Summary
Background
Dementia greatly contributes to poor prognosis in patients with Parkinson's disease (PD). We previously reported that severe olfactory dysfunction may be a good predictor of Parkinson's disease dementia (PDD). In this trial, we investigated whether early administration of donepezil to patients with severe hyposmia can reduce the development of PDD.
Methods
This was a multi-centre, randomized, double-blind, parallel group, placebo-controlled trial in patients with non-demented PD with severe hyposmia (The Donepezil Application for Severe Hyposmic Parkinson's Disease [DASH-PD] study). A total of 201 patients were randomly allocated to receive donepezil or placebo in addition to standard therapy for PD. Patients were followed up every 6 months until the onset of PDD or for a maximum of 4 years. The primary endpoint was the onset of dementia. The secondary endpoint was cognitive impairment measured by Addenbrooke's Cognitive Examination-Revised (ACE-R) and the Clinical Dementia Rating (CDR).
(UMIN000009958: February 2013 to May 2019).
Findings
A total of 201 hyposmic patients with PD were randomly assigned to a treatment: 103 to donepezil and 98 to placebo. Overall, 141 (70%) patients completed the 4-year intervention. During follow-up, 7 of 103 (6.8%) patients in the donepezil group and 12 of 98 (12.2%) patients in the placebo group developed PDD; however, the hazard ratio of PDD incidence was not statistically significant (hazard ratio (HR), 0.609; 95% confidence interval, 0.240 to 1.547; p = 0.2969). At week 208, the patients in the donepezil group had better scores on the ACE-R (p < 0.005) and the CDR (p < 0.005) than those taking placebo.
Interpretation
Administration of donepezil to PD patients with severe olfactory dysfunction for 4 years did not change the incidence of dementia but had a beneficial effect on neuropsychological function, with good tolerability.
Funding
The Ministry of Health Labour and Welfare and the Japan Agency for Medical Research and Development provided funding for this study.
Keywords: Parkinson's disease, Hyposmia, Cholinesterase inhibitor, Dementia, Prevention
Research in context.
Evidence before this study
Dementia is a common and debilitating condition in Parkinson's disease (PD). Finding treatments for the prevention of dementia is an important issue. Several clinical trials have shown beneficial effects of cholinesterase inhibitors on cognition in PD dementia. However, it has not been confirmed whether cholinesterase inhibitors prevent or delay the onset of dementia. To investigate the evidence base for dementia prevention in PD, we searched PubMed up to May 31, 2019 using the search terms “dementia”, “cholinesterase inhibitor” and “prevention”. To our knowledge, there are no randomised, controlled studies aiming to delay or prevent the onset of dementia in PD.
Added value of this study
To our knowledge, the Donepezil Application for Severe Hyposmic Parkinson's Disease [DASH-PD] study is the first randomised, double-blind, placebo-controlled trial to investigate the effect of a very long-term administration of donepezil on conversion to dementia in patients with PD with severe hyposmia. The study is an important addition to the evidence base for dementia prevention in PD, for which high-level evidence is currently lacking.
Implication of all the available evidence
A 4-year administration of donepezil did not change the incidence of dementia in PD patients with severe olfactory dysfunction. On the other hand, long-term donepezil treatment had a consistent beneficial effect on cognition. Additionally, donepezil might alleviate levodopa unresponsive non-motor symptoms.
Alt-text: Unlabelled box
Introduction
Dementia is one of the most common and disabling late-stage complications of Parkinson's disease (PD).1 The rate of dementia increases with age and disease duration of PD, and dementia affects quality of life among both patients and caregivers and increases the risks for nursing home placement and mortality. Recent longitudinal studies demonstrated that approximately half of PD patients had converted to dementia 10 years after diagnosis, and up to 80% eventually developed dementia over the long-term course of the disease. Dementia typically occurs in advanced PD; however, the rate of cognitive decline varies widely among individuals, and some patients develop dementia relatively soon after the onset of motor symptoms.
The pathogenesis of cognitive impairment in PD has been found to be multifactorial, with nigral and extranigral Lewy pathology, especially in limbic and neocortical areas, overlapping Alzheimer-type pathology, and additional vascular lesions being the major contributors.2 In addition to these pathological changes, neurochemical alterations, especially in the cholinergic system, have crucial roles in the development of cognitive dysfunction in PD. Cholinergic deficits are present to some degree in the early stages of PD, worsen with disease progression, and are more prominent in PDD than in Alzheimer's disease.3,4 Several clinical trials have shown beneficial effects of cholinesterase inhibitors on cognition in PDD; however, to date, the life expectancy of PDD patients remains very short despite the use of these medications.5
Early therapeutic intervention that can prevent the onset of PDD may substantially improve the long-term prognosis of patients with Parkinson's disease; thus, risk factors for dementia are currently the focus of considerable attention.6 Previously, we reported that severe olfactory dysfunction was a strong predictor of early cognitive decline in PD,7 and similar results have been reported repeatedly by independent researchers.8,9 It was shown that the severity of olfactory dysfunction correlated well with the degree of cholinergic deficits in PD.10 Therefore, we hypothesized that early administration of a cholinesterase inhibitor to PD patients with severe hyposmia can maintain the integrity of cortical networks and reduce cognitive decline and prevent the development of dementia. In this study, we conducted a placebo-controlled trial to investigate the effects of 4-year administration of donepezil in non-demented PD patients with severe olfactory dysfunction.
Methods
Patients
PD patients with severe hyposmia who had not yet developed PDD were recruited from 21 hospitals in Japan from March 2013 to April 2014. Patients were eligible if they were aged 55‒75 years, developed PD at ≥40 years of age, were at Hoehn-Yahr stage I‒III, had severe olfactory dysfunction (Odor Stick Identification Test for the Japanese [OSIT-J]11≤4), agreed to participate in the study and signed a written informed consent form. Diagnosis of PD was made by board certified expert neurologists.
The exclusion criteria included a history of other neurological and/or psychological disorders that may influence motor and cognitive function; use of anticholinergic drugs within 4 weeks prior to enrolment; sinonasal disease affecting olfaction; depression requiring treatment (a score of ≥3 in “1.3. Depressed Mood” in the Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale [MDS-UPDRS]12; suspected impairment in cognitive function (Mini-Mental State Examination [MMSE]13 in the Addenbrooke's Cognitive Examination-Revised [ACE-R]14; <26, or Clinical Dementia Rating [CDR]15 ≥115; a known allergy to donepezil hydrochloride; heart disease; pulmonary disease; severe peptic ulcer; a history of stereotaxic surgery or deep brain stimulation; and a determination that the patient was inappropriate for the study (as judged by the investigators). During the study, medications for Alzheimer's disease and central anticholinergic drugs were not allowed. This study was approved by the ethics committees of Tohoku University Graduate School of Medicine and the National Hospital Organization, Sendai-Nishitaga Hospital and registered at the UMIN Clinical Trials Registry (UMIN000009958) (see Supplementary material for study protocol). The study was conducted in compliance with the principles of the Declaration of Helsinki and the Ethics Guidelines for Clinical Research.
Sample size calculation
In our previous 3-year longitudinal study, 41.7% (10/24) of PD patients with severe hyposmia developed PDD, whereas none of 20 patients without severe hyposmia developed PDD.7 Thus, we assumed that the PDD incidence would be 40% in the placebo group and expected that donepezil treatment would reduce the incidence by half, resulting in an incidence of 20% in the donepezil group. To detect a 20% difference in the PDD incidence with a two-sided significance level of 0.05 and 80% power, 75 patients were required per treatment group. Assuming a 20% drop-out rate led to a need for 188 patients in total. Thus, we decided to recruit 100 patients per group (i.e., 200 patients in total).
Randomization and masking
Patients were randomly allocated to the donepezil group or the placebo group at a 1:1 ratio. Randomization was performed on the Electronic Data Capture (EDC) system using minimization16 to ensure well-balanced allocation regarding age, disease duration, and institution. According to the treatment allocation, patients received a study drug pack that contained either donepezil or placebo. The placebo drugs were visually identical to donepezil. Both patients and research members (investigators and other staff members involved in management, analysis, or assessment of data) were kept blinded to the treatment allocation. To ensure allocation concealment, the allocation list was sealed in an envelope and was not disclosed except in an emergency.
Procedures
In weeks 1 and 2, patients received donepezil hydrochloride 3 mg or a visually identical placebo once daily, in addition to standard therapy for PD. From week 3 onwards, patients were administered donepezil hydrochloride 5 mg or a visually identical placebo once daily in addition to standard therapy until week 208 (end of the treatment period) or the onset of PDD. The standard therapy for PD is a treatment that complies with Japanese guidelines for the treatment of Parkinson's disease,17 including dopamine replacement therapy. Patients were assessed for the tolerability of up-titration after week 2 (at the time of up-titration from 3 to 5 mg/day) and week 4, and the dose was escalated only when no safety issues were identified.
Patients were assessed for the severity of PD and cognitive impairment at baseline and at every 26-week (6-month) follow-up until the completion of 208 weeks of follow-up or the onset of PDD.
The severity of PD was evaluated using the MDS-UPDRS, a measure to assess impairment or disability in PD patients.12 The MDS-UPDRS comprises four parts (part I, non-motor experiences of daily living; part II, motor experiences of daily living; part III, motor examination; and part IV, motor complications), and each item is rated on a 5-point scale (0‒4), with a higher score indicating greater impairment.
Cognitive impairment was assessed using the ACE-R and the CDR. The ACE-R is a brief assessment tool to detect cognitive impairment and evaluates five domains of cognitive function (attention/orientation, memory, verbal fluency, language, and visuospatial ability).14 The total score ranges from 0 to 100, with a higher score indicating better cognitive functioning. In addition, as the ACE-R incorporates the MMSE, a global measure to screen for cognitive impairment,13 an MMSE score (0‒30, with a higher score indicating better cognitive functioning) can also be obtained. The CDR is a scale for the severity of dementia.15 Based on the evaluation of six domains of cognitive and functional performance (memory, orientation, judgement and problem solving, community affairs, home and hobbies, and personal care), patients’ cognitive impairment is rated as one of five stages: 0 = no dementia, 0.5 = questionable dementia, 1 = mild dementia, 2 = moderate dementia, and 3 = severe dementia. Additionally, the total score of six domains is expressed as the CDR sum of boxes score (0‒18), with a higher score indicating greater cognitive impairment.
The primary outcome was the onset of PDD, which was defined by a combination of an MMSE score <26 and CDR stage ≥1. An MMSE cutoff score of <26 is recommended for the diagnosis of PDD.18 When patients developed PDD, an MRI scan was performed to exclude dementia due to other causes. The judgement of PDD was made by investigators. The secondary outcome measures were the ACE-R score and CDR stage/score at each visit. Safety evaluations included adverse events (AEs), vital signs, laboratory test results, and the MDS-UPDRS score at each visit. AEs were assessed throughout the treatment period.
Statistical analyses
Statistical analyses were performed on an intention to treat approach. The efficacy analysis population was the full analysis set (FAS), which consisted of patients who received at least one dose of study drugs and had data from at least one visit. The safety analysis population consisted of patients who were allocated and received at least one dose of study drugs.
For the primary outcome, the PDD incidence was analysed using a Cox proportional hazard model that included treatment group (donepezil or placebo) as an explanatory variable. For the secondary outcome measures, the ACE-R score and CDR stage/score at each visit were descriptively summarized for each group. Additionally, the change in scores over time was analysed using a general linear model, which included baseline score as a covariate, visit and treatment group as fixed effects, and patient as a random effect. For safety analysis, AEs (coded according to the common terminology criteria for adverse events [CTCAE] v.4.0) were descriptively summarized. The following predefined AEs were summarized descriptively for each group: nausea, vomiting, anorexia, diarrhoea, dizziness, pollakiuria, and others. For the severity of PD, the MDS-UPDRS score at each visit was summarized descriptively for each group. The change in scores over time was analysed using the general linear model, as described above.
All statistical tests were two-sided, with the level of significance set at p < 0.05. All statistical analyses were performed using SAS 9.4 for Windows (SAS Institute, Cary, NC, USA).
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
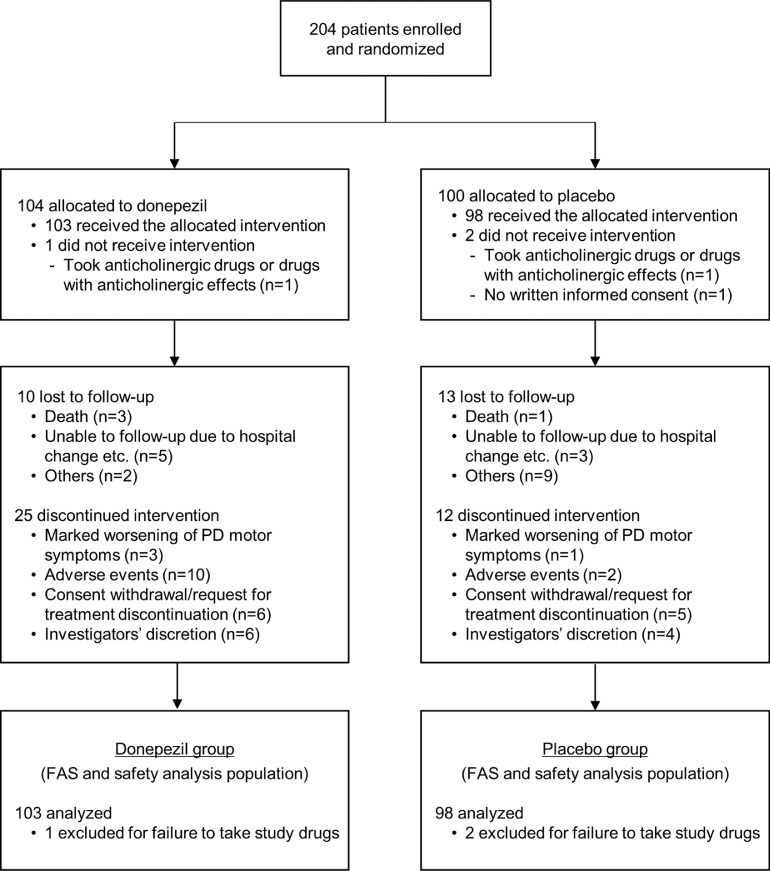
A total of 204 patients underwent randomization: 104 patients were allocated to the donepezil group, and 100 patients were allocated to the placebo group (Figure 1). Of these, 2 patients (1 for each group) who took anticholinergic drugs or drugs with anticholinergic effects within 4 weeks (an exclusion criterion) and 1 patient in the placebo group who orally agreed to participate but did not provide written informed consent did not receive study drugs and were thus excluded from both the FAS and safety analysis populations. Consequently, a total of 201 patients (103 for the donepezil group and 98 for the placebo group) constituted both the efficacy and safety analysis population.
Figure 1.
Trial flow chart.
PD=Parkinson's disease, FAS=full analysis set
The baseline characteristics of the patients (efficacy and safety analysis population) are summarized in Table 1. The donepezil and placebo groups were similar in terms of demographic and clinical characteristics such as age, sex ratio, and PD duration, with no marked differences between the groups, even in the subanalysis of patients who completed the 4-year intervention (Supplementary Table 1). In both groups, approximately 60% of patients were at Hoehn-Yahr stage II, and approximately 30% were at stage III. At baseline, the mean MMSE scores ± standard deviation (SD) were 28.8 ± 1.1 in the donepezil group and 28.6 ± 1.5 in the placebo group. During follow-up, 25 of 103 patients in the donepezil group and 12 of 98 patients in the placebo group discontinued treatment due to marked worsening of PD motor symptoms (n = 3 and 1 in respective groups); AEs (n = 10 and 2); consent withdrawal or patient request for discontinuation (n = 6 and 5); and investigator's discretion (n = 6 and 4) (Figure 1). The overall completion rate for 4-year follow-up was 70.1%. The level of drug adherence was consistently high in both groups (≥94.0%) from weeks 2 through 208. The most common concomitant medications were levodopa and decarboxylase inhibitors (199 patients used at least once), selegiline (101 patients), magnesium oxide (88 patients), pramipexole (83 patients), and zonisamide (70 patients).
Table 1.
Baseline characteristics of patients (efficacy and safety analysis population).
| Characteristics | Donepezil group | Placebo group |
|---|---|---|
| (n = 103) | (n = 98) | |
| Age, years | 67.9 ± 4.6 | 67.9 ± 4.9 |
| Sex, n (%) | ||
| Male | 64 (62.1) | 57 (58.2) |
| Female | 39 (37.9) | 41 (41.8) |
| Height, cm | 160.5 ± 10.2 | 159.4 ± 9.9 |
| Weight, kg | 60.2 ± 11.6 | 57.3 ± 11.5 |
| Body mass index | 23.4 ± 4.0 | 22.4 ± 3.4 |
| Years of education | 13.3 ± 2.3 | 12.9 ± 2.6 |
| PD duration, years | 6.9 ± 4.4 | 6.7 ± 4.1 |
| With complication(s), n (%) | 76 (73.8) | 69 (70.4) |
| Hoehn-Yahr stage, n (%) | ||
| I | 4 (3.9) | 7 (7.1) |
| II | 66 (64.1) | 60 (61.2) |
| III | 33 (32.0) | 31 (31.6) |
| MMSEa | 28.8 ± 1.1 | 28.6 ± 1.5 |
Data are presented as mean ± standard deviation unless otherwise indicated.
PD=Parkinson's disease. MMSE=Mini-Mental State Examination.
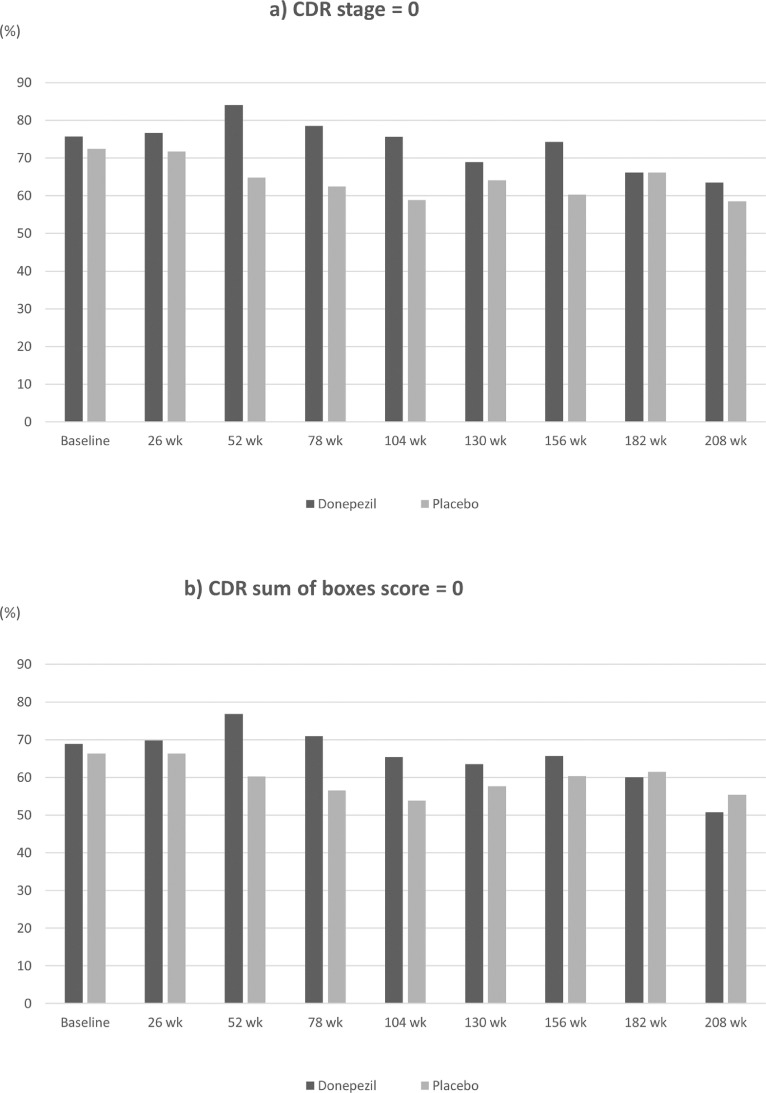
During follow-up, 7 of 103 (6.8%) patients in the donepezil group and 12 of 98 (12.2%) patients in the placebo group developed PDD (primary outcome) (Table 2). Patients who received donepezil had a lower incidence of PDD than those who received placebo; however, the hazard ratio (HR) of PDD incidence was not statistically significant (HR, 0.61; 95% confidence interval, 0.24 to 1.55; p = 0.30). Table 3 summarizes the ACE-R scores at each visit for each group. At baseline, the average ACE-R total scores ± SD were 89.8 ± 6.8 in the donepezil group and 89.6 ± 7.4 in the placebo group, both of which resulted in slightly higher scores at week 208 (94.4 ± 5.4 and 94.1 ± 8.2, respectively). Similarly, the average memory domain scores at week 208 were >3 points higher than those at baseline in both groups. For other domains, the average scores were virtually the same throughout the follow-up period. At every visit, the average scores were comparable between the two groups for all domains, but the average total scores were slightly higher in the donepezil group at several visits, although the difference was quite subtle (Table 3). Analysis using a general linear model revealed that baseline score, visit, and treatment group had significant effects on the ACE-R total scores (p < 0.05) (Table 4), showing that the donepezil group had higher ACE-R total scores than the placebo group over time. At baseline, 75.7% of the donepezil group and 72.4% of the placebo group were at CDR stage 0 (no dementia), and 24.3% and 27.6% of those groups were at stage 0.5 (questionable dementia); no patients were at CDR stage ≥1 in either group. During follow-up, the proportions of patients at CDR stage 0 were higher in the donepezil group than in the placebo group at all visits except for week 182 (Figure 2a). The proportions of patients with a CDR sum of boxes score of 0 (cognitively normal) were also higher in the donepezil group at most visits, although the differences were rather small (Figure 2b). Similar tendencies were also observed for the following CDR domain scores: community affairs, home & hobbies, memory, and orientation (Supplementary Table 2).
Table 2.
Summary of primary endpoint.
| Treatment group (N = 103) |
Placebo group (N = 98) |
Total (N = 201) |
|
|---|---|---|---|
| No. of events | 7 (6.8) | 12 (12.2) | 19 (9.5) |
| No. of censor | 96 (93.2) | 86 (87.8) | 182 (90.5) |
N: Number of participants in FAS.
n: Number of participants.
%: 100*n/N.
Table 3.
The ACE-R scores at every 26-week (6-month) visit.
| Visit | N | ACE-R |
|||||
|---|---|---|---|---|---|---|---|
| Total | Orientation/attention | Memory | Verbal fluency | Language | Visuospatial ability | ||
| (0‒100) | (0‒18) | (0‒26) | (0‒14) | (0‒26) | (0‒16) | ||
| Baseline | Donepezil (n = 103) | 89.8 ± 6.8 | 17.4 ± 0.8 | 20.6 ± 3.7 | 12.1 ± 2.3 | 24.9 ± 1.4 | 14.7 ± 2.0 |
| Placebo (n = 98) | 89.6 ± 7.4 | 17.4 ± 1.0 | 20.5 ± 4.2 | 11.8 ± 2.5 | 24.8 ±1.5 | 15.1 ± 1.4 | |
| Week 26 | Donepezil (n = 86) | 91.2 ± 7.8 | 17.4 ± 1.0 | 21.9 ± 4.2 | 12.1 ± 2.2 | 24.9 ±1.5 | 14.8 ± 2.1 |
| Placebo (n = 92) | 91.3 ± 6.4 | 17.5 ± 1.0 | 21.7 ± 3.8 | 12.1 ± 2.2 | 25.1 ± 1.0 | 15.0 ± 1.7 | |
| Week 52 | Donepezil (n = 82) | 92.6 ± 5.9 | 17.6 ± 0.9 | 22.7 ± 3.3 | 12.2 ± 2.1 | 25.1 ± 1.4 | 15.1 ± 1.4 |
| Placebo (n = 88) | 91.2 ± 7.8 | 17.5 ± 1.3 | 22.0 ± 3.9 | 11.8 ± 2.6 | 25.0 ± 1.2 | 14.9 ± 1.9 | |
| Week 78 | Donepezil (n = 79) | 93.7 ± 5.7 | 17.6 ± 0.7 | 23.2 ± 3.5 | 12.6 ± 1.8 | 25.2 ± 1.3 | 15.2 ± 1.5 |
| Placebo (n = 85) | 92.6 ± 6.9 | 17.5 ± 1.1 | 22.6 ± 3.7 | 12.0 ± 2.3 | 25.2 ± 1.1 | 15.2 ± 1.4 | |
| Week 104 | Donepezil (n = 78) | 93.5 ± 6.1 | 17.6 ± 0.7 | 23.3 ± 3.0 | 12.3 ± 2.1 | 25.2 ± 1.2 | 15.1 ± 1.7 |
| Placebo (n = 80) | 93.3 ± 7.1 | 17.5 ± 1.1 | 22.9 ± 4.0 | 12.5 ± 2.0 | 25.3 ± 1.1 | 15.1 ± 1.5 | |
| Week 130 | Donepezil (n = 74) | 94.4 ± 6.1 | 17.5 ± 0.9 | 23.7 ± 3.3 | 12.5 ± 1.8 | 25.1± 1.2 | 15.4 ± 1.2 |
| Placebo (n = 78) | 92.9 ± 8.7 | 17.4 ± 1.3 | 23.2 ± 3.9 | 11.8 ± 2.9 | 25.3± 1.1 | 15.1 ± 2.0 | |
| Week 156 | Donepezil (n = 70) | 94.5 ± 6.3 | 17.5 ± 0.7 | 24.1± 2.9 | 12.4 ± 2.4 | 25.2 ± 1.3 | 15.2 ± 1.5 |
| Placebo (n = 73) | 93.6 ± 6.7 | 17.6 ± 0.8 | 23.4 ± 3.9 | 12.1 ± 2.3 | 25.2 ± 1.0 | 15.3 ± 1.4 | |
| Week 182 | Donepezil (n = 65) | 94.6 ± 5.3 | 17.7 ± 0.7 | 24.1 ± 2.7 | 12.1 ± 2.5 | 25.3 ± 0.9 | 15.4 ± 1.3 |
| Placebo (n = 65) | 94.2 ± 6.5 | 17.7 ± 0.8 | 24.0 ± 3.3 | 12.1 ± 2.5 | 25.2 ± 1.4 | 15.2 ± 1.5 | |
| Week 208 | Donepezil (n = 63) | 94.4 ± 5.4 | 17.7 ± 0.6 | 24.0 ± 2.8 | 12.2 ± 2.5 | 25.3 ± 0.9 | 15.2 ± 1.2 |
| Placebo (n = 65) | 94.1 ± 8.2 | 17.3 ± 1.7 | 24.0 ± 3.5 | 12.2 ± 2.4 | 25.4 ± 0.8 | 15.2 ± 1.8 | |
Data are presented as mean ± standard deviation.
ACE-R=Addenbrooke's Cognitive Examination-Revised.
Table 4.
Results of the general linear model for the effects on the ACE-R score.
| Effect | ACE-R |
|||||
|---|---|---|---|---|---|---|
| Total | Orient/attent | Memory | Verbal fluency | Language | Visuospatial | |
| Baseline score | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Visit | 0.0004 | 0.4380 | < 0.0001 | 0.2045 | 0.0661 | 0.5948 |
| Treatment group | 0.0339 | 0.0932 | 0.2117 | 0.1057 | 0.7670 | 0.0775 |
| Visit*Treatment group | 0.2985 | 0.1061 | 0.8190 | 0.0828 | 0.3852 | 0.7634 |
Values presented are p values.
ACE-R=Addenbrooke's Cognitive Examination-Revised.
Figure 2.
Proportion of patients with a) a CDR stage of 0 and b) a CDR sum of boxes score of 0 among each treatment group at every 26-week visit.
CDR=Clinical Dementia Rating.
aCDR stage: 0 = no dementia, 0.5 = questionable dementia, 1 = mild dementia, 2 = moderate dementia, and 3 = severe dementia.
bThe CDR sum of boxes score (range: 0-18) is a total score of six domains of cognitive and functional performance (each rated as 0, 0.5, 1, 2, or 3).
The results of the general linear model indicated that baseline score, visit, and treatment group had significant effects on CDR stage, sum of boxes score, and the memory and orientation domains (p < 0.05) (Table 5), showing that cognitively normal patients or patients without cognitive impairment in the memory/orientation domains were more frequent in the donepezil group than in the placebo group over time. The analysis also revealed that baseline score and treatment group had significant effects on the domains community affairs and home & hobbies (p < 0.05), indicating that patients without impairment in these cognitive domains were more frequent in the donepezil group than in the placebo group throughout the follow-up period.
Table 5.
Results of the general linear model for the effects on the CDR stage/scores.
| Effect | CDR stage | CDR sum of boxes |
CDR domains |
|||||
|---|---|---|---|---|---|---|---|---|
| Community affairs | Home & hobbies | Judge & problem solve | Memory | Orientation | Personal care | |||
| Baseline score | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0002 |
| Visit | 0.0086 | 0.0041 | 0.3754 | 0.3397 | 0.5054 | 0.0003 | 0.0003 | 0.0862 |
| Treatment group | 0.0002 | 0.0418 | 0.0103 | 0.0003 | 0.8220 | 0.0003 | 0.0062 | 0.3701 |
| Visit*Treatment group | 0.2931 | 0.2464 | 0.8096 | 0.9781 | 0.9924 | 0.2250 | 0.9949 | 0.9207 |
Values presented are p values.
CDR=Clinical Dementia Rating.
The occurrence of any AE, according to CTCAE v.4.0, was comparable between the donepezil and placebo groups (59.2% and 51.0%, respectively). Most (>90%) AEs were mild or moderate. Serious AEs were reported in 17.5% of the donepezil group and 12.2% of the placebo group. Of the predefined AEs examined, pollakiuria was most common in both groups (10.7% in the donepezil group and 6.1% in the placebo group) (Table 6). For cholinergic AEs, nausea occurred more often in the donepezil group than in the placebo group (8.7% vs. 4.1%); however, anorexia was reported in similar proportions of patients (4.9% vs. 4.1%). Dizziness occurred more frequently among the placebo group (5.1%) than among the donepezil group (2.9%). There were no clinically relevant differences in AEs, vital signs or laboratory test results between the two groups.
Table 6.
Incidence of predefined adverse events.
| Adverse Events | Donepezil group (n = 103) |
Placebo group (n = 98) |
|||||
|---|---|---|---|---|---|---|---|
| n of patients |
(%) | n of events |
n of patients |
(%) | n of events |
Chi-square test p value |
|
| Nausea | 9 | (8.7) | 30 | 4 | (4.1) | 21 | 0.1797 |
| Vomiting | 2 | (1.9) | 6 | 1 | (1.0) | 3 | 0.5903 |
| Anorexia | 5 | (4.9) | 18 | 4 | (4.1) | 21 | 0.7912 |
| Diarrhea | 2 | (1.9) | 6 | 1 | (1.0) | 3 | 0.5903 |
| Dizziness | 3 | (2.9) | 9 | 5 | (5.1) | 18 | 0.4274 |
| Pollakiuria | 11 | (10.7) | 57 | 6 | (6.1) | 24 | 0.2458 |
| Others | 49 | (47.6) | 306 | 42 | (42.9) | 261 | 0.5020 |
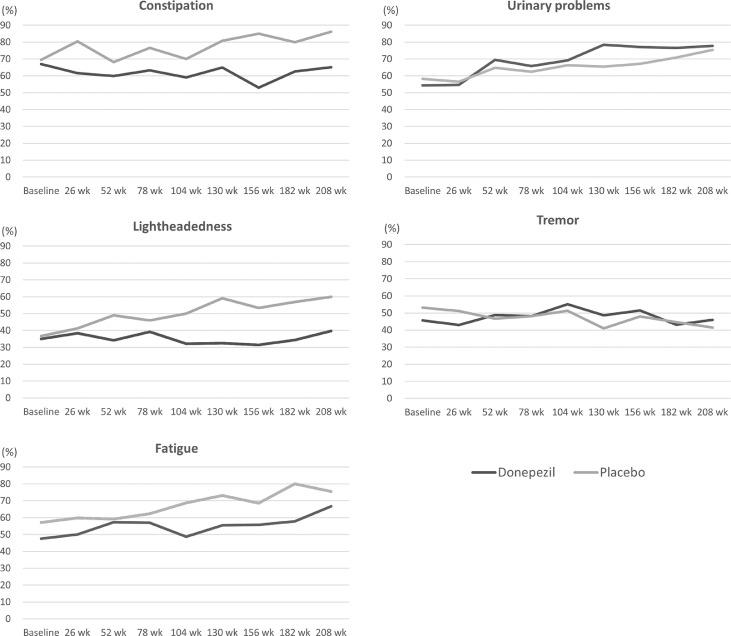
Table 7 summarizes the MDS-UPDRS scores at each visit for each group. At baseline, the average total scores ± SD were 39.9 ± 17.3 in the donepezil group and 41.8 ± 18.0 in the placebo group. The average scores increased over time for both the total score and the score for each part, but the scores at each visit were similar between the two groups. Analysis using the general linear model showed no significant effects of treatment group on with the total score or the score for each part (Supplementary Table 3). To focus on items related to specific symptoms, the proportions of patients who reporting experiencing urinary problems, constipation, lightheadedness on standing, fatigue, and tremor within each group were calculated for each visit (Figure 3). Throughout the follow-up period, constipation, lightheadedness on standing, and fatigue were less common in the donepezil group than in the placebo group. In both groups, urinary problems gradually became more common over time, while tremor was consistently reported by approximately half of patients. The general linear model revealed that treatment group had significant effects on patients’ responses to items related to all five symptoms (p < 0.05) (Table 8), indicating that the donepezil group was more likely to have urinary problems and tremor but less likely than the placebo group to have constipation, lightheadedness on standing, and fatigue.
Table 7.
The MDS-UPDRS scores at every 26-week (6-month) visit.
| Visit | N | MDS-UPDRS |
||||
|---|---|---|---|---|---|---|
| Total | Part I | Part II | Part III | Part IV | ||
| (0‒260) | (0‒52) | (0‒52) | (0‒132) | (0‒24) | ||
| Baseline | Donepezil (n = 103) | 39.9 ± 17.3 | 6.5 ± 4.0 | 8.5 ± 5.4 | 23.6 ± 11.6 | 1.2 ± 2.2 |
| Placebo (n = 98) | 41.8 ± 18.0 | 7.4 ± 4.7 | 8.8 ± 5.8 | 23.9 ± 10.7 | 1.7 ± 3.0 | |
| Week 26 | Donepezil (n = 86) | 44.0 ± 19.7 | 7.0 ± 5.0 | 10.6 ± 6.7 | 24.5 ± 12.2 | 1.8 ±2.8 |
| Placebo (n = 92) | 42.7 ± 20.8 | 7.3 ± 5.2 | 10.5 ± 7.1 | 23.1 ± 11.6 | 1.8 ± 2.7 | |
| Week 52 | Donepezil (n = 82) | 43.7 ± 19.6 | 7.6 ± 5.1 | 10.8 ± 6.6 | 23.2 ± 12.6 | 2.1 ± 2.8 |
| Placebo (n = 88) | 43.2 ± 23.2 | 8.0 ± 5.7 | 11.3 ± 8.2 | 21.7 ± 11.7 | 2.3 ± 3.1 | |
| Week 78 | Donepezil (n = 79) | 46.1 ± 19.8 | 7.9 ± 5.0 | 11.5 ± 7.2 | 24.3 ± 13.4 | 2.4 ± 2.9 |
| Placebo (n = 84) | 44.6 ± 22.4 | 8.1 ± 5.4 | 11.2 ± 7.9 | 22.9 ± 11.8 | 2.4 ± 3.1 | |
| Week 104 | Donepezil (n = 78) | 49.1 ± 21.3 | 8.3 ± 5.7 | 12.2 ± 7.1 | 26.0 ± 12.8 | 2.7 ± 3.5 |
| Placebo (n = 80) | 48.4 ± 24.0 | 9.2 ± 5.4 | 12.9 ± 8.1 | 23.6 ± 13.0 | 2.7 ± 3.7 | |
| Week 130 | Donepezil (n = 74) | 51.3 ± 22.8 | 8.5 ± 5.7 | 13.4 ± 7.6 | 26.3 ± 14.6 | 3.0 ± 3.5 |
| Placebo (n = 78) | 49.8 ± 22.8 | 9.3 ± 5.7 | 13.6 ± 7.9 | 23.9 ± 12.1 | 2.9 ± 3.5 | |
| Week 156 | Donepezil (n = 70) | 48.3 ± 20.7 | 7.8 ± 5.3 | 12.9 ± 7.3 | 25.0 ± 13.3 | 2.6 ± 3.2 |
| Placebo (n = 73) | 50.3 ± 22.1 | 9.8 ± 5.5 | 13.4 ± 7.3 | 24.2 ± 11.9 | 2.9 ± 3.5 | |
| Week 182 | Donepezil (n = 65)a | 52.3 ± 20.9 | 8.8 ± 5.0 | 14.1 ± 7.3 | 26.0 ± 13.3 | 3.2 ± 3.3 |
| Placebo (n = 65) | 53.8 ± 22.2 | 10.4 ± 5.9 | 14.8 ± 7.9 | 25.8 ± 11.4 | 2.8 ± 3.5 | |
| Week 208 | Donepezil (n = 63) | 54.0 ± 20.8 | 9.1 ± 5.3 | 14.8 ± 8.0 | 26.8 ± 13.9 | 3.3 ± 3.2 |
| Placebo (n = 65) | 55.4 ± 22.8 | 11.0 ± 6.6 | 16.0 ± 7.9 | 25.1 ± 11.7 | 3.2 ± 3.6 | |
Data are presented as mean ± standard deviation.
For the total score and Part I and Part II scores, 64 patients were analysed at week 182.
MDS-UPDRS=Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale.
Figure 3.
Proportions of patients who reported urinary problems, constipation, lightheadedness on standing, fatigue and tremor in the MDS-UPDRS among each group.
MDS-UPDRS=Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale.
Table 8.
Results of the general linear model for the effects on the MDS-UPDRS items on urinary problems, constipation lightheadedness on standing, fatigue, and tremor.
| Effects | Urinary problems | Constipation | Lightheadedness | Fatigue | Tremor |
|---|---|---|---|---|---|
| Baseline score | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Visit | 0.0007 | 0.2139 | 0.5777 | 0.0217 | 0.6043 |
| Treatment group | 0.0131 | < 0.0001 | < 0.0001 | 0.0027 | 0.0216 |
| Visit*Treatment group | 0.8274 | 0.3658 | 0.2118 | 0.4965 | 0.8315 |
Values presented are p values.
MDS-UPDRS=Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale.
Discussion
Prevention of cognitive decline is a new challenge in PD research. Advances in the treatment of motor symptoms have significantly prolonged the survival of patients with PD and simultaneously resulted in an increase in the lifetime incidence of dementia.19 Despite its high prevalence, the treatment options for dementia are still limited in patients with PD,1 and the ideal solution will be to prevent or delay the onset of dementia. To our knowledge, DASH-PD is the first randomized controlled trial to assess the effect of very long-term administration of donepezil for dementia prevention in PD patients at high risk of developing dementia. No significant difference in dementia incidence was found between the donepezil and placebo groups. However, compared with the placebo, donepezil had some beneficial effects on general cognitive function assessed by the ACE-R and the CDR, which seems in line with previous studies showing beneficial effects of cholinesterase inhibitors on cognition.20, 21, 22 Furthermore, donepezil treatment improved constipation, lightheadedness on standing and fatigue throughout the follow-up period. Long-term donepezil treatment was well tolerated in this study, and no serious AEs were observed during the 4-year study period, although donepezil treatment slightly worsened tremor severity and urinary problems.
There are several possible explanations for the failure of donepezil to show a preventive effect on dementia in this study. In our previous study, approximately 20% of PD patients with severe olfactory dysfunction converted to dementia during the 3-year observation period.7 However, in this study, the incidence of dementia onset during the 4-year observation period in the placebo and donepezil groups was only 12.2% and 6.8%, respectively. A consistent trend towards a slower rate of cognitive decline was observed in the donepezil group throughout the study period, but the overall low incidence of dementia made it harder to detect the preventive effect of donepezil. Furthermore, the overall completion rates for 4-year follow-up (70.1%) were slightly lower compared to initial expectation (75%), and the result may have an impact on the primary analysis.
This difference in dementia incidence seems to be partly due to the difference in baseline cognitive status in these studies. In the former study, patients with MMSE scores no less than 24 at baseline were enrolled for longitudinal follow-up. On the other hand, in the present study, we set the cutoff MMSE score at 25/26, in line with the current MDS diagnostic criteria for PDD,18 and this change in cutoff scores may have led to a lower dementia conversion rate. Furthermore, practice effects in repeated neuropsychiatric assessments23 may be associated with lower dementia incidence. We performed motor and neuropsychological assessments biannually in this study, and the ACE-R score gradually increased from baseline over time despite progressively worsened daily functioning in PD patients. In contrast to the apparent improvement in the ACE-R score in both groups, analysis of CDR demonstrated that cognitive status was more stable in the donepezil group than in the placebo group. These results indicate that frequent evaluations may cause remarkable practice effects on cognitive test results, especially in the ACE-R, which may obscure cognitive decline and raise the diagnostic threshold of dementia in this study.
In addition, there remains much room for improvement in predictive accuracy for dementia in PD. This study included only severe olfactory dysfunction as a baseline risk marker of rapid dementia conversion, but it now seems insufficient for accurate prognostication. The DASH-PD study was initiated in 2013, and there was no reliable clinical biomarker of dementia in PD at that time.24 Although higher age, severe motor dysfunction and postural instability gait difficulty subtype as well as mild cognitive impairment were known as clinical risk factors for dementia in PD, it was difficult to accurately predict rapid conversion to dementia in daily practice. It has since become clear that non-motor symptoms such as olfactory deficits,8,9 REM sleep behaviour disorder,25 and orthostatic hypotension26 are useful predictors for cognitive decline in PD. Furthermore, various kinds of predictors for dementia have been discovered,1 such as cerebrospinal fluid amyloid β, GBA mutation, posterior predominant cortical hypometabolism27 and atrophy in the basal forebrain cholinergic nucleus.28,29 Recent studies demonstrated that combinations of several predictors showed much better results in the prediction of cognitive outcome.26,30,31 Although assessment based on multiple risk factors may be preferable, the results of the DASH-PD study showing that early long-term administration of donepezil reduced the deterioration of neuropsychological test performance provide valuable information about preventive strategies for PD dementia.
It is noteworthy that donepezil treatment was associated with better outcomes not only in cognitive assessments but also for several non-motor symptoms assessed by the MDS-UPDRS (constipation, lightheadedness on standing and fatigue). There is currently insufficient evidence for pharmacological treatment of these symptoms, especially in advanced PD.32 These non-motor symptoms are common and sometimes have clinical importance almost comparable to that of motor and cognitive dysfunction, and our results may offer another treatment option for PD patients. Furthermore, it is also remarkable that long-term donepezil administration did not exacerbate gait dysfunction in this study. However, these points were not the target of this study must be interpreted with caution because there were no significant effects of treatment group on with the total score or the score for each part of MDS-UPDRS (Supplementary Table 3); thus, further study is needed to confirm these findings.
Our study has some limitations. First, as mentioned above, cognitive decline in this PD population with severe olfactory dysfunction was much lower than expected, which made it harder to meet the primary endpoint requirement. Therefore, future research will need to assess multiple risk factors including genetic risk factors such as GBA variants33 for better prognostication of impending dementia in PD. Second, we used the MMSE, the ACE-R and the CDR to evaluate cognitive dysfunction in this study. In a recently published consensus report, these neuropsychiatric tests were not recommended in the assessment of cognitive dysfunction in PD.34 Thus, the choice of this battery of tests may have limited the ability to detect dementia conversion in this study. Finally, we did not evaluate the cognitive status of participants after the donepezil or placebo was discontinued. Therefore, we cannot make inferences concerning whether improvements in the ACE-R and the CDR scores were merely a temporary benefit or the results of disease modifying effects of donepezil, which needs to be verified in future research targeting PD patients at earlier stages.
In conclusion, 4-year administration of donepezil for PD patients with severe olfactory dysfunction did not change the incidence of dementia but had some beneficial effect on neuropsychological function, with good tolerability.
Contributors
T.B., A.T., and E.M. contributed to the study design. A.M., T.K., and T.I. contributed to the statistical analysis plan. TB, AT, and the DASH-PD study group contributed to the conduct of the study and data collection. A.M., T.K., and T.I. contributed to the data analysis. A.M. and T.I. provided oversight of the safety monitoring. T.B. wrote the first draft. All authors contributed to the interpretation of the data, drafted and edited the manuscript, and approved the final manuscript.
Data sharing statement
The study data will be available to the members of the DASH-PD study group. Individual participant data that underlie the results reported in this Article will be shared after deidentification. These data will not be publicly available.
Declaration of interests
This study was investigator-initiated and we declare no competing interests. T.B. reports research grants from AbbVie, Co., Ltd., and Eisai, Co., Ltd., Honoraria for lectures from Ono Pharmaceutical Co., Ltd., AbbVie, Co., Ltd., Kyowa Kirin, Co., Ltd., Sumitomo Pharma Co., Ltd., Takeda Pharma, Otsuka Pharma, and Eisai, Co., Ltd., outside the submitted work. AT reports scholarship funds from AbbVie Inc., Eisai, Co., Ltd., Kyowa Kirin, Co., Ltd., and Sumitomo Pharma Co., Ltd. Consulting fees from AbbVie Inc., Kyowa Kirin Co., Ltd., Ono Pharmaceutical Co. Ltd., and Sony Co. Honoraria for lectures from AbbVie Inc., Eizai Co. Ltd., Ono Pharmaceutical Co. Ltd., Kyowa Kirin Co., Ltd., Sumitomo Pharma Co., Ltd., and Takeda Pharmaceutical Co., Ltd. A.M., T.K. and T.I. have nothing to disclose. E.M. reports Honoraria for lectures from Eisai, and Sumitomo Pharma Co., Ltd. outside the submitted work.
Acknowledgements
We thank all study participants. Springer Nature Author Service made English editing of the manuscript.
The work was founded by the Project Promoting Clinical Trials for Development of New Drugs from the Ministry of Health Labour and Welfare, Japan and the Japan Agency for Medical Research and Development (16lk0201010h0005), and Grants-in Aid from the Research Committee of CNS Degenerative Diseases, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health, Labour and Welfare Sciences Research Grants, the Ministry of Health, Labour and Welfare, Japan.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101571.
Contributor Information
Atsushi Takeda, Email: takeda.atsushi.nc@mail.hosp.go.jp.
DASH-PD study group:
Kinya Hisanaga, Yoshikazu Ugawa, Nobutaka Hattori, Miho Murata, Kazuko Hasegawa, Gen Sobue, Hidefumi Ito, Ichiro Yabe, Tatsuya Yamamoto, Mutsumi Iijima, Satoshi Orimo, Yasuyuki Okuma, Takahiko Tokuda, Masahiro Sugawara, Tetsuya Maeda, Yoshihiro Suzuki, Yoshinori Ishida, Makoto Tanaka, Hidetsugu Saiki, and Kenichi Kashihara
Appendix. Supplementary materials
References
- 1.Aarsland D, Creese B, Politis M, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13:217–231. doi: 10.1038/nrneurol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halliday GM, Leverenz JB, Schneider JS, Adler CH. The neurobiological basis of cognitive impairment in Parkinson's disease. Mov Disord. 2014;29:634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohnen NI, Kaufer DI, Ivanco LS, et al. Cortical cholinergic function is more severely affected in Parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol. 2003;60:1745–1748. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- 4.Shimada H, Hirano S, Shinotoh H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73:273–278. doi: 10.1212/WNL.0b013e3181ab2b58. [DOI] [PubMed] [Google Scholar]
- 5.Kempster PA, O'Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson's disease: a clinico-pathological study. Brain. 2010;133:1755–1762. doi: 10.1093/brain/awq059. [DOI] [PubMed] [Google Scholar]
- 6.Litvan I, Aarsland D, Adler CH, et al. MDS task force on mild cognitive impairment in Parkinson's disease: critical review of PD-MCI. Mov Disord. 2011;26:1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baba T, Kikuchi A, Hirayama K, et al. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson's disease: a 3 year longitudinal study. Brain. 2012;135:161–169. doi: 10.1093/brain/awr321. [DOI] [PubMed] [Google Scholar]
- 8.Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson's disease: a cohort study. Lancet Neurol. 2017;16:66–75. doi: 10.1016/S1474-4422(16)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fullard ME, Tran B, Xie SX, et al. Olfactory impairment predicts cognitive decline in early Parkinson's disease. Parkinsonism Relat Disord. 2016;25:45–51. doi: 10.1016/j.parkreldis.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohnen NI, Muller ML, Kotagal V, et al. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson's disease. Brain. 2010;133:1747–1754. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito S, Ayabe-Kanamura S, Takashima Y, et al. Development of a smell identification test using a novel stick-type odor presentation kit. Chem Senses. 2006;31:379–391. doi: 10.1093/chemse/bjj042. [DOI] [PubMed] [Google Scholar]
- 12.Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. ``Mini-mental state''. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's cognitive examination revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21:1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 16.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 17.Japanese Society of Neurology . Igaku-Shoin; Tokyo: 2011. Treatment Guideline for Parkinson's Disease. [Google Scholar]
- 18.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 19.Reid WG, Hely MA, Morris JG, Loy C, Halliday GM. Dementia in Parkinson's disease: a 20-year neuropsychological study (Sydney multicentre study) J Neurol Neurosurg Psychiatry. 2011;82:1033–1037. doi: 10.1136/jnnp.2010.232678. [DOI] [PubMed] [Google Scholar]
- 20.Dubois B, Tolosa E, Katzenschlager R, et al. Donepezil in Parkinson's disease dementia: a randomized, double-blind efficacy and safety study. Mov Disord. 2012;27:1230–1238. doi: 10.1002/mds.25098. [DOI] [PubMed] [Google Scholar]
- 21.Mamikonyan E, Xie SX, Melvin E, Weintraub D. Rivastigmine for mild cognitive impairment in Parkinson disease: a placebo-controlled study. Mov Disord. 2015;30:912–918. doi: 10.1002/mds.26236. [DOI] [PubMed] [Google Scholar]
- 22.Wang HF, Yu JT, Tang SW, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson's disease, Parkinson's disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry. 2015;86:135–143. doi: 10.1136/jnnp-2014-307659. [DOI] [PubMed] [Google Scholar]
- 23.Broeders M, De Bie RM, Velseboer DC, Speelman JD, Muslimovic D, Schmand B. Evolution of mild cognitive impairment in Parkinson disease. Neurology. 2013;81:346–352. doi: 10.1212/WNL.0b013e31829c5c86. [DOI] [PubMed] [Google Scholar]
- 24.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson's disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11:697–707. doi: 10.1016/S1474-4422(12)70152-7. [DOI] [PubMed] [Google Scholar]
- 25.Anang JB, Gagnon JF, Bertrand JA, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014;83:1253–1260. doi: 10.1212/WNL.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson BK, Fereshtehnejad SM, Anang JBM, et al. Office-based screening for dementia in Parkinson disease: the montreal Parkinson risk of dementia scale in 4 longitudinal cohorts. JAMA Neurol. 2018;75:704–710. doi: 10.1001/jamaneurol.2018.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baba T, Hosokai Y, Nishio Y, et al. Longitudinal study of cognitive and cerebral metabolic changes in Parkinson's disease. J Neurol Sci. 2017;372:288–293. doi: 10.1016/j.jns.2016.11.068. [DOI] [PubMed] [Google Scholar]
- 28.Ray NJ, Bradburn S, Murgatroyd C, et al. In vivo cholinergic basal forebrain atrophy predicts cognitive decline in de novo Parkinson's disease. Brain. 2018;141:165–176. doi: 10.1093/brain/awx310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulz J, Pagano G, Fernandez Bonfante JA, Wilson H, Politis M. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson's disease. Brain. 2018;141:1501–1516. doi: 10.1093/brain/awy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson's disease: biomarkers and longitudinal progression. Brain. 2017;140:1959–1976. doi: 10.1093/brain/awx118. [DOI] [PubMed] [Google Scholar]
- 31.De Pablo-Fernandez E, Lees AJ, Holton JL, Warner TT. Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol. 2019;76:470–479. doi: 10.1001/jamaneurol.2018.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson's disease-an evidence-based medicine review. Mov Disord. 2019;34:180–198. doi: 10.1002/mds.27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Straniero L, Asselta R, Bonvegna S, et al. The SPID-GBA study: sex distribution, penetrance, incidence, and dementia in GBA-PD. Neurol Genet. 2020;6:e523. doi: 10.1212/NXG.0000000000000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skorvanek M, Goldman JG, Jahanshahi M, et al. Global scales for cognitive screening in Parkinson's disease: critique and recommendations. Mov Disord. 2018;33:208–218. doi: 10.1002/mds.27233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.