Abstract
To seek viable alternatives to antibiotics, we determined the combinatorial effects of Lactobacillus and a quorum quenching enzyme (QQE) on broiler growth performance, antioxidant capacity, immune responses, and cecal microbial populations. In total, 360 one-day-old male broilers (Ross 308) were randomly allotted to 3 dietary treatments, with 12 replicate pens/treatment and 10 birds/replicate pen. Dietary treatments lasted 42 d and comprised: corn-soybean meal basal diet (control group, CON); control plus antibiotic growth promoter supplement group (AGP); and control plus Lactobacillus and QQE supplement group (LQ). Dietary LQ supplementation significantly increased final body weight (BW) and average daily gain (ADG) when compared with CON and AGP groups between 22 and 42 d and 1 to 42 d (P < 0.05). No significant differences were observed for serum superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) levels between treatments (P > 0.05). A higher concentration of total antioxidant capacity (T-AOC) was observed on d 42 in the LQ group (P = 0.06). Feeding LQ significantly increased serum immunoglobulins (IgA and IgG) levels when compared with other treatments (P < 0.05). A statistical trend was also observed for increased cecal butyrate levels (P = 0.06) in the LQ group. Bacterial α-diversity was unaffected by dietary treatments (P > 0.05). However, from principal component analysis (PCoA), the microbial community structure was different between the LQ and AGP groups. Diet supplemented with LQ significantly (P < 0.05) decreased the relative abundance of Synergistota and Proteobacteria and significantly (P < 0.05) increased the proportion of Ruminococcaceae and Faecalibacterium. Thus, supplemental LQ improved growth performance, immune status, and modulated intestinal microbial communities in broilers. We provide a new perceptive on antibiotic substitutes in the poultry industry.
Key words: broiler, Lactobacillus, quorum quenching enzyme, growth performance, microbiota
INTRODUCTION
For many years, antibiotics as feed additives have been used to promote the growth of broiler chickens (Engberg et al., 2000). However, increased antibiotic use has led to the emergence of antibiotic-resistance and antibiotic residues in animal products (Lhermie et al., 2016). Restrictions or total bans on antibiotic additives in feed have gradually been implemented in many countries (Lee et al., 2011; Yadav and Jha, 2019). As a consequence, effective additives need to be generated as alternatives to antibiotics, including feed enzymes, probiotics, prebiotics, and organic acids (Gadde et al., 2017).
Lactobacillus as live microorganism, which was administered in diet of animals, has been demonstrated to improve growth performance (Li et al., 2018b; Wang et al., 2021a). Various studies have indicated that Lactobacillus could benefit nutrient absorption (Wang et al., 2020), antioxidative capacity (Wu et al., 2019a), anti-inflammatory (Wang et al., 2015), intestinal health, and growth performance of broilers (He et al., 2019; Wu et al., 2019b). Wu et al. (2021) reported that dietary Lactobacillus acidophilus (10 × 108 CFU/kg) supplementation during d 1 to 21 consistently elevated body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and jejunum and ileum villus height to crypt depth ratios at 21 d in the presence/absence of an Escherichia coli challenge. Lokapirnasari et al. (2019) determined that the addition of 0.25% Lactobacillus casei and 0.5% Bifidobacterium spp. improved growth performance and egg production in laying hens. A few studies found that growth promoting effect was not significant (Fatufe and Matanmi, 2008), probably due to the kinds of bacterial species/strains, the supplementation methods, processing technologies, different supplementation levels, and environmental systems.
Acyl homoserine lactones (AHLs) are important signal molecules in quorum sensing systems of most gram-negative bacteria (Papenfort and Bassler, 2016). Many pathogenic behaviors of gram-negative bacteria, such as host adhesion, sporulation, exoenzyme production, toxin secretion, biofilm formation, siderophores, and pigment production, are regulated by AHL-mediated Quorum Sensing (QS) (Perez and Hagen, 2010; Rutherford and Bassler, 2012). Quorum quenching enzymes (QQE), which degrade the AHLs, has been successfully used as a novel ecofriendly method to control important gram-negative pathogens in aquaculture (Chu et al., 2011; Torres et al., 2016; Chen et al., 2020; Sikdar and Elias, 2020). Dietary supplementation with quorum quenching Bacillus strains for 35 d, the growth parameters, digestive enzymes activity and survival rate were improved with Asian seabass under normal feeding conditions (Ghanei-Motlagh et al., 2021).
The aim of this study was to evaluate the combinatorial effects of Lactobacillus and QQE on broiler growth performance, antioxidant capacity, immune parameters, and gut microbial populations. We provided the research work on the combinatorial effects of probiotics and enzymes on broilers and explored more ecofriendly antibiotic alternatives.
MATERIALS AND METHODS
Ethics Approval
Experimental procedures were approved by the Animal Welfare Committee of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (Beijing, China). Studies were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Chinese Academy of Agriculture Sciences.
Animals, Experimental Design, and Diets
A total of 360 one-day-old Ross 308 broilers, obtained from a commercial hatchery (Shandong Minhe Animal Husbandry Co., Ltd. Shandong, China), were randomly allotted by weight to 1 of 3 treatment in a completely randomized design. Each treatment consisted of 12 replicate pens with 10 chicks each. Three dietary treatments included 1) basal diet (CON group); 2) basal diet + 45 mg/kg chlortetracycline (15%) + 10 mg/kg kitasamycin (45%) (AGP group); and 3) basal diet + 50 mg/kg Lactobacillus (1 × 1012 CFU/g) + 500 mg/kg QQE (10,000 IU/g) (LQ group). Lactobacillus and QQE products were obtained from Tianjin Biofeed Technology Co., Ltd. (Tianjin, China). The chlortetracycline and kitasamycin were used in broiler diets as AGPs to enhance growth and improve feed efficiency before AGPs were banned, and were usually used as a positive control to evaluate alternatives to AGP (Dong et al., 2011; Han et al., 2020; Pirzado et al., 2021; Gao et al., 2022; Song et al., 2022a).
The corn-soybean basal diets were formulated to meet broiler nutrient requirements as recommended by the National Research Council (1994) and without any AGP and enzymes (Table 1). All chicks were raised on wire-floored cages in the present study. Feed and water were provided ad libitum. Chicks were managed according the guidelines suggested by Ross Broiler Management (Aviagen, 2018). The study lasted 42 d; the starter phase was 1 to 21 d and the grower phase was 22 to 42 d.
Table 1.
Composition and nutrient levels of the basal diet (air-dry base).
| Items | Content |
|
|---|---|---|
| 1 to 21 d of age | 22 to 42 d of age | |
| Ingredients (%) | ||
| Corn | 57.20 | 61.32 |
| Soybean meal | 32.00 | 25.00 |
| Corn gluten meal | 3.50 | 5.00 |
| Soybean oil | 2.60 | 4.00 |
| Limestone | 1.20 | 1.30 |
| CaHPO4 | 1.60 | 1.30 |
| NaCl | 0.25 | 0.20 |
| NaHCO3 | 0.15 | 0.20 |
| Lys 70% | 0.62 | 0.74 |
| Met 98% | 0.14 | 0.15 |
| Thr 98% | 0.14 | 0.19 |
| Choline 50% | 0.10 | 0.10 |
| Premix1 | 0.50 | 0.50 |
| Total | 100.00 | 100.00 |
| Nutrient levels2 | ||
| CP | 21.00 | 19.00 |
| ME (MJ/kg) | 12.54 | 13.16 |
| Ca | 0.90 | 0.85 |
| TP | 0.63 | 0.55 |
| AP | 0.37 | 0.31 |
| Lys | 1.28 | 1.20 |
| Met | 0.46 | 0.46 |
The premix provided the following per kg of diets: VA, 5,000 IU; VD3,10,000 IU; VE,75.0 mg; VK3, 18.8 mg; VB1, 9.8 mg; VB2, 28.8 mg; VB6, 19.6 mg; VB12, 0.1 mg; calcium pantothenate, 58.8 mg; nicotinic acid, 196.0 mg; folic acid, 4.9 mg; biotin, 2.5 mg; Cu (as copper sulfate) 4.0 mg; Fe (as ferrous sulfate) 40.0 mg; Zn (as zinc sulfate), 37.6 mg ; Mn (as manganese sulfate) 50.0 mg; Se (as sodium selenite) 0.2 mg; I (as calcium iodate) 0.2 mg.
The nutrient levels are calculated values.
Growth Performance
Body weight and feed intake per replicates were recorded on d 21 and d 42 after 12 h fast to determined ADG, ADFI, and feed to gain ratio (F/G) of broilers for the periods from d 1 to d 21, from d 22 to d 42, and from d 1 to d 42.
Sample Collection
On d 42, one broiler per replicate was randomly selected for blood collection. Blood was drawn from the wing vein into a 5 mL anticoagulant-free vacuum tube. After resting blood at room temperature for 2 h, serum was generated by centrifuging at 3,000 rpm for 15 min at 4°C, and then was stored at −20°C until required. After that, birds were euthanized by CO2 inhalation to allow for intestinal sample collection. Cecal contents (approximately 2–3 g) were aseptically collected into sterile tubes and immediately snap-frozen in liquid nitrogen, and stored at −80°C for intestinal microbial flora and volatile fatty acid (VFA) analyses.
Serum Biochemical Analyses
Glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), malondialdehyde (MDA), and total antioxidant capacity (T-AOC) serum levels were measured using a Unico7200 ultraviolet-visible spectrometer (Unico Co. Ltd, Shanghai, China) following kit instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Serum immunoglobulins (IgG, IgM, and IgA) were measured using enzyme-linked immunosorbent assay (ELISA) (Zhongshang Boao Biotechnology Co. Ltd. Shanghai, China) according to manufacturer's instructions. Cytokine (tumor necrosis factor-α [TNF-α], interferon-γ [IFN-γ], and interleukin-1β [IL-1β]) serum levels were ELISA assayed according to kit instructions (Kangjia Hongyuan Biotechnology Co. Ltd. Beijing, China).
VFA Levels
Approximately 0.07 g broiler cecal digesta samples were thoroughly mixed with 1.5 mL distilled water. After centrifugation (10,000 rpm for 10 min), 1.35 mL supernatant was mixed with 0.15 mL 25% (w/v) metaphosphoric acid solution at 4°C for 4 h in a shaded environment. The mixture was then centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant was used for VFA (acetate, propionate, isobutyrate, butyrate, isovalerate, and valerate) composition analysis using a gas chromatography method according to Hao et al. (2021).
16S rRNA-Based Microbiota Analysis
Cecal microbial genomic DNA was extracted using the Fast DNA SPIN for soil kit (MP Biomedicals, Solon, OH). The V3–V4 hyper-variable region of bacterial 16S rRNA was amplified using the primer pair: 338F (5’-ACTCCTACGGGAGGCAG CAG-3’) and 806R (5’-GGACTACHVGGGT WTCTAAT-3’) in an ABI Gene Amp 9700 PCR thermocycler (ABI, CA). After amplification and purification, amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina MiSeq PE300 platform (Illumina, San Diego, CA). Raw reads were deposited into the National Center for Biotechnology Information Sequence Read Archive database (Accession Number: PRJNA759712). Raw sequences were processed using the Majorbio I-Sanger Cloud Platform and chimeric sequences removed. Then α and β-diversity analyses were performed to investigate differences in species composition between samples. The Kruskal-Wallis H test was used to identify significant differential bacterial taxa in cecal microbial communities.
Statistical Analysis
Statistical data analysis was conducted using one-way ANOVA with multiple comparisons using Fisher LSD tests (SAS 9.4, Institute, Cary, NC). Pearson correlation analysis was conducted between cecal microbiota (the top 25 relative abundance genus) with growth performance and serum immune parameters of broilers on d 42. The R software (version 3.3.1) was used to graph the data. P-values < 0.05 were considered statistically significant, and 0.05 < P < 0.10 indicated a tendency for significance.
RESULTS
Growth Performance
The effects of LQ in a corn-soybean basal diet on broiler performance at different phases are shown in Table 2. No treatment effects on broiler performance were observed from d 1 to 21 (P > 0.05). The ADG in broilers fed LQ was significantly higher than CON and AGP groups from d 22 to 42, and over the entire supplemental period (d 1–42; P < 0.05). Additionally, broilers in the LQ group were significantly heavier than CON and AGP broilers at d 42 (P < 0.05). The ADFI in the LQ group showed an improved tendency between d 22 and 42 (P = 0.08). No statistical differences were observed for FCR among groups at any treatment phases (P > 0.05).
Table 2.
Effects of dietary supplementation of LQ on growth performance in broilers.1
| Items2 | Treatment3 |
SEM | P value | ||
|---|---|---|---|---|---|
| CON | AGP | LQ | |||
| D 1 BW (g) | 39.96 | 39.89 | 39.73 | 0.69 | 0.97 |
| D 21 BW (g) | 739.16 | 764.08 | 754.01 | 9.30 | 0.18 |
| D 42 BW (g) | 2,435b | 2,415b | 2,563a | 35 | 0.01 |
| D 1–21 | |||||
| ADFI (g/d) | 48.55 | 49.88 | 49.23 | 0.77 | 0.48 |
| ADG (g/d) | 34.96 | 36.21 | 35.71 | 0.45 | 0.16 |
| F/G | 1.39 | 1.38 | 1.38 | 0.02 | 0.85 |
| D 22–42 | |||||
| ADFI (g/d) | 135.58 | 131.73 | 139.55 | 2.35 | 0.08 |
| ADG (g/d) | 77.09b | 75.04b | 82.25a | 1.39 | 0.03 |
| F/G | 1.76 | 1.76 | 1.70 | 0.03 | 0.33 |
| D 1–42 | |||||
| ADFI (g/d) | 91.55 | 90.66 | 94.18 | 1.60 | 0.28 |
| ADG (g/d) | 57.02b | 56.55b | 60.09a | 0.82 | 0.01 |
| F/G | 1.61 | 1.61 | 1.57 | 0.03 | 0.58 |
Values in the same row with different superscripts were significantly different (P < 0.05) while with same superscripts were insignificantly different (P > 0.05).
Values are expressed as means with SEM.
Abbreviations: ADG, average daily gain; ADFI, average daily feed intake; BW, body weight; F/G, feed: gain ratio.
CON, broilers fed a basal diet; AGP, broilers fed a basal diet supplemented with 45 mg/kg chlortetracycline (15%) and 10 mg/kg kitasamycin (45%); LQ, broilers fed a basal diet supplemented with 50 mg/kg Lactobacillus (1 × 1012 CFU/g) and 500 mg/kg quorum quenching enzyme (10,000 IU /g).
Serum Antioxidant Status
Antioxidant data are given in Table 3. On d 42, no significant effects from dietary treatments were observed for SOD, GSH-Px activity, and MDA levels (P > 0.05). T-AOC activity in LQ and AGP groups showed an improved tendency (P = 0.06).
Table 3.
Effects of dietary supplementation of LQ on antioxidant capacity in broilers.1
| Items2 | Treatment3 |
SEM | P value | ||
|---|---|---|---|---|---|
| CON | AGP | LQ | |||
| T-AOC (mmol/L) | 0.29 | 0.41 | 0.42 | 0.04 | 0.06 |
| SOD (U/mL) | 157.48 | 172.00 | 162.06 | 5.68 | 0.21 |
| GSH-Px (U/mL) | 326.33 | 349.40 | 358.46 | 16.10 | 0.37 |
| MDA (nmol/mL) | 5.06 | 3.93 | 4.79 | 0.46 | 0.22 |
Values are expressed as means with SEM.
Abbreviations: GSH-Px, glutathione peroxidase; MDA, Malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
CON, broilers fed a basal diet; AGP, broilers fed a basal diet supplemented with 45 mg/kg chlortetracycline (15%) and 10 mg/kg kitasamycin (45%); LQ, broilers fed a basal diet supplemented with 50 mg/kg Lactobacillus (1 × 1012 CFU/g) and 500 mg/kg quorum quenching enzyme (10,000 IU/g).
Immunoglobulin and Cytokine Serum Levels
As shown in Table 4, when compared with CON and AGP groups, LQ significantly increased IgA and IgG levels (P < 0.05). TNF-ɑ, IFN-γ, and IL-1β serum levels showed no significant differences among groups (P > 0.05).
Table 4.
Effects of dietary supplementation of LQ on immune status in broilers.1
| Items2 | Treatment3 |
SEM | P value | ||
|---|---|---|---|---|---|
| CON | AGP | LQ | |||
| IgA (g/L) | 0.80b | 0.84b | 1.12a | 0.07 | 0.02 |
| IgG (g/L) | 5.45b | 6.10b | 7.45a | 0.44 | 0.02 |
| IgM (g/L) | 0.78 | 0.78 | 0.75 | 0.06 | 0.91 |
| TNF-ɑ (pg/mL) | 72.40 | 69.07 | 79.10 | 3.73 | 0.19 |
| IFN-γ (pg/mL) | 66.02 | 59.04 | 57.99 | 3.08 | 0.17 |
| IL-1β (pg/mL) | 33.19 | 33.07 | 30.63 | 1.52 | 0.43 |
Values in the same row with different superscripts were significantly different (P < 0.05) while with same superscripts were insignificantly different (P > 0.05).
Values are expressed as means with SEM.
Abbreviations: IgA, Immunoglobulin A; IgG, Immunoglobulin G; IgM, Immunoglobulin M; IFN-γ, Interferon-γ; IL-1β, Interleukin-1β; TNF-ɑ, tumor necrosis factor-ɑ.
CON, broilers fed a basal diet; AGP, broilers fed a basal diet supplemented with 45 mg/kg chlortetracycline (15%) and 10 mg/kg kitasamycin (45%); LQ, broilers fed a basal diet supplemented with 50 mg/kg Lactobacillus (1 × 1012 CFU/g) and 500 mg/kg quorum quenching enzyme (10,000 IU /g).
Cecal VFA Concentrations
No significant differences in cecum VFA levels were observed among groups (P > 0.05; Table 5). When compared with CON and AGP groups, broilers in the LQ groups showed an increased tendency in butyrate levels (P = 0.06).
Table 5.
Effects of dietary supplementation of LQ on VFAs in broilers.1
| Items | Treatment2 |
SEM | P value | ||
|---|---|---|---|---|---|
| CON | AGP | LQ | |||
| Acetate (μg/g) | 95.65 | 92.42 | 105.51 | 5.48 | 0.24 |
| Propionate (μg/g) | 40.86 | 38.35 | 45.08 | 5.59 | 0.70 |
| Isobutyrate (μg/g) | 7.00 | 7.09 | 7.00 | 0.34 | 0.98 |
| Butyrate (μg/g) | 19.75 | 16.43 | 23.97 | 2.06 | 0.06 |
| Isovalerate (μg/g) | 5.40 | 5.66 | 5.54 | 0.29 | 0.82 |
| Valerate (μg/g) | 6.20 | 6.42 | 6.74 | 0.31 | 0.47 |
Values are expressed as means with SEM.
CON, broilers fed a basal diet; AGP, broilers fed a basal diet supplemented with 45 mg/kg chlortetracycline (15%) and 10 mg/kg kitasamycin (45%); LQ, broilers fed a basal diet supplemented with 50 mg/kg Lactobacillus (1 × 1012 CFU/g) and 500 mg/kg quorum quenching enzyme (10,000 IU /g).
Cecal Microbiota Diversity
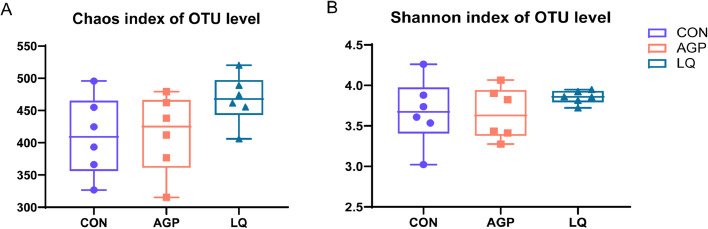
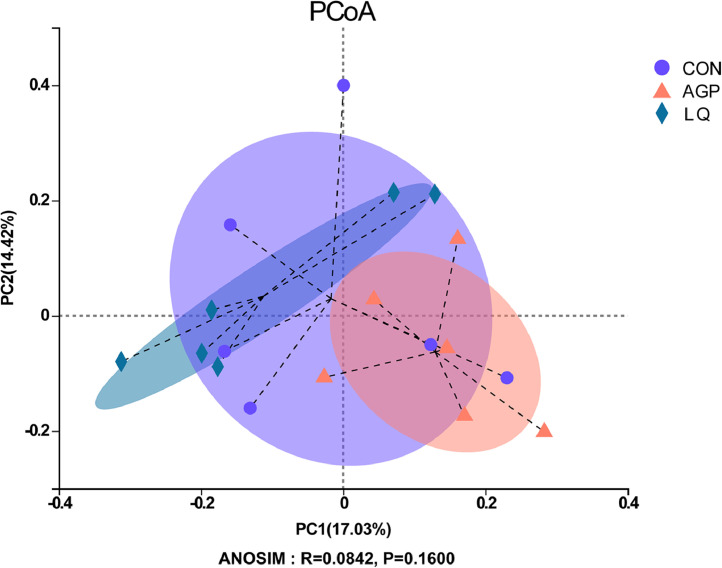
We conducted 16S rRNA gene sequencing of digesta samples to compare differences in cecal microbiota between groups. The rarefaction curves generated from operational taxonomic units showed that sequencing sufficiently captured most operational units in samples (Figure 1). In terms of α-diversity indices, no significant differences (P > 0.05) in Chao and Shannon indices were observed among groups (Figures 2A and 2B). Principal component analysis (PCoA) showed that LQ group samples were separately clustered from bacteria in the AGP group (Figure 3).
Figure 1.
Effects of dietary supplementation of LQ on cecal microbial rarefaction curve of broilers on d 42. CON, broilers fed a basal diet; AGP, broilers fed a basal diet supplemented with 45 mg/kg chlortetracycline (15%) and 10 mg/kg kitasamycin (45%); LQ, broilers fed a basal diet supplemented with 50 mg/kg Lactobacillus (1 × 1012 CFU/g) and 500 mg/kg quorum quenching enzyme (10,000 IU /g).
Figure 2.
Effects of dietary supplementation of LQ on cecal microbial diversity of broilers on d 42. Significant difference was recorded by P < 0.05*. (A) The alpha-diversity of cecal microbiota of Chao index. (B) The alpha-diversity of cecal microbiota of Shannon index.
Figure 3.
Effects of dietary supplementation of LQ on β-diversity based on bray curtis distance of broilers on d 42.
Relative Abundance of Cecal Microflora
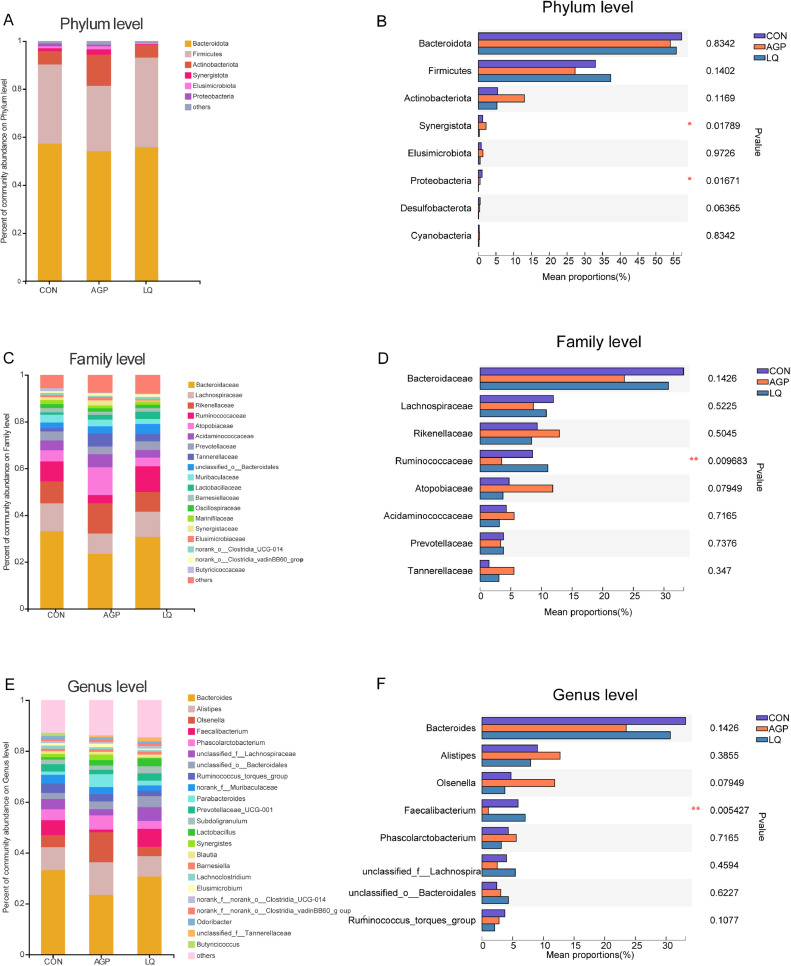
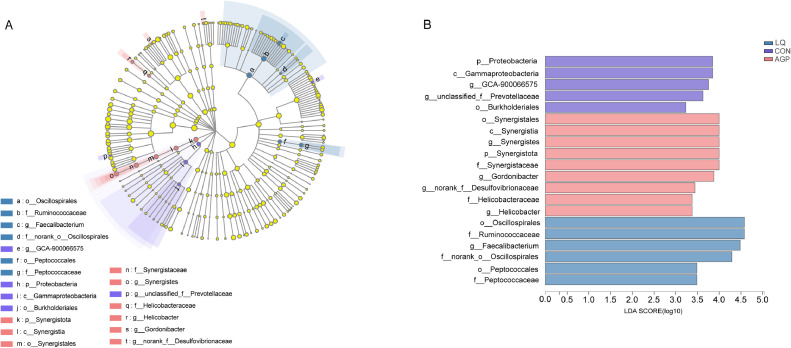
The most abundant phyla in all samples were Bacteroidetes and Firmicutes, followed by Actinobacteriota, Synergistota, Elusimicrobiota, and Proteobacteria (Figure 4A). Also, LQ supplementation significantly decreased Synergistota and Proteobacteria phyla percentages when compared with CON and AGP groups (P < 0.05; Figure 4B). At the family level, Bacteroidaceae, Lachnospiraceae, Rikenellaceae, Ruminococcaceae, Atopobiaceae, Acidaminococcaceae, and Prevotellaceae were the main intestinal flora in all samples (Figure 4C). However, the abundance of the acid-producing bacteria, Ruminococcaceae in the cecum of LQ broilers was significantly higher than other groups (P < 0.05; Figure 4D). At the genus level, Bacteroides and Alistipes were the 2 most dominant genera, followed by Olsenella, Faecalibacterium, Phascolarctobacterium, unclassififiedf-Lachnospiraceae, unclassified-o-Bacteroidales, and Ruminococcus-torques-group (Figure 4E). Additionally, when compared with CON and AGP groups, the beneficial bacteria, Faecalibacterium was significantly increased in the LQ group (P < 0.05; Figure 4F). Linear discriminant analysis effect-size also showed that Ruminococcaceae, Oscillospirates, and Faecalibacterium relative abundance were upregulated in the LQ group, Synergistes, Helicobacter, and Desulfovibrionaceae relative abundance were increased in the AGP group, and Proteobacteria and Gammaproteobacteria relative abundance were relatively higher in the CON group (Figures 5A and 5B).
Figure 4.
Effects of dietary supplementation of LQ on composition of cecal microbiota and differential species identified at phylum, family and genus level of broilers on day 42. (A, C, E) were microbiota composition at phylum, family and genus level, respectively; (B, D, F) were the differential bacteria at phylum, family and genus level. Significant difference was recorded by 0.01 < P ≤ 0.05*, 0.001< P ≤ 0.01**.
Figure 5.
Effects of dietary supplementation of LQ on Linear discriminant analysis effect size (LEfSe) to detect the most significantly abundant cecal microbiota of broilers on d 42 among three groups. (A) Cladoram measured from LEfSe analysis; (B) LDA score generated for differentially abundant microbiota (LDA > 2, P < 0.05).
Correlation Analysis
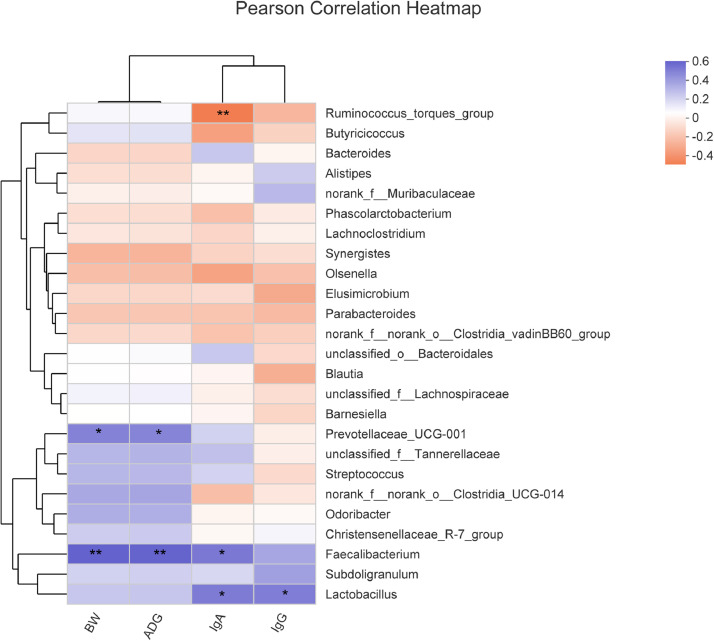
Pearson analysis was conducted to evaluate the associations between cecal microbiota (the top 25 relative abundance genus) with growth performance and serum immune parameters of broilers (Figure 6). According to the heatmap, the growth performance (d 42 BW and 1–42 d ADG) was positively associated with the abundance of Faecalibacterium (P < 0.05) and Prevotellaceae_UCG-001 (P = 0.08). The concentration of serum IgA was positively correlated with the abundance of Faecalibacterium (P = 0.05) and Lactobacillus (P = 0.06), while negatively correlated with the abundance of Ruminococcus_torques_group (P < 0.05). Besides, the positive correlation was found between Lactobacillus and serum IgG (P = 0.07).
Figure 6.
Heatmap of pearson correlation between cecal microbiota (the top 25 relative abundance genus) with growth performance and serum immune parameters of broliers on d 42. Blue suggests a positive correlation, while orange suggests a negative correlation. The intensity of the color indicates the strength of the correlation. The “*” indicates 0.05 < P < 0.10, “**” indicates P < 0.05.
DISCUSSION
Chlortetracycline and kitasamycinas were commonly used as AGPs in commercial farm in China. Various studies have demonstrated that these AGPs had growth-promoting effect on broilers (Dong et al., 2011; Williams et al., 2018; Han et al., 2020; Pirzado et al., 2021). However, in the present study, the AGPs only had a tendency to increase the BW of broilers on d 21, and had no significant effect on BW of broilers on d 42. The results were supported by Bai et al. (2013) who also reported that chlortetracycline only increased the BW of broilers at 21 day of age. The growth-promoting effect of AGPs was decreased when pigs were reared in a clean environment (Cromwell, 2002). The birds in present study were reared in wire-floored cages with a good sanitary condition, which may weaken the growth-promoting effects. The BW of broilers fed LQ diet was higher than that fed AGPs on d 42. Numerous studies found that supplementing with Lactococcus in diets increased the performance of broilers (Panda et al., 2006; Apata, 2008; Forte et al., 2017; Wang et al., 2017). QQE was usually used in aquaculture. Ghanei-Motlagh et al. (2021) found that dietary supplementation with 2 quorum quenching Bacillus strains increased the digestive enzymes activity and growth parameters of Asian seabass before infection with V. harveyi. The increase in BW of broilers fed LQ diet may be the joint action of Lactobacillus and QQE. However, the effects of QQE on the performance of broilers needs to be further studied.
Previous studies showed that probiotic supplementation exerted beneficial effects, including strengthening intestinal barrier function (Wu et al., 2021), stimulating immune systems (Bai et al., 2013), positively modifying intestinal microbiota (Li et al., 2018a), and improving antioxidant activity (Wang et al., 2021b). Antioxidant status within a host serves as an importance to guard against pathogens and maintain homeostasis (Zhu et al., 2015; Fellenberg and Speisky, 2019). In the study, there was an increasing trend of T-AOC in the LQ group and AGP group. Deraz et al. (2019) also found that supplementing with Lactococcus lactis and Lactobacillus plantarum in broiler diets led to increasing T-AOC concentration and decrease MDA in serum. The administration of dietary Lactococcus has been demonstrated to enhance humoral immunity by increasing the serum concentration of immunoglobulins in broilers (Koenen et al., 2004; Wang et al., 2018) and weaned piglets (Dong et al., 2013), which uninfected with pathogens. We also found that the serum concentration of immunoglobulin in LQ group was increased. Moreover, Pearson correlation analysis showed that the relative abundance of Faecalibacterium and Lactobacillus, which were highest in LQ group among 3 groups, were positively correlated with the concentration of immunoglobulin in serum.
The gut microbiota plays an important role in maintaining gut health and enhancing growth (Marchesi et al., 2016; Rowland et al., 2018; Michaudel and Sokol, 2020). The present study revealed that the supplementation of AGP and LQ had no effects on alpha diversity of cecal microflora in broilers. However, the PCoA showed that the beta diversity was different between LQ and AGP group, which suggested that the effect of LQ treatment on gut microflora was different from that of AGPs. Furthermore, the majority of cecal bacteria in broilers were Firmicutes, Bacteroidetes, and Proteobacteria, consistent with previous studies (Choi et al., 2014; Mohd Shaufi et al., 2015). Proteobacteria is not only related to human intestinal diseases, but also to extraintestinal diseases. These diseases are sustained by various degree of inflammation, which thus represents a core aspect of Proteobacteria-related diseases. An increasing amount of data identifies Proteobacteria as a possible microbial signature of disease (reviewed by Rizzatti et al., 2017). The relative abundance of Proteobacteria was decreased in LQ group compared with CON and AGP group, which indicated that the LQ may improve the intestinal health of broilers. Yang et al. (2020) found that dietary supplementation with Lactobacillus reduced the relative abundance of Proteobacteria in cecal content of piglets. Li et al. (2018b) reported that the relative abundance of Proteobacteria was reduced in the ileum by supplementation with L. acidophilus probiotic in bird feed.
The family Ruminococcaceae and genus Faecalibacterium are predominant intestinal butyrate-producing bacteria (Zhou et al., 2018; Liao et al., 2020; Lan et al., 2021; Oladokun et al., 2021). Butyrate provides energy to intestinal epithelial cells, and plays a key role in inhibiting inflammation and promoting intestinal development (Vinolo et al., 2011; Ratajczak et al., 2019; Ranjbar, et al., 2021). Therefore, the Ruminococcaceae and Faecalibacterium have been identified as potentially beneficial microbe (Torok et al., 2011). The abundance of Ruminococcaceae showed highly positive correlations with the body weight (Stanley et al., 2016; Dai et al., 2020) and better feed conversion in broilers (Stanley et al., 2012; Biddle et al., 2013).The Pearson correlation analysis in this study showed that the relative abundance of Faecalibacterium was positively correlated with the BW and ADG of broilers. LQ supplementation significantly increased the relative abundance of Ruminococcaceae and Faecalibacterium, may further increased the BW and ADG of broilers. Previous study found that Lactobacillus supplementation significantly increased the relative abundance of Ruminococcaceae in pigs (Yang et al., 2020). Ghanei-Motlagh et al. (2021) reported that dietary supplementation with 2 quorum quenching Bacillus strains increased the number of total aerobic heterotrophic bacteria, decreased the number of Vibrio spp. in Asian seabass before they were challenged with pathogen. Disrupting bacterial communication (QS) is considered a promising antiviral approach because it can neutralize the pathogens virulence rather than destroys them (Defoirdt, 2018). QQE can reduce the virulence of pathogenic bacteria. It could reduce the colonization of pathogenic bacteria on the intestinal mucosa of broilers, but it also may reduce the colonization of symbiotic bacteria on the mucosa. The effect of QQE on mucosal adhesion bacteria and intestinal health of broilers need to be further studied.
In conclusion, the supplementation of LQ in diets could reduce the relative abundance of Proteobacteria, increase the relative abundance of Ruminococcaceae and Faecalibacteriumin, tend to increase the content of butyric acid, and finally improved the BW and ADG of broilers, which indicated that LQ may be used as a potential alternative to antibiotics in poultry.
ACKNOWLEDGMENTS
This project was financially supported by the Innovation Team of the Chinese Academy of Agricultural Sciences (ASTIP-IAS07) (Beijing, China), Innovation Research Team of China Agriculture Research System (Beijing, China) and fund of Tianjin Biofeed Technology Co. Ltd. (TianJin, China).
DISCLOSURES
The authors declare no financial or personal conflicts of interest.
REFERENCES
- Apata D.F. Growth performance, nutrient digestibility and immune response of broiler chicks fed diets supplemented with a culture ofLactobacillus bulgaricus. J. Sci. Food. Agric. 2008;88:1253–1258. [Google Scholar]
- Aviagen. 2018. Ross Management Book. Aviagen:144, Huntsville, AL. Accessed July 21. https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf.
- Bai S.P., Wu A.M., Ding X.M., Lei Y., Bai J., Zhang K.Y., Chio J.S. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 2013;92:663–670. doi: 10.3382/ps.2012-02813. [DOI] [PubMed] [Google Scholar]
- Biddle A., Stewart L., Blanchard J., Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5:627–640. [Google Scholar]
- Chen B., Peng M., Tong W., Zhang Q., Song Z. The quorum quenching bacterium bacillus licheniformis T-1 protects zebrafish against aeromonas hydrophila infection. Probiotics Antimicrob. Proteins. 2020;12:160–171. doi: 10.1007/s12602-018-9495-7. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Kim G.B., Cha C.J. Spatial heterogeneity and stability of bacterial community in the gastrointestinal tracts of broiler chickens. Poult. Sci. 2014;93:1942–1950. doi: 10.3382/ps.2014-03974. [DOI] [PubMed] [Google Scholar]
- Chu W., Lu F., Zhu W., Kang C. Isolation and characterization of new potential probiotic bacteria based on quorum-sensing system. J. Appl. Microbiol. 2011;110:202–208. doi: 10.1111/j.1365-2672.2010.04872.x. [DOI] [PubMed] [Google Scholar]
- Cromwell G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002;13:7–27. doi: 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- Dai D., Qiu K., Zhang H.J., Wu S.G., Han Y.M., Wu Y.Y., Qi G.H., Wang J. Organic acids as alternatives for antibiotic growth promoters alter the intestinal structure and microbiota and improve the growth performance in broilers. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.618144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoirdt T. Quorum-sensing systems as targets for antivirulence therapy. Trends. Microbiol. 2018;26:313–328. doi: 10.1016/j.tim.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Deraz S.F., Elkomy A.E., Khalil A.A. Assessment of probiotic-supplementation on growth performance, lipid peroxidation, antioxidant capacity, and cecal microflflora in broiler chickens. J. Appl. Pharm. Sci. 2019;9:30–39. [Google Scholar]
- Dong X.F., Gao W.W., Su J.L., Tong J.M., Zhang Q. Effects of dietary polysavone (Alfalfa extract) and chlortetracycline supplementation on antioxidation and meat quality in broiler chickens. Br. Poult. Sci. 2011;52:302–309. doi: 10.1080/00071668.2011.569008. [DOI] [PubMed] [Google Scholar]
- Dong X., Zhang N., Zhou M., Tu Y., Deng K., Diao Q. Effects of dietary probiotics on growth performance, faecal microbiota and serum profiles in weaned piglets. J. Basic. Microbiol. 2013;49:220–226. [Google Scholar]
- Engberg R.M., Hedemann M.S., Leser T.D., Jensen B.B. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci. 2000;79:1311–1319. doi: 10.1093/ps/79.9.1311. [DOI] [PubMed] [Google Scholar]
- Fatufe A.A., Matanmi I.O. The effect of probiotics supplementation on the growth performance of two strains of cockerels. J. Cent. Eur. Agric. 2008;9:405–410. [Google Scholar]
- Fellenberg M.A., Speisky H. Antioxidants: their effects on broiler oxidative stress and its meat oxidative stability. Worlds Poult. Sci. J. 2019;62:53–70. [Google Scholar]
- Forte C., Manuali E., Abbate Y., Papa P., Vieceli L., Tentellini M., Trabalza-Marinucci M., Moscati L. Dietary Lactobacillus acidophilus positively influences growth performance, gut morphology, and gut microbiology in rurally reared chickens. Poult. Sci. 2017;97:930–936. doi: 10.3382/ps/pex396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health. Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gao J., Wang R., Liu J., Wang W., Chen Y., Cai W. Effects of novel microecologics combined with traditional Chinese medicine and probiotics on growth performance and health of broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanei-Motlagh R., Takavar M., Darioush G., Mohammad K., Esmaeil M., Mojtab Z., Mansour E., Simon M. Quorum quenching probiotics modulated digestive enzymes activity, growth performance, gut microflora, haemato-biochemical parameters and resistance against Vibrio harveyi in Asian seabass (Lates calcarifer) Aquaculture. 2021;531 [Google Scholar]
- Han Y., Tang C., Li Y., Yu Y., Zhan T., Zhao Q., Zhang J. Effects of dietary supplementation with clostridium butyricum on growth performance, serum immunity, intestinal morphology, and microbiota as an antibiotic alternative in weaned piglets. Animals (Basel) 2020;10:2287. doi: 10.3390/ani10122287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Ji Z., Shen Z., Wu Y., Zhang B., Tang J., Hou S., Xie M. Effects of total dietary fiber on cecal microbial community and intestinal morphology of growing White Pekin Duck. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T., Long S., Mahfuz S., Wu D., Wang X., Wei X., Piao X. Effects of probiotics as antibiotics substitutes on growth performance, serum biochemical parameters, intestinal morphology, and barrier function of broilers. Animals. 2019;9:985. doi: 10.3390/ani9110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen M.E., Kramer J., Hulst R., Heres L., Jeurissen S.H.M., Boersma W.J.A. Immunomodulation by probiotic lactobacilli in layer- and meat-type chickens. Br. Poult. Sci. 2004;45:355–366. doi: 10.1080/00071660410001730851. [DOI] [PubMed] [Google Scholar]
- Lan J., Chen G., Cao G., Tang J., Li Q., Zhang B., Yang C. Effects of alpha-glyceryl monolaurate on growth, immune function, volatile fatty acids, and gut microbiota in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.W., Li G., Lillehoj H.S., Lee S.H., Jang S.I., Babu U.S., Lillehoj E.P., Neumann A.P., Siragusa G.R. Bacillus subtilis-based direct-fed microbials augment macrophage function in broiler chickens. Res. Vet. Sci. 2011;91:e87–e91. doi: 10.1016/j.rvsc.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Lhermie G., Grohn Y.T., Raboisson D. Addressing antimicrobial resistance: an overview of priority actions to prevent suboptimal antimicrobial use in food-animal production. Front. Microbiol. 2016;7:2114. doi: 10.3389/fmicb.2016.02114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.L., Wang J., Zhang H.J., Wu S.G., Hui Q.R., Yang C.B., Fang R.J., Qi G.H. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front. Physiol. 2018;9:1968. doi: 10.3389/fphys.2018.01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang W., Liu D., Guo Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2018;9:25. doi: 10.1186/s40104-018-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Shao Y., Sun G., Yang Y., Zhang L., Guo Y., Luo X., Lu L. The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers. Poult. Sci. 2020;99:5883–5895. doi: 10.1016/j.psj.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokapirnasari W.P., Pribadi T.B., Arif A.A., Soeharsono S., Hidanah S., Harijani N., Najwan R., Huda K., Wardhani H.C.P., Rahman N.F.N., Yulianto A.B. Potency of probiotics Bifidobacterium spp. and Lactobacillus casei to improve growth performance and business analysis in organic laying hens. Vet. World. 2019;12:860–867. doi: 10.14202/vetworld.2019.860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi J.R., Adams D.H., Fava F., Hermes G.D., Hirschfield G.M., Hold G., Quraishi M.N., Kinross J., Smidt H., Tuohy K.M., Thomas L.V., Zoetendal E.G., Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaudel C., Sokol H. The gut microbiota at the service of immunometabolism. Cell. Metab. 2020;32:514–523. doi: 10.1016/j.cmet.2020.09.004. [DOI] [PubMed] [Google Scholar]
- Mohd Shaufi M.A., Sieo C.C., Chong C.W., Gan H.M., Ho Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut. Pathog. 2015;7:4. doi: 10.1186/s13099-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Oladokun S., Koehler A., MacIsaac J., Ibeagha-Awemu E.M., Adewole D.I. Bacillus subtilis delivery route: effect on growth performance, intestinal morphology, cecal short-chain fatty acid concentration, and cecal microbiota in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A.K., Rao S.V.R., Raju M.V.L.N., Sharma S.R. Dietary supplementation of Lactobacillus sporogenes on performance and serum biochemico-lipid profile of broiler chickens. Poult. Sci. 2006;43:235–240. [Google Scholar]
- Papenfort K., Bassler B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016;14:576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez P.D., Hagen S.J. Heterogeneous response to a quorum-sensing signal in the luminescence of individual Vibrio fischeri. PLoS. One. 2010;5:e15473. doi: 10.1371/journal.pone.0015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirzado S.A., Arain M.A., Huiyi C., Fazlani S.A., Alagawany M., Gouhua L. Effect of zomite on growth performance, immune function and tibia breaking strength of broiler chickens during starter period. Anim. Biotechnol. 2021;3:1–6. doi: 10.1080/10495398.2021.1914644. [DOI] [PubMed] [Google Scholar]
- Ranjbar R., Vahdati S.N., Tavakoli S., Khodaie R., Behboudi H. Immunomodulatory roles of microbiota-derived short-chain fatty acids in bacterial infections. Biomed. Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111817. [DOI] [PubMed] [Google Scholar]
- Ratajczak W., Ryl A., Mizerski A., Walczakiewicz K., Sipak O., Laszczynska M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs) Acta. Biochim. Pol. 2019;66:1–12. doi: 10.18388/abp.2018_2648. [DOI] [PubMed] [Google Scholar]
- Rizzatti G., Lopetuso L.R., Gibiino G., Binda C., Gasbarrini A. Proteobacteria: a common factor in human diseases. Biomed. Res. Int. 2017;2017 doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S.T., Bassler B.L. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold. Spring. Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikdar R., Elias M. Quorum quenching enzymes and their effects on virulence, biofilm, and microbiomes: a review of recent advances. Expert. Rev. Anti. Infect. Ther. 2020;18:1221–1233. doi: 10.1080/14787210.2020.1794815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Li A., Wang Y., Song G., Cheng J., Wang L., Liu K., Min Y., Wang W. Effects of synbiotic on growth, digestibility, immune and antioxidant performance in broilers. Animal. 2022;16 doi: 10.1016/j.animal.2022.100497. [DOI] [PubMed] [Google Scholar]
- Stanley D., Denman S.E., Hughes R.J., Geier M.S., Crowley T.M., Chen H., Haring V.R., Moore R.J. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl. Microbiol. Biotechnol. 2012;96:1361–1369. doi: 10.1007/s00253-011-3847-5. [DOI] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Geier M.S., Moore R.J. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: challenges presented for the identification of performance enhancing probiotic bacteria. Front. Microbiol. 2016;7:187. doi: 10.3389/fmicb.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok V.A., Hughes R.J., Mikkelsen L.L., Perez-maldonado R., Balding K., Macalpine R., Percy N.J., Ophel-Keller K. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl. Environ. Microbiol. 2011;77:5868–5878. doi: 10.1128/AEM.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M., Rubio-Portillo E., Anton J., Ramos-Espla A.A., Quesada E., Llamas I. Selection of the N-Acylhomoserine lactone-degrading bacterium alteromonas stellipolaris PQQ-42 and of its potential for biocontrol in aquaculture. Front. Microbiol. 2016;7:646. doi: 10.3389/fmicb.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinolo M.A., Rodrigues H.G., Nachbar R.T., Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Gong L., Zhou Y., Tang L., Zeng Z., Wang Q., Zou P., Yu D., Li W. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim. Nutr. 2021;7:829–840. doi: 10.1016/j.aninu.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Liu C., Chen M., Ya T., Huang W., Gao P., Zhang H. A novel Lactobacillus plantarum strain P-8 activates beneficial immune response of broiler chickens. Int. Immunopharmacol. 2015;29:901–907. doi: 10.1016/j.intimp.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Wang S., Peng Q., Jia H.M., Zeng X.F., Zhu J.L., Hou C.L., Liu X.T., Yang F.J., Qiao S.Y. Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1. Poult. Sci. 2017;96:2576–2586. doi: 10.3382/ps/pex061. [DOI] [PubMed] [Google Scholar]
- Wang W., Cai H., Zhang A., Chen Z., Chang W., Liu G., Deng X., Bryden W.L., Zheng A. Enterococcus faecium modulates the gut microbiota of broilers and enhances phosphorus absorption and utilization. Animals. 2020;10:1232. doi: 10.3390/ani10071232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Heng C., Zhou X., Cao G., Jiang L., Wang J., Li K., Wang D., Zhan X. Supplemental Bacillus subtilis DSM 29784 and enzymes, alone or in combination, as alternatives for antibiotics to improve growth performance, digestive enzyme activity, anti-oxidative status, immune response and the intestinal barrier of broiler chickens. Br. J. Nutr. 2021;125:494–507. doi: 10.1017/S0007114520002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Y., Xu H., Mei X., Gong L., Wang B., Li W., Jiang S. Direct-fed glucose oxidase and its combination with B. amyloliquefaciens SC06 on growth performance, meat quality, intestinal barrier, antioxidative status, and immunity of yellow-feathered broilers. Poult. Sci. 2018;97:3540–3549. doi: 10.3382/ps/pey216. [DOI] [PubMed] [Google Scholar]
- Williams H.E., Tokach M.D., Dritz S.S., Woodworth J.C., DeRouchey J.M., Nagaraja T.G., Goodband R.D., Pluske J.R., Chitakasempornkul K., Bello N.M., Amachawadi R.G. Effects of chlortetracycline alone or in combination with direct fed microbials on nursery pig growth performance and antimicrobial resistance of fecal Escherichia coli. J. Anim. Sci. 2018;96:5166–5178. doi: 10.1093/jas/sky370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang B., Zeng Z., Liu R., Tang L., Gong L., Li W. Effects of probiotics Lactobacillus plantarum 16 and Paenibacillus polymyxa 10 on intestinal barrier function, antioxidative capacity, apoptosis, immune response, and biochemical parameters in broilers. Poult. Sci. 2019;98:5028–5039. doi: 10.3382/ps/pez226. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhen W., Geng Y., Wang Z., Guo Y. Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci. Rep. 2019;9:10256. doi: 10.1038/s41598-019-46578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Yang K., Zhang A., Chang W., Zheng A., Chen Z., Cai H., Liu G. Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O78. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.J., Wang C.L., Huang K.H., Zhang M.H., Wang J., Pan X.C. Compound Lactobacillus sp. administration ameliorates stress and body growth through gut microbiota optimization on weaning piglets. Appl. Microbiol. Biotechnol. 2020;104:6749–6765. doi: 10.1007/s00253-020-10727-4. [DOI] [PubMed] [Google Scholar]
- Zhou W., Yan Y., Mi J., Zhang H., Lu L., Luo Q., Li X., Zeng X., Cao Y. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from bee collected pollen of chinese wolfberry. J. Agric. Food. Chem. 2018;66:898–907. doi: 10.1021/acs.jafc.7b05546. [DOI] [PubMed] [Google Scholar]
- Zhu W., Li D., Wang J., Wu H., Xia X., Bi W., Guan H., Zhang L. Effects of polymannuronate on performance, antioxidant capacity, immune status, cecal microflora, and volatile fatty acids in broiler chickens. Poult. Sci. 2015;94:345–352. doi: 10.3382/ps/pev006. [DOI] [PubMed] [Google Scholar]