Abstract
COVID-19, a novel coronavirus disease, has provoked a variety of health and safety concerns, and socioeconomic challenges around the globe. The laboratory diagnosis of SARS-CoV-2 was quickly established utilizing nucleic acid amplification techniques (NAAT) after the disease causing virus has been identified, and its genetic sequence has been determined. In addition to NAAT, serological tests based on antibodies testing against SARS-CoV-2 were introduced for diagnostic and epidemiologic studies. Other biochemical investigations include monitoring of peripheral blood cells count, platelets/lymphocyte ratio, coagulation profile, cardiac, and inflammatory markers such as cytokines storm are also crucial in combating COVID-19 pandemic. Further, accurate and reliable laboratory results for SARS-CoV-2 play very important role in the initiation of early treatment and timely management of COVID-19 patients, provide support in clinical decision-making process to control infection, and detection of asymptomatic cases. The Task Force on Coronavirus-19 constituted by International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) has recognized informational framework for epidemiology, pathogenesis, and recommended the PCR-based analysis, serological and biochemical assays for analysis, monitoring, and management of disease. This literature review provides an overview of the currently used diagnostic techniques in clinical laboratories for the diagnosis, treatment monitoring, and management of COVID-19 patients. We concluded that each assays differ in their performance characteristics and the utilization of multiple techniques is necessary for the accurate diagnosis and management of SARS-CoV-2 infection.
Keywords: COVID-19 Diagnostics, molecular testing, biochemical monitoring, serological testing, SARS-CoV-2 antibody response
Introduction
Coronavirus-19 is caused by “Severe acute respiratory syndrome coronavirus-2” (SARS-CoV-2). 1 In September 2020, over 30 million cases of confirmed COVID-19 and more than 900,000 deaths have been reported throughout the world. On 30 January 2020, The WHO declared it as a sixth community health disaster of concern internationally, and WHO announced coronavirus-19 as a virulent disease on 11 March 2020.2,3 Globally, as on 25th March 2022, there have been 6,108,976 deaths among 476,374,234 confirmed cases of COVID-19 reported to WHO. As of 26th March 2022, a total of 11,054,362,790 vaccine doses have been administered. 4 High mortality rate of SARS-CoV-2 was observed in elderly people, immune-compromised, diabetic, cardiac, and hypertensive patients. Many young people and children who are infected with the disease as asymptomatic carriers have mild to moderate symptoms.
Clinical laboratory testing is critical to the global response to this highly pandemic disease, which begins with early diagnosis, treatment, and epidemiological surveillance. Therefore, the proficiency of laboratory professionals is crucial in validating the investigative processes and ensuring analytical performance in provision of health care operations. Reliable and rapid identification of SARS-CoV-2 mutations are significant to control the contagious spread of the virus. 5 The Task Force on coronavirus-19 IFCC provide support to the laboratories performing COVID-19 testing as well as providing evidence-based best recommendations on specific biosafety measures. Task Force also compiled important information about clinical laboratories for diagnosing, prognosticating, and monitoring COVID-19. To make use of genetics, blood serum and enzymatic indicators in the analysis or monitoring of SARS-CoV-2 disease infected persons has been summarized in this review.
Coronavirus-19 is characterized by cytokine storm and coagulation dysfunction which leads to tissue destruction and death. The common mission of health care providers, researchers, and government representatives was to evaluate the best detection technologies for narrative COVID (SARS-CoV-2), and to find out the best treatment for this viral infection. 6 In fact, laboratory diagnosis has played an outstanding role during this COVID-19 pandemic for accurate disease detection, rapid diagnosis, timely treatment, and disease control.6,7 The existing diagnostic markers are of two types: the viral ribonucleic acid (RNA) detection in respiratory tract specimen using RT-PCR is used just before making a particular direct laboratory diagnosis. Direct detection of nucleic acids via RT-PCR amplification of nucleic acids considered the gold standard for viral identification being most frequently used test during this pandemic. 8 Quantitative reverse transcription polymerase chain reaction (qRT-PCR) which is the gold standard method for the detection of SARS-CoV-2. Contrary to this, new approaches gradually rectify the diagnostic difficulties. One of the novel approach called reverse transcription loop-mediated isothermal amplification (RT-LAMP) may also contribute to the cheaper and faster field-based testing. 9 Serological tests are being used as supplemental tools and mainly focused on the determination of immune response antibodies (IgG and IgM) specific to virus produced by the host immune system after 7 days of onset of clinical symptoms, while some specialized laboratories may also have the capability to assess cellular immune response.10,11 D-dimer and platelet/lymphocyte ratio are non-specific laboratory biomarkers that may be decreased during the course of infection and are useful for predicting disease complications. 12 Various biomarkers are connected to the transmittable procedure of SARS coronavirus-2, despite the fact that they are non-specific. Low platelets and lymphocyte counts, decreased serum albumin concentration, and elevated interleukin-6 levels, transaminases, lactate dehydrogenase, ferritin, D-dimer, and C-reactive protein in serum can all be used to predict the severity of COVID-19. Additionally, cytokine storms with increased TNF-α, IL-6, IL-10, and IL-2R as well as a decrease in absolute numbers of CD4+ and CD8+ T lymphocytes have been linked to Severe Coronavirus-19 and 40 instances, including sudden loss of blood flow to brain, numerous limb damage, and rapid deaths. 13
Literature search strategy
Literature related to the topic was searched using different search engines including Web of Science, google scholar, PubMed, Scopus, and Science Direct. The following key words were used to search the published articles and reports: Novel coronavirus 2019, Lab diagnosis, COVID-19, Molecular techniques, Serological assays, and Biochemical markers.
Clinical features of COVID-19
The SARS coronavirus-2 is a single stranded, positive-sense RNA virus from beta family coronavirus that is genetically related to SARS-CoV and bat SARS-like coronaviruses. Each virion has diameter of 50–200 nm and is made up of four structural proteins named as nucleocapsid (N), membrane (M), envelope (E), and spike (S) protein. The nucleocapsid binds to the viral RNA genome, while the spike, envelope, and membrane proteins work together to form the virus envelop. 14 According to the recent research, an ordinary host of SARS-CoV-2 is the bats and the potential intermediate host is Malayan pangolin. 15 Dry cough, fever, dyspnea, fatigue, and myalgia are all the frequent symptoms of COVID-19 infection. Respiratory secretions, headache, hemoptysis, chills, diarrhea, taste, and smell disorders were all reported by a different percentage of individuals.16–18 Guan et al. 19 reported that approximately 80% of confirmed cases have mild symptoms, severe dyspnea (14%), and develop critical conditions (6%) such as numerous organ failure and ARDS (acute respiratory distress syndrome). Incubation period after exposure to infectious agent can range from 2 to 14 days. The death rates are high in patients having comorbidities like diabetes, obesity, heart diseases, chronic respiratory diseases, and malignancies.20,21 Chen et al. 22 reported an increased incidence of medication, involuntary respiration, and Coronavirus-19 associated deaths. Even though the whole human population is exposed to this unique virus, the effects appear to be lesser among children and teenagers under the age of 18 years. 16 Lymphopenia was observed in 70–83% of patients on admission in health care facilities. 23 The persistence of decreased blood lymphocyte number and high blood level of seditious protein are linked to illness severity or deaths, 24 implying that a dangerous reaction or storm protein, as seen in SARS and MERS (Middle east respiratory syndrome), might take part in illness sequence and severity. 25 Alveolar destruction with cellular fibromyxoid exudates, pneumocyte dropping, and development of hyaline membrane in the lung are revealed in postmortem biopsies, confirming the diagnosis of ARDS. 26 The occurrence of SARS-CoV-2 RNA virus in blood, as well as a significant increase in plasma interleukin-6 levels and other immunotherapy like coagulopathy and DIC-related FDP and ferritin, procalcitonin (PCT), and troponin in response to bacterial infection have a strong correlation with clinical severity and mortality.22,27
Storm proteins, a severe form of proteins released by syndrome, is a type of universal provocative reaction produced by monoclonal antibody treatment and infections. It is marked by the discharge of dangerous proteins, which are able to express numerous organ damages.28,29 An in vitro proteins discharge assay, which includes stimulation of releasing plasma interleukin-2, interferon c, and TNF-α have been proposed to predict probable cytokine storm induction.28,30 The potential roles of cytokine storm syndrome in Coronavirus-19 patients' critical illness and mortality have been considered.31,32 ICU patients describe greater plasma interleukin-2, 6, and 7 levels, a colony stimulating factors produced by macrophages, endothelial cells and fibroblasts, antiviral inducible ten proteins, chemo-attractant monocyte protein 1, MIP-1-a, and tumor necrotic factor-α than non-ICU sick person.31,33 Because of the association between elevated level of interleukin-6 and the severity of Coronavirus-19, the IL-6 antagonist tocilizumab have been recommended as a management option for severe COVID-19. 34
Laboratory diagnosis of SARS coronavirus-2
Nucleic acid amplification testing (NAAT)
The direct detection of SARS-CoV-2 RNA through nucleic acid amplification tests (NAATs) is the most prevalent method for diagnosing COVID-19, most often RT-PCR from the upper respiratory tract.35,36 Around the world, several RT-PCR techniques are utilized to increase and identify distinct sections of the SARS-CoV-2 genome. Some of the techniques target two or more genes, including the envelope, spike, and nucleocapsid genes as well as sections of the first open reading frame (ORF), such as RdRp (RNA-dependent RNA polymerase) gene. Next-generation sequencing, isothermal amplification and CRISPR-based assays, are some of the less prevalent types of NAAT. 37 The fast and reliable tools for the primary diagnosis of SARS-CoV-2 are NAAT methods which play important role in isolating, identifying, and controlling the infection. 38 A number of molecular targets for Coronavirus that can be used for PCR assays are present within the positive-sense single stranded RNA genome. The glycoproteins envelope consist of the spike (S), envelop (E), membrane (M), helicase (Hel), and nucleocapsid (N) are among the structural proteins encoded by these genes. There are species specific accessory genes that are essential for viral duplication in addition to polymer protein genes. ORF1a and ORF1b open reading frames are examples of RNA-dependent RNA polymerase (RdRp) and hemagglutinin-esterase. 39 Different molecular-based diagnostics for the rapid detection of COVID-19 are being developed and validated in clinical laboratories. The World Health Organization (WHO) currently recommends the NAATs as gold standard for detecting COVID-19 suspected patients in a laboratory with adequate biosafety (BSL-2). 40 China developed the first version of NAAT that targeted the open reading frame-1ab and nucleocapsid genes of RNA virus, whereas the second version was developed in Germany targeting the N, E, and RdRp genes. Thereafter, many laboratories throughout the world have developed and deployed the real-time RT-PCR testing. 41
The polymerase chain reaction (PCR) characterized by the high specificity, sensitivity, and rapid detection is considered as the “gold standard” among the nucleic acid tests for the detection of some viruses. Real-time RT-PCR as a simple and specific qualitative assay is of great interest today for SARS-CoV-2 detection. 42 The results of an RT-PCR test are dependent on the collection of appropriate respiratory specimens from suspected patients, the use of specified primers and probes, the Ct value, the analysis of fluorescence curves, and the use of appropriate controls. A positive control is used to evaluate the reliability of the primers, reagents, and probes while a negative control is used to test sample cross-contamination. 43 The use of a human specimen control (HSC) recommended by the US Centre for Disease Control and Prevention is an additional step in ensuring the extraction reagents integrity, successful lysis, and minimizing the false negative results through the collection of appropriate human cellular material. 18
Analytical issues of molecular-based detection
Gene target
Molecular assays for SARS-CoV-2 diagnosis are designed for the detection of specific target genes in the virus including 3 of 4 structural proteins, that is, S, E, and N proteins as well as the RdRp gene and ORF1ab region. Therefore, mutation or viral recombination may represent a diagnostic challenge. SARS-CoV-2 has already revealed evidence of active viral recombination. 44 Korber et al. 45 found alterations of 14 amino acids of SARS-CoV-2 in the spike protein in a study that not yet has been peer-reviewed. At the nucleotide level, there are many more synonymous mutations. Such mutations and viral recombination may compromise the RT-PCR accuracy because of mismatches among the sequence of RNA present in the sample and the primer and assay probes, resulting in false negatives. Recent research has linked several newly discovered mutations to various epidemiological characteristics, such as increased transmissibility and a clinical outcome of severe intensity. 46 The improvement of current technologies to selectively distinguish clinically relevant mutations may give helpful information for COVID-19 management if proven by larger epidemiological investigations. Lack of consistency between primer and probe set makes it difficult to compare assay results across platforms. Other analytical challenges include inaccurate cut-off definition, instrument malfunction, inadequate assay validation, and result misinterpretation. 47
Clustered regulatory interspaced short palindromic repeats-based detections
CRISPR-based detection of nucleic acid received a lot of interest in recent years as it is “cheaper, rapid, and better” than conventional PCR methods. 48 CRISPR tests can diagnose infections as precisely as traditional procedures and can simply be converted to a point-of-care diagnostic. The first CRISPR-based COVID-19 diagnostic has been developed by Sherlock Biosciences to receive approval from the FDA EUA (Emergency Use Authorization). This home-based assay is dependent on the CRISPR molecule programming to pick-up the SARS-CoV-2 genetic signature. A detectable signal is released and the CRISPR enzyme is activated, providing the results in an hour if the signature is detected. The University of Connecticut’s All-In-One Dual CRISPR-Cas12a (AIOD-CRISPR) 49 for detection and Stanford University’s PAC-MAN (Prophylactic Antiviral Interspaced Short Palindromic Repeats-Cas13 in human cells) for treatment are two more CRISPR-based projects. The latter has been proved to be efficient in vitro, but before it can be used therapeutically, a safe and effective delivery into human cells in vivo must be established. 50
Viral sequencing
Normal sequencing of a fraction of materials from clinical cases can be valuable for monitoring the alterations in viral genome in addition to confirm the presence of virus that may affect the effectiveness of medical countermeasures, including diagnostic testing. Virus whole genome sequencing can also help with studies on molecular epidemiology. GISAID, which is designed to preserve the rights of the submitting party, is one of many public-access databases for deposition of genomic sequence data. 51
Viral culture
It is not recommended to isolate viruses as a standard diagnostic practice. 52 Due to the unavailability of permissive cell lines, labor-intensive and time consuming technique, and skill requirements, as well as the lack of commercial antisera for culture confirmation, cell culture is not commonly suggested for the isolation of HCoVs. Before the advent of molecular methods, the cell culture was considered the “gold standard” for isolation and characterization of virus. The modified cell lines helped to detect and identify infectious pathogens such as primary lines, preserved lines, diverse cell lines, and transfected lines.53,54 Despite of serious safety concerns, SARS-CoV-2 will grow well in cell lines like Vero, LLCMK2, and primary monkey cells. Viral culture effectively supports the development of therapeutic agents and vaccine.55,56 There is also the development of pharmaceuticals and herbal sprays along with PCR analysis using viral cultures and viral media. Although, various detection methods are used to control the pandemic including fast antigen tests or serological methods and RT-PCR, the actual problem is the control of the disease. 57
Antigen detection tests for COVID-19
The discovery of rapid diagnostic tests for SARS-CoV-2 antigen detection has posed various challenges in the performance and accessibility of tests. Choosing an appropriate test for the detection of COVID-19 affects the rapid control, prophylactic responses, and early detection of virus outbreaks. Early detection of the virus minimizes the transmission opportunities allowing the prompt removal of infections. COVID-19 Ag-RDT is a specific and sensitive antigen test qualitatively detecting the SARS-CoV-2 Ag in nasopharyngeal swab. 58 Ag-RDTs may be based on uncomplicated and more accessible samples that can even be self-collected, like saliva or nasal swab, rather than nasopharyngeal swab which require personal protective equipment (PPEs) and qualified healthcare professional to collect. SARS-CoV-2 proteins formed as a result of viral replication in respiratory secretions are directly detected through Ag-RDTs. 59 Ag-RDTs is inexpensive, do not require infrastructure, easy to perform, and quality results achieved in minutes. Ag-RDT is strongly supported by Ricks et al. for the evaluation of symptomatic individuals making it practical and cost-effective.60,61
Rapid antigen test detect the viral antibodies present or absent in patient’s blood for the rapid diagnosis of viral infection through the principle of lateral flow testing. In this method, Ag-Ab complex migrates through capillary action crossing the membrane and produce a color change through immobilization by capture antibodies. Rapid diagnostic tests are small, inexpensive, and portable tests that work on lateral flow testing principle where samples such as nasal swab, blood, or saliva identify positive or negative results showing color lines. In this assay, gold nanoparticle labeled antibodies (Au-Ab) containing membrane and capture antibodies show two separate lines when sample loaded on the membrane moves along the membrane by capillary action. This encounters first with the Au-Ab and binding of viral antigens form a complex which then advances and is captured by the bound antibodies in second line producing colored lines due to its immobilization that confirm the tests. 57
COVID-19 Ag Respi-Strip CORIS is a rapid diagnostic test evaluated by Lambert-Niclot et al. 62 for SARS-CoV-2 Ag detection in a study population of 138 samples. The assay is based on the sensitization of colloidal gold nanoparticles on a nitrocellulose membrane technology directed against highly conserved nucleoprotein antigens for SARS-CoV-2. Researchers found that 98 samples found positive by RT-PCR, and among them, only 47 samples found positive for SARS-CoV-2 antigens, with 50% sensitivity. The authors concluded that the assay can be used for the diagnosis of disease immediately after the onset of disease up to few days when the virus load is at its peak in the upper respiratory tract. Mertens (2020) 63 conducted a retrospective study on 328 pharyngeal samples in Belgium for the detection of nucleoprotein antigen through immunochromatographic method. They found that COVID-19 Ag Respi-Strip represent a useful rapid antigen assay for SARS-CoV-2 virus for the initial diagnosis of COVID-19 in 15 min when the pandemic is at its peak.
Serology of COVID-19
Serological testing for COVID-19 is the analysis of serum, plasma, or whole blood for immunoglobulins (antibodies) detection, particularly immunoglobulin M (IgM), IgG, and IgA that are specific for antigens of SARS-CoV-2, including the nucleocapsid proteins and spike glycoproteins. 38 Testing procedures range from point-of-care testing (POCT) to fully automated clinical laboratory instruments that uses chemiluminescent immunoassays or enzyme-linked immunosorbent assays. 38
There is a considerable demand for COVID-19 antibody detection tests that are both specific and sensitive. Serology testing is expected to serve a key part in the previous COVID-19 infection diagnosis and in determining the virus incidence in general people. Serological testing might be helpful for the confirmation of suspected cases, particularly in patients tested in the late phase of COVID-19 or with mild to moderate illness, not identified with molecular assays. 64 It is also thought to be important for identifying possible plasma donors recovery, while ongoing immune responses to subsequent COVID-19 infection monitoring and epidemiological surveillance. On the other hand, rapid emergence of a number of immunoassays by diagnostic manufacturers, their value, clinical utility, and diagnostic accuracy mainly remain uncharacterized.
Antibody tests identify the presence of antibodies produced as part of the immune response due to the viral pathogen infection. For SARS-CoV-2 infection detection, the commonly used serological assays include Enzyme-Linked Immunosorbent Assay, neutralization test, rapid diagnostic determinations, and chemiluminescent immunoassay. Neutralization test detect the immunoglobulins in patient’s sample based on their ability to inhibit the replication of SARS-CoV-2 in Vero E6 cell lines. However, antibodies detection specific to viral proteins not involved primarily in replication can be missed using this approach. 65 ELISA technique detects SARS-CoV-2 specific antibodies through colored product formation by binding the secondary labeled antibodies to the primary Ag-Ab complex. In this method, specific viral antigens are coated on the wells and the patient’s blood is added. If antibodies specific to viral antigens are present, forming Ag-Ab complex by binding to the coated antigens followed by the addition of flourochrome labeled secondary antibodies or substrates producing a detectable color change or fluoresces that can be measured through photometer. 66 Chemiluminescent Immunoassay (CLIA) identify the antibodies present in patient’s blood by forming chemiluminescence activity through secondary labeled antibodies binding to the primary Ag-Ab complex. This test quantitatively measures the IgA, IgM, and IgG antibodies. 67
A unique technology of microsphere immunoassay (MIA), which is a variant of ELISA, allow for the multiplexing of several antigens. The method is based on magnetic carboxylated microspheres with attached virus antigens and antibodies present in patients serum against the antigen can be detected through the addition of fluorescent labeled secondary antibody. Both MIA and ELISA are more specific and sensitive than lateral flow immunoassays but require more time for results to be available (3–8 h for MIA and 5 h for ELISA). 68
Analytical issues
The timing and performance features of serological testing to antibody tests are closely associated to molecular testing. Collected literature data suggest that seroconversion occurs 7–14 days following the onset of symptoms.35,69 A published study that uses an ELISA technique reported the median seroconversion of total immunoglobulins, IgM, and IgG in plasma specimen of COVID-19 hospitalized patients on 11, 12, and 14 days, respectively. 70 Another study reported an early detection of IgM within 1–7 days of symptom onset in 85% of COVID-19 confirmed patients. 71 Notably, the IgA response seems to be increased, coincide with IgM, peaks after 18–21 days, and appears more persistently than IgM response. 72
Serological assays now available for various antibodies detection consists of total antibody, IgA, IgG, and IgM. The traditional viral infections immune response involves the development of first IgM, frequently followed by the advent of immunoglobulin A, and then to immunoglobulin G production.73,74 Current research on COVID-19 is contradictory, with some groups finding that IgM is produced first 71 and others claiming that IgM and IgG are produced simultaneously, as shown in other SARS-associated coronaviruses.75,76
Cross-reactivity
Cross-reactivity is a typical problem in serological testing, which is evidenced by about 80% genetic identity shared between SARS-CoV-1 and SARS-CoV-223,77 and common proteins showing similarity in structure with SARS-CoV-1 which caused SARS in 2002–2003. 78 Seroprevalence studies indicated that more than 90% of adults have immunoglobulins to the ordinary coronaviruses even with lower homology with these strains, describing the significance of potential cross-reactivity in COVID-19 in adult population.79,80 Introductory claims in the package inserts provided by the manufacturers are encouraging with most companies claim no cross-reactivity. On the other hand, the virus strains tested varies from manufacturer to manufacturer and there is an urgent need of more peer-reviewed data.
Limitations and advantages of PCR and serological assays
The limitations and advantages of serological tests and nucleic acid tests based on available literature data are summarized in Table 1. Because each type of assay has its own set of benefits and drawbacks, proper interpretation of test findings for pathogen identification and seroconversion is critical for precise analysis, patient administration, and forceful monitoring and observation at various disease stages (Table 2). As given in Table 2, variety and timing are crucial for the assay’s diagnostic performance (specificity and sensitivity) and the accuracy of laboratory results.
Table 1.
Comparative assessment of polymerase chain reaction and serological/immunological tests.
| Advantages/limitations | Nucleic acid tests (e.g., Polymerase chain reaction) | Serological tests (IgG/IgM/Antigens) |
|---|---|---|
| Pros | Specific and sensitive Immediate detection Analytical Verification of current coronavirus disease |
Fast TAT/rapid testing Easy operation/access Economic Sign of infectious coronavirus |
| Cons | Challenge of sample collection (swab) Costly False negative due to sampling and late recovery along with elimination of virus |
Non diagnostic False positive False negative due to relatively late detection at initial phase (5–7 days after onset) until about 2 weeks after infection |
Table 2.
Combined polymerase chain reaction and serological test results interpretations.
| Polymerase chain reaction result | IgG/IgM result | Interpretations |
|---|---|---|
| −ve | −ve | 1. Infection not present 2. Infection at an early stage |
| Positive | Negative | 1. Illness at first week 2. Infection is active; contagious patient and quarantine needed 3. Immunocompromised patient; repeating antibody test in two to 5 days |
| Positive | +ve | 1. Infection is active; contagious patient and quarantine needed 2. Production of antibodies due to immune response |
| −ve | +ve | 1. Infection recovered 2. Illness at late stage or virus elimination from body |
Biochemical markers for monitoring COVID-19
Biomarkers of COVID-19 severity
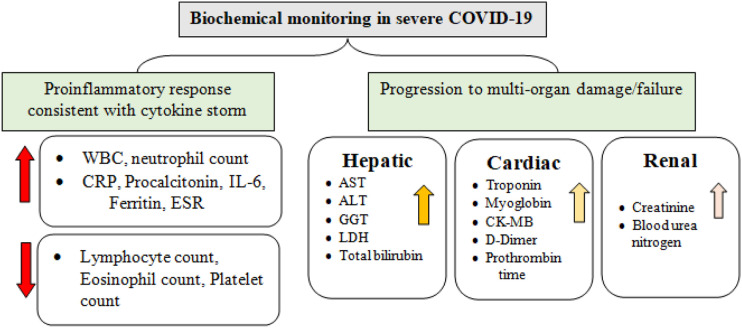
Beyond initial epidemiological surveillance and diagnosis, the medical laboratory plays an important role in this issue. Routine laboratory testing for biochemical, hematological, and immunochemical investigation is critical for determining the severity of disease, identifying appropriate alternative therapy and monitoring treatment response. The abnormalities associated with increased severity of the disease are becoming increasingly clear as the number of COVID-19 patients continues to increase globally (Figure 1).
Figure 1.
Hematological and biochemical markers of coronavirus-19 sequence and strength.
Inflammatory biomarkers
Severe coronavirus-19, implying an immunochemical outline similar to a “cytokine storm,” Has been linked to several inflammatory biomarkers. In summary, pro-inflammatory cytokines, particularly interleukin (IL)-6 and TNF-α, have been demonstrated to be strongly related to death in patients with severe illness.24,81 It is worth noting that pro-inflammatory cytokines appear to represent more than just biomarkers in the course and death of COVID-19. Despite the fact that cytokine testing is not routine in medical laboratories, replacing biochemical markers of irritation such as blood serum, C-reactive protein, and ESR have all been linked to acute COVID-19 and can be used to estimate extreme illness.24,82 In serious COVID-19 cases, high levels of procalcitonin have been found, indicating the development of bacterial co- or supra-infection in seriously sick individuals. 83 Hematological studies imply that high platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) as well as lymphopenia have prognostic significance in adding up to inflammatory biomarkers. In Coronavirus-19 patients, an increase in the D-dimer coagulation limit has been linked to deterioration illness and a high danger of rising a broad range of thrombosis actions, including in situ pulmonary microthrombosis, subterranean vein thrombosis, disseminated intravascular coagulation, and overt pulmonary embolism.82,84
Overall, these conclusions support the hypothesis that severe coronavirus-19 cases are characterized by immense pro-inflammatory responses or cytokine storm, which can lead to MOF (multiple organ failure) in severe cases. As a result, routine laboratory testing will be used to analyze the inflammatory profile of COVID-19 patients, along with early detection of heart, kidney, and liver impairment. Through the identification and quantification of various biomarkers, laboratory test results can aid in the diagnosis, prognosis, and monitoring of patients.
Cardiac biomarkers
Clinical evidence suggests that severe COVID-19 patients have significant cardiovascular impairment.85,86 According to the preliminary findings from a study conducted in China, 12% of all patients and 31% of ICU patients had acute cardiopulmonary damage, as assessed by elevated levels of cardiac troponin I. 87 Patients with increased troponin level have higher chances to be admitted to ICU with increased rate of hospital mortality.22,88,89 Another study series of 187 confirmed COVID-19 cases reported myocardial injury in 27.8% patients resulting in arrhythmias and cardiac dysfunction. 89 Cardiac markers monitoring such as natriuretic peptides and cardiac troponin throughout illness series will be essential for appropriate risk stratification of the patient. A statement recently published by the American College of Cardiology 90 on the role of monitoring cardiovascular biomarkers in COVID-19 patients. “On clinical grounds, doctors are advised to assess troponin only if acute myocardial infarction diagnosis is being evaluated,” they said. 91 This professional guidance is expected to prevent COVID-19 patients from undergoing unnecessary diagnostic tests and to reduce downstream discussion and procedures, such as bedside angiography and echocardiography. 92 Many groups, however, have argued that troponin must not be viewed exclusively as a binary test for the diagnosis of myocardial infarction, but rather as a valuable prognostic marker of both ischemic and non-ischemic causes of cardiac abnormality that can aid in patient triage and appropriate treatment selection. 92 The following pathways are thought to be implicated in coronavirus-19-related cardiovascular complications: (a) severe coronary artery disease, (b) microangiopathy, (c) viral myocarditis, (d) direct myocardial injury, and (e) cytokine-driven myocardial damage. 93 At this time, none of these hypothesized processes has been identified as the principal cause of cardiovascular problems seen in severe coronavirus-19 patients. Additional clinical research is required to identify the major cause of myocardial injury for therapeutic purposes and to guide for laboratory testing.
Hepatic biomarkers
Recent research has discovered that severe COVID-19 individuals have abnormal liver functioning. Many large-scale hospital investigations have found elevated liver enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma glutamyl-transferase (GGT).22,94
A retrospective case assessment of medical and laboratory results from 417 laboratory-confirmed COVID-19 patients was recently conducted by Cai and colleagues. 95 76.3% of the 417 patients had abnormal levels of liver markers, and 21.5% of the patients had liver injury while in the hospital. Whereas alkaline phosphatase (ALP) and GGT are cholangiocyte-related enzymes and only GGT has been linked to severe coronavirus-19, implying that the injury is most likely drug-induced rather than bile duct damage. 95 While it is evident that liver damage is linked to an increased likelihood of having acute COVID-19. The following are some of the possible clinical mechanisms for COVID-19 liver dysfunction: (a) infection resulting through the severe inflammatory response leading to the immune mediated damage, (b) active replication of viruses in the biliary epithelial cells expressing ACE2 resulting in direct cytotoxicity, (c) anoxia caused hypoxic hepatitis, and (d) drug-induced liver damage.96–98 Although the role of basic hepatic situation and the medical method after COVID-19 associated metabolic disorder is unknown, blood test that measure different enzymes, proteins, and other substances made by the liver must be ordered regularly to evaluate relative liver injury in COVID-19 patients in clinical context, particularly for persons getting antiviral treatment.
Kidney biomarkers
COVID-19 has been linked to the higher risk of acute kidney damage (AKI), even though the exact mechanism has yet to be identified. 99 According to previous statistics from the SARS pandemic in 2002–2003, 6.7% of ill persons experienced acute kidney injury (AKI), and SARS-CoV-2 patients with severe renal damage had a mortality rate of up to 91.7%. 100 The examination of renal impairment in coronavirus-19 seems to be highly essential, given the previously emphasized homology between the causative viruses. The prevalence of AKI in COVID-19 appears to be low, but varied, with estimates ranging from 0.5 to 19.1% in various investigations.87,88,101 BUN and blood creatinine are both common indicators of kidney damage. 101 Indeed, the current approach to determine acute kidney injury is primarily reliant on severe changes in serum creatinine, with serum creatinine testing frequency having a significant effect on determination speed.102,103 Sick persons with a higher baseline blood creatinine have higher chances of getting admitted to the intensive care units and to require involuntary exposure to air, according to a current investigation by Cheng et al. 102 These data imply that with COVID-19 care, early detection and management of renal degradation, consists of sufficient physiological balance and restricting medicines toxic to the kidney, may be critical, and that more frequent creatinine tests or other kidney markers may be necessary. In COVID-19, serum and urine albumin, as well as total protein, may be beneficial as prognostic markers. The pathogenesis and causes after kidney impairment in coronavirus-19 individuals are unknown and possibly complex, similar to liver dysfunction. 104
Emerging biomarkers and newly developed technologies
Numerous modern technologies have been produced to fight against COVID-19 that opens up new horizons of biomarker development and research for COVID-19, which are summarized in Table 3.
Table 3.
New technologies and emerging biomarkers for COVID-19.
| Technology | Example | Developer | Source |
|---|---|---|---|
| Omic-based | |||
| Protein interactomics | SARS-CoV-2 protein interaction map | QBI COVID Research Group | 105 |
| Efficient proteomics | Sick person proteome investigation by high throughput mass spectrometry Platform |
European collaborative team | 106 |
| Individual genetics | Person genome sequencing | Genomic England & GenOMICC | 107 |
| Bio-imaging | Coronavirus-19 mass spectrometry combination | MS alliance | 108 |
| AI/ML-based | |||
| Artificial intelligence medicine detection and improvement Stage |
BenevolentA | https://www.benevolent.com/covid-19 | |
| Coronavirus indication reading tracking app | ZOE, Massachusetts General Hospital, and King’s College of London | https://covid.joinzoe.com/us | |
| Structure-based | |||
| M(pro) structure-based fundamental in silicon | Different | 109 | |
| High-throughput in vitro screenings | China collaborative team | 109 | |
| Computational in silica examination of severe acute respiratory syndrome CoV-2 viral targets | University of Pennsylvania & Children’s Hospital of Philadelphia |
110 | |
| Nanotechnology | |||
| Nano-particles | Localized surface Plasmon resonance sensor | Empa, ETH Zurich, & Zurich University Hospital | 111 |
| Types of nanoparticles synthesized by carbon containing polymer | University of California, San Diego | 112 | |
| Sequencing of biopolymers specifically nucleotides | LamPORE | Oxford Nanopore Technologies | 110 |
| Fluorescence microscopy techniques | Nanoimager | ONI | https://oni.bio/covid19 |
| Digital technology | |||
| In coronavirus-19 recognition and translation of spoken language into text by computers | Carnegie Mellon University; and Cambridge University | https://voca.ai/corona-virus/https://covid-19-sounds.org/en/ | |
| COV-19 officially discouraged by governments, physicians and patient care organizations | Apple | https://www.apple.com/newsroom/2020/03/apple-releases-new-covid-19-app-and-website-based-on-CDCguidance/ | |
| For Coronavirus-19 used cardinal directions | Seqster | https://seqster.com/press/pressreleases/seqster-launches-covid-19-compass-based-on-cdc-guidelines-forhealthcare-enterprises | |
| Detect the RNA component of virus by Smartphone-based multiplex 30-min | University of Illinois, Urbana-Champaign | 113 | |
| MBioLIMS | |||
| Bio-repository | Biobanking coronavirus-19 stimulation | LabVantage Solutions | https://www.labvantage.com/your-labtype/biobanking/ |
| wwPDB | Primary protein structure database | Collaborative team from UVA, UAM, Poznan University of Technology, ICHB PAN, and NIH |
https://covid-19.bioreproducibility.org/ |
| Tomography | COVID DPR | Indica Labs and Octo | https://www.biospace.com/article/releases/indica-labs-octo-and-axlework-with-nih-to-launch-a-globalcovid-19-digital-pathology-repository/ |
Abbreviations: ICHB PAN, Institute of Bioorganic Chemistry of the Polish Academy of Sciences; COVID-19; coronavirus disease 2019; AI, artificial intelligence; UVA, University of Virginia; UAM, University of Adam Mickiewicz; QBI, Queensland Brain Institute; MS, mass spectrometry; NIH, National Institutes of Health; LSPR, localized surface plasmon resonance
Significant biomarkers for disease severity prediction
Numerous studies compared the biochemical, hematological, coagulation, and inflammatory markers in critical, severe, and mild disease, and published comparable findings. Biochemical parameters such as ALT, LDH, Ferritin, and CRP were significantly increased in severe cases in comparison to mild cases. 114 With disease progression from mild to critical or severe, a reduction in albumin, prealbumin, and lymphocytes was observed while the opposite trend was seen for LHD, CRP, neutrophil, and WBC count.115,116 In addition to these, inflammatory cytokines including IL-10, TNF-α and IL-2R, fibrinogen degradation product (FDP), D-dimer, prothrombin time (PT), and neutrophil-to-lymphocyte ratio (NLR) have been reported significantly increased.114,116,117 The patient’s age is also an important factor in predicting the disease severity and the age range from 50–51.4 years, 63.9–64 years, and 66 years were reported as the median age in mild,114,115 severe,114,117 and critical 118 COVID-19 patients, respectively.
Several studies analyzed the association of disease severity, outcome, and serum viral load.119–121 Increased IL-6 levels (up to 100 pg/mL) and higher mortality were correlated with serum viral load in critically ill patients. 119 Critically ill patients who require admission in ICU were reported with progressive reduction in lymphocyte count with recurrent lymphopenia, decreased platelets count, reduced eosinophil count, increased liver enzymes, increased procalcitonin, elevated CRP, elevated IL-6 and IL-10, decreased renal function, and high serum total bilirubin. 119 To sum up, with increasing disease severity, progressive worsening of biochemical markers such as decreased lymphocyte count, and higher serum viral load, increased IL-6, IL-10, ferritin, CRP, NLR, and certain coagulation markers were observed. However, age and lymphocytopenia were found to be the most important markers as predictor of disease severity. 122
COVID-19 diagnosis in low, middle, and high income countries
Rapid, accurate, and large-scale diagnosis of COVID-19 is one of the fundamental approaches for the containment of COVID-19 in low, middle, and high income settings. 123 The genome availability of SARS-CoV-2 has led to develop and validate a number of reverse transcriptase RT-PCR diagnostic test kits supplied by different manufacturers for the diagnosis of COVID-19. This test is based on the detection of genes encoding nucleocapsid (N), spike (S), envelop (E), and RNA-dependent RNA polymerase of SARS-CoV-2. 124 Because of unavailability of culture facilities, RT-PCR is the reference method for the confirmation of COVID-19 diagnosis in suspected patients worldwide. RT-PCR has been reported as a reliable tool for the screening and diagnostic confirmation of COVID-19 using upper respiratory (e.g., nasal swab, oropharyngeal swab, nasopharyngeal swab, and throat swab) and lower respiratory (e.g. bronchoalveolar lavage and sputum) samples. In spite of high analytical sensitivity of RT-PCR, the detection range of this technique is limited to 3.2 – < 10 RNA copies per reaction. Various studies conducted inside and outside China have reported false negative results of RT-PCR under certain conditions, thereby missing some COVID-19 cases. These missed cases increase the transmission of the disease in the community. These conditions may include inappropriate or insufficient sample for the isolation of viral RNA, poor transportation of sample, poor storage of processed sample, poor timing for the collection of sample, and poor quality of RT-PCR assay. The scaling up of RT-PCR for the diagnosis of COVID-19 is limited in low- and middle-income countries because of limited number of technical personals, limited number of biosafety level 2/3 laboratories accredited for COVID-19 testing, and limited financial resources.124,125
The above challenges of RT-PCR for COVID-19 testing have necessitated the development of rapid diagnostic tests (RDTs) for the diagnosis of COVID-19 for the possible identification of convalescent and asymptomatic COVID-19 patients undiagnosed by RT-PCR. RDTs for COVID-19 testing are based on antigen–antibody reactions detecting SARS-CoV-2 viral antigen from respiratory sample and IgG and/or IgM antibodies in human blood samples within 15 min.126,127 RDTs unlike RT-PCR require no technical expertise, less expensive equipment, and minimal biosafety. RT-PCR protocols require large sample volume (150–200 μL) and longer run time (average run time of approximately 90 min), whereas RDTs use small sample volume (10–20 μL) and average run time of 15 min only. These advantages of RDTs have attracted serious attention for their application in large-scale COVID-19 testing particularly outside the hospital setting and at peripheral level of health system in low-middle income countries. Worldmeters data showed that fewer tests per population were conducted for COVID-19 testing in African countries compared to other countries. This lower testing for COVID-19 detection indicated relatively fewer case detection. Therefore, the deployment of RDTs kits could be advantageous development especially for low-middle income countries as it can be easy to scale up RDTs for the rapid diagnosis of COVID-19.124,127 RDTs can be used for the determination of immunity level of a community against SARS-CoV-2 and to calculate case fatality rate by identifying active and previous asymptomatic and symptomatic cases in addition to the provision of sero-epidemiological data useful for the determination of magnitude of COVID-19 spread within a population. 124
Considering the limited financial resources, limited availability of infrastructure, and technical human resources, the standard RT-PCR may not be able to manage the testing requirement of low-middle income countries. It is generally agreed that RDTs are useful screening tools for the early detection of symptomatic cases, which is very important for averting the hospital or community transmission and to strengthen active surveillance and contact tracing. Therefore, the use of RDT kits may enhance the screening and detection rate of COVID-19 patients in low-middle income countries. However, it is very important to validate RDT kits in reference to RT-PCR with samples from countries of proposed use.
Biosafety measures
Severe acute respiratory syndrome CoV-2 boundary outbreak, laboratories should take special measures to organize for a number of challenges, including biosafety, specimen handling, process, prioritizing of important tests, and staff arrangement. 128
Face mask, when used in conjunction with other preventive measures like frequent hand washing and social isolation, have been shown to help reduce the increasing illness. Face mask will help prevent the infection spread and SARS-CoV-2 transmission. 129 CDC recommends biosafety instructions during the processing of specimens collected from suspected or confirmed coronavirus-19 patients. 130 When handling clinical specimens, which may all contain potentially infectious components, several worldwide and countrywide healthcare organizations urge that laboratory staff must take conventional measures. While management of specimens that are suspected or confirmed to be infected with SARS-CoV-2, laboratory staff must wash their hands thoroughly and wear special PPEs such as face-masks, face shield, eye safety, fashion accessory, and gowns.130,131
Standard measures should be taken with all blood samples. To reduce the risk of transmission, laboratories should pursue the CDC strategy for sample assortment, management, storage space, and transport. 130 During this pandemic, routine blood testing must be performed as often as feasible on an automated-analytical structure with a stopped up automated-pre-analytical system for de-capping and re-capping. Manual specimen processing, such as opening tubes, jars, pipetting, and aliquoting, should be done in a biosafety level cabinet with the appropriate personal protective equipment. 131 Because respiratory specimen may contain SARS-CoV-2, they should be handled with the compulsory personal protective equipment, which includes N95 or higher-level breathing apparatus (or a mask for face if an oxygen mask is not accessible), eye defense, and a gown. Unless performed in a BSL-3 facility, medical laboratories should not try virus separation from samples taken from suspected coronavirus-19 patients. 130
In addition to biosafety, laboratories should create an institutional approach for urgent situation awareness to deal with the pandemic catastrophe. Laboratory personnel should be aware of the limited resources available and prioritize those tests that are expressly needed to deal with the epidemic, as well as those for vital and serious care. During this outbreak, laboratory guidance should recognize the significant scarcity of laboratory employees and build adequate personnel preparation. In general, laboratories must be well-prepared for adequate supplies of personal protection equipment, reagents, and unpreserved possessions to prioritize critical tests and staff arrangement through this virulent disease. 16
Limitations
The informative data about the diagnosis of SARS-CoV-2 disease discussed in this literature review have certain limitations as the data collected from the published literature with wide diversity of studies and research methodologies, different geographic sites, variable population characteristics, sample size, and the quality of publications that may have confounded our data interpretations. Further investigation and validation of the apparent association between different diagnostic techniques described in this review are required to rule out any misinterpretation of the diagnostic sensitivity and specificity of these laboratory tests and these associations have yet to be repeated using robust statistical tools. It is always important to address the knowledge gaps and pinpoint the factors used to potentially predict the complications of COVID-19 that proclaim additional investigations.
Conclusion
COVID-19 is no exception, with laboratory drug at the heart of analytical interpretation and managed treatment for all human pathologies. The clinical laboratory’s critical role has never been more apparent than in today’s crises. By establishing molecular and anti-HTLV assays for diagnosing severe or previous infection, as well as evaluating the correctness and clinical effectiveness of tests produced quickly in a worldwide crises scenario, medical laboratory medication is a serious support in our reaction to coronavirus-19. Along with the biochemical monitoring of inflammatory markers and MOF, clinical laboratories are already making a significant contribution to patient treatment and risk stratification. Overall, the importance of laboratory medication in medical-care has been demonstrated in this virulent disease and cannot be overstated.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Muhammad Riaz https://orcid.org/0000-0002-5524-7735
References
- 1.Bohn MK, Lippi G, Horvath A, et al. (2020) Molecular, serological, and biochemical diagnosis and monitoring of COVID-19: IFCC taskforce evaluation of the latest evidence. Clinical Chemistry and Laboratory Medicine (CCLM) 58: 1037–1052. [DOI] [PubMed] [Google Scholar]
- 2.Abid K, Bari YA, Younas M, Tahir Javaid S, et al. (2020) <? covid19?> Progress of COVID-19 Epidemic in Pakistan. Asia Pacific Journal of Public Health 32: 154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artik Y, Cesur N, Kenar L, et al. (2021) Biological disasters: an overview of the covid-19 pandemic in the first quarter of 2021. Afet ve Risk Dergisi 4: 163–182. [Google Scholar]
- 4.Artik Y, Varol N, Cesur N, et al. (2022) Hospital disaster and emergency plan in biological disasters (HDEP): coronavirus (SARS-CoV-2) COVID-19 pandemic system model example. Journal of Contemporary Studies in Epidemiology and Public Health 3: ep22003. [Google Scholar]
- 5.Komurcu SZM, Artik Y, Cesur NP, et al. (2022) The evaluation of potential global impact of the N501Y mutation in SARS‐COV-2 positive patients. Journal of Medical Virology 94: 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Guo H. (2020) Biomarkers of COVID-19 and technologies to combat SARS-CoV-2. Advances in Biomarker Sciences and Technology 2: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khizroeva J, Makatsariya AD, Bitsadze VO, et al. (2020) Laboratory monitoring of COVID-19 patients and importance of coagulopathy markers. Obstetrics, Gynecology and Reproduction 14: 132–147. [Google Scholar]
- 8.Organization WH. (2020) Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases: Interim Guidance, 14 January 2020. World Health Organization. [Google Scholar]
- 9.Artik Y, Coşğun AB, Cesur NP, et al. (2022) Comparison of COVID-19 laboratory diagnosis by commercial kits: effectivity of RT-PCR to the RT-LAMP. Journal of medical virology 94: 1998–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandenberg O, Martiny D, Rochas O, et al. (2021) Considerations for diagnostic COVID-19 tests. Nature Reviews Microbiology 19: 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadevall A, Pirofski L-A. (2020) The convalescent sera option for containing COVID-19. The Journal of clinical investigation 130: 1545–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xavier AR, Silva JS, Almeida JPCet al. (2020) COVID-19: clinical and laboratory manifestations in novel coronavirus infection. Jornal Brasileiro de Patologia e Medicina Laboratorial 56: 1–9. [Google Scholar]
- 13.Pizzol JLD, Hora Vpd, Reis AJet al. (2020) Laboratory diagnosis for Covid-19: A mini-review. Revista da Sociedade Brasileira de Medicina Tropical 53: e2020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F, Zhao S, Yu B, et al. (2020) A new coronavirus associated with human respiratory disease in China. Nature 579: 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam TT-Y, Jia N, Zhang Y-W, et al. (2020) Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 583: 282–285. [DOI] [PubMed] [Google Scholar]
- 16.Fang B, Meng QH. (2020) The laboratory’s role in combating COVID-19. Critical reviews in clinical laboratory sciences 57: 400–414. [DOI] [PubMed] [Google Scholar]
- 17.Wang D, Hu B, Hu C, et al. (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, McGoogan JM. (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 19.Guan W-j, Ni Z-y, Hu Y, et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowd JB, Andriano L, Brazel DM, et al. (2020) Demographic science aids in understanding the spread and fatality rates of COVID-19. Proceedings of the National Academy of Sciences 117: 9696–9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson S, Hirsch JS, Narasimhan M, et al. (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. Jama 323: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen T, Wu D, Chen H, et al. (2020) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj 368: m1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou P, Yang X-L, Wang X-G, et al. (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin C, Ziwei MPLZM, Tao SYMY, et al. (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China; clinical infectious diseases; Oxford academic. Clinical Infectious Diseases 71: 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Channappanavar R, Perlman S. (2017) Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. In: Seminars in Immunopathology. New York, NY: Springer, pp. 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Shi L, Wang Y, et al. (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet respiratory medicine 8: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry BM, Vikse J, Benoit S, et al. (2020) Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clinica Chimica Acta 507: 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidal J-M, Kawabata TT, Thorpe R, et al. (2010) In vitro cytokine release assays for predicting cytokine release syndrome: the current state-of-the-science. report of a european medicines agency workshop. Cytokine 51: 213–215. [DOI] [PubMed] [Google Scholar]
- 29.Stebbings R, Findlay L, Edwards C, et al. (2007) Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. The Journal of Immunology 179: 3325–3331. [DOI] [PubMed] [Google Scholar]
- 30.Grimaldi C, Finco D, Fort MM, et al. (2016) Cytokine release: a workshop proceedings on the state-of-the-science, current challenges and future directions. Cytokine 85: 101–108. [DOI] [PubMed] [Google Scholar]
- 31.Mehta P, McAuley DF, Brown M, et al. (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. The lancet 395: 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alhazzani W, Møller MH, Arabi YM, et al. (2020) Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive care medicine 46: 854–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aziz M, Fatima R, Assaly R, et al. (2020) Elevated interleukin-6 and severe COVID-19: a meta-analysis. Journal of medical virology 92: 2283–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo P, Liu Y, Qiu L, , et al. (2020) Tocilizumab treatment in COVID-19: a single center experience. Journal of medical virology 92: 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okba NM, Müller MA, Li W, et al. (2020) Severe acute respiratory syndrome coronavirus 2− specific antibody responses in coronavirus disease patients. Emerging Infectious Diseases 26: 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel A, Jernigan DB. (2020) Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak—United States. December 2019–February 4 2020. Morbidity and Mortality Weekly Report 3169: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Organization WH (2019) Guidance on Regulations for the Transport of Infectious Substances 2019–2020: Applicable from 1 January 2019. World Health Organization. [Google Scholar]
- 38.Carter LJ, Garner LV, Smoot JW, et al. (2020) Assay Techniques and Test Development for COVID-19 Diagnosis. Washington, D.C: ACS Publications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Y-W, Schmitz JE, Persing DH, et al. (2020) Laboratory diagnosis of COVID-19: current issues and challenges. Journal of clinical microbiology 58: e00512–e00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Organization WH (2020) Laboratory Biosafety Guidance Related to Coronavirus Disease 2019 ( COVID-19): Interim Guidance, 12 February 2020. World Health Organization. [Google Scholar]
- 41.Padhye NS. (2020) Reconstructed Diagnostic Sensitivity and Specificity of the RT-PCR Test for COVID-19. MedRxiv. [Google Scholar]
- 42.Tahamtan A, Ardebili A. (2020) Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert review of molecular diagnostics 20: 453–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giri B, Pandey S, Shrestha R, et al. (2021) Review of analytical performance of COVID-19 detection methods. Analytical and Bioanalytical Chemistry 413: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yi H. (2020) 2019 Novel coronavirus is undergoing active recombination. Clinical Infectious Diseases 71: 884–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korber B, Fischer W, Gnanakaran S, et al. (2020) Spike Mutation Pipeline Reveals the Emergence of a More Transmissible Form of SARS-CoV-2. BioRxiv. [Google Scholar]
- 46.Becerra-Flores M, Cardozo T. (2020) SARS-CoV-2 viral spike G614 mutation exhibits higher case fatality rate. International Journal of Clinical Practice 74: e13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippi G, Plebani M. (2020) The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clinical Chemistry and Laboratory Medicine (CCLM) 58: 1063–1069. [DOI] [PubMed] [Google Scholar]
- 48.Maxmen A. (2019) Faster, better, cheaper: the rise of CRISPR in disease detection. Nature 566: 437–438. [DOI] [PubMed] [Google Scholar]
- 49.Ding J, Adiconis X, Simmons SK, et al. (2020) Systematic comparison of single-cell and single-nucleus RNA-sequencing methods. Nature biotechnology 38: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbott TR, Dhamdhere G, Liu Y, et al. (2020) Development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza. Cell 181: 865–876. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stefanelli P, Faggioni G, Presti AL, et al. (2020) Whole genome and phylogenetic analysis of two SARS-CoV-2 strains isolated in Italy in January and February 2020: additional clues on multiple introductions and further circulation in Europe. Eurosurveillance 25: 2000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Organization WH (2020) Laboratory Testing Strategy Recommendations for COVID-19: Interim Guidance, 21 March 2020. World Health Organization. [Google Scholar]
- 53.Yan Y, Chang L, Wang L, et al. (2020) Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): Current status, challenges, and countermeasures. Reviews in medical virology 30: e2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loeffelholz MJ, Tang Y-W. (2020) Laboratory diagnosis of emerging human coronavirus infections–the state of the art. Emerging microbes & infections 9: 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mourya DT, Sapkal G, Yadav PD, et al. (2020) Biorisk assessment for infrastructure & biosafety requirements for the laboratories providing coronavirus SARS-CoV-2/(COVID-19) diagnosis. The Indian Journal of Medical Research 151: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blow JA, Dohm DJ, Negley DL, et al. (2004) Virus inactivation by nucleic acid extraction reagents. Journal of Virological Methods 119: 195–198. [DOI] [PubMed] [Google Scholar]
- 57.Artik Y, Cesur N, Kurtulmus M, , et al. (2022) Clinic evaluation of the destrovir spray effectiveness in SARS-CoV-2 disease. Electronic Journal of General Medicine 19(2): em3572022. [Google Scholar]
- 58.Ulinici M, Covantev S, Wingfield-Digby J, et al. (2021) Screening, diagnostic and prognostic tests for COVID-19: a comprehensive review. Life 11: 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deeks JJ, Raffle AE. (2020) Lateral flow tests cannot rule out SARS-CoV-2 infection. British Medical Journal Publishing Group 371: m4787. [DOI] [PubMed] [Google Scholar]
- 60.Graziadio S, Urwin SG, Cocco P, et al. (2020) Unmet clinical needs for COVID-19 tests in UK health and social care settings. PLoS One 15: e0242125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynard C, Allen JA, Shinkins B, et al. (2022) COVID-19 rapid diagnostics: practice review. Emergency Medicine Journal 39: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lambert-Niclot S, Cuffel A, Le Pape S, et al. (2020) Evaluation of a rapid diagnostic assay for detection of SARS-CoV-2 antigen in nasopharyngeal swabs. Journal of clinical microbiology 58: e00977–e001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mertens P, De Vos N, Martiny D, et al. (2020) Development and potential usefulness of the COVID-19 Ag Respi-Strip diagnostic assay in a pandemic context. Frontiers in medicine 7: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sethuraman N, Jeremiah SS, Ryo A, et al. (2020) Interpreting diagnostic tests for SARS-CoV-2. Jama 323: 2249–2251. [DOI] [PubMed] [Google Scholar]
- 65.Subbaraman N. (2020) Coronavirus tests: researchers chase new diagnostics to fight the pandemic. Nature 2020: 1. [DOI] [PubMed] [Google Scholar]
- 66.Ghaffari A, Meurant R, Ardakani A, et al. (2020) COVID-19 serological tests: how well do they actually perform? Diagnostics 10: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cai X-f, Chen J, li Hu J-, et al. (2020) A peptide-based magnetic chemiluminescence enzyme immunoassay for serological diagnosis of coronavirus disease 2019. The Journal of Infectious Diseases 222: 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ching L, Chang SP, Nerurkar VR, et al. (2020) COVID-19 special column: principles behind the technology for detecting SARS-CoV-2, the cause of COVID-19. Hawai'i Journal of Health & Social Welfare 79: 136–142. [PMC free article] [PubMed] [Google Scholar]
- 69.Yongchen Z, Shen H, Wang X, et al. (2020) Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID-19 patients. Emerging microbes & infections 9: 833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao J, Yuan Q, Wang H, et al. (2020) Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease. Clinical infectious diseases 71: 2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo L, Ren L, Yang S, et al. (2020) Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clinical infectious diseases 71: 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Padoan A, Sciacovelli L, Basso D, et al. (2020) IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: a longitudinal study. Clinica Chimica Acta 507: 164–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gunther N, Hoffmann GW. (1982) Qualitative dynamics of a network model of regulation of the immune system: a rationale for the IgM to IgG switch. Journal of theoretical biology 94: 815–855. [DOI] [PubMed] [Google Scholar]
- 74.Hoffman T, Nissen K, Krambrich J, et al. (2020) Evaluation of a COVID-19 IgM and IgG rapid test; an efficient tool for assessment of past exposure to SARS-CoV-2. Infection Ecology & Epidemiology 10: 1754538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li G, Chen X, Xu A, et al. (2003) Profile of specific antibodies to the SARS-associated coronavirus. New England Journal of Medicine 349: 508–509. [DOI] [PubMed] [Google Scholar]
- 76.Hsueh P-R, Huang L-M, Chen P-J, et al. (2004) Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clinical microbiology and infection 10: 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim D, Lee J-Y, Yang J-S, et al. (2020) The architecture of SARS-CoV-2 transcriptome. Cell 181: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu J, Zhao S, Teng T, et al. (2020) Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses 12: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Letko M, Marzi A, Munster V, et al. (2020) Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nature microbiology 5: 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gorse GJ, Patel GB, Vitale JN, et al. (2010) Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clinical and Vaccine Immunology 17: 1875–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang X, Yu Y, Xu J, et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henry BM, De Oliveira MHS, Benoit S, et al. (2020) Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clinical Chemistry and Laboratory Medicine (CCLM) 58: 1021–1028. [DOI] [PubMed] [Google Scholar]
- 83.Lippi G, Plebani M. (2020) Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clinica Chimica Acta; International Journal of Clinical Chemistry 505: 190–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. (2020) Hematological findings and complications of COVID-19. American Journal of Hematology 95: 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lippi G, Lavie CJ, Sanchis-Gomar F, et al. (2020) Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Progress in Cardiovascular Diseases 63: 390–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tersalvi G, Vicenzi M, Calabretta D, et al. (2020) Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. Journal of cardiac failure 26: 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang C, Wang Y, Li X, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang W, Xu Y, Gao R, et al. (2020) Detection of SARS-CoV-2 in different types of clinical specimens. Jama 323: 1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo T, Fan Y, Chen M, et al. (2020) Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA cardiology 5: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lieberman JA, Pepper G, Naccache SN, et al. (2020) Comparison of commercially available and laboratory-developed assays for in vitro detection of SARS-CoV-2 in clinical laboratories. Journal of Clinical Microbiology 58: e00821–e00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Januzzi J. (2020) Troponin and BNP use in COVID-19. Cardiology magazine 18. [Google Scholar]
- 92.Chapman AR, Bularga A, Mills NL, et al. (2020) High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation 141: 1733–1735. [DOI] [PubMed] [Google Scholar]
- 93.Bansal M. (2020) Cardiovascular disease and COVID-19. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 14: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian S, Hu N, Lou J, et al. (2020) Characteristics of COVID-19 infection in Beijing. Journal of Infection 80: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cai Q, Huang D, Yu H, et al. (2020) COVID-19: abnormal liver function tests. Journal of Hepatology 73: 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang C, Shi L, Wang F-S, et al. (2020) Liver injury in COVID-19: management and challenges. The Lancet Gastroenterology & Hepatology 5: 428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bangash MN, Patel J, Parekh D, et al. (2020) COVID-19 and the liver: little cause for concern. The Lancet Gastroenterology & Hepatology 5: 529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun J, Aghemo A, Forner A, et al. (2020) COVID-19 and Liver Disease. Liver international 40: 1278–1281. [DOI] [PubMed] [Google Scholar]
- 99.Phillips T, Stammers M, Leggatt G, et al. (2021) Acute kidney injury in COVID-19: identification of risk factors and potential biomarkers of disease in a large UK cohort. Nephrology 26: 420–431. [Google Scholar]
- 100.Chu KH, Tsang WK, Tang CS, et al. (2005) Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney International 67: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zaim S, Chong JH, Sankaranarayanan V, et al. (2020) COVID-19 and multiorgan response. Current problems in cardiology 45: 100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng Y, Luo R, Wang K, et al. (2020) Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney International 97: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ge S, Nie S, Liu Z, et al. (2016) Epidemiology and outcomes of acute kidney injury in elderly chinese patients: a subgroup analysis from the EACH study. BMC nephrology 17: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ronco C, Reis T. (2020) Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nature Reviews Nephrology 16: 308–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gordon DE, Jang GM, Bouhaddou M, et al. (2020) A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583: 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Messner CB, Demichev V, Wendisch D, et al. (2020) Ultra-high-throughput clinical proteomics reveals classifiers of COVID-19 infection. Cell systems 11: 11–24. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pathak GA, Singh K, Miller-Fleming TW, et al. (2021) Integrative genomic analyses identify susceptibility genes underlying COVID-19 hospitalization. Nature communications 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Griffin JH, Downard KM. (2021) Mass spectrometry analytical responses to the SARS-CoV2 coronavirus in review. TrAC Trends in Analytical Chemistry 142: 116328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jin Z, Du X, Xu Y, et al. (2020) Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 582: 289–293. [DOI] [PubMed] [Google Scholar]
- 110.Zhang L, Guo H. (2020) Biomarkers of COVID-19 and technologies to combat SARS-CoV-2. Advances in Biomarker Sciences and Technology 2: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qiu G, Gai Z, Tao Y, et al. (2020) Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS nano 14: 5268–5277. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Q, Honko A, Zhou J, et al. (2020) Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano letters 20: 5570–5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun F, Ganguli A, Nguyen J, et al. (2020) Smartphone-based multiplex 30-minute nucleic acid test of live virus from nasal swab extract. Lab on a Chip 20: 1621–1627. [DOI] [PubMed] [Google Scholar]
- 114.Chen G, Wu D, Guo Wet al. (2020) Clinical and immunologic features in severe and moderate coronavirus disease 2019. The Journal of Clinical Investigation 82: 137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qian G-Q, Yang N-B, Ding F, et al. (2020) Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM: An International Journal of Medicine 113: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ji M, Yuan L, Shen W, et al. (2020) Characteristics of disease progress in patients with coronavirus disease 2019 in Wuhan, China. Epidemiology & Infection 148: e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liao D, Zhou F, Luo L, et al. (2020) Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. The Lancet Haematology 7: e671–e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zheng Y, Sun L-J, Xu M, et al. (2020) Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. Journal of Zhejiang University-SCIENCE B 21: 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen X, Zhao B, Qu Y, et al. (2020) Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clinical Infectious Diseases 71: 1937–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Magleby R, Westblade LF, Trzebucki A, et al. (2020) Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clinical Infectious Diseases an official publication of the Infectious Diseases Society of America 73: e4197–e4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Argyropoulos KV, Serrano A, Hu J, et al. (2020) Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. The American journal of pathology 190: 1881–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang X, Tan Y, Ling Y, et al. (2020) Viral and host factors related to the clinical outcome of COVID-19. Nature 583: 437–440. [DOI] [PubMed] [Google Scholar]
- 123.Nkengasong JN, Mankoula W. (2020) Looming threat of COVID-19 infection in Africa: act collectively, and fast. The Lancet 395: 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Olalekan A, Iwalokun B, Akinloye OM, et al. (2020) COVID-19 rapid diagnostic test could contain transmission in low-and middle-income countries. African Journal of Laboratory Medicine 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Younes N, Al-Sadeq DW, Al-Jighefee H, et al. (2020) Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2. Viruses 12: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. (2020) False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One 15: e0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haveri A, Smura T, Kuivanen S, et al. (2020) Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Eurosurveillance 25: 2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yuan D, Gao W, Liang S, et al. (2020) Biosafety threats of the rapidly established labs for SARS-CoV-2 tests in China. Environment international 143: 105964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leung NH, Chu DK, Shiu EY, et al. (2020) Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature medicine 26: 676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.CDC F. CDC 2019-nCoV real-time RT-PCR diagnostic panel. Fact Sheet For Healthcare Providers. [Online] June 12, 2020 . https://www.fda.gov/media/134920/download.2020 [Google Scholar]
- 131.Lippi G, Adeli K, Ferrari M, et al. (2020) Biosafety measures for preventing infection from COVID-19 in clinical laboratories: IFCC Taskforce Recommendations. Clinical Chemistry and Laboratory Medicine (CCLM) 58: 1053–1062. [DOI] [PubMed] [Google Scholar]