Abstract
Purpose
To perform longitudinal analysis of retinal arterial macroaneurysms in 3 patients with adult-onset Coats disease.
Observations
Three eyes of three patients with adult-onset Coats disease were followed longitudinally for 4–15 years. Ultra-widefield images and montage color fundus photographs of affected eyes were analyzed. Size, retinal location, and grading for predominant characteristic (hemorrhagic, exudative, or quiescent) of each individual macroaneurysm were followed longitudinally from the time of onset. Fifty-one individual retinal arterial macroaneurysms were identified. The distance of any lesion-associated hemorrhage or exudation present from the foveal center was measured. Macroaneurysms were located in all quadrants of the retina, with the majority (37/51) graded as hemorrhagic at lesion onset. Hemorrhagic and exudative macroaneurysms that entered the quiescent phase remained quiescent for an average of 26 months. Seven macroaneurysms were found to have hemorrhage or exudation that came within 125 μm of the fovea and all three eyes followed demonstrated a longitudinal decrease in visual acuity despite laser and intravitreal injection therapy. At the initial visit, visual acuities ranged from 20/40 to 20/200, but decreased to 20/80 to 20/320 by the last follow-up visit.
Conclusion and Importance
There are many challenges in treating patients with adult-onset Coats disease. Long-term loss of visual acuity often results from sequelae of hemorrhage and exudation affecting the macula.
Keywords: Coats disease, Adult-onset, Intravitreal anti-vascular endothelial growth factor, Retinal arterial macroaneurysm
1. Introduction
Coats disease is an idiopathic, largely unilateral disorder that targets the retinal vasculature.1 It is characterized by telangiectatic and aneurysmal retinal vessels with intraretinal and subretinal exudation.1 Coats disease shows a strong predilection for males and is often diagnosed in childhood, presenting with vision loss, strabismus, or leukocoria.1, 2, 3 When diagnosed in adulthood, Coats disease has similar retinal vascular abnormalities as those seen in childhood cases, but tends to involve a smaller area, progress more slowly, and result more frequently in hemorrhage close to large vascular dilatations.2,4 Some studies have suggested bilateral involvement in adult-onset disease.4,5 Somatic mutations of implicated genes, such as NDP and ABCA4, have been associated with some cases of pediatric Coats disease.6,7
Tarkkanen et al. previously described the predominance of damage to the arterial system in Coats disease.8,9 While spontaneous regression of aneurysms in Coats disease has been reported, treatment frequently focuses on resolving exudation and preventing disease progression by ablating the abnormal macroaneurysms.3,10,11 Laser photocoagulation, cryotherapy, intravitreal anti-vascular endothelial growth factor (anti-VEGF), drainage of subretinal fluid, and vitrectomy have been used in the treatment of Coats disease.3,5,12 If left untreated, Coats disease can lead to secondary glaucoma and total retinal detachment.1,2,13
Little is known about the long-term progression of macroaneurysms in adult Coats disease and the effectiveness of administered treatments on preserving vision in affected eyes. Focal laser photocoagulation can induce regression of macroaneurysms and previous imaging studies have shown that the affected vessel remains looped 3 months after treatment.2 In this case series, we report the clinical course of 3 patients with adult-onset Coats disease, specifically analyzing the appearance and progression of multiple macroaneurysms in each patient across 4–15 years. These findings provide insight into the pathogenesis of Coats disease and may help guide future management decisions.
2. Findings
The records of 3 patients with predominantly macroaneurysmal Coats disease followed at the National Eye Institute in Bethesda, MD were retrospectively reviewed. Informed consent was given for treatment in standard of care protocols approved by an NIH-based institutional review board. Patients had a tailored treatment approach which included some of the following: laser, sub-Tenons triamcinolone injections and intravitreal injections using bevacizumab, aflibercept, preservative-free triamcinolone, and a dexamethasone intravitreal implant (Ozurdex®). These interventions utilize medications in an off-label manner although their use in treating macroaneurysms of different etiologies has become part of the standard treatment armamentarium. Patients were imaged ∼4 times/year and images consisted of montage Topcon TRC 50-DX (Topcon Medical Systems, Oakland, NJ) 50-degree color fundus photographs for visits prior to 2012 (Fig. 1). Optos 200Tx (Optos, Marlborough, MA) ultra-widefield retinal imaging was introduced in 2012 and became the primary imaging modality in subsequent years. In April 2020, ultra-widefield imaging was transitioned to Optos P200DTx (Optos, Marlborough, MA). Color images and fluorescein angiography (FA), when performed, were analyzed for each affected eye.
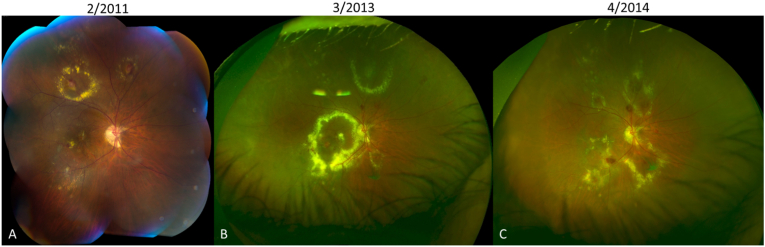
Fig. 1.
Montage color fundus photograph (A) and Optos ultra-widefield retinal images (B and C) showing widespread distribution of macroaneurysms with hemorrhage and/or exudates in all quadrants of the retina and longitudinal progression of activity. In 2011 (A), patient 1 presents with a macroaneurysm in the inferotemporal macula with a small amount of exudation, which is not responsive to focal laser or intravitreal bevacizumab and progresses to a large foveal-involving exudate in 2013 (B). After receiving intravitreal triamcinolone, the exudation is much improved in 2014 (C), but vision is limited by residual subfoveal scarring. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In order to follow the macroaneurysms longitudinally, all images of an eye were aligned spatially to the most recent ultra-widefield image. Corresponding point-landmarks (e.g., vessel bifurcations) across image pairs were manually identified and affine spatial transformation was computed such that the squared distance between the corresponding landmarks was minimized after alignment. This step achieved the alignment, correcting for any differences in translation, rotation, scale, and skew between longitudinally acquired images.
For each eye, the aligned images were chronologically ordered and the macroaneurysms were systematically analyzed using ImageJ.14 Macroaneurysms were defined on color images as a circular dilatation along a blood vessel frequently associated with hemorrhage and/or exudation. FA provided corroborating information when available. The date of onset, diameter at onset, and location at onset of each macroaneurysm were noted based on the color image. Activity of the macroaneurysm as predominantly hemorrhagic, exudative, or quiescent was noted at each time point. To determine the potential of each macroaneurysm to threaten visual acuity, the minimum distance to the fovea from the posterior edge of any associated hemorrhage or exudate was measured.
2.1. Case 1
A 65-year-old Hispanic male with a history of benign prostatic hypertrophy presented in 2009 with a 2-year history of decreased central vision of the right eye due to retinal exudation from an idiopathic vascular lesion, in the context of adult-onset Coats disease. He had previously undergone focal laser photocoagulation treatments with subsequent closure of the vascular lesion and resolving macular lipid and edema. In our clinic, ocular examination revealed best corrected visual acuities (BCVA) of 20/200 in the right eye and 20/32 in the left eye. The anterior segment examination was within normal limits bilaterally, with pseudophakia in the right eye and a cataract in the left eye. Dilated fundus examination and FA of the right eye showed multiple arterial macroaneurysms consistent with Coats disease and indocyanine green angiography revealed an abnormal choroidal vasculature with polypoidal features and a retinal-choroidal anastomosis underlying an area of hemorrhage along the superior arcade.
He was initially treated with focal laser photocoagulation applied to peripheral lesions, but, given the amount of lipid and intraretinal fluid in the macula, was also treated with an anterior sub-Tenon's triamcinolone injection and photodynamic therapy to the proximal complex vascular lesion along the superior arcade (Fig. 1). Over the course of 11 years of follow-up, he continued to develop new macroaneurysms with exudation (Fig. 2), which were treated with ∼20 sessions of focal laser photocoagulation. As the multiple evolving lesions were sometimes accompanied by lipid and fluid that threatened the macular region, non-focal therapies were also pursued. The patient underwent three intravitreal injections of bevacizumab 1.25 mg, one of bevacizumab 2.5 mg, two intravitreal injections of preservative-free triamcinolone 2 mg, followed by two intravitreal injections of dexamethasone intravitreal implant (Ozurdex™). Although intravitreal steroids were successful in decreasing lipid exudation, as seen in Fig. 3, his clinical course was complicated by a steroid-related increase in intraocular pressure (IOP), which was treated with topical antihypertensive medications. The fellow eye developed a central retinal vein occlusion, which was treated with intravitreal bevacizumab and sectoral panretinal photocoagulation. He subsequently also underwent cataract extraction of the fellow eye.
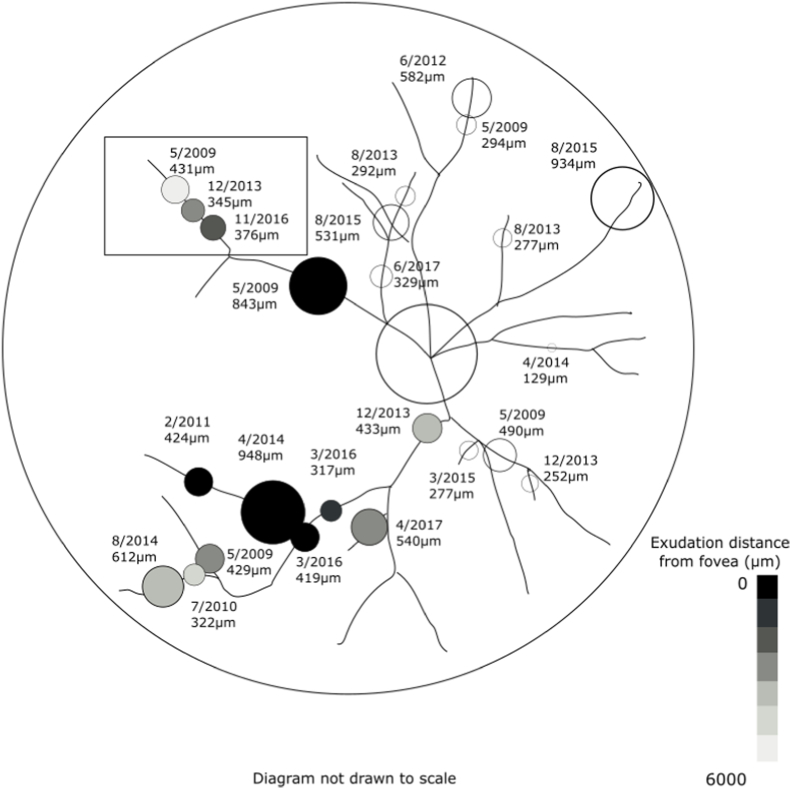
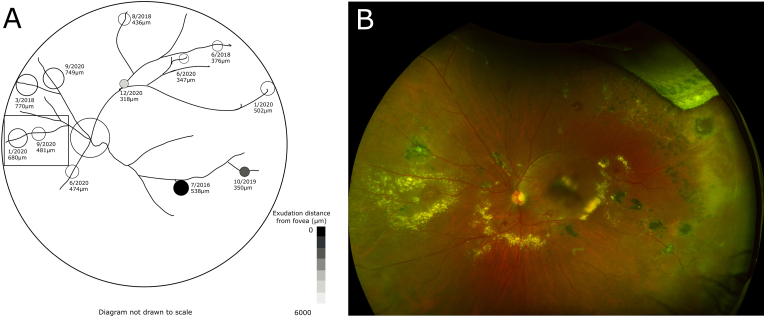
Fig. 2.
Each macroaneurysm is labeled with its date and diameter at appearance from case 1. Proximal progression of macroaneurysms is shown in the boxed area.
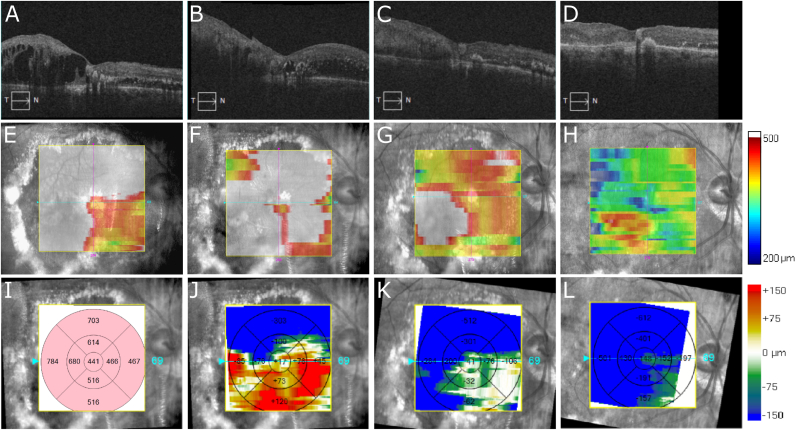
Fig. 3.
Optical coherence tomography scans (OCT; A-D), macular thickness analysis maps (E–H), baseline thickness analysis map from 12/2012 in microns (I), and relative change in macular thickness analysis maps from baseline (J–L) of the right eye of the patient in case 1. In 12/2012 (A and E), there is significant cystoid macular edema (CME). Following 3 injections of intravitreal bevacizumab, there is no improvement of CME in 3/2013 (B,F, and J). One month following a single intravitreal injection of preservative-free triamcinolone 2 mg, CME in 4/2013 is much improved (C, G, and K). With no additional intervention, there is continued response and resolution of CME in 8/2013 (D, H, and L), 5 months following intravitreal triamcinolone. Areas of white in panels E–H correspond to thicknesses greater than 500 μm.
At his most recent visit in September 2020, his right eye had a subfoveal scar with BCVA measuring 20/320, and 20/16 in the left eye.
2.2. Case 2
A 51-year-old African American female with a history of systemic hypertension, gastroesophageal reflux disease, hiatal hernia, and ductal carcinoma in situ of the right breast diagnosed in 2018, first presented to our clinic in 2005 with a 4-year history of hemorrhage and exudation in the left retina and a history of treatment with 4 sessions of focal laser photocoagulation. Ocular examination on presentation revealed BCVAs of 20/16 in the right eye and 20/40 in the left eye. The retinal examination showed multiple retinal arterial macroaneurysms with hard exudates and edema threatening the left fovea. Evaluation with FA identified perifoveal telangiectasias as a source of this leakage, leading to intervention with anterior sub-Tenon's triamcinolone, which did lead to improvement in macular lipid, but was complicated by a steroid-induced IOP elevation necessitating removal of the steroid depot 5 months later. During the subsequent 15-year treatment course, she developed multiple arterial macroaneurysms (Fig. 4), which were treated with ∼30 sessions of focal laser and sectoral panretinal photocoagulation to areas of peripheral nonperfusion. When the exudation from the lesions threatened the fovea despite focal photocoagulation, a course of oral prednisone was started but was poorly tolerated and intravitreal bevacizumab 1.25 mg was tried, both without improvement. While the patient had significant adverse effects from the local steroid, the previous positive disease response to steroid prompted treatment with intravitreal triamcinolone 2 mg and later, two intravitreal dexamethasone implants (Ozurdex®) were performed for significant vision-threatening exudation under close glaucoma follow-up. While the patient developed ocular hypertension of both eyes, her IOP was able to be controlled with topical glaucoma therapy.
Fig. 4.
(A) Each macroaneurysm is labeled with its date and diameter at appearance from case 2. Proximal progression of macroaneurysms is shown in the boxed area. Ultra-widefield retinal imaging from 3/2020 (B) demonstrates macroaneurysms with active hemorrhage superiorly, scarring within the macula and periphery, and temporal vascular sclerosis.
At her most recent visit in March of 2020, BCVA was 20/12.5 in the right eye and 20/80 in the left eye. Although she had no macular exudation, central vision in the left eye was limited by subfoveal scarring and cavitations on optical coherence tomography (OCT).
2.3. Case 3
A 49-year-old African American male with a history of systemic hypertension first presented with blurry central vision in the left eye in 2016. His BCVAs were 20/20 in the right eye and 20/40 in the left eye. Dilated fundus examination of the left eye revealed macular edema secondary to anomalous parafoveal capillaries. The peripheral retina had variably-sized arterial aneurysms in all quadrants and temporal vessel nonperfusion on FA. He was lost to follow-up until 2018, when his BCVA had decreased to 20/200 in the left eye. At that time, examination of the left retina revealed substantial lipid deposition in the macula, which was treated with intravitreal preservative-free triamcinolone 3 mg in an attempt to minimize scarring. Focal laser photocoagulation was applied to peripheral aneurysms. Over the next 2 years (Fig. 5), he received additional treatment with 5 intravitreal injections of aflibercept 2 mg, with only minimal change in retinal exudation and, subsequently, placement of an intravitreal dexamethasone implant (Ozurdex®); this successfully decreased the macular exudation, but resulted in no subjective visual improvement. He underwent ∼8 sessions of focal laser to peripheral aneurysms and scatter laser to areas of peripheral nonperfusion.
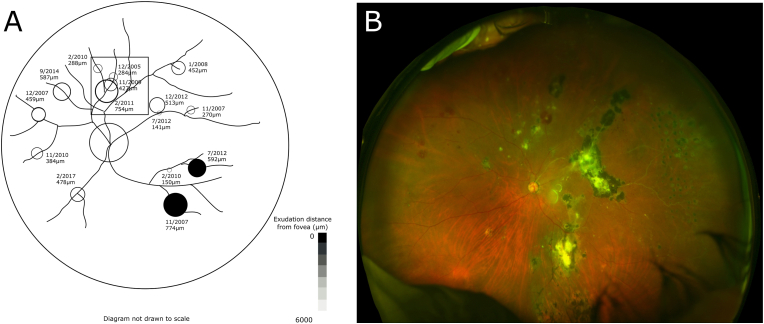
Fig. 5.
(A) Each macroaneurysm is labeled with its date and diameter at appearance from case 3. Proximal progression of macroaneurysms is shown in the boxed area. An ultra-widefield retinal image from 9/2020 (B) demonstrates multiple macroaneurysms with surrounding exudation and actively hemorrhaging macroaneurysms throughout the retina.
At his most recent visit in December 2020, BCVA was 20/20 in the right eye and 20/160 in the left eye. Although lipid reaccumulation in the left macula was noted, vision was limited by subfoveal scarring and he declined further intravitreal injections due to lack of subjective improvement with previous injections.
3. Discussion
Over the course of 4–15 years of follow-up, all 3 patients in this series had development and progression of arterial macroaneurysms. To further understand the spatial impact of the disease on the retina, the macroaneurysms were quantified on color imaging. Macroaneurysms were observed in arteries in all quadrants in all patients and new macroaneurysms were discovered at an average rate of 1.6 per year. At presentation, most aneurysms appeared hemorrhagic, as seen in Table 1. The average size of macroaneurysms at presentation was 459 ± 191 (mean ± standard deviation) μm located 7961 ± 4190 μm from the optic nerve. Macroaneurysms entered a phase of quiescence at an average of 26 ± 17 months from discovery.
Table 1.
Features of macroaneurysms present in case patients.
| Case 1 | Case 2 | Case 3 | Overall | |
|---|---|---|---|---|
| Duration of follow-up (years) | 11 | 15 | 4 | |
| Best corrected visual acuity of affected eye at first presentation | 20/200 | 20/40 | 20/40 | |
| Best corrected visual acuity of affected eye at last follow-up visit | 20/320 | 20/80 | 20/160 | |
| Total # aneurysms (mean distance from the optic nerve [μm]) | 24 (6788 ± 2612) | 15 (6189 ± 1511) | 12 (12522 ± 5713) | 51 (7961 ± 4190) |
| Location | ||||
| # Superotemporal (mean distance from the optic nerve [μm]) | 4 (7159 ± 2579) | 4 (6037 ± 2678) | 5 (15308 ± 6828) | 13 (9948 ± 6218) |
| # Superonasal (mean distance from the optic nerve [μm]) | 8 (7202 ± 3362) | 6 (5903 ± 748) | 4 (12456 ± 891) | 18 (7936 ± 3388) |
| # Inferotemporal (mean distance from the optic nerve [μm]) | 9 (7008 ± 2106) | 3 (6444 ± 1641) | 2 (10809 ± 4540) | 14 (7430 ± 2668) |
| # Inferonasal (mean distance from the optic nerve [μm]) | 3 (4530 ± 724) | 2 (6971 ± 535) | 1 (2281) | 6 (4969 ± 1852) |
| Mean # new aneurysms/year | 2.00 ± 1.81 | 0.94 ± 1.12 | 2.40 ± 2.79 | 1.55 ± 1.75 |
| # of aneurysms within 125 μm of the fovea | 4 | 2 | 1 | 7 |
| Mean diameter at presentation (μm) | 451 ± 210 | 437 ± 192 | 502 ± 156 | 459 ± 191 |
| Predominant characteristic at presentation | ||||
| Hemorrhagic | 15 | 13 | 9 | 37 |
| Exudative | 9 | 2 | 3 | 14 |
| Average length of activity (months) | 24.3 ± 15.7 | 30.4 ± 20.2 | 20.4 ± 13.4 | 26.0 ± 17.1 |
Fig. 2, Fig. 4, Fig. 5 show the distribution of the macroaneurysms and the lesions that caused exudation or hemorrhage that were most vision-threatening. Of the 51 aneurysms present across all three patients, seven had exudation that came within 125 μm of the fovea, as shown by the darkly shaded aneurysms in the figures. Larger macroaneurysms located closest to the macula along the inferior and superior arcades and those associated with greater amounts of exudation resulted in the most visual significance. Tracking the macroaneurysms longitudinally, all three patients showed at least one example of new macroaneurysms developing more proximal to the optic nerve along the same vessel, shown in the boxed regions of Fig. 2, Fig. 4, Fig. 5 and highlighted in Fig. 6.
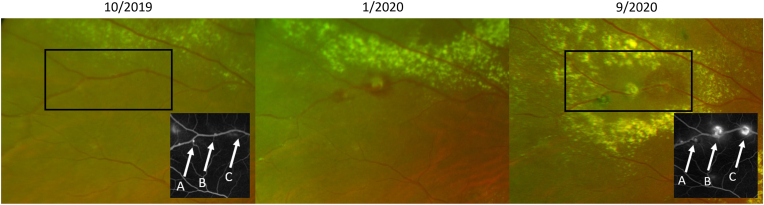
Fig. 6.
Proximal progression of macroaneurysms in case 3. Available fundus fluorescein angiography insets are shown for the regions highlighted by the black rectangles.
Our study found that retinal arterial macroaneurysms occurred in all quadrants of the retina, but the ones that were the most visually significant were frequently located along the arcades closest to the macula. Previous studies have noted that the majority of macroaneurysms tended to develop temporally,1,2,5,15 which may represent a selection bias – patients with Coats disease may not present unless they develop a visually-symptomatic macroaneurysm. With the advent of ultra-widefield imaging, we were able to detect and follow more peripheral aneurysms in this series and demonstrate diffuse distribution of lesions (Fig. 1). Our findings indicate widespread abnormalities in eyes with Coats disease, consistent with pathological findings by Egbert et al.,16 which found no areas of normal retina in the eyes studied. Egbert et al. hypothesized that all retinal vessels in affected eyes possess an inherent defect that variably expresses over time. Patients may initially present with segmental findings clinically, but the disease ultimately progresses into previously uninvolved areas. Macroaneurysms were all detected along arteries in our study, consistent with findings of predominantly arterial changes in previous FA studies.8
Our study used widefield color images to identify macroaneurysms and found that macroaneurysms frequently presented in an active stage with hemorrhage and/or exudation (Fig. 1). Abnormal endothelial permeability is an early event in Coats disease due to structural and/or functional abnormalities, leading to breakdown of the blood-retinal barrier and the subsequent hemorrhage and exudation observed around macroaneurysms.17 Although FA was not used for the primary identification of macroaneurysms in this study, our observation that FA frequently identified more lesions than were visible on color images corroborates the findings of other studies.5 We did not treat lesions visible only on FA and whether those lesions represent early dilatations of future active macroaneurysms and what percentage of those lesions progress is unclear.
Although not universal, we observed that new macroaneurysms frequently occurred more proximally to the nerve along the same vessel as previous macroaneurysms (Fig. 2, Fig. 4, Fig. 5, Fig. 6). Our practice generally favors treatment of new macroaneurysms with focal laser, but due to the multitude of macroaneurysms present in each eye, it was not possible to always determine if/when a macroaneurysm had been previously lasered. Whether this proximal progression represents natural history or a response to focal laser photocoagulation, therefore, remains unknown. However, evidence that the vessel was abnormal prior to the application of laser suggests that there was a pathologic change in the vessel that subsequently evolved to a frank macroaneurysm. Despite concerns about arteriolar occlusion when laser is directly applied to macroaneurysms,18 subsequent FA in these areas of proximal progression showed distal vessel perfusion (Fig. 6). We hypothesize that the presence of a macroaneurysm changes the hemodynamics along the vessel adjacent to the macroaneurysm. Endothelial dysfunction and/or absence have been implicated in the pathogenesis of Coats disease17,19 and might be further stressed in the setting of hemodynamic changes proximal to existing macroaneurysms, leading to the development of new macroaneurysms. Further investigation using other imaging modalities such as OCT angiography, could be helpful to detect macular nonperfusion or capillary dropout after focal laser.20 The endothelial damage and necrosis underlying the pathophysiology of hypertensive retinopathy21 may also promote disease progression in this cohort, given that two of the three patients in this series carry a diagnosis of hypertension. As a result, the retinal vasculature may be more susceptible to hemorrhage in adult-onset disease.2 Other systemic conditions such as hyperlipidemia and diabetes mellitus have also been previously associated with adult-onset Coats disease.2,4,22,23 To our knowledge, there is no known association with other medical conditions reported by the patients in this series.
The patients in this series ultimately developed decreased vision in the affected eye with Coats disease limited by subfoveal scarring frequently following an event of prolonged lipid exudation. That lipid exudation was not necessarily due to lipid from adjacent macroaneurysms; rather, microvascular leakage from parafoveal telangiectatic vessels, as previously described in Coats disease,24 also contributed to vision loss. The exudation was responsive to intraocular steroids, but all patients developed a steroid response (Fig. 3). These patients highlight the clinical dilemma associated with managing Coats disease. Early intervention is needed to decrease foveal lipid exudation, which can be very prolific and lead to scarring in Coats disease. We prefer to treat all visible macroaneurysms with focal laser, as they are easier to target when smaller in size. However, the large number of evolving lesions and substantial exudation may make macroaneurysms difficult to treat to quiescence with laser alone. While other reports have demonstrated improvement in visual acuity with anti-VEGF intravitreal injections,12,22 this effect was not seen in this cohort nor was there a significant effect on the exudation or size of the aneurysm (Fig. 3). This lack of improvement may be attributed to the presence of substantial exudation, which has previously been found to poorly respond to anti-VEGF therapy.12 The results of this series show that greatest attention and aggressive treatment should be given to macroaneurysms along the vascular arcades and closest to the fovea, as activity in these lesions is most likely to have visual consequences.
4. Conclusions
This study is the first to analyze retinal arterial macroaneurysms in adult Coats disease longitudinally and systematically. Using ultra-widefield imaging, we demonstrate widespread distribution of macroaneurysms and provide insight into the progression of these lesions in 3 patients. Despite limitations of the imaging modalities in this retrospective study, this study amplifies descriptive aspects of longitudinal Coats disease. Additional studies are needed to determine why some macroaneurysms regress and others have significant activity. Future work will study a larger cohort of patients to better understand the pathology and treatment outcomes of Coats disease to guide management and preserve vision in affected eyes.
Patient consent
This report does not contain any personal information that could lead to the identification of the patients.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Eye Institute. It was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, and the Colgate-Palmolive Company.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
None; The following authors have no financial disclosures: AD, AT, TD, HW, TK, WW, CC.
Acknowledgements
None.
References
- 1.Dalvin L.A., Udyaver S., Lim L.S., et al. Coats disease: clinical features and outcomes by age category in 351 cases. J Pediatr Ophthalmol Strabismus. Sep 1 2019;56(5):288–296. doi: 10.3928/01913913-20190716-01. [DOI] [PubMed] [Google Scholar]
- 2.Smithen L.M., Brown G.C., Brucker A.J., Yannuzzi L.A., Klais C.M., Spaide R.F. Coats' disease diagnosed in adulthood. Ophthalmology. Jun 2005;112(6):1072–1078. doi: 10.1016/j.ophtha.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L., Ke Y., Wang W., Shi X., Hei K., Li X. The efficacy of conbercept or ranibizumab intravitreal injection combined with laser therapy for Coats' disease. Graefes Arch Clin Exp Ophthalmol. Jul 2018;256(7):1339–1346. doi: 10.1007/s00417-018-3949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rishi E., Rishi P., Appukuttan B., Uparkar M., Sharma T., Gopal L. Coats' disease of adult-onset in 48 eyes. Indian J Ophthalmol. Jul 2016;64(7):518–523. doi: 10.4103/0301-4738.190141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockmann C., Lowen J., Schonfeld S., et al. Vascular findings in primarily affected and fellow eyes of middle-aged patients with Coats' disease using multimodal imaging. Br J Ophthalmol. Oct 2021;105(10):1444–1453. doi: 10.1136/bjophthalmol-2020-317101. [DOI] [PubMed] [Google Scholar]
- 6.Black G.C., Perveen R., Bonshek R., et al. Coats' disease of the retina (unilateral retinal telangiectasis) caused by somatic mutation in the NDP gene: a role for norrin in retinal angiogenesis. Hum Mol Genet. Oct 1999;8(11):2031–2035. doi: 10.1093/hmg/8.11.2031. [DOI] [PubMed] [Google Scholar]
- 7.Saatci A.O., Ayhan Z., Yaman A., Bora E., Ulgenalp A., Kavukcu S. A 12-year-old girl with bilateral Coats disease and ABCA4 gene mutation. Case Rep Ophthalmol. May-Aug 2018;9(2):375–380. doi: 10.1159/000492320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarkkanen A., Laatikainen L. Coat's disease: clinical, angiographic, histopathological findings and clinical management. Br J Ophthalmol. Nov 1983;67(11):766–776. doi: 10.1136/bjo.67.11.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moosavi R.A., Fong K.C., Chopdar A. Retinal artery macroaneurysms: clinical and fluorescein angiographic features in 34 patients. Eye (Lond) Sep 2006;20(9):1011–1020. doi: 10.1038/sj.eye.6702068. [DOI] [PubMed] [Google Scholar]
- 10.Hayasaka S., Katsube T., Yamamoto Y., Setogawa T. Leber's miliary aneurysms in a 63-year-old woman: concurrence of regression and active lesions. Ophthalmologica. 1988;196(4):188–191. doi: 10.1159/000309899. [DOI] [PubMed] [Google Scholar]
- 11.Deutsch T.A.R.M., Jampol L.M. Spontaneous regression of retinal lesions in Coats' disease. Can J Ophthalmol. 1982;17(4):169–172. [PubMed] [Google Scholar]
- 12.Park S., Cho H.J., Lee D.W., Kim C.G., Kim J.W. Intravitreal bevacizumab injections combined with laser photocoagulation for adult-onset Coats' disease. Graefes Arch Clin Exp Ophthalmol. Aug 2016;254(8):1511–1517. doi: 10.1007/s00417-015-3233-6. [DOI] [PubMed] [Google Scholar]
- 13.Shields J.A., Shields C.L., Honavar S.G., Demirci H., Cater J. Classification and management of Coats disease: the 2000 proctor lecture. Am J Ophthalmol. 2001;131(5):572–583. doi: 10.1016/s0002-9394(01)00896-0. [DOI] [PubMed] [Google Scholar]
- 14.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. Jul 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panton R.W., Goldberg M.F., Farber M.D. Retinal arterial macroaneurysms: risk factors and natural history. Br J Ophthalmol. Oct 1990;74(10):595–600. doi: 10.1136/bjo.74.10.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egbert P.R., Chan C.C., Winter F.C. Flat preparations of the retinal vessels in Coats' disease. J Pediatr Ophthalmol. Nov-Dec 1976;13(6):336–339. [PubMed] [Google Scholar]
- 17.Tripathi R., Ashton N. Electron microscopical study of Coat's disease. Br J Ophthalmol. May 1971;55(5):289–301. doi: 10.1136/bjo.55.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer J.C., Ahmad B.U., Blinder K.J., Shah G.K. Laser therapy versus observation for symptomatic retinal artery macroaneurysms. Graefes Arch Clin Exp Ophthalmol. Apr 2015;253(4):537–541. doi: 10.1007/s00417-014-2730-3. [DOI] [PubMed] [Google Scholar]
- 19.Eagle R.C. second ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011. Eye Pathology : An Atlas and Text; p. 310. [Google Scholar]
- 20.Alnawaiseh M., Schubert F., Nelis P., Wirths G., Rosentreter A., Eter N. Optical coherence tomography (OCT) angiography findings in retinal arterial macroaneurysms. BMC Ophthalmol. Jul 22 2016;16:120. doi: 10.1186/s12886-016-0293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tso M.O., Jampol L.M. Pathophysiology of hypertensive retinopathy. Ophthalmology. Oct 1982;89(10):1132–1145. doi: 10.1016/s0161-6420(82)34663-1. [DOI] [PubMed] [Google Scholar]
- 22.Mandura R.A., Alqahtani A.S. Coats' disease diagnosed during adulthood. Cureus. Jul 2021;13(7) doi: 10.7759/cureus.16303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra A., Aggarwal S., Shah S., Negi P., Bharwada R., Desai N. An interesting case of Coats' disease. Med J Armed Forces India. Oct 2015;71(4):384–388. doi: 10.1016/j.mjafi.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes B.F., Odashiro A.N., Maloney S., Zajdenweber M.E., Lopes A.G., Burnier M.N., Jr. Clinical-histopathological correlation in a case of Coats' disease. Diagn Pathol. Aug 30 2006;1:24. doi: 10.1186/1746-1596-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]