticides and heavy metals as metabolites, at least in part, leading to differences in metabolic milieu as seen in lean and non-lean NAFLD24 warrants further investigation. Although we had not assessed liver histology, we feel that advanced fibrosis seen in our study is due to MS as has been validated with liver biopsy in a previous Indian study.22
The prevalence of hepatitis B virus (HBV) was 0.1% and we did not find and any person with hepatitis C virus (HCV) infection. These rates are lower than the WHO estimate of prevalence of 2% HBV and 0.5% HCV in India. A recent meta-analysis on the prevalence of viral hepatitis in India showed25 heterogeneity; some of the variation can be attributed to different testing strategies, sample size and technical factors. There are varying prevalence rates across the districts of Tamil Nadu26 and our study population district had the lowest prevalence rates of 0.27% (HBV) and 0.09% (HCV) raising the possibility that adoption of universal HBV immunization and improved living standards contributed to the low prevalence. In India, the prevalence of auto-immune liver disease,27 primary biliary cholangitis28 and haemochromatosis29 is low, and there are no community-based incidence and prevalence studies on Wilson's disease.30 Hence it is not surprising that our liver screen did not yield any positives.
Our study includes several limitations including the bias based on self-reporting of tobacco and alcohol use; this is unavoidable in any population study. However, we have tried to overcome this bias by relying on estimations of disease prevalence based on objective measurement of different clinical parameters. Achieving a high success rate of elastography measurement shows that with trained operators, fibrosis screening is feasible in community setting. The degree of fibrosis has been shown to be the most reliable factor associated with morbidity and mortality in NAFLD, and elastography is more reliable in people of South Asian descent than other non-invasive markers of liver fibrosis in fatty liver disease,31 thus validating elastography as a pragmatic community screening tool. Our study has a few limitations with sampling; randomised cluster sampling cannot reliably be done as it is sometimes perceived as an offence with social reasons contributing to both willingness and also being a barrier to research participation.32 The use of only HbA1c to determine undiagnosed diabetes may have missed some people who would have been considered to have diabetes based on fasting glucose or 2-hour glucose after a glucose challenge.
Although we did not ascertain steatosis using histology, ultrasound or controlled attenuation parameter, our protocol screening for aetiology of liver disease in subjects with abnormal liver tests helped us to rule out other liver diseases and validate the finding that fatty liver is the common liver disease in an asymptomatic population. One of the limitations of our study is the difficulty in dichotomising the contribution of individual factors (aetiology, BMI, sex, age, AST and diabetes) altering the LSM values. Another limitation of our study is the use of reference standard of LSM values with various aetiologies15 rather than being specific for NAFLD but for community screening, our choice of reference standard with various aetiologies appeared relevant. The cut-offs and thresholds employed was based on already established clinical values, hence no sensitivity analyses has been performed, which is another limitation of our study. The Fibroscan 402 machine used in our study had only M probe measuring fibrosis and not steatosis but with a setting that indicates whether the measurement was unreliable: this we encountered in 0.7% of scans. We may have overestimated fibrosis by using M probe alone in our population with 40% having BMI > 25 but a recent metanalysis33 of individual patient data showed that the difference between M and XL probe values in a given patient may be large, but their mean difference in a given population is quite small. Elastography not being done in a fasting state could have contributed to some high values, but a strict two-hour fasting is often not feasible in clinic visits and point-of-care testing.34 Severe enzyme elevation due to hepatitis/inflammation or cholestasis contributes to high transient elastography values and may have increased our fibrosis estimation. We reviewed the transaminase values of our study population and found only two with extreme enzyme elevation (AST or ALT > 100). Elastography values in these two participants was 6.0 (no significant fibrosis) and protocol liver screen in both was negative. Hence, we feel that hepatitis or cholestasis did not contribute to any bias in our estimation of liver fibrosis.
We conclude by noting that nearly 9% of the studied population have abnormal LFTs. Aetiological screening for abnormal LFTs, other than for fatty liver disease, alcohol, and viral hepatitis, in a community setting is of limited value. Normal LFTs may falsely reassure people with metabolic syndrome and hazardous drinking behaviour. Our findings highlight the prevalence of liver fibrosis in physically active rural population being equivalent to urban counterparts and highlights the association of metabolic risk factors with liver fibrosis in low- and middle-income country. Our study suggests early identification of chronic liver disease (CLD) in the community using elastography screening is a feasible screening strategy.
Contributors
A.R., A.S., S.M., K.S. & J.N. conceived and designed the experiments. A.R., G.V., A.S., S.M. & K.S. involved in data acquisition. G.V., S.M., D.V. & P.N. analyzed the data. A.R., G.V. & J.N. interpreted the results and wrote the manuscript. P.V. & K.S. revised the manuscript. All authors read and approved the final manuscript.
Data sharing statement
Data are available upon reasonable request to the corresponding authors.
Declaration of interests
We declare no competing interests.
Acknowledgments
This study is funded by KMCH Research Foundation, India. We acknowledge Dr Nalla. G. Palanisami and other trustees of KMCH Research Foundation for their continuous support. We acknowledge Echosens/Abbott for provision of Fibroscan machines, SRL labs for processing liver screen. None of the above industries were involved in the design/data interpretation/manuscript writing. We acknowledge our postgraduates, social workers, and student/staff from KMCH allied health services for conduct of the study and KMCH research foundation for funding this study who yet again had no role in the design/data interpretation/manuscript writing.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101553.
Appendix. Supplementary materials
References
- 1.Williams R, Ashton K, Aspinall R, et al. Implementation of the lancet standing commission on liver disease in the UK. Lancet. 2015;386(10008):2098–2111. doi: 10.1016/S0140-6736(15)00680-7. [DOI] [PubMed] [Google Scholar]
- 2.Harrison P, Hogan BJ, Floros L, Davies E, Guideline Development G. Assessment and management of cirrhosis in people older than 16 years: summary of NICE guidance. BMJ. 2016;354:i2850. doi: 10.1136/bmj.i2850. [DOI] [PubMed] [Google Scholar]
- 3.Gines P, Graupera I, Lammert F, et al. Screening for liver fibrosis in the general population: a call for action. Lancet Gastroenterol Hepatol. 2016;1(3):256–260. doi: 10.1016/S2468-1253(16)30081-4. [DOI] [PubMed] [Google Scholar]
- 4.Wilson JMG. World Health Organization; 1968. Jungner, Gunnar, World Health Organization. Principles and Practice of Screening for Disease /J. M. G. Wilson, G. Jungner.https://apps.who.int/iris/handle/10665/37650 [Google Scholar]
- 5.McLernon DJ, Donnan PT, Ryder S, et al. Health outcomes following liver function testing in primary care: a retrospective cohort study. Fam Pract. 2009;26(4):251–259. doi: 10.1093/fampra/cmp025. [DOI] [PubMed] [Google Scholar]
- 6.Tapper EB, Saini SD, Sengupta N. Extensive testing or focused testing of patients with elevated liver enzymes. J Hepatol. 2017;66(2):313–319. doi: 10.1016/j.jhep.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Lilford RJ, Bentham L, Girling A, et al. Birmingham and Lambeth Liver Evaluation Testing Strategies (BALLETS): a prospective cohort study. Health Technol Assess. 2013;17(28):i–xiv. doi: 10.3310/hta17280. 1-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roulot D, Costes JL, Buyck JF, et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut. 2011;60(7):977–984. doi: 10.1136/gut.2010.221382. [DOI] [PubMed] [Google Scholar]
- 9.Abeysekera KWM, Fernandes GS, Hammerton G, et al. Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. Lancet Gastroenterol Hepatol. 2020;5(3):295–305. doi: 10.1016/S2468-1253(19)30419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caballeria L, Pera G, Arteaga I, et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin Gastroenterol Hepatol. 2018;16(7) doi: 10.1016/j.cgh.2017.12.048. 1138–1145.e5. [DOI] [PubMed] [Google Scholar]
- 11.Velmurugan G, Swaminathan K, Mohanraj S, et al. Association of co-accumulation of arsenic and organophosphate insecticides with diabetes and atherosclerosis in a rural agricultural community: KMCH-NNCD-I study. Acta Diabetol. 2020;57(10):1159–1168. doi: 10.1007/s00592-020-01516-6. [DOI] [PubMed] [Google Scholar]
- 12.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 13.Misra A, Chowbey P, Makkar BM, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–170. [PubMed] [Google Scholar]
- 14.2020. IDF Consensus Worldwide Definition of the Metabolic Syndrome.https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html Accessed 16 October 2020. [Google Scholar]
- 15.Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54(4):650–659. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98(5):960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 17.Collaborators GBDA Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim JK, Tate JP, Fultz SL, et al. Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus-infected, and uninfected patients. Clin Infect Dis. 2014;58(10):1449–1458. doi: 10.1093/cid/ciu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velmurugan G, Ramprasath T, Swaminathan K, et al. Gut microbial degradation of organophosphate insecticides-induces glucose intolerance via gluconeogenesis. Genome Biol. 2017;18(1):8. doi: 10.1186/s13059-016-1134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178(1):38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu CS, Kao JH. An update on non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in Asia. Expert Rev Gastroenterol Hepatol. 2017;11(8):759–772. doi: 10.1080/17474124.2017.1342535. [DOI] [PubMed] [Google Scholar]
- 22.Das K, Das K, Mukherjee PS, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51(5):1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 23.Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68(2):359–370. doi: 10.1136/gutjnl-2018-316307. [DOI] [PubMed] [Google Scholar]
- 24.Chen F, Esmaili S, Rogers GB, et al. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology. 2020;71(4):1213–1227. doi: 10.1002/hep.30908. [DOI] [PubMed] [Google Scholar]
- 25.Goel A, Seguy N, Aggarwal R. Burden of hepatitis C virus infection in India: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2019;34(2):321–329. doi: 10.1111/jgh.14466. [DOI] [PubMed] [Google Scholar]
- 26.Shanmugam RP, Balakrishnan S, Varadhan H, Shanmugam V. Prevalence of hepatitis B and hepatitis C infection from a population-based study in Southern India. Eur J Gastroenterol Hepatol. 2018;30(11):1344–1351. doi: 10.1097/MEG.0000000000001180. [DOI] [PubMed] [Google Scholar]
- 27.Choudhuri G, Somani SK, Baba CS, Alexander G. Autoimmune hepatitis in India: profile of an uncommon disease. BMC Gastroenterol. 2005;5:27. doi: 10.1186/1471-230X-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarin SK, Monga R, Sandhu BS, Sharma BC, Sakhuja P, Malhotra V. Primary biliary cirrhosis in India. Hepatobiliary Pancreat Dis Int. 2006;5(1):105–109. [PubMed] [Google Scholar]
- 29.Thakur V, Guptan RC, Hashmi AZ, Sakhuja P, Malhotra V, Sarin SK. Absence of hemochromatosis associated Cys282Tyr HFE gene mutation and low frequency of hemochromatosis phenotype in nonalcoholic chronic liver disease patients in India. J Gastroenterol Hepatol. 2004;19(1):86–90. doi: 10.1111/j.1440-1746.2004.03262.x. [DOI] [PubMed] [Google Scholar]
- 30.Nagral A, Sarma MS, Matthai J, et al. Wilson's Disease: clinical practice guidelines of the Indian National Association for study of the liver, the indian society of pediatric gastroenterology, hepatology and nutrition, and the movement disorders society of India. J Clin Exp Hepatol. 2019;9(1):74–98. doi: 10.1016/j.jceh.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Silva S, Li W, Kemos P, et al. Non-invasive markers of liver fibrosis in fatty liver disease are unreliable in people of South Asian descent. Frontline Gastroenterol. 2018;9(2):115–121. doi: 10.1136/flgastro-2017-100865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Browne JL, Rees CO, van Delden JJM, et al. The willingness to participate in biomedical research involving human beings in low- and middle-income countries: a systematic review. Trop Med Int Health. 2019;24(3):264–279. doi: 10.1111/tmi.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petroff D, Blank V, Newsome PN, et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(3):185–198. doi: 10.1016/S2468-1253(20)30357-5. [DOI] [PubMed] [Google Scholar]
- 34.Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2(2) doi: 10.1016/j.jhepr.2020.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.