Abstract
Psoriasis is a chronic autoimmune disease affecting over 2% of the worldwide population. From an anatomopathological point of view, psoriasis is characterized by immune cells infiltration, epidermal hyperproliferation, and abnormal keratinocyte differentiation. Understanding the pathogenesis of psoriasis will allow clinicians to manage this complex disease. Under these conditions, the application of effective treatments requires a thorough knowledge of all the pathogenetic mechanisms that lead to psoriasis. Numerous immunopathological pathways play crucial roles in the development of new therapies, such as biological therapies, which have been a breakthrough in psoriasis’s treatment. Pharmacogenetics is an essential factor in the patient’s response to treatment. One important pathway targeted by modern treatments is the interleukin (IL)-23/T-helper (Th)17 axis. Like IL-17 inhibitors, IL-23 blockers are a very effective therapy for this autoimmune disease. It is considered that micro-ribonucleic acids (microRNAs) are the starting point for any autoimmune disease. Studying certain microRNA (miR) involved in the inflammatory pathway in psoriasis can find direct targets to future treatments that can even be more specific than actual biological therapies. As such, miR-210 has proven to be up-regulated in psoriasis, also leading to the up-regulation of the Th1/Th17 axis. On the other hand, miR-187 was found to be down-regulated, influencing the outcome of psoriasis by increasing the proliferation of IL-6 stimulated keratinocytes and consecutively generating epidermal thickening. In this review, we are aiming to do an up-to-date briefing of psoriasis histopathology and pharmacogenetic factors that are considered for the accurate evaluation of treatment response.
Keywords: psoriasis, histopathology, pharmacogenetics, microRNA, biological therapies
⧉ Introduction
Psoriasis is an autoimmune disease affecting over 2% [1,2] of the global population without a fully understood pathophysiology path.
The most common form is vulgar psoriasis, being involved in more than 80% of the total cases of psoriasis [3,4]. It is clinically characterized by papulosquamous plaques, with a clear demarcation from normal skin and asymmetrical debut usually involving elbows, scalp, and knees [5,6]. Apart from plaque psoriasis, there are also other clinical forms, such as inverse or flexural psoriasis, characterized by red scales, with a particular shiny aspect, that can sometimes be misdiagnosed with seborrheic dermatitis due to specific localization and often greasy scales [5, 7]. Von Zumbusch psoriasis or general psoriasis, pustular, inverse, and guttate psoriasis are other less frequent forms, while erythroderma is an extremely severe condition that can emerge from any form of psoriasis [8,9].
Clinical evidence plays a key role in the diagnosis approach of this disease [10]. The most important score used in evaluating the severity of the disease, as well as the remission under treatment, is Psoriasis Area and Severity Index (PASI) score. It includes four anatomic sites: head, arms, trunk, and legs, each of those being assessed for typical lesions, such as desquamation, induration, and erythema [11]. The maximum score that can be achieved is 72, equivalent to the most severe and almost complete form of erythroderma [5, 12].
Aim
In this narrative review, we aim to present the latest knowledge in the medical literature regarding the implications of pharmacogenetics in the response to psoriasis’ treatment. The main objectives are to state whether there could be a possible predictable marker in this pathology’s outcome, as well to present the newest treatment directions based on individualized pharmacogenetic treatment, which could be a breakthrough in future medicine.
⧉ Histopathological features and immunopathology
One major approach in understanding the mechanisms behind the disease is to study the histopathological (HP) aspect of the skin. There are three important HP features in psoriasis: leukocyte infiltration, vessel dilation both of which are present in the dermis, and epidermal hyperplasia. Correlations between HP aspects and clinical features are essential for a full understanding of this diseases’ natural outcome. The early macule at the debut of the disease is characterized by a mere lymphocytic infiltrate in the proximity of more prominent blood vessels. The next step in the natural evolution is epidermal hyperplasia and the appearance of scaly papules characterized by parakeratosis. At this point, neutrophilic infiltration appears, which together with parakeratosis form in the horny layer a specific HP lesion: the Munro abscess (Figures 1 and 2). Sometimes, when necrotic epidermal cells form a structure in the shape of a collar surrounding neutrophilic infiltration, a spongiform pustule of Kogoj is formed (Figures 3 and 4). Generally speaking, the classical HP presentation of psoriasis plaques is characterized by elongation of rete ridges, parakeratotic, neutrophilic infiltration, and a more thinned subpapillary plate. The histopathology of the lesions is mostly reversible, starting with fibrosis, and a decrease in the number of neutrophils [5, 13]. Jiang et al. studied the implication of the psoriatic keratinocyte exosome release, as well as their function in the outcome of the disease. Their findings show a clear relationship between keratinocytes exosomes and the expression of proinflammatory factors involved in the development of psoriasis. This can be explained by the role keratinocyte exosomes play in inducing neutrophil activation [14] and by such in the perpetuation of inflammation. The most important conclusion stated in their study is that psoriasis’ inflammation is characterized by a tight “communication” between keratinocyte exosomes and neutrophils [14]. There is a pathological interaction among skin cells, immune cells, and numerous biological signaling molecules [15].
Figure 1.
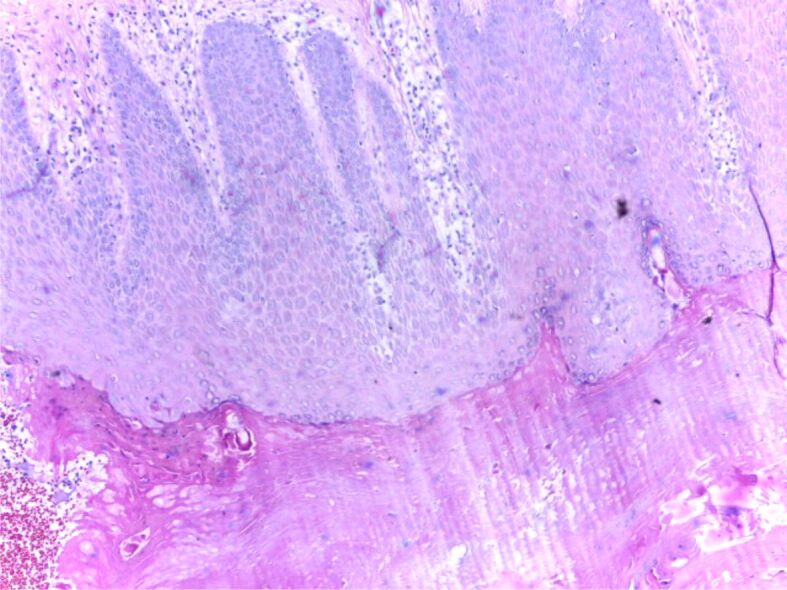
HP patterns of psoriasis, chronic phase: regular acanthosis, hypogranulosis, hyperkeratosis, parakeratosis. HE staining, ×100. HE: Hematoxylin–Eosin; HP: Histopathological
Figure 2.
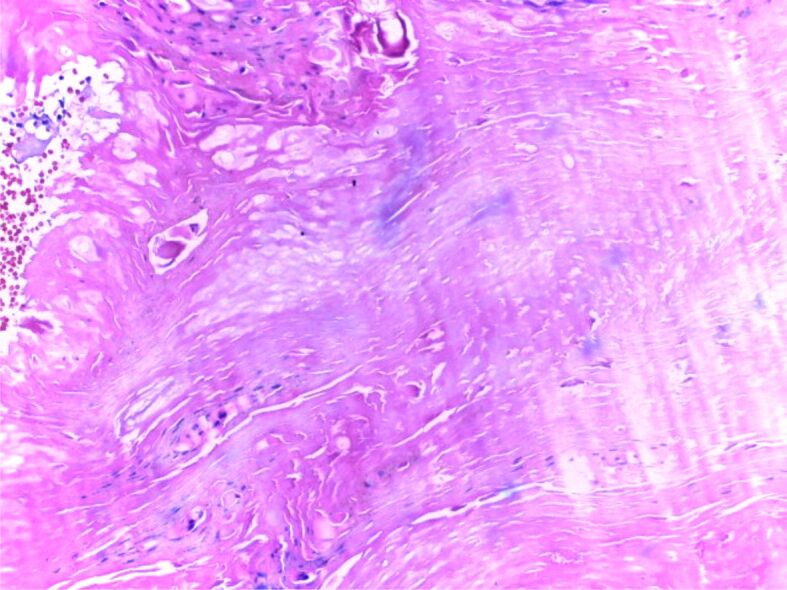
HP patterns of psoriasis, chronic phase: parakeratosis, hyperkeratosis, Munro microabscesses. HE staining, ×200
Figure 3.
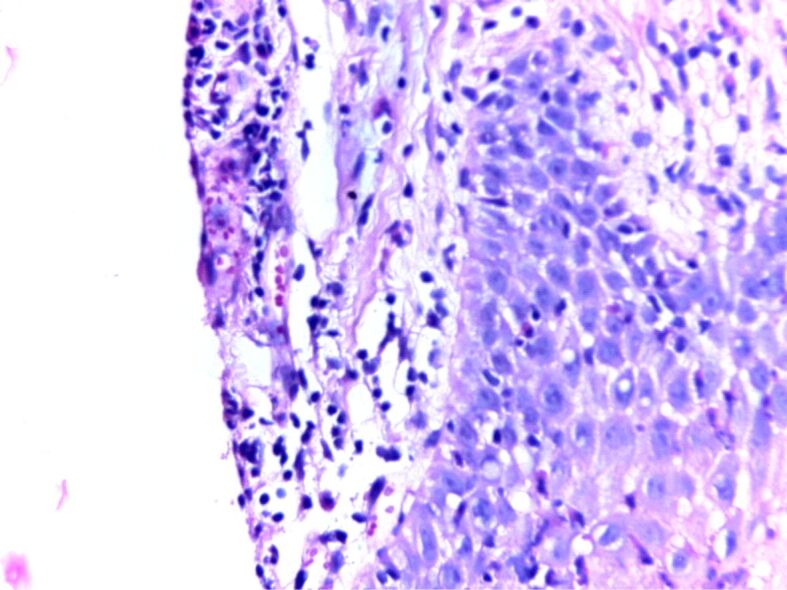
HP patterns of psoriasis, acute phase: congested capillaries, pustules of Kogoj, perivascular lymphocytic infiltrate. HE staining, ×200
Figure 4.
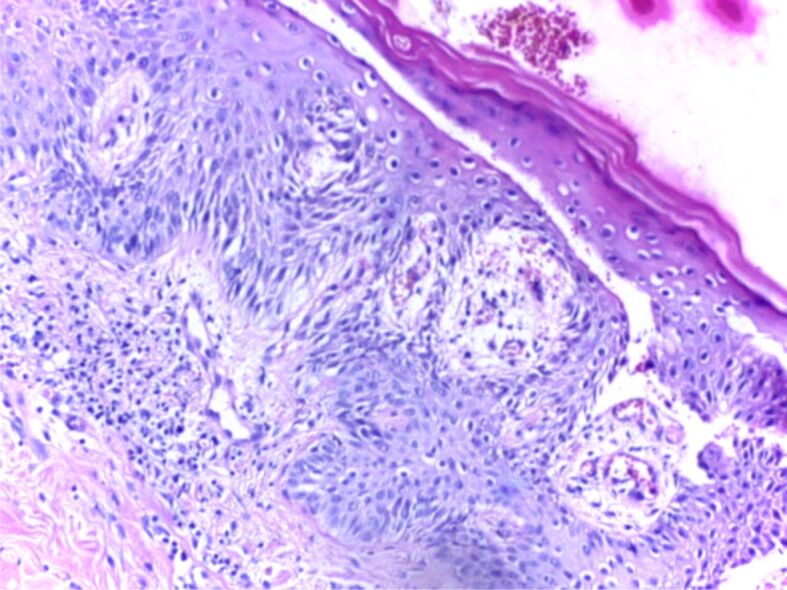
HP patterns of psoriasis, acute phase: elongation and fusion of rete ridges, congested and tortuous capillaries in the edematous dermal papillae, perivascular lymphocytic infiltrate. HE staining, ×200
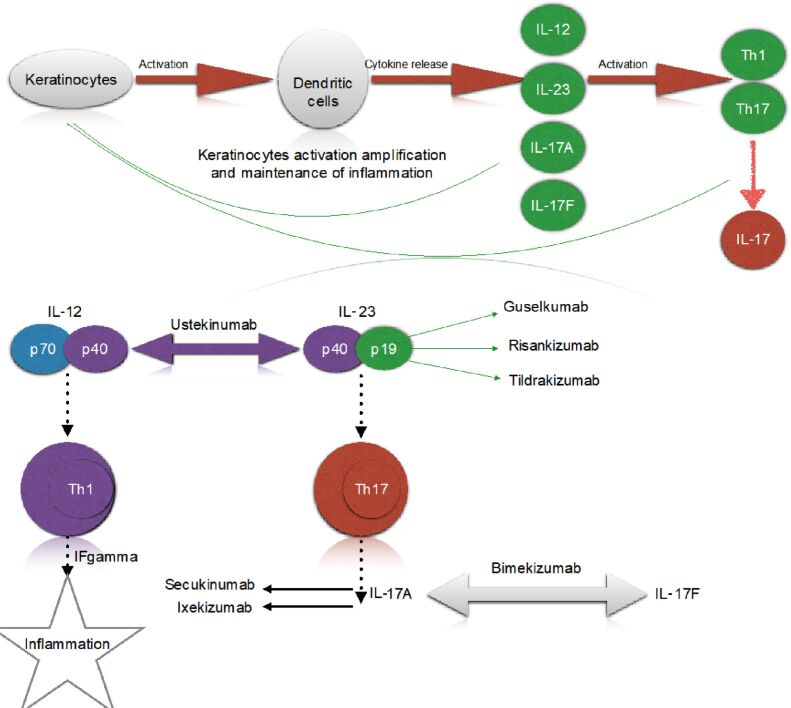
The most studied feature in psoriasis, vessel dilation, is most likely caused by the rise of vascular endothelial growth factor (VEGF). This pathway is signaled by tumor necrosis factor-alpha (TNF-α), targeted by TNF-α blockers, such as Infliximab, Etanercept, Adalimumab, etc. [16,17]. Understanding the immunopathology behind this disease could give us better control of the progression of the lesions and thus a great improvement of patients’ quality of life. Yet very little is known regarding specific predictors of this disease, or the factors that can lead to clinical remission [18]. The immunopathological pathway is triggered in the first phase by keratinocytes which fulfill the role of activating dendritic cells, leading to a cytokine release, including interleukin (IL)-12 and IL-23 [19,20]. Those two ILs will subsequently activate type 1 and type 17 T-helper (Th1, Th17) cells [21]. All these factors (ILs, cytokine increased number, TNF-α) amplify keratinocyte activation and thus maintenance of inflammation [22,23,24].
Most recent studies are based on IL-23/Th17 axis, according to which IL-23 activates Th17, which synthetizes IL-17 [25,26,27,28]. Figure 5 shows a brief overview of the immunopathological pathways triggered in psoriasis, as well as the site of action of most important biological therapies [29,30].
Figure 5.
Immunopathology in psoriasis. IF: Interferon; IL: Interleukin; Th: T-helper
⧉ Involvement of ILs in the onset and evolution of psoriasis
IL-17 plays a major role in the immunopathogenesis of psoriasis [31]. IL-17 is a family of cytokines that includes six different cytokines, the highest potency having the IL-17A cytokine [32]. Important sources for this proinflammatory cytokine are Th17 lymphocytes, gamma delta T (γδT) lymphocytes, and mucosal-associated invariant T (MAIT) cells, type 3 innate lymphoid cells (ILC3s) [33]. IL-17F is more than 50% homologous to IL-17A (55%) and has the same sources (Th17, γδT cell, ILC3s). Although both IL-17A and IL-17F cytokines are more expressed in psoriatic plaque and the serum of psoriatic patients, IL-17F has a higher potency in inducing IL-6 and IL-8 in normal keratinocytes compared to IL-17A [34].
The IL-23/Th17 axis has been identified as a major factor in the pathogenesis of psoriasis. IL-23 has modulatory effects on the maintenance of Th17 cells. IL-23 is produced by dendritic cells, monocytes, and macrophages (antigen-presenting cells) [35]. Like IL-17 inhibitors, IL-23 blockers are a very effective therapy for this autoimmune disease.
⧉ Immunomodulatory treatment of psoriasis
Depending on the form and severity of the disease, treatment can vary from topical to systemic, with biological therapies as the last resort therapy but also the highest efficiency [36,37,38]. The role of biological therapies is to suppress the immune-mediated process that leads to inflammation in most autoimmune diseases. Depending on the pathway targeted by each agent there is a wide variety of biological therapies to be used for treating moderate to severe forms of psoriasis [33, 39,40].
TNF-α blockers were the first agents used as targeted therapy in psoriasis. They were followed by monoclonal antibodies against IL-17, IL-23, and IL-12/IL-23 [41]. TNF-α is an agent produced by both immune cells and non-immune cells, such as T-cells, dendritic cells, macrophages, neutrophils, but especially mature dendritic cells [42]. TNF-α activates the inflammatory pathways mainly in keratinocytes and endothelial cells [43]. Thus, TNF-α blockers modify the nuclear factor-kappa B (NF-κB) pathway through a strong interaction with TNF-α receptors. The result of this interaction is maintaining the inactivity of the NF-κB dimer [44]. TNF influences the expression of intercellular adhesion molecule-1 (ICAM-1) in skin cells by stimulating the adhesion of circulating leukocytes and also stimulating the production of proinflammatory cytokines, such as IL-1 and IL-6 [43]. At present, Etanercept, Infliximab, and Adalimumab are available for the treatment of psoriasis [45]. Certolizumab is only recommended for adult patients with active psoriatic arthritis [46] (Table 1) [47,48,49,50,51,52,53,54,55].
Table 1.
TNF-α inhibitors in psoriasis
|
Drug name/study reference |
Approved indications |
Mechanism of action |
Dosage forms/routes of administration |
|
Etanercept Nguyen & Koo (2009) [47] Michailidou et al. (2014) [48] Papp et al. (2007) [49] |
FDA/EMA Plaque psoriasis Psoriatic arthritis Ankylosing spondylitis Rheumatoid arthritis Polyarticular juvenile idiopathic arthritis |
▪ binds specifically to the cell surface of soluble TNF; ▪ blocks the interaction of TNF-α and TNF-β with their receptors; ▪ down-regulates the expression of E-selectin, ICAM-1 (adhesion molecules that are responsible for leukocyte migration); ▪ decreases serum levels of IL-1, IL-6, and matrix metalloproteinase. |
Solution for parenteral administration or powder for solution for injection (subcutaneous) |
|
Infliximab Netherlands Yearbook (1985) [50] ZYPREXA FDA (2009) [51] Gall & Kalb (2008) [52] |
FDA/EMA Psoriasis Psoriatic arthritis Ankylosing spondylitis Ulcerative colitis Crohn’s disease Rheumatoid arthritis |
▪ binds both soluble and membrane-bound TNF-α; ▪ decreases epidermal T-cell infiltration; ▪ down-regulates angiopoietin and thus modulate angiogenesis; ▪ decreases keratinocyte differentiation. |
Powder for concentrate for solution for infusion (intravenous use) Solution for parenteral administration (subcutaneous) |
|
Adalimumab AMGEVITA EMA (2017) [53] HUMIRA FDA (2021) [54] Markus et al. (2019) [55] |
FDA/EMA Psoriasis Psoriatic arthritis Axial spondyloarthritis Crohn’s disease Ulcerative colitis Hidradenitis suppurativa Rheumatoid arthritis Juvenile idiopathic arthritis Uveitis |
▪ binds both soluble and membrane-bound TNF-α; ▪ decreases inflammatory cytokines; ▪ blocks TNF-α signaling and inflammatory cascade. |
Solution for parenteral administration (subcutaneous) |
EMA: European Medicines Agency; FDA: Food and Drug Administration; ICAM-1: Intercellular adhesion molecule-1; IL: Interleukin; TNF: Tumor necrosis factor
IL-17 is a key factor in the immunopathogenesis of psoriasis [31]. IL-17 is a family of cytokines involved in the chronic inflammation in psoriasis consisting of six different cytokines, among which IL-17A has the highest potency [32]. The most important sources for this proinflammatory cytokine are Th17 lymphocytes, γδT lymphocytes, as well as MAIT cells, and ILC3s [33]. IL-17F is more than 50% homologous to IL-17A (55%) and has the same sources (Th17, γδT cell, ILC3s). Although both IL-17A and IL-17F cytokines are more expressed in psoriatic plaque and the serum of psoriatic patients, IL-17F has a higher potency in inducing IL-6 and IL-8 in normal keratinocytes compared to IL-17A [34].
Currently, there are IL-17 inhibitors available for the treatment of moderate-severe plaque psoriasis: two monoclonal antibodies targeting IL-17A (Secukinumab, Ixekizumab), one targeting both IL-17A and IL-17F (Bimekizumab), and one against IL-17 receptors (Brodalumab) [31, 56] (Table 2) [48, 57,58,59,60,61,62,63,64,65]. The importance of a direct treatment target of specific cytokines involved in this pathology cannot be understated, leading not only to an important minimization of the adverse effects of immunosuppressive therapy but also to impressive improvements in HP lesions [66].
Table 2.
New generation biological agents in psoriasis (I)
|
Drug name/study reference |
Approved indications |
Mechanism of action |
Dosage forms/routes of administration |
|
Secukinumab Deodhar et al. (2019) [57] Krueger et al. (2019) [58] Mercurio et al. (2020) [59] |
FDA/EMA Plaque psoriasis Psoriatic arthritis Ankylosing spondylitis |
▪ selectively inhibits IL-17A; ▪ influences the immune function of keratinocytes; ▪ inhibits the release of chemokines and antimicrobial peptides; ▪ decreases neutrophil accumulation and psoriasis inflammation. |
Solution for parenteral administration or lyophilized powder for reconstitution (subcutaneous) |
|
Ixekizumab Genovese et al. (2020) [60] Michailidou et al. (2019) [48] Craig & Warren (2020) [61] |
FDA Plaque psoriasis Psoriatic arthritis Ankylosing spondylitis |
▪ blocks the action of IL-17A, binding this cytokine with high affinity and specificity; ▪ decreases the activation and proliferation of keratinocytes |
Solution for parenteral administration (subcutaneous) |
|
Bimekizumab Oliveira et al. (2021) [62] Freitas & Torres (2021) [63] |
EMA Plaque psoriasis |
▪ inhibits both IL-17A and IL-17F. |
Solution for parenteral administration (subcutaneous) |
|
Brodalumab Rivera-Oyola et al. (2020) [64] Puig (2017) [65] |
FDA/EMA Plaque psoriasis |
▪ binds IL-17A receptor (has high affinity); ▪ locks the biological activities of IL-17 family cytokines (IL-17A, IL-17A/F, IL-17F, and IL-25). |
Solution for parenteral administration (subcutaneous) |
EMA: European Medicines Agency; FDA: Food and Drug Administration; IL: Interleukin
The next step in psoriasis’ pathophysiology pathway is the IL-23/Th17 axis, which has been identified as a major factor in the persistent chronic inflammation, characteristic of this pathology. IL-23 has modulatory effects on the maintenance of Th17 cells, and by this, in the constant production of IL-17. IL-23 is mainly produced by dendritic cells, monocytes, and macrophages (antigen-presenting cells) [67]. Like IL-17 inhibitors, IL-23 blockers are a very effective therapy for this autoimmune disease (Table 3) [35, 67,68,69].
Table 3.
New generation biological agents in psoriasis (II)
|
Drug name/study reference |
Approved indications |
Mechanism of action |
Dosage forms/routes of administration |
|
Tildrakizumab Sakkas et al. (2019) [67] |
Moderate-to-severe plaque psoriasis |
▪ binds (with high affinity) to the p19 subunit of IL-23; ▪ inhibits IL-23 signaling. |
Solution for parenteral administration (subcutaneous) |
|
Guselkumab Tsukazaki & Kaito (2020) [35] Nogueira & Torres (2019) [68] |
Moderate-to-severe plaque psoriasis Active psoriatic arthritis |
▪ inhibits IL-23 specifically. |
Solution for parenteral administration (subcutaneous) |
|
Risankizumab Blair (2020) [69] |
Moderate-to-severe plaque psoriasis |
▪ selectively bind to the IL-23 p19 subunit; ▪ inhibits the interaction with the specific receptor; ▪ decreases expression of IL-17A, IL-17F, IL-21, and IL-22. |
Solution for parenteral administration (subcutaneous) |
IL: Interleukin
The concentration of IL-12/IL-23p40 and IL-23p19 micro-ribonucleic acid (microRNA, miR) was observed to be higher in lesions compared to normal skin. Also, IL-17A, IL-17F, and IL-22, the cytokines induced by IL-12 and IL-23 are increased in lesions vs. non-lesioned skin.
IL-12 and IL-23 stimulate the activity of Janus kinase 2 (JAK2) and tyrosine kinase 2 (TYK2) and activate the signal transducer and activator of transcription (STAT) family of transcription factors, IL-12 especially STAT4 and IL-23 STAT3 [70].
IL-23 is a proinflammatory cytokine produced by dendritic cells and activated macrophages. IL-23 is considered to produce negative effects through the production of inflammatory mediators, such as IL-17, IL-22, TNF-α, and thus pathological consequences occur [71]. The effect of IL-23 in psoriasis is considered to be the consequence of its effect on IL-17, an important cytokine with a role in the cascade of inflammation [72]. The most important biological therapies used nowadays in psoriasis, as well as their site of action, are presented in Table 4 [37, 73,74,75,76].
Table 4.
Biological therapies in psoriasis and their site of action
|
Biological therapies/study reference: Trembath et al. (1997) [75]; Capon et al. (2002) [76] | |||
|
TNF-α |
Adalimumab (approved) |
Etanercept (approved) |
Infliximab (approved) |
|
IL-12/ IL-23p40 |
Ustekinumab (approved) |
Briakinumab (withdrew from market due to severe adverse effects) |
– |
|
IL-23p19 |
Tildrakizumab (phase III) |
Risankizumab (phase II) |
Guselkumab (phase III) |
|
IL-17A |
Secukinumab (approved) |
Ixekizumab (phase III) |
Brodalumab (unstudied) |
IL: Interleukin; TNF-α: Tumor necrosis factor-alpha
⧉ Genetic changes in psoriasis
Psoriasis’ genetic background involves several genes, psoriasis susceptibility (PSORS)1–7, but according to literature human leukocyte antigen (HLA)-Cw6 represents a highly important disease allele at PSORS1 [77]. PSORS1 is located in the major histocompatibility complex (MHC) region on chromosome 6p, and it is thought to play a major role in the immunopathological pathway of this disease [78,79,80]. The specific roles of this allele are not yet fully elucidated, but original studies targeting HLA-Cw6 status showed implications in the severity of the disease, quicker onset, and most important, variations in terms of response to treatment [80].
One major pathological factor is represented by HLA-Cw6, which is considered to be highly associated with psoriasis susceptibility alleles. This review aims to state the possible connection between the level of HLA-Cw6 and the response to different types of biological agents [74].
Burlando et al. stated that there might be a connection between the high expression of HLA-Cw6 and a longer-lasting response to treatments targeting TNF-α, IL-17, and IL-12/IL-23. Therefore, HLA-Cw6 could play the role of a predictor for the achievement and maintenance of clinical remission. Throughout their study, their main aim was the PASI score and patients’ comorbidities. Thus, patients with a severe PASI score (more than 15) were treated with Secukinumab or Ixekinumab, biological agents targeting IL-17, while younger patients or patients who due to traveling were unable to follow treatments involving more frequent doses were given Ustekinumab, targeting IL-12/IL-23. Last but not least, patients with a moderate PASI score were treated with anti-TNF-α agents, such as Adalimumab, Etanercept, Infliximab). The response to treatment showed yet no link between the base level of HLA-Cw6 and the maintenance of PASI90 after being reached at week 16, which suggested that HLA-Cw6 status could not be considered an “overall prognostic factor” [74]. On the other hand, when taken into consideration separately, drugs targeting IL-12/IL-23 and IL-17 showed a better response in those with an HLA-Cw6 positive (POS) status, while drugs targeting TNF-α were opposingly showing more efficiency on those with an HLA-Cw6 negative (NEG) status [81,82]. Costanzo et al. state that the efficiency of Secukinumab on patients with psoriasis is independent of HLA-Cw6 status. These findings are yet contrary to other studies which suggest a link between HLA-Cw6 and the response to other biological agents, such as Adalimumab and Ustekinumab [80]. For their study, the response to treatment assessed by the PASI90 score reached on week 16 was similar in POS and NEG groups [74, 81]. Similar results at week 16 were found by Burlando et al. who did not find any difference between POS and NEG groups [74]. On the other hand, after evaluating the rate of maintenance for PASI90 at week 48, showed a higher rate of response for those in the POS group, so as opposed to no relationship between treatment response and IL-17 treatment, drugs targeting IL-23/IL-12 and TNF-α pathways might have a better long-lasting response for patients with an HLA-Cw6 POS status. On the other hand, though, Gallo et al. found a worse response to treatment for drugs targeting TNF-α pathway on HLA-Cw6 POS groups only for PASI75 [73].
Dand et al. have conducted a study a longitudinal study based on data from 1326 patients which concluded that HLA-Cw6 is a predictive biomarker on the response of biological therapies with Adalimumab and Ustekinumab. Moreover, the state that patients with HLA-Cw6 NEG are more likely to respond to Adalimumab than to Ustekinumab. Furthermore, patients with HLA-Cw6 POS are more likely to have inactive psoriasis, and thus could be treated in the long term with Ustekinumab, which also has the advantage of longer doses intervals. One important aspect suggested by their study is that HLA-Cw6 status only interacts with the biological drug, and not with the biological native status, and so the different rate of response between Adalimumab and Ustekinumab could be explained [73].
Another possible role of HLA-Cw6 status is the link to certain clinical forms of psoriasis. Thus, Mallon et al. found a direct implication of HLA-Cw6 POS status and the development of guttae psoriasis. This clinical form of psoriasis is usually diagnosed in young adults and is developed consequently to a streptococci infection. In most cases, the streptococcal infection is hard to be proven due to prior antibiotic therapies. Their findings show a 100% carriage of HLA-Cw6 allele in patients diagnosed with guttate psoriasis, compared to only 20% in the healthy group. It is not yet understood the functional role of HLA-C in psoriasis, but there is most certainly a consensus regarding its implications in the progression and outcome of the disease [83] (Table 5).
Table 5.
Link between HLA-Cw6 status and response to biological treatment according to literature
|
Study reference |
HLA-Cw6 POS |
HLA-Cw6 NEG |
|
Dand et al. (2019) [73] |
▪ more likely to develop unactive psoriasis; ▪ no advantage was found on using Adalimumab over Ustekinumab. |
▪ more likely to respond to Adalimumab than to Ustekinumab; ▪ 46.6% of patients with NEG status have severe psoriasis. |
|
|
▪ selecting the treatment based on HLA-Cw6 status could improve the response to treatment, reaching PASI90 in the first 12 months for over 30% of the patients; ▪ stratifying patients over HLA status, rather than aged (psoriasis type I/II) could be a more useful approach for a better treatment response. |
|
|
Costanzo et al. (2018) [81] |
▪ response to Secukinumab showed no link to the HLA-Cw6 status, as POS and NEG groups reached PASI90 at week 16 on close percentages. |
|
|
Burlando et al. (2020) [74] |
▪ similar response for reaching PASI90 at week 16 on HLA-Cw6 POS and NEG groups. |
|
|
|
▪ higher rate of PASI90 maintenance at week 48. |
|
|
Gallo et al. (2013) [84] |
▪ worse response for HLA-Cw6 at PASI75 for Adalimumab and Etanercept. |
|
HLA: Human leukocyte antigen; NEG: Negative; PASI: Psoriasis Area and Severity Index; POS: Positive
⧉ Molecular lesions in psoriasis and future alternative therapies
Another high interest point in literature is the study of molecular mechanisms triggered by microRNAs. In psoriasis, one of the most discussed genes is miR-210. It is believed that once a patient genetically predisposed to psoriasis is exposed to environmental factors, activated dendritic cells stimulate the production of IL-23, IL-6, as well as IL-12, and TNF-α. The previously mentioned ILs will consecutively activate autoregressive Th1, Th17, Th22 cells, which will finally result in producing proinflammatory cytokines that will in the end amplify the immune response in psoriasis [85]. MicroRNAs are non-coding RNA molecules that have the role of negatively regulating gene expression at the post-transcriptional level [86]. There are several microRNAs that are considered to be of interest regarding psoriasis patients. Wu et al. have studied the importance of miR-210 overexpression over the inflammatory pathways triggered in psoriasis, moreover, they also stated a link between increased levels of inflammatory cytokines, such as IL-23, and the overexpression of miR-210 in cluster of differentiation (CD)4+ T-cells in patients with active psoriasis lesions and higher severity index (PASI score). These findings alongside all studies regarding microRNAs in psoriasis are of vital importance in the future development of targets treatments. As such, intradermal injections of miR-210 inhibitor could be a viable alternative to biological treatments especially for patients with a poor answer or severe adverse effects. Another important finding of their study was that miR-210 might have an affinity to upregulate Th1 and Th17 in vivo cell differentiation and at the same time downregulate the differentiation of Th2. This pathological pathway triggered by miR-210 led to an increase in the severity of psoriasis lesions proving that the higher the expression of miR-210, the higher the severity scores. Furthermore, the most important outcome of their study showed that suppressing miR-210 leads to an improvement of skin lesions in psoriasis [85].
Some numerous other microRNAs are somehow linked to the pathophysiology of psoriasis lesions. Another recent study conducted by Tang et al. showed that downregulation of miR-187 is related to the activity of the disease. Moreover, they proved that stimulating the expression of miR-187 can improve the outcome of the disease by inhibiting the proliferation of IL-6 stimulated keratinocytes, which led to a decrease in the epidermal hyperplasia in a mouse model. The pathway behind this important discovery is the inhibition of CD276 by miR-187. CD276 is an immunoregulatory protein, with yet unknown implications in the hyper-proliferation of keratinocytes in psoriasis skin. To assess the possibility of a future treatment targeting miR-187, they administrated miR-187 agomir to mouses with induced psoriasis lesions. The outcomes showed a decrease in the epidermal thickening. Also, the decrease of acanthosis after HP analysis proved a good response to the experimental treatment. Dermal cell infiltration showed no modification consecutive to this treatment, however. Another important marker for cell proliferation is the expression ok Ki67, which was considerably decreased after miR-187 agomir was administrated [87].
Bian et al. studied the importance of decreased levels of miR-340 in patients with active psoriasis. They also used mice with Imiquimod (IMQ)-induced psoriasis that presented in the first place with low levels of miR-340. Consecutive, they administrated miR-340 agomir and which led to a decrease in the epidermal thickening similar to the results after miR-187 agomir was administrated. They also proved that experimental treatment with miR-340 agomir leads to an important downregulation of the expression of IL-17A, and thus limits the local inflammation [88].
Another important aspect that must be evaluated regarding pharmacogenetic profile for patients diagnosed with psoriasis is whether the levels of microRNAs vary after therapies. A study conducted by Wipasiri et al. in 2020 investigates the downregulation of miR-155 in patients with symptomatic psoriasis (PASI score over 10) before and after being treated with Methotrexate and narrow-band ultraviolet B (UVB). They compared levels of miR-155, miR-135b, as well as miR-125b in normal skin, as well as in skin affected by psoriasis. The most relevant increase was for the levels of miR-155 before the beginning of the therapy. miR-155 is responsible according to them for inhibiting apoptosis of keratinocytes in psoriasis skin, as well as for increasing cell viability in the same conditions, leading to the abnormal keratinocytic proliferation that characterizes skin affected by psoriasis. They concluded that after patients were treated with Methotrexate and narrow-band UVB levels of miR-155 were importantly diminished [89]. This can easily explain the rapid clinical response after immunosuppressive therapy is started, due to Methotrexate’s capacity to increase keratinocyte apoptosis by stimulating apoptosis markers, such as caspase-3 and caspase-9 [90].
Even though studies are yet at an incipient level, it is clear that there is a strong connection between the expression of certain microRNAs and the development of psoriasis lesions. Future studies must be carried out to be able to find alternative directed treatments especially for patients with a poor response to biological agents, which are considered nowadays the last resort therapy (Table 6).
Table 6.
Possible future treatments based on microRNAs
|
MicroRNA/study reference |
Up/down regulation in psoriasis |
Physiopathological implications |
Future implications |
|
miR-210 Wu et al. (2018) [85] |
Up |
▪ increased IL-23; ▪ upregulation of Th1 and Th17 in vivo. |
Possible intradermal injections with miR-210 inhibitor as adjuvant therapies? |
|
miR-187 Tang et al. (2019) [87] |
Down |
▪ increased proliferation of IL-6 stimulated keratinocytes; ▪ epidermal thickening. |
Decrease in epidermal thickening after intradermal injection with miR-187 agomir. |
|
miR-340 Bian et al. (2018) [88] |
Down |
▪ increased levels of IL-17A. |
Decrease in epidermal thickening after intradermal injection with miR-340 agomir. |
|
miR-155 Soonthornchai et al. (2021) [89] |
Up |
▪ inhibition of keratinocyte apoptosis in psoriasis. |
Narrow-band UVB can lower level of miR-155 and thus decrease epidermal thickening. |
|
miR-369-3p Hou et al. (2016) [91] |
Up |
▪ correlated to the disease’s severity (PASI). |
Possible intradermal administration of miR-369-3p inhibitor. |
|
miR-205 An et al. (2017) [92] |
Down |
▪ linked to epidermal thickening in psoriasis similar to the development of keloid lesion due to VEGF production. |
Upregulation of miR-205 can improve epidermal thickening, as well as keloid lesions. |
IL: Interleukin; miR: Micro-ribonucleic acid (RNA); PASI: Psoriasis Area and Severity Index; Th: T-helper; UVB: Ultraviolet B; VEGF: Vascular endothelial growth factor
⧉ Conclusions
Psoriasis is an autoimmune disease with a complex pathogenetic background. Mainly, psoriasis represents a clinical diagnostic, due to the clinical specific aspects. The HP examination is mandatory to be performed any time the clinical aspects or response to therapy is uncommon. MicroRNAs are considered the starting point to any HP pathway behind all autoimmune diseases and seem to be the answer to our problem. Studying certain microRNA involved in the inflammatory pathway in psoriasis can find direct targets to future treatments that can even be more specific than actual biological therapies. Data in the literature shows that intradermal injection of either agomirs or antagomirs directed to specific microRNAs can dramatically improve the morphopathology of psoriasis affected skin. These possible future treatments can play a major role not necessarily by replacing actual biological treatments, but most probably by potentiating their beneficial effects. The most important breakthrough in modern pharmacology is to personalize the treatment to every patient. Individually treating each patient is thought to enhance the desired effect of the therapy, as well as to decrease to a minimum possible the incidence of adverse effects. Furthermore, being able to link the response to treatment to the genetic status of the patient, can improve the outcomes of therapy.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Raychaudhuri SP, Farber EM. The prevalence of psoriasis in the world. J Eur Acad Dermatol Venereol. 2001;15(1):16–17. doi: 10.1046/j.1468-3083.2001.00192.x. [DOI] [PubMed] [Google Scholar]
- 2.Christophers E. Psoriasis - epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 3.Langley RGB, Krueger GG, Griffiths CEM. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):ii18–ii23; discussion 1124–ii25. doi: 10.1136/ard.2004.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Burgdorf WHC, Plewig G, Wolff HH, Landthaler M, editors. Braun-Falco’s Dermatology. 3. Berlin-Heidelberg: Springer-Verlag; 2009. pp. 506–527.https://link.springer.com/book/9783540293125 [Google Scholar]
- 6.Reich K, Krüger K, Mössner R, Augustin M. Epidemiology and clinical pattern of psoriatic arthritis in Germany: a prospective interdisciplinary epidemiological study of 1511 patients with plaque-type psoriasis. Br J Dermatol. 2009;160(5):1040–1047. doi: 10.1111/j.1365-2133.2008.09023.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferrándiz C, Pujol RM, García-Patos V, Bordas X, Smandía JA. Psoriasis of early and late onset: a clinical and epidemiologic study from Spain. J Am Acad Dermatol. 2002;46(6):867–873. doi: 10.1067/mjd.2002.120470. [DOI] [PubMed] [Google Scholar]
- 8.Finlay AY, Coles E. The effect of severe psoriasis on the quality of life of 369 patients. Br J Dermatol. 1995;132(2):236–244. doi: 10.1111/j.1365-2133.1995.tb05019.x. [DOI] [PubMed] [Google Scholar]
- 9.Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, Mehta NN, Finlay AY, Gottlieb AB. Psoriasis. Nat Rev Dis Primers. 2016;2:16082–16082. doi: 10.1038/nrdp.2016.82. [DOI] [PubMed] [Google Scholar]
- 10.Finlay AY, Kelly SE. Psoriasis - an index of disability. Clin Exp Dermatol. 1987;12(1):8–11. doi: 10.1111/j.1365-2230.1987.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 11.Krueger GG, Feldman SR, Camisa C, Duvic M, Elder JT, Gottlieb AB, Koo J, Krueger JG, Lebwohl M, Lowe N, Menter A, Morison WL, Prystowsky JH, Shupack JL, Taylor JR, Weinstein GD, Barton TL, Rolstad T, Day RM. Two considerations for patients with psoriasis and their clinicians: what defines mild, moderate, and severe psoriasis? What constitutes a clinically significant improvement when treating psoriasis. J Am Acad Dermatol. 2000;43(2 Pt 1):281–285. doi: 10.1067/mjd.2000.106374. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 13.Keijsers RRMC, Hendriks AGM, van Erp PEJ, van Cranenbroek B, van de Kerkhof PCM, Koenen HJPM, Joosten I. In vivo induction of cutaneous inflammation results in the accumulation of extracellular trap-forming neutrophils expressing RORγt and IL-17. J Invest Dermatol. 2014;134(5):1276–1284. doi: 10.1038/jid.2013.526. [DOI] [PubMed] [Google Scholar]
- 14.Jiang M, Fang H, Shao S, Dang E, Zhang J, Qiao P, Yang A, Wang G. Keratinocyte exosomes activate neutrophils and enhance skin inflammation in psoriasis. FASEB J. 2019;33(12):13241–13253. doi: 10.1096/fj.201900642R. [DOI] [PubMed] [Google Scholar]
- 15.Hu SCS, Yu HS, Yen FL, Lin CL, Chen GS, Lan CCE. Neutrophil extracellular trap formation is increased in psoriasis and induces human β-defensin-2 production in epidermal keratinocytes. Sci Rep. 2016;6:31119–31119. doi: 10.1038/srep31119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroda K, Sapadin A, Shoji T, Fleischmajer R, Lebwohl M. Altered expression of angiopoietins and Tie2 endothelium receptor in psoriasis. J Invest Dermatol. 2001;116(5):713–720. doi: 10.1046/j.1523-1747.2001.01316.x. [DOI] [PubMed] [Google Scholar]
- 17.Markham T, Mullan R, Golden-Mason L, Rogers S, Bresnihan B, FitzGerald O, Fearon U, Veale DJ. Resolution of endothelial activation and down-regulation of Tie2 receptor in psoriatic skin after Infliximab therapy. J Am Acad Dermatol. 2006;54(6):1003–1012. doi: 10.1016/j.jaad.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M, Gratton D, Kunynetz RA, Poulin Y, Rosoph LA, Stingl G, Bauer WM, Salter JM, Falk TM, Blödorn-Schlicht NA, Hueber W, Sommer U, Schumacher MM, Peters T, Kriehuber E, Lee DM, Wieczorek GA, Kolbinger F, Bleul CC. Evidence that a neutrophil–keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol. 2015;24(7):529–535. doi: 10.1111/exd.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 20.van der Fits L, Mourits S, Voerman JSA, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 21.Sakkas LI, Bogdanos DP. Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun Rev. 2017;16(1):10–15. doi: 10.1016/j.autrev.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Liang Y, Sarkar MK, Tsoi LC, Gudjonsson JE. Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr Opin Immunol. 2017;49:1–8. doi: 10.1016/j.coi.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J Exp Med. 2005;202(1):135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabat R, Philipp S, Höflich C, Kreutzer S, Wallace E, Asadullah K, Volk HD, Sterry W, Wolk K. Immunopathogenesis of psoriasis. Exp Dermatol. 2007;16(10):779–798. doi: 10.1111/j.1600-0625.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- 25.Campa M, Mansouri B, Warren R, Menter A. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis. Dermatol Ther (Heidelberg) 2016;6(1):1–12. doi: 10.1007/s13555-015-0092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bissonnette R, Fuentes-Duculan J, Mashiko S, Li X, Bonifacio KM, Cueto I, Suárez-Fariñas M, Maari C, Bolduc C, Nigen S, Sarfati M, Krueger JG. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J Dermatol Sci. 2017;85(1):20–26. doi: 10.1016/j.jdermsci.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, Ruether A, Schreiber S, Weichenthal M, Gladman D, Rahman P, Schrodi SJ, Prahalad S, Guthery SL, Fischer J, Liao W, Kwok PY, Menter A, Lathrop GM, Wise CA, Begovich AB, Voorhees JJ, Elder JT, Krueger GG, Bowcock AM, Abecasis GR, Collaborative Association Study of Psoriasis Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nat Genet. 2009;41(2):199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, Housseau F, Yu H, Pardoll DM, Drake CG. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179(7):4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 29.Santini SM, Lapenta C, Donati S, Spadaro F, Belardelli F, Ferrantini M. Interferon-α-conditioned human monocytes combine a Th1-orienting attitude with the induction of autologous Th17 responses: role of IL-23 and IL-12. PLoS One. 2011;6(2):e17364–e17364. doi: 10.1371/journal.pone.0017364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silfvast-Kaiser A, Paek SY, Menter A. Anti-IL17 therapies for psoriasis. Expert Opin Biol Ther. 2019;19(1):45–54. doi: 10.1080/14712598.2019.1555235. [DOI] [PubMed] [Google Scholar]
- 32.Singh R, Balogh EA, Feldman SR. Update on IL-17 inhibitors for psoriasis. Curr Dermatol Rep. 2020;9(4):339–352. https://link.springer.com/article/10.1007/s13671-020-00322-1 [Google Scholar]
- 33.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb AB, Koo JYM, Lebwohl M, Lim HW, Van Voorhees AS, Beutner KR, Bhushan R. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61(3):451–485. doi: 10.1016/j.jaad.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Reis J, Vender R, Torres T. Bimekizumab: the first dual inhibitor of interleukin (IL)-17A and IL-17F for the treatment of psoriatic disease and ankylosing spondylitis. BioDrugs. 2019;33(4):391–399. doi: 10.1007/s40259-019-00361-6. [DOI] [PubMed] [Google Scholar]
- 35.Tsukazaki H, Kaito T. The role of the IL-23/IL-17 pathway in the pathogenesis of spondyloarthritis. Int J Mol Sci. 2020;21(17):6401–6401. doi: 10.3390/ijms21176401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daudén E, Carretero G, Rivera R, Ferrándiz C, Llamas-Velasco M, de la Cueva P, Belinchón I, Gómez-García FJ, Herrera-Acosta E, Ruiz-Genao DP, Ferrán-Farrés M, Alsina M, Baniandrés-Rodríguez O, Sánchez-Carazo JL, Sahuquillo-Torralba A, Fernández-Freire LR, Vilar-Alejo J, García-Donoso C, Carrascosa JM, Herrera-Ceballos E, López-Estebaranz JL, Botella-Estrada R, Segovia-Muñoz E, Descalzo MA, García-Doval I, BIOBADADERM Study Group Long-term safety of nine systemic medications for psoriasis: a cohort study using the Spanish Registry of Adverse Events for Biological Therapy in Dermatological Diseases (BIOBADADERM) Registry. J Am Acad Dermatol. 2020;83(1):139–150. doi: 10.1016/j.jaad.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Gudjónsson JE, Kárason A, Antonsdóttir AA, Rúnarsdóttir EH, Gulcher JR, Stefánsson K, Valdimarsson H. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118(2):362–365. doi: 10.1046/j.0022-202x.2001.01656.x. [DOI] [PubMed] [Google Scholar]
- 38.Samarasekera E, Sawyer L, Parnham J, Smith CH, Guideline Development Group Assessment and management of psoriasis: summary of NICE guidance. BMJ. 2012;345:e6712–e6712. doi: 10.1136/bmj.e6712. [DOI] [PubMed] [Google Scholar]
- 39.Mehlis SL, Gordon KB. The immunology of psoriasis and biologic immunotherapy. J Am Acad Dermatol. 2003;49(2 Suppl):S44–S50. doi: 10.1016/s0190-9622(03)01134-4. [DOI] [PubMed] [Google Scholar]
- 40.Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002;46(1):1–23; quiz 23-26. doi: 10.1067/mjd.2002.120568. [DOI] [PubMed] [Google Scholar]
- 41.Bugaut H, Aractingi S. Major role of the IL17/23 axis in psoriasis supports the development of new targeted therapies. Front Immunol. 2021;12:621956–621956. doi: 10.3389/fimmu.2021.621956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mylonas A, Conrad C. Psoriasis: classical vs. paradoxical. The Yin–Yang of TNF and type I interferon. Front Immunol. 2018;9:2746–2746. doi: 10.3389/fimmu.2018.02746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gisondi P, Bellinato F, Girolomoni G, Albanesi C. Pathogenesis of chronic plaque psoriasis and its intersection with cardio-metabolic comorbidities. Front Pharmacol. 2020;11:117–117. doi: 10.3389/fphar.2020.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69(2):89–94. doi: 10.1016/j.jdermsci.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Muñoz-Aceituno E, Martos-Cabrera L, Ovejero-Benito MC, Reolid A, Abad-Santos F, Daudén E. Pharmacogenetics update on biologic therapy in psoriasis. Medicina (Kaunas) 2020;56(12):719–719. doi: 10.3390/medicina56120719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goel N, Stephens S. Certolizumab pegol. MAbs. 2010;2(2):137–147. doi: 10.4161/mabs.2.2.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen TU, Koo J. Etanercept in the treatment of plaque psoriasis. Clin Cosmet Investig Dermatol. 2009;2:77–84. doi: 10.2147/ccid.s3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michailidou A, Trenz HJ, de Wilde P. In: The Internet and European integration: pro- and anti-EU debates in online news media. 1. Michailidou A, Trenz HJ, de Wilde P, editors. Leverkusen Germany: Verlag Barbara Budrich; 2014. Annex I: Sampling of articles for quantitative and qualitative coding; pp. 167–172.https://www.jstor.org/stable/j.ctvdf0dxq?turn_away=true [Google Scholar]
- 49.Papp KA, Keystone EC, Shear NH. Mechanism of action, pharmacokinetics, and drug interactions of Etanercept in dermatology. J Cutan Med Surg. 2007;11(2 Suppl):S3–S13. https://journals.sagepub.com/doi/abs/10.2310/7750.2006.00069?journalCode=cmsa [Google Scholar]
- 50.*** Annex I. Netherlands Yearbook of International Law. 1985;16:279–300. https://www.cambridge.org/core/journals/netherlands-yearbook-of-international-law/article/abs/annex-i/DA9A8831E27E96F6B11B00EC8A5ED16D [Google Scholar]
- 51.***. ZYPREXA Food and Drug Administration (FDA) Label. Eli Lilly and Company, Indianapolis, IN, USA, 2009. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020592s051,021086s030,021253s036lbl.pdf[ Accessed 14.01.2022]
- 52.Gall JS, Kalb RE. Infliximab for the treatment of plaque psoriasis. Biologics. 2008;2(1):115–124. doi: 10.2147/btt.s2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.European Medicines Agency (EMA). AMGEVITA Assessment Report. International non-proprietary name: Adalimumab, Committee for Medicinal Products for Human Use (CHMP), EMA/106922/2017, Procedure No. EMEA/H/C/004212/0000, 26 January 2017. Available at: https://www.ema.europa.eu/en/documents/assessment-report/amgevita-epar-public-assessment-report_en.pdf[ Accessed 14.01.2022]
- 54.***. HUMIRA FDA Label. AbbVie Inc. North Chicago, IL, USA, 2021. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125057s417lbl.pdf[ Accessed 14.01.2022]
- 55.Markus R, McBride HJ, Ramchandani M, Chow V, Liu J, Mytych D, Fanjiang G. A review of the totality of evidence supporting the development of the first Adalimumab biosimilar ABP 501. Adv Ther. 2019;36(8):1833–1850. doi: 10.1007/s12325-019-00979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, Vanvoorden V, Madden C, White K, Cioffi C, Blauvelt A. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475–486. doi: 10.1016/S0140-6736(21)00126-4. [DOI] [PubMed] [Google Scholar]
- 57.Deodhar A, Mease PJ, McInnes IB, Baraliakos X, Reich K, Blauvelt A, Leonardi C, Porter B, Das Gupta A, Widmer A, Pricop L, Fox T. Long-term safety of Secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther. 2019;21(1):111–111. doi: 10.1186/s13075-019-1882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krueger JG, Wharton KA, Schlitt T, Suprun M, Torene RI, Jiang X, Wang CQ, Fuentes-Duculan J, Hartmann N, Peters T, Koroleva I, Hillenbrand R, Letzkus M, Yu X, Li Y, Glueck A, Hasselberg A, Flannery B, Suárez-Fariñas M, Hueber W. IL-17A inhibition by Secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol. 2019;144(3):750–763. doi: 10.1016/j.jaci.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 59.Mercurio L, Failla CM, Capriotti L, Scarponi C, Facchiano F, Morelli M, Rossi S, Pagnanelli G, Albanesi C, Cavani A, Madonna S. Interleukin (IL)-17/IL-36 axis participates to the crosstalk between endothelial cells and keratinocytes during inflammatory skin responses. PLoS One. 2020;15(4):e0222969–e0222969. doi: 10.1371/journal.pone.0222969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Genovese MC, Mysler E, Tomita T, Papp KA, Salvarani C, Schwartzman S, Gallo G, Patel H, Lisse JR, Kronbergs A, Leage SL, Adams DH, Xu W, Marzo-Ortega H, Lebwohl MG. Safety of Ixekizumab in adult patients with plaque psoriasis, psoriatic arthritis and axial spondyloarthritis: data from 21 clinical trials. Rheumatology (Oxford) 2020;59(12):3834–3844. doi: 10.1093/rheumatology/keaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Craig S, Warren RB. Ixekizumab for the treatment of psoriasis: up-to-date. Expert Opin Biol Ther. 2020;20(6):549–557. doi: 10.1080/14712598.2020.1729736. [DOI] [PubMed] [Google Scholar]
- 62.Oliveira DG, Faria R, Torres T. An overview of Bimekizumab for the treatment of psoriatic arthritis: the evidence so far. Drug Des Devel Ther. 2021;15:1045–1053. doi: 10.2147/DDDT.S267405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freitas E, Torres T. Bimekizumab: the new drug in the biologics armamentarium for psoriasis. Drugs Context. 2021;10:2021-4–1. doi: 10.7573/dic.2021-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rivera-Oyola R, Stanger R, Litchman GH, Thibodeaux Q, Koo J, Fried R, Goldenberg G, Han G, Hsu S, Kircik L, Knuckles M, Murina A, Weinberg J, Wu JJ, Lebwohl M. The use of Brodalumab in three patients with psoriasis and psychiatric comorbidities. J Clin Aesthet Dermatol. 2020;13(12):44–48. [PMC free article] [PubMed] [Google Scholar]
- 65.Puig L. Brodalumab: the first anti-IL-17 receptor agent for psoriasis. Drugs Today (Barcelona) 2017;53(5):283–297. doi: 10.1358/dot.2017.53.5.2613690. [DOI] [PubMed] [Google Scholar]
- 66.Grän F, Kerstan A, Serfling E, Goebeler M, Muhammad K. Current developments in the immunology of psoriasis. Yale J Biol Med. 2020;93(1):97–110. [PMC free article] [PubMed] [Google Scholar]
- 67.Sakkas LI, Zafiriou E, Bogdanos DP. Mini Review: New treatments in psoriatic arthritis. Focus on the IL-23/17 axis. Front Pharmacol. 2019;10:872–872. doi: 10.3389/fphar.2019.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nogueira M, Torres T. Guselkumab for the treatment of psoriasis – evidence to date. Drugs Context. 2019;8:212594–212594. doi: 10.7573/dic.212594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blair HA. Risankizumab: a review in moderate to severe plaque psoriasis. Drugs. 2020;80(12):1235–1245. doi: 10.1007/s40265-020-01357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teng MWL, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, Cua DJ. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21(7):719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 71.Pastor-Fernández G, Mariblanca IR, Navarro MN. Decoding IL-23 signaling cascade for new therapeutic opportunities. Cells. 2020;9(9):2044–2044. doi: 10.3390/cells9092044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menter A, Krueger GG, Paek SY, Kivelevitch D, Adamopoulos IE, Langley RG. Interleukin-17 and interleukin-23: a narrative review of mechanisms of action in psoriasis and associated comorbidities. Dermatol Ther (Heidelberg) 2021;11(2):385–400. doi: 10.1007/s13555-021-00483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dand N, Duckworth M, Baudry D, Russell A, Curtis CJ, Lee SH, Evans I, Mason KJ, Alsharqi A, Becher G, Burden AD, Goodwin RG, McKenna K, Murphy R, Perera GK, Rotarescu R, Wahie S, Wright A, Reynolds NJ, Warren RB, Griffiths CEM, Smith CH, Simpson MA, Barker JN, BADBIR Study Group. BSTOP Study Group. PSORT Consortium HLA-C*06:02 genotype is a predictive biomarker of biologic treatment response in psoriasis. J Allergy Clin Immunol. 2019;143(6):2120–2130. doi: 10.1016/j.jaci.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 74.Burlando M, Russo R, Clapasson A, Carmisciano L, Stecca A, Cozzani E, Parodi A. The HLA-Cw6 dilemma: is it really an outcome predictor in psoriasis patients under biologic therapy? A monocentric retrospective analysis. J Clin Med. 2020;9(10):3140–3140. doi: 10.3390/jcm9103140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trembath RC, Clough RL, Rosbotham JL, Jones AB, Camp RDR, Frodsham A, Browne J, Barber R, Terwilliger J, Lathrop GM, Barker JNWN. Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum Mol Genet. 1997;6(5):813–820. doi: 10.1093/hmg/6.5.813. [DOI] [PubMed] [Google Scholar]
- 76.Capon F, Munro M, Barker J, Trembath R. Searching for the major histocompatibility complex psoriasis susceptibility gene. J Invest Dermatol. 2002;118(5):745–751. doi: 10.1046/j.1523-1747.2002.01749.x. [DOI] [PubMed] [Google Scholar]
- 77.Capon F, Novelli G, Semprini S, Clementi M, Nudo M, Vultaggio P, Mazzanti C, Gobello T, Botta A, Fabrizi G, Dallapiccola B. Searching for psoriasis susceptibility genes in Italy: genome scan and evidence for a new locus on chromosome 1. J Invest Dermatol. 1999;112(1):32–35. doi: 10.1046/j.1523-1747.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- 78.Shen M, Lim SWD, Tan ES, Oon HH, Ren EC. HLA correlations with clinical phenotypes and risk of metabolic comorbidities in Singapore Chinese psoriasis patients. Mol Diagn Ther. 2019;23(6):751–760. doi: 10.1007/s40291-019-00423-z. [DOI] [PubMed] [Google Scholar]
- 79.Nair RP, Stuart PE, Nistor I, Hiremagalore R, Chia NVC, Jenisch S, Weichenthal M, Abecasis GR, Lim HW, Christophers E, Voorhees JJ, Elder JT. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet. 2006;78(5):827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wiśniewski A, Matusiak Ł, Szczerkowska-Dobosz A, Nowak I, Kuśnierczyk P. HLA-C*06:02-independent, gender-related association of PSORS1C3 and PSORS1C1/CDSN single-nucleotide polymorphisms with risk and severity of psoriasis. Mol Genet Genomics. 2018;293(4):957–966. doi: 10.1007/s00438-018-1435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costanzo A, Bianchi L, Flori ML, Malara G, Stingeni L, Bartezaghi M, Carraro L, Castellino G, SUPREME Study Group Secukinumab shows high efficacy irrespective of HLA-Cw6 status in patients with moderate-to-severe plaque-type psoriasis: SUPREME Study. Br J Dermatol. 2018;179(5):1072–1080. doi: 10.1111/bjd.16705. [DOI] [PubMed] [Google Scholar]
- 82.Li K, Huang CC, Randazzo B, Li S, Szapary P, Curran M, Campbell K, Brodmerkel C. HLA-C*06:02 allele and response to IL-12/23 inhibition: results from the Ustekinumab phase 3 psoriasis program. J Invest Dermatol. 2016;136(12):2364–2371. doi: 10.1016/j.jid.2016.06.631. [DOI] [PubMed] [Google Scholar]
- 83.Mallon E, Bunce M, Savoie H, Rowe A, Newson R, Gotch F, Bunker CB. HLA-C and guttate psoriasis. Br J Dermatol. 2000;143(6):1177–1182. doi: 10.1046/j.1365-2133.2000.03885.x. [DOI] [PubMed] [Google Scholar]
- 84.Gallo E, Cabaleiro T, Román M, Solano-López G, Abad-Santos F, García-Díez A, Daudén E. The relationship between tumour necrosis factor (TNF)-α promoter and IL12B/IL-23R genes polymorphisms and the efficacy of anti-TNF-α therapy in psoriasis: a case-control study. Br J Dermatol. 2013;169(4):819–829. doi: 10.1111/bjd.12425. [DOI] [PubMed] [Google Scholar]
- 85.Wu R, Zeng J, Yuan J, Deng X, Huang Y, Chen L, Zhang P, Feng H, Liu Z, Wang Z, Gao X, Wu H, Wang H, Su Y, Zhao M, Lu Q. MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J Clin Invest. 2018;128(6):2551–2568. doi: 10.1172/JCI97426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang L, He S, Zhu Y, Feng B, Su Z, Liu B, Xu F, Wang X, Liu H, Li C, Zhao J, Zheng X, Li C, Sun C, Lu C, Zheng G. Downregulated miR-187 contributes to the keratinocytes hyperproliferation in psoriasis. J Cell Physiol. 2019;234(4):3661–3674. doi: 10.1002/jcp.27135. [DOI] [PubMed] [Google Scholar]
- 88.Bian J, Liu R, Fan T, Liao L, Wang S, Geng W, Wang T, Shi W, Ruan Q. miR-340 alleviates psoriasis in mice through direct targeting of IL-17A. J Immunol. 2018;201(5):1412–1420. doi: 10.4049/jimmunol.1800189. [DOI] [PubMed] [Google Scholar]
- 89.Soonthornchai W, Tangtanatakul P, Meephansan J, Ruchusatsawat K, Reantragoon R, Hirankarn N, Wongpiyabovorn J. Down-regulation of miR-155 after treatment with narrow-band UVB and Methotrexate associates with apoptosis of keratinocytes in psoriasis. Asian Pac J Allergy Immunol. 2021;39(3):206–213. doi: 10.12932/AP-031218-0451. [DOI] [PubMed] [Google Scholar]
- 90.Elango T, Thirupathi A, Subramanian S, Ethiraj P, Dayalan H, Gnanaraj P. Methotrexate treatment provokes apoptosis of proliferating keratinocyte in psoriasis patients. Clin Exp Med. 2017;17(3):371–381. doi: 10.1007/s10238-016-0431-4. [DOI] [PubMed] [Google Scholar]
- 91.Hou RX, Liu RF, Zhao XC, Jia YR, An P, Hao ZP, Li JQ, Li XH, Yin GH, Zhang KM. Increased miR-155-5p expression in dermal mesenchymal stem cells of psoriatic patients: comparing the microRNA expression profile by microarray. Genet Mol Res. 2016;15(3) doi: 10.4238/gmr.15038631. [DOI] [PubMed] [Google Scholar]
- 92.An G, Liang S, Sheng C, Liu Y, Yao W. Upregulation of microRNA-205 suppresses vascular endothelial growth factor expression-mediated PI3K/Akt signaling transduction in human keloid fibroblasts. Exp Biol Med (Maywood) 2017;242(3):275–285. doi: 10.1177/1535370216669839. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]