Abstract
Background: Screening programs using Papanicolaou smear along with human papillomavirus (HPV) testing led to a significantly decrease of cervical cancer rates. Nevertheless, both assessments have limited specificity for revealing cervical high-grade lesions. The main problem is how to identify the real precursor of cervical squamous cell carcinomas (SCC), namely high-grade squamous intraepithelial lesions (HSIL). Aim: The aim of our study was to conclude if ProEx C might be used as a marker for high-grade cervical intraepithelial neoplasia (CIN). Materials and Methods: In this study, we detected the immunochemical expression of anti-ProEx C antibody in liquid-based cytology (LBC) samples. We analyzed a total number of 125 cervical cytology specimens. Results: In 48% of all cases, ProEx C was found to be positive. The percentage increased from 0% in negative for intraepithelial lesion or malignancy (NILM) cases to 100% in SCC cases. Conclusions: ProEx C may be utilized to improve the accuracy of cytological diagnosis on cervical smears, according to the findings of this study. This marker is also useful in detecting unrevealed high-grade lesions on atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesions (LSIL) smears, being very useful in establishing the conduct of these cases.
Keywords: liquid-based cytology, human papillomavirus, ProEx C, immunocytochemistry
⧉ Introduction
Most cervical malignancies are known to develop from precursor lesions as squamous intraepithelial lesions (SIL) associated with high-risk human papillomavirus (HR-HPV) [1]. SIL are categorized as low or high grade according on their likelihood of progressing to carcinoma if left untreated. Whereas low-grade SIL (LSIL) are infrequently followed by a cancer diagnosis, high-grade SIL (HSIL) are thought to be the true precursor to cervical squamous cell carcinomas (SCC) [2]. The requirement of a proper diagnosis of the latter is essential for both optimal care and to stop the disease’s natural development. Since the Papanicolaou (Pap) test cannot provide sufficient sensitivity (Sn) for detecting HSIL, women with moderate cytological atypia must receive colposcopy and cervical biopsies to acquire satisfactory test Sn. High specificity (Sp) is the intended outcome at this phase to avoid false-positive results that could result in patient psychological stress, unneeded conization, or hysterectomy [3]. Consequently, a method for diagnosing high-grade cervical disease in conjunction with cervical cytology and molecular testing for HR-HPV infection is required. This method should be capable to detect high-grade cervical disease and to differentiate high-grade disease from other mimics or from conditions that are not considered real precursors of SCC, such as early-stage HPV infection and mild dysplasia.
In clinical practice, an improved screening algorithm for cervical cancer is undeniably essential. An appropriate screening test should identify the consequence of HR-HPV infection subsequently to cervical cell changes. Moreover, it should precisely identify the patients in which disease progression to neoplasia occurs [4].
HPV oncoproteins are directly involved in the progression of dysplasia and cervical cancer, according to previous research. E6 acts through interaction with p53 tumor suppressor protein and E7 through interaction with pRb, p107 and p130 retinoblastoma proteins [5]. These induce aberrant transcription of S-phase proteins, such as topoisomerase II alpha (TOP2A) and minichromosome maintenance 2 (MCM2) protein. ProEx C is an immunocytochemical (ICC) marker that can represent an adjuvant test for equivocal cytodiagnostics and can help to detect high-grade cervical lesions [6], having a high Sn and Sp in detecting aberrant induction of the S phase.
ProEx C contains three antibodies: two clones of MCM2 and TOP2A. MCM2 proteins are involved in the early stages of deoxyribonucleic acid (DNA) replication. TOP2A, the third antibody included in ProEx C, is a nuclear enzyme involved in DNA replication, helping to maintain the accurate conformation of DNA. These are overexpressed in the aberrant induction of the S phase, being overexpressed in dysplastic and malignant cells [7], but the presence of immunolabeling may also be possible in some morphologically normal cells. For this reason, normal-looking cells, even if they react positively to these markers, should be reported as negative [8,9].
Aim
Our study aim was to identify the ICC expression of ProEx C and to investigate the expression of this marker in cervical lesions.
⧉ Materials and Methods
Within the study, we analyzed a total number of 125 patients who were cytologically investigated, tested for HPV and had biopsy under colposcopic control. The Pap smears were immunocytochemically stained using anti-ProEx C (BD Diagnostics–TriPath, Burlington, North Carolina, USA) antibodies.
Liquid-based cytology (LBC)
The samples collected from patients using a Wallach® Papette, which was then directly immersed into a container with PapSpin Collection Fluid stabilizer. Cytospin 4 was used to perform LBC (PapSpin®) (Thermo Shandon). Cyto Check densitometer was used to monitor the cell density. We performed regular cell dilution using manufacturer’s procedure. Cells from the megafunnel were deposited by cytocentrifugation on the glass slide. Two slides per case were prepared: one slide was fixed and stained with Pap stain, respectively one slide was used for ICC staining. The Bethesda reporting system was used to assess the cytological specimens [10].
HPV DNA testing
Thirty-seven HPV types with high and low risk were identified individually at the Ştefan S. Nicolau Institute of Virology, Bucharest, Romania, by using the Linear Array HPV Genotyping Test (Roche Diagnostics). Specifically, these types were 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73 (MM9), 81, 82 (MM4), 83 (MM7), 84 (MM8), IS39, and CP6108.
The method is centered on polymerase chain reaction (PCR) amplification of a fragment of HPV L1 gene followed by hybridization; this kit is registered for use in the European Union. The tests were carried out in accordance with the manufacturer’s instructions. Each run included an internal control, as well as HPV-negative and HPV-positive controls [11].
Cervical biopsy specimens
To establish the exact degree of dysplasia, a cervical biopsy was performed under colposcopic control. We applied 5% Acetic acid at the exocol and endocol level. The biopsy was performed on the aceto-white areas. When no aceto-white areas were observed, the biopsy was performed at the squamous-cylindrical junction in the upper quadrant. Sections were stained with Hematoxylin–Eosin (HE), and the diagnosis was written using SIL terminology [12].
ProEx C ICC staining
The BD ProEx™ C Antibody Reagent containing mouse monoclonal anti-MCM2 and anti-TOP2A antibodies was used for ICC staining. They were purified from tissue culture supernatant, and consequently diluted in buffered saline solution, having 0.09% Sodium Azide and protein stabilizers.
The testing was also done respecting the manufacturer’s protocol. SureDetect™ SiHa (human cervical tumor cell) Cell Control was available from TriPath Imaging®, Inc. A cervical cancer cell line was contained in each sample, prepared in the same way as the clinical specimens. As a negative control, a mouse immunoglobulin G (IgG) was utilized. Each staining step comprised both positive and negative control slides.
The evaluation of ProEx C immunostaining
All histopahological (HP) samples were assessed to confirm the absence or nonspecific staining and to validate the nuclear staining of ProEx C. SiHa cell line was used as an internal positive control. The evaluation of tissue specimens was done using a three-step algorithm. According to 2001 Bethesda System, we firstly considered the adequacy of the specimen [10]. In the next phase, we evaluated the brown nuclear staining in the squamous cells. Finally, we analyzed if the stained cells revealed changes comply with the diagnostic criteria. We considered positive the slides in which the three steps were fulfilled [13].
Statistical analysis
Sn, Sp, positive predictive values (PPV), negative predictive values (NPV) and accuracy (Ac) were assessed to evaluate the diagnostic precision of the ProEx C, using HP exam as the “gold standard”. HP diagnoses of HSIL were considered positive results [14].
⧉ Results
Cytological results
The cytological diagnosis of the 125 patients included in our research were as follows: 18 (14.4%) cases were negative for intraepithelial lesion or malignancy (NILM), eight (6.4%) cases with atypical squamous cells of undetermined significance (ASC-US), 43 (34.4%) cases LSIL, 52 (41.6%) cases HSIL and in four (3.2%) cases SCC.
HP analysis
HP results revealed that 22 (17.6%) cases were benign lesions, 46 (36.8%) cases were LSIL, 49 (39.2%) cases were HSIL, and eight (6.4%) cases were SCC. Table 1 displays the distribution of cytological diagnoses in accordance with HP diagnosis.
Table 1.
The distribution of cytological diagnosis, according to HP diagnosis
|
Cytological diagnosis |
HP diagnosis |
|||
|
B |
LSIL |
HSIL |
SCC |
|
|
NILM |
15 |
3 |
0 |
0 |
|
ASC-US |
4 |
3 |
1 |
0 |
|
LSIL |
3 |
38 |
2 |
0 |
|
HSIL |
0 |
2 |
46 |
4 |
|
SCC |
0 |
0 |
0 |
4 |
ASC-US: Atypical squamous cells of undetermined significance; B: Benign lesions; HP: Histopathological; HSIL: High-grade squamous intraepithelial lesion; LSIL: Low-grade squamous intraepithelial lesion; NILM: Negative for intraepithelial lesion or malignancy; SCC: Squamous cell carcinoma
HPV genotyping results
Our results proved single HPV infections in 68 (54.4%) cases and multiple HPV infections in other 41 (32.8%) cases; 16 (12.8%) cases were HPV-negative. In the group with single HPV infection, 11 (16.17%) cases presented with low-risk HPV (LR-HPV) and 57 (83.82%) cases with HR-HPV. Since all cases of multiple type HPV infections contained at least one oncogenic type, they were all included in the HR-HPV group for evaluation.
Following that, the HPV genotyping findings divided into groups based on HPV risk. Of all 125 included patients, we found 98 (78.4.4%) cases with HR-HPV and 11 (8.8%) cases with LR-HPV, the rest being negative. Furthermore, we divided the HPV risk group in relation to the diagnostic category. HR-HPV infection was found in 11.11% of NILM cases (2/18), 50% of ASC-US cases (4/8) and 83.72% of LSIL cases (36/43). All HSIL and SCC cases displayed HR-HPV genotypes.
ICC results
In this study, a positive result for ProEx C was found in 48% from all the cases (Table 2), namely 12.5% from ASC-US cases (Figure 1), 18.6% from LSIL cases (Figure 2), 90.4% from HSIL cases (Figure 3), and 100% from SCC cases (Figure 4). ProEx C was found to be negative in all NILM cases.
Table 2.
ProEx C biomarker detection rate in the cytological smear in liquid medium
|
Cytological diagnosis |
Total cases |
ProEx C positive |
|
|
n |
% |
||
|
NILM |
18 |
0 |
0.0 |
|
ASC-US |
8 |
1 |
12.5 |
|
LSIL |
43 |
8 |
18.6 |
|
HSIL |
52 |
47 |
90.4 |
|
SCC |
4 |
4 |
100 |
|
Total |
125 |
60 |
48 |
ASC-US: Atypical squamous cells of undetermined significance; B: Benign lesions; HSIL: High-grade squamous intraepithelial lesion; LSIL: Low-grade squamous intraepithelial lesion; n: No. of cases; NILM: Negative for intraepithelial lesion or malignancy; SCC: Squamous cell carcinoma
Figure 1.
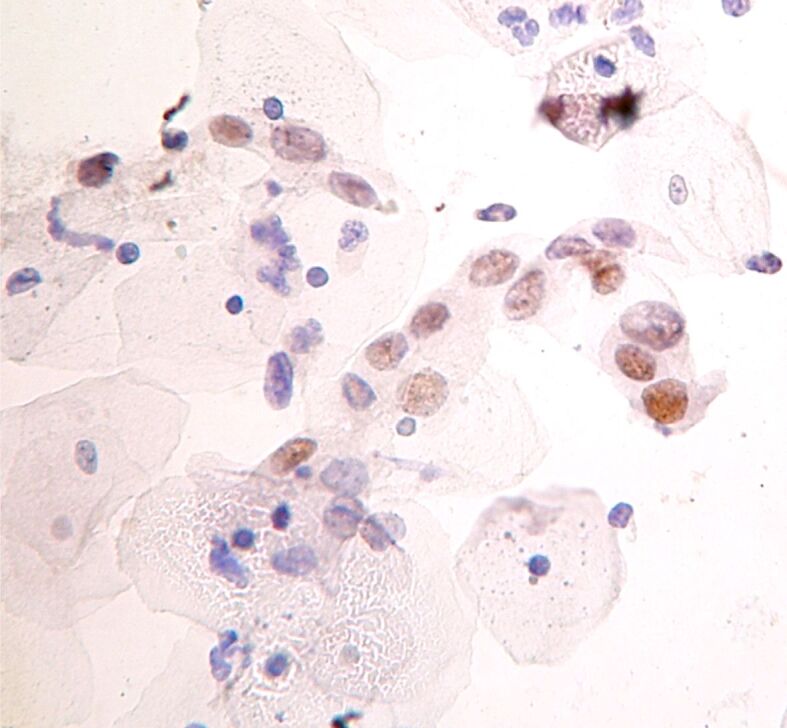
ASC-US: nuclear marking for ProEx C (immunocytochemistry, ×400). ASC-US: Atypical squamous cells of undetermined significance
Figure 2.
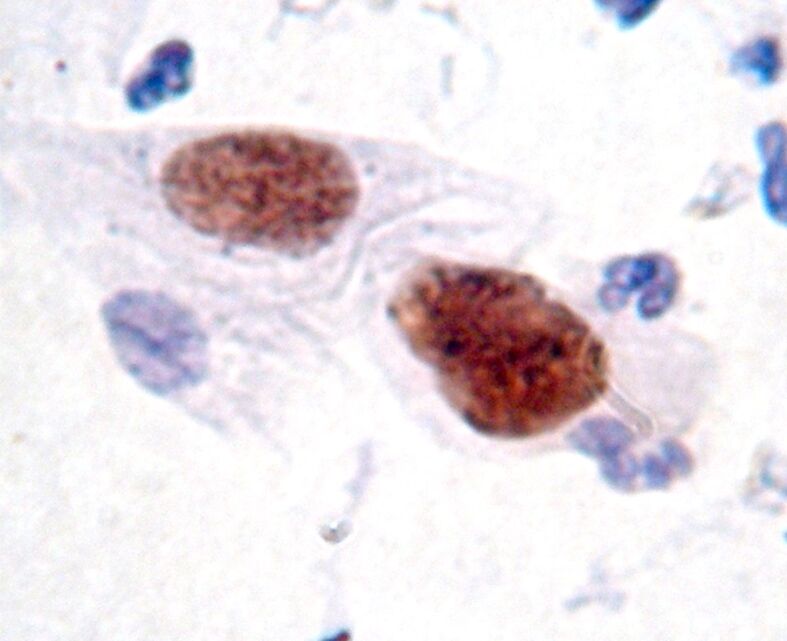
LSIL: nuclear labeling for ProEx C in dysplastic cells (immunocytochemistry, ×1000). Low-grade squamous intraepithelial lesion
Figure 3.
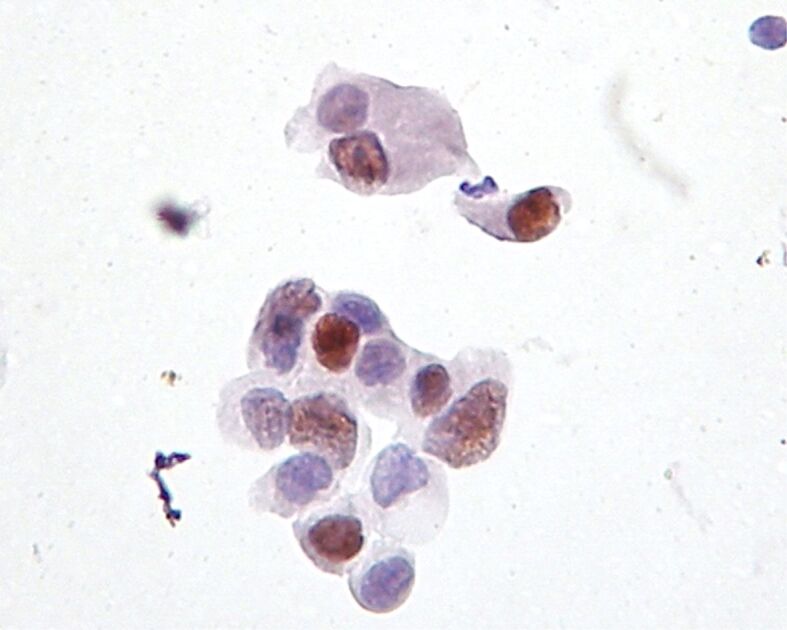
HSIL: nuclear labeling for ProEx C in dysplastic cells (immunocytochemistry, ×400). HSIL: High-grade squamous intraepithelial lesion
Figure 4.
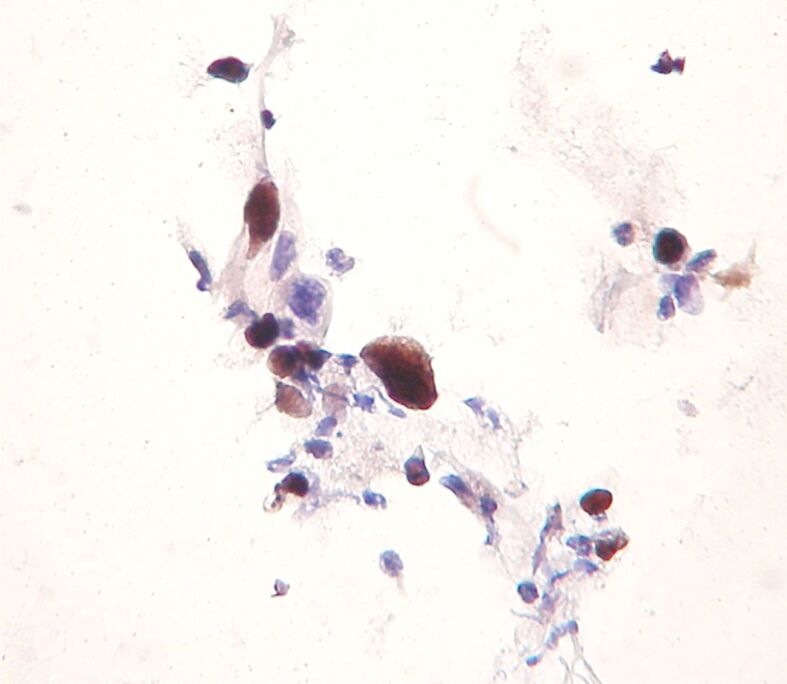
SCC: nuclear labeling for ProEx C in dysplastic cells (immunocytochemistry, ×400). SCC: Squamous cell carcinoma
60.2% of positive HR-HPV patients presented positive ProEx C biomarker, while in negative HR-HPV patients, only one positive ProEx C case (3.7%) was registered (p=0.0005) (Table 3).
Table 3.
Correlation between HPV risk and ProEx C positivity in study patients
|
ProEx C |
HPV |
Statistical significance |
|||
|
HR-HPV (+) |
Statistical significance |
||||
|
n |
% |
n |
% |
||
|
Positive |
59 |
60.2 |
1 |
3.7 |
χ2=12.11; GL=1; p=0.0005 |
|
Negative |
39 |
39.8 |
26 |
96.3 |
|
HR-HPV: High-risk human papillomavirus; n: No. of cases
The association of HR-HPV infection with ProEx C positivity induces a relatively viral oncogenic risk of 1.28 times higher [relative risk (RR): 1.28; 95% confidence interval (CI): 1.11–1.49]. The outcomes of HP follow-up on LBC specimens in relation to ProEx C immunostaining are shown in Table 4.
Table 4.
HP follow-up results of the LBC samples in correlation with ProEx C immunostaining
|
ProEx C |
B |
LSIL |
HSIL (+) |
|
Negative |
22 |
39 |
4 |
|
Positive |
0 |
7 |
53 |
B: Benign lesions; HP: Histopathological; HSIL: High-grade squamous intraepithelial lesion; LBC: Liquid-based cytology; SIL: Low-grade squamous intraepithelial lesion
Statistical results
For the ProEx C, the presence of the immunostaining was considered as a positive result. The diagnostic test results revealed that Sn 93%, Sp 87%, PPV 88.33%, NPV 92.2%, and the Ac was 90%. The log-rank analysis was statistically relevant for this antibody (p<0.0001) (Table 5).
Table 5.
Comparative analysis of the Ac of the ICC diagnosis in the detection of HSIL (+) lesions
|
ICC test |
Sn [%] |
Sp [%] |
PPV [%] |
NPV [%] |
Ac [%] |
p |
|
ProEx C (+) |
93.0 |
87.0 |
88.33 |
92.2 |
90.0 |
<0.0001 |
Ac: Accuracy; HSIL: High-grade squamous intraepithelial lesion; ICC: Immunocytochemical; NPV: Negative predictive value; Sn: Sensitivity; Sp: Specificity; PPV: Positive predictive value
⧉ Discussions
ProEx C represents a cocktail of antibodies which play together an important role in regulating DNA replication in eukaryotes [15,16]. E6 and E7 HR-HPV oncoproteins act at cell cycle checkpoints causing a lengthy and abnormal induction of the S phase. During cell cycle aberrant transcription triggering, MCM2 and TOP2A levels increase in proliferating cells. Previous studies proved an overexpression of MCM2 and TOP2A in various dysplastic and malignant cell types, including HR-HPV-induced cervical neoplasia [15].
ProEx C appears to be an ICC marker that can provide an alternative or an auxiliary test for cervical cytology of uncertain significance (ASC) and can help detect high-grade intraepithelial lesions [17,18].
Within our study, correlation of HR-HPV infection with the presence of the ProEx C immunolabel showed positivity in 60.2% of HR-HPV (+) patients, whereas only one ProEx C positive case was reported in negative HR-HPV patients. All these observations are supported by statistical analysis, which confirmed significant differences (p=0.0005) between HR-HPV (positive versus negative) and ProEx positive immunolabeling. Overall, the results obtained support the interest focused on the role of HR-HPV and positive ProEx C in the development of viral oncogene risk.
In 2006, Shroyer et al. analyzed and proved the performance of ProEx C in 40 prospectively clinical specimens collected from patients who were evaluated at the University of Colorado at Denver and Health Sciences Center. The findings of this study validated previous research stating that ProEx C score was negative in NILM cases and positive in HSIL cases [16].
Within a study performed by Siddiqui et al. (2008), using the HP diagnosis as a “gold standard”, the authors obtained a Sn of 98.04% in the case of ProEx C and 82.35% in HR-HPV genotyping, respectively; additionally, the Sp varied between 74.50% and 73.15% [13].
A study by Tambouret, in 2008, concluded that the ICC label for ProEx C combined with any degree of cytological atypia has a Sn of 92% and a Sp of 84% in the detection of cervical intraepithelial neoplasia (CIN) grade 2. Moreover, ProEx C can also be useful in identifying cases with risk progression [19].
A study of Halloush et al. (2008) proved that ProEx C had an improved performance in distinguishing NILM versus HSIL/SCC compared with p16INK4a. Because it offers only a nuclear staining, it is an easier to interpret marker even in inadequate samples [20].
Another retrospective study from 2009 validated this marker, demonstrating the PPV of ProEx C in diagnosing high-grade cervical lesions on biopsies [21].
Also, in 2009, in the study of Walts & Bose, 26% of CIN2–3 and 6% of CIN1 cases expressed ProEx C. In the progression group, the expression was higher compared with that in the persistence and regression groups indicating that ProEx C ICC is a suitable indicator for progression to HSIL [22].
Studies regarding MCM2 and TOP2A are contradictory. Some authors reported their utility for screening of precancerous lesions and cervical cancer, while others consider that the expression level of MCM2 cannot predict post-treatment response and tumor stage [23,24].
Few studies have followed patients, analyzing their survival and prognosis. In this regard, the appropriate mechanisms and pathways are less understood. Yang et al. claimed, in 2020, that in addition to influencing other genes, they may also play a key role in post-translational modification or activation of certain other pathways, resulting in a prolonged patient survival, which requires more research in the area [25].
In 2020, Ding et al. reported the first study proving that when compared to p16INK4a and Ki67, ProEx C staining is an independent risk factor for LSIL development over two years. The research provides a method for identifying LSIL patients who are at a higher risk of developing cancer [26].
The results of our study showed that ProEx C has two important roles: the first is to facilitate the identification of atypical cells on the smear, especially cells with high-grade lesions and the second is to interpret the presence of a brown nuclear marking of a cell atypical being correlated with an increased risk of high-grade injury.
ProEx C immunolabeling has occasionally been observed in benign endocervical cells and metaplastic cells. These may be a potential reason for diagnostic error, so it is very important to correlate cell morphology with the result of ProEx C labeling. The exact cause of staining of these cells has not yet been demonstrated. A plausible explanation could be the presence of inflammation and its mediators. For this reason, it is important to interpret the ProEx C label carefully because not every stained core should be interpreted as a positive result. Only when a cell is morphologically assessed and shows cytological atypia, nuclear staining should be stated as a positive result.
In the present study, an increase in ProEx C positivity was found from smears with ASC-US to LSIL, HSIL, and SCC, respectively. ProEx C was absent in all cases of NILM. The use of the ICC label for ProEx C showed moderate Sn, whereas the Sp and PPV are superior to other markers.
In the absence of a reliable HP diagnosis of a high-grade lesion, a positive ProEx C result in patients with ASC-US or LSIL indicates overexpression of MCM2 and TOP2A and the need for closer follow-up of these cases due to the risk of progression of lesions in the near future [27].
ProEx C positivity was frequently found in patients with high-grade intraepithelial lesions and rarely in patients with mild dysplasia. The use of the ICC label for ProEx C on cervical smears showed Sp and Ac, greatly reducing the percentage of false-positive results. The presence of the ProEx C marking on ASC-US/LSIL smears raises the suspicion of a high-grade intraepithelial lesion, and careful monitoring of these cases is required.
⧉ Conclusions
The present study suggests that ProEx C may be used to increase the Ac of cytological diagnosis on cervical smears. This marker is also useful in detecting unidentified high-grade lesions on ASC-US/LSIL smears, being very useful in establishing the conduct of these cases. In the future, this marker can be used in addition to cervical cytology to increase the Sn and Sp of this test.
Conflict of interests
The authors declare that they have no conflict of interests.
Acknowledgments
Acknowledgments
This research was carried out in collaboration with doctors from the Cuza-Vodă Obstetrics–Gynecology Hospital, Iaşi, Romania, and from the Department of Molecular Virology, Ştefan S. Nicolau Institute of Virology, Bucharest, Romania. The study was partially founded by research project PNII 41-030/2007.
References
- 1.Kim G. Harald zur Hausen’s experiments on human papilloma-virus causing cervical cancer (1976–1987) Embryo Project Encyclopedia, Arizona State University, School of Life Sciences, Center for Biology and Society. 2017 http://embryo.asu.edu/handle/10776/11444 [Google Scholar]
- 2.Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, Huh WK, Kim JJ, Moscicki AB, Nayar R, Saraiya M, Sawaya GF, Wentzensen N, Schiffman M, 2019 ASCCP Risk-Based Management Consensus Guidelines Committee ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24(2):102–131. doi: 10.1097/LGT.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khieu M, Butler SL. In: StatPearls, editor. Treasure Island FL USA: StatPearls Publishing; 2022. High grade squamous intraepithelial lesion (HSIL. [PubMed] [Google Scholar]
- 4.Raţiu AC, Secoşan CA, Balint O, Sas I, Grigoraş D, Ilina RŞ, Jianu AM, Motoc AGM, Pirtea LC. The importance of immunocytochemistry in the detection of high-grade cervical lesions. Rom J Morphol Embryol. 2017;58(4):1151–1156. [PubMed] [Google Scholar]
- 5.Buitrago-Pérez A, Garaulet G, Vázquez-Carballo A, Paramio JM, García-Escudero R. Molecular signature of HPV-induced carcinogenesis: pRb, p53 and gene expression profiling. Curr Genomics. 2009;10(1):26–34. doi: 10.2174/138920209787581235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanati S, Huettner P, Ylagan LR. Role of ProExC: a novel immunoperoxidase marker in the evaluation of dysplastic squamous and glandular lesions in cervical specimens. Int J Gynecol Pathol. 2010;29(1):79–87. doi: 10.1097/PGP.0b013e3181ae81a0. [DOI] [PubMed] [Google Scholar]
- 7.Depuydt CE, Makar AP, Ruymbeke MJ, Benoy IH, Vereecken AJ, Bogers JJ. BD-ProExC as adjunct molecular marker for improved detection of CIN2+ after HPV primary screening. Cancer Epidemiol Biomarkers Prev. 2011;20(4):628–637. doi: 10.1158/1055-9965.EPI-10-0818. [DOI] [PubMed] [Google Scholar]
- 8.Pinto AP, Schlecht NF, Woo TYC, Crum CP, Cibas ES. Biomarker (ProEx C, p16INK4A, and MiB-1) distinction of high-grade squamous intraepithelial lesion from its mimics. Mod Pathol. 2008;21(9):1067–1074. doi: 10.1038/modpathol.2008.101. [DOI] [PubMed] [Google Scholar]
- 9.Conesa-Zamora P, Doménech-Peris A, Ortiz-Reina S, Orantes-Casado FJ, Acosta-Ortega J, García-Solano J, Pérez-Guillermo M. Immunohistochemical evaluation of ProEx C in human papilloma-virus-induced lesions of the cervix. J Clin Pathol. 2009;62(2):159–162. doi: 10.1136/jcp.2008.061408. [DOI] [PubMed] [Google Scholar]
- 10.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T, Young N, Forum Group Members, Bethesda 2001 Workshop The Bethesda System 2001: terminology for reporting the results of cervical cytology. JAMA. 2002;287(16):2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 11.Donà MG, Ronchetti L, Giuliani M, Carosi M, Rollo F, Congiu M, Mazza D, Pescarmona E, Vocaturo A, Benevolo M. Performance of the linear array HPV genotyping test on paired cytological and formalin-fixed, paraffin-embedded cervical samples. J Mol Diagn. 2013;15(3):373–379. doi: 10.1016/j.jmoldx.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FAR, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS, Spitzer M, Moscicki AB, Franco EL, Stoler MH, Schiffman M, Castle PE, Myers ER, ACS–ASCCP–ASCP Cervical Cancer Guideline Committee American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqui MT, Hornaman K, Cohen C, Nassar A. ProEx C immunocytochemistry and high-risk human papillomavirus DNA testing in Papanicolaou tests with atypical squamous cell (ASC-US) cytology: correlation study with histologic biopsy. Arch Pathol Lab Med. 2008;132(10):1648–1652. doi: 10.5858/2008-132-1648-PCIAHH. [DOI] [PubMed] [Google Scholar]
- 14.Sprent P. In: International Encyclopedia of Statistical Science. Lovric M, editor. Berlin–Heidelberg: Springer; 2011. Fisher exact test; pp. 524–525.https://link.springer.com/referenceworkentry/10.1007/978-3-642-04898-2_253 [Google Scholar]
- 15.Takagi M, Sueishi M, Saiwaki T, Kametaka A, Yoneda Y. A novel nucleolar protein, NIFK, interacts with the forkhead associated domain of Ki-67 antigen in mitosis. J Biol Chem. 2001;276(27):25386–25391. doi: 10.1074/jbc.M102227200. [DOI] [PubMed] [Google Scholar]
- 16.Shroyer KR, Homer P, Heinz D, Singh M. Validation of a novel immunocytochemical assay for topoisomerase II-alpha and minichromosome maintenance protein 2 expression in cervical cytology. Cancer. 2006;108(5):324–330. doi: 10.1002/cncr.22171. [DOI] [PubMed] [Google Scholar]
- 17.Sun H, Shen K, Cao D. Progress in immunocytochemical staining for cervical cancer screening. Cancer Manag Res. 2019;11:1817–1827. doi: 10.2147/CMAR.S195349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tosuner Z, Türkmen İ, Arici S, Sönmez C, Turna S, Onaran Ö. Immunocytoexpression profile of ProExC in smears interpreted as ASC-US, ASC-H, and cervical intraepithelial lesion. J Cytol. 2017;34(1):34–38. doi: 10.4103/0970-9371.197605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tambouret RH. Valeur diagnostique du ProEx™ C en cytopathologie gynécologique [Diagnostic importance of ProEx C in gynecologic cytopathology] Ann Pathol. 2008;28 Spec No 1(1):S92–S93. doi: 10.1016/j.annpat.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Halloush RA, Akpolat I, Jim Zhai Q, Schwartz MR, Mody DR. Comparison of ProEx C with p16INK4a and Ki-67 immunohistochemical staining of cell blocks prepared from residual liquid-based cervicovaginal material: a pilot study. Cancer. 2008;114(6):474–480. doi: 10.1002/cncr.23951. [DOI] [PubMed] [Google Scholar]
- 21.David O, Cabay RJ, Pasha S, Dietrich R, Leach L, Guo M, Mehrotra S. The role of deeper levels and ancillary studies (p16INK4a and ProExC) in reducing the discordance rate of Papanicolaou findings of high-grade squamous intraepithelial lesion and follow-up cervical biopsies. Cancer. 2009;117(3):157–166. doi: 10.1002/cncy.20020. [DOI] [PubMed] [Google Scholar]
- 22.Walts AE, Bose S. p16, Ki-67, and BD ProExC immunostaining: a practical approach for diagnosis of cervical intra-epithelial neoplasia. Hum Pathol. 2009;40(7):957–964. doi: 10.1016/j.humpath.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Amaro Filho SM, Nuovo GJ, Cunha CB, Ramos Pereira Lde O, Oliveira-Silva M, Russomano F, Pires A, Nicol AF. Correlation of MCM2 detection with stage and virology of cervical cancer. Int J Biol Markers. 2014;29(4):e363–e371. doi: 10.5301/jbm.5000081. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Li J, Chen J, Shan Q, Dai H, Xie H, Zhou L, Xu X, Zheng S. MCM family in HCC: MCM6 indicates adverse tumor features and poor outcomes and promotes S/G2 cell cycle progression. BMC Cancer. 2018;18(1):200–200. doi: 10.1186/s12885-018-4056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang HJ, Xue JM, Li J, Wan LH, Zhu YX. Identification of key genes and pathways of diagnosis and prognosis in cervical cancer by bioinformatics analysis. Mol Genet Genomic Med. 2020;8(6):e1200–e1200. doi: 10.1002/mgg3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding L, Song L, Zhao W, Li X, Gao W, Qi Z, Wang J. Predictive value of p16INK4a, Ki-67 and ProExC immunoqualitative features in LSIL progression into HSIL. Exp Ther Med. 2020;19(4):2457–2466. doi: 10.3892/etm.2020.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown CA, Bogers J, Sahebali S, Depuydt CE, De Prins F, Malinowski DP. Role of protein biomarkers in the detection of high-grade disease in cervical cancer screening programs. J Oncol. 2012;2012:289315–289315. doi: 10.1155/2012/289315. [DOI] [PMC free article] [PubMed] [Google Scholar]


