Abstract
Background: The specific mechanism of action of each anesthetic drug on the immune system is still incompletely known. It is important to know how the various anesthetics used in minimally invasive surgery (MIS) act on the inflammatory response because the choice of the anesthetic agent can influence the patient’s immune system. Aim: Evaluation of the effect of anesthetic drugs used for total intravenous anesthesia (Propofol and Midazolam) on the inflammatory response after minimally invasive gynecological surgery. Patients, Materials and Methods: The inflammatory response in 20 female patients who underwent minimally invasive gynecological surgery under which intravenous anesthesia was performed. Depending on the combination of anesthetics used, we subdivided the study group into two groups, Group 1 consisting of the patients (n=10) who were given for total intravenous anesthesia, the combination with Midazolam+Fentanyl, and Group 2 (n=10) the patients who received the combination of Propofol+Fentanyl, respectively. Surgical interventional procedures included day surgery: diagnostic and operative hysteroscopy, endometrial ablation, surgical treatment of vulvar disorders. Serological profiling of patients was performed by dosing the serum concentration of nucleotide-binding domain (NOD) and leucine-rich repeat protein 3 (NLRP3) inflammasomes, interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), IL-10 before and two hours after the surgical procedure. Results: In our study, we found that in both groups of patients (Midazolam+Fentanyl – Group 1, Propofol+Fentanyl – Group 2), NLRP3 and cytokines concentrations in the serum were higher after MIS than those before MIS. Conclusions: It appears that both Midazolam and Fentanyl and Propofol and Fentanyl have an immunomodulatory action due to the anti-inflammatory effect of both anesthetics. Therefore, anesthesiologists must choose an anesthetic method that uses individualized anesthetic agents, depending on the patient’s immune status and disease.
Keywords: Propofol, Midazolam, minimally invasive surgery, cytokines, inflammatory response
⧉ Introduction
The beneficial or harmful effects of anesthetic drugs on the immune system have been known for over a century [1]. But, despite all the advances made in immunology and anesthesiology, the specific mechanism of action of each anesthetic drug on the immune system is still incompletely known. Anesthetic drugs act on each subpopulation of immune cells, inducing changes in neutrophils, mononuclear phagocytes, lymphocytes, natural killer (NK) cells, T-lymphocytes, B-lymphocytes, but also on cytokines.
Cytokine synthesis occurs through cellular activation, some being considered as regulators of innate immunity, others as regulators of the adaptive immune response, they act in postoperative immunosuppression when there is an imbalance between pro- and anti-inflammatory mediated responses [2].
It is important to know how the various anesthetics used in minimally invasive surgery (MIS) act on the inflammatory response because the choice of the anesthetic agent can influence the patient’s immune system. It should also be noted that surgical stress is added to the effect of anesthetic drugs. It induces, through the neuroendocrine system, as well as through activation/inhibition of proinflammatory and anti-inflammatory cytokines an immunosuppressive effect in the perioperative period [3].
Potential danger associated with this immunosuppression relate to an increased risk of infection and tumor metastasis (which should be considered when MIS in a malignant process), while the anti-inflammatory effects of some anesthetics may lead to benefits in the conditions in which we have systemic and local inflammation. Given that immune suppression and the presence of excessive inflammatory mediators expose the patient to several perioperative complications, anesthetists should select anesthetic techniques and anesthetic drugs, considering both the clinical situation and the patient’s immune status, morbidity, and optimal prognosis. Different anesthetic techniques can influence the stress response, especially by activating intraoperative and postoperative cytokines, with changes in postoperative evolution. But, for an immunocompetent patient, the immunosuppressive effect of anesthetic drugs should not have significant consequences, especially when it comes to MIS and short-lived surgery.
Propofol are the most commonly used anesthetic drugs for sedation, as well as for total intravenous anesthesia in minimally invasive gynecological surgery. Midazolam, a commonly used benzodiazepine, acts by regulating the proinflammatory function of macrophages [4]. As an anesthetic drug, Midazolam is used in premedication and procedural sedation. Midazolam can minimize the proinflammatory response to anesthesia [5]. It seems that Midazolam can induce apoptosis and can suppress the progression of cancer cells, having antitumorigenic properties, which should be considered in choosing the anesthetic drug [6].
Propofol is a frequently used intravenous anesthetic drug, causing a rapid induction of anesthesia, but also a rapid recovery after anesthesia. Information about the effect of Propofol on cytokines is still controversial, but most studies agree that this anesthetic drug has antioxidant and anti-inflammatory properties [7,8,9]. Opioids affect the synthesis of cytokines and immunoglobulins, as well as the activation of NK cells and phagocytosis, with immunomodulatory effects [10,11].
But most studies have found that synthetic opioids (Fentanyl, used in our study as a narcotic analgesic agent) used in general anesthesia, cause only temporary immunomodulatory effects [1,2, 12].
Studies in recent years have used frequently, as an index to assess the immune response in the inflammatory process, immunocytes. It has also been observed that different rates of immunocytes can be used to assess the extent of inflammation, such as neutrophil-to-lymphocyte (NLR) count ratio, platelet-to-lymphocyte (PltLR) count ratio and monocyte-to-lymphocyte (MLR) count ratio, as well as mean platelet volume-to-platelet (MPV/Plt) count ratio [13,14,15,16]. These indices are not only affected by surgical stress, but also by the type of used anesthesia [17].
Studies on the mechanisms involved in innate immunity have shown the involvement of multiprotein complexes (inflammasomes). Inflammasomes are protein complexes with key roles in intracellular signaling (mediation or even involvement in the transmission of intracellular signaling), as well as involvement in the activation of cysteine-dependent aspartate-directed protease-1 (caspase-1), which will lead to the conversion of inactive precursors of interleukin (IL)-1β (pro-IL-1β) and IL-18 (pro-IL-18), in active forms [18,19].
Aim
This study aimed to evaluate the effect of anesthetic drugs used for total intravenous anesthesia (Propofol and Midazolam) on the inflammatory response after minimally invasive gynecological surgery. For this purpose, the serum concentrations of IL-6 a proinflammatory cytokine, IL-10 an anti-inflammatory cytokine, tumor necrosis factor-alpha (TNF-α), the inflammasome nucleotide-binding domain (NOD) and leucine-rich repeat protein 3 (NLRP3) and the rates of immunocytes (NLR, PltLR and MLR) were investigated.
⧉ Patients, Materials and Methods
Subjects and clinical assessment
This prospective study included the evaluation through a panel of biomarkers of the evolution of the immune response in 20 female patients who underwent minimally invasive gynecological surgery under which intravenous anesthesia was performed.
The patients included in this study were diagnosed in the OpenMed Private Hospital and in the Department of Gynecology of the Filantropia Municipal Clinical Hospital, Craiova, Dolj County, Romania.
The introduction of the cases in the study was based on certain criteria. The inclusion criteria were over 18 years of age, obtaining informed consent to participate in the study, American Society of Anesthesiologists (ASA) grade I or II, operation duration less than 60 minutes, the type of intervention to be minimally invasive.
Exclusion criteria were predicted duration of a surgery over 60 minutes, allergy to anesthetic drugs used, possible alteration of the immune response by the presence of genetic diseases, liver or kidney diseases, endocrinopathy, diabetes mellitus, morbid obesity, analgesic or immunosuppressive therapy with any indication, the patient’s refusal to sign informed consent.
Depending on the combination of anesthetics used, we subdivided the study group into two groups, Group 1 consisting of the patients (n=10) who were given for total intravenous anesthesia, the combination with Midazolam+Fentanyl, and Group 2 (n=10) the patients who received the combination of Propofol+Fentanyl, respectively.
Group 1 anesthesia was induced by administration of Fentanyl 3 μg/kg, three minutes before the administration of Midazolam, then Midazolam – a target-controlled infusion 0.15–0.2 mg/kg, continued with 0.03–0.1 mg/kg to maintain the effect.
Group 2 anesthesia was induced by administration of Fentanyl 3 μg/kg, three minutes before the administration of Propofol, then Propofol – a target-controlled infusion of Propofol 1.5 mg/kg/h to 4.5 mg/kg/h, then 0.1–0.2 mg/kg/min to maintain the effect. Spontaneous respiration, administration of 98% O2 on the nasal cannula, 4 L/min.
Surgical interventional procedures included day surgery: diagnostic and operative hysteroscopy, endometrial ablation. Even if we cannot eliminate surgery stress to have only the effect of anesthetics on the inflammatory response, we wanted to introduce in the study only the cases subjected to MIS with as little tissue trauma as possible.
We performed a histopathological (HP) study on endometrial fragments obtained during the hysteroscopy and endometrial ablation procedure. Depending on the results obtained, we classified the lesions in simple and complex endometrial hyperplasia, with and without atypia and in endometrial carcinoma. HP aspects were compared and interpreted with the other investigations, to follow a possible association between systemic inflammation and local inflammation.
Intraoperative monitoring was standard, with electrocardiogram, peripheral oxygen saturation (SpO2), noninvasive arterial pressure.
Sample collection
Blood samples were obtained before anesthetic induction and two hours after the end of the surgical procedure, from all subjects into tubes without additives (the vacutainer from Becton Dickinson) by venous approach, in the morning before meals. The tubes were kept in upright positions for 30 minutes at room temperature (RT) to allow clot to form, then centrifuged at 3000×g for 10 minutes. After the clot was removed, the sera were distributed in several cryotubes and stored at temperatures below -20°C until evaluation. At the time of processing the samples, we left the cryotubes to accommodate at RT, not allowing the remaining samples to re-freeze.
Peripheral venous blood was collected into separator vacutainers with Ethylenediaminetetraacetic acid (EDTA) anticoagulant and was used to perform complete blood counting (CBC) cells: neutrophils, basophils, eosinophils, lymphocytes, monocytes, red blood cells, platelets.
Immunological investigations
Immunological investigations were performed with the support of the Department of Immunology, University of Medicine and Pharmacy of Craiova. The technique used was represented by enzyme-linked immunosorbent assay (ELISA), the sandwich variant, quantitative, respecting the working instructions specified in the kits by the manufacturer. The ELISA method was performed with a standard optical analyzer, at 450 nm wavelength.
Specifically-designed commercial test kits were used for each of the mediators: NLRP3 (Cat# OKEH03368, assay range: 0.312–20 ng/mL) – Aviva Systems Biology, San Diego, USA; TNF-α (Cat# BMS223-4, assay range: 7.8–500 pg/mL), IL-6 (Cat# BMS213-2, assay range: 1.56–100 pg/mL), IL-10 (Cat# BMS215-2, assay range: 3.15–200 pg/mL) – Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA.
HP investigations
Tissue fragments were fixed in 10% neutral buffered formalin for 24 hours before routine embedding in paraffin. For fixation, the tissue fragments were cut into the multiple longitudinal slices of 1–2 cm and kept in 10% neutral buffered formalin for several days. After fixation, tissue samples were taken from representative areas. For the HP study, there were used the Hematoxylin–Eosin (HE).
Complete blood counting (CBC)
To differentiate and count the neutrophils, basophils, eosinophils, lymphocytes, monocytes, red blood cells and platelets, we used an automatic hematology analyzer, able to give us an extended leukocyte formula 5 diff, by flow cytometry and Coulter’s principle. Using these determinations, we calculated the different rates of immunocytes: NLR, MLR and PltLR.
Ethical issue
All stages of our study were conducted in accordance with both the ethical standards for human studies recommended by the responsible institutional committees and the Helsinki Declaration of 1975, revised in 2008. Also, to carry out this project, we have the agreement of the Ethics, Academic and Scientific Deontology Committee of the University of Medicine and Pharmacy of Craiova (Approval No. 135/17.09.2021).
Statistical analysis
The patients’ information obtained from medical documents were uploaded and filtered, to be further statistically processed, in Microsoft Excel files (Data Analysis package). As a program dedicated to statistical processing, we used the free trial version of GraphPad Prism 5. Significant differences between the means of the parameters investigated in the groups included in the study were analyzed with the Mann–Whitney U-test (for the analysis of two differences) and the Kruskal–Wallis test (for the analysis of several differences). The values of the tests that have p≤0.05 were considered statistically significant, the tests were on two-sided.
⧉ Results
Clinical features of the enrolled patients
The two groups of patients were similar, the demographic data being homogeneous, depending on age or urban/rural areas, we did not register statistically significant differences (Table 1).
Table 1.
Demographics and clinical characteristics of enrolled patients
|
Characteristics |
Midazolam+Fentanyl Group 1 (n=10) |
Propofol+Fentanyl Group 2 (n=10) |
|
Age (years) (mean ± SD) |
42.89±7.52 |
42.67±6.65 |
|
BMI (kg/m2) (mean ± SD) |
25.70±2.07 |
26.20±2.04 |
|
Urban/rural areas, n |
5/4 |
4/5 |
|
ASA grade, n (%): |
|
|
|
▪ ASA I |
7 (70%) |
8 (80%) |
|
▪ ASA II |
3 (30%) |
2 (20%) |
|
HP lesions, n (%): |
|
|
|
▪ Endometrial hyperplasia without atypia |
5 (50%) |
6 (60%) |
|
▪ Endometrial hyperplasia with atypia |
4 (40%) |
3 (30%) |
|
▪ Endometrioid endometrial carcinoma |
1 (10%) |
1 (10%) |
ASA: American Society of Anesthesiologists; BMI: Body mass index; HP: Histopathological; n: No. of cases; SD: Standard deviation
Evaluating the medical comorbidities of the patients before the anesthesia, by using the ASA Physical Status Classification level, we noticed that in both analyzed groups ASA I predominated (Group 1 – seven cases, 70%; Group 2 – eight cases, 80%).
Depending on the minimally invasive gynecological surgery, in Group 1 (the patients who received the combination of Midazolam+Fentanyl), in most cases (eight cases, 80%) were performed the diagnosis and operative hysteroscopy (four cases, 40%) and the surgical treatment of vulvar disorders (four cases, 40%), respectively. Instead, patients from Group 2 (the patients who received the combination of Propofol+Fentanyl) benefited from the diagnosis and operative hysteroscopy in 60% of the cases.
Analyzing the mean rates of immunocytes, we did not find statistically significant differences in the two investigated groups. The combinations used, Midazolam+Fentanyl and Propofol+Fentanyl, respectively, did not cause statistically significant changes in these rates of immunocytes after performing minimally invasive gynecological surgery.
In Midazolam+Fentanyl group, when we compared the mean rates of immunocytes between after performing the MIS vs before performing MIS, we obtained: NLR (2.49±0.27 vs 2.40±0.32, p=0.622), PltLR (123.00±50.85 vs 126.00±54.98, p=1.000), MLR (0.21±0.04 vs 0.19±0.11, p=0.342) (Table 2). And in the Propofol+Fentanyl group, when comparing the mean of the immunocytes rates, after performing the MIS vs before performing the MIS, we found: NLR (2.29±0.73 vs 1.98±0.87, p=0.426), PltLR (152.40±51.13 vs 127.90±68.93, p=0.472), MLR (0.24±0.07 vs 0.20±0.08, p=0.141).
Table 2.
The mean rates of immunocytes in the two investigated groups
|
Characteristics |
Midazolam+Fentanyl Group 1 (n=10) |
p-value |
Propofol+Fentanyl Group 2 (n=10) |
p-value |
||
|
Before MIS |
After MIS |
Before MIS |
After MIS |
|||
|
Mean ± SD |
Mean ± SD |
Mean ± SD |
Mean ± SD |
|||
|
NLR |
2.49±0.27 |
2.40±0.32 |
0.622 |
2.29±0.73 |
1.98±0.87 |
0.426 |
|
PltLR |
123.00±50.85 |
126.00±54.98 |
1.000 |
152.40±51.13 |
127.90±68.93 |
0.472 |
|
MLR |
0.21±0.04 |
0.19±0.11 |
0.342 |
0.24±0.07 |
0.20±0.08 |
0.141 |
MIS: Minimally invasive surgery; MLR: Monocyte-to-lymphocyte count ratio; NLR: Neutrophil-to-lymphocyte count ratio; PltLR: Platelet-to-lymphocyte count ratio; SD: Standard deviation
HP analysis
HP analysis of the obtained tissue fragments revealed HP aspects characteristic for endometrial hyperplasia. But the correlation with non-specific inflammatory indices showed that local inflammatory changes, manifested by the more or less frequent presence of lymphocytes on HP preparations, are not expressed in the serum values of these inflammatory markers (Figures 1,2,3,4,5).
Figure 1.

Simple endometrial hyperplasia without atypia with moderate diffuse lymphocytic infiltrate. Hematoxylin–Eosin (HE) staining, ×200
Figure 2.
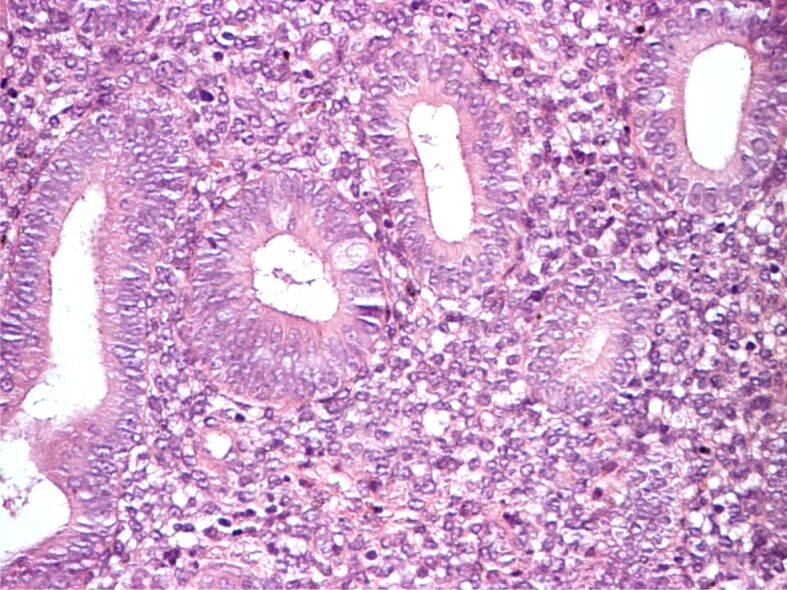
Simple endometrial hyperplasia with atypia. HE staining, ×200
Figure 3.
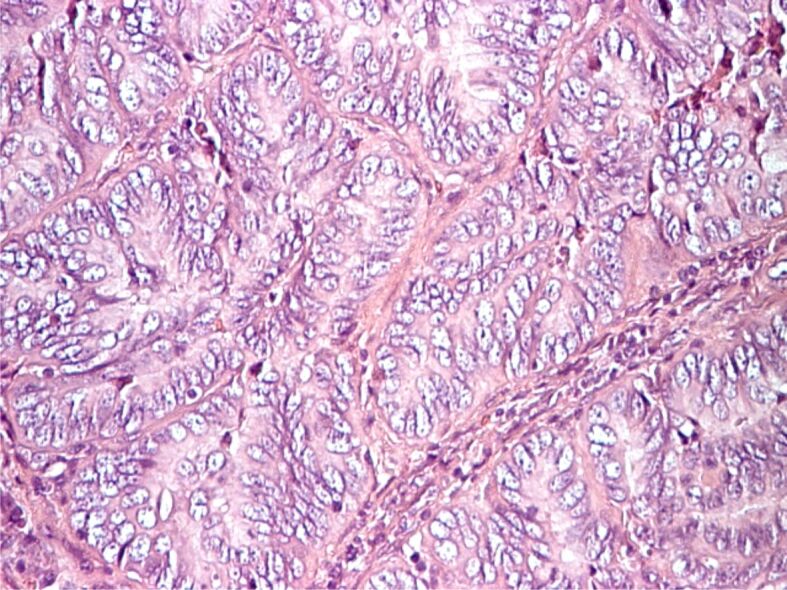
Complex endometrial hyperplasia without atypia, periglandular lymphocyte band. HE staining, ×200
Figure 4.

Complex endometrial hyperplasia with atypia, periglandular lymphocytic infiltrates with occasional intraepithelial extension. HE staining, ×200
Figure 5.
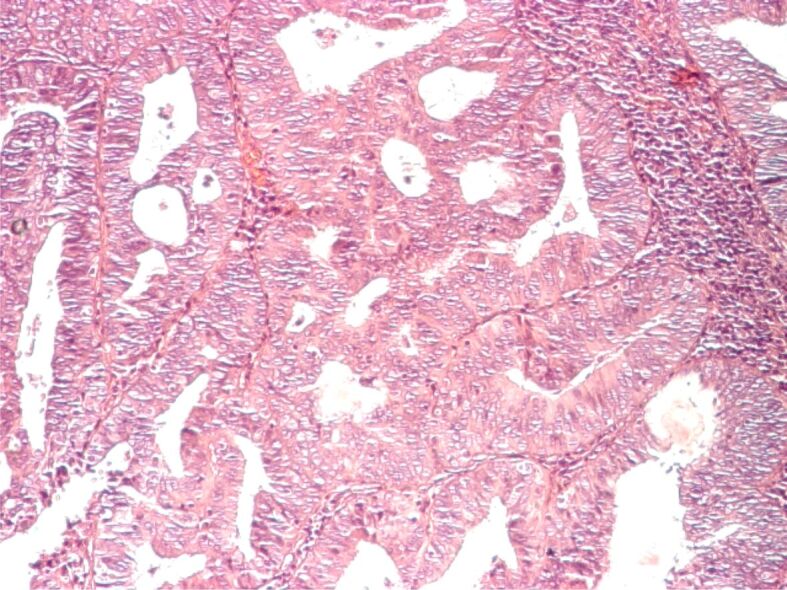
Well-differentiated endometrioid adenocarcinoma, rich periglandular lymphocytic infiltrate. HE staining, ×100
NLRP3 and cytokines concentrations
In our study, we found that in both groups of patients (Midazolam+Fentanyl – Group 1, Propofol+Fentanyl – Group 2), NLRP3 and cytokines concentrations in the serum were higher after MIS than those before MIS (Tables 3 and 4).
Table 3.
NLRP3 and cytokines concentrations in the serum of patients for Group 1, the patients who received the combination Midazolam+Fentanyl
|
Parameter |
Midazolam+Fentanyl Group 1 (n=10) |
||||
|
Before MIS |
After MIS |
p-value |
|||
|
Mean ± SD |
95% CI |
Mean ± SD |
95% CI |
||
|
NLRP3 [ng/mL] |
13.74±2.35 |
12.06–15.42 |
16.93±3.17 |
14.66–19.19 |
0.049* |
|
TNF-α [pg/mL] |
15.77±4.12 |
12.82–18.71 |
19.54±3.54 |
17.10–22.07 |
0.044* |
|
IL-6 [pg/mL] |
11.63±3.75 |
8.95–14.31 |
13.13±3.72 |
10.46–15.79 |
0.436 |
|
IL-10 [pg/mL] |
18.12±3.12 |
15.89–20.35 |
21.78±3.50 |
19.28–24.29 |
0.035* |
CI: Confidence interval; IL: Interleukin; MIS: Minimally invasive surgery; NLRP3: Nucleotide-binding domain (NOD) and leucine-rich repeat protein 3; SD: Standard deviation; TNF-α: Tumor necrosis factor-alpha. *Statistically significant
Table 4.
NLRP3 and cytokines concentrations in the serum of patients for Group 2, the patients who received the combination Propofol+Fentanyl
|
Parameter |
Propofol+Fentanyl Group 2 (n=10) |
||||
|
Before MIS |
After MIS |
p-value |
|||
|
Mean ± SD |
95% CI |
Mean ± SD |
95% CI |
||
|
NLRP3 [ng/mL] |
14.82±1.54 |
13.72–15.91 |
16.11±1.54 |
15.00–17.21 |
0.037* |
|
TNF-α [pg/mL] |
12.83±4.79 |
9.39–16.26 |
16.43±4.66 |
13.09–19.76 |
0.089 |
|
IL-6 [pg/mL] |
12.34±4.51 |
9.11–15.56 |
14.89±4.12 |
11.94–17.84 |
0.143 |
|
IL-10 [pg/mL] |
14.58±3.00 |
12.43–16.73 |
17.37±2.89 |
15.30–19.45 |
0.043* |
CI: Confidence interval; IL: Interleukin; MIS: Minimally invasive surgery; NLRP3: Nucleotide-binding domain (NOD) and leucine-rich repeat protein 3; SD: Standard deviation; TNF-α: Tumor necrosis factor-alpha. *Statistically significant
Analyzing the results obtained for Group 1, the patients who received the combination Midazolam+Fentanyl, we observed that the serum concentrations of NLRP3, TNF-α and IL-10 have higher values, statistically significant differences, after MIS than those determined before this: NLRP3 [16.93 ng/mL, 95% confidence interval (CI): 14.66–19.19, p=0.049], TNF-α (19.54 pg/mL, 95% CI: 17.10–22.07, p=0.044), and IL-10 (21.78 pg/mL, 95% CI: 19.28–24.29, p=0.035) (Table 3).
In the analysis of Group 2 (the patients who received the combination of Propofol+Fentanyl), we found that only the serum concentrations of NLRP3 (16.11 ng/mL, 95% CI: 15.00–17.21, p=0.037) and IL-10 (17.37 pg/mL, 95% CI: 15.30–19.45, p=0.043), have statistically significant differences, after performing the MIS vs before performing MIS (Table 4).
In Figures 6,7,8,9, we highlighted the NLRP3, TNF-α, IL-6, and IL-10 concentrations in serum of patients who underwent MIS, in the two groups enrolled in the study.
Figure 6.
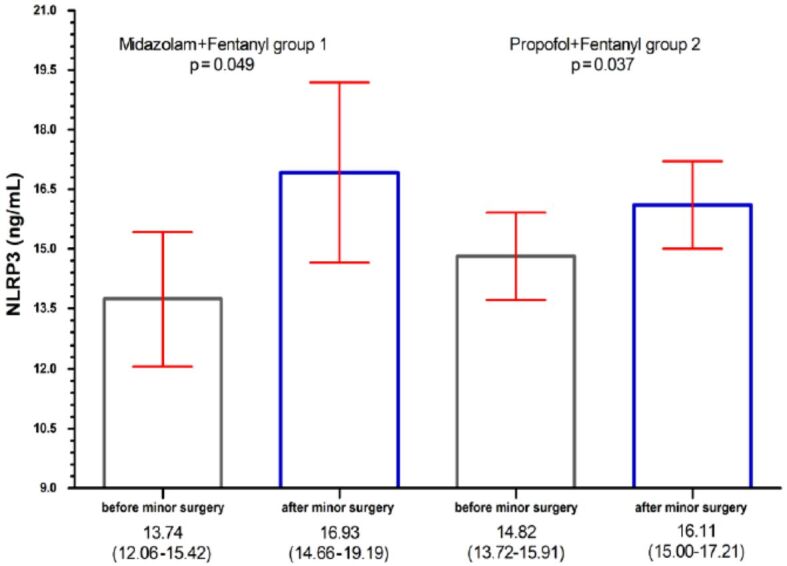
NLRP3 concentrations in serum of patients who underwent minimally invasive surgery: bar per column represent the concentrations of NLRP3 [ng/mL]; red horizontal lines represent the 95% confidence interval (CI). NLRP3: Nucleotide-binding domain (NOD) and leucine-rich repeat protein 3
Figure 7.

TNF-α concentrations in serum of patients who underwent minimally invasive surgery: bar per column represent the concentrations of TNF-α [pg/mL]; red horizontal lines represent the 95% confidence interval (CI). TNF-α: Tumor necrosis factor-alpha
Figure 8.
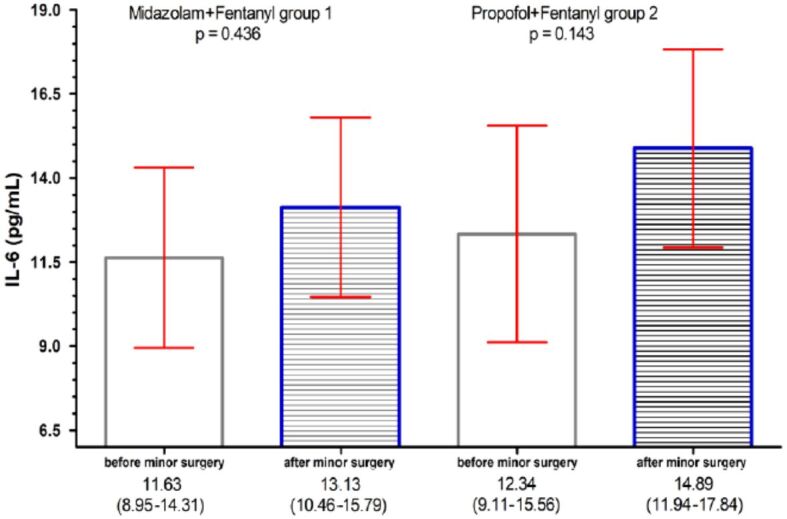
IL-6 concentrations in serum of patients who underwent minimally invasive surgery: bar per column represent the concentrations of IL-6 [pg/mL]; red horizontal lines represent the 95% confidence interval (CI). IL-6: Interleukin-6
Figure 9.
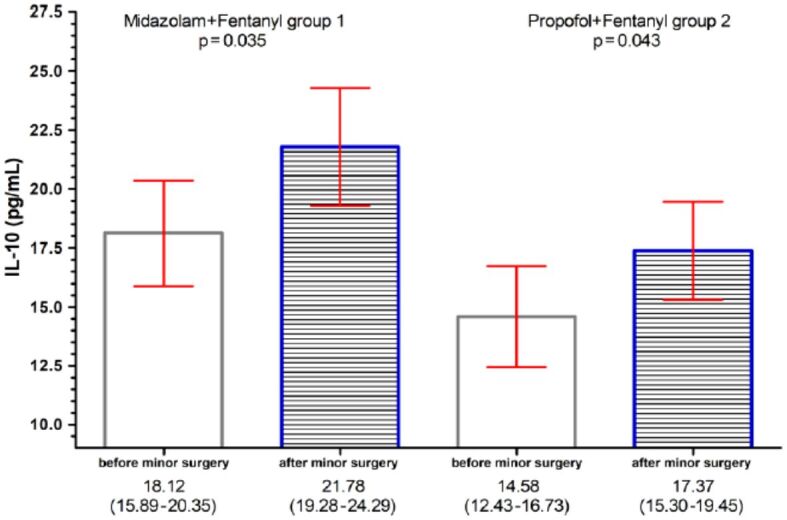
IL-10 concentrations in serum of patients who underwent minimally invasive surgery: bar per column represent the concentrations of IL-10 [pg/mL]; red horizontal lines represent the 95% confidence interval (CI). IL-10: Interleukin-10
⧉ Discussions
In our study, we wanted to see what is the effect of general total anesthesia with Propofol and Midazolam on the inflammatory response, only in minimally invasive gynecological interventions, in day surgery. Even in this type of intervention, surgical stress cannot be eliminated, as it is involved in increasing the tendency towards inflammation and reducing the number of lymphocytes in the post-intervention period, increases the chance of developing infections [20].
Published studies have shown that NLR and PltLR can be considered reliable markers of systemic inflammation [21]. Studies to date have investigated more types of anesthetic agents, finding that total intravenous anesthesia influences less serum growth of immune mediators than other types of anesthesia [22]. Maybe that’s why in our study analyzing mean rates of immunocytes we did not find statistically significant differences depending on the type of anesthetic used in minimally invasive gynecological surgery.
Kubyshkin et al. showed the role that the inflammatory process has in the progression of the degree of endometrial hyperplasia, the inflammatory changes being more accentuated in the presence of the malignant transformation of the endometrial hyperplasia with atypia [23]. It has also been shown that inflammatory markers have higher values in endometrial hyperplasia with atypia than in those without atypia [24]. But in our study, we did not find an accord between local and systemic inflammatory changes.
Surgical stress can cause an immunosuppressive status, on which anesthetics and analgesic drugs act by directly affecting immunocompetent cells, by increasing the inflammatory response [25].
Activation of inflammatory mediators is also done by inflammasomes, which are an intracellular multiprotein complex known as a modulator of inflammation [26].
We observed that the serum concentration of NLRP3 inflammasomes in the study performed, in both groups of patients (Midazolam+Fentanyl and Propofol+Fentanyl), were higher after a MIS than before. The increase of NLRP3 inflammasome in both groups of anesthetics used shows that there is still a high inflammatory response during MIS.
NLRP3 inflammasome is structured by NLRP3 protein, procaspase-1 and another protein that has a domain in which it recruits caspase and is associated with apoptosis, called ASC [27]. Procaspase-1, the inactive form, is activated in caspase-1, which is responsible for activating the inactive forms of IL-1β (pro-IL-1β) and IL-18 (pro-IL-18) in their active, mature forms, which trigger the inflammatory response [28]. As Kelley et al. [29], the NLRP3 inflammasome can be activated by a multitude of stimuli, the activation of NLRP3 inflammasomes being a mechanism of self-defense against invasive factors and stress. The activation of NLRP3 results in the production of proinflammatory cytokines, cytokines having a key role in the action of anesthetic drugs and for systemic inflammatory responses [30].
But how NLRP3 responds to signaling stimuli and how NLRP3 inflammasome formation is initiated is not fully understood to date. Ma et al. [31], in an animal study, founded that in the case of neuroinflammation, when there is excessive activation of NLRP3 in the cerebral cortex, Propofol can reduce the inflammatory response, thus reducing brain damage. Midazolam also decreases NLRP3-mediated proinflammatory cytokines secretion, according to the study of Feng et al. [32].
The immune response is affected in anesthesia by inhibiting/releasing cytokines that can affect the inflammatory response, along with the body’s metabolic and immune response to surgical stress, influencing the profile of inflammatory factors and especially of cytokines [12, 33,34,35].
The increase in serum concentration of NLRP3 in both groups studied should be reflected by the increase in the proinflammatory cytokines IL-6, TNF-α, cytokines studied by us in both groups, Midazolam anesthesia, and Propofol anesthesia. But the results of our study showed that only in the case of the Midazolam group, the proinflammatory proteins TNF-α, not IL-6, showed a statistically significant increase, while in the Propofol group we did not have this increase.
Serum vascular endothelial growth factor (VEGF) has a strong angiogenic effect, this effect causing neovascularization and endothelial regeneration induced by ischemia. VEGF promotes angiogenesis in neoplasms and has an important role in pathogenesis, progression and neoplastic metastasis [36,37,38]. Low levels of VEGF in neoplasm may reduce the risk of invasion, proliferation and metastasis [39,40].
These results are partially in contradiction with the studies of Helmy & Al-Attiyah [41], Lu et al. [42], Xiao et al. [43], which show that Midazolam significantly reduces the level of proinflammatory cytokines after administration.
A recent 2021 study by Lotfy et al. [5] on a population of pediatric patients with intra-abdominal infection showed that the infusion of Midazolam and not Propofol decreases serum cytokine levels. A series of studies by Lisowska et al. [9] and Marik [44] have shown that Propofol decreases serum concentrations of IL-1, TNF-α, and IL-6 and increases the concentration of IL-10, so this anesthetic drug may have antioxidant and anti-inflammatory properties.
This situation was encountered in the study, because in the Propofol group we did not find statistically significant serum values of the TNF-α, and IL-6. In the study of Ke et al. [45], focused on the comparison between total anesthesia with Propofol vs inhalational technique, it has been shown that in the case of total anesthesia with Propofol, IL-6 and TNF-α on the one hand but also IL-10 showed significantly elevated plasma concentrations during surgery. But, at the end of the intervention, the plasma values of IL-6, TNF-α decreased considerably, while the level of IL-10 remained at high values in the anesthetic technique with Propofol compared to the inhalation technique. It has been suggested that total intravenous anesthesia with Propofol and Remifentanil inhibits the inflammatory response. This finding is in contradiction with the study of Mazoti et al. [46] who found no difference in plasma cytokine profiles in Isoflurane vs Propofol anesthesia in patients who underwent minimally invasive procedures.
On the other hand, the study conducted by Fekkes et al. [47] shows that an increase in IL-6 during surgery could be determined by the duration and extent of the surgical procedure so that Propofol would not influence the IL-6 response.
However, the increase in serum IL-10 in both the Midazolam group and the Propofol group shows that the two anesthetics confer an anti-inflammatory effect.
Research in recent years, included more and more, investigation of NLR, MLR, PltLR ratio and systemic immune-inflammation index (SII=NLR×platelets) as new biological markers, useful in assessing the inflammatory condition of the organism, but also the possibility of assessing the activity of the disease. Several scientific reports have highlighted the usefulness of NLR and PltR as important non-invasive tests in monitoring immune status in patients with autoimmune diseases, neoplasms, rheumatoid arthritis, osteoarthritis, myocardial infarction [48,49,50,51,52,53,54,55].
We also set out in this study to investigate whether these ratios change before or after the minimally invasive intervention are performed, or if after administration of the two combinations of anesthetics (Midazolam+Fentanyl and Propofol+Fentanyl) significant changes occur. We observed that in both groups of patients (Midazolam+Fentanyl and Propofol+Fentanyl), did not encounter statistically significant differences after performing the MIS vs before performing MIS.
Given that in the study we collected data from an only healthcare provider, that we have a small sample size group of patients, the retrospective and descriptive nature of the study, makes this study have limitations and the results highlighted in our study do not have a statistically significant power. Additional studies are needed in which to reproduce the results obtained on a larger population sample, both in our reference center and at national level in the other university centers. It is difficult to perform a systemic comparative analysis due to the heterogeneity of the type of parameters studied: surgical mode, pediatric or adult populations, the type of disease for which the surgical procedure was performed, the type of hospitalization, in vitro or in vivo research, etc. Therefore, the results obtained by us in the performed study are valid only for the minimally invasive gynecological surgery studied, having no other research focused on this type of surgical procedure.
⧉ Conclusions
It has long been suspected that anesthetic drugs may affect the functionality of the immune system, but these effects are only minor and temporary in a patient with a healthy immune system. It appears that both Midazolam+Fentanyl and Propofol+Fentanyl groups have an immunomodulatory action due to the anti-inflammatory effect of both anesthetics. Therefore, anesthesiologists must choose an anesthetic method that uses individualized anesthetic agents, depending on the patient’s immune status and disease.
Conflict of interest
The authors declare that they have no conflict of interests.
Authors’ contribution
Anda Lorena Dijmărescu and Marius Bogdan Novac equally contributed to the manuscript.
Acknowledgments
This work was supported by the S.C. Open Medical S.R.L., Craiova, grant of the University of Medicine and Pharmacy of Craiova, Romania, Project No. 26/35C/14.09.2021.
References
- 1.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22(3):263–277. doi: 10.1007/s00540-008-0626-2. [DOI] [PubMed] [Google Scholar]
- 2.Colucci DG, Puig NR, Hernandez-Pando R. Influence of anaesthetic drugs on immune response: from inflammation to immunosuppression. OA Anaesthetics. 2013;1(3):21–21. https://www.oapublishinglondon.com/article/1091# [Google Scholar]
- 3.Cruz FF, Rocco PRM, Pelosi P. Anti-inflammatory properties of anesthetic agents. Crit Care. 2017;21(1):67–67. doi: 10.1186/s13054-017-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Memiş D, Hekimoğlu S, Vatan İ, Yandım T, Yüksel M, Süt N. Effects of Midazolam and Dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth. 2007;98(4):550–552. doi: 10.1093/bja/aem017. [DOI] [PubMed] [Google Scholar]
- 5.Lotfy MA, Ayaad MG, Elsawaf MI, Atyia GF. Continuous Midazolam infusion can minimize the proinflammatory response to anesthesia and surgery for pediatric patients with intra-abdominal infection: comparative study versus continuous Propofol infusion. Egypt J Anaesth. 2021;37(1):337–342. https://www.tandfonline.com/doi/full/10.1080/11101849.2021.1955532 [Google Scholar]
- 6.Wang C, Datoo T, Zhao H, Wu L, Date A, Jiang C, Sanders RD, Wang G, Bevan C, Ma D. Midazolam and Dexmedetomidine affect neuroglioma and lung carcinoma cell biology in vitro and in vivo. Anesthesiology. 2018;129(5):1000–1014. doi: 10.1097/ALN.0000000000002401. [DOI] [PubMed] [Google Scholar]
- 7.Hu XL, Tang HH, Zhou ZG, Yin F, Liu WJ. The effect of Sevoflurane inhalation anesthesia only and Propofol total intravenous anesthesia on perioperative cytokine balance in lung cancer patients] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011;27(6):659–661. [PubMed] [Google Scholar]
- 8.González-Correa JA, Cruz-Andreotti E, Arrebola MM, López-Villodres JA, Jódar M, De La Cruz JP. Effects of Propofol on the leukocyte nitric oxide pathway: in vitro and ex vivo studies in surgical patients. Naunyn Schmiedebergs Arch Pharmacol. 2008;376(5):331–339. doi: 10.1007/s00210-007-0220-4. [DOI] [PubMed] [Google Scholar]
- 9.Lisowska B, Szymańska M, Nowacka E, Olszewska M. Anesthesiology and the cytokine network. Postepy Hig Med Dosw (Online) 2013;67:761–769. doi: 10.5604/17322693.1061412. [DOI] [PubMed] [Google Scholar]
- 10.Finley MJ, Happel CM, Kaminsky DE, Rogers TJ. Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell Immunol. 2008;252(1-2):146–154. doi: 10.1016/j.cellimm.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Hashimi M, Scott SWM, Thompson JP, Lambert DG. Opioids and immune modulation: more questions than answers. Br J Anaesth. 2013;111(1):80–88. doi: 10.1093/bja/aet153. [DOI] [PubMed] [Google Scholar]
- 12.Schneemilch CE, Schilling T, Bank U. Effects of general anaesthesia on inflammation. Best Pract Res Clin Anaesthesiol. 2004;18(3):493–507. doi: 10.1016/j.bpa.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Pantzaris ND, Platanaki C, Pierrako C, Karamouzos V, Velissaris D. Neutrophil-to-lymphocyte ratio relation to sepsis severity scores and inflammatory biomarkers in patients with community-acquired pneumonia: a case series. J Transl Int Med. 2018;6(1):43–46. doi: 10.2478/jtim-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elbaset MA, Zahran MH, Hashem A, Ghobrial FK, Elrefaie E, Badawy M, Shokeir AA, Ibrahim MA. Could platelet to leucocytic count ratio (PLR) predict sepsis and clinical outcomes in patients with emphysematous pyelonephritis. J Infect Chemother. 2019;25(10):791–796. doi: 10.1016/j.jiac.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Detournay O, Mazouz N, Goldman M, Toungouz M. IL-6 produced by type I IFN DC controls IFN-γ production during the MLR by blocking the suppressive effect of regulatory T cells. Blood. 2004;104(11):3797–3797. https://ashpublications.org/blood/article/104/11/3797/78038/IL-6-Produced-by-Type-I-IFN-DC-Controls-IFN [Google Scholar]
- 16.Djordjevic D, Rondovic G, Surbatovic M, Stanojevic I, Udovicic I, Andjelic T, Zeba S, Milosavljevic S, Stankovic N, Abazovic D, Jevdjic J, Vojvodic D. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically ill and injured patients: which ratio to choose to predict outcome and nature of bacteremia. Mediators Inflamm. 2018;2018:3758068–3758068. doi: 10.1155/2018/3758068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ní Eochagáin A, Burns D, Riedel B, Sessler DI, Buggy DJ. The effect of anaesthetic technique during primary breast cancer surgery on neutrophil–lymphocyte ratio, platelet–lymphocyte ratio and return to intended oncological therapy. Anaesthesia. 2018;73(5):603–611. doi: 10.1111/anae.14207. [DOI] [PubMed] [Google Scholar]
- 18.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 19.Boldeanu MV, Siloşi I, Bărbulescu AL, Sandu RE, Geormăneanu C, Pădureanu V, Popescu-Drigă MV, Poenariu IS, Siloşi CA, Ungureanu AM, Dijmărescu AL, Boldeanu L. Host immune response in chronic hepatitis C infection: involvement of cytokines and inflammasomes. Rom J Morphol Embryol. 2020;61(1):33–43. doi: 10.47162/RJME.61.1.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi J, Shono Y, Hirabayashi H, Kamimura M, Nakagawa H, Ebara S, Kato H. Usefulness of white blood cell differential for early diagnosis of surgical wound infection following spinal instrumentation surgery. Spine (Phila Pa 1976) 2006;31(9):1020–1025. doi: 10.1097/01.brs.0000214895.67956.60. [DOI] [PubMed] [Google Scholar]
- 21.Bingöl Tanrıverdi T, Tercan M, Güsun Halitoğlu A, Kaya A, Patmano G. Comparison of the effects of low-flow and normal-flow Desflurane anaesthesia on inflammatory parameters in patients undergoing laparoscopic cholecystectomy. Turk J Anaesthesiol Reanim. 2021;49(1):18–24. doi: 10.5152/TJAR.2020.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng L, Hagan KB, Villarreal J, Keerty V, Chen J, Cata JP. Scalp block for glioblastoma surgery is associated with lower inflammatory scores and improved survival. Minerva Anestesiol. 2017;83(11):1137–1145. doi: 10.23736/S0375-9393.17.11881-X. [DOI] [PubMed] [Google Scholar]
- 23.Kubyshkin AV, Aliev LL, Fomochkina II, Kovalenko YP, Litvinova SV, Filonenko TG, Lomakin NV, Kubyshkin VA, Karapetian OV. Endometrial hyperplasia-related inflammation: its role in the development and progression of endometrial hyperplasia. Inflamm Res. 2016;65(10):785–794. doi: 10.1007/s00011-016-0960-z. [DOI] [PubMed] [Google Scholar]
- 24.Cakmak B, Gulucu S, Aliyev N, Ozsoy Z, Nacar M, Koseoglu D. Neutrophil–lymphocyte and platelet–lymphocyte ratios in endometrial hyperplasia. Obstet Gynecol Sci. 2015;58(2):157–161. doi: 10.5468/ogs.2015.58.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin OAI, Salah HE. The effect of general or spinal anaesthesia on pro- and anti-inflammatory intracellular cytokines in patients undergoing appendicectomy using flowcytometric method. Egypt J Anaesth. 2011;27(2):121–125. https://www.tandfonline.com/doi/full/10.1016/j.egja.2011.04.005 [Google Scholar]
- 26.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu A, Wu H. Structural mechanisms of inflammasome assembly. FEBS J. 2015;282(3):435–444. doi: 10.1111/febs.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rai RC. Host inflammatory responses to intracellular invaders: review study. Life Sci. 2020;240:117084–117084. doi: 10.1016/j.lfs.2019.117084. [DOI] [PubMed] [Google Scholar]
- 29.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328–3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalaycı D, Dikmen B, Kaçmaz M, Taşpınar V, Ornek D, Turan O. Plasma levels of interleukin-10 and nitric oxide in response to two different Desflurane anesthesia flow rates. Braz J Anesthesiol. 2014;64(4):292–298. doi: 10.1016/j.bjane.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Ma J, Xiao W, Wang J, Wu J, Ren J, Hou J, Gu J, Fan K, Yu B. Propofol inhibits NLRP3 inflammasome and attenuates blast-induced traumatic brain injury in rats. Inflammation. 2016;39(6):2094–2103. doi: 10.1007/s10753-016-0446-8. [DOI] [PubMed] [Google Scholar]
- 32.Feng H, Liu Y, Zhang R, Liang Y, Sun L, Lan N, Ma B. TSPO ligands PK11195 and Midazolam reduce NLRP3 inflammasome activation and proinflammatory cytokine release in BV-2 cells. Front Cell Neurosci. 2020;14:544431–544431. doi: 10.3389/fncel.2020.544431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boldeanu L, Dijmărescu AL, Radu M, Siloşi CA, Popescu-Drigă MV, Poenariu IS, Siloşi I, Boldeanu MV, Novac MB, Novac LV. The role of mediating factors involved in angiogenesis during implantation. Rom J Morphol Embryol. 2020;61(3):665–672. doi: 10.47162/RJME.61.3.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boldeanu L, Dijmărescu AL, Novac MB, Rotaru LT, Pădureanu V, Neamţu SD, Siloşi CA, Geormăneanu C, Boldeanu MV, Siloşi I, Novac LV. Evaluation of iNOS -2087A>G polymorphism in recurrent pregnancy loss. Rom J Morphol Embryol. 2019;60(4):1137–1142. [PubMed] [Google Scholar]
- 35.Alsina E, Matute E, Ruiz-Huerta AD, Gilsanz F. The effects of Sevoflurane or Remifentanil on the stress response to surgical stimulus. Curr Pharm Des. 2014;20(34):5449–5468. doi: 10.2174/1381612820666140325105723. [DOI] [PubMed] [Google Scholar]
- 36.Boldeanu L, Siloşi CA, Pădureanu V, Dijmărescu AL, Manolea MM, Tabacu MC, Boldeanu MV, Popescu-Drigă MV, Poenariu IS, Pădureanu R, Novac LV, Novac MB. Determination of VEGFR-2 (KDR) -604A>G polymorphism in recurrent pregnancy loss. Rom J Morphol Embryol. 2018;59(4):105301059–105301059. [PubMed] [Google Scholar]
- 37.Siminel MA, Neamţu CO, Diţescu D, Forţofoiu MC, Comănescu AC, Novac MB, Neamţu SD, Gluhovschi A. Apert syndrome – clinical case. Rom J Morphol Embryol. 2017;58(1):2770280–2770280. [PubMed] [Google Scholar]
- 38.Pădureanu V, Boldeanu MV, Streaţă I, Cucu MG, Siloşi I, Boldeanu L, Bogdan M, Enescu AŞ, Forţofoiu M, Enescu A, Dumitrescu EM, Alexandru D, Şurlin VM, Forţofoiu MC, Petrescu IO, Petrescu F, Ioana M, Ciurea ME, Săftoiu A. Determination of VEGFR-2 (KDR) 604A>G polymorphism in pancreatic disorders. Int J Mol Sci. 2017;18(2):439–439. doi: 10.3390/ijms18020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sen Y, Xiyang H, Yu H. Effect of thoracic paraspinal block-Propofol intravenous general anesthesia on VEGF and TGF-β in patients receiving radical resection of lung cancer. Medicine (Baltimore) 2019;98(47):e18088–e18088. doi: 10.1097/MD.0000000000018088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moldoveanu AL, Oprescu DN, Catanescu Novac V, Dragan I, Novac M. Conference Proceedings of 5th Romanian Congress of the Romanian Society of Ultrasound in Obstetrics and Gynecology. Târgu Mureş Romania: 2017. The role of fetal Doppler monitoring in preeclampsia with/without intrauterine growth delay; pp. 401–405.https://publons.com/journal/175171/5th-romanian-congress-of-the-romanian-society-of-u/ [Google Scholar]
- 41.Helmy SA, Al-Attiyah RJ. The immunomodulatory effects of prolonged intravenous infusion of Propofol versus Midazolam in critically ill surgical patients. Anaesthesia. 2001;56(1):4–8. doi: 10.1046/j.1365-2044.2001.01713.x. [DOI] [PubMed] [Google Scholar]
- 42.Lu HB, Jia YP, Liang ZH, Zhou R, Zheng JQ. Effect of continuous infusion of Midazolam on immune function in pediatric patients after surgery. Genet Mol Res. 2015;14(3):10007–10014. doi: 10.4238/2015.August.21.7. [DOI] [PubMed] [Google Scholar]
- 43.Xiao D, Zhang D, Xiang D, Liu QI, Liu Y, Lv L, Xing X. Effects of Fentanyl, Midazolam and their combination on immune function and mortality in mice with sepsis. Exp Ther Med. 2015;9(4):1494–1500. doi: 10.3892/etm.2015.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marik PE. Propofol: an immunomodulating agent. Pharmacotherapy. 2005;25(5 Pt 2):28S–33S. doi: 10.1592/phco.2005.25.5_part_2.28s. [DOI] [PubMed] [Google Scholar]
- 45.Ke JJ, Zhan J, Feng XB, Wu Y, Rao Y, Wang YL. A comparison of the effect of total intravenous anaesthesia with Propofol and Remifentanil and inhalational anaesthesia with Isoflurane on the release of pro- and anti-inflammatory cytokines in patients undergoing open cholecystectomy. Anaesth Intensive Care. 2008;36(1):74–78. doi: 10.1177/0310057X0803600113. [DOI] [PubMed] [Google Scholar]
- 46.Mazoti MA, Braz MG, de Assis Golim M, Braz LG, Dias NH, Salvadori DM, Braz JR, Fecchio D. Comparison of inflammatory cytokine profiles in plasma of patients undergoing otorhinological surgery with Propofol or Isoflurane anesthesia. Inflamm Res. 2013;62(10):879–885. doi: 10.1007/s00011-013-0643-y. [DOI] [PubMed] [Google Scholar]
- 47.Fekkes D, Hol JW, Stolker RJ. Anesthesia with Propofol does not reduce interleukin-6 release in response to abdominal surgery of varying severity. J Clin Gynecol Obstet. 2016;5(4):106–111. https://www.jcgo.org/index.php/JCGO/article/view/413 [Google Scholar]
- 48.Hao X, Li D, Wu D, Zhang N. The relationship between hematological indices and autoimmune rheumatic diseases (ARDs), a meta-analysis. Sci Rep. 2017;7(1):10833–10833. doi: 10.1038/s41598-017-11398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8(1):10566–10566. doi: 10.1038/s41598-018-28646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. 2019;39(4):345–357. doi: 10.3343/alm.2019.39.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo Y, Deng R, Zhong Q, Luo D, Li X, Chen X, Tao S, Feng Z, Jiayi L, Huang Y, Li J, Liu W. The prognostic value of inflammation markers in postoperative gliomas with or without adjuvant treatments. Medicine (Baltimore) 2021;100(25):e26437–e26437. doi: 10.1097/MD.0000000000026437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walzik D, Joisten N, Zacher J, Zimmer P. Transferring clinically established immune inflammation markers into exercise physiology: focus on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune–inflammation index. Eur J Appl Physiol. 2021;121(7):1803–1814. doi: 10.1007/s00421-021-04668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, Chen CQ, He YL, Cai SR. Systemic immune–inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–6272. doi: 10.3748/wjg.v23.i34.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taşoğlu Ö, Bölük H, Şahin Onat Ş, Taşoğlu İ, Özgirgin N. Is blood neutrophil–lymphocyte ratio an independent predictor of knee osteoarthritis severity. Clin Rheumatol. 2016;35(6):1579–1583. doi: 10.1007/s10067-016-3170-8. [DOI] [PubMed] [Google Scholar]
- 55.Xu N, Tang XF, Yao Y, Zhao X, Chen J, Gao Z, Yang Y, Gao RL, Xu B, Yuan JQ. Predictive value of neutrophil to lymphocyte ratio in long-term outcomes of left main and/or three-vessel disease in patients with acute myocardial infarction. Catheter Cardiovasc Interv. 2018;91(S1):551–557. doi: 10.1002/ccd.27495. [DOI] [PubMed] [Google Scholar]


