Abstract
During the past decade, we have entered an era of biologics for the treatment of Crohn’s disease and ulcerative colitis. The therapeutic goal of inflammatory bowel disease (IBD) management has evolved from symptom control and clinical remission to mucosal healing or even deep remission. Histological remission for ulcerative colitis and transmural healing of Crohn’s disease are potential future goals. With the adoption of the treat-to-target concept, and given the need for tight control of IBD activity, therapeutic drug monitoring (TDM) is an important element of precision medicine. TDM involves the measurement of serum biologics and anti-drug antibodies levels, to confirm whether the right drug with the right dosage was prescribed to reach the right serum levels. TDM may help clinicians adjust biologics based on objective biomarkers instead of using empirical dosage escalation or making symptom-based therapeutic adjustments. Well-established reactive TDM algorithms have been proposed, and emerging evidence supports the clinical application of a proactive TDM strategy to enhance the duration of effective biologics and improve clinical outcomes. Recently, the proactive TDM strategy was shown to avoid the secondary loss of response to biologics, and improve long-term clinical outcomes in IBD patients. This review summarizes data from trials, and practice guidelines, on the clinical application of proactive and reactive TDM strategies for the daily care of biologic-treated IBD patients.
Keywords: Biologics, Crohn’s disease, Inflammatory bowel disease, Therapeutic drug monitoring, Ulcerative colitis
INTRODUCTION
Effective biologics are now available for the management of Crohn’s disease (CD) and ulcerative colitis (UC). Before the era of biologics therapy for inflammatory bowel disease (IBD), up to 70% of patients with CD, and 30% of those with UC, underwent surgery at some stage during the disease course.1-4 Biologics can modify the disease course and reduce the likelihood of surgery in IBD patients.5,6 The therapeutic goal of IBD has changed from clinical remission to mucosal healing or even deep remission.7 Following the adoption of the treat-to-target concept, and given the need for tight control of disease activity, confirmation of mucosal healing, histological remission, and the normalization of biomarkers has become increasingly important in the daily care of both CD and UC patients.7-9
Optimizing the serum biologics levels under the guidance of therapeutic drug monitoring (TDM) is important for the precision care of IBD patients.10-12 TDM involves the objective measurement of serum biologics and anti-drug antibody (ADAb) levels during the induction and maintenance phase of biologics therapy. TDM provides information on pharmacokinetics and pharmacodynamics at the individual patient level, which maximizes the efficacy and duration of the effectiveness of biologics. This enables the objective clarification of the causes of primary non-response (PNR), or secondary loss of response (LOR), and allows proactive optimization of serum drug levels through dose titration or dosage interval adjustment to avoid ADAb-related secondary LOR.
Three biologics classes have been approved for the treatment of CD and UC patients: anti-tumor necrosis factor α (anti-TNF-α), anti-integrin, and anti-interleukin agents. Differences in immunogenicity for the development of ADAbs have been observed among the various classes of biologics.13-15 Thus, different needs of immune modulator combinations and different TDM strategies may be required. Two main TDM strategies for IBD have been proposed; reactive (for patients with active IBD disease activity), and proactive (for patients with quiescent disease).
In this review, we discuss “How, When, and for Whom” TDM should be used in IBD patients taking biologics differing in immunogenicity.
THERAPEUTIC OUTCOMES AND BIOLOGICS LEVELS
1. Rationale for TDM
The rationale of biologics TDM is based on the exposure-response relationship, which indicates the presence of a positive correlation between therapeutic outcomes with serum biologics levels, the difference in clearance, or metabolism of biologics (either immune- or non-immune-mediated pharmacokinetic mechanisms), and the possibility for mechanistic failure.16 Three classes of biologics are currently approved by the U.S. Food and Drug Administration and European Medicines Agency for the induction and maintenance treatment of IBD: anti-TNF-α (infliximab [IFX], adalimumab [ADA], certolizumab pegol [CZP], and golimumab [GOL]), anti-integrin (vedolizumab [VDZ]), and anti-cytokine (ustekinumab [UST]) agents. The physicians usually prescribed the approved biologics with the standard dosage, and hope to achieve the maximal therapeutic goals (including clinical response/remission, biomedical remission, endoscopic healing, mucosal healing, transmural healing, or even histological healing). As an important ingredient of precision medicine for IBD care, the TDM may guide the physician to prescribe the right drug with the right dosage to have the best performance of the biologics objectively.
2. Anti-TNF-α agents
The anti-TNF-α agents are approved to be effective for both inducing and maintaining remission in moderate-to-severe CD and UC patients. Up to 30% of IBD patients do not gain any benefit from anti-TNF-α agents (PNR), and another 50% who initially respond will lose the response during treatment (secondary LOR).16,17
The exposure-response relationship between therapeutic outcomes and anti-TNF-α agents, especially IFX, is well established.18-22 Undetectable IFX trough level is reported to associate with higher colectomy rate in acute severe UC, and a higher IFX level is also noted to associate with longer remission and better endoscopic scores in moderate-to-severe CD.18,19
Both the post-hoc analysis of ACT I/II trials in UC patients and the ACCENT I trial in CD patients supported the exposure-response relationship regarding the therapeutic outcomes.20,21 The IFX trough levels >5.1 μg/mL at week 14 were positively correlated with the week 30 clinical response in UC patients, and ≥3.5 μg/mL at week 14 is predictive of the week 54 clinical response in CD patients.20,21 A serum IFX trough level above 5 μg/mL was reported to have longer IFX retention either monotherapy or combination therapy.22
A recent cross-section study also demonstrated a positive correlation between IFX trough levels during the maintenance phase and serum erythrocyte sedimentation rate (ESR) in pediatric IBD patients.23 A trough IFX level >1.58 µg/mL was demonstrated to predict ESR <18 mm/hr in pediatric IBD patients.23
Different treatment goals, different diseases and different time points may require different biologics target levels in reported data.20,21,24,25 The IFX trough levels ≥18.6 μg/mL and ≥10.6 μg/mL at weeks 2 and 6, respectively, in the post-hoc analysis of the ACT I/II trials were associated with a week 8 Mayo endoscope subscore (MES) of ≤1. The week 14 IFX trough levels ≥5.1 μg/mL and ≥6.7 μg/mL, respectively, were predictive of a week 30 MES ≤1 and =0 in UC patients.24 The TAILORIX study post-hoc analysis demonstrated that IFX trough levels >23.1 μg/mL and >10.0 μg/mL at weeks 2 and 6, respectively, are associated with week 12 endoscopic remission in CD patients.25
To achieve the perianal fistula response in CD patients, a subgroup post-hoc analysis of ACCENT II study demonstrated the serum IFX trough levels >13.9 μg/mL at week 6 was correlated with a week 14 complete fistula response.26
In the CLASSIC I trial, the week 4 ADA trough levels were higher in patients with clinical response in CD patients.27 The exposure-response relationship between serum ADA trough level and clinical remission was also identified in CLASSIC I/II CD patients at several time points.27
A cross-section designed study in both CD and UC patients treated with ADA showed that the serum ADA trough level was significantly higher in patients with clinical remission than in those without clinical remission (6.02 μg/mL vs 3.20 μg/mL, p=0.012).28 Subjects with mucosal healing also had higher median ADA trough levels than those without (6.50 μg/mL vs 4.20 μg/mL, p<0.005). A serum ADA trough level >4.90 μg/mL was reported to predict mucosal healing in both CD and UC patients.28 Another cross-sectional study of IBD patients treated with ADA or IFX demonstrated that the ADA trough levels >7.1 μg/mL (p=0.004) and IFX trough levels >5 μg/mL (p<0.001) predicted mucosal healing with 85% specificity.29
The Personalized Anti-TNF therapy in Crohn's Disease Study (PANTS) study of bio-naïve active luminal CD patients (655 and 955 patients treated with ADA and IFX, respectively) demonstrated that PNR was associated with low week 14 biologics trough levels (odds ratio [OR], 0.13; p<0.001 for ADA and OR, 0.35; p<0.001 for IFX).13 Low ADA and IFX trough levels at week 14 were also predictive of no clinical remission at week 54 (OR, 0.03; p<0.001 for ADA and OR, 0.29; p<0.001 for IFX).13
Analysis of CZP quartiles, performed during the MUSIC trial, showed that CD patients with the CZP trough levels in the lowest quartile at week 8 had a lower probability of week 10 endoscopic response and clinical remission (p=0.002 and p=0.03, respectively) than others with serum CZP higher quartiles levels.30 Higher CZP trough levels during the maintenance phase were demonstrated to correlate with a higher probability of clinical remission.31 The receiver operating characteristic (ROC) curve analysis of CD patients treated with CZP, pooled data from nine clinical trials, showed that CZP trough levels >36.1 μg/mL at week 6 was predictive of week 26 clinical improvement.31
The data of the GOL exposure-response relationship is relatively limited. In the PURSUIT-SC study of GOL in UC patients, the week 6 GOL though levels were significantly higher in patients with clinical response than non-clinical response (2.96 μg/mL vs 1.55 μg/mL, p<0.001), remission than non-remission (3.14 μg/mL vs 2.13 μg/mL, p<0.001), and mucosal healing versus non-mucosal healing (3.14 μg/mL vs 1.70 μg/mL, p<0.001). An optimal week 6 GOL trough level >2.5 μg/mL, and week 44 GOL trough level >1.4 μg/mL predicted better therapeutic outcomes in UC patients.32
3. Anti-integrin agent
VDZ, a recombinant humanized α4β7 integrin IgG1 monoclonal antibody, was approved for both moderate-to-severe adult UC and CD patients.14,33 The VDZ GEMINI I trial showed that UC patients with the highest quartile of week 6 serum VDZ trough level (>37.5 μg/mL) had a higher probability of clinical remission as compared to those with VDZ levels in the lowest quartile (<17 μg/mL) (remission rate, 37.0% vs 5.6%).14 In the GEMINI II CD trial, CD patients with the highest quartile of VDZ trough level (>33.3 μg/mL) also had higher clinical remission rates than those in the lowest quartile (<16.7 μg/mL) (remission rate, 22.0% vs 6.1%) at week 6.33 In a prospective study, a week 14 VDZ trough level >16.55 µg/mL was associated with the duration of VDZ persistence in UC patients.34 In a retrospective study, VDZ trough concentrations >28.9 µg/mL, >20.8 µg/mL, and >12.6 µg/mL at weeks 2, 6, and 14, respectively, were associated with the week 14 clinical response rate in UC patients.35 A week 14 VDZ cutoff trough level >17 µg/mL in UC patients was required for the goal of mucosal healing.35 A week 2 VDZ trough level >35.2 µg /mL was predictive of week 6 biomedical remission in CD patients.35
4. Anti-interleukin agent
UST, a recombinant humanized IgG1 monoclonal antibody against the subunit p40 of interleukin-12 and -23, was approved for both adult CD and UC patients.36-38 CD patients with UST trough levels in the two highest versus two lowest quartiles in the UNITI-I/II trials were reported to have higher clinical remission rate (p<0.039 for UNITI-I and p=0.007 for II trial). The UNITI-I/II post-hoc ROC analysis identified the week 8 UST trough level >3.3 μg/mL is associated with clinical remission (area under the curve=0.57, p=0.001). In the UST maintenance study of CD patients, IM-UNITI, the clinical remission rate was significantly higher in patients with UST trough levels in the two highest quartiles (p=0.002).15 A recent report demonstrated that CD patients UST trough levels >15.9 μg/mL at week 4 was associated with a significant decrease in fecal calprotectin at week 8.39
5. The current recommended biologics trough level by practice guidelines
Reported exposure-response relationships between biologics and clinical treatment outcomes are summarized in Table 1, and the recommended target serum biologic trough levels by the American Gastroenterological Association (AGA), Building Research in Inflammatory Bowel Disease Globally (BRIDGe), and European Crohn’s and Colitis Organisation (ECCO)-European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) practice consensus and guidelines are also summarized in Table 2.10-12,16,40
Table 1.
Therapeutic Outcomes by Biologic Trough Levels in Crohn’s Disease and Ulcerative Colitis
| Disease | Biologics | Week | Trough levels (μg/mL) | Therapeutic outcomes |
|---|---|---|---|---|
| Crohn’s disease | Infliximab21 | 14 | ≥3.5 | Clinical response (week 54) |
| Infliximab25 | 2 | >23.1 | Endoscopic remission (week 12) | |
| 6 | >10.0 | Endoscopic remission (week 12) | ||
| Infliximab26 | 6 | >13.9 | Complete fistula response (week 14) | |
| 14 | >4.8 | Complete fistula response (week 14) | ||
| Infliximab29 | >5 | Mucosal healing | ||
| Infliximab13 | 14 | >7 | Clinical remission (week 54) | |
| Adalimumab13 | 14 | >12 | Clinical remission (week 54) | |
| Adalimumab28 | >4.9 | Mucosal healing | ||
| Adalimumab29 | >7.1 | Mucosal healing | ||
| Certolizumab pegol31 | 6 | >31.8 | Clinical response (week 6) | |
| 6 | >36.1 | Fecal calprotectin <250 mg/g and Crohn’s Disease Activity Index ≤150 (week 26) | ||
| 12 | >14.8 | Clinical response (week 26) | ||
| Vedolizumab33 | 6 | >33.3 | Clinical remission (week 6) | |
| Vedolizumab35 | 2 | >35.2 | Biomedical remission (week 6) | |
| Ustekinumab39 | 8 | >3.3 | Clinical remission (week 8) | |
| Ustekinumab40 | 8 | >7.2 | Biological remission (week 8) | |
| Ulcerative colitis | Infliximab20 | 14 | >5.1 | Clinical response (week 30) |
| Infliximab24 | 2 | ≥18.6 | Mayo endoscope subscore ≤ 1 (week 8) | |
| 6 | ≥10.6 | Mayo endoscope subscore ≤ 1 (week 8) | ||
| 8 | ≥34.9 | Mayo endoscope subscore ≤ 1 (week 8) | ||
| 14 | ≥5.1 | Mayo endoscope subscore ≤ 1 (week 30) | ||
| 14 | ≥6.7 | Mayo endoscope subscore = 0 (week 30) | ||
| 30 | ≥2.3 | Mayo endoscope subscore ≤ 1 (week 30) | ||
| 30 | ≥3.8 | Mayo endoscope subscore = 0 (week 30) | ||
| Adalimumab28 | >4.9 | Mucosal healing | ||
| Golimumab32 | 2 | >8.9 | Clinical response (week 6) | |
| 6 | >2.5 | Clinical response (week 6) | ||
| Vedolizumab14 | 6 | >37.5 | Clinical remission (week 6) | |
| Vedolizumab34 | 6 | >16.55 | Vedolizumab persistence (1 year) | |
| Vedozinumab35 | 2 | >28.9 | Clinical response (week 14) | |
| 6 | >20.8 | Clinical response (week 14) | ||
| 14 | >17.0 | Mucosal healing (week 14) | ||
| 14 | >12.6 | Clinical response (week 14) |
Table 2.
Trough Levels of Biologics Recommended by Current Clinical Practice Guidelines
| Drugs | Phase | Trough level (µg/mL) | Reference |
|---|---|---|---|
| Infliximab | Post-induction phase (week 14) | ≥7 | Papamichael et al.11 |
| Maintenance phase | ≥3 | Papamichael et al.11 | |
| Maintenance phase | ≥5 | Feuerstein et al.10 | |
| Maintenance phase | ≥5 | Vande Casteele et al.16 | |
| Induction phase (week 2) | ≥25 | van Rheenen et al.40 | |
| Induction phase (week 6) | ≥15 | van Rheenen et al.40 | |
| Post-induction phase (week 14) | ≥5 | van Rheenen et al.40 | |
| Adalimumab | Induction phase (week 4) | ≥7 | Papamichael et al.11 |
| Maintenance phase | ≥7.5 | Feuerstein et al.10 | |
| Maintenance phase | ≥5 | Papamichael et al.11 | |
| Maintenance phase | ≥7.5 | Vande Casteele et al.16 | |
| Induction phase (week 4) | ≥7.5 | van Rheenen et al.40 | |
| Maintenance phase (week 8) | ≥7.5 | van Rheenen et al.40 | |
| Certolizumab pegol | Induction phase (week 6) | ≥32 | Papamichael et al.11 |
| Maintenance phase | ≥15 | Papamichael et al.11 | |
| Maintenance phase | ≥20 | Feuerstein et al.10 | |
| Maintenance phase | ≥20 | Vande Casteele et al.16 | |
| Golimumab | Induction phase (week 6) | ≥2.5 | Papamichael et al.11 |
| Maintenance phase | ≥1 | Papamichael et al.11 | |
| Vedolizumab | Induction phase (week 6) | >20 | Shukla et al.12 |
| Maintenance phase (week 14 and beyond) | >12 | Shukla et al.12 | |
| Ustekinumab | Induction phase (week 8) | >4 | Shukla et al.12 |
| Maintenance phase (week 16 and beyond) | >2 | Shukla et al.12 |
IMMUNOGENICITY OF BIOLOGICS
1. Biologics with relatively high immunogenicity
The PANTS study demonstrated that 62.8% (95% confidence interval [CI], 59.0% to 66.3%) of IFX and 28.5% (95% CI, 24.0% to 32.7%) of ADA-treated CD patients developed ADAbs.13 Suboptimal week 14 IFX or ADA trough levels predicted PNR, the development of ADAbs, and subsequent low serum biologics levels.13 The combination of immune modulator decreased the risk of ADAbs for both IFX and ADA (hazard ratio [HR], 0.39; 95% CI, 0.32 to 0.46; p<0.001 for IFX and HR, 0.44; 95% CI, 0.31 to 0.64; p<0.001 for ADA). A previous case series also demonstrated that the add-on of immune modulator (azathioprine or methotrexate) therapy in IFX-treated patients may boost the IFX trough level and lead to the disappearance of low-level ADAbs.41 A genome-wide association study demonstrated that CD patients carrying HLA-DQA1*05 are associated with the development of ADAbs to IFX and ADA during the biologics therapy.42
Up to 20% of ADA-treated patients were reported to develop neutralizing ADAbs after a median time of 34 weeks, and patients with ADAb were noted to have lower week 4 ADA trough levels.43 The ADA trough levels <5 μg/mL at week 4 was associated with PNR (HR, 25.1; 95% CI, 5.6 to 111.9; p=0.002), and secondary LOR (OR, 3.0; 95% CI, 1.04 to 9.09; p=0.034).43
Hence, IFX and ADA have relatively high immunogenicity, and maintaining optimal trough levels via concomitant use of an immune modulator may prevent the development of ADAbs. Patients carrying HLA-DQA1*05 genotype are prone to the development of ADAbs targeting IFX and ADA, may have a special need for the concomitant immune modulator (methotrexate or azathioprine) and TDM guided therapy.
2. Biologics with relatively low immunogenicity
VDZ and UST were reported to have relatively low immunogenicity for the development of ADAbs.14,15,33 The prevalence of ADAb against VDZ was 3.7% and 4.1% in patients with UC and CD, respectively, in the GEMINI I and II trials.14,33 A cohort of 179 VDZ-treated IBD patients further confirmed the low immunogenicity of VDZ, with 4 (2.2%) subjects developing a transient ADAb against VDZ.44
The UNITI-I/II and IM-UNITI trial data revealed prevalence rates of ADAb against UST of 3.1% and 2.6% at weeks 8 and 24, respectively.15 The UST trough level quartile analysis showed that the prevalence rates of ADAb against UST were 5.7%, 0.6%, 2.9%, and 3.4%, respectively, at week 8 in UST level quartiles 1 to 4 (p=0.042).15 Thus, the prevalence of ADAb against UST is inversely correlated with the UST level at week 8. UST trough level quartile analysis at week 24 showed that the prevalence rates of ADAb against UST were 6.4%, 2.1%, 0%, and 2.1%, respectively, in UST level quartiles 1 to 4 (p=0.227).15
A study of psoriasis patients on UST therapy detected ADAb against UST in 6.5% of patients at a mean time of 13 months of UST treatment, and the development of ADAb targeting UST was significantly correlated with lower serum UST trough concentrations in psoriasis patients (p<0.001).45
TDM: HOW, WHEN, AND FOR WHOM
1. Mechanism of treatment failure
Three major mechanisms of biologic treatment failure have been proposed by the AGA consensus (Table 3).16 Subjects with non-immune-mediated pharmacokinetic failure may present with PNR during the induction phase, or secondary LOR during the maintenance phase. The mechanisms of non-immune-mediated pharmacokinetic failure may associate with noncompliance, an excessive inflammatory burden, low serum albumin level, rapid drug clearance, gastrointestinal loss, different drug distribution, and excessive drug wastage.13,16,46-48 The check of patient’s adherence is a key step in the management of non-immune-mediated pharmacokinetic failure.47 The presence of a neutralizing ADAb against biologics may result in ADAb-mediated pharmacokinetic failure, presenting as secondary LOR during the maintenance phase of biologics treatment.13,16,21 While subjects with mechanistic failure may present with PNR during the induction phase, their IBD may be driven by inflammatory mechanisms not blocked by the applied biologics.16
Table 3.
| Drug trough level | Anti-drug antibody | Phase of treatment | Cause of failure | |
|---|---|---|---|---|
| Non-immune mediated pharmacokinetic failure | Suboptimal | Undetectable | Primary non-responder at induction phase |
|
| Secondary loss of response at maintenance phase |
|
|||
| Anti-drug antibodies mediated pharmacokinetic failure | Suboptimal | Detectable | Secondary loss of response at maintenance phase | Neutralizing anti-drug antibodies |
| Mechanistic failure | Optimal | Undetectable | Primary non-responder at induction phase | Inflammatory mechanisms not blocked by the applied biologics |
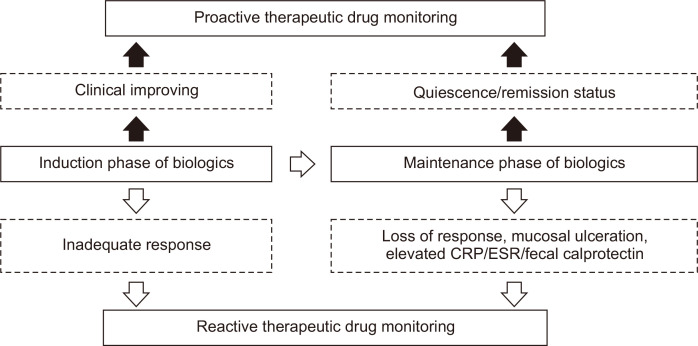
2. When to perform TDM: proactive versus reactive
Two main biologics TDM strategies for IBD, the reactive TDM (in patients with active IBD disease activity), and the proactive (IBD patients with a quiescent disease or clinical remission status), have been proposed (Fig. 1).11,12,16,40 Studies based on reactive TDM of anti-TNF-α, a group of relatively high-immunogenicity biologics, estimated non-immune-mediated pharmacokinetic failure, ADAb-mediated pharmacokinetic failure, and mechanistic failure rates of 51%, 19%, and 30%, respectively.16,46,48 Non-immune-mediated pharmacokinetic failure is characterized by inadequate control of IBD disease activity, suboptimal serum biologic levels, and an absence of ADAbs.16 Since there are pretty evidence demonstrating the suboptimal anti-TNF-α levels is a significant independent predictor for the development of neutralizing ADAbs, non-immune-mediated pharmacokinetic failure in anti-TNF-α treated IBD patients can transition to ADAb-mediated pharmacokinetic failure subsequently.13,16,42,43
Fig. 1.
Reactive and proactive therapeutic drug monitoring strategies for biologics during the treatment course of inflammatory bowel disease.
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
For the management of PNR and secondary LOR, the reactive TDM has proved to be a more cost-effective strategy compared to empiric dose escalation. In a randomized, controlled, single-blind, multicenter study, adult patients with IFX secondary LOR were randomized to an IFX empiric dose intensification group (5 mg/kg every 4 weeks) (n=36) or reactive TDM group (n=33). The study demonstrated the strategy of reactive TDM was more cost-effective than dose intensification and may have utility as an intervention after secondary IFX failure.49
A retrospective observational study demonstrated that IFX-treated IBD patients under the proactive TDM care were more likely to remain on IFX than others without proactive TDM (p<0.001). Patients with a serum IFX trough levels >5 μg/mL versus <5 μg/mL (HR, 0.03; p<0.001) was demonstrated to achieve a higher probability of retention on IFX therapy.50 A retrospective multicenter study of IBD patients (n=167 for CD and n=97 for UC) receiving IFX maintenance therapy demonstrated that the proactive TDM strategy significantly decreased the risk of treatment failure, IBD-related surgery, IBD-related hospitalization, neutralizing ADAb to IFX, and serious infusion reaction (HR=0.16, 0.30, 0.16, 0.25, and 0.17; p<0.001, 0.017, <0.001, 0.025, and 0.023, respectively).51 Another multicenter retrospective cohort study of 382 ADA treated IBD patients (n=311 for CD and n=71 for UC) demonstrated that the proactive TDM strategy with pre-empty dosage adjustment to therapeutic window significantly decrease the risk of ADA treatment failure (HR, 0.4; 95% CI, 0.2 to 0.9; p=0.022).52
The prospective controlled Trough Level Adapted Infliximab Treatment (TAXIT) study optimized the trough concentration of IFX to 3–7 μg/mL before randomization to the proactive TDM and empiric dose escalation groups.53 The proactive TDM group was demonstrated to have a higher relapse-free survival rate than the empiric dose escalation group (p=0.017).54 The recent PAILOT trial reported that the proactive TDM strategy in children who initially responded to ADA achieved a higher clinical remission rate than those managed with the reactive TDM strategy (82% vs 48%, p=0.002) in a randomized controlled design study.54
The recent BRIDGe TDM consensus recommended both proactive and reactive TDM for relatively high-immunogenicity biologics, such as anti-TNF-α, to check the trough drug levels, and for ADAbs in responders and non-responders during the induction and maintenance phases.11 Since the number of biologics approved for pediatric IBD patients is extremely limited, the duration of effectiveness of those that are available is very important. The ECCO-ESPGHAN guidelines also recommend both proactive TDM (followed by dose optimization) and reactive TDM strategies to guide the treatment of children receiving IFX and ADA.40
Exposure-response relationships between serum drug levels and clinical outcomes are evident for both VDZ and UST. Higher serum trough levels of both VDZ and UST were correlated with the clinical response, and with clinical, biochemical, and endoscopic remission.15,33-35,39 No studies have compared proactive and reactive TDM for IBD patients treated with VDZ and UST. The prevalence of ADAbs against UST negatively correlated with serum UST trough levels.15,45 Proactive TDM and optimization of drug levels before the development of neutralizing ADAb for UST is likely to be important, but more evidence is still needed. ADAbs against VDZ and UST were seen in less than 5% of cases, and these agents have relatively low immunogenicity.14,15,33 The BRIDGe TDM consensus guidelines currently only recommend reactive TDM strategy for these relatively low-immunogenicity biologics, to check trough drug levels and ADAbs in non-responders in the induction (PNR) and maintenance (secondary LOR) phases.11
3. Proactive TDM and reactive TDM for whom
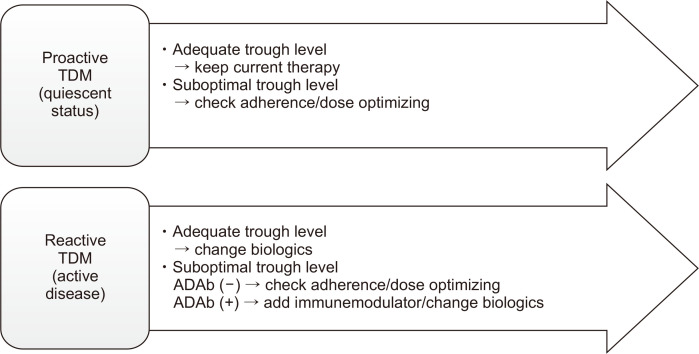
Proactive TDM is indicated for IBD patients with HLA-DQA1*05 carriage receiving relatively high immunogenicity biologics (such as anti-TNF-α), without a concomitant immune modulator (azathioprine or methotrexate). It may also be indicated for children, and for patients with a high inflammatory burden, low serum albumin level, or previous failure of biologics.11,12,16,40 Proactive TDM in these subjects who are high risk for the development for ADAb and have limited biologics choice at the quiescent status in the induction and maintenance phase may get the chance to optimize the trough biologics serum levels within the goal therapeutic window. Proactive TDM strategy could avoid the transition from non-immune-mediated to ADAb-mediated pharmacokinetic failure in high-risk patients by optimizing the biologics levels to prevent the development of neutralizing ADAbs.
The reactive TDM strategy is indicated for all IBD patients with active disease in the induction and maintenance phases, for all three classes of biologics, to assist in the assessment and treatment adjustment for active disease.11,12,16,40 Since the immunogenicity of VDZ and UST is relatively low, as is the likelihood of transitioning from non-immune-mediated pharmacokinetic failure to ADAb-mediated pharmacokinetic failure, reactive TDM alone may be sufficient for VDZ- and UST-treated patients.
Base on reported data and practice guidelines, a proposed algorithm after proactive TDM in quiescent patients and reactive TDM in patients with active disease with biologics was summarized in Fig. 2.11,12,16,40,55,56
Fig. 2.
Proposed treatment algorithms for proactive and reactive therapeutic drug monitoring (TDM) in patients receiving biologics.
ADAb, anti-drug antibody.
LIMITATION
The use of different assays for the measurement of biologic and ADAb levels may result in different reference values. However, this review did not discuss this issue.
CONCLUSIONS
The advent of biologics has improved the management of IBD, and the treatment goals have changed from a clinical response and clinical remission to mucosal healing, or even deep remission. With the adoption of precision medicine for the management of IBD, objective non-invasive biomarkers have become increasingly important. Given the exposure-response relationship between biologic trough levels and clinical outcomes, TDM is now increasingly recommended by practice guidelines of different societies.11,12,16,40,54,56
Well-established reactive TDM algorithms for biologics guide dose escalation, augmentation of therapy, and the switching of biologics. Particularly for biologics with high immunogenicity, proactive TDM can prevent PNR, increase the duration of effective biologics, avoid secondary LOR, improve clinical outcomes, and achieve the best benefit of patients.57
In summary, TDM is a key element of precision medicine for IBD patients in the context of treat-to-target, given the need for tight control of IBD disease activity. Both proactive and reactive TDM strategies are well-established for anti-TNF-α biologics, while the reactive TDM strategy is generally recommended for non-anti-TNF-α biologics. The proactive TDM strategy for non-anti-TNF-α biologics with relatively low immunogenicity has potential clinical benefit, but more evidence is needed.39,55
ACKNOWLEDGEMENTS
This work was supported by a grant from National Taiwan University Hospital (number: NTUH 110-S4800). The funder had no role in study design, data interpretation, or manuscript writing.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV., Jr Surgery in a population-based cohort of Crohn's disease from Olmsted County, Minnesota (1970-2004) Am J Gastroenterol. 2012;107:1693–1701. doi: 10.1038/ajg.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn's disease: a population-based cohort study. Gastroenterology. 2008;135:1106–1113. doi: 10.1053/j.gastro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 3.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV., Jr Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burisch J, Katsanos KH, Christodoulou DK, et al. Natural disease course of ulcerative colitis during the first five years of follow-up in a European population-based inception cohort: an Epi-IBD study. J Crohns Colitis. 2019;13:198–208. doi: 10.1093/ecco-jcc/jjy154. [DOI] [PubMed] [Google Scholar]
- 5.Jenkinson PW, Plevris N, Siakavellas S, et al. Temporal trends in surgical resection rates and biologic prescribing in Crohn's disease: a population-based cohort study. J Crohns Colitis. 2020;14:1241–1247. doi: 10.1093/ecco-jcc/jjaa044. [DOI] [PubMed] [Google Scholar]
- 6.Olivera P, Spinelli A, Gower-Rousseau C, Danese S, Peyrin-Biroulet L. Surgical rates in the era of biological therapy: up, down or unchanged? Curr Opin Gastroenterol. 2017;33:246–253. doi: 10.1097/MOG.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 7.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD). Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn's disease. Clin Gastroenterol Hepatol. 2015;13:1042–1050. doi: 10.1016/j.cgh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390:2779–2789. doi: 10.1016/S0140-6736(17)32641-7. [DOI] [PubMed] [Google Scholar]
- 10.Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S American Gastroenterological Association Institute Clinical Guidelines Committee, author. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–834. doi: 10.1053/j.gastro.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:1655–1668. doi: 10.1016/j.cgh.2019.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla R, Ananthakrishnan A. Therapeutic drug monitoring of non-anti-tumor necrosis factor biologics. Clin Gastroenterol Hepatol. 2021;19:1108–1110. doi: 10.1016/j.cgh.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341–353. doi: 10.1016/S2468-1253(19)30012-3. [DOI] [PubMed] [Google Scholar]
- 14.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 15.Adedokun OJ, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn's disease. Gastroenterology. 2018;154:1660–1671. doi: 10.1053/j.gastro.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 16.Vande Casteele N, Herfarth H, Katz J, Falck-Ytter Y, Singh S. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. 2017;153:835–857. doi: 10.1053/j.gastro.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 17.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 18.Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 19.Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1248–1254. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147:1296–1307. doi: 10.1053/j.gastro.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 21.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721–1727. doi: 10.1136/gutjnl-2012-304094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21:182–197. doi: 10.1097/MIB.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 23.Choi SY, Kang B, Choe YH. Serum infliximab cutoff trough level values for maintaining hematological remission in pediatric inflammatory bowel disease. Gut Liver. 2019;13:541–548. doi: 10.5009/gnl18129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vande Casteele N, Jeyarajah J, Jairath V, Feagan BG, Sandborn WJ. Infliximab exposure-response relationship and thresholds associated with endoscopic healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2019;17:1814–1821. doi: 10.1016/j.cgh.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 25.Dreesen E, D'Haens G, Baert F, et al. Infliximab exposure predicts superior endoscopic outcomes in patients with active Crohn's disease: pharmacokinetic-pharmacodynamic analysis of TAILORIX. J Crohn'. s Colitis. 2018;12 Suppl 1:S063–S064. doi: 10.1093/ecco-jcc/jjx180.084. [DOI] [Google Scholar]
- 26.Vande Casteele N, Papamichael K, Jeyarajah J, Osterman MT, Cheifetz AS. Adequate infliximab exposure during the induction phase is associated with early complete fistula response in patients with fistulizing Crohn's disease: a post-hoc analysis of the ACCENT-2 trial. J Crohn'. s Colitis. 2019;13 Suppl 1:S053–S054. doi: 10.1093/ecco-jcc/jjy222.079. [DOI] [Google Scholar]
- 27.Chiu YL, Rubin DT, Vermeire S, et al. Serum adalimumab concentration and clinical remission in patients with Crohn's disease. Inflamm Bowel Dis. 2013;19:1112–1122. doi: 10.1097/MIB.0b013e3182813242. [DOI] [PubMed] [Google Scholar]
- 28.Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:80–84. doi: 10.1016/j.cgh.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNF-α therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14:550–557. doi: 10.1016/j.cgh.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn's disease. Clin Gastroenterol Hepatol. 2014;12:423–431. doi: 10.1016/j.cgh.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 31.Vande Casteele N, Feagan BG, Vermeire S, et al. Exposure-response relationship of certolizumab pegol induction and maintenance therapy in patients with Crohn's disease. Aliment Pharmacol Ther. 2018;47:229–237. doi: 10.1111/apt.14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adedokun OJ, Xu Z, Marano CW, et al. Pharmacokinetics and exposure-response relationship of golimumab in patients with moderately-to-severely active ulcerative colitis: results from Phase 2/3 PURSUIT Induction and Maintenance Studies. J Crohns Colitis. 2017;11:35–46. doi: 10.1093/ecco-jcc/jjw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 34.Guidi L, Pugliese D, Panici Tonucci T, et al. Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease. United European Gastroenterol J. 2019;7:1189–1197. doi: 10.1177/2050640619873784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dreesen E, Verstockt B, Bian S, et al. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16:1937–1946. doi: 10.1016/j.cgh.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 36.Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med. 2012;367:1519–1528. doi: 10.1056/NEJMoa1203572. [DOI] [PubMed] [Google Scholar]
- 37.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375:1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 38.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–1214. doi: 10.1056/NEJMoa1900750. [DOI] [PubMed] [Google Scholar]
- 39.Verstockt B, Dreesen E, Noman M, et al. Ustekinumab exposure-outcome analysis in Crohn's disease only in part explains limited endoscopic remission rates. J Crohns Colitis. 2019;13:864–872. doi: 10.1093/ecco-jcc/jjz008. [DOI] [PubMed] [Google Scholar]
- 40.van Rheenen PF, Aloi M, Assa A, et al. The medical management of paediatric Crohn's disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. 2021;15:171–194. doi: 10.1093/ecco-jcc/jjaa161. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:444–447. doi: 10.1016/j.cgh.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Sazonovs A, Kennedy NA, Moutsianas L, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn's disease. Gastroenterology. 2020;158:189–199. doi: 10.1053/j.gastro.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 43.Baert F, Kondragunta V, Lockton S, et al. Antibodies to adalimumab are associated with future inflammation in Crohn's patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut. 2016;65:1126–1131. doi: 10.1136/gutjnl-2014-307882. [DOI] [PubMed] [Google Scholar]
- 44.Bian S, Dreesen E, Tang HT, et al. Antibodies toward vedolizumab appear from the first infusion onward and disappear over time. Inflamm Bowel Dis. 2017;23:2202–2208. doi: 10.1097/MIB.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 45.Chiu HY, Chu TW, Cheng YP, Tsai TF. The association between clinical response to ustekinumab and immunogenicity to ustekinumab and prior adalimumab. PLoS One. 2015;10:e0142930. doi: 10.1371/journal.pone.0142930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013;19:2568–2576. doi: 10.1097/MIB.0b013e3182a77b41. [DOI] [PubMed] [Google Scholar]
- 47.Ooi CJ, Hilmi I, Banerjee R, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn's disease in Asia. Intest Res. 2019;17:285–310. doi: 10.5217/ir.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol. 2015;13:522–530. doi: 10.1016/j.cgh.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 49.Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63:919–927. doi: 10.1136/gutjnl-2013-305279. [DOI] [PubMed] [Google Scholar]
- 50.Vaughn BP, Martinez-Vazquez M, Patwardhan VR, Moss AC, Sandborn WJ, Cheifetz AS. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20:1996–2003. doi: 10.1097/MIB.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol. 2017;15:1580–1588. doi: 10.1016/j.cgh.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papamichael K, Juncadella A, Wong D, et al. Proactive therapeutic drug monitoring of adalimumab is associated with better long-term outcomes compared with standard of care in patients with inflammatory bowel disease. J Crohns Colitis. 2019;13:976–981. doi: 10.1093/ecco-jcc/jjz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–1329. doi: 10.1053/j.gastro.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 54.Assa A, Matar M, Turner D, et al. Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children with Crohn's disease compared with reactive monitoring. Gastroenterology. 2019;157:985–996. doi: 10.1053/j.gastro.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508–1524. doi: 10.1053/j.gastro.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 57.Sparrow MP, Papamichael K, Ward MG, et al. Therapeutic drug monitoring of biologics during induction to prevent primary non-response. J Crohns Colitis. 2020;14:542–556. doi: 10.1093/ecco-jcc/jjz162. [DOI] [PMC free article] [PubMed] [Google Scholar]