Abstract
The intestinal microbiota has attracted tremendous attention in the field of the poultry industry due to its critical role in the modulation of nutrient utilization, immune system, and consequently the improvement of the host health and production performance. Accumulating evidence implies intestinal microbiota of laying hens is a potential mediator to improve the prevalent issues in terms of egg quality decline in the late phase of laying production. However, the regulatory effect of intestinal microbiota on egg quality in laying hens remains elusive, which requires consideration of microbial baseline composition and succession during their long lifespans. Notable, although Firmicutes, Bacteroidetes, and Proteobacteria form the vast majority of intestinal microbiota in layer hens, dynamic intestinal microbiota succession occurs throughout all laying periods. In addition to the direct effects on egg safety, intestinal microbiota and its metabolites such as short-chain fatty acids, bile acids, and tryptophan derivatives, are suggested to indirectly modulate egg quality through the microbiota-gut-liver/brain-reproductive tract axis. These findings can extend our understanding of the crosstalk between intestinal microbiota and the host to improve egg quality and safety. This paper reviews the compositions of intestinal microbiota in different physiological stages of laying hens and their effects on egg quality and proposes that intestinal microbiota may become a potential target for modulating egg quality and safety by nutritional strategies in the future.
Key words: intestinal microbiota, microbial succession, short-chain fatty acids, egg quality, layer hen
INTRODUCTION
As a cost-effective and superior resource of food protein, eggs are widely produced and consumed globally. The world egg production reached 76.50 Mt in 2018, with an increase of 50.12% from 50.96 Mt in 2000 (FAOSTAT, 2020). Egg production has met consumer demand while the improvement of egg quality is essential at present. In addition to eggshell quality, albumen quality, yolk quality, egg flavor, and egg safety are also concerned. However, due to the prolonged and high-intensity egg laying, adverse changes in intestinal health, liver metabolism, and oviducal immune function result in poor eggshell and albumen quality in the late phase of laying production (Wang et al., 2018; Feng et al., 2020), causing huge economic losses. Additionally, given the perception of animal welfare, the laying hen housing system is changing from cage rearing to cage-free laying hen management (Kidd and Anderson, 2019). This will increase laying hen exposure to pathogenic bacteria and parasites, challenging the physiology, performance, egg quality, and egg safety of laying hens (Jeni et al., 2021; Ricke et al., 2022). The disorder of intestinal microbiota indued by the invasion of pathogenic bacteria will pose serious threats to food safety due to the presence of foodborne pathogens in eggs (Gantois et al., 2009; Salihu et al., 2015). Thus, over the past 2 decades, numerous studies have tried various nutritional approaches to improve egg quality and safety, which found amino acids (Khattak and Helmbrecht, 2019), vitamins and minerals (Alagawany et al., 2020), natural plant bioactive compounds (Feng et al., 2021), prebiotics and probiotics (Khan et al., 2020), and enzyme (Lei et al., 2018) play vital roles in modulating production performance and egg quality of laying hens. Notably, improved egg quality was usually accompanied by the alteration of intestinal microbiota in these studies, suggesting critical interactions between the intestinal microbiota and nutritional responses, as well as the physiological health of layers.
It has been reported that the intestinal microbiota of layer hens had positive effects on the improvement of nutrient utilization (Dai et al., 2020; Wang et al., 2020a), intestinal barrier (Khan et al., 2020; Miao et al., 2020), production performance (Shang et al., 2020; Wang et al., 2020c), as well as egg quality (Zhan et al., 2019; Feng et al., 2021). Indeed, intestinal microbes and their metabolites act as signaling molecules linking the gut, liver, brain, and reproductive tract (Nicholson et al., 2012; Cryan et al., 2020; Agus et al., 2021), which in turn have a direct or indirect impact on poultry health and egg quality. Thus, intestinal microbiota may act as a potential target for modulating egg quality and safety by nutritional strategies in the future. However, numerous studies imply that poultry age is the primary driver-force of changes in intestinal microbiota (Ballou et al., 2016; Park et al., 2017; Shi et al., 2019; Dai et al., 2020), which requires consideration of microbial baseline composition and succession during long lifespans to shape it effectively. Currently, the composition and function of the intestinal microbiota of poultry have been well characterized and clarified in broilers (Huang et al., 2018; Shang et al., 2018). Nevertheless, layers have longer lifespans, different genotypes, dietary requirements, and rearing conditions than broilers (Kers et al., 2018). Previous studies on layer microbiota were limited to a specific age and its regulatory effects on egg quality and safety remain unclear.
This review is hereby focused on the colonization characteristics of intestinal microbiota at different physiological stages of layer hens and their effects on egg quality and safety. This can extend our understanding of intestinal microbiota and host crosstalk, contributing to the development of nutrition strategies with targeted microbiota interventions to improve egg quality and safety of laying hens.
INTESTINAL MICROBIOTA IN DIFFERENT STAGES OF LAYER HENS
Origin and Succession of Intestinal Microbiota in Laying Hens
It was previously accepted that chick embryos were sterile and the initial intestinal microbiota of chicks originated from a posthatch environment (van der Wielen et al., 2002). However, recent studies have argued the theory of sterility and reported the presence of diverse microbes in chick embryos using 16S rRNA sequencing technology (Ding et al., 2017; Lee et al., 2019; Akinyemi et al., 2020; Shterzer et al., 2020; Dai et al., 2021). Ding et al. (2017) collected a total of 12 fecal samples from the maternal hens, 51 embryo samples, and 113 chick samples under aseptic conditions and characterize the intestinal microbiota of maternal hens, embryos, and chicks. They found that there was a correlation coefficient of 0.40 in the microbiota composition between maternal and offspring embryonic intestines (Ding et al., 2017), indicating that maternal microbiota affects the microbial colonization in its offspring. A wide variety of microbes colonize in the oviduct of laying hens, such as Firmicutes, Proteobacteria, Bacteroidetes, and Acidobacteria (Lee et al., 2019; Wen et al., 2021). Interestingly, Shterzer et al. (2020) characterized the microbial composition along the oviduct and the intestine (jejunum and cecum) of broiler breeders at 37 wk of age based on amplicon sequence variant taxonomic assignment. They found a large overlap significant overlap between the intestinal microbiota and the oviducal microbiota, with 55 and 53% of amplicon sequence variants in the jejunum and cecum, respectively, shared with the magnum. Additionally, they concluded that higher microbe abundance in the jejunum and cecum resulted in a higher probability of being presented in the oviduct by the semi-log nonlinear regression analysis (Shterzer et al., 2020). Considering that the end of the reproductive and digestive systems of chicken open at the cloaca, intestinal microbes should travel the oviduct up to the infundibulum and establish a full oviductal microbiota (Lee et al., 2019; Shterzer et al., 2020). Because of the close contact between the egg and oviduct for about 24 h during egg formation (Hincke et al., 2012), microbes can be directly transmitted into the yolk, albumen, eggshell membrane, and eggshell. Recently, Lee et al. (2019) sampled oviducts, cloaca, eggs, and chicken embryos from specific pathogen-free layers to assess the impact of oviducal microbiota on the fertilized eggs and embryos by the SourceTracker analysis. They found that a total of 21 shared core genuses were identified among the oviduct, cloaca, eggshell, albumen, and the chick embryo (Lee et al., 2019). The eggshell microbiota contributed about 63% of the chick embryo and albumen microbiota, in comparison to the oviduct microbiota contributed 28% of the eggshell microbiota (Lee et al., 2019). This finding suggested the transfer of maternal oviduct microbiota to the embryo mainly occurs through the albumen and eggshell. It is consistent with the view that the fetus initially colonizes the microbiota from the uterus in mammals (Collado et al., 2016; Bi et al., 2021). Consequently, the vertical transmission route of microbiota has been established in poultry, starting from the maternal gut and passing through the oviduct to its offspring. However, microbial transmission appears to be selective from the maternal oviduct to the embryo. It is challenging to transfer Lactobacillus from the oviducal magnum to chick embryos (Lee et al., 2019), whereas pathogenic microorganisms such as Salmonella, Escherichia coli, and Campylobacter can be transmitted vertically (Kizerwetter-Świda and Binek, 2008). Studies on how microbes colonize from the egg to the embryonic intestine are still limited. It seems that chick embryos not only obtain microbes through the amniotic fluid but also absorb the yolk so that microbes in the egg can colonize the embryonic intestine.
The Embryonic Stage
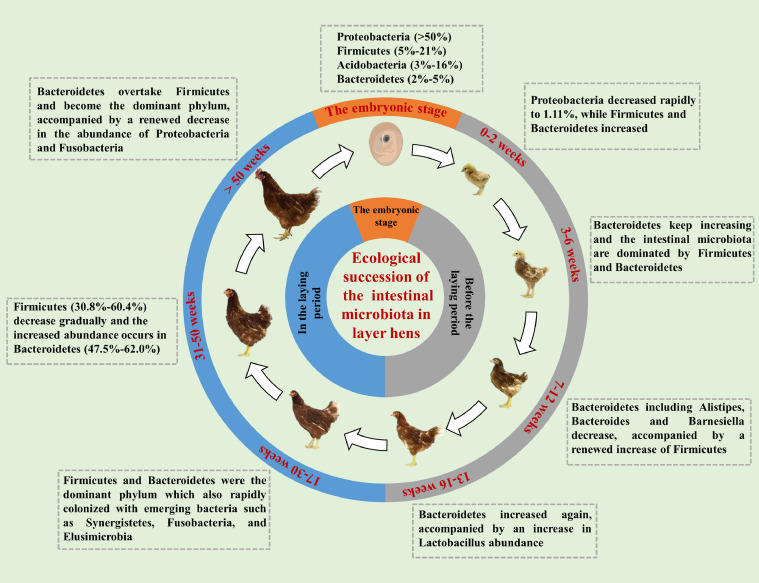
The embryonic intestine microbiota was predominantly composed of Proteobacteria and Firmicutes at the phylum level. Proteobacteria showed the highest abundance and accounted for more than 50% of all species (Ding et al., 2017), followed by Firmicutes (5%–21%), Acidobacteria (3%–16%), and Bacteroidetes (2%–5%) (Ding et al., 2017; Akinyemi et al., 2020). However, inconsistent results at the genus level were found in recent studies. Ding et al. (2017) reported that the core genus of embryonic gut microbiota was Halomonas (79%) while Pseudomonas (71%) and Ochrobactrum (23%) dominated embryonic gut microbiota in other studies (Lee et al., 2019; Akinyemi et al., 2020). In addition to poultry breeds and hatching environments, the different hypervariable regions of the 16S rRNA gene for PCR also cause inconsistent results (Sperling et al., 2017). Moreover, there are dynamic alterations in the gut microbiota of chick embryos across the embryonic stage. An increased microbial population at the early embryonic stage was reported in chickens (Ding et al., 2017; Akinyemi et al., 2020), especially the Proteobacteria which was also the dominant bacterial taxa in the embryonic intestine of humans and mammals without intra-amniotic infection (Seferovic et al., 2019; Bi et al., 2021). Akinyemi et al. (2020) revealed that microbial functions of the chick embryos were enriched in the membrane transport, cellular processing and signal transduction, carbohydrate metabolism, amino acid metabolism, and genetic information pathways. Among them, the membrane transport pathway is closely related to host immune cell activation, while cellular processing and signal transduction is involved in the antigen presentation (Cella et al., 1997; Odamaki et al., 2016). Therefore, one hypothesis was that the high abundance of Proteobacteria could be associated with the development of the immune organ and system rather than permanent colonization (Hamburger and Hamilton, 1992; Oakley et al., 2014). Numerous pieces of evidence suggest that the microbial community is an essential driver of intestinal innate immune programming (Romano-Keeler et al., 2014; Levy et al., 2017), and plays a critical role in the differentiation and maturation of epithelial cells (Ivanov et al., 2009; Sommer and Bäckhed, 2013). Besides, aerobic lipid oxidation of yolk was the dominant way to supply energy for chick embryos accompanied by the extremely low carbohydrate metabolism (Yu et al., 2018), which can also explain the low abundance of Firmicutes at the embryonic stage (Polansky et al., 2015). However, the limited oxygen and other gas exchange capacity of chick embryos resulted in the shift of energy metabolism from aerobic oxidation to anaerobic glycolysis after the age of embryos 19 (Uni and Ferket, 2004). Simultaneously, the microbial population dropped drastically at the late embryonic stage and was accompanied by the absence of some microbes in chicks and maternal hens (Ding et al., 2017; Akinyemi et al., 2020). Interestingly, most of the microbes that gradually disappeared during the dramatic succession were facultative aerobes (Awad et al., 2016), suggesting these microbes were temporarily harbored in embryos and were influenced by the physiological metabolism shift during embryonic development (Ding et al., 2017).
Before the Laying Period
The chicken intestinal microbiota is a microenvironment with continuous succession to maintain dynamic balance (Lu et al., 2003). The intestinal microbial composition in chickens is influenced by several factors such as genotypes, sex, dietary patterns, and the rearing environment (Zhu et al., 2002). At present, the composition and function of the intestinal microbiota have been well characterized and clarified in broiler chicks (Huang et al., 2018; Shang et al., 2018), which have different microbial diversity and community composition compared to those of layers (Pandit et al., 2018). The early microbial succession of the broiler intestine can be divided into 3 stages: the first stage is dominated by vertical transmission and rapid colonization of Streptococcus, Shigella, and Escherichia coli. The second stage is characterized by the rapid growth of Lachnospiraceae and Ruminococcaceae from 4 d posthatch. In the third stage, the relative abundance of Clostridium decreases with the appearance of Bacilliales, Bacteroidales, and Pseudomonadales from 10 d posthatch (Jurburg et al., 2019). Nevertheless, studies about intestinal microbiota composition and succession in layers are limited due to longer lifespans.
To consider changes in physiological characteristics and nutritional requirements of pullets, the prelaying period (0–16 wk) is divided into 3 stages, including digestive and immune system development (0–6 wk), bone development (7–12 wk), and reproductive system development (13–16 wk) (Adil and Magray, 2012). Likewise, the composition of the intestinal microbiota of pullets undergoes characteristic succession with changes in host growth and development (Li et al., 2018). After the embryonic microbial succession, the low microbial diversity in newly-hatched layer chicks is predominantly composed of Proteobacteria and Firmicutes, including Enterobacteriaceae, Clostridiaceae, Streptococcus, Enterococcaceae, Lachnospiraceae, and Ruminococcaceae (Ballou et al., 2016; Dai et al., 2020). After 2 wk posthatch, the relative abundance of Proteobacteria in the cecal microbiota decreased from 52.33% to 1.11%, while the relative abundance of Firmicutes increased from 17.13% to 67.38%. Meanwhile, the relative abundance of Bacteroidetes increased from 0.31% to 24.49% (Ballou et al., 2016; Dai et al., 2020). The development of the intestinal immune system has been shown to parallel the development of intestinal microbiota (Ekino et al., 1980). Therefore, short-term colonization of the intestine with a high abundance of Proteobacteria in layer chicks may be associated with activating the intestinal immune response and promoting immune system development (Oakley et al., 2014). Furthermore, a previous study found that the early succession of intestinal microbiota was synchronized with intestinal development of layer chicks, and the enriched Firmicutes, Lactobacillus, Enterococcus, and Ruminiclostridium were identified as biomarkers for accelerated intestinal development by linear discriminant analysis (Dai et al., 2020). In addition, Firmicutes can ferment dietary nondigestible carbohydrates into short-chain fatty acids (SCFA) such as butyric acid, providing energy to enhance the intestinal barrier (Peng et al., 2009). Lachnospiraceae and Ruminococcaceae were also confirmed to be highly correlated with feed efficiency in poultry (Singh et al., 2012). Accordingly, the predominant bacterial taxa rapidly shift from Proteobacteria to Firmicutes in the early intestinal microbiota, contributing to carbohydrate absorption to provide more energy for the intestinal development of layer chicks (Polansky et al., 2015; Dai et al., 2020). The relative abundance of Bacteroidetes in the cecum of layer chicks increases over wk 3 to 6 posthatch. Subsequently, a relatively stable intestinal microbiota dominated by Firmicutes and Bacteroidetes is established (Dai et al., 2020; Liu et al., 2020b). Bacteroidetes can also provide energy for the host intestine by utilizing complex polysaccharides to produce propionic and butyric acids (Gibiino et al., 2018). Bacteroidetes proliferate with the increasing complexity and maturity of intestinal microbiota, and its abundance gradually becomes consistent with that of Firmicutes. This finding indicates that the microbial energy supply pathway gradually shifts from mainly relying on the butyric acid produced by Firmicutes to synergistic effects, which may be adapted to the physiological needs of layers with slower growth (Polansky et al., 2015). In addition, one of the characteristics of Firmicutes is the ability to express L-fucose isomerase, whereas Bacteroidetes express xylose isomerase. The site-specific or age-specific proteoglycan expressions of the fucosylation or xylosylation in intestinal mucus may be the conditions for microbial colonization (Polansky et al., 2015; Richards et al., 2019). In general, the intestinal microbial succession of layer chicks is first characterized by the vertical transmission of Proteobacteria as the dominant microbes, followed by the rapid colonization of Firmicutes and Bacteroidetes during the brooding period.
During the pullet period, the abundance of Bacteroidetes in layers, including Alistipes, Bacteroides and Barnesiella gradually decrease in layers, accompanied by a renewed increase in the abundance of Firmicutes (Li et al., 2018; Neijat et al., 2019). The critical period for the rapid development of the bones in layers occurs at 7 to 12 wk posthatch (Adil and Magray, 2012). An increase in Firmicutes indicates a corresponding increase in the content of butyric acid as a fermentation product. Butyric acid can bind to the G protein-coupled receptor 43 (GPR43) on dendritic cells and activate Wnt signaling pathway in bone marrow stromal cells by increasing the production of TGF-β by Tregs, causing their proliferation and differentiation into osteoblasts (Zaiss et al., 2019). Therefore, the periodic increase in the abundance of butyric acid-producing bacteria may reflect the physiological needs of rapidly developing bones during the pullet period. In addition, the physiology of layers changes significantly near the laying period, as evidenced by the enhancement of lipid metabolism and nutrient utilization (Li et al., 2017), driving the succession of intestinal microbiota. A more mature and stable intestinal microflora has been established at 16 wk of layers’ age. The relative abundance of Bacteroidetes increased again, accompanied by an increase in Lactobacillus abundance and microbial community diversity. These changes can reduce intestinal lumen pH to inhibit colonization of pathogenic bacteria while enhancing nutrient utilization, consequently supporting the increased egg production in the laying period (Videnska et al., 2014; Neijat et al., 2019).
In the Laying Period
Age-linked variations in the microbial composition of layers intestinal have been defined in recent studies (Videnska et al., 2014; Li et al., 2018; Liu et al., 2020b; Xiao et al., 2021). The composition of the gut microbiota fluctuates substantially before the laying period while it is relatively stable in the laying period, which may be attributed to the management system of laying hens (Joat et al., 2021). Laying hens acclimatize to the rearing conditions after moving to the production shed, which remains constant till the end of the production cycle. However, the endocrine and sex hormone changes at the onset of laying may potentially influence the intestinal microbiota in laying hens (Lumpkins et al., 2008). For example, Lactobacillus, Lactococcus, and Bifidobacterium were more abundant in laying hens with low body weight and high production performance (Zhao et al., 2013; Wang et al., 2020c). Although Firmicutes was still the dominant phylum in the early laying period, Bacteroidetes overtook Proteobacteria as the second most abundant phylum (Joat et al., 2021). Moreover, the intestine of laying hens was also rapidly colonized with some bacteria that were not present before the laying period, such as Synergistetes, Fusobacteria, and Elusimicrobia with 1.0 to 2.0% relative abundance (Videnska et al., 2014). Compared with the brooding and pullet period, a broader microbial diversity of the intestine is observed in the laying period (Videnska et al., 2014). In the peak production period (30–50 wk), Bacteroidetes (47.5%–62.0%), Firmicutes (30.8%–60.4%), Proteobacteria (2.0%–10.0%), and Fusobacteria (2.0%–5.0%) formed the vast majority of microbiota (Guo et al., 2018; Hamid et al., 2019; Miao et al., 2020; Shang et al., 2020; Joat et al., 2021). Notably, the relative abundance of Firmicutes decreases gradually in the mid laying period, whereas the increased abundance occurs in Bacteroidetes. Accordingly, Ruminococcaceae (16.7%–28.8%), Rikenellaceae (12.5%–30.0%), Bacteroidaceae (12.5%–27.5%), Lachnospiraceae (6.7%–21.2%), and Lactobacillaceae (2.5% –10.0%) dominate gut microbiota at the family level. Among them, the most abundant bacterial genuses are Bacteroides (11.1%–32.5%), Rikenella (10.1%–15.0%), Lachnospira (5.0%–8.0%), and Lactobacillus (2.0%–10.0%) (Guo et al., 2018; Hamid et al., 2019; Miao et al., 2020; Shang et al., 2020; Joat et al., 2021). In addition, the relative abundance of Bacteroidetes overtake Firmicutes and become the dominant phylum in the late laying period, accompanied by a renewed decrease in the abundance of Proteobacteria and Fusobacteria (Joat et al., 2021). Collectively, Firmicutes, Bacteroidetes, and Proteobacteria form the vast majority of intestinal microbiota across the whole laying period. Furthermore, the relative abundance of Bacteroides increase in the early laying period, accompanied by a decrease in the relative abundance of Firmicutes, until they reach a balance in the peak production period. In the late phase, the relative abundance of Bacteroides exceeds that of Firmicutes (Figure 1).
Figure 1.
Intestinal microbial compositions in different stages of layer hens.
EFFECTS OF THE INTESTINAL MICROBIOTA OF LAYING HENS ON EGG QUALITY AND SAFETY
Vertical Microbiota Transmission Directly Affects Egg Quality and Safety
Egg contaminations with pathogenic bacteria such as Escherichia and Salmonella can lead to foodborne infection in human consumers (Wen et al., 2021). In addition to environmental factors, the microbiome on the eggshell surface assists in establishing maternal-offspring contact by vertical microbiota transmission (van Veelen et al., 2018). The laying hen can pass intestinal and cloacal microbes to its offspring during oviposition, as the egg is exposed to cecal secretions in the cloaca. Previous studies reported that Firmicutes were the dominant bacterial taxa on the eggshell surface, making up approximately 50% of the total phylum (Neira et al., 2017; Shi et al., 2020). The cloacal microbial community of laying hens is also largely dominated by Firmicutes (Wen et al., 2021), suggesting vertical microbiota transmission is key to the contamination of eggshell and egg safety (Trudeau et al., 2020). Therefore, modulating the intestinal microbiota will be an effective way to reduce the risk of spreading foodborne pathogens in humans. As the first barrier, the eggshell microbiota plays an important role in preventing pathogenic bacteria through competitive exclusion. Compared with eggs that did not contact the cloaca and were removed by dissection, the oviposited egg surface had a distinct microbiota and a higher bacterial load with a density of 0.75 per 1800 μm2 (Bunker et al., 2021). Generally, fungi can multiply rapidly by catabolizing nutrients in eggs and producing harmful toxins and an unpleasant odor (Chang et al., 2021). Recently, Bunker et al. (2021) confirmed that eggshell microbes had antifungal properties by fungal attachment assays. Microbes can produce hydrolytic enzymes such as chitinase or protease to degrade mycelia and disrupt fungal growth (Gutiérrez-Román et al., 2015). In addition, it was found that microbes on the eggshell surface like Salmonella and Escherichia directly affect the eggshell quality and accelerate the formation of egg translucency through bacterial penetration (Chousalkar et al., 2010). Conversely, the eggshell characteristics and antimicrobial molecules may determine the eggshell surface microbiota (Réhault-Godbert, 2021). Certain matrix proteins distributed from the outer cuticle to the inner membranes of eggshells reinforce the antimicrobial properties of eggs (Gautron et al., 2007). The association between ovocalyxin-32 and egg quality, including eggshell color, eggshell strength, eggshell stiffness, blood, and meat spots, was revealed by quantitative trait locus searching (Takahashi et al., 2010). Moreover, ovocalyxin-36 is a prominent protein expressed in the oviduct at the shell mineralization stage (Gautron et al., 2007). As it is similar to lipopolysaccharide-binding proteins and bactericidal permeability-increasing proteins, ovocalyxin-36 is related to the natural defense of eggshells (Yin et al., 2020). The purified ovocalyxin-36 can inhibit the growth of Staphylococcus aureus and demonstrate immune regulatory functions in the LPS challenged model (Kovacs-Nolan et al., 2014). Compared with the magnum and uterus, some of the avian beta-defensin family members including AvBD-1, AvBD-7, AvBD-3, and AvBD-10 were over-expressed in the oviducal isthmus (Yin et al., 2020). This implies that the eggshell membrane also plays a defensive function against further pathogenic invasion (Hincke et al., 2012). In addition, pigments were secreted from the oviducal epithelium into the uterine fluid and then deposited on the eggshell (Sparks, 2011). A recent study found that the abundance of oviducal Staphylococcus was positively associated with the darker brownness of eggshells (Wen et al., 2021). Notably, Staphylococcus has been demonstrated to biosynthesize uroporphyrin and coproporphyrin (De la Fuente et al., 1986), which were part of pigments affecting the eggshell color. Overall, the eggshell not only is a barrier reducing the contamination chances of eggs but also provides potential crosstalk between microbes and host to modulate egg quality and safety.
Notably, microbes in the internal contents of eggs can be passed down from the maternal oviduct during egg development (Trevelline et al., 2018). Proteobacteria were the most abundant phylum in the internal contents of eggs (Vieira et al., 2019), which coincided with the highest abundance of Proteobacteria in the oviduct of laying hens (Wen et al., 2021). When laying hens are infected with pathogenic bacteria like Salmonella, intestinal Salmonella will spread to the oviduct through the cloaca due to the unique anatomical structure of poultry (Gantois et al., 2009). Furthermore, Salmonella alters the expression of TLRs, NLRs, AvβDs, and cytokine family genes in the oviduct, resulting in the decline of egg quality (Zhang et al., 2019). Finally, the presence of foodborne pathogens in eggs poses serious threats to food safety (Gantois et al., 2009; Salihu et al., 2015). Additionally, early microbial colonization of chick embryos by vertical transmission is an essential driver of intestinal innate immune programming in heredity. Thus, future studies are required to understand the mechanism of vertical microbiota transmission to improve egg quality and safety by shaping the oviducal microbiota.
Effects of Microbial Metabolites on Egg Quality
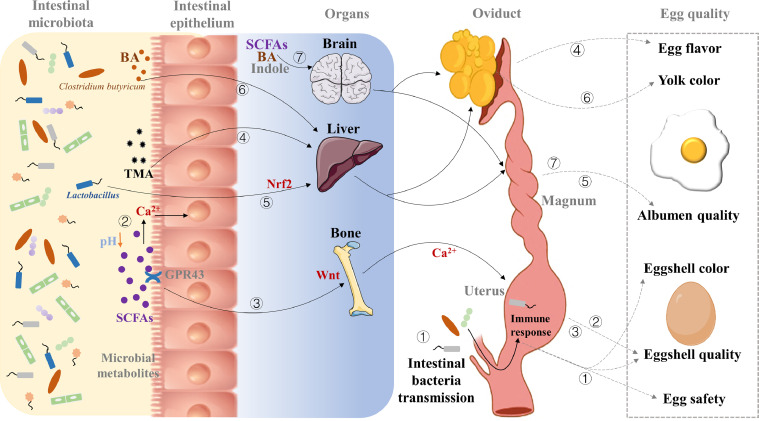
Microbiota-derived metabolites produced mainly through saccharolytic fermentation of carbohydrates, notably short-chain fatty acids (SCFAs) such as acetate, propionate, butyrate, and lactate have been implicated in host-microbiota crosstalk (Morrison and Preston, 2016). SCFAs can improve intestinal nutrient utilization by stimulating intestinal epithelial cell proliferation and differentiation, and further increasing the intestinal villus height and absorptive surface area (De Vadder et al., 2014). Calcium is the most abundant mineral element in eggshells, accounting for 37% of the dry weight of eggshells. The declined utilization of intestinal calcium was recognized as the dominant reason for the poor eggshell quality in the late phase production of laying hens (Al-Batshan et al., 1994). The improvement in intestinal structure induced by SCFAs is favorable to calcium absorption, increasing calcium deposition into the eggshell and improving eggshell thickness (Feng et al., 2021). However, there is almost void of calcium in the intestinal contents about 4 to 5 h after feeding. To avoid dietary calcium deficiency during intense eggshell calcification, the development of medullary bone occurs at the onset of sexual maturity. Large amounts of calcium are stored in the medullary bone and are released into the blood for eggshell formation during the night hours of laying hens’ photoperiod (Bar et al., 1998). Nevertheless, long-term and intense laying behavior will cause osteoporosis simultaneously with poor eggshell quality in the late phase of laying hens (Hanna, 2019). SCFAs have been shown to play an important role in the prevention and treatment of bone metabolism-related diseases (Zaiss et al., 2019). SCFAs directly induce metabolic reprogramming of osteoclast precursor cells, resulting in enhanced glycolysis and decreased oxidative phosphorylation, thereby inhibiting osteoclast differentiation (Montalvany-Antonucci et al., 2019). On the other hand, SCFAs like butyric acid can bind to GPR43 to promote the differentiation of auxiliary CD4+ cells to Treg, and activate Wnt signaling in the bone marrow stromal cells to proliferate and differentiate into osteoblasts (Zaiss et al., 2019). Therefore, the regulation of SCFAs on bone resorption and bone formation is of great significance for improving the homeostasis of bone metabolism, eggshell quality, and prolonging the laying phase of aged laying hens. Another potential mechanism involves SCFAs lowering the pH of the host intestinal lumen and increasing calcium solubility, thus further increasing dietary calcium utilization in laying hens (Gultemirian et al., 2014). Overall, SCFAs as microbial metabolites affect calcium utilization and deposition by regulating the homeostasis of systemic calcium metabolism, thereby improving eggshell quality (Figure 2).
Figure 2.
Effects of the intestinal microbiota of layers on egg quality and safety. Suggested mechanisms of microbial action are as follows: ① Intestinal bacteria can spread to the oviduct through the cloaca leading to egg contaminations, and accelerating the formation of egg translucency through bacterial penetration. In addition, oviducal bacteria biosynthesize pigments affecting the eggshell color. Moreover, oviducal pathogenic bacteria activate the TLR4/NF-κB signaling pathway inducing the synthesis of IL-1β and IL-6, which affects the eggshell ultrastructure by inhibiting the protein expression of a calcium-binding protein and Ca2+ transport. ② Short-chain fatty acids (SCFAs) produced by intestinal microbiota reduce the luminal pH and enhances calcium solubility, utilization, and deposition to improve eggshell thickness. ③ SCFAs bind to G protein-coupled receptor 43 (GPR43) to activate Wnt signaling in the bone marrow stromal cells to improve the Ca2+ homeostasis of bone metabolism and eggshell quality. ④ Trimethylamine (TMA) produced by intestinal microbiota is deposited in egg yolks, resulting in an unpleasant fishy odor. ⑤ Intestinal probiotics can activate the Nrf2 signaling pathway in the liver to regulate the antioxidant status and metabolic functions, which may improve albumen quality. ⑥ Bile acids (BA) produced by intestinal microbiota modulate lipid metabolism and intestinal absorption to improve the egg yolk color. ⑦ SCFAs, BA, and indole derivatives can directly interact with intrinsic enteric neurons and gut-innervating vagal and spinal afferents to regulate the secretion of estradiol, which regulates the secretion of proteins in the oviducal magnum and finally completes the regulation of albumen quality.
The presence of a fishy odor in eggs seriously affects the egg quality and flavor, which is associated with abnormally elevated trimethylamine (TMA) levels. Since excessive TMA cannot be metabolized, it is gradually deposited in egg yolks, resulting in an unpleasant fishy odor (Honkatukia et al., 2005). In addition to chicken monooxygenase 3 enzyme activity, the abnormally increased TMA level is also mainly derived from the degradation of choline and other dietary TMA precursors by intestinal microbiota (Honkatukia et al., 2005; Rath et al., 2017), and the reduction of TMA oxide through bacterial reductase (Barrett and Kwan, 1985). Studies found that the TMA level in egg yolks was positively correlated with the TMA level in the cecum (Wang et al., 2016), and the fishy odor in eggs disappeared after the cecum of laying hens was removed (Pearson et al., 1983). Further studies found that Firmicutes and Proteobacteria were positively correlated with the TMA level in the cecum, whereas Bacteroidetes were negatively correlated with the TMA levels (Long et al., 2017). Analyses of bacterial genomes revealed that the gene cluster of choline utilization is widely distributed across Firmicutes and Proteobacteria, but it is absent in Bacteroidetes (Martínez-del Campo et al., 2015). Firmicutes and Proteobacteria are commonly considered TMA-producing bacteria (Long et al., 2017). Therefore, the intestine microbiota-mediated TMA formation suggests its essential role and potential targets to improve egg flavor (Figure 2). This hypothesis is also supported by the decreased fishy eggs by antibiotics supplementation to disturb intestinal microbiota (Zentek, 2003).
Moreover, microbial metabolites can modulate lipid metabolism to affect egg quality. The disturbance of lipid metabolism is common in the late phase production of laying hens (Wang et al., 2020b). The liver is the main organ of lipid metabolism in poultry, and induced fat accumulation and hepatic dysfunction can affect not only the yolk color but also the cholesterol deposition in egg yolks (Qiu et al., 2021). Due to extremely low contents of dietary cholesterol, almost all of the cholesterol is synthesized endogenously in the liver and enriched into egg yolks via the blood. Furthermore, enzymatic oxidation of cholesterol in the liver generates numerous distinct bile acids (BA), which are metabolized in the intestine by the gut microbiota (Wahlström et al., 2016). In addition to its function as a detergent facilitating digestion and absorption of dietary lipids, BA can also act as an effective ligand activating farnesoid X receptor (FXR) to modulate lipid metabolism (de Aguiar Vallim et al., 2013). Recent studies found that supplemental Clostridium butyricum modulated lipid metabolism of aged laying hens via shaping BA profiles and enhancing intestinal absorption to improve the egg yolk color (Wang et al., 2020a,b) (Figure 2). The alteration of BA induced by the intestinal microbes is dependent on the secretion of bile salt hydrolase (BSH) to generate unconjugated free BA through deconjugation (Wahlström et al., 2016). Similarly, the BSH produced by Lactobacillus can regulate bile acid enterohepatic circulation to improve cholesterol metabolism, which subsequently contributes to reducing the cholesterol levels in serum and egg yolks (Choe et al., 2012; Hou et al., 2020).
Effects of Microbial-Mediated Immune Responses in the Oviducal Mucosa on Eggshell Quality
It is well documented that microbes can be sensed by the pattern recognition receptors of immune cells such as macrophages and dendritic cells to establish interactions with the host (Agostinis et al., 2019). This process is involved in regulating innate and adaptive immune responses in the host endometrium. Notably, the maintenance of host mucosal homeostasis requires a dynamic balance between anti-inflammatory and proinflammatory cytokines generated in the microenvironment (Maillard and Snapper, 2010). The expression of IL-1β and IL-6 proinflammatory cytokines and CX3CL1 chemokine in the oviducal uterine mucosa of laying hens is increased during eggshell mineralization. Furthermore, the expression of TGF-β2 anti-inflammatory cytokine is also significantly increased during the initial stage of eggshell mineralization (Elhamouly et al., 2018). However, when attacked by pathogenic bacteria or certain gram-negative bacteria, the innate immune system of oviducal tissue recognizes microbial-associated molecular patterns including molecules from the microbial cell wall (peptidoglycan) and cell membrane (lipopolysaccharide) through Toll-like receptors. This induces the synthesis of proinflammatory cytokines IL-1β and IL-6 by activating transcription factors NF-κB and AP-1 (Yoshimura, 2015), resulting in an imbalance between anti-inflammatory and proinflammatory factors in uterine mucosal tissues. Further studies revealed that the proinflammatory cytokines IL-1β and IL-6 affected the eggshell ultrastructure by inhibiting the protein expression of a calcium-binding protein (CABP-D28K) and Ca2+ transport in the oviduct uterine mucosa of laying hens (Nii et al., 2017). This is probably due to the critical roles of IL-1β and IL-6 in promoting hematopoiesis and protein degradation (Narsale and Carson, 2014), interfering with the effect of matrix proteins and the efficiency of inorganic ion supply during eggshell mineralization. Furthermore, the dysregulation of organic matrix protein synthesis and the compromised immune function in the oviducal uterus of aged laying hens result in the variation of eggshell ultrastructure and mechanical properties. The latter may be the main reason for the decline in eggshell quality in the late phase production of laying hens (Feng et al., 2020). Recently, Feng et al. (2021) found that dietary oregano essential oil improved epithelial barrier functions and mucosal immune status by altering microbial composition and decreasing Shigella abundance, thus favoring eggshell quality of late-phase laying hens. The balance of intestinal microbiota can effectively reduce the transfer of pathogenic bacteria to the oviduct causing inflammation and immune responses. Thus, we hypothesize that microbial-mediated immune responses in the oviducal mucosa have a potential impact on the eggshell quality, contributing to the nutritional improvement of eggshell quality via oviducal microbiota of laying hens.
Effects of the Microbiota-Gut-Liver/Brain-Reproductive Tract Axis on Egg Quality
After intensive metabolism during peak production, attenuation of the antioxidant function in aged laying hens results in excessive reactive oxygen species disrupting the balance of the host redox system (Liu et al., 2018). Numerous age-related diseases are associated with elevated levels of oxidatively modified proteins (Stadtman, 2001). Thus, impaired liver metabolism in aged laying hens may lead to poor albumen quality, including the decrease in albumen height and Haugh unit (Wang et al., 2018). It was found that dietary probiotics or tea polyphenols improved albumen quality by altering intestinal microbiota composition and its metabolites to regulate the antioxidant status and metabolic functions of the liver (Wang et al., 2018; Zhan et al., 2019; Wang et al., 2020b). In fact, the intestine and liver are anatomically and physiologically connected, which has been called the gut-liver axis (Ohtani and Kawada, 2019). As an important gut-liver axis mediated factor, the microbiota affects the intestine and distant organs via systemic circulation. For example, the intestinal microbiota induced upregulation of the Nrf2 antioxidant and xenobiotic response of the liver in a germ-free mouse model. Moreover, oral delivery of Lactobacillus protected against oxidative liver injury by producing 5-methoxyindoleacetic acid to potently activate Nrf2 in the liver (Saeedi et al., 2020). Indeed, bacterial tryptophan catabolites like indole can be sensed by enteroendocrine cells to activate enteric and vagal neuronal pathways, achieving the remote regulation of the liver (Ye et al., 2021). Likewise, BA are important microbial metabolites involving the regulation of lipid metabolism and inflammatory response, and key mediators in the gut-liver crosstalk. BA promoted fatty acid oxidation in the liver by activating intestinal and hepatic FXR and inducing the expression of the peroxisomal proliferator-activated receptor (Schneider et al., 2018). It further inhibits the expression of key genes for lipid synthesis, thereby reducing plasma triglyceride and cholesterol levels (Xi and Li, 2020), suggesting the potential of bile acids as important targets for regulating egg yolk lipids.
In addition, it is well-known that the development of ovarian follicles and production performance in laying hens are mainly regulated by the hypothalamus (gonadotropic releasing hormone)-pituitary (luteinizing and follicle-stimulating hormone)-gonad (estrogen) axis. Significant differences in the intestinal microbiota of laying hens with different production performance have been found in the previous study (Elokil et al., 2020). Interestingly, fecal microbiota transplantation from high-yield hens improved production performance in low-yield hens (Wang et al., 2020c). Notably, the abundance of some intestinal microbes was positively correlated with serum follicle-stimulating hormone, luteinizing hormone, and estradiol levels (Liu et al., 2020a), indicating the microbiota-brain crosstalk is involved in regulating the production performance in laying hens. Generally, intestinal microbes and their metabolites, like SCFAs, BA, and indole derivatives, can signal via enteroendocrine cells and enterochromaffin cells to regulate the secretion of neuropeptides (Agirman and Hsiao, 2021). Moreover, they can directly interact with intrinsic enteric neurons and gut-innervating vagal and spinal afferents to regulate neurotransmitters such as aminobutyric acid and 5-hydroxytryptamine (Cryan et al., 2020). Furthermore, the hypothalamus can modulate oviducal health in laying hens through the brain-reproductive tract axis, in particular, regulating the secretion of proteins in the oviducal magnum by changing the secretion of estradiol, and finally completing the regulation of albumen quality (Gaub et al., 1990). Overall, the microbiota-gut-liver/brain axis may be proposed as a new and systematic strategy to improve egg quality and safety (Figure 2), but further research to understand the mechanisms underlying is still needed.
CONCLUSIONS AND FUTURE RESEARCH
The analysis of the colonization and succession patterns of intestinal microbiota is critical for our understanding of intestinal microbiota ecology and targeted microbiota interventions. The available literature suggests that microbial succession in different physiological stages of layers seems to correspond to changes in organ development and metabolic functions, including driving immune system development, promoting nutrient utilization and bone development, and maintaining production performance. In addition to direct effects on the egg quality and safety via vertical transmission route in the intestine-oviduct-egg, intestinal microbiota and its metabolites such as SCFAs, BA, and tryptophan derivatives are indirectly involved in regulating egg quality through the microbiota-gut-liver/brain-reproductive tract axis. Nevertheless, the connection between intestinal microbiota and egg quality remain rather tentative, due to numerous conclusions being summarized using correlation analysis in previous studies. Therefore, a comprehensive understanding of interactions between intestinal microbiota and egg quality is needed. We suggest that future research should focus on the critical periods of albumen secretion and eggshell mineralization to explore relevant molecules and signaling pathways based on the microbiota-gut-liver/brain-reproductive tract axis. It will contribute to extending our understanding of intestinal microbiota and host crosstalk to improve egg quality and safety in the late phase production of laying hens.
Acknowledgments
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China (31872396), China Agriculture Research System (CARS-40-K12), and the Agricultural Science and Technology Innovation Program (ASTIP) of the Chinese Academy of Agricultural Sciences.
Author Contributions: Dong Dai: Literature collection, Writing-original draft preparation. Guang-hai Qi and Jing Wang: Writing-Reviewing and editing, supervision, funding acquisition. Hai-jun Zhang, Kai Qiu and Shu-geng Wu: Reviewing and editing.
DISCLOSURES
The authors declare that there is no conflict of interest.
REFERENCES
- Adil S., Magray S. Impact and manipulation of gut microflora in poultry: a review. J. Anim. Vet. Adv. 2012;11:873–877. [Google Scholar]
- Agirman G., Hsiao E.Y. Snapshot: the microbiota-gut-brain axis. Cell. 2021;184 doi: 10.1016/j.cell.2021.03.022. 2524-2524. [DOI] [PubMed] [Google Scholar]
- Agostinis C., Mangogna A., Bossi F., Ricci G., Kishore U., Bulla R. Uterine immunity and microbiota: a shifting paradigm. Front. Immunol. 2019;10:2387. doi: 10.3389/fimmu.2019.02387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agus A., Clément K., Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70:1174–1182. doi: 10.1136/gutjnl-2020-323071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi F.T., Ding J., Zhou H., Xu K., He C., Han C., Zheng Y., Luo H., Yang K., Gu C., Huang Q., Meng H. Dynamic distribution of gut microbiota during embryonic development in chicken. Poult. Sci. 2020;99:5079–5090. doi: 10.1016/j.psj.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R., Tiwari R., Yatoo M.I., Karthik K., Michalak I., Dhama K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health - a comprehensive review. Vet. Q. 2020;41:1–29. doi: 10.1080/01652176.2020.1857887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Batshan H., Scheideler S., Black B., Garlich J., Anderson K. Duodenal calcium uptake, femur ash, and eggshell quality decline with age and increase following molt. Poult. Sci. 1994;73:1590–1596. doi: 10.3382/ps.0731590. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Mann E., Dzieciol M., Hess C., Schmitz-Esser S., Wagner M., Hess M. Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with campylobacter jejuni infection. Front. Cell Infect. Microbiol. 2016;6:154. doi: 10.3389/fcimb.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J., Koci M.D. Development of the chick microbiome: how early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:2. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar A., Vax E., Striem S. Effects of age at onset of production, light regime and dietary calcium on performance, eggshell traits, duodenal calbindin and cholecalciferol metabolism. Brit. Poult. Sci. 1998;39:282–290. doi: 10.1080/00071669889268. [DOI] [PubMed] [Google Scholar]
- Barrett E., Kwan H. Bacterial reduction of trimethylamine oxide. Annu. Rev. Microbiol. 1985;39:131–149. doi: 10.1146/annurev.mi.39.100185.001023. [DOI] [PubMed] [Google Scholar]
- Bi Y., Tu Y., Zhang N., Wang S., Zhang F., Suen G., Shao D., Li S., Diao Q. Multiomics analysis reveals the presence of a microbiome in the gut of fetal lambs. Gut. 2021;70:853–864. doi: 10.1136/gutjnl-2020-320951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker M.E., Elliott G., Heyer-Gray H., Martin M.O., Arnold A.E., Weiss S.L. Vertically transmitted microbiome protects eggs from fungal infection and egg failure. Anim. Microbiome. 2021;3:43. doi: 10.1186/s42523-021-00104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Sallusto F., Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Chang W.C.W, Wu H.Y., Kan H.L., Lin Y.C., Tsai P.J., Chen Y.C., Pan Y.Y., Liao P.C. Discovery of spoilage markers for chicken eggs using liquid chromatography-high resolution mass spectrometry-based untargeted and targeted foodomics. J. Agr. Food. Chem. 2021;69:4331–4341. doi: 10.1021/acs.jafc.1c01009. [DOI] [PubMed] [Google Scholar]
- Choe D., Loh T., Foo H., Hair-Bejo M., Awis Q. Egg production, faecal ph and microbial population, small intestine morphology, and plasma and yolk cholesterol in laying hens given liquid metabolites produced by lactobacillus plantarum strains. Brit. Poult. Sci. 2012;53:106–115. doi: 10.1080/00071668.2012.659653. [DOI] [PubMed] [Google Scholar]
- Chousalkar K.K., Flynn P., Sutherland M., Roberts J.R., Cheetham B.F. Recovery of salmonella and escherichia coli from commercial egg shells and effect of translucency on bacterial penetration in eggs. Int. J. Food Microbiol. 2010;142:207–213. doi: 10.1016/j.ijfoodmicro.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Collado M.C., Rautava S., Aakko J., Isolauri E., Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., O'riordan K.J., Sandhu K., Peterson V., Dinan T.G. The gut microbiome in neurological disorders. Lancet. Neurol. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- Dai D., Wu S.G., Zhang H.J., Qi G.H., Wang J. Dynamic alterations in early intestinal development, microbiota and metabolome induced by in ovo feeding of L-arginine in a layer chick model. J. Anim. Sci. Biotechnol. 2020;11:19. doi: 10.1186/s40104-020-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D., Zhang H.J., Qiu K., Qi G.H., Wang J., Wu S.G. Supplemental L-arginine improves the embryonic intestine development and microbial succession in a chick embryo model. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.692305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Aguiar Vallim T.Q., Tarling E.J., Edwards P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente R., Schleifer K., Götz F., Köst H-P. Accumulation of porphyrins and pyrrole pigments by Staphylococcus aureus ssp. Anaerobius and its aerobic mutant. FEMS Microbiol. Lett. 1986;35:183–188. [Google Scholar]
- De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Ding J., Dai R., Yang L., He C., Xu K., Liu S., Zhao W., Xiao L., Luo L., Zhang Y., Meng H. Inheritance and establishment of gut microbiota in chickens. Front. Microbiol. 2017;8:1967. doi: 10.3389/fmicb.2017.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekino S., Nawa Y., Tanaka K., Matsuno K., Fujii H., Kotani M. Suppression of immune response by isolation of the bursa of fabricius from environmental stimuli. Aust. J. Exp. Biol. Med. Sci. 1980;58:289–296. doi: 10.1038/icb.1980.28. [DOI] [PubMed] [Google Scholar]
- Elhamouly M., Nii T., Isobe N., Yoshimura Y. Expression of pro- and anti-inflammatory cytokines and chemokines during the ovulatory cycle and effects of aging on their expression in the uterine mucosa of laying hens. Cytokine. 2018;111:303–308. doi: 10.1016/j.cyto.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Elokil A.M.Magdy, Melak S., Ishfaq H., Bhuiyan A., Cui L., Jamil M., Zhao S., Li S. Faecal microbiome sequences in relation to the egg-laying performance of hens using amplicon-based metagenomic association analysis. Animal. 2020;14:706–715. doi: 10.1017/S1751731119002428. [DOI] [PubMed] [Google Scholar]
- FAOSTAT. 2020. FAO Statistics, Food and Agriculture Organization of the United Nations. http://www.fao.org/.
- Feng J., Lu M., Wang J., Zhang H.J., Qiu K., Qi G.H., Wu S.G. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 2021;12:1–15. doi: 10.1186/s40104-021-00600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Zhang H.J., Wu S.G., Qi G.H., Wang J. Uterine transcriptome analysis reveals MRNA expression changes associated with the ultrastructure differences of eggshell in young and aged laying hens. BMC Genomics. 2020;21:1–15. doi: 10.1186/s12864-020-07177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantois I., Ducatelle R., Pasmans F., Haesebrouck F., Gast R., Humphrey T.J., Van Immerseel F. Mechanisms of egg contamination by Salmonella enteritidis. FEMS Microbiol. Rev. 2009;33:718–738. doi: 10.1111/j.1574-6976.2008.00161.x. [DOI] [PubMed] [Google Scholar]
- Gaub M.P., Bellard M., Scheuer I., Chambon P., Sassone-Corsi P. Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell. 1990;63:1267–1276. doi: 10.1016/0092-8674(90)90422-b. [DOI] [PubMed] [Google Scholar]
- Gautron J., Murayama E., Vignal A., Morisson M., Mckee M.D., Réhault S., Labas V., Belghazi M., Vidal M.L., Nys Y., Hincke M.T. Cloning of ovocalyxin-36, a novel chicken eggshell protein related to lipopolysaccharide-binding proteins, bactericidal permeability-increasing proteins, and plunc family proteins. J. Biol. Chem. 2007;282:5273–5286. doi: 10.1074/jbc.M610294200. [DOI] [PubMed] [Google Scholar]
- Gibiino G., Lopetuso L.R., Scaldaferri F., Rizzatti G., Binda C., Gasbarrini A. Exploring bacteroidetes: metabolic key points and immunological tricks of our gut commensals. Dig. Liver. Dis. 2018;50:635–639. doi: 10.1016/j.dld.2018.03.016. [DOI] [PubMed] [Google Scholar]
- Gultemirian M.D.L., Corti H.R., Chaia A.P., Apella M.C. Fermentation in vitro of a mixture of dietary fibers and cane molasses by the cecal microbiota: application on mineral absorption through the laying hen's colonic epithelium. Anim. Feed Sci. Tech. 2014;191:76–82. [Google Scholar]
- Guo J.R., Dong X.F., Liu S., Tong J.M. High-throughput sequencing reveals the effect of Bacillus subtilis cgmcc 1.921 on the cecal microbiota and gene expression in ileum mucosa of laying hens. Poult. Sci. 2018;97:2543–2556. doi: 10.3382/ps/pey112. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Román M.I., Holguín-Meléndez F., Dunn M.F., Guillén-Navarro K., Huerta-Palacios G. Antifungal activity of serratia marcescens cffsur-b2 purified chitinolytic enzymes and prodigiosin against mycosphaerella fijiensis, causal agent of black sigatoka in banana (Musa spp. BioControl. 2015;60:565–572. [Google Scholar]
- Hamburger V., Hamilton H.L. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hamid H., Zhang J.Y., Li W.X., Liu C., Li M.L., Zhao L.H., Ji C., Ma Q.G. Interactions between the cecal microbiota and non-alcoholic steatohepatitis using laying hens as the model. Poult. Sci. 2019;98:2509–2521. doi: 10.3382/ps/pey596. [DOI] [PubMed] [Google Scholar]
- Hanna, D. E. 2019. The effects of butyric acid on performance parameters, egg quality and nutrient utilization in young white leghorn hens.
- Hincke M.T., Nys Y., Gautron J., Mann K., Rodriguez-Navarro A.B., Mckee M.D. The eggshell: structure, composition and mineralization. Front. Biosci. (Landmark Ed). 2012;17:1266–1280. doi: 10.2741/3985. [DOI] [PubMed] [Google Scholar]
- Honkatukia M., Reese K., Preisinger R., Tuiskula-Haavisto M., Weigend S., Roito J., Mäki-Tanila A., Vilkki J. Fishy taint in chicken eggs is associated with a substitution within a conserved motif of the fmo3 gene. Genomics. 2005;86:225–232. doi: 10.1016/j.ygeno.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Hou G., Peng W., Wei L., Li R., Yuan Y., Huang X., Yin Y. Lactobacillus delbrueckii interfere with bile acid enterohepatic circulation to regulate cholesterol metabolism of growing–finishing pigs via its bile salt hydrolase activity. Front. Nutr. 2020;7 doi: 10.3389/fnut.2020.617676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Zhang Y., Xiao K., Jiang F., Wang H., Tang D., Liu D., Liu B., Liu Y., He X., Liu H., Liu X., Qing Z., Liu C., Huang J., Ren Y., Yun L., Yin L., Lin Q., Zeng C., Su X., Yuan J., Lin L., Hu N., Cao H., Huang S., Guo Y., Fan W., Zeng J. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome. 2018;6:211. doi: 10.1186/s40168-018-0590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., Tanoue T., Imaoka A., Itoh K., Takeda K., Umesaki Y., Honda K., Littman D.R. Induction of intestinal th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeni R.E., Dittoe D.K., Olson E.G., Lourenco J., Seidel D.S., Ricke S.C., Callaway T.R. An overview of health challenges in alternative poultry production systems. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joat N., Van T.T.H., Stanley D., Moore R.J., Chousalkar K. Temporal dynamics of gut microbiota in caged laying hens: a field observation from hatching to end of lay. Appl. Microbiol. Biotechnol. 2021;105:4719–4730. doi: 10.1007/s00253-021-11333-8. [DOI] [PubMed] [Google Scholar]
- Jurburg S.D., Brouwer M.S.M., Ceccarelli D., Van Der Goot J., Jansman A.J.M., Bossers A. Patterns of community assembly in the developing chicken microbiome reveal rapid primary succession. Microbiologyopen. 2019;8:e00821. doi: 10.1002/mbo3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kers J.G., Velkers F.C., Fischer E.A., Hermes G.D.A., Stegeman J.A., Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Moore R.J., Stanley D., Chousalkar K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020;86 doi: 10.1128/AEM.00600-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak F., Helmbrecht A. Effect of different levels of tryptophan on productive performance, egg quality, blood biochemistry, and caecal microbiota of hens housed in enriched colony cages under commercial stocking density. Poult. Sci. 2019;98:2094–2104. doi: 10.3382/ps/pey562. [DOI] [PubMed] [Google Scholar]
- Kidd M.T., Anderson K.E. Laying hens in the US market: an appraisal of trends from the beginning of the 20th century to present. J. Appl. Poult. Res. 2019;28:771–784. [Google Scholar]
- Kizerwetter-Świda M., Binek M. Bacterial microflora of the chicken embryos and newly hatched chicken. J. Anim. Feed Sci. 2008;17:224–232. [Google Scholar]
- Kovacs-Nolan J., Cordeiro C., Young D., Mine Y., Hincke M. Ovocalyxin-36 is an effector protein modulating the production of proinflammatory mediators. Vet. Immunol. Immunopathol. 2014;160:1–11. doi: 10.1016/j.vetimm.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Lee S., La T.M., Lee H.J., Choi I.S., Song C.S., Park S.Y., Lee J.B., Lee S.W. Characterization of microbial communities in the chicken oviduct and the origin of chicken embryo gut microbiota. Sci. Rep. 2019;9:6838. doi: 10.1038/s41598-019-43280-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X., Lee K., Kim I. Performance, egg quality, nutrient digestibility, and excreta microbiota shedding in laying hens fed corn-soybean-meal-wheat-based diets supplemented with xylanase. Poult. Sci. 2018;97:2071–2077. doi: 10.3382/ps/pey041. [DOI] [PubMed] [Google Scholar]
- Levy M., Blacher E., Elinav E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017;35:8–15. doi: 10.1016/j.mib.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Li H., Zhang Z., Zhu W., Zhang T., Guo W., Cai Y., Zhu Z., Zhang L. Functional analysis of liver transcriptome before and after white leghorn hen begin laying eggs. Acta Veterinaria et Zootechnica Sinica. 2017;48:1624–1634. [Google Scholar]
- Li X., Wu S., Li X., Yan T., Duan Y., Yang X., Duan Y., Sun Q., Yang X. Simultaneous supplementation of bacillus subtilis and antibiotic growth promoters by stages improved intestinal function of pullets by altering gut microbiota. Front. Microbiol. 2018;9:2328. doi: 10.3389/fmicb.2018.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Tang J., Feng F. Medium-chain α-monoglycerides improves productive performance and egg quality in aged hens associated with gut microbiota modulation. Poult. Sci. 2020;99:7122–7132. doi: 10.1016/j.psj.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.T., Lin X., Mi Y.L., Zeng W.D., Zhang C.Q. Age-related changes of yolk precursor formation in the liver of laying hens. J Zhejiang Univ-Sc B. 2018;19:390–399. doi: 10.1631/jzus.B1700054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.L., Yan T., Li X.Y., Duan Y.L., Yang X., Yang X.J. Effects of bacillus subtilis and antibiotic growth promoters on the growth performance, intestinal function and gut microbiota of pullets from 0 to 6 weeks. Animal. 2020:1619–1628. doi: 10.1017/S1751731120000191. [DOI] [PubMed] [Google Scholar]
- Long C., Wang J., Zhang H., Wu S., Qi G. Effects of dietary rapeseed meal supplementation on cecal microbiota in laying hens with different flavin-containing monooxygenase 3 genotypes. Poult. Sci. 2017;96:1748–1758. doi: 10.3382/ps/pew449. [DOI] [PubMed] [Google Scholar]
- Lu J., Idris U., Harmon B., Hofacre C., Maurer J.J., Lee M.D. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkins B.S., Batal A.B., Lee M. The effect of gender on the bacterial community in the gastrointestinal tract of broilers. Poult. Sci. 2008;87:964–967. doi: 10.3382/ps.2007-00287. [DOI] [PubMed] [Google Scholar]
- Maillard M.H., Snapper S.B. Cytokines and chemokines in mucosal homeostasis. Inflamm. Bowel Dis. 2010:119–156. [Google Scholar]
- Martínez-Del Campo A., Bodea S., Hamer H.A., Marks J.A., Haiser H.J., Turnbaugh P.J., Balskus E.P. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. MBio. 2015;6 doi: 10.1128/mBio.00042-15. e00042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L., Gong Y., Li H., Xie C., Xu Q., Dong X., Elwan H.A.M., Zou X. Alterations in cecal microbiota and intestinal barrier function of laying hens fed on fluoride supplemented diets. Ecotoxicol. Environ. Saf. 2020;193 doi: 10.1016/j.ecoenv.2020.110372. [DOI] [PubMed] [Google Scholar]
- Montalvany-Antonucci C., Duffles L., De Arruda J., Zicker M., De Oliveira S., Macari S., Garlet G., Madeira M., Fukada S., Andrade J.I. Short-chain fatty acids and ffar2 as suppressors of bone resorption. Bone. 2019;125:112–121. doi: 10.1016/j.bone.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsale A.A., Carson J.A. Role of il-6 in cachexia–therapeutic implications. Curr. Opin. Support. Pa. 2014;8:321. doi: 10.1097/SPC.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijat M., Habtewold J., Shirley R.B., Welsher A., Barton J., Thiery P., Kiarie E. Bacillus subtilis strain DSM 29784 modulates the cecal microbiome, concentration of short-chain fatty acids, and apparent retention of dietary components in shaver white chickens during grower, developer, and laying phases. Appl. Environ. Microbiol. 2019;85 doi: 10.1128/AEM.00402-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neira C., Laca A., Laca A., Díaz M. Microbial diversity on commercial eggs as affected by the production system. A first approach using pgm. Int. J. Food Microbiol. 2017;262:3–7. doi: 10.1016/j.ijfoodmicro.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Nii T., Isobe N., Yoshimura Y. Effects of interleukin-1β and-6 on the expression of ion transporters involved in eggshell mineralization in cultured hen uterine mucosal tissue. J. Poult. Sci. 2017;55 doi: 10.2141/jpsa.0170138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Odamaki T., Kato K., Sugahara H., Hashikura N., Takahashi S., Xiao J.Z., Abe F., Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani N., Kawada N. Role of the gut–liver axis in liver inflammation, fibrosis, and cancer: a special focus on the gut microbiota relationship. Hepatol. Commun. 2019;3:456–470. doi: 10.1002/hep4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R.J., Hinsu A.T., Patel N.V., Koringa P.G., Jakhesara S.J., Thakkar J.R., Shah T.M., Limon G., Psifidi A., Guitian J., Hume D.A., Tomley F.M., Rank D.N., Raman M., Tirumurugaan K.G., Blake D.P., Joshi C.G. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome. 2018;6:115. doi: 10.1186/s40168-018-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Perrotta A., Hanning I., Diaz-Sanchez S., Pendleton S., Alm E., Ricke S. Pasture flock chicken cecal microbiome responses to prebiotics and plum fiber feed amendments. Poult. Sci. 2017;96:1820–1830. doi: 10.3382/ps/pew441. [DOI] [PubMed] [Google Scholar]
- Pearson A.W., Greenwood N.M., Butler E.J., Fenwick G.R., Curl C.L. Rapeseed meal and egg taint: effects of B. campestris meals, progoitrin and potassium thiocyanate on trimethylamine oxidation. J. Sci. Food Agr. 1983;34:965–972. [Google Scholar]
- Peng L., Li Z.R., Green R.S., Holzman I.R., Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of amp-activated protein kinase in caco-2 cell monolayers. J. Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky O., Sekelova Z., Faldynova M., Sebkova A., Sisak F., Rychlik I. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl. Environ. Microbiol. 2015;82:1569–1576. doi: 10.1128/AEM.03473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu K., Zhao Q., Wang J., Qi G.H., Wu S.G., Zhang H.J. Effects of pyrroloquinoline quinone on lipid metabolism and anti-oxidative capacity in a high-fat-diet metabolic dysfunction-associated fatty liver disease chick model. Int. J. Mol. Sci. 2021;22:1458. doi: 10.3390/ijms22031458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath S., Heidrich B., Pieper D.H., Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5:54. doi: 10.1186/s40168-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réhault-Godbert, S. 2021. The eggshell microbiome. Innate immunity in a biomineralized context: trade-offs or synergies?.
- Richards P., Fothergill J., Bernardeau M., Wigley P. Development of the caecal microbiota in three broiler breeds. Front. Vet. Sci. 2019;6:201. doi: 10.3389/fvets.2019.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke S.C., Dittoe D.K., Olson E.G. Microbiome applications for laying hen performance and egg production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano-Keeler J., Moore D.J., Wang C., Brucker R.M., Fonnesbeck C., Slaughter J.C., Li H., Curran D.P., Meng S., Correa H., Lovvorn H.N., Tang Y.W., Bordenstein S., George A.L., Weitkamp J.H. Early life establishment of site-specific microbial communities in the gut. Gut Microbes. 2014;5:192–201. doi: 10.4161/gmic.28442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi B.J., Liu K.H., Owens J.A., Hunter-Chang S., Camacho M.C., Eboka R.U., Chandrasekharan B., Baker N.F., Darby T.M., Robinson B.S. Gut-resident lactobacilli activate hepatic nrf2 and protect against oxidative liver injury. Cell Metab. 2020;31:956–968.e5. doi: 10.1016/j.cmet.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihu M., Garba B., Isah Y. Evaluation of microbial contents of table eggs at retail outlets in Sokoto metropolis, Nigeria. Sokoto. J. Vet. Sci. 2015;13:22–28. [Google Scholar]
- Schneider K.M., Albers S., Trautwein C. Role of bile acids in the gut-liver axis. J. Hepatol. 2018;68:1083–1085. doi: 10.1016/j.jhep.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Seferovic M.D., Pace R.M., Carroll M., Belfort B., Major A.M., Chu D.M., Racusin D.A., Castro E.C.C., Muldrew K.L., Versalovic J., Aagaard K.M. Visualization of microbes by 16s in situ hybridization in term and preterm placentas without intraamniotic infection. Am. J. Obstet. Gynecol. 2019;221 doi: 10.1016/j.ajog.2019.04.036. 146.e1-e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang H., Zhao J., Dong X., Guo Y., Zhang H., Cheng J., Zhou H. Inulin improves the egg production performance and affects the cecum microbiota of laying hens. Int. J. Biol. Macromol. 2020;155:1599–1609. doi: 10.1016/j.ijbiomac.2019.11.137. [DOI] [PubMed] [Google Scholar]
- Shang Y., Kumar S., Oakley B., Kim W.K. Chicken gut microbiota: importance and detection technology. Front. Vet. Sci. 2018;5:254. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wu S., Li W., Liu D., Ma G., Zhang Z., Yuan L., Li H., Zhang S., Mushtaq N. Microbiota analysis of eggshells in different areas and during different storage time by non-cultural methods. Curr. Microbiol. 2020;77:3842–3850. doi: 10.1007/s00284-020-02212-y. [DOI] [PubMed] [Google Scholar]
- Shi Z., Rothrock M.J., Ricke S.C. Applications of microbiome analyses in alternative poultry broiler production systems. Front. Vet. Sci. 2019;6:157. doi: 10.3389/fvets.2019.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shterzer N., Rothschild N., Sbehat Y., Stern E., Nazarov A., Mills E. Large overlap between the intestinal and reproductive tract microbiomes of chickens. Front. Microbiol. 2020;11:1508. doi: 10.3389/fmicb.2020.01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.M., Shah T., Deshpande S., Jakhesara S.J., Koringa P.G., Rank D.N., Joshi C.G. High through put 16s rrna gene-based pyrosequencing analysis of the fecal microbiota of high fcr and low fcr broiler growers. Mol. Biol. Rep. 2012;39:10595–10602. doi: 10.1007/s11033-012-1947-7. [DOI] [PubMed] [Google Scholar]
- Sommer F., Bäckhed F. The gut microbiota–masters of host development and physiology. Nat. Rev. Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Sparks NH. Eggshell pigments–from formation to deposition. Avian Biol. Res. 2011;4:162–167. [Google Scholar]
- Sperling J.L., Silva-Brandão K.L., Brandão M.M., Lloyd V.K., Dang S., Davis C.S., Sperling F.H., Magor K.E. Comparison of bacterial 16s rRNA variable regions for microbiome surveys of ticks. Ticks Tick Borne Dis. 2017;8:453–461. doi: 10.1016/j.ttbdis.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Stadtman E.R. Protein oxidation in aging and age-related diseases. Ann. Ny. Acad. Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Sasaki O., Nirasawa K., Furukawa T. Association between ovocalyxin-32 gene haplotypes and eggshell quality traits in an f2 intercross between two chicken lines divergently selected for eggshell strength. Anim. Genet. 2010;41:541–544. doi: 10.1111/j.1365-2052.2010.02034.x. [DOI] [PubMed] [Google Scholar]
- Trevelline B.K., Macleod K.J., Knutie S.A., Langkilde T., Kohl K.D. In ovo microbial communities: a potential mechanism for the initial acquisition of gut microbiota among oviparous birds and lizards. Biol. Lett. 2018;14 doi: 10.1098/rsbl.2018.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau S., Thibodeau A., Côté J.C., Gaucher M.L., Fravalo P. Contribution of the broiler breeders' fecal microbiota to the establishment of the eggshell microbiota. Front. Microbiol. 2020;11:666. doi: 10.3389/fmicb.2020.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni Z., Ferket R. Methods for early nutrition and their potential. World Poult. Sci. J. 2004;60:101–111. [Google Scholar]
- Van Der Wielen P.W., Keuzenkamp D.A., Lipman L.J., Van Knapen F., Biesterveld S. Spatial and temporal variation of the intestinal bacterial community in commercially raised broiler chickens during growth. Microb. Ecol. 2002;44:286–293. doi: 10.1007/s00248-002-2015-y. [DOI] [PubMed] [Google Scholar]
- Van Veelen H.P.J., Salles J.F., Tieleman B.I. Microbiome assembly of avian eggshells and their potential as transgenerational carriers of maternal microbiota. ISME J. 2018;12:1375–1388. doi: 10.1038/s41396-018-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnska P., Sedlar K., Lukac M., Faldynova M., Gerzova L., Cejkova D., Sisak F., Rychlik I. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira D.A., Cabral L., Noronha M.F., Junior G.V., Sant'ana A.S. Microbiota of eggs revealed by 16s rRNA-based sequencing: from raw materials produced by different suppliers to chilled pasteurized liquid products. Food Control. 2019;96:194–204. [Google Scholar]
- Wahlström A., Sayin S.I., Marschall H.U., Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Wang J., Long C., Zhang H., Zhang Y., Wang H., Yue H., Wang X., Wu S., Qi G. Genetic variant in flavin-containing monooxygenase 3 alters lipid metabolism in laying hens in a diet-specific manner. Int. J. Biol. Sci. 2016;12:1382. doi: 10.7150/ijbs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.W., Wang J., Zhang H.J., Wu S.G., Qi G.H. Effects of Clostridium butyricum on production performance and intestinal absorption function of laying hens in the late phase of production. Anim. Feed Sci. Tech. 2020;264 [Google Scholar]
- Wang W.W., Wang J., Zhang H.J., Wu S.G., Qi G.H. Supplemental Clostridium butyricum modulates lipid metabolism through shaping gut microbiota and bile acid profile of aged laying hens. Front. Microbiol. 2020;11:600. doi: 10.3389/fmicb.2020.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.C., Wang X.H., Wang J., Wang H., Zhang H.J., Wu S.G., Qi G.H. Dietary tea polyphenol supplementation improved egg production performance, albumen quality, and magnum morphology of hy-line brown hens during the late laying period. J. Anim. Sci. 2018;96:225–235. doi: 10.1093/jas/skx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu L., Sun X., Wan X., Sun G., Jiang R., Li W., Tian Y., Liu X., Kang X. Characteristics of the fecal microbiota of high- and low-yield hens and effects of fecal microbiota transplantation on egg production performance. Res. Vet. Sci. 2020;129:164–173. doi: 10.1016/j.rvsc.2020.01.020. [DOI] [PubMed] [Google Scholar]
- Wen C., Li Q., Lan F., Li X., Li G., Yan Y., Wu G., Yang N., Sun C. Microbiota continuum along the chicken oviduct and its association with host genetics and egg formation. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y., Li H. Role of farnesoid x receptor in hepatic steatosis in nonalcoholic fatty liver disease. Biomed. Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109609. [DOI] [PubMed] [Google Scholar]
- Xiao S.S., Mi J.D., Mei L., Liang J., Feng K.X., Wu Y.B., Liao X.D., Wang Y. Microbial diversity and community variation in the intestines of layer chickens. Animals (Basel). 2021;11 doi: 10.3390/ani11030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Bae M., Cassilly C.D., Jabba S.V., Thorpe D.W., Martin A.M., Lu H.Y., Wang J., Thompson J.D., Lickwar C.R. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2021;29:179–196. doi: 10.1016/j.chom.2020.11.011. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z., Lian L., Zhu F., Zhang Z.H., Hincke M., Yang N., Hou Z.C. The transcriptome landscapes of ovary and three oviduct segments during chicken (Gallus gallus) egg formation. Genomics. 2020;112:243–251. doi: 10.1016/j.ygeno.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y. Avian β-defensins expression for the innate immune system in hen reproductive organs. Poult. Sci. 2015;94:804–809. doi: 10.3382/ps/peu021. [DOI] [PubMed] [Google Scholar]
- Yu L.L., Gao T., Zhao M.M., Lv P.A., Zhang L., Li J.L., Jiang Y., Gao F., Zhou G.H. In ovo feeding of l-arginine alters energy metabolism in post-hatch broilers. Poult. Sci. 2018;97:140–148. doi: 10.3382/ps/pex272. [DOI] [PubMed] [Google Scholar]
- Zaiss M.M., Jones R.M., Schett G., Pacifici R. The gut-bone axis: how bacterial metabolites bridge the distance. J. Clin. Invest. 2019;129:3018–3028. doi: 10.1172/JCI128521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentek J. Egg taint–a problem of practical importance. Lohmann Inf. 2003;28:1–4. [Google Scholar]
- Zhan H., Dong X., Li L., Zheng Y., Gong Y., Zou X. Effects of dietary supplementation with clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult. Sci. 2019;98:896–903. doi: 10.3382/ps/pey436. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen Y., Gu T., Xu Q., Zhu G., Chen G. Effects of Salmonella enterica serovar enteritidis infection on egg production and the immune response of the laying duck Anas platyrhynchos. PeerJ. 2019;7:e6359. doi: 10.7717/peerj.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Wang G., Siegel P., He C., Wang H., Zhao W., Zhai Z., Tian F., Zhao J., Zhang H., Sun Z., Chen W., Zhang Y., Meng H. Quantitative genetic background of the host influences gut microbiomes in chickens. Sci. Rep. 2013;3:1163. doi: 10.1038/srep01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.Y., Zhong T., Pandya Y., Joerger R.D. 16s rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl. Environ. Microbiol. 2002;68:124–137. doi: 10.1128/AEM.68.1.124-137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]