Abstract
With diversity of hosts range, most identified nematode species still lack the crucial connection between morphological and molecular make-up, which is important for precisely classifying the specimens. The present study provides the complete description of Rhabdias africanus in the new host record, Sclerophrys regularis. Fifty toad specimens were collected, and a high prevalence of R. africanus infection (74%) was observed. Morphology and ultrastructure were observed using light and scanning electron microscopes. Morphological characteristics, including peculiarities of the head, the shape and position of the lips, and the number of labial papillae, were described. The length of the body, the esophageal length, the distance from an anterior end to the nerve ring, and the tail length were reduced in the studied samples relative to previously described specimens. Furthermore, some variable matrices that have not previously been described, e.g., ovarian part widening, the nerve ring and its location, and eggs with different stages of larvae, were included in the present study. Genus and species identification was confirmed by comparing partial 12S (619 bp) and ITS (878 bp) gene sequences to those of Rhabdias species deposited in GenBank. The studied species showed a 99.34% resemblance to R. africanus from South Africa. We assume our findings will aid in the molecular identification of adult and larval stages of this genus in amphibians. We strongly recommend further studies on the environmental factors that promote Rhabdias infection and transmission.
Keywords: Rhabdias, Toad, Morphology, ITS, 12S gene, Egypt
1. Introduction
Nematodes are a highly diverse worm taxon, that includes both free living and parasitic species (Martínez-Arce et al., 2020). One of nematodes genus, Rhabdias Stiles and Hassall, 1905 which belongs to Rhabdiasidae, comprises more than 80 known species of lung-dwelling parasites of cold blooded animals including amphibians and reptiles (Kuzmin et al., 2020). Rhabdias is cosmopolitan in nature: it exhibits an alternating life cycle between parasitic and free-living generations (Baker, 1979). Nematode parasites of amphibians and reptiles predominantly affect the gastrointestinal tracts of their hosts, although the respiratory tract, lungs, blood, and body cavity may also be affected (Halajian et al., 2013). These nematode parasites can cause a range of changes and damage to the host physiology and metabolism; such effects have been reported for lungworms (Rhabdias spp.) infecting toads (Finnerty et al., 2018, Pizzatto and Shine, 2012). The prevalence of nematodes can potentially compromise the fitness of amphibian and reptilian hosts and may ultimately affect their survival (Kelehear et al., 2009). Indeed, a drastic decline in amphibian populations has recently been attributed to the prevalence of nematode parasites (Leary et al., 2018).
Parasitic individuals are usually protandrous hermaphrodites that first produce spermatozoa, or sometimes spermatozoa and egg development simultaneously (Runey et al., 1978). The developed eggs from the host lungs are first transported to the gastrointestinal tract and then defecated at which point they hatch in the soil and begin a free living life cycle (Langford and Janovy Jr, 2009). Several species of Rhabdias have been reported from tropical and subtropical Africa (Baker, 1982, Baker, 1987, Hugot et al., 2001, Junker et al., 2010, Kuzmin et al., 2017, Svitin et al., 2018, Tkach et al., 2014). However, data on the nematodes that parasitize the cold blooded fauna of Egypt are scarce (Moravec et al., 1987).
Sclerophrys regularis also known as the Egyptian toad and Ptychadena mascareniesis are stable species considered to be of least concern globally (IUCN, 2016). Sclerophrys regularis is a widely distributed African species found in Senegal, Egypt, Sudan, Ethiopia, and Congo (IUCN, 2016).
The current study, we provide an evidence for the infection of Rhabdias africanus in the lungs of Egyptian toad S. regularis, as a new host in Egypt. Morphological, ultrastructural, and molecular analyses were conducted. Morphological identification was assumed to provide new information on the species from this host group in Egypt. Furthermore molecular analysis based on sequences from the complete ITS region and partial 12S gene of nuclear ribosomal DNA.
2. Materials and methods
2.1. Animal sampling
Fifty toad samples were collected manually at night using the Visual Acoustic Encounter Survey method (Scott et al., 1994). Samples were collected from moist fields and gardens in Assiut Governorate, Egypt, in May 2019 and November 2020. They were transferred alive to the Laboratory of Parasitology, Faculty of Science, Assiut University, Egypt, where were euthanized using 20% benzocaine gel (Anbesol; Pfizer, Inc., New York, NY, USA), dissected, and examined for parasites.
2.2. Light microscopy analysis
For light microscopy observations, the nematodes were isolated from the lungs, fixed in triethanolamine fixative for 24 h, and then preserved in a mixture of 70% ethanol and 5% glycerin. The nematodes were cleared in lactophenol and examined on temporary slides using an Optica microscope equipped with a digital colored video camera (Optica 4083.B9 Digital Camera, Italy). Different regions of an organism were drawn by a light microscope using a camera lucida and photographed by a digital camera. Measurements of 25 specimens from five samples are given in Table 1. All measurements were provided in micrometers unless otherwise indicated and the results are expressed as means ± standard deviation (SD).
Table 1.
Morphometric characters of Rhabdias africanus from Sclerophrys regularis in Upper Egypt. All measurements in micrometers unless otherwise indicated.
| R. aegyptiaca | Rhabdias sp. | R. bufonis | R. bufonis | R. africanus | Rhabdias species |
|---|---|---|---|---|---|
| El–Garhy and Garo | Saad et al (2009) | Moravec et al (1987) | Morsy et al (2018) | Present study | Author |
| −2006 | |||||
| –– | N = 30 | N = 10 | N = 28 | N = 40 | No. of worms |
| 8–10 | 5.2–12.5 | 2.99–13.02 | 5.64 ± 0.03 | 9.67 ± 2.97 | body length (mm) |
| (3.22–9.86) | (6.61–13.96) | ||||
| –– | 225–400 | –– | –– | 291.4 ± 105.8 | body width oesophago-intestinal junction |
| (200.3–444.1) | |||||
| 300–500 | 200–700 | 136–476 | 230 ± 20 | 311 ± 97.6 | Body width at vulva |
| (90–480) | (210.3–470.9) | ||||
| –– | –– | –– | –– | 228.8 ± 44.6 | Body width at anus |
| (177.6–258.8) | |||||
| –– | 10–32 | 15 | 18 ± 2 | 34.4 ± 7.9 | buccal cavity length |
| (13–31) | (22.5–34.4) | ||||
| –– | –– | 21 | 24 ± 2 | 17.3 ± 4.4 | buccal cavity depth |
| (16–27) | (10.8–20.2) | ||||
| 550 | 250–500 | 288–510 | 180 ± 20 | 503.9 ± 40.5 | oesophagus length |
| (270–630) | (442.9–542.7) | ||||
| –– | –– | –– | –– | 39.4 ± 3.6 | Ovary width at anterior part |
| (35.5–44.3) | |||||
| –– | –– | –– | –– | 53.4 ± 8.6 | Ovary width at end of muscular part |
| (40.3–64.6) | |||||
| –– | –– | –– | –– | 68.4 ± 10.7 | Ovary width at glandular part |
| (57.7–84.4) | |||||
| –– | 90–110 | 168–240 | –– | 141.5 ± 86.2 | Nerve ring from anterior end |
| (43.9–207.3) | |||||
| –– | 3.75–5.5 | 1.47–5.98 | –– | 5.9 ± 0.9 | Vulva ring from anterior end (mm) |
| (5–6.7) | |||||
| –– | –– | –– | –– | 3 ± 1 | Uterus (mm) |
| (2.3–3.7) | |||||
| 66 | 100–120 | 117–144 | 126 ± 2 | 101.1 ± 13.2 | Egg length (n = 20) |
| (120–132) | (65.2–114.7) | ||||
| –– | 60–80 | 51–72 | 51 ± 20 | 51.4 ± 10.5 | Egg width (n = 20) |
| (39–81) | (33.2–70.4) | ||||
| 170–200 | 230–400 | 144–420 | 320 ± 20 | 319.3 ± 44.2 | Tail length |
| (131–435) | (259–371.5) |
2.3. Ultrastructure analysis using scanning electron microscope
Specimens were first fixed in 4% glutaraldehyde and 0.1 M sodium cacodylate buffer (pH 7.3) for 6 h at 4 °C; they were then fixed in 1% osmium tetroxide for 2 h at 4 °C. Afterwards, they were washed three times in 0.1 M sodium phosphate buffer for 15 min/time. The specimens were dehydrated in a graded alcohol series (50%, 70%, and 90%) for 30 min at each concentration and then at 100% alcohol for 2 days, after which they were transferred to pure isoamyl acetone for 2 days. The samples were then processed in a critical point drying drier with a Polaron apparatus and Freon 13. Subsequently, the specimens were coated with gold in a Technics Hummer V (JEOL- 1100 E) ion-sputtering device and observed with a Jeol scanning electron microscope (JSM 5400 LV) at 10 KV (at the electron microscopy unit of Assiut University).
2.4. Sequencing and phylogenetic analysis
For molecular analysis, live specimens were preserved directly in 95% ethanol. Genomic DNA was extracted at the Molecular Biological Unit in Assiut University according to the method of Tkach et al. (1999) using the QIAGEN extraction kit (QIAamp DNA Minikit; QIAGEN, Hilden, Germany) following the manufacturer’s protocol. DNA concentration was estimated using a Nano Drop spectrophotometer (Fisher Scientific) and the samples were stored at − 20 °C until further PCR analysis. Fragments of the nuclear DNA region of the internal transcribed spacer (ITS) region was amplified by PCR according to the method of Kuzmin et al (2017). Amplification was performed in an Eppendorf Master Gradient thermal cycler (Veriti 96-well Thermal Cycler, 9902; Singapore) using the forward primer ritf (5′-GCG GCT TAA TTT GAC TCA ACA CGG-3′) and reverse primer 1500R (5′- GCT ATC CTG AGG GAA ACT TCG-3′). The PCR reaction was performed at 94 °C for 5 min for denaturation; followed by 40 cycles at 94 °C for 1 min, 53 °C for 1 min, and 72 °C for 1 min for amplification; and an extension at 72 °C for 5 min. Aliquots of 10 μL from each PCR reaction were subjected to a 1.5% agarose gel in a horizontal cell (Compact M, Biometric, Germany) and stained with ethidium bromide; DNA fragments were then observed using ultraviolet illumination.
According to most Afrotropical Rhabdias species have been deposited in Genbank, we sequenced partial ribosomal 12S amplicons (Junker et al., 2010) using primers 12SF (5′ GTTC CAGAATAATCGGCT A-3′) and 12SR (5′ ATTGACGGATG (AG) TTTGTACC-3′) reported by Casiraghi et al (2004). The obtained sequences were compared with the most Rhabdias species represented in GeneBank using the Basic Local Alignment Search Tool engine from the National Center of Biotechnology Information website.
Phylogenetic analysis was performed via DNASTRA MegAlgin software 5.01@DNA. Cluster X and GeneDoc were used to determine the alignment and percentage of sequence dissimilarity. The phylogenetic tree was generated via the Cluster W method using the Kimura 2-parameter algorithm with bootstrap analysis of 1,000 replicates; Pneumonema tiliquae and Anisakis simplex were used as the outgroup for construction of the phylogenetic tree (Tkach et al., 2014). New sequences were deposited in GenBank with accession numbers MZ351256.
3. Results
3.1. Taxonomic summary
Type host: Egyptian toad, Sclerophrys regularis.
Site of infection: Lungs.
Locality: Assiut Governorate, Upper Egypt (27° 10′ 39′' N, 031° 11′ 09′' E).
Prevalence and intensity of infection: 37 of 50 (74%) hosts examined infected with an intensity of two to three worms per host.
Deposition: Permanent slides and 70% of preserved specimens were deposited at the Parasitology Laboratory, Zoology Department, Faculty of Science, Assiut University, Egypt.
3.2. Promorph features of Rhabdias africanus
Morphometric measurements of adult hermaphrodites (based on 40 gravid specimens) are presented in Table 1.
The body is slender and outstretched, with S-shape form in fixated status, 9.67 ± 2.97 (6.61–13.96) mm in length, 311 ± 97.6 (210–470.9) µm width at midbody (location of vulva). Body covered with swollen cuticle, especially at anterior end, smooth or finely annulated with regular transverse folds at anterior end and irregular folds at caudal end (Fig. 1, Fig. 2, Fig. 3).
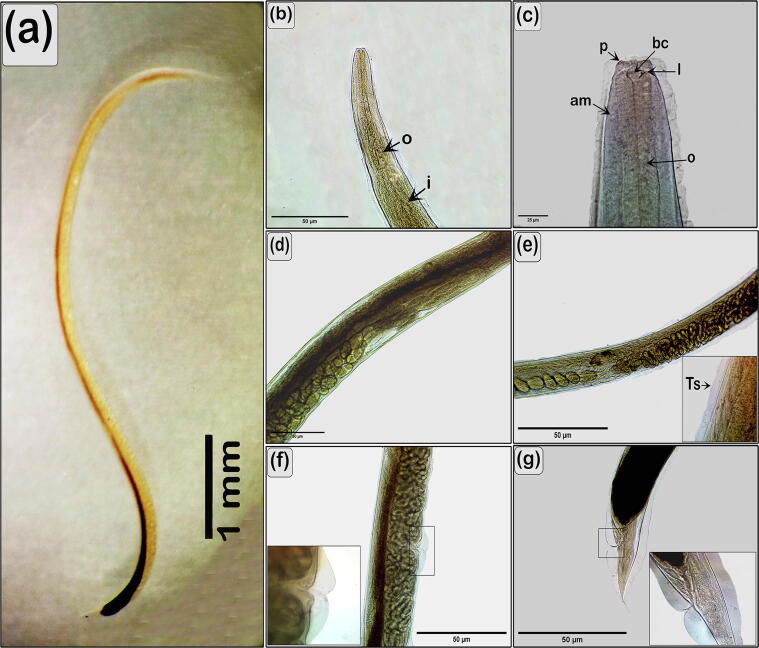
Fig. 1.
Light micrographs of R. africanus. (A) Lateral view of gravid female. (B) Anterior part of the body lateral view. (C) Magnification of the anterior end showing esophagus (o), lip (l), papillae (p), buccal cavity (bc), amphids (am). (D) Mid-region of the body showing dark part of the intestines and part of the genital system (ovary, oviduct, and uterus contained eggs). (E) Mid-region of the body showing uterus filled with larvae, magnification of the cuticle showing transverse striations (Ts). (F) Vulva. (G) Tail region, high power showing cuticle inflation.
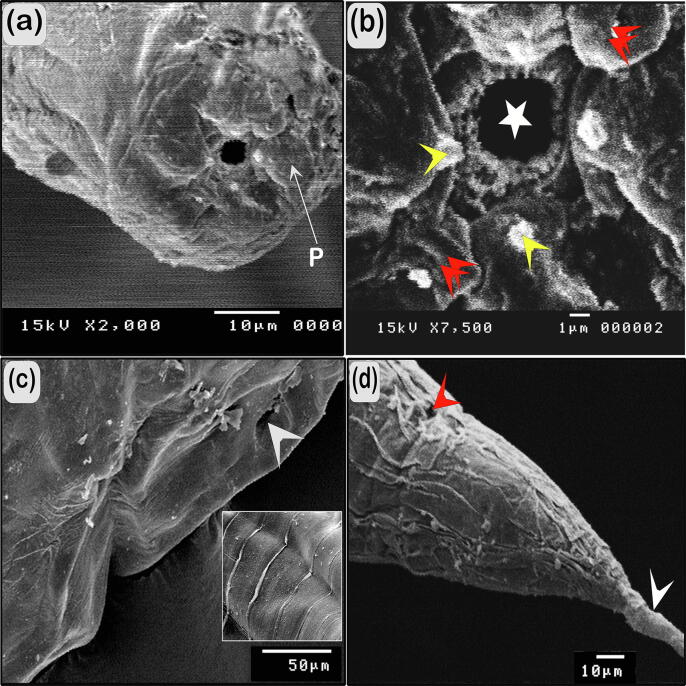
Fig. 2.
Scanning electron micrographs of R. africanus. (A) Apical view of the cephalic end, six papillae (p). (B) Magnified apical view of the cephalic end showing spherical oral opening surrounding by four true submedian lips and two of lateral pseudolabia (red arrowhead) appear in simi-spherical and papillae (yellow arrowhead) on the top of each lip. (C) Ventro-lateral view of the vulva opening (white arrowhead), high power showing transverse folds of the cuticle. (D) Lateral view of posterior end of the body showing the surface of cuticle, anus (red arrowhead), and tail (white arrowhead).
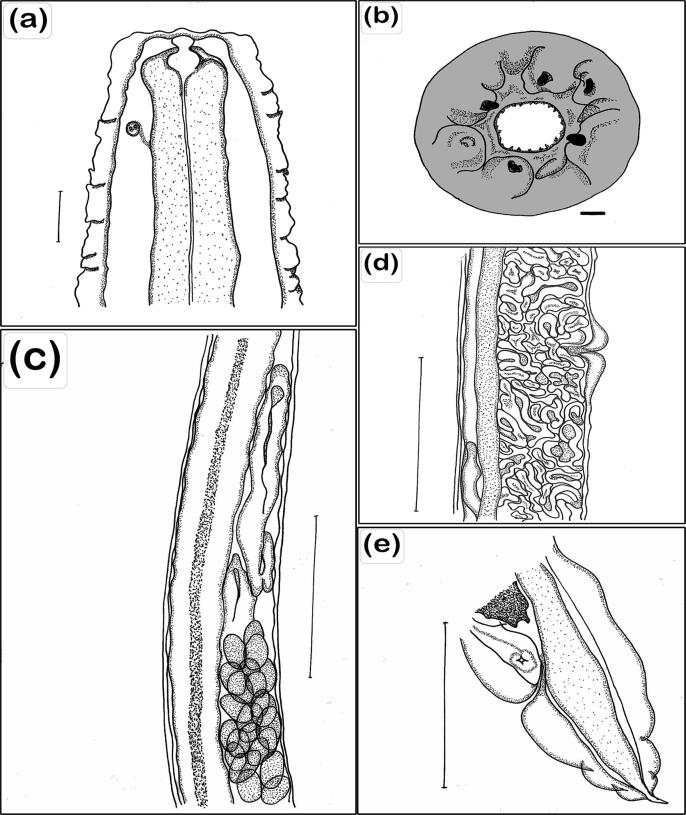
Fig. 3.
Camera lucida drawing of R. africanus showing (A) Anterior region, lateral view, with lateral amphids, scale bar 25 µm. (B) Head end, apical view, scale bar 1 µm. (C) Anterior ovary, loop of uterus containing eggs, scale bar 50 µm. (D) Vulva opening, uterus with larvae, scale bar 50 µm. (E) Tail end, lateral view, cuticle inflation, scale bar 50 µm.
Apical anterior of body is almost round in lateral and apical views, mediated by spherical mouth opening covered with sclerotized walls as a thin layer of cuticle. Oral opening surrounded by two pairs of true submedian lips (two subdorsal and two subventral) and pair of lateral outgrowths of cuticle arising around the true lips (described as lateral pseudolabia, appearing semispherical in apical and lateral apical views). Amphids located clearly in lateral view, appearing almost pore-like. Buccal capsule (esophastoma) infundibuliform in lateral view, 34.4 ± 7.9 (22.5–34.4) mm length, 17.3 ± 4.4 (10.8–20.2) mm in depth, with distinct cheilo–gymno–stegostom. Phasmid pore spherical-shaped, open somewhat at mid-length of tail.
3.3. Interior features
Esophagus cylindrical-shaped (Rhabditoid type), 503.9 ± 40.5 (442.9–542.7) µm in length, consisting of shorter anterior part (muscular) and longer glandular posterior part (procorpus and metacorpus). Anterior end of esophagus (muscular part) consisting of isthmus and basal bulb, metacorpus observed as a muscular part and forming slightly-swollen median bulb. Nerve ring surrounding esophagus at boundary between muscular and glandular parts positioned 141.5 ± 86.2 (43.9–207.3) µm in length from anterior end of body. Excretory glands located near posterior margin of nerve ring.
In the digestive system, the intestine represents the majority region and its color tends to be bloody red. Anterior part of intestine is clear, wide, and straight with a muscular sphincter thicker than the anterior part separating it from the rectum region. Intestinal lumen occupying whole body space in the front, at anterior of the genital system, and in the posterior region behind the genital system, containing dark black contents. Rectum short, lined with thick cuticle (compared with that lining mouth opening).
Genital system is amphidelphic type with limbs of similar length and shape, vulva located median to the gonads. Vulva is transverse, 5.9 ± 0.9 (5–6.7) mm in length from anterior end. Vulva pore pre-equatorial with slightly elevated around vulva opening as lips in lateral view. Ovaries are straight-reflexed with U-bends, reflexed parts almost overlapping at midbody, widening 68.4 ± 10.7 (57.7–84.4) and 53.4 ± 8.6 (40.3–64.6) µm at glandular part and muscular part, respectively. Uterus is tubular-shaped 3.0 ± 1.0 (2.3–3.7) mm in length, straight, thin-walled, filled with numerous different size eggs measured in uteri at 101.1 ± 13.2 (65.2–114.7) long by 51.4 ± 10.5 (33.2–70.4) wide. Eggs containing different stages of larvae from embryo to fully developed larvae. Oviducts are tubular-shaped, short, and straight. Vagina is short, transverse, 5.9 ± 0.9 (5–6.7) mm from anterior end of body. Tail conical-shaped, 319.3 ± 44.2 (259–371.5) µm in length with acute terminus, tapering distinctly in its distal part, narrowed cuticular inflations.
3.4. Remarks
Rhabdias africanus differs from Rhabdias bufonis described from Sclerophrys regularis in Egypt (Morsy et al., 2018) by the shape and length of the body (greater than9.86 mm in R. bufonis), the rounded oral opening, lateral amphibs, labial papillae with tapered tips, pre-equatorial vulva, and overlapping ovaries.
3.5. Phylogenic analysis
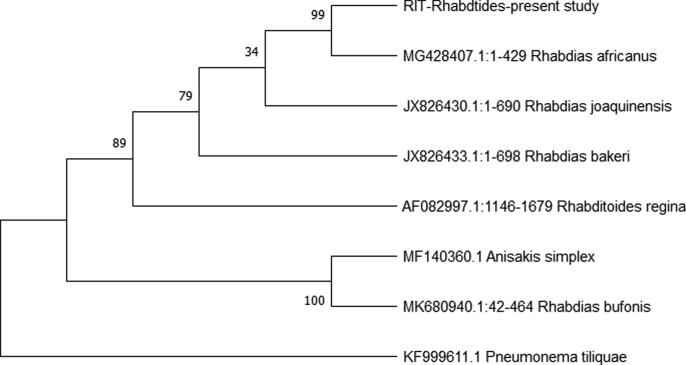
The morphological identification of R. africanus was confirmed by molecular assessment of the 12S and ITS genes. The sequenced regions of the nuclear ribosomal DNA from the present R. africanus specimens and previously published sequences from other Rhabdias species were aligned, with the P. tiliquae sequence used as an outgroup. The alignment was 619 and 878 bp in length for the 12S and ITS genes, respectively. In the resultant phylogenetic tree, the present specimens was confirmed as R. africanus (Accession no.: MZ351256) and was 99.34% sequence similarity with R. africanus (Accession no.: MG428407) collected in South Africa (Fig. 4).
Fig. 4.
Phylogenetic relationships of the present specimen, R. africanus, among five texa of Rhabdias species deposited in Genbank resulting from the Kimura 2-parameter algorithm with bootstrap analysis of 1,000 replicates; Pneumonema tiliquae and Anisakis simplex were used as the outgroup.
4. Discussion
In the last decade, studies on the helminth parasites of amphibians have been received much attention (Akani et al., 2011, Dos Santos et al., 2013, Düşen and Oğuz, 2010). Globally, about 40 species of Rhabdias infect the lungs of amphibians and reptiles (Tkach et al., 2014). In Egypt, Rhabdias species have been reported from bufonids in subtropical areas (Moravec et al., 1987, Morsy et al., 2018). In the present study, we provided a morphological description of R. africanus, as a new record from Egypt, with molecular characterization based on the 18S rRNA gene and ITS-28S region.
Morphological characteristics including body size, morphology of the buccal capsule and esophagus, and the configuration of the apex of intestine can be used for species differentiation (Tkach et al., 2006). In addition, Rhabdias species can be divided according to labial structure into three subgroups: species lacking lips, species with two pseudolabia, and species with six lips (Baker, 1978). Three species of Rhabdias are limited to Africa and represented by a single host genus, Sclerophrys (formerly included in Bufo) R. bufonis (Moravec et al., 1987), R. africanus (Kuzmin, 2001), R. picardiae (Junker et al., 2010). Our recovered specimens were morphometrically compared to various species of the same genus previously recorded in the tropical region these specimens were morphometrically similar to R. africanus (Kuzmin, 2001).
The current specimens were assigned to R. africanus based on similarities in the shape and size of the body, peculiarities of head morphology, the presence of subdorsal and subventral lips, the barrel-shaped of the buccal capsule, and the cuticular swelling in the anterior and caudal end of the body, which were previously reported by Kuzmin (2001). Differences between the present specimens and the previous description of R. africanus by Kuzmin (2001), were noted in relation to the length of the body and some organs. For example, body length was 12.45–19.80 mm in the previous description vs. 6.61–13.96 mm in the present study, and the length from the anterior end to the nerve ring was 170–230 µm in the previous description vs. 43.9–207.3 µm in the present study. Furthermore, in the previous description, the esophageal length was 575–710 µm vs. 442.9–542.7 µm in our specimens, and tail length is 250–400 in the previous description vs. 259–371.5 µm in our specimens. The noted differences in the proportions of the body and esophagus length may be attributable to the number of samples examined, maturation stage, and parasitism of different hosts (Lhermitte-Vallarino and Bain, 2004). In addition, the process of flattening the specimens on temporary mounts during the examination might have affected (Mueller et al., 2018). Furthermore, differences in the size of the host itself and the size of its internal organs might have affected on the size of parasites (Borkin et al., 2016). Interestingly, our study included detailed descriptions of the ovarian parts widening, the nerve ring and its location, and eggs with different stages of larvae, which have not previously been described in the literature.
We combined light microscopy with scanning electron microscopy observations to assess the apical structures of Rhabdias, particularly, the number and position of the labial papillae. Kuzmin (2001) described four lips overhanging and surrounding the oral opening, with two lateral pseudolabia in R. africanus. This description was in accordance with the present specimens for six elevations of the body wall were observed surrounding the oral opening with protruding tips. The entity and number of papillae on R. africanus follows the template of circumoral structures in some free-living Rhabditidae (Chitwood and Chitwood, 1938). This arrangement has been reported in several Rhabdias species, e.g., R. bufonis (Morsy et al., 2018), R. picardiae (Svitin et al., 2018), and R. ranae (Tkach et al., 2006). Difference in the circumoral structure could be explained by the growth of adult worms and migration at the cephalic end resulting in the relocation of the apical features (Baker, 1979).
Due to relatively high levels of interspecific variation, phylogenetic sequences are valuable for species identification and differentiation. We found that there is 99 % similarity between the currently investigated R. africanus sequences and those previously deposited in GenBank. On the other hand, comparison of the partial small subunits of the 18S rRNA gene sequence of the present specimens revealed genetic divergence with the Egyptian Rhabdias spp. previously deposited in GenBank.
Only three species of Rhabdias have been described in Egypt based on light microscopy: R. bufonis (Moravec et al., 1987, Morsy et al., 2018), Rhabdias aegyptiaca (Elgarhy and Garo, 2006), and Rhabdias sp. (Saad et al., 2009). However, many characteristics were observed that differed from those of the comparable species recorded in Egypt including the shape of the oral opening, the presence of lips and submedian oral papillae, and the pre-equatorial position of the vulva. The two South African species, i.e., R. picardiae and R. bufonis, are more morphologically similar to each other than to R. africanus (Junker et al., 2010). The host of R. bufonis was collected in Giza Governorate in Egypt, whereas the hosts of R. africanus were collected in the South African Lowveld Bushveld in Kruger National Park (Kuzmin, 2001).
5. Conclusion
Parasitic Rhabdias spp. demonstrates a high level of host specificity in amphibians. Consequently, more data are needed to determine the preferred host for R. africanus at various localities. This is the first report for R. africanus in Egypt and therefore represents an important reference for future studies. Further observations in the field and studies of experimental infection will help to clarify the level of specificity of Rhabdias spp. to different hosts.
Ethical approval
All procedures in the present study were approved by the National Ethical Committee of the Faculty of Science, Assiut University, Egypt. The minimum number of animals required to obtain valid results was used.
CRediT authorship contribution statement
Sara Salah Abdel-Hakeem: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Project administration. Yousef Abdal Jalil Fadladdin: Validation, Formal analysis, Resources, Data curation, Supervision, Funding acquisition. Atef M. El-Sagheer: Software, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision. Asmaa Adel: Methodology, Software, Investigation, Resources, Writing – original draft, Writing – review & editing, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors pay thanks to Dr. Yasser Farhat Mahmoud Karar, Faculty of Science South Valley University, Egypt for his valuable help throughout the study and Egyptian Knowledge Bank for English language editing.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Sara S. Abdel-Hakeem, Email: sara_assiut86@aun.edu.eg.
Yousef A. Fadladdin, Email: yfadladdin@kau.edu.sa.
Atef M. El-Sagheer, Email: Atefelsagheer@azhar.edu.eg.
Asmaa Adel, Email: asmaa.adel@sci.svu.edu.eg.
References
- Akani G., Luiselli L., Amuzie C., Wokem G. Helminth community structure and diet of three Afrotropical anuran species: a test of the interactive-versus-isolationist parasite communities hypothesis. Web Ecol. 2011;11:11–19. [Google Scholar]
- Baker M. Morphology and taxonomy of Rhabdias spp. (Nematoda: Rhabdiasidae) from reptiles and amphibians of southern Ontario. Can. J. Zool. 1978;56:2127–2141. [Google Scholar]
- Baker M. The free-living and parasitic development of Rhabdias spp. (Nematoda: Rhabdiasidae) in amphibians. Can. J. Zool. 1979;57:161–178. [Google Scholar]
- Baker M. Systematics and zoogeography of three new nematode parasites of the frog Breviceps sylvestris sylvestris FitzSimons from South Africa. Can. J. Zool. 1982;60:3134–3142. [Google Scholar]
- Baker M. Rhabdias collaris n. sp. (Nematoda: Rhabdiasidae) from frogs of Tanzania. Syst. Parasitol. 1987;9:199–201. [Google Scholar]
- Borkin L., Goncharov A., Litvinchuk S. THE Egyptian toad, Sclerophrys regularis (REUSS, 1833) AT Sharm EL-Sheikh, with comments on amphibians of the Sinai Peninsula. Russ. J. Herpetol. 2016;23:283–292. [Google Scholar]
- Casiraghi M., Bain O., Guerrero R., Martin C., Pocacqua V., Gardner S.L., Franceschi A., Bandi C. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Int. J. Parasitol. 2004;34:191–203. doi: 10.1016/j.ijpara.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Chitwood B., Chitwood M. Section I. Part II. An Introduction to Nematology. Section I; Part II: 1938. An Introduction to Nematology. [Google Scholar]
- Dos Santos V.G.T., Amato S.B., Borges-Martins M. Community structure of helminth parasites of the “Cururu” toad, Rhinella icterica (Anura: Bufonidae) from southern Brazil. Parasitol. Res. 2013;112:1097–1103. doi: 10.1007/s00436-012-3236-8. [DOI] [PubMed] [Google Scholar]
- Düşen S., Oğuz M. Metazoan endoparasites of three species of anurans collected from the Middle Black Sea Region of Turkey. Helminthologia. 2010;47:226–232. [Google Scholar]
- Elgarhy M., Garo K. Rhabdias aegyptiaca sp. n. (Nematoda: Rhabdiasidae) from the lungs of Bufo regularis (Amphibia: Bufonidae) in Egypt: light and scanning electron microscopic study. J. Union Arab Biol. Cairo. A, Zool. 2006;26:127–137. [Google Scholar]
- Finnerty P.B., Shine R., Brown G.P. The costs of parasite infection: effects of removing lungworms on performance, growth and survival of free-ranging cane toads. Funct. Ecol. 2018;32:402–415. [Google Scholar]
- Halajian A., Bursey C.R., Goldberg S.R., Luus-Powell W. Helminths of six species of anurans from the Republic of South Africa: Amietophrynus garmani, Amietophrynus gutturalis, Amietophrynus maculatus, Schismaderma carens (Bufonidae), Amietia angolensis, and Strongylopus grayii (Pyxicephalidae), with a review of South African anuran helminths. Compar. Parasitol. 2013;80:80–95. [Google Scholar]
- Hugot J.-P., Baujard P., Morand S. Biodiversity in helminths and nematodes as a field of study: an overview. Nematology. 2001;3:199–208. [Google Scholar]
- IUCN, 2016. Amphibian Specialist Group. 2016. Sclerophrys regularis. The IUCN Red List of Threatened Species 2016. e.T54747A107349840.
- Junker K., Lhermitte-Vallarino N., Barbuto M., Ineich I., Wanji S., Bain O. New species of Rhabdias (Nematoda: Rhabdiasidae) from Afrotropical anurans, including molecular evidence and notes on biology. Folia Parasitol. 2010;57:47–61. doi: 10.14411/fp.2010.007. [DOI] [PubMed] [Google Scholar]
- Kelehear C., Webb J., Shine R. Rhabdias pseudosphaerocephala infection in Bufo marinus: lung nematodes reduce viability of metamorph cane toads. Parasitology. 2009;136:919–927. doi: 10.1017/S0031182009006325. [DOI] [PubMed] [Google Scholar]
- Kuzmin Y. Rhabdias africanus sp. nov. (Nematoda: Rhabdiasidae)—A new nematode species from the South African toads (Amphibia: Bufonidae) Acta Parasitol. 2001;46:148–150. [Google Scholar]
- Kuzmin Y., Halajian A., Tavakol S., Luus-Powell W.J., Tkach V.V. Description and phylogenetic position of a new species of Rhabdias Stiles et Hassall, 1905 (Nematoda: Rhabdiasidae) from the banded rubber frog, Phrynomantis bifasciatus (Smith)(Amphibia: Microhylidae), in South Africa. Folia Parasitol. 2017;64:1–8. doi: 10.14411/fp.2017.035. [DOI] [PubMed] [Google Scholar]
- Kuzmin Y., Svitin R., Harnoster F., Du Preez L. Description and molecular characterisation of a new nematode species parasitic in the lungs of Strongylopus grayii (Smith)(Anura: Pyxicephalidae) in South Africa. Syst. Parasitol. 2020;97:369–378. doi: 10.1007/s11230-020-09917-5. [DOI] [PubMed] [Google Scholar]
- Langford G.J., Janovy J., Jr Comparative life cycles and life histories of North American Rhabdias spp. (Nematoda: Rhabdiasidae): lungworms from snakes and anurans. J. Parasitol. 2009;95:1145–1155. doi: 10.1645/GE-2044.1. [DOI] [PubMed] [Google Scholar]
- Leary C.J., Ralicki H.F., Laurencio D., Crocker-Buta S., Malone J.H. Assessing the links among environmental contaminants, endocrinology, and parasites to understand amphibian declines in montane regions of Costa Rica. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0191183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhermitte-Vallarino N., Bain O. Morphological and biological study of Rhabdias spp. (Nematoda) from African chameleons with description of a new species. Parasite. 2004;11:15–31. doi: 10.1051/parasite/200411115. [DOI] [PubMed] [Google Scholar]
- Martínez-Arce A., Jesús-Navarrete D., Leasi F. DNA barcoding for delimitation of putative Mexican marine nematodes species. Diversity. 2020;12:107. [Google Scholar]
- Moravec F., Baruš V., Ryšavý B. Some parasitic nematodes, excluding Heterakidae and Pharyngodonidae, from amphibians and reptiles in Egypt. Folia Parasitol. 1987;34:255–267. [Google Scholar]
- Morsy K., Mohamed S.A., Abdel-Ghaffar F., El-Fayoumi H., Abdel-Haleem H. Rhabdias bufonis (Rhabdiasidae) from the lung of the African common toad, Amietophrynus regularis (Bufonidae) in Egypt: new data on the basis of light and scanning electron microscopic study. Peer J. 2018;6 doi: 10.7717/peerj.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M.I., Morais D.H., Costa-Silva G.J., Aguiar A., Avila R.W., da Silva R.J. Diversity in the genus Rhabdias (Nematoda, Rhabdiasidae): evidence for cryptic speciation. Zoolog. Scr. 2018;47:595–607. [Google Scholar]
- Pizzatto L., Shine R. Lungworm infection modifies cardiac response to exercise in cane toads. J. Zool. 2012;287:150–155. [Google Scholar]
- Runey W., Runey G., Lauter F. Gametogenesis and fertilization in Rhabdias ranae Walton 1929: I. the parasitic hermaphrodite. J. Parasitol. 1978;64:1008–1014. [PubMed] [Google Scholar]
- Saad A.H., Aziz A.A., Yahie A., El-Ghareeb A. Programmed cell death in the liver of different species of anuran amphibians during metamorphosis. Aust. J. Basic Appl. Sci. 2009;3:4644–4655. [Google Scholar]
- Scott N., Crump M., Zimmerman B., Jaeger R., Inger R., Corn P., Woodward B., Dodd C., Scott D., Shaffer H. Paperback Edition. Smithsonian Books; 1994. Standard techniques for inventory and monitoring. [Google Scholar]
- Stiles, CW., Hassall, A., 1905. The determination of generic types and a list of roundworm genera with their original and type species, U.S. Dept. Agric., Bureau of Animal Industry, Bull. No. 79, 150 PP.
- Svitin R., Kuzmin Y., du Preez L. Molecular and morphological characterisation of Rhabdias picardiae Junker, Lhermitte-Vallarino et Bain, 2010 (Nematoda: Rhabdiasidae) from Delaland’s River Frog, Amietia delalandii (Duméril et Bibron, 1841)(Amphibia: Pyxicephalidae) in South Africa. Acta Parasitol. 2018;63:55–64. doi: 10.1515/ap-2018-0007. [DOI] [PubMed] [Google Scholar]
- Tkach V., Grabda-Kazubska B., Pawlowski J., Swiderski Z. Molecular and morphological evidence for close phylogenetic affinities of the genera Macrodera, Leptophallus, Metaleptophallus and Paralepoderma [Digenea, Plagiorchiata] Acta Parasitol. 1999;44:170–179. [Google Scholar]
- Tkach V.V., Halajian A., Kuzmin Y. Phylogenetic affinities and systematic position of Entomelas sylvestris Baker, 1982 (Nematoda: Rhabdiasidae), a parasite of Breviceps sylvestris FitzSimons (Amphibia: Brevicipitidae) in South Africa. Syst. Parasitol. 2014;87:293–298. doi: 10.1007/s11230-014-9469-4. [DOI] [PubMed] [Google Scholar]
- Tkach V.V., Kuzmin Y., Pulis E.E. A new species of Rhabdias from lungs of the wood frog, Rana sylvatica, in North America: the last sibling of Rhabdias ranae? J. Parasitol. 2006;92:631–636. doi: 10.1645/GE-625R.1. [DOI] [PubMed] [Google Scholar]