Abstract
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the digestive tract, originating from structures differentiating towards Cajal cells. Due to their morphology and localization, the extragastrointestinal stromal tumors (EGISTs) can be a diagnostic challenge. We investigated a series of 51 EGISTs diagnosed in our institutions, aiming to explore the immunophenotypes and to analyze the process and the utility of the antibodies required for a positive diagnosis. Immunohistochemical examinations were done for pan-cytokeratin (pan-CK), Ki67, discovered on GIST1 (DOG1) protein and platelet-derived growth factor receptor alpha (PDGFRA), as necessary. The main tumor site was abdominal wall in 43 (84%) cases, most of the tumors showed spindle cell cellularity, followed by mixed and epithelioid type. Twenty-six cases revealed a full conventional immunohistochemical profile with DOG1 positivity. In 10 cases, c-KIT expression was absent but with the preservation of cluster of differentiation (CD)34 positivity, and eight cases were positive for PDGFRA. In our study, we found a subgroup of eight cases presenting in extra-abdominal settings (including one in lung and two in the head-and-neck area). We concluded EGISTs represent a histopathological and immunohistochemically challenging subgroup testing more often negative for c-KIT mutations and positive for PDGFRA compared to GIST. DOG1 remains the marker of choice regardless of tumor site, while CD34 and CD117 should be considered as adjuvants.
Keywords: EGIST, DOG1, PDGFRA, extra-abdominal, gastrointestinal stromal tumor
⧉ Introduction
Gastrointestinal stromal tumors (GISTs) are tumors of the digestive tract stroma with the actual cell-of-origin unknown but differentiating to interstitial cells of Cajal [1]. The diagnosis is laborious and needs confirmation by fine-needle biopsy with a complete histopathological (HP) exam on surgical specimens, including immunohistochemistry (IHC) tests [2,3].
HP features that define GIST and extragastrointestinal stromal tumors (EGISTs) are somehow similar: they are nodular proliferations, varying in size (from millimetric tumors to more than 10 cm), usually presenting homogeneous solid growths. Central pseudocyst formation and intratumoral hemorrhage are frequently mentioned [4]. Histopathologically, the tumors are well circumscribed, with spindle, epithelioid or mixed cellularity, with pale to eosinophilic cytoplasm and central nucleus molded to the cell shape. The invasive pattern is rare, more frequently found in tumors with succinate dehydrogenase (SDH) gene mutations [5,6]. The surrounding stroma is scant, with incidental collagen globules (skenoid fibers) or severe sclerosis. Areas of necrosis and hemorrhage are more commonly seen in aggressive tumors [7].
Even though the differential diagnosis is relatively simple for GIST, the EGIST differential is broad, encompassing tumors with similar morphology or similar immunophenotype, including leiomyomas, leiomyosarcomas, schwannomas, malignant peripheral nerve sheath tumors, inflammatory myofibroblastic tumors, dedifferentiated liposarcomas [8,9,10,11]. The GIST and EGIST diagnosis require most of the time extensive ancillaries testing including IHC. On this perspective, the vast majority (95% of all GISTs) are cluster of differentiation (CD)117-positive, accompanied by diffuse and intense positive expression for CD34, discovered on GIST1 (DOG1) and vimentin. The tumors express only partial positivity for alpha-smooth muscle actin (α-SMA), and have weak, partial, or absent desmin expression. A small fraction of GISTs have altered immunophenotypes, testing positive for platelet-derived growth factor receptor (PDGFR) and negative for CD117, meanwhile continuing to show positive expression for DOG1 [12]. In these situations, the preserved DOG1 expression in EGISTs ensures the reliability of the HP diagnosis.
GISTs harbor mutations involving the c-KIT oncogene (transcription product the CD117 receptor) or the PDGFR alpha (PDFGRA). A fraction of these tumors entails mutations in SDH complex (with subunits A, B, C and D) with consecutive SDH-B negative IHC testing (regardless of the mutated SDH subunit) [13]. In the context of molecular and immunohistochemical advances in the last decade, the diagnosis of EGIST remains an elaborate process.
Aim
The objectives of the study were to evaluate the pathological and immunohistochemical profile of EGISTs, aiming to identify particularities of morphological or immunohistochemical profiles. Secondary, we investigated IHC markers utility in the diagnosis to emphasize its relevance in the context of an EGIST.
⧉ Materials and Methods
We performed retrospective research of the database and slides from the archives of Victor Babeş National Institute of Pathology (INCDVB) and of University Emergency Hospital (SUUB), Bucharest, Romania. Criteria for the case definition included patients whose clinical or HP report included the term EGIST and related ones, diagnosed between 2005–2021. Clinical and HP data were collected from patient’s medical records, including demographic data, tumor site and size and additional information like recurrence, metastases, and molecular biology testing results for PDGFR and SDH. HP slides were reviewed by two pathologists documenting the HP type, particular microscopic features and the immunophenotypic profile. IHC examinations were performed using an indirect protocol on Leica Bond II platforms, with Leica Novocastra, Agilent and Abcam ready-to-use antibodies: anti-pan-cytokeratin (pan-CK) cocktails (MNF116, clone MA1-26237; AE1/AE3, clone ab80826; OC15E and CK19, clone b170), as well as anti-epithelial membrane antigen (EMA, clone E29), anti-vimentin (clone SRL33), anti-S100 (polyclonal), anti-α-SMA (clone alpha sm-1), anti-desmin (clone DE-R-11), anti-CD34 (clone QBEnd/10), anti-CD117 (clone EP10) and anti-Ki67 (clone K2). Additional testing for DOG1 and PDGFRA was redone as necessary (damaged slides or missing) using rabbit monoclonal clones SP31 and D13C6, respectively. The antigen epitopes unmasking was done by wet heat-induced epitope retrieval (HIER) Bond Epitope Retrieval Solution 1 and 2. Detection was made using polymer kit (DS9800/Bond Polymer-Refine-Detection) and 3,3’-Diaminobenzidine (DAB). The counterstaining was performed using Mayer’s Hematoxylin. HP input was correlated with the gross features, clinical data, as well as molecular biology reports. Each batch of slides was run simultaneous with parallel external positive control tissue slides.
⧉ Results
The database research for the two institutions yielded a batch of 59 cases. Eight cases were eliminated due to fragmentary or absence of data, or considered as loco-regional recurrence recurrences, or metastases, resulting a series of 51 EGIST cases (n=51). Age at the time of diagnosis ranged between 26 and 80 years (average 56.15 years). Male/female (M/F) sex ratio was 1:2 (0.57). Average tumor size was 8.4 cm, with values between 3.5 and 17 cm. The main tumor site was in the abdominal region of 42 (82%) cases.
We grouped these cases based on tumors location as abdominal ones (42 cases) and extra-abdominal ones (nine cases). Furthermore, the abdominal group was divided into an intra-abdominal group (21 cases) and a group formed by the abdominal walls and retroperitoneal area (21 cases). The intra-abdominal group included the following anatomical sites: bursae omentalis (two cases); intraperitoneal surface (two cases); liver parenchyma and ovary (three cases on each site); mesentery (six cases); omentum (four cases) and pancreas (the remaining one case). Abdominal wall and retroperitoneal area were the primary site for 21 cases (seven cases located into the walls and 14 cases in the retroperitoneal area). The extra-abdominal group cases (18%) were subdivided into two subgroups, four cases neighboring abdominal region (7.8%; rectovaginal septum, bladder, femoral triangle, and kidney; one case each) and five cases distantly located (9.8%; lung, skeletal muscle, submandibular region, tonsil, vaginal walls; one case each for each location). Graphical representation of localization is highlighted into Figure 1.
Figure 1.
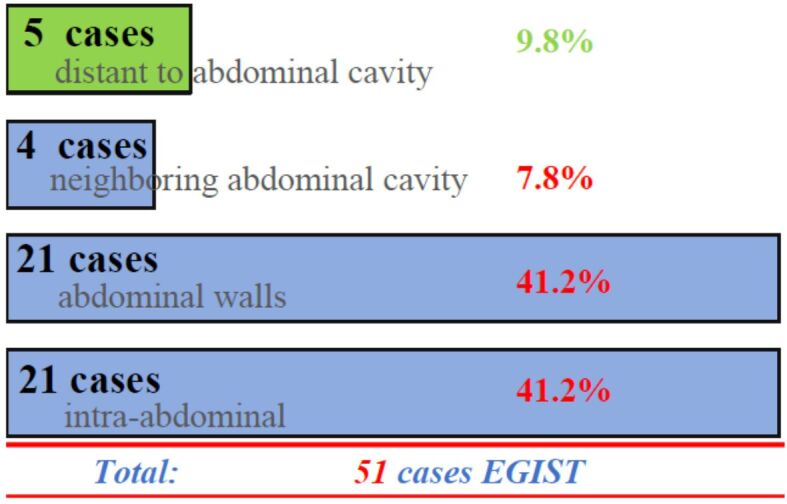
Regional distribution of the tumor series in our study. The vast majority are closely related to the abdominal cavity, 42 (82%) cases. Five (10%) cases were in sites unrelated to the abdominal cavity such as the submandibular region, lung, amygdala; while the remaining four (8%) cases were in close vicinity or related to the peritoneal serosa, like rectovaginal septum, the bladder, kidney, and the Scarpa’s triangle region. EGIST: Extragastrointestinal stromal tumors
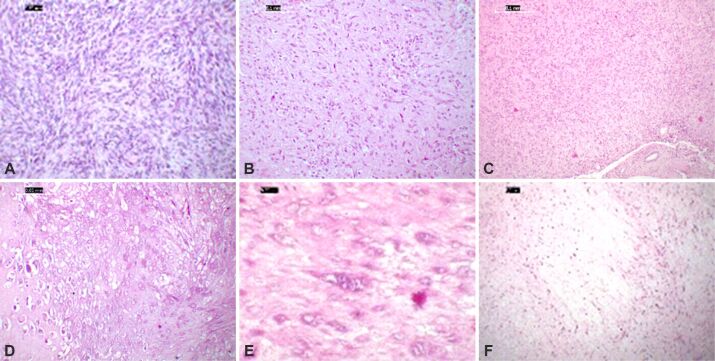
Morphologically, most of the tumors showed spindle cell cellularity (31 cases, 60.7%), followed by mixed type and epithelioid cellularity in equal proportions (10 cases each; 19.6%; Figure 2A,2B,2C). Particular HP aspects were found in seven cases: three cases presenting areas of myxoid degeneration, one case presenting hyaline chondroid metaplasia, and three cases showing scattered multinucleated tumor cells (Figure 2D,2E,2F). Common to all tumors investigated was a reduced amount of stroma, skenoid fibers were scarce only a few tumors exhibiting this feature (three cases).
Figure 2.
Microphotographs of EGIST morphological aspects showing spindle cell morphology in retroperitoneal region (A), a mixed type’ cellularity in the omentum (B) and an epithelioid pattern in a liver tumor (C). Moderate nuclear pleomorphism and active mitotic areas are common. Less frequent features: chondroid metaplasia (D) or multinucleation (E), while myxoid change is not uncommon (F). HE staining: (A and D) ×200; (B, C and F) ×100; (E) ×400. EGIST: Extragastrointestinal stromal tumors
Five of the nine cases in extra-abdominal tumors group (five cases distantly located) showed a spindle cell morphology with moderate to marked cytonuclear atypia, and an increased mitotic activity, with a Ki67 index ranging between 15–50%. The remaining four comported epithelioid and mixed type morphology with Ki67 index values between 15–20%.
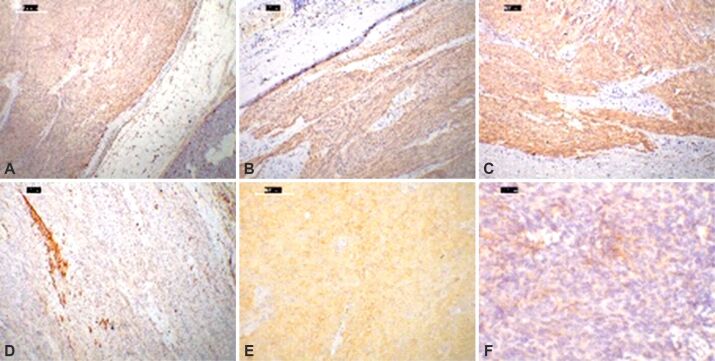
The immunohistochemical profile for half of the studied cases (50%; 26 cases) was conventional: positive expressions for vimentin, CD117 and CD34 with concurrent positivity for DOG1. For 10 (20%) cases, c-KIT expression was absent, while CD34 expression was preserved. Eight of the 10 cases showed positive expression for PDGFRA (15% of the studied group). Regarding CKs expression, the IHC testing for pan-CK was largely negative, but there were certain cases with variable staining: OC15E – one case, CK19 – one case, EMA – one case with variable positive expression (Figure 3A,3B,3C,3D,3E,3F).
Figure 3.
Immunohistochemical stainings of diverse EGIST locations: (A) Micrograph with anti-CD34 antibody moderate immunostaining in tumor cells and vascular channels in the nearby adipose tissue and pancreas, while anti-CD117 antibody (B) and anti-DOG1 antibody (C) show diffuse, strong positivity and scattered tumor cells and fascicles for α-SMA (D); (E) Micrograph of a mesentery-located epithelioid EGIST, DOG1 negative (not showed), PDGFRA positive; (F) EMA can be focally and patchy expressed in a retroperitoneal spindle cell-shaped EGIST. DAB, Hematoxylin counterstaining: (A–C) ×40; (D) ×100; (E) ×200; (F) ×400. α-SMA: Alpha-smooth muscle actin; CD: Cluster of differentiation; DAB: 3,3’-Diaminobenzidine; DOG1: Discovered on gastrointestinal stromal tumor (GIST) 1; EGIST: Extragastrointestinal stromal tumors; EMA: Epithelial membrane antigen; PDGFRA: Platelet-derived growth factor receptor alpha
DOG1 expression was absent in four (8%) cases resulting in a test sensitivity of 90%. Three of the four negative cases showed a positive expression for PDGFRA with negative CD117, and one case was positive for both PDGFRA and CD117. Tumor proliferation rate using anti-Ki67 antibodies was 15%, with a range between 3% and 65%.
⧉ Discussions
Historically, the term GIST was first attributed by Mazur & Clark [13], in 1983, for tumors arising in the gastrointestinal tract that on electron microscopy showed both aspects of smooth muscle and nerve cell-like organelles. The finding of c-KIT mutations in these tumors by Hirota et al. [14], in 1998, led to reclassification of mesenchymal tumors in the digestive tract fusing terms like gastrointestinal autonomic nerve sheet tumors and leiomyoblastoma and excluding gastric schwannoma from this category.
Currently, EGIST remains a rare neoplastic proliferation [15] that raises the issue of the cell of origin. To date, English language literature supports rare single case reports or small case series of these tumors, most frequently located in the omentum, mesentery, and retroperitoneum. However, less common anatomical locations have been also reported, such as the rectovaginal septum, salpinx, liver, pancreas mediastinum, pharynx, and gallbladder [16,17,18,19,20,21,22,23,24,25]. Several hypotheses have been proposed to elucidate the mechanisms of distant sites tumorigenesis. The two most popular theories concern neighborhood seeding or a common precursor to Cajal cells with altered differentiation/maturation. It is generally accepted that the origin of the tumor resides in a digestive GIST, whom, due to local non-mutational phenomena, produces implants or direct regional seedings (due mechanical or vascular factors). In our study, most of the tumors were closely related to the abdominal cavity (82% of cases were located intra-abdominal). If we add to this category the ones located in the near vicinity of abdominal region meaning the neighboring abdominal group (four cases) the proportion rise to 90%. This suggest that the direct regional seeding mechanism is a viable one. The distant located group do not have a direct explanation for a cell of origin. A Cajal-like cell was till now not confidently put into evidence on these sites. Different scenario has been proposed like hamartomatous tissue going a mutational pathway as well as aberrant differentiation of a common connective tissue cell progenitor. Besides the limitations of data EGIST series, or long-term surviving data, this grey zone of origin warrants further studies.
Microscopically, our study revealed the usual epithelioid/spindle cell morphology, but we identified the first EGIST with chondroid metaplasia – a feature reported only in GIST to our knowledge. Multinuclearity was sparse, with single isolated cells. Myxoid–edematous stromal degeneration was encountered, similar to the same features seen in GIST. Five of the nine cases unrelated to the abdominal cavity (lung, amygdala, submandibular region) exhibited, as common feature, a spindle cell morphology, and an increased mitotic activity, with a moderate to marked cytonuclear atypia.
In our study, 50% of the cases proved a conventional IHC profile: DOG1 expression, along with positivity for vimentin, CD117 and CD34. Ten (20%) cases had absent c-KIT expression, and the diagnosis was facilitated by the preservation of CD34 and DOG1 expressions. Only a 16% (eight cases) of EGISTs expressed PDGFRA compared to a 70% reported in cases of GIST [26,27].
DOG1 sensitivity was 90% with four (8%) cases negative for the CD117. The literature reports a sensitivity of up to 99% but it varies with the clone (K9 has lower sensitivity compared to SP31) [28]. Subsequent studies have shown an increased specificity for the SP31 clone compared to the K9 clone. Besides technical difficulties due to clone type, three of the four DOG1 negative cases were positive for PDGFRA – indicating a possible gene mutation in PDGFRA protein. The remaining one case was positive for CD117 aiding the diagnosis in the context of negative expression for anti-S100, CD45 and CK.
Although the GIST is a relatively straight away diagnosis, we found that an EGIST requires a more elaborate differential. Considering the evaluation process for unknown primary tumors, the step-by-step procedure proposed by Lin & Liu (2014) remains the optimal approach confirming the cell line differentiation is primary [29]. For this purpose, the first battery of IHC tests should incorporate the concurring use of pan-CK (AE1/AE3), S100 and a lymphoid lineage marker (CD45). A diffuse intense expression of pan-CKs is exceptional in mesenchymal tumors. False negative CK expression should be a pitfall to consider in small biopsy specimens involving differentiated and metaplastic carcinomas. Intense expression for S100 advocate for a tumor with melanocytic or nervous differentiation, circumstance that demands using additional markers (Melan A, CD56) to allow further classification. Therefore, considering the literature and the authors previous studies, it is desirable to add an anti-DOG1 antibody to the second or third batch of IHC testing. This avoids the misdiagnosis of a melanoma or germ tumor with partial c-KIT expression or a modified expression for S100 [30,31,32].
For tumors with mixed and spindle cell cellularity, it is recommended to review in the differential diagnosis neoplastic entities according to the site and semiology aspects. An in deep differential diagnosis should include in the IHC tests arguments against synovial sarcoma, a liposarcoma, and a sclerosing rhabdomyosarcoma. While the location and the monotonous morphology are good clues for the low-grade synovial sarcomas, CD34 positivity seen in liposarcomas implies further testing for DOG1 and CD117. The distinction between EGISTs and peritoneal fibromatosis remains sensitive, because of the shared location and the similar HP appearance. In addition, CD34 and CD117 are frequently positive in both entities. A nuclear positivity for β-catenin aids in distinguishing the diagnosis [33]. Solitary fibrous tumor should have a similar approach due its frequent involvement of mesenteric and serosal surface of the colon, but in these cases a signal transducer and activator of transcription 6 (STAT6) testing is diagnostic [34]. Due to the increased number of CD117-positive GISTs compared to PDGFRA (mostly negative) [35] alternatively uses of PDGFRA are not sustained. PDGRFA positivity was reported in intimal sarcomas expanding the differential diagnosis for PDGRFA-positive EGIST [36].
In total, the number of immunohistochemical tests used for diagnosing an EGIST in our series case resulted in an average of 10 tests/case, ranging between six and 24 tests for each case. The number of tests used in extra-abdominal situated cases was much higher (on average 16 tests), compared to the rest of the group.
⧉ Conclusions
In our study, we were able to highlight the variable morphological immunophenotype spectrum of EGISTs, increasing the awareness of these entities sites that cannot be directly related to the abdominal region. The IHC marker of choice for EGIST is DOG1, along with an additional marker to highlight the differentiations towards a Cajal-like cell profile (CD117 and secondary PDGFRA). CD34, CD10 and α-SMA have low use for the cell line confirmation, being more helpful for additional differential diagnostic panels. HP diagnosis remains the most practical and reliable tool with lower costs compared to molecular biology (extended sequencing).
Conflict of interests
Conflict of interests
The authors declare no conflict of interests.
Research Ethics Statement
The research was conducted in a concordance with the Approval of Bioethics Commissions from Victor Babeş National Institute of Pathology and from University Emergency Hospital, Bucharest, Romania.
References
- 1.Dei Tos AP, Hornick JL, Miettinen M, Wanless IR, Wardelmann E. In: Soft tissue and bone tumours. 5. World Health Organization (WHO) Classification of Tumours Editorial Board, editor. Lyon France: International Agency for Research on Cancer (IARC) Press; 2020. Gastrointestinal stromal tumour; pp. 216–220.https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Soft-Tissue-And-Bone-Tumours-2020 [Google Scholar]
- 2.González-Cámpora R, Delgado MD, Amate AH, Gallardo SP, León MS, Beltrán AL. Old and new immunohistochemical markers for the diagnosis of gastrointestinal stromal tumors. Anal Quant Cytol Histol. 2011;33(1):1–11. [PubMed] [Google Scholar]
- 3.Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24(26):2806–2817. doi: 10.3748/wjg.v24.i26.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonopoulos P, Leonardou P, Barbagiannis N, Alexiou K, Demonakou M, Economou N. Gastrointestinal and extragastrointestinal stromal tumors: report of two cases and review of the literature. Case Rep Gastroenterol. 2014;8(1):61–66. doi: 10.1159/000354724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miettinen M, Lasota J. Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs) - a review. Int J Biochem Cell Biol. 2014;53:514–519. doi: 10.1016/j.biocel.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, George S, Trent JC, von Mehren M, Wright JA, Schiffman JD, Raygada M, Pacak K, Meltzer PS, Miettinen MM, Stratakis C, Janeway KA, Helman LJ. Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: a report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016;2(7):922–928. doi: 10.1001/jamaoncol.2016.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyler R, Davies E, Tan D, Hodson J, Taniere P, Thway K, Jafri M, Almond M, Ford S, Strauss D, Hayes A, Smith M, Desai A. Tumor necrosis is significantly associated with reduced recurrence-free survival after curative resection of gastrointestinal stromal tumors. J Surg Oncol. 2021;123(2):432–438. doi: 10.1002/jso.26294. [DOI] [PubMed] [Google Scholar]
- 8.Miyahira CK, Bonfitto M, de Lima Farto JF, de Figueiredo Calili A, da Silva Sousa NR, de Figueiredo Calili AP. Extragastrointestinal stromal tumor: a differential diagnosis of compressive upper abdominal tumor. Case Rep Surg. 2018;2018:1052960–1052960. doi: 10.1155/2018/1052960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kataoka TR, Yamashita N, Furuhata A, Hirata M, Ishida T, Nakamura I, Hirota S, Haga H, Katsuyama E. An inflammatory myofibroblastic tumor exhibiting immunoreactivity to KIT: a case report focusing on a diagnostic pitfall. World J Surg Oncol. 2014;12:186–186. doi: 10.1186/1477-7819-12-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawal G, Zaheer S, Ahluwalia C, Dhawan I. Malignant peripheral nerve sheath tumor of the transverse colon with peritoneal metastasis: a case report. J Med Case Rep. 2019;13(1):15–15. doi: 10.1186/s13256-018-1896-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riddle ND, Gonzalez RJ, Bridge JA, Antonia S, Bui MM. A CD117 and CD34 immunoreactive sarcoma masquerading as a gastrointestinal stromal tumor: diagnostic pitfalls of ancillary studies in sarcoma. Cancer Control. 2011;18(3):152–159. doi: 10.1177/107327481101800302. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher CDM, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33(5):459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 13.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7(6):507–519. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 15.Du CY, Shi YQ, Zhou Y, Fu H, Zhao G. The analysis of status and clinical implication of KIT and PDGFRA mutations in gastrointestinal stromal tumor (GIST) J Surg Oncol. 2008;98(3):175–178. doi: 10.1002/jso.21104. [DOI] [PubMed] [Google Scholar]
- 16.Hatipoğlu E. Extragastrointestinal stromal tumor (EGIST): a 16-year experience of 13 cases diagnosed at a single center. Med Sci Monit. 2018;24:3301–3306. doi: 10.12659/MSM.907654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goukassian ID, Kussman SR, Toribio Y, Rosen JE. Secondary recurrent multiple EGIST of the mesentery: a case report and review of the literature. Int J Surg Case Rep. 2012;3(9):463–466. doi: 10.1016/j.ijscr.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanamori K, Yamagata Y, Honma Y, Date K, Wada T, Hayashi T, Otsuki S, Sekine S, Yoshikawa T, Katai H, Nishida T. Extra-gastrointestinal stromal tumor arising in the lesser omentum with a platelet-derived growth factor receptor alpha (PDGFRA) mutation: a case report and literature review. World J Surg Oncol. 2020;18(1):183–183. doi: 10.1186/s12957-020-01961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu W, Zheng C, Li R, Feng X, Zheng G, Zheng Z, Xiong W, Lin G, Zhou Y, Wang W, Zhao Y, Li Y. Retroperitoneal extragastrointestinal stromal tumors have a poor survival outcome: a multicenter observational study. Cancer Manag Res. 2020;12:10491–10504. doi: 10.2147/CMAR.S278612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiq MA, East D, Hock YL, Warfield AT. Gastrointestinal stromal tumour of the pharynx. J Laryngol Otol. 2004;118(4):315–316. doi: 10.1258/002221504323012120. [DOI] [PubMed] [Google Scholar]
- 21.Park JK, Choi SH, Lee S, Min KO, Yun SS, Jeon HM. Malignant gastrointestinal stromal tumor of the gallbladder. J Korean Med Sci. 2004;19(5):763–767. doi: 10.3346/jkms.2004.19.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X, Forster J, Damjanov I. Primary malignant gastrointestinal stromal tumor of the liver. Arch Pathol Lab Med. 2003;127(12):1606–1608. doi: 10.5858/2003-127-1606-PMGSTO. [DOI] [PubMed] [Google Scholar]
- 23.Lee YH, Chong GO, Hong DG. Is gastrointestinal stromal tumor (GIST) originating from the rectovaginal septum GIST or extra-GIST (EGIST)? A case report with literature review. Eur J Gynaecol Oncol. 2015;36(6):750–754. [PubMed] [Google Scholar]
- 24.Elgeidie A, El-Magd ESA, El-Maaty SRA, El-Hawary AK. Pancreatic gastrointestinal stromal tumor: a case report. Int J Surg Case Rep. 2016;29:67–70. doi: 10.1016/j.ijscr.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster R, Solano S, Mahoney J, Fuller A, Oliva E, Seiden MV. Reclassification of a tubal leiomyosarcoma as an eGIST by molecular evaluation of c-KIT. Gynecol Oncol. 2006;101(2):363–366. doi: 10.1016/j.ygyno.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Zheng S, Chen LR, Wang HJ, Chen SZ. Analysis of mutation and expression of c-kit and PDGFR-alpha gene in gastrointestinal stromal tumor. Hepatogastroenterology. 2007;54(80):2285–2290. [PubMed] [Google Scholar]
- 27.Peterson MR, Piao Z, Weidner N, Yi ES. Strong PDGFRA positivity is seen in GISTs but not in other intra-abdominal mesenchymal tumors: immunohistochemical and mutational analyses. Appl Immunohistochem Mol Morphol. 2006;14(4):390–396. doi: 10.1097/01.pai.0000203038.33414.a3. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt AC, Popp AC, Cohen C, Lawson D, Siddiqui MT. Differential expression of two different DOG-1 antibodies: utility in detecting gastrointestinal stromal tumors. J Histotechnol. 2010;33(2):71–75. https://www.tandfonline.com/doi/abs/10.1179/his.2010.33.2.71 [Google Scholar]
- 29.Lin F, Liu H. Immunohistochemistry in undifferentiated neoplasm/tumor of uncertain origin. Arch Pathol Lab Med. 2014;138(12):1583–1610. doi: 10.5858/arpa.2014-0061-RA. [DOI] [PubMed] [Google Scholar]
- 30.Ali L, Moldovan V, Derewicz D, Ginghina O, Sajin M, Costache M. Metastatic melanoma presenting as undifferentiated tumour of unknown primary site: an immunohistochemical algorithm. Arch Balk Med Union. 2019;54(4):672–679. https://umbalk.org/metastatic-melanoma-presenting-as-undifferentiated-tumour-of-unknown-primary-site-an-immunohistochemical-algorithm/ [Google Scholar]
- 31.Omholt K, Grafström E, Kanter-Lewensohn L, Hansson J, Ragnarsson-Olding BK. KIT pathway alterations in mucosal melanomas of the vulva and other sites. Clin Cancer Res. 2011;17(12):3933–3942. doi: 10.1158/1078-0432.CCR-10-2917. [DOI] [PubMed] [Google Scholar]
- 32.Lyu J, Wu Y, Li C, Wang R, Song H, Ren G, Guo W. Mutation scanning of BRAF, NRAS, KIT, and GNAQ/GNA11 in oral mucosal melanoma: a study of 57 cases. J Oral Pathol Med. 2016;45(4):295–301. doi: 10.1111/jop.12358. [DOI] [PubMed] [Google Scholar]
- 33.Wronski M, Ziarkiewicz-Wroblewska B, Slodkowski M, Cebulski W, Gornicka B, Krasnodebski IW. Mesenteric fibromatosis with intestinal involvement mimicking a gastrointestinal stromal tumour. Radiol Oncol. 2011;45(1):59–63. doi: 10.2478/v10019-010-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Göck T, Jones DT, Mawrin C, Schittenhelm J, Becker A, Heim S, Simon M, Herold-Mende C, Mechtersheimer G, Paulus W, König R, Wiestler OD, Pfister SM, von Deimling A. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2–STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013;125(5):651–658. doi: 10.1007/s00401-013-1117-6. [DOI] [PubMed] [Google Scholar]
- 35.Sui XL, Wang H, Sun XW. Expression of DOG1, CD117 and PDGFRA in gastrointestinal stromal tumors and correlations with clinicopathology. Asian Pac J Cancer Prev. 2012;13(4):1389–1393. doi: 10.7314/apjcp.2012.13.4.1389. [DOI] [PubMed] [Google Scholar]
- 36.Sai S, Imamura Y, Kiyota N, Jimbo N, Toyoda M, Funakoshi Y, Chayahara N, Hyogo Y, Takenaka K, Suto H, Minami H. Relationship between PDGFR expression and the response to Pazopanib in intimal sarcoma of the pulmonary artery: a case report. Mol Clin Oncol. 2021;14(1):6–6. doi: 10.3892/mco.2020.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]