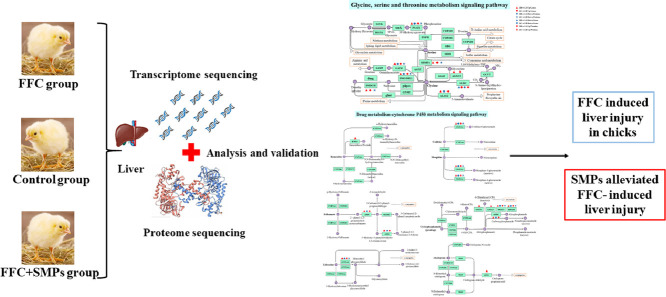
Abstract
Excessive and nonstandard use of florfenicol (FFC) can damage animal body, pollute ecological environment, and even harm human health. The toxic and side effects of FFC directly affect the production performance of poultry and the safe supply of chicken-related food. Salvia miltiorrhaza polysaccharides (SMPs) are natural macromolecular compounds, and were proved to have the effect of protecting animal liver. We used transcriptome and proteome sequencing technologies to study the effect of FFC on specific signal transduction pathways in chick livers and further explored the regulatory effect of SMPs on the above same signal pathways, and finally revealed the intervention effect and mechanism of SMPs on FFC-induced changes of liver function. The screened sequencing results were verified by qPCR and PRM methods. The results showed that FFC changed significantly 9 genes and 5 proteins in drug metabolism-cytochrome P450 signaling pathway, and the intervention of SMPs adjusted the expression levels of 5 genes and 4 proteins of the above factors. In glycine, serine and threonine metabolism signaling pathway, 8 genes and 8 proteins were significantly changed due to FFC exposure, and SMPs corrected the expression levels of 5 genes and 6 proteins to a certain extent. In conclusion, SMPs alleviated FFC-induced liver metabolic disorder in chicks by regulating the drug and amino acid metabolism pathway. This study is of great significance for promoting the healthy breeding of broilers and ensuring the safe supply of chicken-related products.
Key words: Florfenicol, Salvia miltiorrhaza polysaccharide, metabolic disorder, high throughput sequencing, chick
Graphical abstract
INTRODUCTION
Broiler feeding has the characteristics of high feed return, quick turnover, and more profit. Chicken is rich in nutrients and has a unique flavor. Antibiotics are widely used in poultry industry to prevent and treat broiler diseases. However, the nonstandard use of antibiotics has led to the slow growth of broilers, bacterial drug resistance, antibiotic residues, decline of meat quality, and other problems in poultry farming. Therefore, the search for safe drugs that can alleviate the toxic and side effects of veterinary antibiotics became a research hotspot. Chinese herbal medicines have the advantages of wide sources, low toxicity, and small side effects, which are worthy of in-depth study.
Florfenicol (FFC) is a synthetic monofluoro derivative of thiamphenicol, which belongs to the amide alcohol antibiotics. FFC has a significant therapeutic effect on livestock and poultry bacterial diseases caused by sensitive bacteria (Schwarz et al., 2004). In the course of treatment, FFC has the characteristics of easy absorption, wide antibacterial spectrum and strong antibacterial ability (van de Riet et al., 2003).
With the continuous development of livestock and poultry breeding, FFC has been widely used. However, a large number of studies have shown that the ingestion of FFC has adverse effects on animals and leads to excessive drug residues. Wang et al. (2021) found that FFC had certain nephrotoxicity to broilers, can inhibit the expression of related factors in Nrf2 signaling pathway, lead to lipid peroxidation in broiler kidneys, and accelerate abnormal apoptosis of renal tissue cells. Yun et al. (2020) demonstrated that oral FFC could affect the functions of intestinal mucosal barrier, immune system and intestinal flora in mice. Another study found that excessive drug residues were detected in the tissues of shrimp fed with FFC by gas chromatography with meta-nitrochloramphenicol (mCAP) as an internal standard proposed by Pfenning Allen P (Li et al., 2018).
FFC not only causes adverse effects on animals themselves, but also pollutes the ecological environment. Studies have shown that animals exposed to FFC can directly affect soil microbial composition and cause drug resistance (Liu et al., 2021b). Another study has shown that the residue of FFC can destroy the metabolism of microorganisms in the environment and change the nitrogen cycle (Zhou et al., 2021). FFC eventually enters human body through bioaccumulation and food chain. After a certain amount of FFC residue being ingested by the human body, it may damage the normal body function and lead to the occurrence of various diseases (Li et al., 2019b). It can be seen that excessive use of FFC can not only have adverse effects on animals, but also pollute the environment and even harm human health. Therefore, it is necessary to study the toxic effects of FFC on animals and explore new drugs that can alleviate its toxic and side effects.
As the main metabolic site and detoxification organ of the body, liver plays an important role in cleaning up the residue of FFC and other drugs. But, liver is vulnerable to the toxicity of their toxic and side effects. Shah et al. (2016) exposed 40 and 60 mg/kg b.w. of FFC to 25 kg mature healthy goats, and found that 2 doses of FFC both significantly changed the liver function indexes such as UREA, CRE, TP, ALP, SGOT, SGPT, GGT, and BIL in the blood of goats. Another study found that the average residual concentrations of FFC in muscle and liver of broilers were 311.42 ± 186.56 and 2,585.44 ± 1,759.71 µg/kg, respectively, both higher than their respective maximum residue limits (MRLs) (Nasim et al., 2016). This residue can be transmitted to humans through meat consumption, resulting in serious adverse effects on human health.
Polysaccharide is one of the main active components of Salvia miltiorrhiza. It was found that polysaccharides showed a variety of biological activities, such as immune regulation, anti-cancer and antioxidation, and had the advantages of less adverse reactions and high safety (Schlemmer et al., 2021). Salvia miltiorrhiza polysaccharides (SMPs) are natural high molecular polymers containing aldehyde or ketone groups formed by the connection of multiple monosaccharides through glycosidic bonds. It plays a key pharmacodynamic role in Salvia miltiorrhiza and has important clinical application value. Its production and usage are increasing year by year (Feng et al., 2021). Geng et al. (2015) studied the cardioprotective effect of SMPs on isoproterenol (ISO)-induced myocardial infarction (MI) in rats. The results showed that long-term oral administration of SMPs could enhance the endogenous antioxidant and lipid-lowering functions of the body, and then effectively alleviate ISO-caused heart injury of rats. Han et al. (2019) found that SMPs significantly increased the content of reduced glutathione in livers, increased the levels of serum total protein and albumin, thus improved the antioxidant capacity and alleviated the degree of liver injury.
Our previous studies found that SMPs may affect lipid metabolism, inflammation, antioxidant and drug metabolism pathways in livers of chicks at the overall biological function level (Han et al., 2021; Geng et al., 2022). However, the previous studies were not in-depth and did not clearly indicate the directional regulation of SMPs on specific signaling pathways and the interaction between genes and proteins in the changed signaling pathways. Therefore, transcriptome and proteome sequencing methods were used to study the mechanism of FFC inducing disorders of liver function and the pathway of SMPs alleviating functional disorder by acting on the same signal pathway. Quantitative real-time PCR (qPCR) and parallel reaction monitoring (PRM) were used to verify the sequencing results. This study mainly screened and analyzed the drug metabolism-cytochrome P450 signaling pathway and glycine, serine and threonine metabolism signaling pathway, and the significantly changed genes and proteins were studied as a whole to explore the biological pathway by which SMPs alleviates FFC-induced disorders of liver function in chicks. The results of this experiment will provide new ideas for the development of new drugs that can reduce the hepatotoxicity of antibiotics, which is of great significance to ensure the safety of chicken-related food and increase animal welfare. It is expected to provide a theoretical basis for the clinical application of safe and effective Chinese herbal extracts in broiler breeding industry.
MATERIALS AND METHODS
Drugs and Reagents
SMPs (purity ≥ 95%) were provided by Hangzhou Zhengda Youthbao Pharmaceutical Co., Ltd. (Hangzhou, China), and consisted of 5 different monosaccharides including mannose, rhamnose, arabinose, glucose, and galactose, with the molar ratio of 2.36 : 2.21 : 1.25 : 1.17 : 1.24.
FFC solution (commercially available clinical over-the-counter veterinary medicine, purity ≥ 10%) was purchased from Shenniu Biological Technical Co., Ltd. (Dezhou, China).
Eastep Super Total RNA Extraction Kit was purchased from Promega Biotechnology Co., Ltd. (Beijing, China). PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time) and SYBR Premix Dimer Eraser Reagent Kit (Perfect Real Time) were purchased from Takara Biotechnology Co., Ltd. (Dalian, China).
Animals and Experimental Design
One hundred and twenty one-day-old AA broilers were raised in a standard chicken house and were provided with adequate tap water and standard feed during the experiment. They were randomly divided into control group, FFC group, and FFC+SMPs group with 8 repetitions in each group and 5 chicks in each repetition. Chicks in the control group were given tap water and standard feed, those in the FFC group were given tap water containing 0.15 g/L (the clinical therapeutic dose recommended in the instructions of FFC) FFC and standard feed, and those in the FFC+SMPs group were given tap water containing 0.15 g/L FFC and 5 g/L SMPs and standard feed. Starting from 1 d of age, the drugs were administered continuously for 5 d. On the 6th d of the experiment, 10 chicks from each group were randomly selected, euthanized by intraperitoneal injection of sodium pentobarbital, and fresh liver tissues were aseptically extracted. Three liver tissues from each group were selected and labeled as LA1, LA2, LA3 (control group); LB1, LB2, LB3 (FFC group); LC1, LC2, LC3 (FFC+SMPs group). They were placed in enzyme-free tubes for transcriptome and proteome sequencing to construct transcriptome proteome database. Samples for sequencing were immediately placed in liquid nitrogen. The remaining liver tissues was wrapped in tin foil and stored in a -80°C ultra-low temperature refrigerator for use in testing other test indicators.
The chicks and standard feed used in the experiment was purchased from Hebei Dawu Agricultural Group Poultry Co., Ltd. (Baoding, China), and the transcriptome and proteome sequencing were completed by PERSONAL Company (Nanjing, China).
All experimental steps were approved by the Animal Protection and Welfare Committee of Hebei Agricultural University, China. All methods of animals used in this study were carried out in accordance with the relevant guidelines and regulations of the Animal Protection and Welfare Committee of Hebei Agricultural University (Permission number: AUH-2021259).
Transcriptome Sequencing
In this study, Oligo (dT) magnetic beads were used to enrich mRNA with polyA structure in total RNA, and the mRNA was interrupted to fragments with a length of about 300 bp by ion interruption. The first strand of cDNA was synthesized with 6-base random primers and reverse transcriptase, and the second strand of cDNA was synthesized with the first strand of cDNA as the template. After the construction of the gene bank, PCR amplification was used to enrich the library fragments, and the library was selected according to the fragment size, which was 450 bp. After RNA extraction, purification, and library construction of the samples, the next-generation sequencing technology (NGS) was used to perform paired-end (PE) sequencing on these libraries based on the Illumina HiSeq sequencing platform. The original deplantable data (raw data) were filtered, and then the high-quality sequences (clean data) were compared to the reference genome of the species. According to the comparison results, the expression of each gene was calculated. On this basis, the samples were further analyzed by expression difference analysis, enrichment analysis, and cluster analysis.
The differential expression levels of genes were analyzed. The conditions for screening differentially expressed genes were as follows: the expression difference multiple |log2 (fold change)| > 1, and the significance P value < 0.05.
Proteome Sequencing
In this experiment, TMT-labeled quantitative proteomics technology was used to carry out the research. Firstly, proteins from chick livers were extracted and protein samples were prepared using SDT lysate. The protein samples were trypsinized by Filter Aided Proteome Preparation (FASP). Around 100 μg peptide segment was taken from each sample for TMT labeling. SCX chromatographic classification and liquid chromatographic classification were performed. According to the steps of tandem mass spectrometry (LC-MS/MS) data collection, protein identification and quantitative analysis, differential expression protein screening and bioinformatics (GO, KEGG), the differentially expressed proteins were screened with the change of expression multiple of more than 1.2 times (up-regulated more than 1.2 times and down-regulated less than 0.833 times) as the standard.
The differential expression levels of proteins were analyzed. The conditions for screening differentially expressed proteins were: ratio > 1.2 or ratio < 0.833, and the significant P value < 0.05.
Detection of Candidate Genes by qPCR
Total RNA was extracted from the liver of each chick according to Eastep Super Total RNA Extraction Kit instructions. The purity and concentration of RNA were detected by spectrophotometer at 260/280 nm to ensure that the quality of samples met the test requirements. Reverse transcription experiments were performed with Primescript RT Reagent Kit with gDNA Eraser (Perfect Real Time). The synthesis of primers was entrusted to Sangon Biotech Co. Ltd., (Shanghai, China) and the primer sequences of differential genes were shown in Table 1. The β-actin was used as a reference gene. The sequences of all primers were specifically verified and were in line with normal distribution. The reaction condition of qPCR was 90°C for 30 s (predenaturation); 95°C 5 s, 60°C 20 s, 72°C 30 s (PCR reaction); 72°C 30 s (dissolution curve analysis), a total of 40 cyclic reactions were carried out. After the reaction, the threshold cycle (Ct) value of the target genes in the control group, FFC group and FFC+MPs group were obtained. The results were analyzed by 2−△△CT method, ΔΔ Ct = (Ct (target, test) – Ct (reference, test)) – (Ct (target, calibrator) – Ct (reference, calibrator)) (Chen et al., 2022; Zhang et al., 2022).
Table 1.
Special primers of genes.
| Gene name | Primer sequence (5’-3’) | Product length (bp) |
|---|---|---|
| CYP1A2 | F:GGACACGGTGCTGAATGGCTAC R:TGAGGAAACGCTCTGGGTTGAAAG |
125 |
| FMO3 | F:CACCAAGTGCTGCCTGGAAGAG R:GTGAAGACAGTGCGGTAGATGCTAG |
129 |
| ADH6 | F:GGATTGTGGAGAGCATTGGAGAAGG R:CAGGCAGTAGTTGGAGTCAGGATTC |
125 |
| AOX1 | F:ACATGGTGGAATTGAGTTGGGACAG R:GAAGCACACGCATTAGGAACAGTTG |
135 |
| UGT1A1 | F:CCCAGACCAGTGATGCCCAATATG R:GAGCCCAGGGAGAAGACAACAATAC |
134 |
| GSTT1 | F:AAGTGGAAGGTGCTACCGAGGAG R:TACGAGGTCTGCCAAGGAGATGTC |
113 |
| MGST1 | F:CAGATGTTGAACGTGTACGCAGAG R:TGGACAGATCAGGGCCACAG |
105 |
| HPGDS | F:GCCGCTACCTGTTTGCCTATG R:CAATTGCTAGGCTCTGGTGAATGA |
125 |
| GSTA3 | F:TTGGATAAGGCCGCAAACAGATA R:AATGCACGTCTGCTCTGCTCA |
106 |
| DMGDH | F:ACAGCAGCCAAAGCAAGGGAATC R:CAAATCATACAGCCCGCTAGTCCTC |
111 |
| DMGDHL1 | F:TGGTGACCGAACGCATTGAAGG R:TCGCCTTGGAGACGGAGATACAC |
85 |
| GATM | F:CACCCACCAGTGCCACTCATTC R:ATCAACCATCACACGCTTCTCATCC |
105 |
| AGXT2 | F:GCGTTGTCTTTAGGCAGCAGAAATG R:TCTTCAGGAACAAAGTCGCAAGGAG |
82 |
| SHMT1 | F:GGCAGCAGCACTGATGGAGATG R:TACGCAGGTCCAGGAGAATGAGG |
80 |
| PSAT1 | F:AGGAGGGAAGACAGGCGGATTATG R:CTGGGTCAGGAATGCTTGTATAGGC |
132 |
| ALAS2 | F:CATCTCTGGAACGCTCGGCAAG R:CCAGCAGCATACGAACGGACAG |
96 |
| GCLC | F:CATCTCTGGAACGCTCGGCAAG R:CCAGCAGCATACGAACGGACAG |
96 |
| β-action | F:GCATCTCCAGCGTGAAGAAGGTC R:AGCATCCACCGTCTCCATAGCC |
107 |
Detection of Candidate Proteins by PRM
Firstly, lysates (Beijing Solarbio Technology Co., Ltd., Beijing, China) were added into the homogenate of chick liver tissues, and the protein solution was prepared by the steps of ultrasonic, boiling water bath and centrifugation. The protein was quantitatively analyzed by BCA method. Then, it was treated with protease (Boster Biological Technology Co., Ltd., California, USA). After enzymatic hydrolysis, the peptide was desalted, lyophilized, and redissolved. The concentration of peptide was determined in OD 280. PRM quantitative analysis of target peptide was performed according to the screening results of pre-experiment. The peptide information suitable for PRM analysis was imported into the software xcalibur for PRM method setting. There were 3 samples, in which 2 μg peptide was taken from each sample and 20 fmol standard peptide was added. HPLC system easynlc with nanoliter flow rate was used for chromatographic separation. PRM detection was carried out on 9 samples respectively, and the software skyline 3.5.0 was used to perform data analysis on PRM original files.
Statistics Analysis
Statistical analysis was performed by using SPSS 19.0 (IBM Corp., Armonk, NY). All data obtained were presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) followed by Tukey's test was used for multiple comparisons. Compared with the control group, * means P < 0.05, ** means P < 0.01. Compared with the FFC group, # means P < 0.05, ## means P < 0.01.
RESULTS
The Number of Significant Differentially Expressed Genes and Proteins
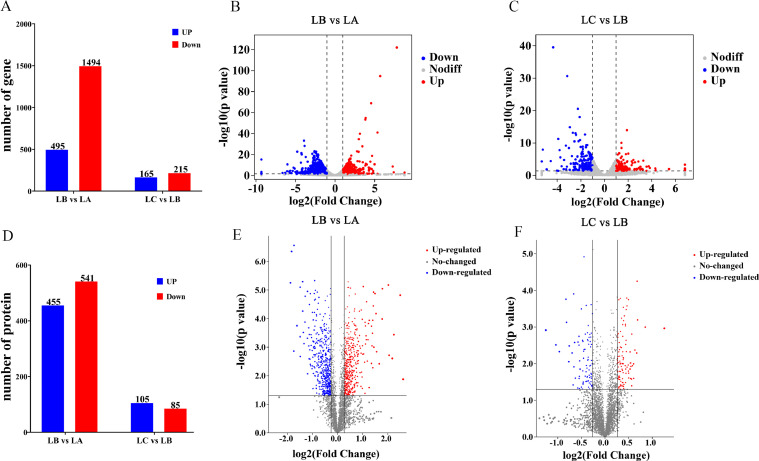
Compared with the control group, a total of 1,989 genes with significant differences were screened from the FFC group, of which 495 genes were upregulated and 1,494 genes were downregulated. Compared with FFC group, the intervention of SMPs significantly changed 380 genes, of which 165 differential genes were upregulated, and 215 differential genes were down regulated (Figure 1A–C).
Figure 1.
The number of significant differentially expressed genes and proteins in chick livers. (A) The number of significant differentially expressed genes between 3 groups. (Blue represents upregulated genes and red represents down-regulated genes.) (B) Effect of FFC exposure on differentially expressed genes. (C) Effect of SMPs intervention on differentially expressed genes induced by FFC. (D) The number of significant differentially expressed proteins between 3 groups. (E) Effect of FFC exposure on differentially expressed proteins. (F) Effect of SMPs intervention on differentially expressed proteins induced by FFC. (The abscissa of the volcano map is log2(Fold Change) and the ordinate is the negative logarithm of the difference significance level based on 10. The 2 vertical dashed lines in the figure are the thresholds for the expression of multiple of difference. The horizontal dashed line represents the threshold of significance level. Red represents up-regulated genes and proteins, blue represents down-regulated genes and proteins, and gray represents nonsignificantly differentially expressed genes and proteins.)
Early exposure to FFC significantly changed the expression of 996 proteins in chick livers, of which 455 significantly different proteins were upregulated and 541 significantly different proteins were down regulated. The expression levels of 190 proteins in FFC+MPs group were significantly different from those in FFC group, of which 105 significantly different proteins were upregulated and 85 significantly different proteins were down regulated (Figure 1D–F).
GO Analysis and KEGG Analysis of Transcriptome
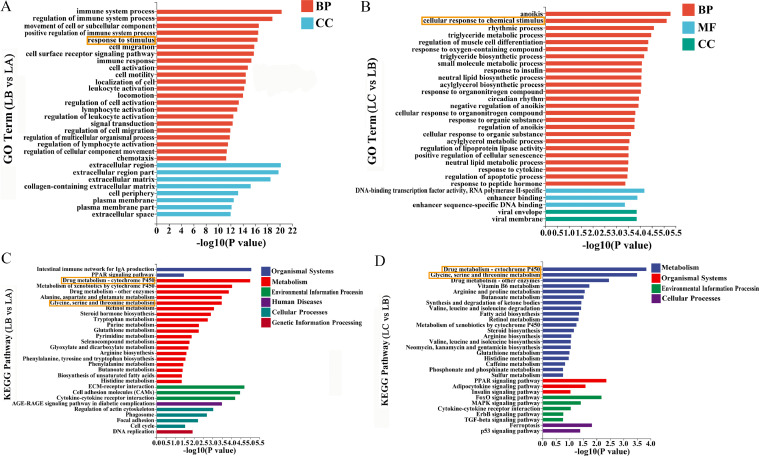
Compared with the control group, a total of 10,709 GO Term items were screened out from the GO enrichment results of FFC group, including 8,100 items belonging to Biological Process (BP), 859 items belonging to Cellular Component (CC), and the remaining 1,750 items belonging to Molecular Function (MF). However, compared with FFC group, a total of 5,502 GO Term items were screened out from the GO enrichment results of FFC+SMPs group, including 4,277 items belonging to BP, 428 items belonging to CC, and the remaining 797 items belonging to MF. GO enrichment results showed that the differential genes in this experiment were mainly enriched in BP items. We further found that FFC exposure and SMPs intervention mainly affected the response to stimulus and the cellular response to chemical stimulus (Figure 2A and 2B).
Figure 2.
GO analysis and KEGG analysis of transcriptome data in chick livers. (A) GO analysis of transcriptome data between control group and FFC group. (B) GO analysis of transcriptome data between FFC group and FFC+SMPs group. (C) KEGG analysis of transcriptome data between control group and FFC group. (D) KEGG analysis of transcriptome data between FFC group and FFC+SMPs group.
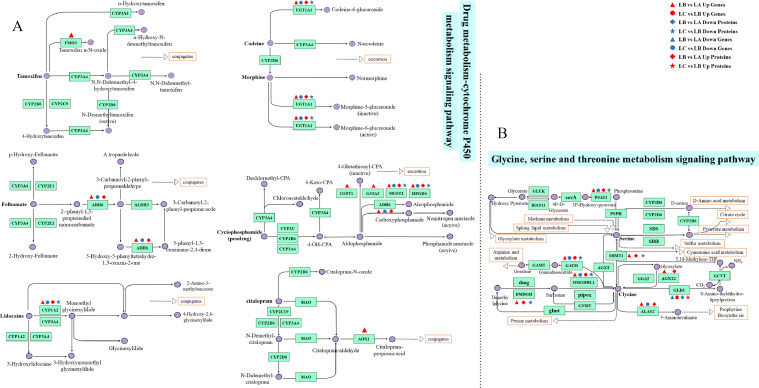
According to the KEGG enrichment analysis results of differentially expressed genes, the top 30 pathways with the smallest P value, that is the most significant enrichment, were selected for display. Compared with the control group, a total of 148 enrichment pathways were screened out in KEGG enrichment results of FFC group. Compared with FFC group, 105 enrichment pathways were screened out in FFC+SMPs group. Among the above pathways, the top 30 typical pathways with the highest proportion of significantly different genes were mainly distributed in metabolism. FFC and SMPs significantly changed the drug metabolism- cytochrome P450 signaling pathway and glycine, serine and threonine metabolism signaling pathway (Figure 2C and 2D).
GO Analysis and KEGG Analysis of Proteome
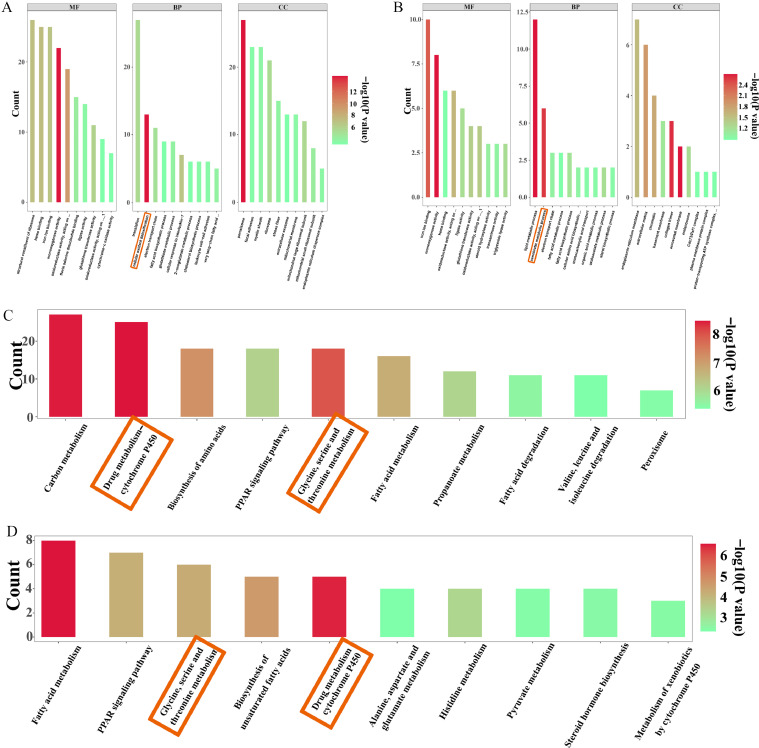
Compared with the control group, a total of 2,794 GO Term items were screened out from the GO enrichment results of differentially expressed proteins in FFC group, including 1,494 items belonging to BP, 357 items belonging to CC, and the remaining 489 items belonging to MF. However, compared with the FFC group, a total of 611 GO Term items were screened out from the GO enrichment results of differentially expressed proteins in the FFC+SMPs group, including 354 items belonging to BP, 107 items belonging to CC, and the remaining 150 items belonging to MF. GO enrichment results showed that the differential proteins in this experiment were mainly enriched in BP items. We found that FFC treatment and SMPs intervention mainly affected cellular oxidant detoxification and xenobiotic metabolic process (Figure 3A and 3B).
Figure 3.
GO analysis and KEGG analysis of proteome data in chick livers. (A) GO analysis of proteome data between control group and FFC group. (B) GO analysis of proteome data between FFC group and FFC+SMPs group. (C) KEGG analysis of proteome data between control group and FFC group. (d) KEGG analysis of proteome data between FFC group and FFC+SMPs group.
According to the KEGG enrichment analysis results of differentially expressed proteins, the top 30 pathways with the smallest p value, that is, the most significant enrichment, are selected for display. Compared with the control group, 135 significantly enriched signal pathways were screened from the KEGG enrichment results of FFC group. Compared with FFC group, 94 significantly enriched signal pathways were screened from FFC+MPs group. Among the above pathways, the top 30 typical pathways with the highest enrichment proportion of significantly different proteins were mainly distributed in the category of metabolism. Among them, drug metabolism-cytochrome P450 signaling pathway and glycine, serine and threonine metabolism signaling pathway were significantly regulated by FFC and SMPs (Figure 3C and 3D).
The Significantly Differentially Expressed Genes and Proteins in Drug Metabolism-Cytochrome P450 Signaling Pathway
Results of Combined Transcriptome and Proteome Analysis
Transcriptome results showed that compared with the control group, there were 9 significantly upregulated differential genes in the drug metabolism-cytochrome P450 signaling pathway in the livers of chicks after drinking FFC solution, namely CYP1A2, FMO3, ADH6, AOX1, UGT1A, GSTT1, MGST1, HPGDS, and GSTA3. The intervention of SMPs significantly down regulated the expression levels of 5 differential genes in the drug metabolism-cytochrome P450 signaling pathway in the livers of FFC treated chicks, namely CYP1A2, ADH6, UGT1A1, MGST1, and HPGDS.
Proteomic results showed that 5 proteins CYP1A2, ADH6, UGT1A, MGST1, and HPGDS, were significantly upregulated in FFC treated chick livers drug metabolism-cytochrome P450 signaling pathway. The expression levels of 4 proteins CYP1A2, UGT1A1, MGST1, and HPGDS of the drug metabolism-cytochrome P450 signaling pathway in the livers of chicks treated with SMPs were significantly down-regulated compared with those of FFC group (Figure 4A).
Figure 4.
The significant differentially expressed genes and proteins in the drug metabolism-cytochrome P450 signaling pathway and the glycine, serine and threonine signaling pathway in chick livers between 3 groups. (A) The significant differentially expressed genes and proteins in the drug metabolism-cytochrome P450 signaling pathway. (B) The significant differentially expressed genes and proteins in the glycine, serine and threonine metabolism signaling pathway.
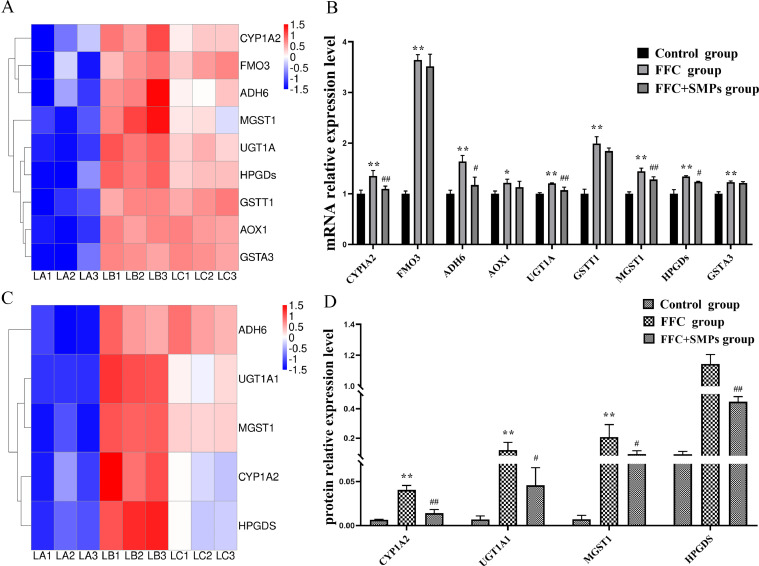
Results of qPCR Verification
Compared with the control group, the mRNA expression levels of CYP1A2, FMO3, ADH6, AOX1, UGT1A, GSTT1, MGST1, HPGDS, and GSTA3 were significantly upregulated in FFC group. However, the expression levels of CYP1A2, ADH6, UGT1A1, MGST1, and HPGDS mRNA in FFC+SMPs group were significantly lower than those in FFC group. The results of qPCR were consistent with those of transcriptome sequencing, confirming that the transcriptome sequencing data were accurate (Figure 5A and 5B).
Figure 5.
Validation of significant differentially expressed genes and proteins in drug metabolism-cytochrome P450 signaling pathway in chick livers between 3 groups. (A) Heat maps of 9 significantly differentially expressed genes. (B) The mRNA relative expression levels of CYPIA2, FMO3, ADH6, AOX1, UGT1A, GSTT1, MGST1, HPGDS, and GSTA3 by qPCR. (C) Heat maps of 5 significantly differentially expressed proteins. (D) The protein expression levels of CYP1A2, UGT1A1, MGST1, and HPGDS by PRM. Compared with the control group, * means P < 0.05, ** means P < 0.01. Compared with the FFC group, # means P < 0.05, ## means P < 0.01.
Results of PRM Verification
PRM technique was used to validate the protein levels of CYPIA2, UGT1A, MGST1, and HPGDS. There were significant differences of 2 specific peptides in each protein. After drinking FFC, the protein expression levels of CYP1A2, UGT1A, MGST1, and HPGDS in livers of chicks were significantly upregulated. However, compared with the FFC group, the expression levels of CYP1A2, UGT1A, MGST1, and HPGDS protein were significantly downregulated in FFC+SMPs group. The overall trend of RRM results and proteomics results was consistent. Since PRM results are secondary identification based on proteomics, PRM has a high accuracy. However, the coelution of peptides of significantly different proteins would produce quantitative ratio compression effect, resulting in lower quantitative difference than actual difference (Figure 5C and D).
The Significantly Differentially Expressed Genes and Proteins in Glycine, Serine, and Threonine Metabolism Signaling Pathway
Results of Combined Transcriptome and Proteome Analysis
Transcriptome results showed that the expression levels of DMGDH, DMGDHL1, GATM, AGXT2, SHMT1, PSAT1, and ALAS2 genes in the glycine, serine and threonine metabolism signaling pathway of the chick livers exposed to FFC were significantly upregulated, and the expression level of GCLC gene was significantly down regulated. After the intervention of SMPs, the expression levels of DMGDHL1, GATM, PSAT1, and ALAS2 genes in glycine, serine and threonine metabolism signaling pathway were significantly down regulated, and the expression level of GCLC gene was significantly upregulated.
The proteomic results showed that compared with the control group, there were 8 differential proteins in the glycine, serine and threonine metabolism signaling pathway in the livers of FFC group, among which the protein expression levels of DMGDH, DMGDHL1, GATM, AGXT2, SHMT1, PSAT1, and ALAS2 were significantly upregulated, and the protein expression level of GCLC was significantly down regulated. The expression levels of DMGDH, DMGDHL1, GATM, SHMT1 and PSAT1 proteins in glycine, serine and threonine metabolism signaling pathway in FFC+MPs group were significantly lower than those in FFC group, and the expression level of GCLC protein was significantly higher (Figure 4B).
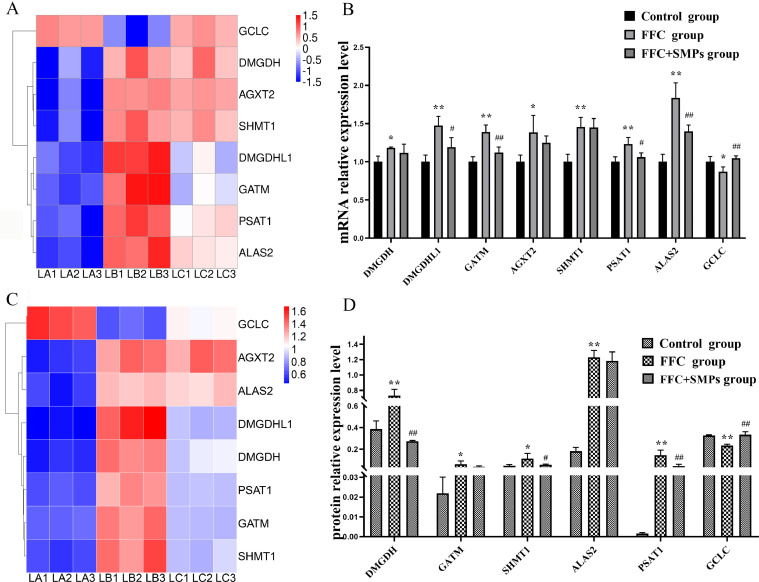
Results of qPCR Verification
Compared with the control group, the mRNA expression levels of DMGDH, DMGDHL1, GATM, AGXT2, SHMT1, PSAT1, and ALAS2 were significantly upregulated and the expression levels of GLDC mRNA were significantly down-regulated in FFC group. Compared with the FFC group, the expression levels of DMGDHL1, GATM, PSAT1, and ALAS2 mRNA were significantly down-regulated and the expression level of GLDC mRNA was significantly upregulated in FFC+SMPs group. The results of qPCR were consistent with those of transcriptome, thus confirming the accuracy of transcriptome (Figure 6A and 6B).
Figure 6.
Validation of significant differentially expressed genes and proteins in glycine, serine and threonine metabolism signaling pathway in chick livers between 3 groups. (A) Heat maps of 8 significantly differentially expressed genes. (B) The mRNA expression levels of DMGDH, DMGDHL1, GATM, AGXT2, SHMT1, PSAT1, ALAS2, and GCLC by qPCR. (C) Heat maps of 8 significantly differentially expressed proteins. (D) The protein expression levels of DMGDH, ALAS2, GATM, SHMT1, PSAT1, and GCLC by PRM. Compared with the control group, * means P < 0.05, ** means P < 0.01. Compared with the FFC group, # means P < 0.05, ## means P < 0.01.
Results of PRM Verification
PRM technique was used to validate the expression levels of DMGDH, GATM, SHMT1, PSAT1, ALAS2, and GCLC proteins. Compared with the control group, the protein expression levels of DMGDH, GATM, SHMT1, PSAT1, and ALAS2 were significantly upregulated and the levels of GCDC protein was significantly downregulated in FFC group. SMPs significantly reduced the protein expression levels of DMGDH, SHMT1, and PSAT1 activated by FFC, and significantly increased the expression levels of GCDC protein inhibited by FFC. The general trend agreement between RRM result and proteomics result is high (Figure 6C and 6D).
DISCUSSION
Drug exposure is easy to produce adverse effects on animals. As an important place for metabolism and biological transformation of endogenous and exogenous substances, liver plays an important role in detoxification. Several recent studies have found that animal poisoning can damage the liver and lead to liver metabolic disorder in chicks (Li et al., 2021b; Liu et al., 2021c). Drug metabolic enzymes or transporters catalyze the metabolic process of drugs in the liver, and finally improve the water solubility of drugs and excrete them from the liver. This metabolic process can be divided into 3 stages (Board and Anders, 2021). The first stage usually includes oxidation, reduction, hydrolysis and other reactions, which is mainly catalyzed by phase I metabolic enzymes such as cytochrome P450 enzymes (CYPs) and flavin containing monooxygenases (FMOs) (Wei et al., 2000). The second stage is the binding reaction catalyzed by transferase, which is mainly completed by phase II metabolic enzymes such as glutathione S-transferase (GSTs) and uridine diphosphate glucuronosyltransferases (UGTs). The third stage is the expulsion of metabolites from the liver mediated by transporters. It is mainly completed by phase II metabolic enzymes such as ATP-binding cassette protein (ABCs) and solute carrier transporter (SCLs) (Li et al., 2019a).
The significantly differentially expressed CYP1A2, FMO3, ADH6, and AOX1 screened from the sequencing results belong to phase I metabolic enzymes. CYP1A2 is an important subtype of CYP1 family. CYP1 family is a monooxygenase mainly existing in the liver, and its common metabolic substrates are generally molecular compounds of polycyclic aromatic hydrocarbons such as polychlorinated biphenyls and nitrogen-containing compounds (Mao et al., 2019). FFC is one of the metabolic substrates of CYP1 family. FMO3 is a subtype of FMOs, which is widely distributed in the endoplasmic reticulum and has many catalytic substrates. FMOs catalyze the metabolic process of FFC with FAD as cogroup, NADPH as cofactor, and molecular oxygen as cosubstrate, and eventually form R-SOH and H2O (Phillips and Shephard, 2020). ADH6 is one of the 7 genotypes of alcoholi dehydrogenase (ADH), which strictly uses NAD/NADH as a coenzyme when it is involved in catalyzing the oxidation and reduction of various alcohols and aldehydes. ADH is a general defense system against exogenous alcohols and aldehydes (Tête et al., 2020). AOX1 (aldehyde oxidase1) is a flavin protease containing molybdenum, which has a wide range of substrates and weak specificity. The structure with catalytic function in AOX1 contains a dimer composed of 2 identical subunits, and each subunit contains 3 conserved domains with different functions, which can catalyze the process of aldehyde group to generate corresponding carboxylic acid and the decomposition of nitrogen-containing heterocyclic compounds (Schumann et al., 2009; Romão et al., 2017). The aldehyde group does not appear in the molecular formula of FFC, but in the intermediate produced in the process of ADH metabolism. Sequencing and validation results showed that FFC resulted in the up regulation of expression levels of the above 4 phase I metabolic enzymes, which over stimulated the first stage metabolic function of chick livers, and may lead to the excessive accumulation of toxic metabolites in chick livers, resulting in liver injury. Study showed that ethanol and isoamyl alcohol increased the level of drug metabolism enzyme CYP3A in hepatocytes. The increased CYP3A could catalyze the demethylation of cocaine to form toxic metabolites and increase the risk of hepatotoxicity (Kostrubsky et al., 1995). The above finding corroborate with the FFC-induced hepatic drug metabolism disorder in our study. However, through down regulating the expression levels of CYP1A1 and ADH6, SMPs inhibited part of the phase I metabolic function of chick livers, antagonized the surge of metabolic level induced by FFC, reduced the accumulation of toxic metabolites, and may alleviate liver injury.
UGTIA1, GSTT1, MGST1, GSTA3, and HPGDS belong to phase II metabolic enzymes in the liver. UGTIA1 plays an important role in the metabolism of drugs and bilirubin (Sugatani et al., 2001). It can accelerate the metabolism of drugs and promote the combination of unconjugated bilirubin and glucuronic acid to form water-soluble conjugated bilirubin that can be excreted with bile (Liu et al., 2021a). Bilirubin is toxic, but it also has antioxidant function and can inhibit the oxidation of linoleic acid and phospholipids. Sequencing and validation results showed that the gene and protein expression levels of UGTIA1 were significantly increased after livers exposure to FFC, which may lead to dysfunction of liver metabolism of drugs and bilirubin. The intervention of SMPs significantly reduced the expression level of UGTIA1, which was close to the normal level, and alleviated the stimulation of FFC on liver metabolism. GSTT1, GSTA3, and MGST1 are 3 different types of GSTs. GSTs can be stimulated by exogenous substances to increase the expression level (Hashim et al., 2020; Buratti et al., 2021). Some studies have shown that antibiotics can change the expression level and activity level of GST (Savcı et al., 2020; Ayna et al., 2021). GST took part in excess toxic substance-induced poisoning mechanism in animals (Li et al., 2021a). GSTs can catalyze the binding of metabolites and accelerate the transport of the conjugates in cells. This process catalyzes the GSH-dependent conversion reaction. GSTs can also transform the metabolites produced in the first stage to form highly hydrophilic compounds with low chemical activity, which are then excreted with bile or urine (Vaish et al., 2020). Some substances can bypass the first stage and be directly metabolized in the second stage under the action of metabolic enzymes such as GSTs. The results showed that FFC induced the up regulation of the expression levels of GSTT1, GSTA3, and MGSTT1 in the livers of chicks, abnormally enhanced the phase II metabolism, resulting in the excessive metabolism of drugs, which may produce a large number of toxic metabolites. Our previous results showed that FFC significantly increased the activity of GST in the liver of chicks, stimulated the detoxification function of glutathione signaling pathway, over-activated the metabolic process, and caused a serious burden on the liver (Liu et al., 2022). This study is consistent with our previous experimental results. SMPs significantly reduced the expression level of MGST1 in the livers, partially weakened the hyperactivity of drug metabolic function, and played a certain role in alleviating liver metabolic disorder.
Glycine is the simplest amino acid, and is involved in the synthesis of purines, porphyrins, creatine, and glyoxylic acid. Glycine can be excreted with bile or urine by combining with a variety of substances. The significant difference factors DMGDH, DMGDHL1, GATM, and AGXT2 screened from the sequencing results of transcriptome and proteome are related to the synthesis of glycine, while GCLC is related to the degradation of glycine. DMGDH is a mitochondrial matrix enzyme involved in choline metabolism. It removes a methyl group from dimethyl glycine to form the modified amino acid sarcosine (Binzak et al., 2001). Then sarcosine is converted to glycine under the action of DMGDHL1. GATM can catalyze the transfer of guanidine from L-arginine to glycine, which is the rate limiting step of creatine synthesis (Sandell et al., 2003; Sun et al., 2021). With alanine as the amino donor, AGXT2 catalyzes the conversion of the intermediate metabolite glyoxylic acid to glycine (Seppälä et al., 2014). GCLC is involved in the main pathway of glycine degradation and provides tetrahydrofolic acid derivatives for the biosynthesis of various cellular substances (Ramani et al., 2012). The expression levels of DMGDH, DMGDHL1, GATM, and AGXT2 genes and proteins in FFC treated livers were significantly increased, and the expression level of GCLC was significantly decreased. The significant changes in the transcriptome levels and proteome levels of the above 5 factors led to a large amount of glycine synthesis in chick livers, inhibited the degradation process of glycine and induced glycine metabolic disorder. Compared with FFC alone, the combination of SMPs and FFC significantly decreased the expression levels of GATM, DMGDHL1, DMGDH, and increased the expression level of GCLC, which not only reduced the synthesis of glycine, but also increased the degradation of glycine. It can be seen that SMPs antagonized the metabolic disorder of glycine caused by FFC to a certain extent, and reduced the burden of glycine synthesis and metabolism in the liver.
Serine is a neutral aliphatic hydroxyl amino acid, which is a nonessential amino acid. Serine synthesis pathway is an important branch of glycolysis reaction, which is completed by 3-step enzymatic reaction. SHMT is in the second step of enzymatic reaction and can catalyze the transamination of 3-phosphohydroxypyruvate (pPYR) to produce phosphoserine (pSER), and alpha-ketoglutarate (αKG). SHMT1 is responsible for encoding cytoplasmic isozymes, and SHMT2 is responsible for encoding mitochondrial isozymes (Anderson and Stover, 2009; Macfarlane et al., 2011). SHMT can also catalyze the mutual transformation between serine, glycine, and tetrahydrofolate. Tetrahydrofolate can enter the process of folate cycle. PSAT1, an enzyme dependent on vitamin B6, catalyzed the reversible conversion of 3-phosphate hydroxypyruvate to 3-phosphate serine, and was the second rate-limiting enzyme in the biosynthesis of serine, playing an important role in the biosynthesis of serine (Hwang et al., 2016; Lin et al., 2019). The synthetic pathway of serine is the aerobic assimilation pathway of reducing carbon-1 compounds into the central metabolic system, which plays an important role in the metabolic process of chicks. FFC upregulated the expression levels of PSAT1 and SHMT1 in chick livers and synthesized excessive serine. Although serine plays an important role in animal body, the content of serine has its own dynamic balance in liver. Serine synthesized in large quantities would stimulate its metabolic response in chick livers. In order to maintain the dynamic balance of serine synthesis and degradation, chick liver was forced to accelerate the degradation of serine, which seriously increased its burden. After oral administration of SMPs, the expression levels of PSAT1 and SHMT1 in the livers of FFC treated chicks was significantly down regulated, which partially reduced the rate of serine synthesis. The results showed that SMPs were helpful to restore the normal metabolic level of serine in the liver.
ALAS2 is a key enzyme in heme synthesis and promotes hematopoietic cell differentiation by increasing hemoglobin synthesis (Whatley et al., 2008). Heme is oxidizing. According to literature reports, excessive heme can cause oxidative damage to cells (Tilley, 2004). FFC increased the expression level of ALAS2 and promoted the synthesis of excessive heme, which may cause oxidative damage to the livers histocyte of chicks. However, the combined use of SMPs and FFC reduced the expression level of ALAS2 and alleviated the oxidative damage.
In conclusion, the early application of the recommended dose of FFC excessively stimulated the phase I and phase II drug metabolism, increase the synthesis of glycine and serine and reduce their degradation, thus breaking the metabolic balance in chick livers. Chick livers were difficult to maintain the normal metabolic process, resulting in metabolic disorder, increased metabolic burden, and might cause liver damage. The intervention of SMPs inhibited the phase I and phase II metabolic function of liver and the hyperactivity of glycine and serine metabolic reaction induced by FFC, corrected the metabolic disorder of drugs and amino acids to a certain extent, reduced the accumulation of toxic metabolites in chick livers, and then maintained the normal function of liver.
ACKNOWLEDGMENTS
Authors’ contributions: Wanyu Shi conceived and designed the study, and he rigorously revised the manuscript. Wei Liu participated in the design of the experiment, conducted most of the experiments, and analyzed the results. Ying Liu helped collect samples and participated in the qPCR experiment. Siyuan Fang and Weiyu Yao participated in the collation of transcriptome sequencing data. Xiao Wang and Yongzhan Bao participated in the collation of proteome sequencing data. All authors read and approved the final manuscript.
This study was supported by Key Research and Development Plan Project of Hebei Province of China (No. 22326622D), Natural Science Foundation of Hebei Province of China (No. C2021204026) and Graduate Innovation Funding Project of Hebei Province of China (No. CXZZBS2021037).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101989.
Appendix. Supplementary materials
References
- Anderson D.D., Stover P.J. SHMT1 and SHMT2 are functionally redundant in nuclear de novo thymidylate biosynthesis. PLoS One. 2009;4:e5839. doi: 10.1371/journal.pone.0005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayna A., Khosnaw L., Temel Y., Ciftci M. Antibiotics as inhibitor of glutathione S-transferase: biological evaluation and molecular structure studies. Curr. Drug Metab. 2021;22:308–314. doi: 10.2174/1389200222666210118102700. [DOI] [PubMed] [Google Scholar]
- Binzak B.A., Wevers R.A., Moolenaar S.H., Lee Y.M., Hwu W.L., Poggi-Bach J., Engelke U.F., Hoard H.M., Vockley J.G., Vockley J. Cloning of dimethylglycine dehydrogenase and a new human inborn error of metabolism, dimethylglycine dehydrogenase deficiency. Am. J. Hum. Genet. 2001;68:839–847. doi: 10.1086/319520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board P.G., Anders M.W. Moonlighting in drug metabolism. Drug Metab. Rev. 2021;53:76–99. doi: 10.1080/03602532.2020.1858857. [DOI] [PubMed] [Google Scholar]
- Buratti F.M., Darney K., Vichi S., Turco L., Di Consiglio E., Lautz L.S., Béchaux C., Dorne J.C.M., Testai E. Human variability in glutathione-S-transferase activities, tissue distribution and major polymorphic variants: meta-analysis and implication for chemical risk assessment. Toxicol. Lett. 2021;337:78–90. doi: 10.1016/j.toxlet.2020.11.007. [DOI] [PubMed] [Google Scholar]
- Chen D., Liang J., Jiang C., Wu D., Huang B., Teng X., Tang Y. itochondrion participated in effect mechanism of manganese poisoning on heat shock protein and ultrastructure of testes in chickens [e-pub ahead of print] Biol. Trace Elem Res. 2022 doi: 10.1007/s12011-022-03259-7. Accessed July 6, 2022. [DOI] [PubMed] [Google Scholar]
- Feng J., Set Byeol K., Hee Jung L., Su Min S., Soon Sung L., Hong Won S. Effects of Salvia miltiorrhiza Bunge extract and its single components on monosodium urate-induced pain in vivo and lipopolysaccharide-induced inflammation in vitro. J. Tradit. Chinese Med. 2021;41:219–226. [PubMed] [Google Scholar]
- Geng Y., Lu C., Jin G., Li S., Cui Y., Han C., Shi W., Bao Y. Study on the mechanism of Salvia miltiorrhiza polysaccharides in relieving liver injury of broilers induced by florfenicol. Environ. Sci. Pollut. Res. Int. 2022;29:3372–3385. doi: 10.1007/s11356-021-15687-4. [DOI] [PubMed] [Google Scholar]
- Geng Z.H., Huang L., Song M.B., Song Y.M. Protective effect of a polysaccharide from Salvia miltiorrhiza on isoproterenol (ISO)-induced myocardial injury in rats. Carbohydr. Polymers. 2015;132:638–642. doi: 10.1016/j.carbpol.2015.06.086. [DOI] [PubMed] [Google Scholar]
- Han C., Wang X., Zhang D., Wei Y., Cui Y., Shi W., Bao Y. Synergistic use of florfenicol and Salvia miltiorrhiza polysaccharide can enhance immune responses in broilers. Ecotoxicol. Environ. Saf. 2021;210 doi: 10.1016/j.ecoenv.2020.111825. [DOI] [PubMed] [Google Scholar]
- Han C., Wei Y., Wang X., Ba C., Shi W. Protective effect of Salvia miltiorrhiza polysaccharides on liver injury in chickens. Poult. Sci. 2019;98:3496–3503. doi: 10.3382/ps/pez153. [DOI] [PubMed] [Google Scholar]
- Hashim Z., Ilyas A., Zarina S. Therapeutic effect of hydrogen peroxide via altered expression of glutathione S-transferase and peroxiredoxin-2 in hepatocellular carcinoma. Hepatobiliary Pancreat Dis. Int. 2020;19:258–265. doi: 10.1016/j.hbpd.2020.03.006. [DOI] [PubMed] [Google Scholar]
- Hwang I.Y., Kwak S., Lee S., Kim H., Lee S.E., Kim J.H., Kim Y.A., Jeon Y.K., Chung D.H., Jin X., et al. Psat1-dependent fluctuations in α-ketoglutarate affect the timing of ESC differentiation. Cell Metab. 2016;24:494–501. doi: 10.1016/j.cmet.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Kostrubsky V.E., Strom S.C., Wood S.G., Wrighton S.A., Sinclair P.R., Sinclair J.F. Ethanol and isopentanol increase CYP3A and CYP2E in primary cultures of human hepatocytes. Arch. Biochem. Biophy. 1995;322:516–520. doi: 10.1006/abbi.1995.1495. [DOI] [PubMed] [Google Scholar]
- Li N., Zhou T., Wu F., Wang R., Zhao Q., Zhang J.Q., Yang B.C., Ma B.L. Pharmacokinetic mechanisms underlying the detoxification effect of Glycyrrhizae Radix et Rhizoma (Gancao): drug metabolizing enzymes, transporters, and beyond. Expert Opin. Drug Metab. Toxicol. 2019;15:167–177. doi: 10.1080/17425255.2019.1563595. [DOI] [PubMed] [Google Scholar]
- Li W., Guo F., Jiang X., Li Y., Li X., Yu Z. Compound ammonium glycyrrhizin protects hepatocytes from injury induced by lipopolysaccharide/florfenicol through oxidative stress and a MAPK pathway. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2019;225 doi: 10.1016/j.cbpc.2019.108585. [DOI] [PubMed] [Google Scholar]
- Li W., Li Y., Jiang X., Li X., Yu Z. Compound ammonium glycyrrhizin protects hepatocytes from injury induced by lipopolysaccharide/florfenicol through a mitochondrial pathway. Molecules. 2018;23:2378. doi: 10.3390/molecules23092378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Ali Shah S.W., Zhou Q., Yin X., Teng X. The contributions of miR-25-3p, oxidative stress, and heat shock protein in a complex mechanism of autophagy caused by pollutant cadmium in common carp (Cyprinus carpio L.) hepatopancreas. Environ. Pollut. 2021;287 doi: 10.1016/j.envpol.2021.117554. [DOI] [PubMed] [Google Scholar]
- Li Z., Miao Z., Ding L., Teng X., Bao J. Energy metabolism disorder mediated ammonia gas-induced autophagy via AMPK/mTOR/ULK1-Beclin1 pathway in chicken livers. Ecotoxicol. Environ. Saf. 2021;217 doi: 10.1016/j.ecoenv.2021.112219. [DOI] [PubMed] [Google Scholar]
- Lin J.Y., Zhang C.H., Zheng L., Li H.J., Zhu Y.M., Fan X., Li F., Xia Y., Huang M.Z., Yang S.H., Qi X.L., Huo H.Z., Chen H.S., Lou X.L., Luo M. Establishment and assessment of the hepatic venous pressure gradient using biofluid mechanics (HVPG(BFM)): protocol for a prospective, randomised, non-controlled, multicentre study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-028518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Yu Q., Li Z., Zhang L., Hu M., Wang C., Liu Z. UGT1A1 dysfunction increases liver burden and aggravates hepatocyte damage caused by long-term bilirubin metabolism disorder. Biochem. Pharmacol. 2021;190 doi: 10.1016/j.bcp.2021.114592. [DOI] [PubMed] [Google Scholar]
- Liu J., Yu F., Call D.R., Mills D.A., Zhang A., Zhao Z. On-farm soil resistome is modified after treating dairy calves with the antibiotic florfenicol. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141694. [DOI] [PubMed] [Google Scholar]
- Liu W., Wang X., Liu Y., Fang S., Wu Z., Han C., Shi W., Bao Y. Effects of early florfenicol exposure on glutathione signaling pathway and PPAR signaling pathway in chick liver. Ecotoxicol. Environ. Saf. 2022;237 doi: 10.1016/j.ecoenv.2022.113529. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yu M., Cui J., Du Y., Teng X., Zhang Z. Heat shock proteins took part in oxidative stress-mediated inflammatory injury via NF-κB pathway in excess manganese-treated chicken livers. Ecotoxicol. Environ. Saf. 2021;226 doi: 10.1016/j.ecoenv.2021.112833. [DOI] [PubMed] [Google Scholar]
- Macfarlane A.J., Perry C.A., McEntee M.F., Lin D.M., Stover P.J. Shmt1 heterozygosity impairs folate-dependent thymidylate synthesis capacity and modifies risk of Apc(min)-mediated intestinal cancer risk. Cancer Res.. 2011;71:2098–2107. doi: 10.1158/0008-5472.CAN-10-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Wang J., Wang Q., Yang L., Li Y., Lin H., Peng Y., Zheng J. Nitidine chloride-induced CYP1 enzyme inhibition and alteration of estradiol metabolism. Drug Metab. Dispos. 2019;47:919–927. doi: 10.1124/dmd.119.086892. [DOI] [PubMed] [Google Scholar]
- Nasim A., Aslam B., Javed I., Ali A., Muhammad F., Raza A., Sindhu Z.U. Determination of florfenicol residues in broiler meat and liver samples using RP-HPLC with UV-visible detection. J. Sci. Food Agric. 2016;96:1284–1288. doi: 10.1002/jsfa.7220. [DOI] [PubMed] [Google Scholar]
- Phillips I.R., Shephard E.A. Flavin-containing monooxygenase 3 (FMO3): genetic variants and their consequences for drug metabolism and disease. Xenobiotica. 2020;50:19–33. doi: 10.1080/00498254.2019.1643515. [DOI] [PubMed] [Google Scholar]
- Ramani K., Tomasi M.L., Yang H., Ko K., Lu S.C. Mechanism and significance of changes in glutamate-cysteine ligase expression during hepatic fibrogenesis. J. Biol. Chemi. 2012;287:36341–36355. doi: 10.1074/jbc.M112.370775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romão M.J., Coelho C., Santos-Silva T., Foti A., Terao M., Garattini E., Leimkühler S. Structural basis for the role of mammalian aldehyde oxidases in the metabolism of drugs and xenobiotics. Curr. Opin. Chem. Biol. 2017;37:39–47. doi: 10.1016/j.cbpa.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Sandell L.L., Guan X.J., Ingram R., Tilghman S.M. Gatm, a creatine synthesis enzyme, is imprinted in mouse placenta. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4622–4627. doi: 10.1073/pnas.0230424100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savcı A., Koçpınar E.F., Budak H., Çiftci M., Şişecioğlu M. The effects of amoxicillin, cefazolin, and gentamicin antibiotics on the antioxidant system in mouse heart tissues. Protein Pept. Lett. 2020;27:614–622. doi: 10.2174/0929866526666191112125949. [DOI] [PubMed] [Google Scholar]
- Schlemmer W., Selinger J., Hobisch M.A., Spirk S. Polysaccharides for sustainable energy storage - a review. Carbohydr. Polymers. 2021;265 doi: 10.1016/j.carbpol.2021.118063. [DOI] [PubMed] [Google Scholar]
- Schumann S., Terao M., Garattini E., Saggu M., Lendzian F., Hildebrandt P., Leimkühler S. Site directed mutagenesis of amino acid residues at the active site of mouse aldehyde oxidase AOX1. PLoS One. 2009;4:e5348. doi: 10.1371/journal.pone.0005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Kehrenberg C., Doublet B., Cloeckaert A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004;28:519–542. doi: 10.1016/j.femsre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Seppälä I., Kleber M.E., Lyytikäinen L.P., Hernesniemi J.A., Mäkelä K.M., Oksala N., Laaksonen R., Pilz S., Tomaschitz A., Silbernagel G., et al. Genome-wide association study on dimethylarginines reveals novel AGXT2 variants associated with heart rate variability but not with overall mortality. Eur. Heart J. 2014;35:524–531. doi: 10.1093/eurheartj/eht447. [DOI] [PubMed] [Google Scholar]
- Shah J.M., Qureshi T.A., Shah T., Shah Q.A., Arain M.A., Bhutto Z.A., Saeed M., Siyal F.A. Impact of therapeutic and high doses of florfenicol on kidney and liver functional indicators in goat. Vet. World. 2016;9:1135–1140. doi: 10.14202/vetworld.2016.1135-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugatani J., Kojima H., Ueda A., Kakizaki S., Yoshinari K., Gong Q.H., Owens I.S., Negishi M., Sueyoshi T. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology. 2001;33:1232–1238. doi: 10.1053/jhep.2001.24172. [DOI] [PubMed] [Google Scholar]
- Sun C., Lin S., Li Z., Liu H., Liu Y., Wang K., Zhu T., Li G., Yin B., Wan R. iTRAQ-based quantitative proteomic analysis reveals the toxic mechanism of diclofenac sodium on the kidney of broiler chicken. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2021;249 doi: 10.1016/j.cbpc.2021.109129. [DOI] [PubMed] [Google Scholar]
- Tête A., Gallais I., Imran M., Legoff L., Martin-Chouly C., Sparfel L., Bescher M., Sergent O., Podechard N., Lagadic-Gossmann D. MEHP/ethanol co-exposure favors the death of steatotic hepatocytes, possibly through CYP4A and ADH involvement. Food Chem. Toxicol. 2020;146 doi: 10.1016/j.fct.2020.111798. [DOI] [PubMed] [Google Scholar]
- Tilley B. Red blood cell physiology, Epoetin alfa, and iron. Nephrol. Nurs. J. 2004;31:75–78. [PubMed] [Google Scholar]
- Vaish S., Gupta D., Mehrotra R., Mehrotra S., Basantani M.K. Glutathione S-transferase: a versatile protein family. Biotech. 2020;10:321. doi: 10.1007/s13205-020-02312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Riet J.M., Potter R.A., Christie-Fougere M., Burns B.G. Simultaneous determination of residues of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in farmed aquatic species by liquid chromatography/mass spectrometry. J. AOAC Int. 2003;86:510–514. [PubMed] [Google Scholar]
- Wang X., Han C., Cui Y., Li S., Jin G., Shi W., Bao Y. Florfenicol causes excessive lipid peroxidation and apoptosis induced renal injury in broilers. Ecotoxicol. Environ. Saf. 2021;207 doi: 10.1016/j.ecoenv.2020.111282. [DOI] [PubMed] [Google Scholar]
- Wei P., Zhang J., Egan-Hafley M., Liang S., Moore D.D. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- Whatley S.D., Ducamp S., Gouya L., Grandchamp B., Beaumont C., Badminton M.N., Elder G.H., Holme S.A., Anstey A.V., Parker M., et al. C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload. Am. J. Hum. Genet. 2008;83:408–414. doi: 10.1016/j.ajhg.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S., Guo Y., Yang L., Zhang X., Shen W., Wang Z., Wen S., Zhao D., Wu H., Chen J., et al. Effects of oral florfenicol on intestinal structure, function and microbiota in mice. Arch. Microbiol. 2020;202:161–169. doi: 10.1007/s00203-019-01731-y. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cui J., Wang Y., Lin X., Teng X., Tang Y. Complex molecular mechanism of ammonia-induced apoptosis in chicken peripheral blood lymphocytes: miR-27b-3p, heat shock proteins, immunosuppression, death receptor pathway, and mitochondrial pathway. Ecotoxicol. Environ. Saf. 2022;236 doi: 10.1016/j.ecoenv.2022.113471. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Xie X., Feng F., Huang S., Sun Y. Impact of acyl-homoserine lactones on the response of nitrogen cycling in sediment to florfenicol stress. Sci.Total Environ. 2021;785 doi: 10.1016/j.scitotenv.2021.147294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.