Abstract
Colorectal carcinoma represents a major cause of mortality and 0.2–12% of resected colonic polyps have malignant cells inside. We performed a retrospective study of patients with resected polyps during a period of 13 years. A total of 905 patients had 2033 polyps removed; 122 polyps (109 patients) had malignant cells. Prevalence of malignant polyps with submucosal invasion was 1.23% and for all polyps with malignant cells was 6%; malignant polyps had a larger size (23.44 mm mean diameter) vs benign polyps (9.63 mm); the risk of malignancy was increased in polyps larger than 10 mm, in lateral spreading lesions and in Paris types 0-Ip, 0-Isp, in sigmoid, descending colon and rectum, in sessile serrated adenoma and traditional serrate adenoma subtypes of serrated lesions and in tubulovillous and villous adenoma. In 18 cases surgery was performed, in 62 patients only colonoscopic follow-up was made and in 35 patients no colonoscopic follow-up was recorded. From initially endoscopic resected polyps, recurrence was noted in seven (11.3%) cases; there was a trend toward association with depth of invasion, piecemeal resection, right and rectum location, sessile and lateral spreading type and pathological subtype. In surgical group, post-therapeutic staging was available in 11 cases; nodal involvement was noted in three (27.27%) cases; none had lymphatic or vascular invasion in endoscopically resected polyps. Four patients with no macroscopic local recurrence underwent surgery with no residual tumor. The rate of metastasis was 16.67% in surgical group and 1.61% in endoscopic group. Evaluation of lymph node (LN) invasion was available for 11 operated patients, with LN invasion (N1) in three patients, local residual tumoral tissue in one patient with incomplete resection and no residual tumor (R0 resection) in four patients with endoscopic resection before surgery.
Keywords: malignant polyp, serrated lesion, desmin, alpha-smooth muscle actin, endoscopic mucosal resection
⧉ Introduction
Colorectal carcinoma (CRC) represents a major cause of morbidity and mortality worldwide [1], being the second most common cancer in women and third most common cancer in men worldwide [2]. 95% of CRC appear inside an adenoma [3,4]: the probability of high-grade dysplasia and carcinoma is related to the number, size (especially above 1 cm), villous structure and age above 60 years [3]. Adenoma detection and resection can reduce CRC carcinoma mortality [5], but 0.2–12% of resected colonic polyps already have malignant cells inside [6].
A carcinoma found inside a CRC polyp can be confined to the epithelium [in situ (IS) carcinoma], to the mucosa [intramucosal (IM) carcinoma] or can surpass the muscularis mucosae and invade the submucosa [submucosal (SM) carcinoma]. Polyps with high-grade dysplasia or with epithelial (IS) involvement are classified today as non-invasive high-grade neoplasia (NIHGN) by Vienna pathological classification and have no risk of nodal involvement or metastasis [3, 7] because lymphatic channels are located below the mucosal layer and are very rarely found above the submucosa; for this reason, a carcinoma located in the epithelium or in lamina propria (IS carcinoma) has only exceptionally a risk of lymphatic or venous invasion [8]. The term IM carcinoma [7,8] was used before, but some authors avoid this diagnosis because of confusion regarding the term of carcinoma for the patients and difficulty of differentiation between high-grade non-invasive dysplasia and carcinoma. Colorectal polyps with malignant cells which surpass muscularis mucosae are named malignant polyps [3, 7]; they are included in the term of “early CRC” and have a risk of lymphatic or even hematogenic dissemination.
The macroscopic aspect of polyps can sometimes predict malignancy or even depth of invasion. Several classifications can be useful: Paris classification based on conventional endoscopy [9,10,11], Kudo (Table 1), Nice and Sano classification based on chromoendoscopy or virtual chromoendoscopy [narrow-band imaging (NBI), I-Scan, flexible spectral imaging color enhancement (FICE)] [12,13,14,15]. Macroscopic signs associated with high risk of malignancy are [10]: non-lifting sign in lesions with no previous attempted resection, Paris 0-IIc lesions, non-granular lateral spreading lesions, pit-pattern Kudo V, NBI pattern NICE III (NBI International Colorectal Endoscopic Classification), Sano capillary pattern type 3. Some polyps have macroscopic confounding appearance (large polyps without malignant lesions, depressed polyps with benign structure at pathology). Pedunculated polyps (type 0-Ip and 0-Isp) have a more favorable prognosis because of interposed stalk between the polyp and the base. Many malignant pedunculated polyps can be safely managed by endoscopic resection [7, 16], but a metachronous carcinoma can be found on follow-up [16].
Table 1.
|
Paris classification |
Kudo classification |
||
|
Type 0-Is |
Sessile lesions (>2.5 mm or the height of closed biopsy forceps) |
Type 1 |
Round pits |
|
Type 0-Ip |
Pedunculated lesions |
Type 2 |
Stellar or papillary pits |
|
Type 0-Isp |
Semipedunculated polyps |
Type 3 |
Tubular or roundish pits |
|
Type 0-IIa |
Slightly elevated (<2.5 mm) |
▪ 3S |
▪ small pits |
|
Type 0-IIb |
Really flat |
▪ 3L |
▪ large pits |
|
Type 0-IIc |
Slightly depressed (<1.2 mm) |
Type 4 |
Branch-like or gyrus-like pits |
|
Type 0-III |
Excavated polyps |
Type 5 |
Non-structural pits |
The main risk in case of colorectal malignant polyps is the risk of carcinoma recurrence; several mechanisms can be involved. There is a possibility of an incomplete resection (macroscopic or microscopic) of the polyp, which can facilitate the local recurrence of carcinoma. Malignant cells appeared inside the polyp can spread into the local lymphatic vessels, followed by lymph node (LN) metastasis. Rarely, distant metastases can occur even in carcinoma which invaded only submucosa. Several factors can influence carcinoma recurrence [3, 17]: morphological factors (size, pedunculated or sessile type, pit pattern), surface (border invasion) and depth invasion, type of resection, LN invasion, grade of differentiation [8].
Risk of local recurrence and of SM invasion can be predicted by morphological factors; large polyps, sessile polyps and non-protruding or depressed lesions have a greater risk of recurrence and incomplete resection compared with pedunculated lesions and the probability of SM invasion is greater for large lesions, for lateral spreading lesions (especially granular type vs non-granular type) and for depressed lesions compared to protruding polyps. An analysis of outcomes from trials regarding endoscopic management of large non-pedunculated polyps reveal a rate of complete resection close to 90%, a 3-month recurrence between 14.5–27%, a rate of malignancy between 4.4–6.9% and a need for surgery between 9–16.3% [10]. Even in pedunculated polyps, where resection can be made at the 1/2 of the stalk, a great diameter can be associated with higher risk of recurrence. Some macroscopic aspects can be associated with a higher risk of recurrence/or LN invasion [18]: irregular surface or border, pit-pattern Kudo 3L/5, depressed center, pedunculated polyps with short and immobile stalk, positive lifting-sign.
Several pathological factors can predict the risk of recurrence and/or LN invasion: resection border (above 1 mm but even 0.1 mm in some studies) [3, 6, 8, 18,19,20], depth of invasion [3, 6,7, 21] (Table 2), pedunculated vs sessile type [3, 6,7, 21], differentiation grading (higher risk in G3 grade and in mucinous, signet ring and micropapillary carcinoma) [7,8], lymphovascular invasion [1, 3, 8] and tumoral budding [1, 8, 22,23]. LN involvement is very important because in case of resected colorectal T1 SM carcinoma polyps a LN invasion can demand surgical intervention, while cases with no LN involvement can be managed only by local close surveillance [1]. Some studies suggest that up to 16% of T1 CRCs may have LN metastasis [1], but the average risk is 6.7% [19].
Table 2.
Classification of depth invasion in malignant polyps
|
Haggitt classification: head/neck/stalk/base |
Kitajima classification: baseline of the head or line between head/neck |
Kudo–Kikuchi classification |
|
|
Type 1 |
Head |
▪ above |
▪ sm1: <1/3 of sm or ≤3 mm |
|
Type 2 |
Neck |
▪ ≤3 mm below line |
▪ sm2 |
|
Type 3 |
Stalk |
▪ >3 mm below line |
▪ sm3: >2/3 sm invaded |
|
Type 4 |
Adjacent border wall/all sessile |
|
|
sm: Submucosal
Type of resection can influence the risk of recurrence [3]; en bloc resection (EBR) is ideal for all polyps and especially for malignant polyps and can be done by classical polypectomy or endoscopic mucosal resection (EMR). For sessile larger polyps and for lateral spreading tumors (LST), EBR cannot be made by EMR and requires endoscopic SM dissection (ESD), which is not available in all centers and is associated with more extensive procedure and a higher risk of perforation.
Lesions with low risk of recurrence can be defined as follows [18]: pedunculated polyps with uninvaded stalk, low depth of invasion (Haggitt score 1–3/Kikuchi sm1/Kitajima maximum A3-B/C-1), free resection border above 2 mm, grading G1–G2, no lymphovascular invasion and EBR [3]. Increased risk lesions [6, 18] include: sessile polyps, with irregular surface/border, Kudo 3L/5, depressed center, pedunculated polyps with short and immobile stalk, positive lifting-sign, Haggitt 4/Kikuchi sm3/Kitajima >A3-B/C-1, resection border <1 mm/invaded, lymphovascular invasion or G3 grading [3].
Aim
The objectives of the study were: to estimate the prevalence of malignant polyps in patients with removed colon polyps, to assess risk factors associated with malignancy inside polyps (size, macroscopic type, location, pathological type of the polyp, age, gender), to assess the depth of invasion [intraepithelial (IE), IS, IM or SM invasion], to evaluate the risk factors for depth invasion, to evaluate the risk for local recurrence and nodal invasion.
⧉ Patients, Materials and Methods
We performed a retrospective study of patients with colonoscopy performed at the Research Center of Gastroenterology and Hepatology, University of Medicine and Pharmacy of Craiova, and at the Clinic of Gastroenterology, Emergency County Hospital, Craiova, Romania, during a period of 13 years (2006–2018). Informed consent was obtained for every polypectomy. Polyp removal was made by polypectomy, by EMR in cases of sessile large polyps or in lateral spreading lesions; several small polyps were removed by multiple biopsies (MB) but after 2012 snare polypectomy was preferred instead. EBR was preferred, although piecemeal resection (PMR) was sometimes necessary in large lesions. Macroscopic examination noted Paris type or the lateral spreading type; Kudo pattern for a proportion of patients was also evaluated and in cases with large sessile or lateral spreading lesions resected by EMR the presence or absence of “lifting sign” was noted (Figures 1,2,3,4,5,6,7,8).
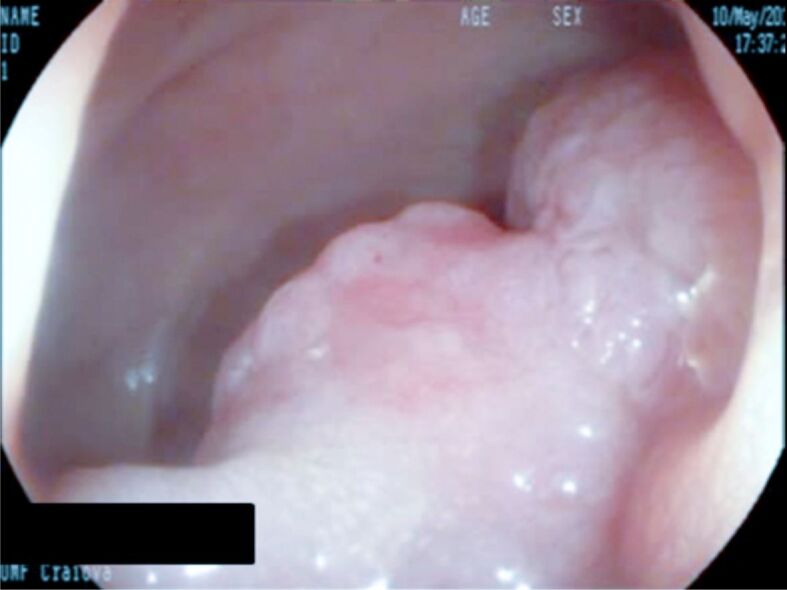
Figure 1.
Sessile polyp located to the right colon. EMR was performed. EMR: Endoscopic mucosal resection
Figure 2.
Same polyp: aspect after EMR. Pathology exams revealed a sessile serrated lesion with intraepithelial carcinoma (non-invasive high-grade dysplasia)
Figure 3.
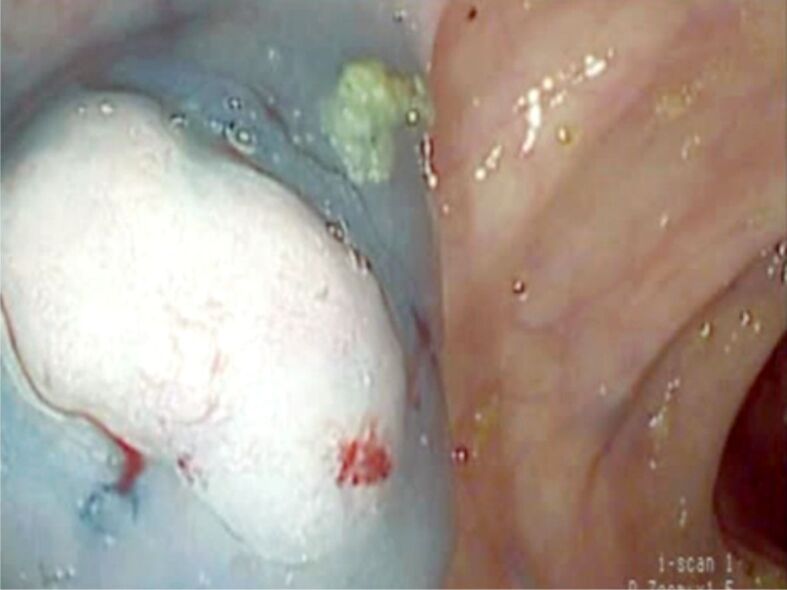
Large pedunculated polyp located to the sigmoid colon. Polypectomy was performed with previous adrenaline injection at the peduncle to prevent bleeding
Figure 4.
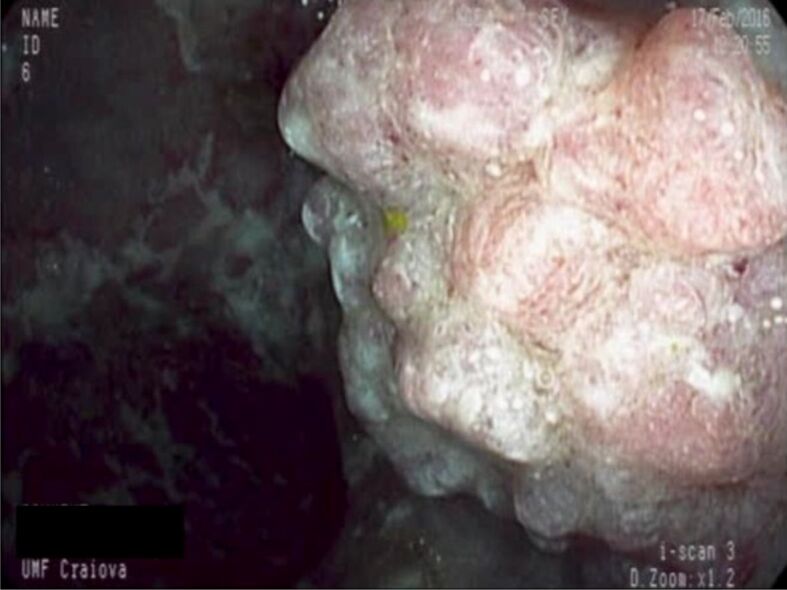
Large sessile polyp in a patient with ulcerative colitis. Pathology exam revealed a VA with SM carcinoma. SM: Submucosal; VA: Villous adenoma
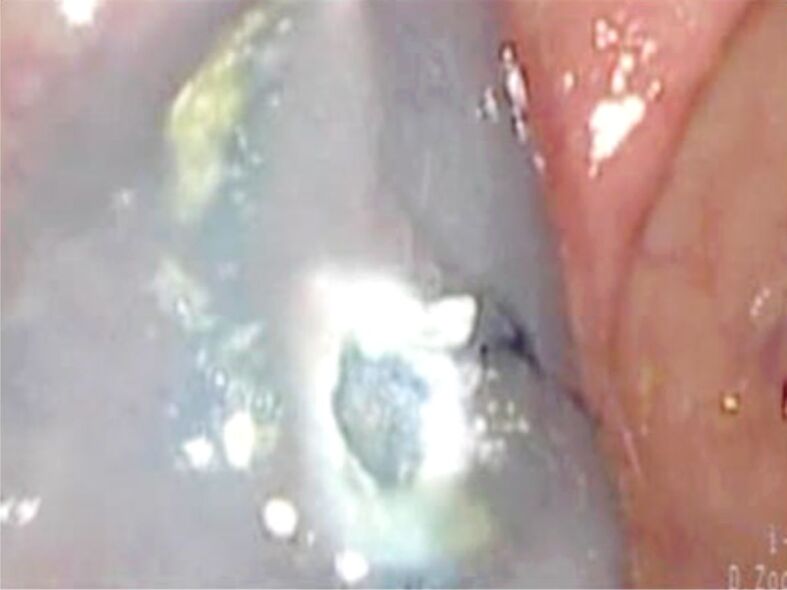
Figure 5.
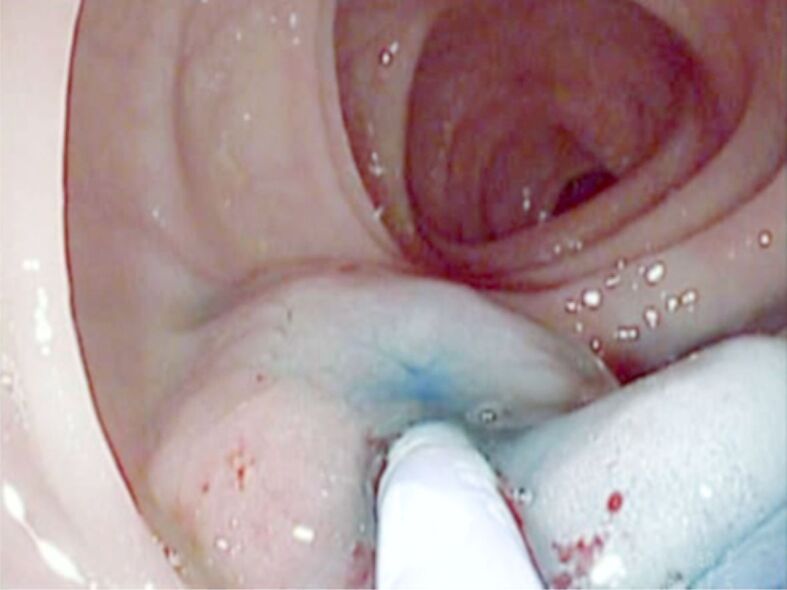
Flat (Paris 0-IIa) polyp located to the sigmoid colon. SM lifting using Methylene Blue and 1/10 000 diluted adrenaline was performed
Figure 6.
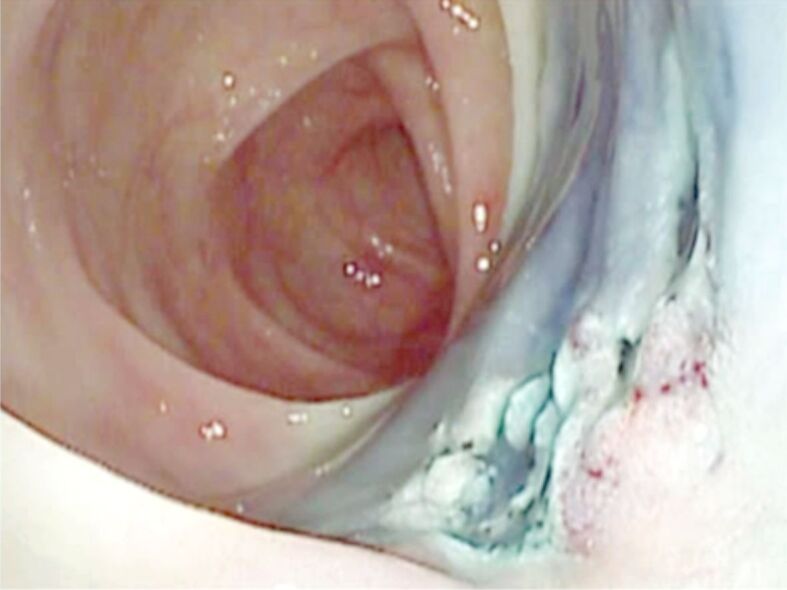
Same polyp: aspect after EMR. The polyp could not be completely resected because some region was not lifted by saline injection. Pathology exams revealed a TVA with SM carcinoma. TVA: Tubulovillous adenoma
Figure 7.
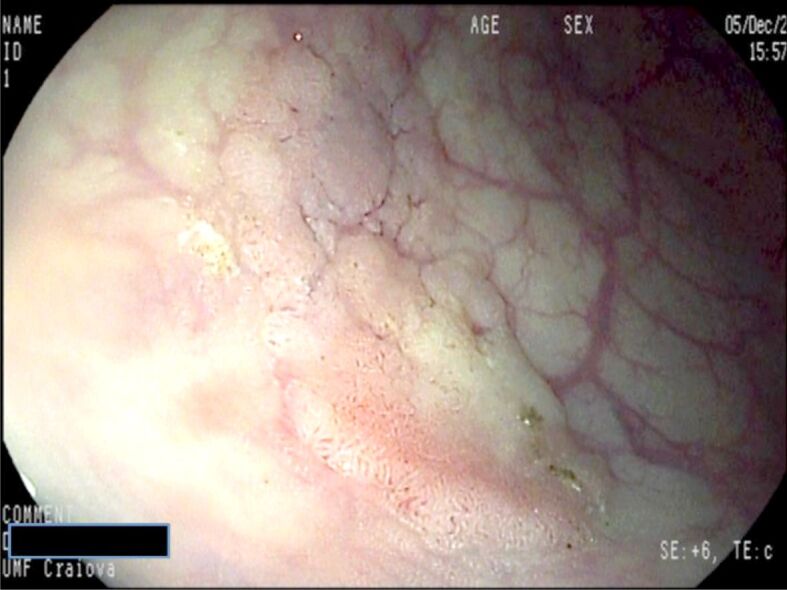
Flat (Paris 0-IIb) polyp barely visible located to the descending colon. I-Scan image
Figure 8.
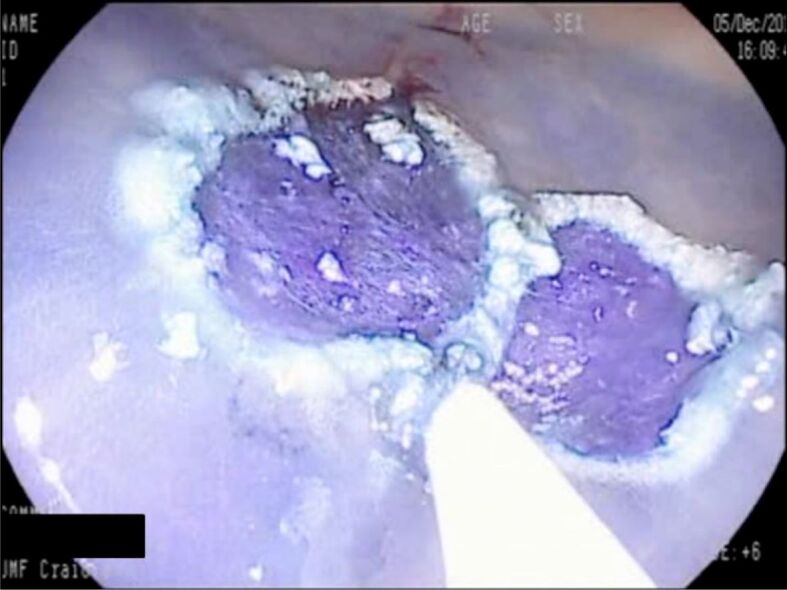
Same polyp after resection: SM lifting with 1/10 000 diluted adrenaline with Methylene Blue (I-Scan exam). Pathology exam revealed a TVA with intramucosal carcinoma
2033 polyps from 905 patients were resected and pathological examination was performed in all resected specimens. Pathological examination noted the type of polyp (adenoma, serrated or hyperplastic lesion, carcinoma), the subtype of adenoma (tubular, tubulovillous, villous), the presence and grade (low- or high-grade) dysplasia and the presence of carcinoma; revised Vienna classification [24] was used for dysplasia and carcinoma presence (Table 3). In case of carcinoma presence, the depth of invasion was evaluated (IE, IS, IM or SM invasion) and immunohistochemistry using desmin and alpha-smooth muscle actin (α-SMA) was performed to facilitate differentiation between mucosal and SM invasion; the presumed complete or incomplete resection, grade of differentiation (G1–G2–G3 or signet ring carcinoma), lymphatic and vascular invasion.
Table 3.
Vienna Classification for dysplasia and carcinoma in gastrointestinal epithelial tumors [24]
|
Type 1 |
Negative for neoplasia |
Optional follow-up |
|
Type 2 |
Indefinite for neoplasia |
Follow-up |
|
Type 3 |
Mucosal low-grade neoplasia |
Endoscopic resection or follow-up |
|
Low-grade adenoma |
|
|
|
Low-grade dysplasia |
|
|
|
Type 4 |
Mucosal high-grade neoplasia |
|
|
4.1 High-grade adenoma/dysplasia |
Endoscopic or surgical local resection |
|
|
4.2 Non-invasive carcinoma (carcinoma in situ) |
|
|
|
4.3 Suspicious for invasive carcinoma |
|
|
|
4.4 Intramucosal carcinoma |
|
|
|
Type 5 |
Submucosal invasion by carcinoma |
Surgical resection |
Cases with non-lifting sign were referred to surgery, and also some cases with incomplete polypectomy (i-pEMR) because some parts of the polyp cannot be lifted in case of piecemeal EMR (pEMR). After polypectomy and pathology confirmation of malignancy, therapeutic options were presented to every patient with preferred endoscopic management in low-risk and surgical management in high-risk cases. Patients with low-risk factors who accept endoscopic management were followed by colonoscopy and a second polypectomy was made in case of recurrence or previous incomplete polypectomy; a follow-up program using computed tomography (CT) scan and tumoral markers [carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9)] was also implemented.
Cases with high risk factors were divided into surgical subgroup and colonoscopy follow-up subgroup (patients who refused surgery and those with significant comorbidities).
The objectives of the study were: to estimate the prevalence of malignant polyps in patients with removed colon polyps, to assess risk factors associated with malignancy inside polyps (size, macroscopic type, location, pathological type of the polyp, age, gender), to assess the depth of invasion (IE, IS, IM or SM invasion), to evaluate the risk factors for depth invasion, to evaluate the risk for local recurrence and nodal invasion.
Statistical analysis was made using IBM Statistical Package for the Social Sciences (SPSS) Statistics Faculty Packs, odds ratio (OR) calculation for estimation of risk, unpaired Student’s t-test for continuous parameters and χ2 (chi-squared) contingency table for categorical parameters.
⧉ Results
A total number of 905 patients had colonoscopic polyps removed or only biopsied; pathological examination showed a total of 122 polyps with malignant cells (109 patients) and 1911 benign polyps (796 patients). Thirty-eight polyps had NIHGN (former IE carcinoma), 46 were IM or IS carcinoma, 25 polyps had SM invasive carcinoma (malignant polyps) and in 13 polyps the depth of invasion was not specified (NS). The rate of malignant polyps with SM invasion in our study was 1.23%, the rate of polyps with malignant cells was 6%. Characteristics of malignant polyps are showed in Table 4.
Table 4.
Characteristics of malignant polyps
|
Age, mean [years] |
62.62/60.75 (p=0.06) |
|
Age group (years): 20–29/30–39/40–49/50–59/60–69/70–79/>80, n |
1/3/6/30/46/19/4 |
|
Residence: U/R, n |
84/25 |
|
Sex: M/F, n |
68/41 |
|
Diameter, mean (min.–max.) [mm] |
23.44 (4–90) |
|
Diameter interval (mm): 0–9/10–19/20–29/30–39/>40, n |
10/39/34/20/19 |
|
Invasion depth: SM/IM/IS/IE/NS, n |
25/31/15/38/13 |
|
Macroscopic Paris type: S/SP/P/LST/IIb, n |
32/20/54/15/1 |
|
Microscopic type: SSA/TSA/TA/TVA/VA/T/AD, n |
8/7/107/65/5/3/34 |
|
Lymphovascular invasion: Lymphatic only/vascular only/both, n |
2/1/1 |
|
Side: right/left/rectum, n |
19/69/34 |
|
Resection type/biopsy: EBR/pEMR/i-pEMR/MB/OB, n |
75/28/9/3/7 |
AD: Adenomatous; EBR: En bloc resection; F: Female; i-pEMR: Incomplete piecemeal endoscopic mucosal resection (pEMR); IE: Intraepithelial; IM: Intramucosal; IS: In situ; LST: Lateral spreading tumors; M: Male; MB: Multiple biopsies; n: No. of cases; NS: Not specified; OB: Only biopsies; P: Pedunculated; R: Rural; S: Sessile; SM: Submucosal; SP: Semipedunculated; SSA: Sessile serrated adenoma; T: Tumoral; TA: Tubular adenoma; TSA: Traditional serrated adenoma; TVA: Tubulovillous adenoma; U: Urban; VA: Villous adenoma
The rate of malignancy in colorectal polyps was higher in patients from urban residence (OR 0.77, p=0.29 NS), in polyps larger than 10 mm (OR 7.2, p<0.00001), in Paris types 0-Ip, 0-Isp and in lateral spreading lesions (as compared with sessile and flat lesions) and for left-side and rectum location. The presence of concomitant carcinoma in resected polyps was higher in both sessile serrated adenoma (SSA, 17.02%) and traditional serrated adenoma (TSA, 29.17%) subtypes of serrated lesions and also in tubulovillous adenoma (TVA, 13.32%) and villous adenoma (VA, 17.78%), while in hyperplastic polyps, tubular adenoma and adenomatous polyps the frequency of carcinoma was low (below 5%). Malignant polyps had a higher mean diameter (23.44 mm) compared to benign polyps (9.63 mm) and for all types of polyps mean diameter was higher for malignant polyps. Mean age was slightly higher (62.62 years) in malignant vs benign polyps (60.75 years, p<0.00001) (Table 5).
Table 5.
Comparison between malignant and benign colorectal polyps
|
Age, mean – malignant/benign [years] |
62.62/60.75 (p<0.00001) |
|
Age group (years): <40/40–49/50–59/60–69/70–79/>80 [%] |
10.9/6.3/13.7/16.3/11.9/21 |
|
Residence (OR): U/R |
0.75 (p=0.29, NS) |
|
Sex (OR): M/F |
1.02 (p=0.94, NS) |
|
Diameter, mean – malignant/benign [mm] |
23.44/9.63 |
|
Malignancy rate/diameter (mm): 0–9/10–19/20–29/30–39/>40 [%] |
0.81/7.6/22.22/29.41/30.65 |
|
Macroscopic Paris type: S/SP/P/LST/IIb [%] |
2.28/14.13/13.77/35.29/1.75 |
|
Microscopic type: SSA/TSA/TA/TVA/VA/T [%] |
17.02/29.17/7.04/13.32/17.78/0.9 |
|
Side: right/left/rectum [%] |
3.11/8.1/12.55 |
F: Female; LST: Lateral spreading tumors; M: Male; NS: Not significant; OR: Odds ratio; P: Pedunculated; R: Rural; S: Sessile; SP: Semipedunculated; SSA: Sessile serrated adenoma; T: Tumoral; TA: Tubular adenoma; TSA: Traditional serrated adenoma; TVA: Tubulovillous adenoma; U: Urban; VA: Villous adenoma
The risk of malignant polyps was greater in patients with two or more polyps (16.76%) compared to those with one polyp (8.79%, OR 2.09, p=0.0004). 15.57% of malignant polyps were located in the right colon, 56.56% were located in the left colon and 27.87% were located in the rectum. In left-sided and rectum polyps, a high rate of malignancy was encountered in serrated lesions regardless of TSA or SSA (11.76–41.67%) and in TVA and VA (14.18–25%), while in right-sided located polyps only villous polyps and SSA (7.14% and 7.4%) had a moderate risk of malignancy. Only 3.11% of polyps located in right colon were malignant, while 8.1% from left colon and 12.55% from rectum were malignant.
All malignant polyps were analyzed by a pathologist who evaluated the depth of the invasion as IE neoplasia (IEN), IM, IS or SM carcinoma. Sometimes muscularis mucosae was not present in pathological sample the invasion depth assessment cannot be made, and these cases were assessed as NS. In some cases, the polyp cannot be removed because of in-depth invasion, in many cases a non-lifting sign was present and therefore only biopsies (OB) were performed. Some cases were EBR, and others (especially in large lesions) were PMR; in some cases, incomplete resection was noted. The repartition of cases by depth invasion is illustrated in Table 6.
Table 6.
Depth invasion in malignant polyps: correlation with various parameters
|
|
SM |
IS |
IM |
IE |
|
Macroscopic Paris type: S/P/SP/LST/IIb, n |
7/10/6/2/0 |
3/6/3/3/0 |
5/17/4/5/0 |
11/20/5/2/0 |
|
Side: right/left/rectum, n |
1/18/6 |
1/8/6 |
4/18/9 |
9/22/7 |
|
Diameter (mm): 0–9/10–19/20–29/30–39/>40, n |
0/5/9/7/4 |
1/6/4/0/4 |
3/8/8/6/6 |
5/16/8/5/4 |
|
Microscopic type: SSA/TSA/VA/TVA/T/AD, n |
3/1/2/10/1/8 |
2/3/0/9/0/1 |
1/1/2/17/1/9 |
2/0/0/23/0/13 |
|
Resection type/biopsy: EBR/pEMR/i-pEMR/MB/OB, n |
13/8/1/0/3 |
11/1/3/0/0 |
23/7/1/0/0 |
24/9/0/32/2 |
AD: Adenomatous; EBR: En bloc resection; i-pEMR: Incomplete piecemeal endoscopic mucosal resection (pEMR); IE: Intraepithelial; IM: Intramucosal; IS: In situ; LST: Lateral spreading tumors; MB: Multiple biopsies; n: No. of cases; OB: Only biopsies; P: Pedunculated; S: Sessile; SM: Submucosal; SP: Semipedunculated; SSA: Sessile serrated adenoma; T: Tumoral; TSA: Traditional serrated adenoma; TVA: Tubulovillous adenoma; VA: Villous adenoma
Mean diameter of malignant polyps was 23.44 mm, there were no significant statistical differences between SM malignant polyps (25.20 mm) and those with only IM/IS invasion (26.65 mm) or with IM/IS/IEN (23.21 mm) (p=0.71 and 0.56, respectively). Mean diameter for NIHGN was 19.05 mm, lower than SM (p=0.02) or IM/IS carcinoma (p=0.03). Most malignant polyps (91.8%) had a diameter of 10 mm or above.
Most SM malignant polyps were Paris 0-Is, 0-Ip or 0-Isp lesions, whereas most lateral spreading lesions had predominant IM invasion. Most malignant polyps were located to left colon and rectum. TVAs were predominant regardless of depth of invasion. Most lesions were resected by polypectomy or EMR (en bloc or piecemeal), three small adenomas were removed by biopsy and confirmed later as malignant and in seven cases because of suspected deep invasion OB were performed.
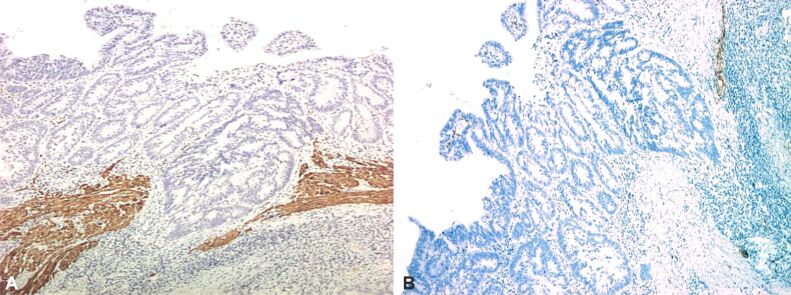
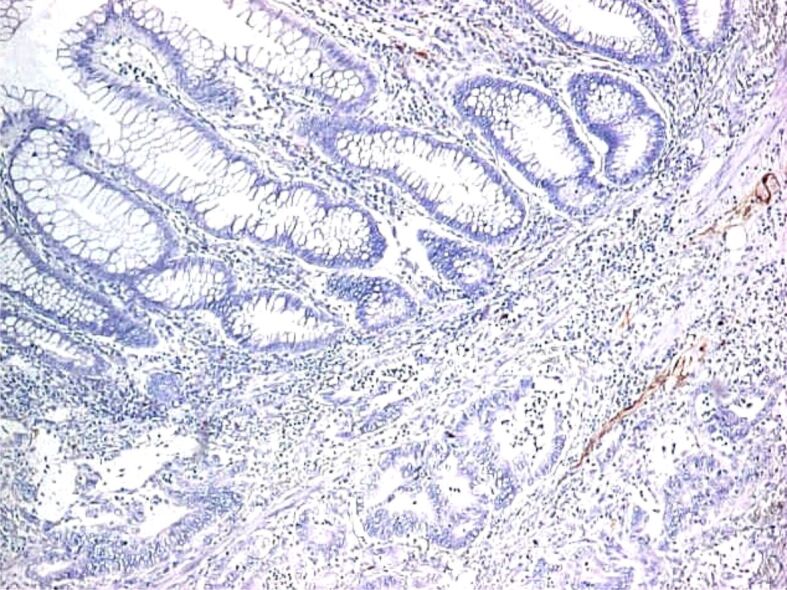
Proper assessment of muscularis mucosae is important for evaluation of deepest portion of SM invasion. In some specimens, muscularis mucosae was poorly visible or even not visible at all. Normally muscularis mucosae expresses desmin and α-SMA [25] (Figures 9,10,11,12,13,14,15,16,17), while stromal pericryptal and non-pericryptal myofibroblasts are α-SMA positive and may have focal desmin-positive immunostaining [25], especially at the crypt basis [26]. In another study, stromal cells in the early invasive area, designed as bundles of eosinophilic spindle cells on routine HE staining and partly continuous with the muscularis mucosae, showed α-SMA expression, but a loss of desmin expression [26]. The desmin immunostaining can be used for the assessment of the deepest point of invasion similar to esophageal adenocarcinoma [27]. In some cases, because of fragmentation, particularities of sectioning and thermal artifacts generated by electrocoagulation, muscularis mucosae cannot be readily visible or can have a hyperplastic appearance similar to muscularis propria, such in urinary bladder tumors [28]. Some invasive tumors can be associated with myofibroblastic spindle cell stromal response called desmoplastic reaction, which can be confounded in some specimens with muscularis mucosae. In these cases, a panel of α-SMA and desmin can help differentiate the muscularis mucosae (α-SMA and desmin positive) from muscularis propria and myofibroblasts (α-SMA positive and desmin negative) [28] although some fibroblasts can have a weak and predominant basal crypt located desmin-positive staining [25,26].
Figure 9.
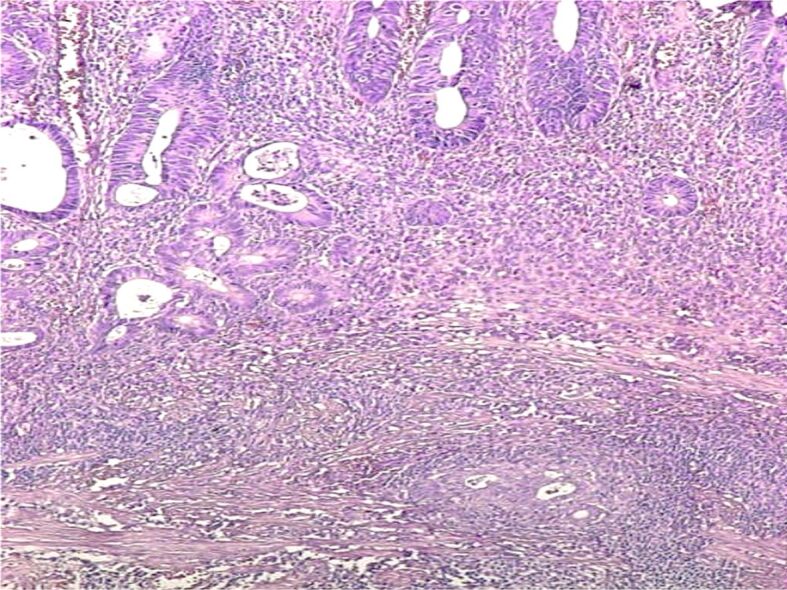
Malignant polyp with the invasion of submucosa (HE staining, ×40). HE: Hematoxylin–Eosin
Figure 10.
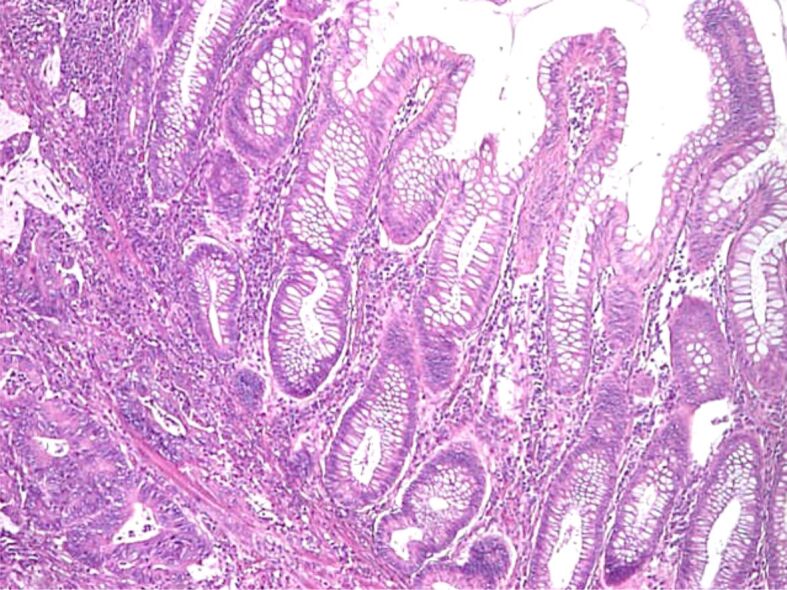
Malignant polyp: invasion and surpass of muscularis mucosae (HE staining, ×40)
Figure 11.
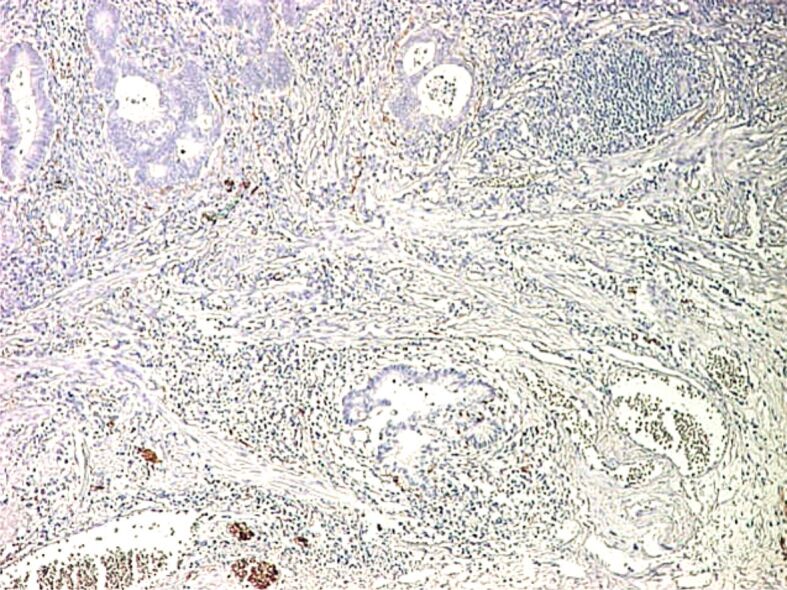
Malignant polyp (adenocarcinoma with SM invasion): positive IHC staining for S100 protein in nervous fibers from submucosa (×40). IHC: Immunohistochemical
Figure 12.
Malignant polyp: positive IHC staining for S100 protein in nervous fibers which confirm SM invasion (×40)
Figure 13.
(A and B) Malignant polyp: absent IHC staining for desmin in area of tumoral invasion of muscularis mucosae (×40).
Figure 14.
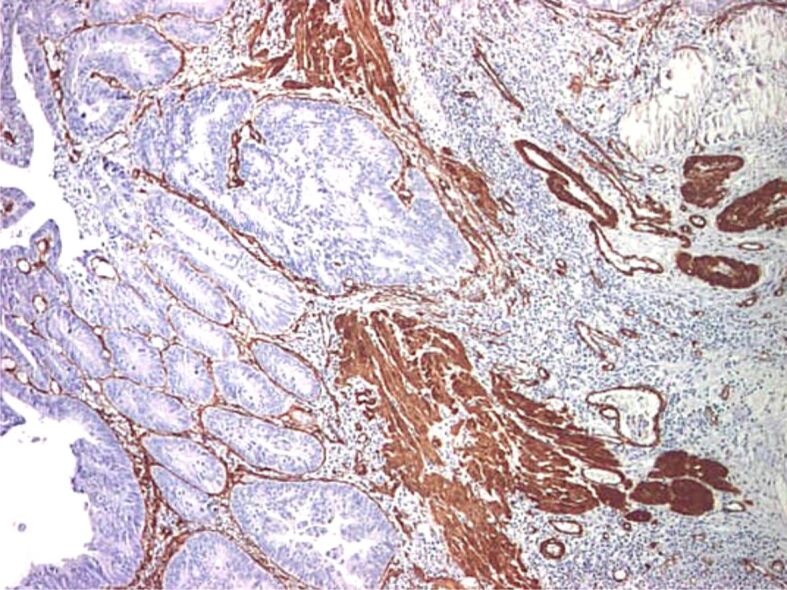
Malignant polyp with invasion of the muscularis mucosae: intense and diffuse positive IHC staining for α-SMA in muscularis mucosae and myofibroblasts (×40). α-SMA: Alpha-smooth muscle actin
Figure 15.
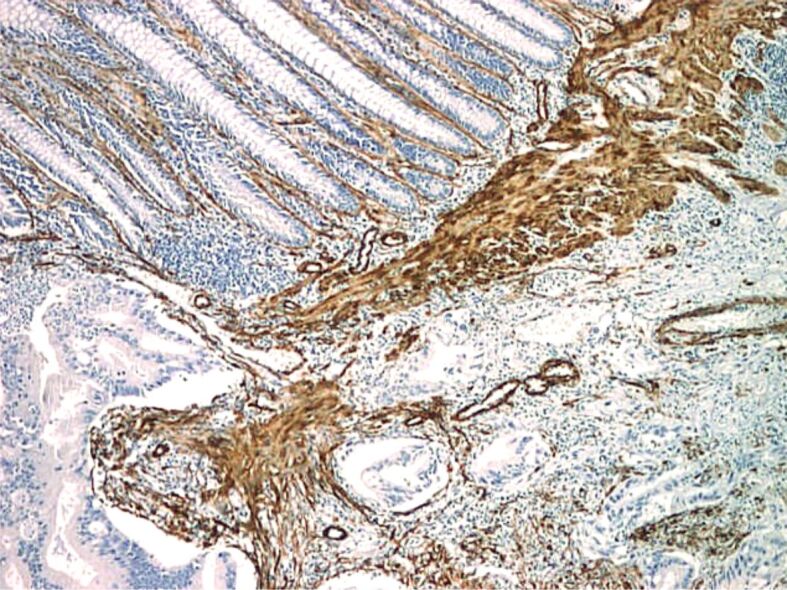
Malignant polyp (adenocarcinoma with SM invasion): intense and diffuse positive IHC staining for α-SMA in muscularis mucosae and myofibroblasts (×40)
Figure 16.
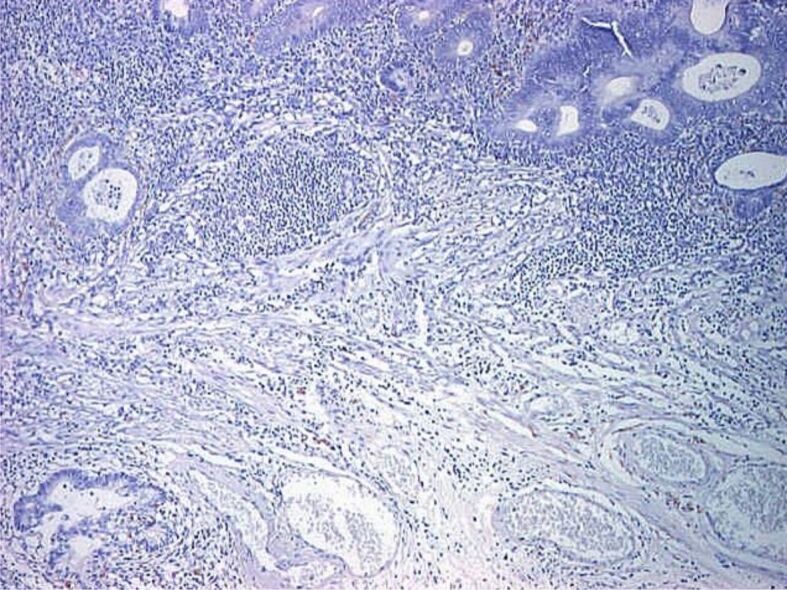
Malignant polyp (adenocarcinoma with SM invasion): absent IHC staining for desmin in muscularis mucosae (×40)
Figure 17.
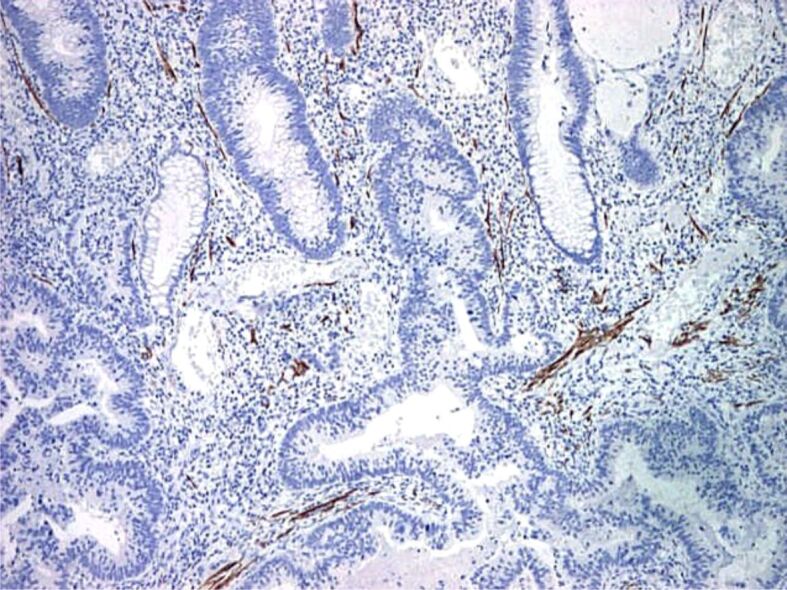
Malignant polyp with invasion and surpass of muscularis mucosae (×40): absent IHC staining for desmin in invasion area of muscularis mucosae (×40)
Lymphovascular invasion was confirmed in three patients; from 25 polyps with SM invasion, grading evaluation was available in 24 polyps and only highly or moderate differentiated tumors were found (G1 15 cases and G2 nine cases, respectively). Non-lifting sign was present in six cases, stalk invasion was noted in one case and macroscopic aspect of depressed center was noted in another case. For the cases where biopsies were taken first, when pathological aspect was adenomatous, even with carcinoma inside, mucosectomy was performed or tried. In case of failed polypectomy (non-lifting sign) or when only partial polypectomy was possible and SM invasion was present, the patient was referred to surgical department. In polyps with incomplete resection and IM or IE carcinoma, colonoscopy was repeated after 1–2 months and another endoscopic resection was performed.
If polypectomy (classical or mucosectomy) was successful, the attitude was guided by the pathological parameters and further management was established together with the patient. Cases with invaded border margin, non-lifting sign, incomplete resection or lymphovascular invasion were considered to have a high risk of recurrence or lymphatic invasion, surgical intervention was therefore advocated; the same indication was for cases with incomplete resected polyps and SM invading carcinoma. In patients with clear free border, with no lymphovascular invasion periodic follow-up colonoscopy combined with tumoral markers and CT scan was recommended. First colonoscopic evaluation was made at 3–6 months after polypectomy; for cases with no recurrence, follow-up by colonoscopy was performed at interval of 1–3 years guided by the size of the resected polyp, by the appearance of a new polyp, by age and family history of carcinomas. The mean period of evaluation was 8.5 years (limits 3–12 years). Patient distribution by outcome is figured in Table 7.
Table 7.
Endoscopic vs surgical group
|
Surgical group I (non-lifting sign, depressed center, invaded stalk) (8 patients) |
Endoscopic group (101 patients) |
||||||||
|
Outcome OK (3 patients) |
Outcome poor 2 patients M1hep, 1 patient alive at 7 years |
Lost to follow-up (3 patients) |
i-pEMR (6 patients) |
EMR (70 patients) |
pEMR (25 patients) |
||||
|
Endoscopic group management | |||||||||
|
Demand surgery (4 patients) |
Follow-up (62 patients) |
Lost to follow-up (35 patients) |
|||||||
|
Local recurrence (7 patients) |
Another polyp (2 patients) |
Metastasis (1 patient) Metastasis other tumor (1 patient) |
Outcome OK (51 patients) |
5 deaths other diseases Unknown (29 patients) |
|||||
|
Surgical group II (10 patients) – 1 patient death, 1 patient metastasis, 5 patients OK |
Second endoscopy intervention (3 patients) |
|
|||||||
i-pEMR: Incomplete piecemeal endoscopic mucosal resection (pEMR).
The favorable outcome was considered when patients had no local recurrence during follow-up, no LN metastasis and no need for surgery. The unfavorable outcome was considered when the patients had local recurrence of carcinoma at the site of previous polypectomy, when the CT scan showed LN invasion or tumoral markers showed a potential of recurrent disease or surgery was needed during follow-up to remove a recurrent carcinoma.
From the 109 patients, a total of 18 patients had performed surgery, 62 patients performed colonoscopic follow-up with no surgical treatment and 35 patients had no colonoscopic follow-up. Only eight patients had surgery after first endoscopy (incomplete resection, non-lifting sign, polyps with depressed invaded center, invaded stalk); follow-up was possible in five cases, two with liver metastasis and three with no signs of recurrence (other three were lost to follow-up). Another 10 patients had surgery after local recurrence (six patients), because of deep SM invasion (one patient), one patient because of concomitant right colon tumor and two patients because of personal option (one patient with NIHGN and another with complete EMR). Six from 10 patients who performed secondary surgery were followed with no signs of recurrence, one developed liver metastases and one died because of chemotherapy complications.
A total of 62 patients with malignant polyps had only endoscopic management. One case with a giant rectal lateral spreading lesion had a local recurrence managed by colonoscopy, with a concomitant ongoing renal carcinoma with a contraindication for surgery. Two patients had another benign polyp removed during follow-up and one patient developed liver metastases and died. Another patient died because of liver metastases from another tumor and the rest of 51 patients were alive and with no recurrence.
From the 35 patients who had no follow-up colonoscopy, five deaths from other causes were recorded (liver cirrhosis, hepatocellular carcinoma, necrotising fasciitis, respiratory failure and cardiac arrest at home three months after colonoscopy).
From the 62 patients with first curative endoscopic resection and follow-up endoscopies (first at 3–6 months), recurrence was noted in seven (11.29%) cases whilst 55 cases had no local recurrence. There was no statistically significant association with depth of invasion (OR 1.52, p=0.73 for SM vs IM+IS+NIHGN and OR 4.7, p=0.31 for SM+IS+IM vs NIHGN), location (OR 3.56, p=0.24 right-sided vs left-sided and OR 2.2, p=0.34 rectum vs other location), and pathological type of the polyps (serrated lesions vs traditional adenoma OR 2.12, p=0.52 and villous + tubulovillous vs other type OR 1.54, p=0.62), possible because of small study sample. There was however a statistical association with PMR vs EBR (OR 6.47, p=0.03), and LST macroscopic type (OR 18, p=0.004) but not sessile type (OR 1.02, p=0.89). Mean diameter of resected polyps with local recurrence was 33.14 mm.
From the 18 patients who performed surgery, definitive post-therapeutic staging was available in 11 patients; nodal involvement being noted in three (27.27%) cases, from which none had lymphatic or vascular invasion. Only four patients with no local macroscopic recurrence underwent surgery with no local microscopic recurrence (colonoscopic resection R0).
Three cases with liver and pulmonary metastases related to the malignant polyp were noted, two (11.11%) from surgical sample study and one (1.81%) from colonoscopic sample. There was no correlation with macroscopic type, depth of invasion, pathological type of the polyps or grading.
Looking at rectal polyps, there were 34 malignant polyps; mean diameter was 26.83 mm. Rectal polyps had a slightly more frequent villous or tubulovillous structure than other locations (70.83% vs 55.43%), and had more frequent metastases (8.82% vs 2.56%, OR 3.68, p=0.27) and need for surgery (29.41% vs 20.37%, OR 2.19, p=0.16), although without statistical significance.
⧉ Discussions
The prevalence of SM invading malignant polyps in our study was 1.23% (2.54% of patients), while the total prevalence of polyps with malignant cells was 6% (12% of patients); after excluding NIHGN and patients with undetermined depth of invasion, the prevalence of polyps with malignancy was 3.5% (7.3% of patients), comparable to literature data, where a prevalence of polyps with adenocarcinoma of 3–4.6% was reported in screening studies [3]. Some studies showed an increased prevalence during the time (0.37% in the first years to 10.2% in the last years, mean rate 3.5%) [29], but we did not observe that effect.
62.4% of patients with malignant polyps were males, similar to benign polyps’ group (60.28%); in the literature, the majority of subjects with malignant polyps were males, prevalence ranging from 51–88% [19, 30,31,32,33,34,35]. The presence of three or more polyps is significantly higher in male patients (p=0.012) and is associated with a higher risk for HGD (p<0.0001). Mean age in malignant polyps was 62.6 years, slightly higher than benign polyps (60.8 years) and similar to literature data (mean 60–73 years) [19, 30,31, 34,35]; a study of 2077 patients with T1N0M0 carcinoma showed a mean age between 66–69 years [32]. In our study, 25.69% of cases had 50–59 years, 41.28% had 60–69 years and 19.27% had 70–79 years, similar to other studies [32]. Elders (65 years and above) have a higher rate of HGD and carcinoma as compared to younger population (p=0.0165).
The mean diameter of malignant polyps was 23.44 mm (4–90 mm) and for benign polyps 9.63 mm (3–70 mm) (p<0.0001); the difference was statistically significant but there are some larger benign polyps in our study. In various small studies, the mean diameter of malignant polyps was between 18.6–25 mm [4, 20, 31, 36], one large study found a mean diameter of 20.6±13.1 mm for LN negative and of 19.6±9.9 mm for LN positive [35], and another large study found a mean diameter of 17 mm [37]. The risk of malignancy increases with size: 0.81% below 10 mm, 14.1% above 10 mm, 25.8% above 20 mm and 30% above 30 mm, comparable to other studies [17, 30, 37]. The risk of malignancy in polyps greater than 2 cm diameter was estimated in one study at up to 46%, while in polyps smaller than 1 cm the risk was less than 2% [21].
26.23% of malignant polyps were sessile, 44.26% were pedunculated and 16.39% were semipedunculated. 12.3% of malignant polyps had aspect of lateral spreading lesions (LST) while one (0.82%) polyp was small flat lesion. Most studies found that malignant polyps were predominantly pedunculated although some studies found a predominance of sessile lesions [33]; a meta-analysis of 31 studies (1900 patients) showed that pedunculated polyps were 65.7% and sessile polyps 34.3% [38]. Pedunculated malignant polyps are considered traditionally to have a better prognosis than sessile lesions [16, 39], because of en bloc frequent resection and the possibility to resect at half distance of the stalk, which can prevent recurrent disease. In sessile malignant polyps, recurrence rate was 3% after surgical resection and 8.6% after endoscopic resection on 411 malignant polyps [39], higher than in pedunculated lesions, although in a study of pedunculated malignant polyps’ LN metastasis was noted in 11% and recurrent cancer in 10% [30].
56.56% of malignant polyps in our study were located to the left colon, 27.87% in the rectum and 15.57% in the right colon. Most malignant polyps had a left-side location [20, 32, 40] and in one large study with 19 743 patients [37] 55% of malignant polyps were located to the left colon, followed by right colon (31%) and transverse colon and flexures (13%).
In our study, malignant polyps were seven (5.74%) TSAs, eight (6.56%) SSAs and 107 traditional adenomas (65 tubulovillous, five villous, three tubular and in 34 cases only adenoma structure was described). Rate of malignancy was 29.17% for TSAs, 17.02% for SSA, 13.7% for villous and tubulovillous lesions, 0.9% for tubular polyps. The data for traditional adenomas are similar to that from the literature, where a greater risk for malignancy was noted in VA [20, 37], with one study showed that 66% of malignant polyps had a villous component [37]. For serrated polyps (TSAs and SSAs), the rate of malignancy in our study was much higher than in literature data (1.3% for SSAs and 0.7% for TSAs) [41], although SSAs of the right colon have been frequently incriminated into the etiology of interval cancers between screening colonoscopies [41]. We found G1 grading in 60.53% of patients, G2 in 39.47% and no G3 carcinoma, similar to literature data with mainly G1 and G2 malignant polyps (90–95%) [4, 19,20, 33, 35] and only 4% G3 cases in a database study of over 19 000 patients [37]. The prevalence of G3 grading was higher in surgery vs polypectomy groups [32, 34].
Only three (2.46%) patients had lymphatic invasion, while vascular invasion was noted in one patient without lymphatic invasion and in another patient (1.64%) who had also lymphatic invasion. Several studies found lymphatic invasion in 6.67–31.13% [4, 34,35, 42] and venous invasion was noted in 5–35.36% [3,4, 19, 35]. Lymphatic invasion and LN involvement are not identical; a study showed that only two from seven patients with local lymphatic invasion had LN metastasis and also that from seven patients with positive LN involvement who are treated by surgery, only two had lymphatic invasion [4].
In 62 cases followed by colonoscopy, local recurrence was noted in seven (11.3%) cases, and metastases were noted in one (1.62%) patient with malignant rectal polyp. Only macroscopic incomplete resection because of non-lifting sign (especially in rectal malignant polyps) and SM deep invasion were identified as risk factors. Lymphovascular invasion was very rare and was not associated with bad outcome, and no G3 grading was seen. The risk of recurrence is estimated in the literature between 0.8–48.4% [31, 33,34, 39, 42,43,44,45] and is related to several factors, such as type of polyp, resection border, depth of invasion, grading, lymphovascular invasion and budding. The risk is lower after EBR, in polyps with border margin above 1–2 mm (although even a 0.1 mm margin is safe in some studies) [19, 43, 46], in pedunculated lesions (0.8–10%) [7, 30] and in endoscopic vs surgical group [39]. The risk is higher for PMR (12–14%) vs ESD (2%) [10] and a recurrence of benign adenoma component was also noted for PMR [47].
In our study, evaluation of LN invasion was available in 11 patients and LN invasion (LN=1) was noted in three (27.27%) patients; one has deep SM invasion. The rate of nodal involvement in several studies was between 6.2% and 8.6% [37, 42, 48,49]. The risk of nodal invasion is correlated with several factors, such as greater width and depth invasion [31, 35], invasion of basis of the stalk or of basis of sessile polyps (12–25%) [34], G3 [46], and grade 2/3 of tumoral budding [22], pathological incomplete removal, lymphatic invasion (OR 9.2, p=0.02) [42] and vascular invasion (OR 7 for colon and OR 12 for rectal location) [18]. Nodal metastasis was more frequent in rectum (15%) compared with left (8%) or right location (3%) [34]. In our study, we couldn’t find a significant risk factor for nodal invasion because of the small number of cases with nodal involvement.
The classification of patients in high-risk vs low-risk groups is important in clinical practice because patients with favorable pathology have a risk of recurrence close to 1% [3], while in case of unfavorable pathology the risk of relapse or residual lesions estimated between 10% and 39% [3]. However, a large study in USA found that even in patients with favorable pathological factors who performed surgery the rate of LN invasion was 5.5% [37], and National Comprehensive Cancer Network (NCCN) Guideline stated that even in patients with favorable pathological factors the risk of LN metastasis in sessile malignant polyps is 10%, so colectomy can be added as possible management [50]. The need for further refining of surgery criteria is argumented by the fact that in 94% of operated patients no malignant or even adenomatous lesions were found [18].
In our study, rectal malignant polyps have more frequent metastases (8.82%) and need for surgery (29.41%) than other locations. As compared to other locations, rectal polyps are larger (p=0.0014), have non-adenomatous structure (p=0.026). Moreover, villous pattern of adenomas is seen rather in the rectum than other locations (p<0.0001) and cancer is also present in the rectum more frequent (p=0.0008). On the other hand, polyps in the rectum are less frequent serrated type (p=0.0365). Rectal malignant polyps represent a particular situation because the risk of distant metastases may be greater than in colonic malignant polyps. Surgery is usually recommended for high-risk rectal malignant polyps, but even in these cases colonoscopic management can achieve success in more than 80% of cases, and a large study of malignant polyps which were treated by polypectomy vs surgery showed similar outcome for surgical vs colonoscopy-managed patients [32].
We had a case with an IE malignant polyp resected with local recurrence and SM invasion (possible because of an initial underdiagnosis), which imposes surgical intervention with favorable outcome, and another patient with a rectal malignant polyp (IS carcinoma) who developed liver metastases despite complete local resection and no local recurrence. There are several reported cases with unfavorable outcome despite no local recurrence and no LN metastasis because of rare hematological dissemination even in T1 tumors [20], a missed SM invasion or tumoral deposits in subserosa (already stage III tumors) [13].
⧉ Conclusions
The rate of malignant polyps was 1.23% and of polyps with malignant cells inside was 6%. The rate of malignancy was higher in polyps 10 mm and above, in pedunculated, semipedunculated and in lateral spreading lesions, in left and rectal location and in patients with two or more polyps. Serrated lesions (TSA, SSA) and polyps with villous component had a malignancy rate superior to other types. Most SM lesions were sessile, pedunculated and semipedunculated, while in LST lesions most have only IM invasion. Lymphovascular invasion was rare. Most patients were managed endoscopically; recurrence rate was 11.3%. Main predictive factors for surgery were incomplete resection and deep SM invasion. Rectal malignant polyps have more frequent metastases and need for surgery. There is clearly a need for improvements regarding patients’ selection in surgical or follow-up groups.
Conflict of interest
The authors declare that they have no conflict of interests.
Authors’ contribution
Sevastiţa Iordache and Sergiu Marian Cazacu had equal contributions.
References
- 1.Freeman HJ. Early stage colon cancer. World J Gastroenterol. 2013;19(46):8468–8473. doi: 10.3748/wjg.v19.i46.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Zoghbi M, Cummings LC. New era of colorectal cancer screening. World J Gastrointest Endosc. 2016;8(5):252–258. doi: 10.4253/wjge.v8.i5.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bujanda L, Cosme A, Gil I, Arenas-Mirave JI. Malignant colorectal polyps. World J Gastroenterol. 2010;16(25):3103–3111. doi: 10.3748/wjg.v16.i25.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonçalves BM, Fontainhas V, Caetano AC, Ferreira A, Gonçalves R, Bastos P, Rolanda C. Oncological outcomes after endoscopic removal of malignant colorectal polyps. Rev Esp Enferm Dig. 2013;105(8):454–461. doi: 10.4321/s1130-01082013000800003. [DOI] [PubMed] [Google Scholar]
- 5.Bonnington SN, Rutter MD. Surveillance of colonic polyps: are we getting it right. World J Gastroenterol. 2016;22(6):1925–1934. doi: 10.3748/wjg.v22.i6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aarons CB, Shanmugan S, Bleier JIS. Management of malignant colon polyps: current status and controversies. World J Gastroenterol. 2014;20(43):16178–16183. doi: 10.3748/wjg.v20.i43.16178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciocalteu A, Gheonea DI, Saftoiu A, Streba L, Dragoescu NA, Tenea-Cojan TS. Current strategies for malignant pedunculated colorectal polyps. World J Gastrointest Oncol. 2018;10(12):465–475. doi: 10.4251/wjgo.v10.i12.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geramizadeh B, Marzban M, Owen DA. Malignant colorectal polyps; pathological consideration (a review) Iran J Pathol. 2017;12(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 9.*** The Paris endoscopic classification of superficial neoplastic lesions esophagus stomach and colon November 30 to December 1 2002. Gastrointest Endosc. 2003;58(6 Suppl):S3–S43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 10.Rutter MD, Chattree A, Barbour JA, Thomas-Gibson S, Bhandari P, Saunders BP, Veitch AM, Anderson J, Rembacken BJ, Loughrey MB, Pullan R, Garrett WV, Lewis G, Dolwani S. British Society of Gastroenterology/Association of Coloproctologists of Great Britain and Ireland guidelines for the management of large non-pedunculated colorectal polyps. Gut. 2015;64(12):1847–1873. doi: 10.1136/gutjnl-2015-309576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saiki H, Nishida T, Yamamoto M, Hayashi S, Shimakoshi H, Shimoda A, Amano T, Sakamoto A, Otake Y, Sugimoto A, Takahashi K, Mukai K, Matsubara T, Nakajima S, Fukui K, Inada M, Yamamoto K, Tokuda R, Adachi S. Frequency of coexistent carcinoma in sessile serrated adenoma/polyps and traditional serrated adenomas removed by endoscopic resection. Endosc Int Open. 2016;4(4):E451–E458. doi: 10.1055/s-0042-103239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Ali SM, Umm-a-OmarahGilani S, Liu J, Li YQ, Zuo XL. Kudo’s pit pattern classification for colorectal neoplasms: a meta-analysis. World J Gastroenterol. 2014;20(35):12649–12656. doi: 10.3748/wjg.v20.i35.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung EJ, Ryu CG, Paik JH, Hwang DY. Undetermined margins after colonoscopic polypectomy for malignant polyps: the need for radical resection. Anticancer Res. 2015;35(12):6887–6891. [PubMed] [Google Scholar]
- 14.Ikematsu H, Matsuda T, Emura F, Saito Y, Uraoka T, Fu KI, Kaneko K, Ochiai A, Fujimori T, Sano Y. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol. 2010;10:33–33. doi: 10.1186/1471-230X-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu CH, Chen TH, Hsu CM, Su MY, Chiu CT, Wu RC, Lai CC. Linked-color imaging combined with the NICE classification system for optical diagnosis of colon polyps: new image-enhanced endoscopic technology for pathological prediction. Ther Clin Risk Manag. 2017;13:1317–1321. doi: 10.2147/TCRM.S147155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman HJ. Long-term follow-up of patients with malignant pedunculated colon polyps after colonoscopic polypectomy. Can J Gastroenterol. 2013;27(1):20–24. doi: 10.1155/2013/380389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fragaki M, Voudoukis E, Chliara E, Dimas I, Mpitouli A, Velegraki M, Vardas E, Theodoropoulou A, Karmiris K, Giannikaki L, Paspatis G. Complete endoscopic mucosal resection of malignant colonic sessile polyps and clinical outcome of 51 cases. Ann Gastroenterol. 2019;32(2):174–177. doi: 10.20524/aog.2018.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor JM, Hosie KB. In: Colonoscopy and colorectal cancer screening - future directions. Bustamante M, editor. London UK: IntechOpen Ltd; 2013. The malignant polyp polypectomy or surgical resection; pp. 140–160. [Google Scholar]
- 19.Naqvi S, Burroughs S, Chave HS, Branagan G. Management of colorectal polyp cancers. Ann R Coll Surg Engl. 2012;94(8):574–578. doi: 10.1308/003588412X13373405387771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pizarro-Moreno A, Cordero-Fernández C, Garzón-Benavides M, Cayuela A, Bozada-García JM, Sobrino-Rodríguez S, López-Ruiz T, Caballero-Gómez J, Zulueta T, Márquez-Galán JL. Malignant colonic adenomas. Therapeutic criteria. Long-term results of therapy in a series of 42 patients in our healthcare area. Rev Esp Enferm Dig. 2009;101(12):830–836. doi: 10.4321/s1130-01082009001200002. [DOI] [PubMed] [Google Scholar]
- 21.Hall JF. Management of malignant adenomas. Clin Colon Rectal Surg. 2015;28(4):215–219. doi: 10.1055/s-0035-1564434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oka S, Tanaka S, Nakadoi K, Kanao H, Chayama K. Risk analysis of submucosal invasive rectal carcinomas for lymph node metastasis to expand indication criteria for endoscopic resection. Dig Endosc. 2013;25(Suppl 2):21–25. doi: 10.1111/den.12089. [DOI] [PubMed] [Google Scholar]
- 23.Hagen CE, Farooq A. Histologic evaluation of malignant polyps and low-stage colorectal carcinoma. Arch Pathol Lab Med. 2019;143(12):1450–1454. doi: 10.5858/arpa.2019-0291-RA. [DOI] [PubMed] [Google Scholar]
- 24.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51(1):130–131. doi: 10.1136/gut.51.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med. 2002;126(7):829–836. doi: 10.5858/2002-126-0829-ISOMIN. [DOI] [PubMed] [Google Scholar]
- 26.Ban S, Kamada K, Mitsuki N, Goto Y, Shimizu Y, Takahama M, Shibata T. Phenotypic change of muscularis mucosae in early invasive colorectal adenocarcinoma. J Clin Pathol. 2000;53(11):878–881. doi: 10.1136/jcp.53.11.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Künzli HT, Belghazi K, Pouw RE, Meijer SL, Seldenrijk CA, Weusten B, Bergman J. Endoscopic management and follow-up of patients with a submucosal esophageal adenocarcinoma. United Eur Gastroenterol J. 2018;6(5):669–677. doi: 10.1177/2050640617753808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Council L, Hameed O. Differential expression of immunohistochemical markers in bladder smooth muscle and myofibroblasts, and the potential utility of desmin, smoothelin, and vimentin in staging of bladder carcinoma. Mod Pathol. 2009;22(5):639–650. doi: 10.1038/modpathol.2009.9. [DOI] [PubMed] [Google Scholar]
- 29.Reggiani-Bonetti L, Di Gregorio C, Pedroni M, Domati F, Barresi V, Marcheselli L, Ponz de Leon M. Incidence trend of malignant polyps through the data of a specialized colorectal cancer registry: clinical features and effect of screening. Scand J Gastroenterol. 2013;48(11):1294–1301. doi: 10.3109/00365521.2013.838301. [DOI] [PubMed] [Google Scholar]
- 30.Backes Y, Moons LM, Novelli MR, van Bergeijk JD, Groen JN, Seerden TC, Schwartz MP, de Vos Tot Nederveen Cappel WH, Spanier BW, Geesing JM, Kessels K, Kerkhof M, Siersema PD, Offerhaus GJ, Milne AN, Lacle MM. Diagnosis of T1 colorectal cancer in pedunculated polyps in daily clinical practice: a multicenter study. Mod Pathol. 2017;30(1):104–112. doi: 10.1038/modpathol.2016.165. [DOI] [PubMed] [Google Scholar]
- 31.Brown IS, Bettington ML, Bettington A, Miller G, Rosty C. Adverse histological features in malignant colorectal polyps: a contemporary series of 239 cases. J Clin Pathol. 2016;69(4):292–299. doi: 10.1136/jclinpath-2015-203203. [DOI] [PubMed] [Google Scholar]
- 32.Cooper GS, Xu F, Barnholtz Sloan JS, Koroukian SM, Schluchter MD. Management of malignant colonic polyps: a population-based analysis of colonoscopic polypectomy versus surgery. Cancer. 2012;118(3):651–659. doi: 10.1002/cncr.26340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang EJ, Kim DD, Cho CH. Value and interpretation of resection margin after a colonoscopic polypectomy for malignant polyps. J Korean Soc Coloproctol. 2011;27(4):194–201. doi: 10.3393/jksc.2011.27.4.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butte JM, Tang P, Gonen M, Shia J, Schattner M, Nash GM, Temple LKF, Weiser MR. Rate of residual disease after complete endoscopic resection of malignant colonic polyp. Dis Colon Rectum. 2012;55(2):122–127. doi: 10.1097/DCR.0b013e3182336c38. [DOI] [PubMed] [Google Scholar]
- 35.Kawachi H, Eishi Y, Ueno H, Nemoto T, Fujimori T, Iwashita A, Ajioka Y, Ochiai A, Ishiguro S, Shimoda T, Mochizuki H, Kato Y, Watanabe H, Koike M, Sugihara K. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Mod Pathol. 2015;28(6):872–879. doi: 10.1038/modpathol.2015.36. [DOI] [PubMed] [Google Scholar]
- 36.Seitz U, Bohnacker S, Seewald S, Thonke F, Brand B, Bräiutigam T, Soehendra N. Is endoscopic polypectomy an adequate therapy for malignant colorectal adenomas? Presentation of 114 patients and review of the literature. Dis Colon Rectum. 2004;47(11):1789–1796; discussion 1796–1797. doi: 10.1007/s10350-004-0680-2. [DOI] [PubMed] [Google Scholar]
- 37.Wasif N, Etzioni D, Maggard MA, Tomlinson JS, Ko CY. Trends, patterns, and outcomes in the management of malignant colonic polyps in the general population of the United States. Cancer. 2011;117(5):931–937. doi: 10.1002/cncr.25657. [DOI] [PubMed] [Google Scholar]
- 38.Hassan C, Zullo A, Risio M, Rossini FP, Morini S. Histologic risk factors and clinical outcome in colorectal malignant polyp: a pooled-data analysis. Dis Colon Rectum. 2005;48(8):1588–1596. doi: 10.1007/s10350-005-0063-3. [DOI] [PubMed] [Google Scholar]
- 39.Lopez A, Bouvier AM, Jooste V, Cottet V, Romain G, Faivre J, Manfredi S, Lepage C. Outcomes following polypectomy for malignant colorectal polyps are similar to those following surgery in the general population. Gut. 2019;68(1):111–117. doi: 10.1136/gutjnl-2016-312093. [DOI] [PubMed] [Google Scholar]
- 40.Di Gregorio C, Bonetti LR, de Gaetani C, Pedroni M, Kaleci S, Ponz de Leon M. Clinical outcome of low- and high-risk malignant colorectal polyps: results of a population-based study and meta-analysis of the available literature. Intern Emerg Med. 2014;9(2):151–160. doi: 10.1007/s11739-012-0772-2. [DOI] [PubMed] [Google Scholar]
- 41.Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49(3):270–297. doi: 10.1055/s-0043-102569. [DOI] [PubMed] [Google Scholar]
- 42.Boenicke L, Fein M, Sailer M, Isbert C, Germer CT, Thalheimer A. The concurrence of histologically positive resection margins and sessile morphology is an important risk factor for lymph node metastasis after complete endoscopic removal of malignant colorectal polyps. Int J Colorectal Dis. 2010;25(4):433–438. doi: 10.1007/s00384-009-0836-6. [DOI] [PubMed] [Google Scholar]
- 43.Gill MD, Rutter MD, Holtham SJ. Management and short-term outcome of malignant colorectal polyps in the north of England. Presented at NREG Symposium 11 October 2011. Colorectal Dis. 2013;15(2):169–176. doi: 10.1111/j.1463-1318.2012.03130.x. [DOI] [PubMed] [Google Scholar]
- 44.Fasoli R, Nienstedt R, De Carli N, Monica F, Guido E, Valiante F, Armelao F, de Pretis G. The management of malignant polyps in colorectal cancer screening programmes: a retrospective Italian multi-centre study. Dig Liver Dis. 2015;47(8):715–719. doi: 10.1016/j.dld.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Sharma V, Junejo MA, Mitchell PJ. Current management of malignant colorectal polyps across a regional United Kingdom Cancer Network. Dis Colon Rectum. 2020;63(1):39–45. doi: 10.1097/DCR.0000000000001509. [DOI] [PubMed] [Google Scholar]
- 46.Berg KB, Telford JJ, Gentile L, Schaeffer DF. Re-examining the 1-mm margin and submucosal depth of invasion: a review of 216 malignant colorectal polyps. Virchows Arch. 2020;476(6):863–870. doi: 10.1007/s00428-019-02711-9. [DOI] [PubMed] [Google Scholar]
- 47.Mouchli MA, Ouk L, Scheitel MR, Chaudhry AP, Felmlee-Devine D, Grill DE, Rashtak S, Wang P, Wang J, Chaudhry R, Smyrk TC, Oberg AL, Druliner BR, Boardman LA. Colonoscopy surveillance for high risk polyps does not always prevent colorectal cancer. World J Gastroenterol. 2018;24(8):905–916. doi: 10.3748/wjg.v24.i8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rickert A, Aliyev R, Belle S, Post S, Kienle P, Kähler G. Oncologic colorectal resection after endoscopic treatment of malignant polyps: does endoscopy have an adverse effect on oncologic and surgical outcomes. Gastrointest Endosc. 2014;79(6):951–960. doi: 10.1016/j.gie.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Kimura YJ, Kudo SE, Miyachi H, Ichimasa K, Kouyama Y, Misawa M, Sato Y, Matsudaira S, Oikawa H, Hisayuki T, Mori Y, Kudo T, Ogata N, Kodama K, Wakamura K, Hayashi T, Katagiri A, Baba T, Hidaka E, Ishida F, Hamatani S. Head invasion’ is not a metastasis-free condition in pedunculated T1 colorectal carcinomas based on the precise histopathological assessment. Digestion. 2016;94(3):166–175. doi: 10.1159/000450942. [DOI] [PubMed] [Google Scholar]
- 50.Barton MK. Surgical management of malignant colonic polyps often suboptimal. CA Cancer J Clin. 2011;61(2):63–64. doi: 10.3322/caac.20109. [DOI] [PubMed] [Google Scholar]