Abstract
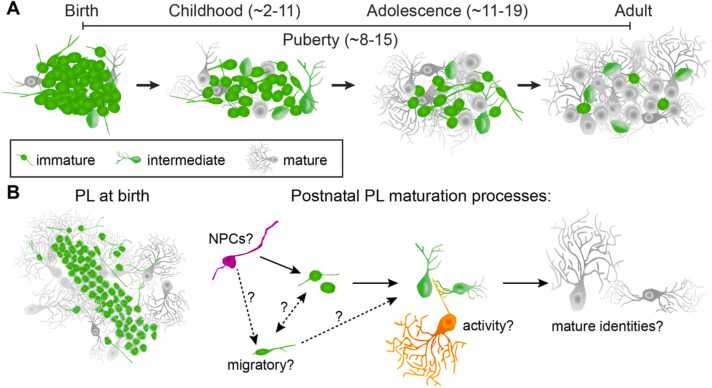
The human amygdala is critical for emotional learning, valence coding, and complex social interactions, all of which mature throughout childhood, puberty, and adolescence. Across these ages, the amygdala paralaminar nucleus (PL) undergoes significant structural changes including increased numbers of mature neurons. The PL contains a large population of immature excitatory neurons at birth, some of which may continue to be born from local progenitors. These progenitors disappear rapidly in infancy, but the immature neurons persist throughout childhood and adolescent ages, indicating that they develop on a protracted timeline. Many of these late-maturing neurons settle locally within the PL, though a small subset appear to migrate into neighboring amygdala subnuclei. Despite its prominent growth during postnatal life and possible contributions to multiple amygdala circuits, the function of the PL remains unknown. PL maturation occurs predominately during late childhood and into puberty when sex hormone levels change. Sex hormones can promote developmental processes such as neuron migration, dendritic outgrowth, and synaptic plasticity, which appear to be ongoing in late-maturing PL neurons. Collectively, we describe how the growth of late-maturing neurons occurs in the right time and place to be relevant for amygdala functions and neuropsychiatric conditions.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; BrdU, 5-bromo-2-deoxyuridine; AB, accessory basal nucleus; BA, basal nucleus of the amygdala; BCL-2, B-cell lymphoma 2; BLA, basolateral nucleus of the amygdala; BLVM, basolateral ventromedial amygdala; DCX, doublecortin; LA, lateral nucleus of the amygdala; LPL, lateral paralaminar nucleus; MPL, medial paralaminar nucleus; NPC, neural progenitor cell; PL, paralaminar nucleus; PSA-NCAM, polysialylated neural cell adhesion molecule; TBR1, T-box brain 1; tLV, temporal lobe lateral ventricle
Keywords: Paralaminar nucleus, Amygdala, Development, Neurogenesis, Migration, Primates
Graphical Abstract
1. Introduction
During embryonic brain development, neurons are born in germinal zones along the walls of the cerebral ventricles, a birthplace that is far removed from their ultimate final destinations throughout the human brain. Over weeks to months during gestation, neurons migrate long distances to reach these locations, finally settling in around birth and in the months after (Kostović et al., 2019, Sanai et al., 2011, Paredes et al., 2016a). After neurons arrive at their final destinations, they begin to downregulate genes associated with migration, extend axons and dendrites, and form and prune synapses, a process that continues throughout childhood (2–11 years) and into adolescence (11–19 years) (Goddings, 2014, Huttenlocher and Dabholkar, 1997, Kostović et al., 2019, Østby, 2009). Even though synaptogenesis and pruning are ongoing, connectivity between regions is established before birth (though refinement of connectivity continues) and most neurons are mature in the sense that they fire action potentials and are functionally wired with each other (Budday et al., 2015, Raybaud et al., 2013).
In contrast to this typical trajectory of neuron development, there exists a unique subset of neurons that follow a different timeline. Although born at embryonic ages, these neurons retain immature molecular and morphological features throughout childhood, adolescence, and even into adulthood, so we refer to them as late-maturing neurons. Electrophysiological studies of late-maturing neurons in mice show that in their immature stages, they receive sparse synaptic inputs and have infrequent action potentials suggesting they are not yet functionally integrated within neural circuits (Benedetti, 2020, Klempin et al., 2011). These neurons are distributed in parts of the neocortex and allocortex in many mammalian species (Ghibaudi and Bonfanti, 2022) including mice (Rotheneichner et al., 2018, Rubio et al., 2016), rats (Seki and Arai, 1991), bats (Chawana et al., 2016), cats (Martí-Mengual et al., 2013), tree shrews (Ai et al., 2021), rabbits (Luzzati et al., 2009, Ponti et al., 2006), guinea pigs (Xiong et al., 2008), sheep (Piumatti et al., 2018), non-human primates (Fasemore et al., 2018, Zhang, 2009), and humans (Coviello et al., 2022, Sorrells et al., 2021).
Some species also have a population of late-maturing neurons within a subregion of the amygdala called the paralaminar nucleus (PL). Late-maturing neurons in the PL have been observed in tree shrews (Ai et al., 2021), bats (Crosby and Humphrey, 1939), cats (Sah et al., 2003), rabbits (Luzzati et al., 2009), sheep (Piumatti et al., 2018), non-human primates (Chareyron et al., 2012, Fudge, 2004, Zhang, 2009), and humans (Avino, 2018, Liu et al., 2018, Martí-Mengual et al., 2013, Sorrells et al., 2019, Yachnis et al., 2000). The PL is an especially prominent feature of the non-human primate and human amygdala that exhibits significant neuron maturation between childhood and adolescence (Avino, 2018, Sorrells et al., 2019), placing it in the right time and place to be important for the development of amygdala structure and function.
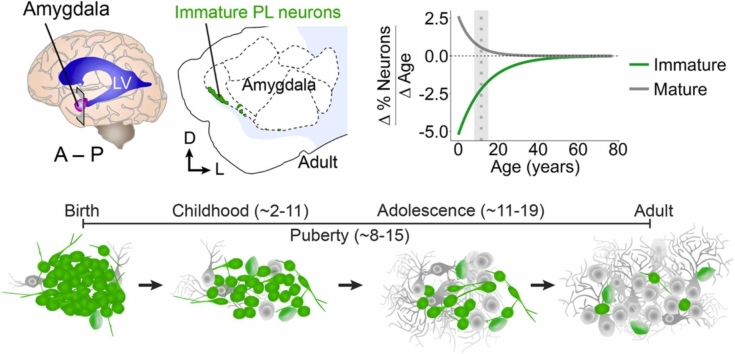
In this review, we consider the evidence for ongoing PL neuron developmental processes during childhood (~2–11 years) and adolescence (~11–19 years) in humans. Puberty (~8–15 years) (Blakemore et al., 2010) occurs in the midst of this range of ages, when there are sex-specific hormonal changes that can influence neuron development. We will consider evidence that PL neurons are born during gestation and undergo protracted postnatal development and discuss ways that the PL could impact developmental increases in the number of mature neurons in nearby amygdala subnuclei. We will consider the evidence available from studies of the primate PL, alongside evidence from other species and brain regions that contain immature neurons, to develop hypotheses of the possible functional significance of the recruitment of immature PL neurons into established amygdala circuits.
2. The paralaminar nucleus of the amygdala
2.1. Nomenclature and anatomy
The PL is a distinctive region of the primate amygdala that was recognized as early as the 1950s using simple histological preparations (Sanides, 1957). Nissl and Golgi staining of the human amygdala revealed a region with small, compact cells clustered tightly together (Sanides, 1957, Braak and Braak, 1983) that contains about 3 million neurons in total, or ~20% of the neurons in the adult human amygdala (García-Amado and Prensa, 2012). The nomenclature describing this region has been highly variable, as reviewed by deCampo and Fudge (deCampo and Fudge, 2012). Some of this variability may be due to the heterogeneous architecture of the PL: it consists of both a lamina, or a planar collection of cells, on its medial side which we refer to as the medial PL (MPL). It also consists of multiple lateral nuclei, or tight groupings of neuronal somas, which we refer to as the lateral PL (LPL) (Fig. 1). The MPL has been called the granular nucleus (Braak and Braak, 1983), the basomedial nucleus (Sims and Williams, 1990), or considered part of the ventral and/or “parvocellular” division of the basolateral nucleus due to its anatomical location and the small size of the cells (García-Amado and Prensa, 2012, Price and Amaral, 1981, Campbell et al., 2021). The LPL has been considered part of the intercalated islands (Sims and Williams, 1990), which are also small, densely associated clusters of neuronal somas but in fact contain different types of neurons than the PL (Zikopoulos et al., 2016).
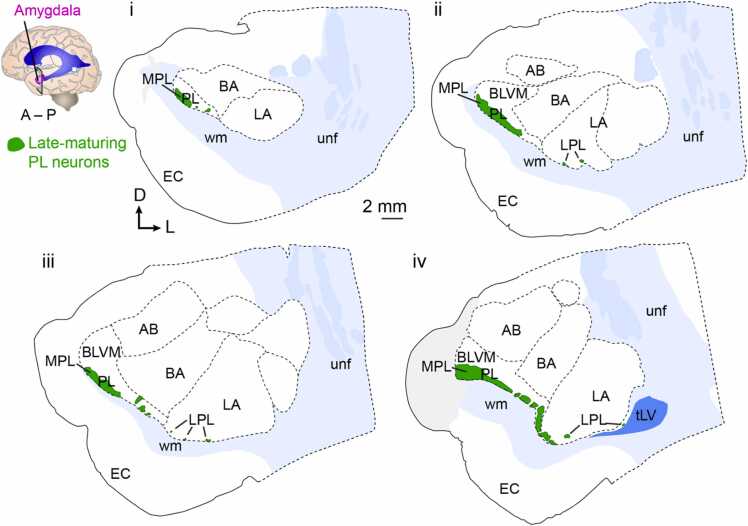
Fig. 1.
Adult human PL anatomy. Serial coronal sections of the adult, 27 year old human amygdala. Sections indicate the location of late-maturing neurons in the amygdala paralaminar nucleus and surrounding regions and are spaced anterior to posterior across 2 mm intervals. Light gray shading indicates damaged tissue areas. AB: accessory basal nucleus, BA: basolateral amygdala, BLVM: basolateral ventromedial division, EC: entorhinal cortex, LA: lateral amygdala, LPL: lateral paralaminar nucleus, MPL: medial paralaminar nucleus, PL: paralaminar nucleus, tLV: temporal lateral ventricle, unf: uncinate fasciculus, wm: white matter.
The MPL is identifiable as a large field of immature neurons along the ventral edge of the basal amygdala (BA). The MPL begins anterior to the basal and lateral amygdala (LA), collectively referred to as the basolateral amygdala (BLA) (Fig. 1). The posterior boundary of the MPL extends beyond the BLA above the uncus of the hippocampus to the stria terminalis. The ventromedial boundary of the MPL is a large white matter fiber tract (Fig. 1). This white matter represents the rostral end of the angular bundle and the Allen Institute reference atlas for the human brain identifies this tract as an arm extending from the uncinate fasciculus (Ding et al., 2017). Fibers from this white matter tract appear to innervate the MPL, though it is unknown whether they are terminating on late-maturing MPL neurons. In the MPL, the immature neurons close to the white matter are densely packed whereas the immature neurons along the dorso-lateral edge are more dispersed, blending into the adjacent accessory basal (AB) and BA nuclei (Avino, 2018, Fudge, 2004, Sorrells et al., 2019). At more caudal and lateral levels, the ventral edge of the MPL comes in close proximity to the ventricular-subventricular zone (V-SVZ) of the temporal lobe lateral ventricle.
The LPL, by contrast, is a collection of multiple discrete, dense cell clusters along the ventral boundary of the lateral amygdala and, in the more posterior levels, dorsal to the temporal lateral ventricle (Fig. 1). The locations of the LPL nuclei are heterogeneous within an individual amygdala: some are located within the gray matter of the BLA, with one island consistently observed at the boundary of the BA and LA. Many of the LPL clusters that are in the gray matter of the BA or LA occur at small crests along the dorsal side of the white matter tract ventral to the BLA (Fig. 1). These crests correspond to fibers emanating from the white matter that extend and surround the LPL nuclei (Sorrells et al., 2019, Supplementary Fig. 3). Some LPL nuclei are embedded deeply within the white matter ventral to the BLA. Still other LPL clusters are within the V-SVZ on the dorsolateral side of the temporal lateral ventricle. The LPL nuclei are also heterogeneous between individuals, not always found in the same anatomical location or in the same size or number across people (Sorrells et al., 2019). Despite the heterogeneous anatomical features of the MPL and LPL, both of these subregions contain late-maturing neurons.
2.2. Late-maturing neurons and other cell types in the PL
The most distinctive cell type in the PL is neurons with immature morphological and molecular features. At birth these cells make up ~90% of the cells within the region and decline with age as we will discuss in Section 3.1. In both the MPL and LPL, these cells have small somas (around 10 µm in diameter) and are found individually as well as densely clustered together. Some clusters are so densely packed that there is essentially no space between individual neuronal nuclei. Other clusters contain larger cells intermixed with small cells tightly associated in groups or organized into larger parallel columns or chains. These small cells have the ultrastructural features of immature neurons, including few/simple processes, small nuclei with compacted chromatin, limited cytosol, and few organelles. Clusters of immature neurons form adherens junctions with each other (Sorrells et al., 2019). Immunostaining in the macaque PL found these cells to express B-cell lymphoma 2 (BCL-2) (Fudge, 2004, Bernier et al., 2002), which could facilitate their survival by preventing apoptosis (Ceizar, 2016, Farlie et al., 1995). In the primate PL, the BCL-2+ cells frequently co-express class III ꞵ-tubulin (TUJ1) (Fudge, 2004); these two proteins also co-express in the human PL (Yachnis et al., 2000). In addition to these markers, late-maturing neurons co-express the immature neuron markers doublecortin (DCX) and polysialylated neural cell adhesion molecule (PSA-NCAM) (Martí-Mengual et al., 2013, Sorrells et al., 2019). These molecules are important for cells to remodel their cytoskeleton for migration, growth, or synaptogenesis (Bai, 2008, Bonfanti, 2006, Francis et al., 1999, Gleeson et al., 1999, Ulfig and Chan, 2004). In contrast to these small immature neurons, the larger mature neurons in the PL express NEUN and are DCX–PSA-NCAM–. Some cells of intermediate size co-express DCX, PSA-NCAM, and NEUN, which could represent an intermediate stage of maturation (Sorrells et al., 2019). Single cell RNA-sequencing of human amygdala samples from 4 to 15 years of age identified a cluster of immature neurons that differentially express many of the genes (e.g. DCX, BCL-2, MAP2) that were also identified by immunostaining, as well as novel genes (e.g. ROBO1, BCL11B, SOX11) that are related to ongoing neural developmental processes (Sorrells et al., 2019). Together these observations reveal a nucleus with a large population of immature neurons that undergo structural growth and maturation across postnatal life.
The majority of late-maturing neurons in the PL appear to be excitatory (Sorrells et al., 2019). At 13 years of age, 54.4% of immature DCX+ cells express T-box brain 1 (TBR1), an excitatory neuron transcription factor important for cortical excitatory neuron development (Hevner et al., 2001). At this same age, 97.6% of the mature NEUN+TBR1+ neurons express VGLUT2. Only about 3% of all cells in the PL express the GABAergic marker GAD67, and the number of cells in the PL expressing GAD67 or other interneuron markers such as SST, nNOS, or CR does not change with age (Sorrells et al., 2019). Together these observations indicate that the majority of late-maturing neurons in the PL are excitatory. Some VGLUT2+ neurons express the calcium binding protein calbindin (CB) and some do not (Sorrells et al., 2019). The functional significance of these varied expression patterns is unknown, and could represent different types of excitatory neurons, or cells in different stages of maturation. For instance, adult-born excitatory neurons in the rodent hippocampus only express CB once mature (Kempermann et al., 2004), so CB+ neurons in the human PL may be more mature than those that do not.
The PL is not composed only of late-maturing neurons. A small percentage of neurons in the PL are not late-maturing, as there are mature neurons (DCX–NEUN+) present at birth in both the MPL and LPL (Sorrells et al., 2019). Along the temporal lateral ventricle, parts of the PL are in close proximity to the ependymal cells that line the ventricle walls, and glia are also found throughout the PL (Fig. 2). Astrocytes labeled with GFAP, Vimentin, or BLBP are present within the MPL and LPL, however very few of these cells are dividing after birth. Of the dividing (Ki-67+) cells present in the region at birth, a large fraction (~36%) are OLIG2+, suggesting they are likely oligodendrocyte precursor cells (OPCs) (Sorrells et al., 2019). The abundance of oligodendrocytes increases throughout the amygdala, including within the PL, between birth and adulthood in monkeys (Chareyron et al., 2012). Glia play critical roles in neuron maturation, from neuron birth, migration, growth, and synaptogenesis (Allen and Lyons, 2018) and likely influence the rate and timing of PL neuron maturation, but it is currently unknown if or how glia contribute to the uniquely delayed developmental trajectory of this region.
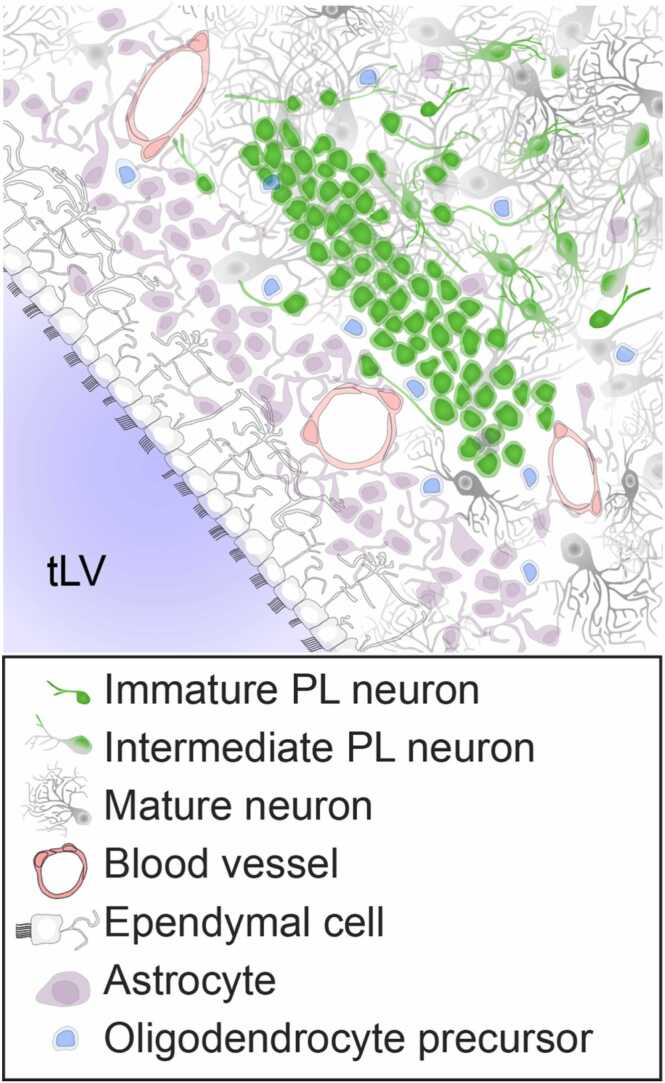
Fig. 2.
Cell types in the PL and local environment. A cluster of immature PL neurons is depicted adjacent to the dorsolateral wall of the temporal lobe lateral ventricle, at an anterior-posterior level corresponding to approximately (iv) in Fig. 1. The arrangement of immature PL neurons and the prevalence of other cell types varies depending on anatomical location. The type of dense cluster depicted here is consistent with those present in the LPL and is representative of what might be observed during puberty. Neurons of intermediate maturity (co-expressing DCX, PSA-NCAM, and NEUN) are localized facing the basal or lateral amygdala (top right of field of view). tLV: temporal lobe lateral ventricle.
2.3. Developmental trajectory of the PL and neighboring amygdala subnuclei
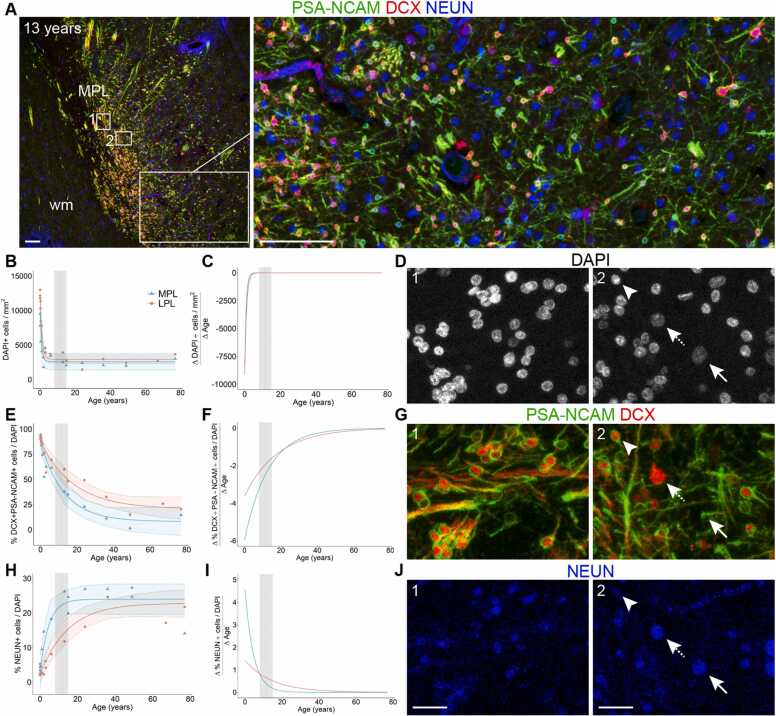
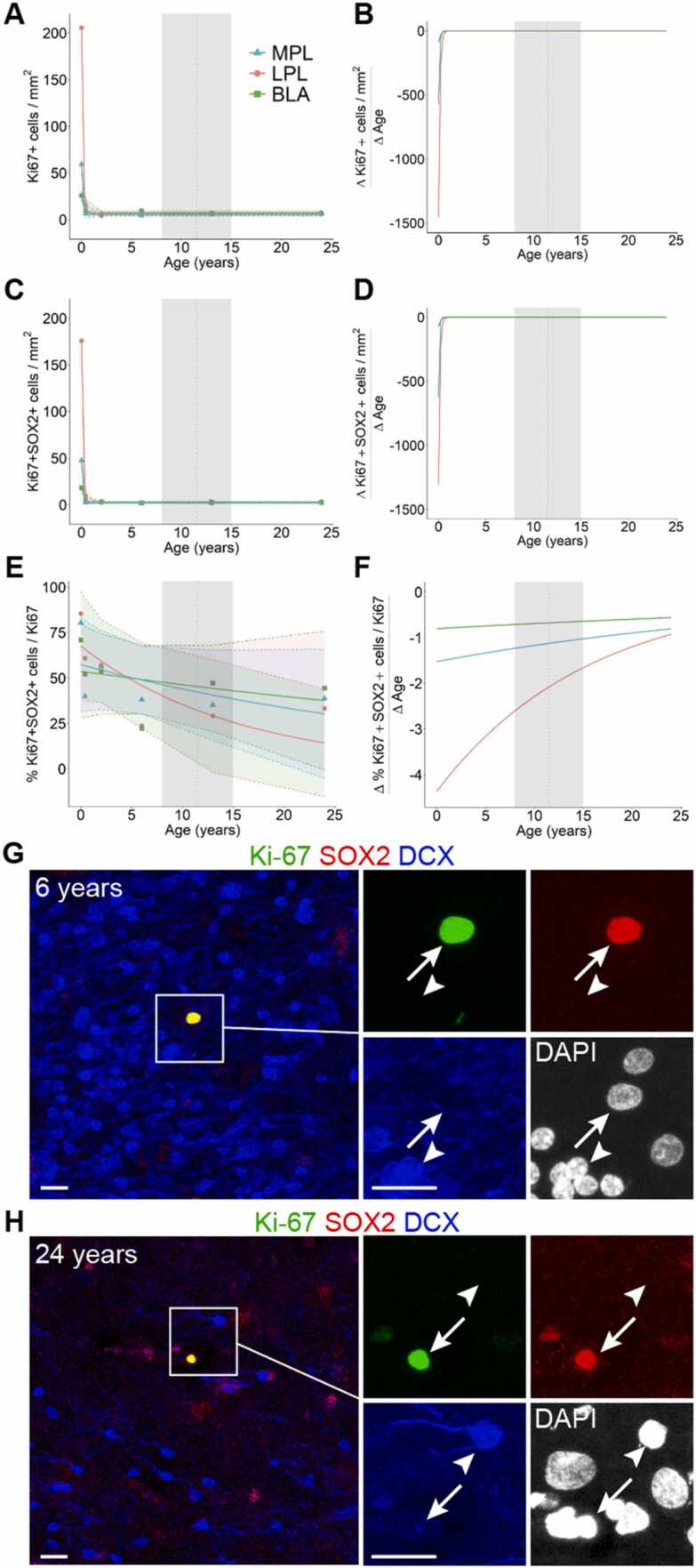
Investigations following the developmental trajectory of the PL across lifespan have used both postmortem histology and in vivo neuroimaging. In postmortem brain samples, changes in PL composition can be observed by quantifying small, immature (DCX+PSA-NCAM+) and large, mature (NEUN+, DCX–PSA-NCAM–) cells in the region across ages (Sorrells et al., 2019) (Fig. 3A,D,G,J). Quantifications of these cells in the MPL and LPL between birth and 77 years were fitted with nonlinear exponential one-phase decay curves (data from Sorrells et al., 2019). We plotted the differential of these curves to determine the rate of change for the cellular composition over age (pooled between sexes)(Fig. 3C,F,I). The overall cellular density decreases sharply between birth and 2 years of age and remains relatively steady through adulthood, as assessed by nuclear staining for 4′,6-diamidino-2-phenylindole (DAPI) (Fig. 3B,C). In contrast, the fraction of immature (DCX+PSA-NCAM+) neurons as a percentage of total cells (DAPI+ nuclei) changes more gradually across the span of childhood and adolescence. The decrease in the percentage of immature neurons is most rapid after birth and begins to slow around puberty (~8–15 years of age). The percentage of immature neurons in the MPL declines more rapidly than the LPL, indicating earlier maturation (Fig. 3E,F). The percentage of mature (NEUN+) neurons follow a similar trajectory, with more rapid increases earlier in life compared to later in development, particularly for the MPL, which begins to stabilize during puberty (Fig. 3H,I). Stereological estimates of neuron types across postnatal ages support these observations. In humans between 2 and 48 years of age, the number of Nissl-stained neurons with mature morphology increases from about 2.5–3.5 million in the BA (including the PL). Across these same ages, the number of BCL-2+ neurons in the PL decreased from about 3.5 million to less than 1 million (Avino et al., 2018). In macaques, the total number of neurons (both mature and immature) does not change in the amygdala with age (birth to 9 years) (Chareyron et al., 2012). Instead, the ratio of immature neurons to mature neurons decreased with age in the PL, supporting the idea of delayed neuronal development.
Fig. 3.
Age related changes in PL cell types. A, 13 year old human amygdala medial paralaminar region immunostained for DCX, PSA-NCAM, and NEUN. (B–D), DAPI+ nuclei in the MPL and LPL quantified from birth to 77 years, plotted as a density with 95% confidence intervals (B), as a rate of change (C), and example histology at 13 years (D) from insets 1 and 2 in panel (A). (E–G) DCX+PSA-NCAM+ cells quantified from birth to 77 years, plotted as a percentage of DAPI+ cells with 95% confidence intervals (E), as a rate of change (F), and example histology at 13 years (G) from insets 1 and 2 in panel (A). (H–J) NEUN+ cells quantified from birth to 77 years, plotted as a percentage of DAPI+ cells with 95% confidence intervals (H), as a rate of change (I), and example histology at 13 years (J) from insets 1 and 2 in panel (A). The shaded region of the graphs represent the age range of puberty across girls and boys (8–15 years, mean age of 11.5). Example of a mature NEUN+ cell that does not express DCX or PSA-NCAM (arrow) and a putative intermediate maturity cell that is DCX+ and NEUN+ (dashed arrow), and a small immature neuron that is DCX+PSA-NCAM+ (arrowhead). Scale bars: 100 µm (A); 20 µm (D,G,J). Data re-analyzed from Sorrells et al., 2019.
The expression of immature markers like DCX could be influenced by several factors. The age-related decline of DCX+ neurons in the PL suggests that its expression is downregulated with maturity. However, it is unknown how long it takes an individual PL neuron to mature and if it is longer than a typical timeframe for a neuron. In primates, a developing neuron expresses DCX for as long as 6 months (compared to ~2–3 weeks in rats), though final stages of maturation continue after DCX is turned off (Snyder, 2019, Kohler et al., 2011, Brown et al., 2003). An immature neuron in the PL may remain in stasis until it matures over the course of 6 or more months beginning in childhood, adolescence, or adulthood. Alternatively, its development and the duration of DCX expression may be protracted well beyond 6 months, possibly over the span of years. The decrease in DCX+ cells in the PL over age could also result from these cells dying. It is not known to what extent programmed cell death occurs in the PL across the lifespan. Programmed cell death in the PL has been hypothesized to occur based on stereological estimates of immature and mature neuron numbers between infancy and adulthood in non-human primates (Chareyron et al., 2021); however, direct measurements of apoptosis in late-maturing PL neurons have not been conducted. Late-maturing neurons in the human and non-human primate PL express the anti-apoptotic protein BCL-2 (Avino, 2018, Sorrells et al., 2019), which might be predicted to prevent or limit the extent of cell death (Ceizar, 2016, Farlie et al., 1995). The number of immature neurons in the PL may also decrease with age if there is a subpopulation of immature neurons migrating from the PL, a possibility we will discuss in Section 3.2.
As the resolution of magnetic resonance imaging (MRI) has increased, segmentation algorithms have been better able to distinguish the PL from the BA and LA (Saygin et al., 2017). This has not yet been used to follow volumetric changes in the PL across age; however, the above described changes in the density and cellular composition of the PL during development may make it difficult to consistently define this region across different ages. A recent cross-sectional MRI study of 10–17 year old girls and boys used an MRI segmentation approach that identifies the ventral division of the basolateral nucleus (BLVPL), which encompasses the PL and closely corresponds with the basolateral ventromedial (BLVM) amygdala in Fig. 1 (Campbell et al., 2021; Tyszka and Pauli, 2016). This study identified sex-specific changes in the volume of the BLVPL. The volume of the BLVPL decreased in boys, but remained unchanged in girls, a change that was more strongly associated with chronological age than pubertal stage (Campbell et al., 2021). The same segmentation of BLVPL also shows that the density of neurites (axons and dendrites) increases in this region between 8 and 22 years of age in both boys and girls (Azad et al., 2021). It is presently unknown how decreased volume alongside increased density of neuropil relate to the changes in cellular density and neuronal maturation in the PL, and the implications of these findings for sex-specific development of the PL.
Histological studies of the human PL so far have not had sufficient sample sizes of males and females to investigate sex differences in the PL, but the observed variability in neuron numbers between individuals suggests that multiple factors including sex could be relevant to its maturation. There is some evidence that sex hormone receptors are expressed in the PL. In adult humans, estrogen receptor alpha (ERɑ) is lowly expressed in the PL, BA, and LA, but highly expressed in dorsomedial areas closer to the cortex, including the AB (Österlund et al., 2002, Perlman et al., 2004). ERβ shows the opposite pattern with high expression in the PL, BA, and LA, and low expression in the dorsomedial parts of the amygdala (Österlund et al., 2000). In contrast, androgen receptor (AR) expression in and around the PL in adult male cynomolgus monkeys is low, as is expression of cytochrome P450 aromatase, the enzyme responsible for estrogen biosynthesis from testosterone (Roselli et al., 2001). It is currently unknown whether the late-maturing neurons in the PL express sex hormone receptors, and if so, at what stage of maturation and/or chronological age. We will discuss in Section 3 some ways in which sex hormones such as estrogens and androgens may influence developmental processes in the PL.
3. Developmental processes in the PL
It is evident that PL neuron maturation is ongoing throughout childhood and adolescence (Avino, 2018, Sorrells et al., 2019), however, the dynamics of neuron birth, migration, and circuit integration remain to be characterized. In this section, we will consider the evidence about when neurogenesis occurs for PL neurons and whether immature neurons migrate from the PL into neighboring nuclei throughout life. We will also discuss evidence for possible activity-dependent influences on the PL that come from the surrounding postnatal environment in which it matures, which changes with age.
3.1. Is PL neurogenesis ongoing postnatally?
In the human PL at birth, large groups of immature neurons are already present. This suggests that many of the immature neurons observed at older ages were likely born during gestation, though it has been hypothesized that at least some may come from ongoing postnatal neurogenesis (Bernier et al., 2002). Evidence for the gestational formation of the PL comes from observations at 22 gestational weeks (GW) identifying cells expressing molecular markers of PL neurons in the region where the PL will form. The PL forms immediately adjacent to the anterior tip of the large germinal zone for cortical inhibitory interneurons, the caudal ganglionic eminence (CGE). CGE progenitor cells and immature neurons express the transcription factors COUPTFII, SP8, and PROX1. Neurons in the PL also express COUPTFII but are SP8– and PROX1–, indicating that the PL is distinct from the CGE. At 22 GW, the COUPTFII+ cells in this region express TBR1 (Sorrells et al., 2019), a marker of cortical excitatory neurons (Hevner et al., 2001). Cells expressing COUPTFII but not SP8 or PROX1 are found in the region where the PL forms at 22 GW, birth, and also at postnatal ages (Sorrells et al., 2019). Together these observations indicate that cells with the postnatal molecular identity of PL excitatory neurons are present by mid-gestational ages. However, the continued presence after birth of a large population of immature neurons in the PL, and the concurrent postnatal increases in mature neuron number in amygdala subnuclei, present the possibility that new neurons could continue to be generated in this region late in embryogenesis or postnatally.
There is some evidence that embryonic neurogenesis may continue in the PL up until birth, although it is not definitive. At birth, the MPL and LPL both contain a high density of SOX2+ cells (Sorrells et al., 2019), a transcription factor that is expressed in neural progenitor cells (Graham et al., 2003). Quantifications of cells in the cell cycle (Ki-67+) and Ki-67+SOX2+ cells in the MPL, LPL, and BLA between birth and 24 years were fitted with nonlinear exponential one-phase decay curves (Fig. 4, data from Sorrells et al., 2019). We plotted the differential of these curves to determine the rate of change for the cellular composition across these ages (pooled between sexes). At birth the PL contains a higher density of Ki-67+ (Fig. 4A) and Ki-67+SOX2+ (Fig. 4C) cells compared to the rest of the BLA, as well as Ki-67+ cells co-expressing BLBP or vimentin, additional markers of neural progenitor cells (Sorrells et al., 2019). Many of these cells were observed closely opposed to immature neurons (Sorrells et al., 2019), suggesting that it is possible that they are undergoing neurogenic divisions still at birth in humans. However, the number of these Ki-67+ cells (Fig. 4A-B) and Ki-67+SOX2+ (Fig. 4C-F) cells declined rapidly in the first year of infancy (Sorrells et al., 2019).
Fig. 4.
Age related changes in PL cells in the cell cycle. A,B, Ki-67+ cells quantified from birth to 24 years in the MPL, LPL, and BLA, plotted as a density (A), as a rate of change (B). C,D, Ki-67+SOX2+ cells quantified from birth to 24 years in the MPL, LPL, and BLA, plotted as a density (C), as a rate of change (D). E,F, The percentage of Ki-67+ cells also expressing SOX2 between birth and 24 years in the MPL, LPL, and BLA, plotted as a percent (E), as a rate of change (F). The shaded region of the graphs represent the age range of puberty across girls and boys (8–15 years, mean age of 11.5). G, 6 year old and H, 24 year old human amygdala medial paralaminar region immunostained for Ki-67, SOX2, and DCX. Insets show single channels of Ki-67+SOX2+ cells (arrow) near DCX+ immature neurons (arrowhead). Scale bars: 20 µm. Data re-analyzed from Sorrells et al., 2019.
Evidence of postnatal or adult neurogenesis in the primate PL is more limited. In the human PL at 6 years (Fig. 4 G) and 24 years (Fig. 4H), Ki-67+SOX2+ cells are present adjacent to immature DCX+ neurons (Sorrells et al., 2019). However, these cells are not directly in contact with each other, as might be expected if the immature DCX+ cells were recently derived from cell division. At these ages it is more likely that the Ki-67+SOX2+ cells in the PL are dividing to produce glial cells (Mercurio et al., 2019), as their prevalence is similar to the rest of the BLA where DCX+ cells are not observed. The presence of Ki-67+ cells co-labeled with other neural progenitor markers like vimentin or BLBP is rare at ages older than birth (Sorrells et al., 2019), further supporting the conclusion that these division events may not be neurogenic in the postnatal and adult human PL. In the macaque amygdala, the total number of neurons stays consistent between birth and adulthood whereas the ratio of mature to immature neurons increases in the PL, arguing that even if new neurons are being born postnatally in this region, these events are rare (Chareyron et al., 2012).
Several studies in non-human primates using 5-bromo-2-deoxyuridine (BrdU) to label newly-synthesized DNA found evidence of labeled neurons in the amygdala. In adult squirrel monkeys (3–6 years old) and cynomolgus monkeys (6–12 years old), BrdU was observed co-localizing with the neuronal markers NEUN, TUJ1, or MAP-2, as well as BCL-2 (Bernier et al., 2002). In 4 year old marmosets, there are dense DCX+ cells in the PL that disperse into the basal nuclei, and these regions contain a few BrdU+DCX+ cells (Marlatt et al., 2011). While these results could indicate the birth of new neurons in the adult primate amygdala, further characterization is needed to validate if these BrdU+ cells are newly-born neurons or are other cell types in the PL. BrdU administration is a powerful technique to experimentally label DNA at a fixed time-point; however, several follow-up questions remain to be addressed: (1) Is the BrdU labeling in these samples due to neural progenitor cell division or DNA repair? BrdU can also be incorporated into a cell during DNA repair, not only during cell division (Breunig et al., 2007, Sorrells et al., 2018, Sorrells et al., 2021). To distinguish these possibilities, if the labeled neurons are the result of adult neurogenesis, BrdU would also be expected to label the neural progenitor cells (NPCs) producing these new neurons (i.e. with a short-chase). If these progenitors are present, they remain to be discovered and their identification would be an important piece of evidence of postnatal neurogenesis in this region. (2) Is the labeled DNA present in neurons, or could it be DNA from a neighboring cell? Satellite glial cells that incorporate BrdU during cell divisions are found closely attached to neurons and give the appearance of co-localized cells (Rakic, 2002). Orthogonal views of z-stacks are needed to reveal when the BrdU labeling is not within but on top of the neuron, however, the true localization of staining can be difficult to interpret without using a nuclear stain like DAPI to confirm that the BrdU signal is within the cell of interest (Takemura, 2005). (3) Are the BrdU+DCX+ cells indeed neurons? One possible explanation for BrdU labeling in DCX+ cells is that DCX is not a reliable marker for immature neurons. DCX is also expressed in oligodendrocytes (Boulanger and Messier, 2017, Ehninger et al., 2011, Franjic et al., 2022, Paredes et al., 2018, Takemura, 2005) and microglia (Boulanger and Messier, 2017, Franjic et al., 2022, Sorrells et al., 2018), which are known to divide postnatally. DCX has also been observed to be expressed in interneurons (Franjic et al., 2022, Xiong, 2008) or re-expressed in mature neurons (Ohira et al., 2019). Therefore, DCX co-labeling with BrdU does not necessarily indicate neurogenesis, particularly in the absence of a neuron-specific marker.
An exciting alternative approach to assessing neurogenesis takes advantage of the changing biospheric levels of radioactive 14C across lifespan. As biospheric carbon is incorporated into DNA during cell division, the detection of altered 14C levels is an indirect way to estimate cell genesis. Using this approach, a recent study evaluated NEUN-sorted nuclei from the amygdala of 6 adult humans and concluded that new neurons are generated in the amygdala at a rate of > 2.7% each year (Roeder et al., 2022), similar to what was previously estimated in the hippocampus using 14C (Spalding et al., 2013). The high rates of neurogenesis estimated with this technique are not supported by histological evidence, which consistently shows far fewer cells in the cell cycle in the adult human amygdala (Sorrells et al., 2019), hippocampus (Sorrells et al., 2018, Sorrells et al., 2021), or caudate (Ernst, 2014, Wang, 2014). Similar to BrdU, 14C could also be incorporated into NEUN-sorted nuclei from processes independent of cell division like DNA repair or methylation/de-methylation. The use of 14C to estimate rates of neurogenesis, while potentially powerful, is indirect and remains to be validated in animal models. Thus far, the data in humans and non-human primates point to the immature neurons in the PL being generated during normal embryonic stages and undergoing delayed maturation rather than new neuron generation. Together these results indicate that, although it is possible that a few neurons in the PL are postnatally or adult-born, this process is rare in primates.
Much of what we know about the function of sex hormones on adult birth, survival, growth, and integration of neurons comes from studies of adult neurogenesis in rodents; therefore in the PL we would predict that sex hormones have similar effects on the post-mitotic phases of neuron maturation. Sex hormones facilitate the survival of newly-born neurons in the rodent dentate gyrus (Mahmoud et al., 2016). Androgens such as testosterone and dihydrotestosterone enhance the survival of immature hippocampal neurons in adult male rodents (Duarte-Guterman, 2019, Hamson et al., 2013, Spritzer and Galea, 2007), and estrogens have similar effects in adult female rodents (Barha et al., 2009, Mazzucco et al., 2006). Estrogens and androgens also promote morphological maturation of adult-born granule cell neurons, evidenced by increased dendritic complexity and spine density (Hatanaka et al., 2015, Kight and McCarthy, 2020, Sheppard et al., 2019). In the rodent medial amygdala, androgens increase the number of excitatory synapses during puberty (Cooke and Woolley, 2009, Cooke, 2011). Androgens during puberty also impact process complexity of astrocytes in the medial amygdala (Johnson et al., 2013, Johnson et al., 2012), which could have effects on how astrocytes influence axon guidance and synapse formation (Allen and Lyons, 2018, Farhy-Tselnicker and Allen, 2018). Sex hormones in primates may similarly promote PL neuron maturation through direct effects on immature neurons and/or indirect effects via glial cells.
Low or non-existent levels of neurogenesis in the primate PL after birth is in agreement with the hypothesis that postnatal neurogenesis is rare in long-lived species with large brains. Ongoing neurogenesis may be limited in these species due to the biological cost of maintaining pools of progenitor cells and the difficulty that new neurons would face when trying to traverse vast distances to their final destinations while navigating through a complex postnatal environment full of vasculature and neuropil (Palazzo et al., 2018, Paredes et al., 2016b). Instead of postnatal neurogenesis, delayed neuron maturation may be more common in these brains, with immature neurons that mature in place and/or migrate short distances to integrate with established circuits.
3.2. Do immature PL neurons migrate into adjacent amygdala subnuclei?
The large pool of immature neurons in the PL is a reservoir of cells that could be recruited locally within the PL as well as into nearby amygdala regions via migration. If a subset of these excitatory neurons do indeed migrate within the human and non-human primate amygdala, it would be quite remarkable given that neuronal migration primarily happens during gestation and shortly after birth (Sanai et al., 2011, Paredes et al., 2016a). After infancy, immature neurons face a multitude of challenges to migration including large distances to traverse as the brain grows in size, and structural obstacles such as white matter tracts. During postnatal life, substrates and guidance factors for migration are also depleted (Paredes et al., 2016b). Cortical excitatory neurons migrate along radial glial fibers during embryonic development, but these processes are progressively dismantled during postnatal ages. These limitations would be expected to also impact the ability of immature excitatory neurons in the PL to migrate into neighboring regions; however, there is some evidence in humans that this may happen.
Evidence of the possibility of continued postnatal migration in the human PL includes the observation of DCX+PSA-NCAM+ cells with putative morphological features of migratory neurons, such as a single elongated (sometimes bifurcated) leading process, limited amounts of cytosol, and an elongated nucleus (Sorrells et al., 2019). These cells are in contrast to presumed non-migratory cells which also express DCX and PSA-NCAM, but have multiple processes and small, rounded nuclei. In addition to individual cells with migratory morphologies, some DCX+PSA-NCAM+ cells in the human and non-human primate amygdala appear organized in chains (Avino, 2018, Martí-Mengual et al., 2013, Zhang, 2009), possibly suggesting chain migration that occurs in areas like the subventricular zone and rostral migratory stream (García-Verdugo et al., 1998). However, the somas of the DCX+ cells in these chains frequently have rounded morphologies, making it unclear whether the chains actually represent migratory structures or have some other significance. The overall number of migratory neurons in the PL decreases rapidly between birth and 2 years of age, though some are still noticeably present in adults 24–77 years of age (Sorrells et al., 2019). Some of the neurons with migratory morphology in the PL may be roaming around locally. Others have leading processes that are oriented dorsally towards other regions of the amygdala, suggesting that migration from the PL could contribute to the developmental growth of adjacent amygdala subnuclei (Avino, 2018, deCampo and Fudge, 2012, Zhang, 2009).
Further evidence of postnatal migration in the PL comes from comparing the anatomical location of neurons with migratory morphology and stereological estimates of mature neuron number changes in amygdala subnuclei. The majority of neurons with migratory morphology are observed in the MPL (Sorrells et al., 2019), which is ventrally adjacent to the BA and AB. Between 2 and 48 years of age, the number of mature neurons increases from about 2.5–3.5 million in the BA (including the PL), and from about 1.1–1.3 million in the AB (Avino et al., 2018). There are also migratory neurons around LPL clusters, though fewer than in the MPL (Sorrells et al., 2019). White matter fibers surrounding some LPL clusters may be a physical barrier limiting neuron migration into the lateral nucleus. Additionally, the LPL clusters are smaller than the MPL in terms of cell number and volume, so its relative contribution to adjacent nuclei such as the LA may also be smaller. Consistent with these predictions, the number of mature neurons in the LA only increases by ~100,000 between 2 and 48 years and the central nucleus, which is far-removed from the MPL or LPL, does not increase with age (Avino et al., 2018). Many of these nuclei, including the BA, AB, and LA, are greatly expanded in volume (proportional to brain size) in non-human primates and humans compared to rodents (Chareyron et al., 2011), suggesting that extended developmental processes may be ongoing in these regions of the primate amygdala.
Neurons in the human PL display a maturation gradient with more NEUN+ mature neurons (large morphological complexity, fewer DCX+ cells) on the dorsolateral side facing the BLA. This difference is most notable in the MPL, but can also be observed in some LPL clusters. Cells that are farther from the ventral boundary of the MPL are also much less densely packed, and give the appearance of diffusing into the basal and lateral nuclei (Fig. 3A) (Sorrells et al., 2019). Neurons with migratory morphology are found within this anatomical gradient of maturity, which could indicate that late-developing MPL neurons disperse into the basal amygdala where they then finish their maturation. In adult macaque PL, the immature neurons (BCL-2+ cells) also display an anatomical distribution where those with immature morphologies are most densely located closest to the white matter alongside the MPL lamina but become morphologically more mature along the ventral-to-dorsal gradient into the basal nucleus (Fudge, 2004). This distribution could suggest that immature neurons are migrating ventral-to-dorsal from the MPL into the basal amygdala, where they then continue to mature, but it is also possible that the neurons remain in place and the dorsal ones mature earlier than the ventral ones, generating the appearance of a gradient.
Molecular markers expressed in late-maturing neurons provide further evidence for the possibility of migration. DCX and PSA-NCAM play a role in neuronal migration mechanisms (Bai, 2008, Ulfig and Chan, 2004). DCX destabilizes microtubules, permitting polymerization and rearrangement necessary for migration and plasticity (Gleeson et al., 1999, Francis et al., 1999), while PSA-NCAM facilitates migration by limiting cell-cell adhesions (Bonfanti, 2006). Single cell RNA-sequencing analysis of the human PL also shows that the transcription factor SOX11 is expressed in the cluster of cells corresponding to late-maturing neurons (Sorrells et al., 2019). Like DCX and PSA-NCAM, SOX11 is important for neuronal migration. In the mouse cortex during prenatal and early postnatal development, high expression levels of Sox11 promote neuron migration, but when Sox11 is downregulated, migration stops and dendrite morphogenesis begins (Hoshiba et al., 2016). Similarly, in the forebrain of juvenile zebra finches, migratory neurons only begin to develop dendritic processes after they stop migrating (Scott et al., 2012). These neurons use somal translocation to migrate along an existing process, rather than following a path pre-defined by blood vessels or radial glia, and travel in a short-range “wandering” pattern. This type of relatively short-range local migration may be more widely utilized by large-brained species like humans and non-human primates where increased brain size and physical complexity present challenges for cells to travel long distances (Paredes et al., 2016b). If there are immature neurons traveling from the PL to nearby amygdala nuclei, they are relatively close to their final destinations and finish developing once they settle down, increasing the number of mature neurons in these nuclei.
Despite this evidence, it is possible that postnatal neuron migration is not actually occurring in the PL. Cells with inferred migratory morphology could instead be in the process of growing through a transitional stage that resembles some structural features of migratory neurons. Increases in the total numbers of neurons in neighboring nuclei might still occur, but as the result of displacement of PL neurons through non-migratory means like the growth of other nearby cells. This is consistent with increases in neurite density (including axons and dendrites) measured between 8 and 22 years old in the human BLVPL discussed in Section 3.2 (Azad et al., 2021). Additionally, the number of oligodendrocytes also increases throughout the amygdala in macaques, which may also account for volumetric increases in individual subnuclei (Chareyron et al., 2012). Importantly, these possibilities are not mutually exclusive, and all of these mechanisms may be ongoing in differing degrees across ages alongside neuron migration.
The possibility of migration in the PL raises multiple intriguing questions. For instance, is there a structural substrate such as vasculature or radial glia that these neurons use to migrate? Are the non-migratory immature neurons at various ages a unique subset that remain in place, or are they triggered to migrate later? Additionally, is there a role for sex hormones in PL neuron migration? In humans, there are sex differences in the volumetric changes of individual amygdala subnuclei, including the PL (Campbell et al., 2021). In rodents and birds, estrogen has been shown to be important for neuronal migration during embryogenesis. Mice lacking ERβ have fewer neurons in the embryonic cortex, without changes in rates of neurogenesis. Fewer cortical neurons were likely the result of increased cell death as well as impaired migration, as radial glia processes in the cortex were fragmented in the absence of ERβ (Wang et al., 2003). In addition to these effects on the substrates for migration, there is evidence that estrogens can directly affect cell migration. In vitro administration of estradiol-17β (E2) to slice cultures from the embryonic mouse hypothalamus rapidly increased the speed of neuronal movement (Knoll et al., 2007). Similarly, in slice cultures from the adult avian ventricular zone, where neurogenesis is ongoing, estrogen administration promoted migration within the culture (Williams et al., 1999). Regardless of the final destination of migrating PL neurons, the factors contributing to their choice to settle and begin to develop are unknown. In the next section we consider the possible role of activity in the maturation of these cells.
3.3. Does activity influence PL maturation?
Activity-dependent maturation is a feature of many developing circuits, established by pioneering work in the visual system (Wiesel, 1982). Activity into and within the PL may influence the developmental trajectory of late-maturing neurons. Interestingly, white matter tracts associated with the amygdala including the uncinate fasciculus and the cingulum show increased neurite density and mature connectivity patterns during puberty (Azad, 2021, Jalbrzikowski et al., 2017). If these axonal projections with protracted development make contacts within the PL, their delayed inputs could result in late maturation of activity-dependent aspects of PL neuron growth. Even if these inputs are not direct, their targeting of other regions that project to the PL could still influence activity in the PL. As discussed in Section 2.1, there is a white matter tract along the ventral border of the PL with fibers that appear to innervate the MPL and LPL islands (Sorrells et al., 2019). This white matter tract may include axons from the uncinate fasciculus, which increases in neurite density between 8 and 22 years of age (Azad et al., 2021). Other white matter tracts that connect with the amygdala also increased in neurite density during these ages, including the cingulum, the ventral amygdalofugal pathway, and the anterior commissure; these changes did not differ by sex (Azad et al., 2021). The cingulum in particular may be part of the white matter ventral to the PL at caudal levels (Jones et al., 2013). However, it is currently unknown whether these white matter tracts are specifically connected to the PL. Puberty likely influences white matter development in these amygdala-associated tracts. Diurnal rises in luteinizing hormone (LH) are one of the first endocrine changes associated with puberty, and higher levels of LH correlate with increased white matter density in 9 year old boys and girls, including in the anterior cingulum (Peper et al., 2008).
The PL receives inputs from parts of the hippocampus (Fudge et al., 2012), as we will discuss in more detail in Section 4.1. Amygdala connectivity with the hippocampus increases from childhood to adulthood in humans (Saygin et al., 2015), and there is some evidence that hippocampal inputs to PL neurons impact their maturation. Macaques with neonatal (12–16 days after birth) hippocampal lesions had more (nearly 1.5-fold) mature neurons in the PL when examined in adulthood (~9 years old) (Chareyron et al., 2016). A similar ~1.5x increase in mature neurons in the PL occurred with adult (~6–9 years old) lesions. Interestingly, the total number of immature PL neurons increased following the neonatal but not adult lesions, suggesting that the loss of hippocampal innervation could impact PL neuron maturation differentially in early development and in adulthood. The mechanisms of how hippocampal lesions affect the numbers of immature and mature neurons in the PL are unknown, but if they involve activity-dependent maturation, then these inputs must directly (or indirectly) inhibit maturation. Alternatively, the increased numbers of immature and mature neurons in the PL could result from lesion effects that limit neuron migration out of the PL, as opposed to changes in the rate of maturation (Chareyron et al., 2021, Chareyron et al., 2016). Hippocampal lesions are a large-scale and long-term manipulation where compensatory mechanisms have an opportunity to emerge that may facilitate neuronal maturation.
Further clues that activity could promote maturation of PL neurons comes from work in non-human primates that investigated the impact of maternal separation during infancy on gene expression specifically within the PL (deCampo, et al., 2017). In this study, infant macaques were maternally separated for 1 week or for 1 month, and expression changes were examined for an array of genes involved in neuron maturation processes, including fate commitment, migration, and neurite outgrowth. Interestingly, the only gene that showed significant expression changes was TBR1, which was reduced in both maternal separation conditions. TBR1, as previously described in Section 2.2, is expressed in late-maturing neurons in the human PL (Sorrells et al., 2019). This transcription factor may be a mechanistic link between activity and PL neuron maturation. In rodent studies, Tbr1 is upregulated by neuronal activation (Chuang et al., 2014) and is dose-dependently required for axon growth in the amygdala (Huang et al., 2014). Therefore, reduced TBR1 expression in the PL may suggest reduced activity-dependent neuronal maturation, though this has not been tested. Insight into activity-dependent factors influencing PL neuron development remains limited, but there may be additional clues from the piriform cortex and hippocampal dentate gyrus in rodents, two other regions with immature neurons that develop within established brain circuits.
As mentioned in the Introduction (Section 1), there are also late-maturing excitatory neurons in the rodent piriform cortex (Rubio et al., 2016, Seki and Arai, 1991). These neurons are born embryonically and retain immature markers like Dcx and Psa-Ncam in adults 3 months of age (Gómez-Climent et al., 2008, Rotheneichner et al., 2018, Rubio et al., 2016, Varea et al., 2009). After 3 months, most late-maturing piriform cortex neurons no longer express these markers and have mature electrophysiological properties such as less hyperexcitability, suggesting functional integration (Benedetti, 2020, Rotheneichner et al., 2018). Signaling through multiple neurotransmitter systems, including glutamate (Nacher et al., 2002), norepinephrine (Vadodaria et al., 2017), and dopamine (Coviello et al., 2020), is capable of modifying the expression of immature molecular signatures like Dcx and Psa-Ncam in these cells. Excitatory inputs from the olfactory bulb to the piriform cortex also impact the expression of these markers (Gómez-Climent et al., 2011, He et al., 2014). Additionally, enzymatic removal of polysialic acid (Psa) from Ncam in the piriform cortex of adult mice leads to a higher density of NeuN+ cells, increased numbers of dendritic spines and density of dendrites on Dcx+ cells, and more Dcx+ cells displaying an axon initial segment (Coviello et al., 2021). These findings suggest that the removal of Psa may create a more permissive environment for immature neurons to develop synaptic contacts, which may encourage their maturation. In experimental models where it is possible to confirm functional integration through lineage tracing and live cell slice physiology, there is potential to determine how circuit activity influences maturation in the rodent piriform cortex, mechanisms that may be similar in the primate PL.
Activity also plays a role in guiding the trajectory of immature neuron development in the rodent dentate gyrus, where excitatory granule cells are generated through adult neurogenesis. Here, neurons take about 3–4 weeks to mature after being born from a progenitor in the subgranular zone (Overstreet-Wadiche and Westbrook, 2006). Like in the PL and piriform cortex, maturation is evident as Dcx and Psa-Ncam expression decreases and NeuN expression increases, alongside progressively more complex dendritic morphology and mature electrophysiological properties (Overstreet-Wadiche and Westbrook, 2006). At stages when the adult-born neurons express Dcx and Psa-Ncam and have simple morphologies, they depolarize in response to GABA due to chloride gradients established by the ion transporter Nkcc1. This GABA-evoked depolarization is critical to their morphological and functional maturation: when Nkcc1 expression is attenuated in immature granule cells, they instead hyperpolarize in response to GABA and subsequently fail to develop complex dendritic arborizations (Ge et al., 2006). In contrast, GABA-A receptor agonism promotes dendritic arborization in the immature neurons (Ge et al., 2006). GABA-evoked depolarization in newly-born hippocampal neurons may facilitate maturation by “unsilencing” excitatory synapses on these cells. Immature granule cells express NMDA receptors (NMDA-R) on their dendritic processes but not AMPAs receptors (AMPA-R); these are referred to as “silent synapses” as they alone are not capable of transducing glutamatergic signals into membrane depolarization (Toni and Schinder, 2015). Concurrent GABA-mediated depolarization and NMDA-R activation by presynaptic glutamate release causes rapid incorporation of AMPA-Rs into the postsynaptic membrane (Chancey, 2013, Ge et al., 2007, Tozuka et al., 2005). The subsequent appearance of AMPA-R mediated excitatory postsynaptic currents (EPSCs) occurs in the absence of other maturational changes like increased morphological complexity, so it is likely that synapse unsilencing is a precipitating factor for maturation and functional integration. The process of granule cell maturation is also sensitive to external conditions. Mice exposed to an enriched environment have enhanced survival of newborn granule cells without changes in progenitor cell proliferation, as well as an increase in the percentage of immature neurons with AMPA-R EPSCs (Chancey et al., 2013). By contrast, chronic stress decreases survival and dendritic complexity (Chen et al., 2015). It is currently unknown whether late-maturing neurons in the primate PL utilize similar maturation mechanisms.
4. Evidence for the function of late-maturing PL neurons
The prominence of a large and complex array of immature cells in the human amygdala throughout life presents the compelling mystery of their functional significance. It is possible that these cells are merely a vestige of development with no functional purpose. In this situation, the immature neurons might have once been a part of a migratory route into the amygdala and surrounding cortical regions, but were left behind at the conclusion of gestation as migratory substrates and guidance cues diminish. If so, their functional significance would be expected to be minimal, and perhaps their loss through programmed cell death is an inevitable conclusion. Instead, the data seem to support that rather than being developmentally removed, they are specifically retained in an immature state, expressing factors like BCL-2 which may prevent programmed cell death. PL neuron maturation occurs at the right time and place to be critically important for developing amygdala circuits, yet the function of these cells and this region of the amygdala remains a mystery.
In general, functional maturation of amygdala circuitry between childhood and adulthood impacts social interactions and emotional processing. Puberty in particular is a time when the salience of social information is increased, facilitating new interests in romantic and sexual partnerships in adolescence (Scherf et al., 2013). For instance, blood-oxygen-level-dependent (BOLD) signals of amygdala reactivity to facial cues of both positive and negative valence are heightened during puberty and adolescence relative to childhood and adulthood (Hare et al., 2008, Pfeifer et al., 2011, Vijayakumar et al., 2019). Individuals in earlier stages of puberty also showed heightened reactivity to neutral faces compared with age-matched peers in later stages of puberty (Forbes et al., 2011), highlighting the transiently enhanced salience of social cues. Amygdala functional maturation also involves greater emotional regulation abilities (Frere et al., 2020). Adults perform better on a cognitive task requiring inhibitory control under high emotional arousal states than adolescents and show increased functional connectivity between the amygdala and regions of the frontal cortex including the anterior cingulate cortex, medial prefrontal cortex, and dorsolateral prefrontal cortex (Ravindranath et al., 2020). The amygdala and frontal cortex are connected by some of the white matter tracts that undergo protracted maturation, such as the uncinate fasciculus and the cingulum (Azad, 2021, Jalbrzikowski et al., 2017). Increased emotional control may involve age-related decreases in amygdala connectivity with other frontal regions, such as the ventromedial prefrontal cortex (Jalbrzikowski et al., 2017). Decreased amygdala reactivity to fearful faces in adults (21–40 years) compared to adolescents (9–17 years) also involves stronger hippocampal-amygdala connectivity, suggesting that this pathway is also important for mature emotional regulation and contextualization (Guyer et al., 2008).
Currently, no functional data specifically about late-maturing PL neurons exists, so it is unknown if or how these neurons impact these social and emotional processes that mature during puberty in the amygdala. Clues into the function of late-maturing PL neurons can be gained from their connectivity with other brain regions that have known functions. Late-maturing neurons may also be a mechanism for life-long structural plasticity in the brain (Benedetti and Couillard-Despres, 2022, König et al., 2016, La Rosa et al., 2019, La Rosa et al., 2020, La Rosa and Bonfanti, 2018). In this section we will discuss this hypothesis, including its implications for amygdala circuits and how it has been investigated in other systems where immature neurons are known to integrate into already established circuits, such as adult neurogenesis in the rodent hippocampus. Lastly, we will explore insights into the function of late-maturing PL neurons based on how they may be disrupted in neuropsychiatric disorders.
4.1. Input and output connectivity of the PL
Our current understanding of PL connectivity comes from stereotactic microinjections of retrograde or anterograde tracers in macaques, which reveal reciprocal connections between the PL and parts of the hippocampus, as well as between the PL and other amygdala subnuclei. The rostral hippocampus, specifically, the portion of CA1 that is in the uncus (referred to as CA1′) and the rostral prosubiculum, is a major source of inputs/afferents to the PL (Fudge et al., 2012). These regions, along with others comprising the anterior hippocampus, are active during recall of autobiographical memories and during imagination of fictitious events (reviewed by Zeidman and Maguire) (Zeidman and Maguire, 2016). Intriguingly, some of the anterograde-labeled projections from the anterior hippocampus were closely associated with PSA-NCAM+ neurons within the PL (Fudge et al., 2012), so it will be interesting to determine if or when these fibers form synapses with late-maturing PL neurons, and whether this occurs in different stages of PL neuron maturity.
The PL sends outputs/efferents to the entorhinal cortex (Insausti et al., 1987, Pitkänen et al., 2002). There are also reciprocal connections between the PL and the perirhinal and parahippocampal cortices (Stefanacci et al., 1996). Within the amygdala, the PL receives inputs from the LA (Pitkänen and Amaral, 1998) and sends outputs to the BA (Amaral and Insausti, 1992). The PL projections to the BA are glutamatergic and are also one of the main sources of inputs to this nucleus (Amaral and Insausti, 1992). The input and output connectivity patterns of the PL place it in a position to potentially integrate autobiographical memory (by the hippocampus) and emotional valence encoding (by the amygdala). The amygdala is important for valence encoding of emotional stimuli (Jin et al., 2015), and its connectivity with the hippocampus is highly relevant to mood state (Kirkby et al., 2018) and emotional memory (Hamann et al., 1999, McDonald and Mott, 2017). Altered connectivity between these two regions is also evident in neuropsychiatric conditions such as major depressive disorder (MDD) (Tang et al., 2018) and post-traumatic stress disorder (PTSD) (Sripada et al., 2012), or following adverse experiences like childhood neglect (Cheng et al., 2021). We will discuss a possible role for the PL in neuropsychiatric disorders in more depth in Section 4.3.
The PL may also receive inputs and/or send outputs via white matter tracts that connect the amygdala to other regions of the brain, such as the uncinate fasciculus or the cingulum. These white matter tracts connect the amygdala with the hippocampus and with areas of the frontal lobe (Jones et al., 2013, Colnat-Coulbois et al., 2010, Mori et al., 2017). Connectivity between the basal amygdala and the prosubiculum can also involve the parahippocampal cingulum (Bubb et al., 2018), which provides additional evidence that the cingulum may be involved in PL connectivity given that the rostral prosubiculum projects onto late-maturing neurons in the PL (Fudge et al., 2012). The parahippocampal cingulum in particular is involved in emotional processing and episodic memory, and structural abnormalities are observed in MDD, autism spectrum disorders (ASD), obsessive compulsive disorder (OCD), and schizophrenia (Bubb et al., 2018). The uncinate fasciculus also plays a role in anxiety (Modi et al., 2013), depression (Zhang et al., 2012), and emotional pattern separation (Granger et al., 2021), a function we will discuss in more detail in the following section (Section 4.2). PL connectivity with the hippocampus, other amygdala nuclei, and potentially other brain regions - possibly via the uncinate fasciculus and/or the cingulum - places this region as an important node in emotional circuits.
Our current understanding of PL connectivity comes from tracing studies in adult nonhuman primates and neuroimaging in adult humans. It remains to be identified at which ages and cellular maturation stages these connections are established. PL neurons in an immature state are present across the lifespan, and while many continue to mature during puberty, a sizable number (~20–25% of all cells in the region) are still present in this region in adults. Those that mature prior to adulthood would be predicted to contribute to developmental plasticity in PL circuits, like those identified between the hippocampus and amygdala. However, it is not known what function, if any, the immature neurons that are still observed in adults might confer, or whether they even remain capable of maturing. These could be neurons that never received maturation signals at earlier ages (e.g., input activity as discussed in Section 3.3). It is also possible that these immature cells participate in cell-cell signaling with neurons and glia in their environment, influencing PL circuits indirectly while remaining phenotypically immature. In the following section, we will discuss ways that the recruitment of immature neurons into established circuits can affect the plasticity of established circuits.
4.2. Circuit plasticity arising from the introduction of new neurons
Plasticity in the brain comes from changes in the number and/or strength of synaptic connections through the addition or deletion of dendritic spines or axonal boutons, rearrangement of dendritic branches, or inserting or removing AMPA receptors from a postsynaptic membrane (Holtmaat and Svoboda, 2009). These mechanisms are modifications to existing neurons throughout postnatal life, but plasticity can also come about by introducing entirely new neurons that have not previously been functionally wired into an established circuit. Postnatal neurogenesis (such as in the rodent hippocampal dentate gyrus) and delayed neuron maturation (such as in the primate PL) are both ways in which neurons can be added to and rearrange existing networks (La Rosa et al., 2019). The functions of adult neurogenesis and delayed neuron maturation in other species and brain regions may provide insight into similar plasticity functions performed by late-maturing neurons in the primate amygdala.
Adult hippocampal neurogenesis supports cognitive and emotional learning in rodents. New dentate gyrus neurons facilitate pattern separation, the ability to distinguish between two similar contexts. Adult mice with enhanced levels of hippocampal neurogenesis performed better in a contextual fear conditioning paradigm, in that they showed appropriate freezing behavior in the chamber where fear learning took place, but showed reduced freezing in a similar, but not identical, chamber (Sahay et al., 2011). These findings indicate that increased hippocampal neurogenesis was sufficient to enhance the mice’s ability to not over-generalize the cues in the fear learning paradigm to other contexts. This pattern separation ability has implications for anxiety-related disorders such as PTSD, where cognitive and emotional responses to a trigger (such as a loud explosive sound) are generalized from fearful contexts (like a war zone) to safe contexts (like a celebratory fireworks show) (Kheirbek et al., 2012). These implications are supported by research showing that increasing neurogenesis prevents mice from developing anxiety- and depression-like behaviors after repeated corticosterone administration to model chronic stress (Hill et al., 2015).
Pattern separation relies on sparse activation of neurons in the dentate gyrus so that a relatively few number of neurons encode the specific features of a context, avoiding representational overlap and generalization of contextual cues. Newly-born neurons are thought to support this sparse encoding in two ways, the evidence for which is reviewed by Anacker and Hen (2017). The first is that new, naive neurons can take on additional contextual information without interfering with existing representations already encoded by neurons in the dentate gyrus. This also allows for new memory traces to update past learning, a process known as cognitive flexibility. For instance, adult mice with increased neurogenesis are better able to remember the new location of an escape platform from a water maze when it was moved from its previously-learned location (Berdugo-Vega et al., 2021). The second way that neurogenesis supports sparse encoding is that at a microcircuit level, when newly-born neurons functionally mature and integrate, they preferentially excite inhibitory interneurons, which in turn inhibit pre-existing excitatory neurons. This enhanced inhibitory control facilitates pattern separation by limiting the number of neurons that activate in response to contextual cues (Anacker and Hen, 2017). This sparse activation is also important because neurons in the dentate gyrus primarily receive excitatory inputs, are excitatory themselves, and are densely packed together, so tightly-regulated inhibition is necessary to prevent nonspecific and runaway excitatory activity (Anacker and Hen, 2017).
Similar to the dentate gyrus, the PL contains excitatory neurons that are densely packed, especially along the ventral border of the MPL and within LPL islands. In addition to receiving excitatory inputs from the anterior hippocampus (Fudge et al., 2012), the PL is composed predominantly of excitatory neurons, as discussed in Section 2.2 (Sorrells et al., 2019). Late-maturing PL neurons could facilitate emotional flexibility similar to how neurogenesis in the rodent dentate gyrus neurons facilitates cognitive flexibility. Work in mice shows that discrete populations of neurons within the BLA encode cues of innate or learned negative vs. positive valence (Beyeler et al., 2018), and negative stimuli can be relearned as positive and vice versa (Zhang and Li, 2018). Interestingly, hippocampal-amygdala connectivity and the uncinate fasciculus are important for emotional pattern separation (Granger et al., 2021, Zheng, 2019). The possible involvement of the PL in connectivity between the hippocampus and the amygdala and emotional flexibility also raises the question of how late-maturing neurons in the PL may be relevant to neuropsychiatric disorders, especially those involving emotional dysregulation.
4.3. Late-maturing neurons in neurodevelopmental and neuropsychiatric conditions
There has been increasing interest in recent years about how PL neurons may be impacted in neuropsychiatric disorders. Changes to the PL in pathological contexts can provide clues as to the link between late-maturing neurons and the functions that are disrupted in those conditions. Roughly half of all lifetime mental health conditions start by mid-teenage years (Kessler et al., 2007), so developmental changes in childhood and puberty that precede these years may relate to disorder onset in adolescence. Altered amygdala structure and/or function is also a common feature across many neurodevelopmental and neuropsychiatric disorders (Schumann et al., 2011). We will discuss evidence for amygdala changes in a number of these pathological contexts, including early life adversity, autism spectrum disorders (ASD), and major depressive disorder (MDD), and how late-maturing PL neurons may also be affected in these contexts. Since PL neuron maturation begins in early life and is protracted through puberty and adolescence, events prior to puberty (such as early life adversity) could impact the developmental trajectory of the PL during puberty and impact its function into adolescence and adulthood.
Evidence that early life adversity may impact the maturation rate of neurons in the PL comes from non-human primate research. A key study (introduced in Section 3.3) examined expression changes of neuron maturation genes in the PL after infant maternal separation in macaques for 1 week or 1 month. Following both durations of maternal separation, TBR1 mRNA expression was reduced in the PL (deCampo et al., 2017). As observed in humans, TBR1 protein is frequently expressed in DCX+PSA-NCAM+ late-maturing PL neurons, as well as in some NEUN+ mature neurons in the PL (Sorrells et al., 2019). DCX and NCAM1 were also reduced, though not significantly (deCampo et al., 2017). It is still unknown whether these changes result in altered PL neuron maturation, but the correlation between early life stress and gene expression changes suggests that neuron maturation in this region may be impacted. Interestingly, reduced TBR1 expression in the PL was correlated with reduced time spent in social interaction behaviors at 3 months old, suggesting the possibility of a link between the PL and the development of social behavior (deCampo et al., 2017). In a different study, adult 4 year old marmosets did not show any changes in the number of DCX+ cells in the amygdala as a whole following 2 weeks of psychosocial stress (isolation and social defeat stress) (Marlatt et al., 2011). These results are consistent with the idea of developmental critical periods for stress effects (Bale and Neill Epperson, 2015), but species and methodology differences between these few studies point to the need for further investigation of the hypothesis that early life stress may have long-lasting influences on postnatal amygdala maturation. Altered structural development of the amygdala has been shown in a longitudinal human MRI study of youth who experience caregiving adversity during the first 3 years of life (VanTieghem et al., 2021). In this study, caregiving adversity was associated with a slower rate of volumetric increases between approximately 4 and 10 years of age, followed by lower overall amygdala volumes through early adulthood (~20 years old). These studies show that early life adversity impacts the developmental trajectory of the amygdala, possibly including altered PL neuron maturation.
The maturation of neurons in the PL may also be affected in autism spectrum disorders (ASD). As mentioned in Section 3.2, the total number of mature neurons in the amygdala (specifically the BA, including the PL, and the AB) increases from infancy to adulthood in neurotypical individuals; this does not occur in individuals with ASD, where the total number of mature neurons does not change or may even decrease (Avino et al., 2018). These differences could come from altered maturation rates of PL neurons. However, the number of immature (BCL-2+) neurons in the PL declines at a similar rate in the PL in neurotypical individuals compared to individuals with ASD. It is possible that the migratory capacity and/or survival of late-maturing neurons is impaired in ASD as opposed to their maturation. Clues as to the cellular developmental processes affected in ASD might be obtained from using single nuclei RNA-sequencing to examine genes in immature neurons that are differentially expressed between control and ASD brains (Sorrells et al., 2019). However, it is also important to consider influences of conditions that are often comorbid with ASD, which include MDD.
There is some evidence that immature neuron marker expression is altered in the amygdala in MDD. In adults with MDD, DCX and PSA-NCAM protein levels were increased in the BLA of depressed patients, specifically those who did not die by suicide (Maheu et al., 2013). It is unknown whether increased DCX and PSA-NCAM expression was specifically within late-maturing neurons in the PL, but if so, these findings could represent neuron development that is further delayed or stunted in MDD. If late-maturing neurons in the amygdala are indeed involved in emotional flexibility similar to how hippocampal neurogenesis is involved in cognitive flexibility in rodents, impaired maturation could hinder this function. Poor emotional flexibility is associated with depression (Wen and Yoon, 2019), and enhanced flexibility following cognitive behavior therapy (CBT) predicts a reduction in symptoms among patients with MDD (Yasinski et al., 2020).
Despite these findings, we currently do not understand the cause-effect relationships between altered PL maturation and neuropsychiatric disorders. If PL neuron maturation is indeed altered in MDD, for instance, does this contribute to depression, or does a depressed brain state affect maturation? Whether altered maturation is a cause or effect of different neuropsychiatric conditions, these data from early life adversity, ASD, and MDD suggest a possible functional role for late-developing PL neurons in behaviors that are disrupted in these contexts, such as social interactions and emotional processing.
5. Conclusion
Late-maturing neurons in the primate PL are well-positioned in time (protracted maturation) and space (within amygdala-hippocampal circuitry) to be important for social and emotional functions that begin to develop in childhood and are refined during puberty and into adolescence. Remarkably, developmental processes including neuron growth, survival, migration, and activity-dependent maturation may be ongoing to differing degrees in the PL during these ages (Fig. 5). Future research on late-maturing PL neurons has the important task of investigating: (1) To what extent, if any, are these different developmental processes occurring? (2) At what ages are they taking place? (3) What are the mechanisms that influence these processes, and therefore the unusual developmental trajectory of the PL? (4) What are the functional implications of PL maturation? In this article, we have summarized the current evidence available for answering these questions, and presented the many remaining gaps in our knowledge of this unique brain region and form of neurodevelopment.
-
(1)
To what extent, if any, are these different developmental processes occurring? Some developmental processes, such as neurogenesis, are likely rare past birth in the PL (Section 3.1). However, it is still a possibility that some of the late-maturing neurons are born at later ages. Identifying neural progenitor cells at different ages would help to answer this question. Intriguingly, most studies of the PL note the possibility that neuron migration is ongoing postnatally based on observations of neurons with migratory morphology and increases in the volume and number of mature neurons in amygdala subnuclei adjacent to the PL (Avino, 2018, Martí-Mengual et al., 2013, Sorrells et al., 2019, Zhang, 2009) (Section 3.2). Identification of their eventual destinations and migration substrates would help to clarify this process. There may also be activity-dependent influences on late-maturing neuron structural/functional integration (Section 3.3). Longitudinal imaging studies are needed to follow the development of these inputs and regions that send them including the hippocampus, other areas of the amygdala, and/or more distant brain regions.
-
(2)
At what ages are developmental processes in the PL taking place? Though it is evident that the maturation of the PL is ongoing from childhood to adulthood (Fig. 3), it is not known which developmental processes are taking place at what ages, and if the extent of these processes varies by age. For instance, is neuron migration more frequent or faster in early childhood? Are different inputs to the PL (i.e., from the hippocampus or other amygdala subnuclei), differentially active throughout the lifespan? Do the immature neurons that are still present in the adult PL ever mature?
-
(3)
What are the mechanisms that influence developmental processes in the PL, and therefore its unusual trajectory of maturation? The molecular and cellular mechanisms of PL neuron maturation remain mostly unknown, including whether these mechanisms vary across age. What signal(s) presence or absence prevents these cells from growing along a normal timeline? Glial cells and sex hormones are known to influence cell developmental processes such as neuron survival, migration, and synapse formation and may have an important role. Estrogen and androgen receptors are expressed in specific subregions of the amygdala including some within the PL (Österlund et al., 2000, Österlund et al., 2002, Perlman et al., 2004, Roselli et al., 2001), so these hormones are well-positioned to influence the growth of the PL especially during puberty when levels of these hormones rise. The development of the PL could also be influenced by changes in the psychosocial environment between childhood, puberty, and adolescence, such as early life adversity (deCampo et al., 2017). Late-maturing neurons may in turn play a functional role in social and emotional behaviors that shape this external environment across developmental ages.
-
(4)
What are the functional implications of PL maturation? Studies of unique forms of neurodevelopment in other species and brain regions, such as adult neurogenesis in the rodent hippocampus or late-maturing neurons in the piriform cortex, offer insights about the possible function of late-maturing PL neurons in humans and non-human primates. For instance, PL neurons are in a position to modulate plasticity in amygdala circuits and play a role in valence encoding and emotional pattern separation. Further investigation of the PL with known neuropsychiatric diagnoses will inform these hypotheses.
Fig. 5.
PL maturation processes. A, Depiction of the growth of the PL between birth and adult ages. At birth the PL clusters are very dense and primarily composed of immature neurons. By late childhood ages, many of these immature neurons remain, although some mature neurons are present. During teenage years and into adult ages, the development of the PL plateaus. B, At birth, the PL is composed of dense clusters of immature neurons. As the PL cells mature during postnatal life, multiple processes associated with their maturation may be ongoing. Although a higher proportion of Ki-67+SOX2+ cells are present in the PL compared to the rest of the BLA at birth, it is not known if these are indeed neural precursor cells (NPCs) and whether these cells persist into postnatal ages. Cells with migratory morphology are present in the PL across postnatal ages, but it is not known if these cells are truly migrating, if they are born directly from NPCs, or if the immature neurons in the region lacking migratory morphology are able to re-activate their ability to migrate. As the neurons in the PL mature, activity of inputs to these regions and these cells likely sculpts their maturation, but the mechanisms of this remain to be discovered. In the mature PL it is not known whether late-maturing neurons adopt a similar identity to neurons that developed on a normal timeline, or whether there are a variety of subtypes of neurons that mature late.
These open questions about the development and function of the PL are addressable by integrating observations from histology, in vivo MRI, and animal model systems. Histological investigations of the cellular environment within and around the human PL will reveal cellular developmental processes that are ongoing in this region across different ages. These histological studies will be powerfully augmented by in vivo MRI in humans and non-human primates, especially with frequent developmental sampling (i.e., every few months or years whenever possible). As MRI segmentation of the amygdala becomes increasingly refined and able to identify the PL (Saygin et al., 2017), we will be able to assess how the volume, activity, and connectivity of the PL changes longitudinally across age in living humans and how this developmental trajectory relates to behaviors and experiences. Within-subjects fMRI of the amygdala has enabled investigations into the connectivity patterns of multiple functional networks (Sylvester et al., 2020); when combined with subnuclei segmentation that includes the PL (Saygin et al., 2017), these types of investigations have the potential to test causal hypotheses about the function of this region. Ultimately, the development of animal model systems to study late-maturing neurons will permit direct tests of mechanistic cause-effect relationships. Cross-species comparisons of the PL across the mammalian evolutionary tree (Ai, 2021, Crosby and Humphrey, 1939, deCampo and Fudge, 2012, Luzzati et al., 2009, Piumatti et al., 2018, Sah et al., 2003), or the prominence of it relative to other amygdala subnuclei in species where it is present, could provide exciting new opportunities to investigate previously unanswerable questions about the ontogeny and function of this unique brain region and form of neurodevelopment.
Funding
This work was supported by the National Institute of Mental Health (NIMH), United States, Grant R21MH125367; a Competitive Medical Research Fund (CMRF) award from the University of Pittsburgh, United States; and start-up funds from the University of Pittsburgh, United States.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Dr. David Lewis for providing the human brain tissue used to generate the amygdala traces presented in Fig. 1.
References
- Ai J.-Q., et al. Doublecortin-expressing neurons in Chinese tree shrew forebrain exhibit mixed rodent and primate-like topographic characteristics. Front. Neuroanat. 2021;15 doi: 10.3389/fnana.2021.727883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen N.J., Lyons D.A. Glia as architects of central nervous system formation and function. Science. 2018;362:181–185. doi: 10.1126/science.aat0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D.G., Insausti R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Exp. Brain Res. 1992;88:375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- Anacker C., Hen R. Adult hippocampal neurogenesis and cognitive flexibility — linking memory and mood. Nat. Rev. Neurosci. 2017;18:335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avino T.A., et al. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc. Natl. Acad. Sci. 2018;115:3710–3715. doi: 10.1073/pnas.1801912115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad A., et al. Microstructural properties within the amygdala and affiliated white matter tracts across adolescence. Neuroimage. 2021;243 doi: 10.1016/j.neuroimage.2021.118489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., et al. The role of DCX and LIS1 in migration through the lateral cortical stream of developing forebrain. Dev. Neurosci. 2008;30:144–156. doi: 10.1159/000109859. [DOI] [PubMed] [Google Scholar]
- Bale T.L., Neill Epperson C. Sex differences and stress across the lifespan. Nat. Neurosci. 2015;vol. 18:1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha C.K., Lieblich S.E., Galea L.A.M. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J. Neuroendocr. 2009;21:155–166. doi: 10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- Benedetti B., et al. Functional integration of neuronal precursors in the adult murine piriform cortex. Cereb. Cortex. 2020;30:1499–1515. doi: 10.1093/cercor/bhz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti B., Couillard-Despres S. Why Would the Brain Need Dormant Neuronal Precursors? Frontiers in Neuroscience. 2022;16(877167) doi: 10.3389/fnins.2022.877167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdugo-Vega G., Lee C.-C., Garthe A., Kempermann G., Calegari F. Adult-born neurons promote cognitive flexibility by improving memory precision and indexing. Hippocampus. 2021;31:1068–1079. doi: 10.1002/hipo.23373. [DOI] [PubMed] [Google Scholar]
- Bernier P.J., Bedard A., Vinet J., Levesque M., Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc. Natl. Acad. Sci. USA. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A., et al. Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Rep. 2018;22:905–918. doi: 10.1016/j.celrep.2017.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog. Neurobiol. 2006;80:129–164. doi: 10.1016/j.pneurobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Boulanger J.J., Messier C. Doublecortin in oligodendrocyte precursor cells in the adult mouse brain. Front. Neurosci. 2017;11:143. doi: 10.3389/fnins.2017.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuronal types in the basolateral amygdaloid nuclei of man. Brain Res. Bull. 1983;11:349–365. doi: 10.1016/0361-9230(83)90171-5. [DOI] [PubMed] [Google Scholar]
- Breunig J.J., Arellano J.I., Macklis J.D., Rakic P. Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell. 2007;1:612–627. doi: 10.1016/j.stem.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Brown J.P., et al. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Bubb E.J., Metzler-Baddeley C., Aggleton J.P. The cingulum bundle: anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018;92:104–127. doi: 10.1016/j.neubiorev.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budday S., Steinmann P., Kuhl E. Physical biology of human brain development. Front. Cell. Neurosci. 2015;9 doi: 10.3389/fncel.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C.E., et al. Restructuring of amygdala subregion apportion across adolescence. Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2020.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron L.J., Banta Lavenex P., Amaral D.G., Lavenex P. Stereological analysis of the rat and monkey amygdala. J. Comp. Neurol. 2011;519:3218–3239. doi: 10.1002/cne.22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron L.J., Lavenex P.B., Amaral D.G., Lavenex P. Postnatal development of the amygdala: a stereological study in macaque monkeys. J. Comp. Neurol. 2012;520:1965–1984. doi: 10.1002/cne.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceizar M., et al. Bcl-2 is required for the survival of doublecortin-expressing immature neurons. Hippocampus. 2016;26:211–219. doi: 10.1002/hipo.22504. [DOI] [PubMed] [Google Scholar]
- Chancey J.H., et al. GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J. Neurosci. 2013;33:6614–6622. doi: 10.1523/JNEUROSCI.0781-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron L.J., Amaral D.G., Lavenex P. Selective lesion of the hippocampus increases the differentiation of immature neurons in the monkey amygdala. Proc. Natl. Acad. Sci. USA. 2016;113:14420–14425. doi: 10.1073/pnas.1604288113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron L.J., Banta Lavenex P., Amaral D.G., Lavenex P. Life and death of immature neurons in the juvenile and adult primate amygdala. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22136691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawana R., et al. The distribution of Ki-67 and doublecortin immunopositive cells in the brains of three microchiropteran species, Hipposideros fuliginosus, Triaenops persicus, and Asellia tridens. Anat. Rec. 2016;299:1548–1560. doi: 10.1002/ar.23460. [DOI] [PubMed] [Google Scholar]
- Chen C.-C., Huang C.-C., Hsu K.-S. Chronic social stress affects synaptic maturation of newly generated neurons in the adult mouse dentate gyrus. Int. J. Neuropsychopharmacol. 2015;19:yv097. doi: 10.1093/ijnp/pyv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T.W., et al. Characterizing the impact of adversity, abuse, and neglect on adolescent amygdala resting-state functional connectivity. Dev. Cogn. Neurosci. 2021;47 doi: 10.1016/j.dcn.2020.100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang H.-C., Huang T.-N., Hsueh Y.-P. Neuronal excitation upregulates Tbr1, a high-confidence risk gene of autism, mediating Grin2b expression in the adult brain. Front. Cell. Neurosci. 2014;8:280. doi: 10.3389/fncel.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnat-Coulbois S., et al. Tractography of the amygdala and hippocampus: anatomical study and application to selective amygdalohippocampectomy. J. Neurosurg. 2010;113:1135–1143. doi: 10.3171/2010.3.JNS091832. [DOI] [PubMed] [Google Scholar]
- Cooke B.M. Synaptic reorganisation of the medial amygdala during puberty. J. Neuroendocr. 2011;23:65–73. doi: 10.1111/j.1365-2826.2010.02075.x. [DOI] [PubMed] [Google Scholar]
- Cooke B.M., Woolley C.S. Effects of prepubertal gonadectomy on a male-typical behavior and excitatory synaptic transmission in the amygdala. Dev. Neurobiol. 2009;69:141–152. doi: 10.1002/dneu.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coviello S., et al. PSA depletion induces the differentiation of immature neurons in the piriform cortex of adult mice. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coviello S., et al. Phenotype and distribution of immature neurons in the human cerebral cortex layer II. Front. Neuroanat. 2022;16 doi: 10.3389/fnana.2022.851432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coviello S., Gramuntell Y., Castillo-Gomez E., Nacher J. Effects of dopamine on the immature neurons of the adult rat piriform cortex. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.574234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby E.C., Humphrey T. Studies of the vertebrate telencephalon. I. The nuclear configuration of the olfactory and accessory olfactory formations and of the nucleus olfactorius anterior of certain reptiles, birds, and mammals. J. Comp. Neurol. 1939;71:121–213. [Google Scholar]
- deCampo D.M., et al. Maternal deprivation alters expression of neural maturation gene tbr1 in the amygdala paralaminar nucleus in infant female macaques. Dev. Psychobiol. 2017;59:235–249. doi: 10.1002/dev.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCampo D.M., Fudge J.L. Where and what is the paralaminar nucleus? A review on a unique and frequently overlooked area of the primate amygdala. Neurosci. Biobehav. Rev. 2012;36:520–535. doi: 10.1016/j.neubiorev.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S.-L., et al. Comprehensive cellular-resolution atlas of the adult human brain. J. Comp. Neurol. 2017;525:407. doi: 10.1002/cne.24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Guterman P., et al. Androgens enhance adult hippocampal neurogenesis in males but not females in an age-dependent manner. Endocrinology. 2019;160:2128–2136. doi: 10.1210/en.2019-00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D., et al. Enriched environment and physical activity reduce microglia and influence the fate of NG2 cells in the amygdala of adult mice. Cell Tissue Res. 2011;345:69–86. doi: 10.1007/s00441-011-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A., et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Farhy-Tselnicker I., Allen N.J. Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev. 2018;13:7. doi: 10.1186/s13064-018-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlie P.G., Dringen R., Rees S.M., Kannourakis G., Bernard O. bcl-2 transgene expression can protect neurons against developmental and induced cell death. Proc. Natl. Acad. Sci. USA. 1995;92:4397–4401. doi: 10.1073/pnas.92.10.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasemore T.M., et al. The distribution of Ki-67 and doublecortin-immunopositive cells in the brains of three strepsirrhine primates: Galago demidoff, Perodicticus potto, and Lemur catta. Neuroscience. 2018;372:46–57. doi: 10.1016/j.neuroscience.2017.12.037. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Phillips M.L., Silk J.S., Ryan N.D., Dahl R.E. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev. Neuropsychol. 2011;36:429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis F., et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Franjic D., et al. Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron. 2022;110(452–469) doi: 10.1016/j.neuron.2021.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frere P.B., et al. Sex effects on structural maturation of the limbic system and outcomes on emotional regulation during adolescence. Neuroimage. 2020;210 doi: 10.1016/j.neuroimage.2019.116441. [DOI] [PubMed] [Google Scholar]
- Fudge J.L. Bcl-2 immunoreactive neurons are differentially distributed in subregions of the amygdala and hippocampus of the adult macaque. Neuroscience. 2004;127:539–556. doi: 10.1016/j.neuroscience.2004.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge J.L., deCampo D.M., Becoats K.T. Revisiting the hippocampal-amygdala pathway in primates: association with immature-appearing neurons. Neuroscience. 2012;212:104–119. doi: 10.1016/j.neuroscience.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Amado M., Prensa L. Stereological analysis of neuron, glial and endothelial cell numbers in the human amygdaloid complex. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Verdugo J.M., Doetsch F., Wichterle H., Lim D.A., Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J. Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Ge S. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S., Pradhan D.A., Ming G.-L., Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Ghibaudi M., Bonfanti L. How Widespread Are the “Young” Neurons of the Mammalian Brain? Frontiers in Neuroscience. 2022;16(918616) doi: 10.3389/fnins.2022.918616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson J.G., Lin P.T., Flanagan L.A., Walsh C.A. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Goddings A.-L., et al. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Climent M.A., et al. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb. Cortex. 2008;18:2229–2240. doi: 10.1093/cercor/bhm255. [DOI] [PubMed] [Google Scholar]
- Gómez-Climent M.Á. Olfactory bulbectomy, but not odor conditioned aversion, induces the differentiation of immature neurons in the adult rat piriform cortex. Neuroscience. 2011;181:18–27. doi: 10.1016/j.neuroscience.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Graham V., Khudyakov J., Ellis P., Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Granger S.J., et al. Integrity of the uncinate fasciculus is associated with emotional pattern separation-related fMRI signals in the hippocampal dentate and CA3. Neurobiol. Learn. Mem. 2021;177 doi: 10.1016/j.nlm.2020.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E. A developmental examination of amygdala response to facial expressions. J. Cogn. Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S.B., Ely T.D., Grafton S.T., Kilts C.D. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat. Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hamson D.K. Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology. 2013;154:3294–3304. doi: 10.1210/en.2013-1129. [DOI] [PubMed] [Google Scholar]
- Hare T.A. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka Y. Rapid increase of spines by dihydrotestosterone and testosterone in hippocampal neurons: dependence on synaptic androgen receptor and kinase networks. Brain Res. 2015;1621:121–132. doi: 10.1016/j.brainres.2014.12.011. [DOI] [PubMed] [Google Scholar]
- He X. Olfactory experience modulates immature neuron development in postnatal and adult guinea pig piriform cortex. Neuroscience. 2014;259:101–112. doi: 10.1016/j.neuroscience.2013.11.056. [DOI] [PubMed] [Google Scholar]
- Hevner R.F. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Hill A.S., Sahay A., Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology. 2015;40:2368–2378. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A., Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hoshiba Y., et al. Sox11 balances dendritic morphogenesis with neuronal migration in the developing cerebral cortex. J. Neurosci. 2016;36:5775–5784. doi: 10.1523/JNEUROSCI.3250-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.-N., et al. Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nat. Neurosci. 2014;17:240–247. doi: 10.1038/nn.3626. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Insausti R., Amaral D.G., Cowan W.M. The entorhinal cortex of the monkey: III. Subcortical afferents. J. Comp. Neurol. 1987;264:396–408. doi: 10.1002/cne.902640307. [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M., et al. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol. Psychiatry. 2017;82:511–521. doi: 10.1016/j.biopsych.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Zelano C., Gottfried J.A., Mohanty A. Human amygdala represents the complete spectrum of subjective valence. J. Neurosci. 2015;35:15145–15156. doi: 10.1523/JNEUROSCI.2450-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.T., Schneider A., DonCarlos L.L., Breedlove S.M., Jordan C.L. Astrocytes in the rat medial amygdala are responsive to adult androgens. J. Comp. Neurol. 2012;520:2531–2544. doi: 10.1002/cne.23061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.T., Breedlove S.M., Jordan C.L. Androgen receptors mediate masculinization of astrocytes in the rat posterodorsal medial amygdala during puberty. J. Comp. Neurol. 2013;521:2298–2309. doi: 10.1002/cne.23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K., Christiansen K.F., Chapman R.J., Aggleton J.P. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: implications for neuropsychological investigations. Neuropsychologia. 2013;51:67–78. doi: 10.1016/j.neuropsychologia.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Jessberger S., Steiner B., Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kessler R.C. Age of onset of mental disorders: a review of recent literature. Curr. Opin. Psychiatry. 2007;20:359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek M.A., Klemenhagen K.C., Sahay A., Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat. Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kight K.E., McCarthy M.M. Androgens and the developing hippocampus. Biol. Sex Differ. 2020;11:30. doi: 10.1186/s13293-020-00307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby L.A., et al. An amygdala-hippocampus subnetwork that encodes variation in human mood. Cell. 2018;175(1688–1700) doi: 10.1016/j.cell.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Klempin F., Kronenberg G., Cheung G., Kettenmann H., Kempermann G. Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS ONE. 2011;vol. 6 doi: 10.1371/journal.pone.0025760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll J.G., Wolfe C.A., Tobet S.A. Estrogen modulates neuronal movements within the developing preoptic area-anterior hypothalamus. Eur. J. Neurosci. 2007;26:1091–1099. doi: 10.1111/j.1460-9568.2007.05751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S.J., Williams N.I., Stanton G.B., Cameron J.L., Greenough W.T. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc. Natl. Acad. Sci. USA. 2011;108:10326–10331. doi: 10.1073/pnas.1017099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König R., et al. Distribution and fate of DCX/PSA-NCAM expressing cells in the adult mammalian cortex: a local reservoir for adult cortical neuroplasticity? Front. Biol. 2016;11:193–213. [Google Scholar]
- Kostović I., Sedmak G., Judaš M. Neural histology and neurogenesis of the human fetal and infant brain. Neuroimage. 2019;188:743–773. doi: 10.1016/j.neuroimage.2018.12.043. [DOI] [PubMed] [Google Scholar]
- La Rosa C., Bonfanti L. Brain plasticity in mammals: an example for the role of comparative medicine in the neurosciences. Front. Vet. Sci. 2018;5:274. doi: 10.3389/fvets.2018.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa C., Ghibaudi M., Bonfanti L. Newly generated and non-newly generated ‘immature’ neurons in the mammalian brain: a possible reservoir of young cells to prevent brain aging and disease? J. Clin. Med. 2019;8:685. doi: 10.3390/jcm8050685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa C., Parolisi R., Bonfanti L. Brain structural plasticity: from adult neurogenesis to immature neurons. Front. Neurosci. 2020;14:75. doi: 10.3389/fnins.2020.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.Y.W. Doublecortin-expressing cell types in temporal lobe epilepsy. Acta Neuropathol. Commun. 2018;6:60. doi: 10.1186/s40478-018-0566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati F., Bonfanti L., Fasolo A., Peretto P. DCX and PSA-NCAM expression identifies a population of neurons preferentially distributed in associative areas of different pallial derivatives and vertebrate species. Cereb. Cortex. 2009;19:1028–1041. doi: 10.1093/cercor/bhn145. [DOI] [PubMed] [Google Scholar]
- Maheu M.E., Davoli M.A., Turecki G., Mechawar N. Amygdalar expression of proteins associated with neuroplasticity in major depression and suicide. J. Psychiatr. Res. 2013;47:384–390. doi: 10.1016/j.jpsychires.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Mahmoud R., Wainwright S.R., Galea L.A.M. Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Front. Neuroendocrinol. 2016;41:129–152. doi: 10.1016/j.yfrne.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Marlatt M.W., et al. Distinct structural plasticity in the hippocampus and amygdala of the middle-aged common marmoset (Callithrix jacchus) Exp. Neurol. 2011;230:291–301. doi: 10.1016/j.expneurol.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Martí-Mengual U., Varea E., Crespo C., Blasco-Ibáñez J.M., Nacher J. Cells expressing markers of immature neurons in the amygdala of adult humans. Eur. J. Neurosci. 2013;37:10–22. doi: 10.1111/ejn.12016. [DOI] [PubMed] [Google Scholar]
- Mazzucco C.A., et al. Both estrogen receptor α and estrogen receptor β agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141:1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- McDonald A.J., Mott D.D. Functional neuroanatomy of amygdalohippocampal interconnections and their role in learning and memory. J. Neurosci. Res. 2017;95:797–820. doi: 10.1002/jnr.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio S., Serra L., Nicolis S.K. More than just stem cells: functional roles of the transcription factor Sox2 in differentiated glia and neurons. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20184540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi S., et al. Individual differences in trait anxiety are associated with white matter tract integrity in fornix and uncinate fasciculus: preliminary evidence from a DTI based tractography study. Behav. Brain Res. 2013;238:188–192. doi: 10.1016/j.bbr.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Mori S., et al. Elucidation of white matter tracts of the human amygdala by detailed comparison between high-resolution postmortem magnetic resonance imaging and histology. Front. Neuroanat. 2017;11:16. doi: 10.3389/fnana.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J., Alonso-Llosa G., Rosell D., McEwen B. PSA-NCAM expression in the piriform cortex of the adult rat. modulation by NMDA receptor antagonist administration. Brain Res. 2002;927:111–121. doi: 10.1016/s0006-8993(01)03241-3. [DOI] [PubMed] [Google Scholar]
- Ohira K., Hagihara H., Miwa M., Nakamura K., Miyakawa T. Fluoxetine-induced dematuration of hippocampal neurons and adult cortical neurogenesis in the common marmoset. Mol. Brain. 2019;12:69. doi: 10.1186/s13041-019-0489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østby Y., et al. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J. Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Österlund M.K., Grandien K., Keller E., Hurd Y.L. The human brain has distinct regional expression patterns of estrogen receptor α mRNA isoforms derived from alternative promoters. J. Neurochem. 2002;75:1390–1397. doi: 10.1046/j.1471-4159.2000.0751390.x. [DOI] [PubMed] [Google Scholar]
- Österlund M.K., Gustafsson J.A., Keller E., Hurd Y.L. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J. Clin. Endocrinol. Metab. 2000;85:3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche L.S., Westbrook G.L. Functional maturation of adult-generated granule cells. Hippocampus. 2006;16:208–215. doi: 10.1002/hipo.20152. [DOI] [PubMed] [Google Scholar]
- Palazzo O., La Rosa C., Piumatti M., Bonfanti L. Do large brains of long-living mammals prefer non-newly generated, immature neurons? Neural Regen. Res. 2018;13:633–634. doi: 10.4103/1673-5374.230282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes M.F. Extensive migration of young neurons into the infant human frontal lobe. Science. 2016;354 doi: 10.1126/science.aaf7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes M.F. Does adult neurogenesis persist in the human hippocampus? Cell Stem Cell. 2018;vol. 23:780–781. doi: 10.1016/j.stem.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes M.F., Sorrells S.F., Garcia-Verdugo J.M., Alvarez-Buylla A. Brain size and limits to adult neurogenesis. J. Comp. Neurol. 2016;524:646–664. doi: 10.1002/cne.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper J.S., et al. Cerebral white matter in early puberty is associated with luteinizing hormone concentrations. Psychoneuroendocrinology. 2008;33:909–915. doi: 10.1016/j.psyneuen.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Perlman W.R., Webster M.J., Kleinman J.E., Weickert C.S. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biol. Psychiatry. 2004;56:844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A., Amaral D.G. Organization of the intrinsic connections of the monkey amygdaloid complex: projections originating in the lateral nucleus. J. Comp. Neurol. 1998;398:431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pitkänen A., Kelly J.L., Amaral D.G. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the entorhinal cortex in the macaque monkey. Hippocampus. 2002;12:186–205. doi: 10.1002/hipo.1099. [DOI] [PubMed] [Google Scholar]
- Piumatti M. Non-newly generated, ‘immature’ neurons in the sheep brain are not restricted to cerebral cortex. J. Neurosci. 2018;38:826–842. doi: 10.1523/JNEUROSCI.1781-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G., Aimar P., Bonfanti L. Cellular composition and cytoarchitecture of the rabbit subventricular zone and its extensions in the forebrain. J. Comp. Neurol. 2006;498:491–507. doi: 10.1002/cne.21043. [DOI] [PubMed] [Google Scholar]
- Price J.L., Amaral D.G. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J. Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat. Rev. Neurosci. 2002;3:65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- Ravindranath O. Influences of affective context on amygdala functional connectivity during cognitive control from adolescence through adulthood. Dev. Cogn. Neurosci. 2020;45 doi: 10.1016/j.dcn.2020.100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybaud C., Ahmad T., Rastegar N., Shroff M., Al Nassar M. The premature brain: developmental and lesional anatomy. Neuroradiology. 2013;55(Suppl. 2):S23–S40. doi: 10.1007/s00234-013-1231-0. [DOI] [PubMed] [Google Scholar]
- Roeder S.S. Evidence for postnatal neurogenesis in the human amygdala. Commun. Biol. 2022;5:366. doi: 10.1038/s42003-022-03299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli C.E., Klosterman S., Resko J.A. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J. Comp. Neurol. 2001;439:208–223. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- Rotheneichner P., et al. Cellular plasticity in the adult murine piriform cortex: continuous maturation of dormant precursors into excitatory neurons. Cereb. Cortex. 2018;28:2610–2621. doi: 10.1093/cercor/bhy087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio A. Characterization and isolation of immature neurons of the adult mouse piriform cortex. Dev. Neurobiol. 2016;76:748–763. doi: 10.1002/dneu.22357. [DOI] [PubMed] [Google Scholar]
- Sah P., Faber E.S.L., de Armentia M.L., Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sahay A., et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N., et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanides F. Histological structure of the amygdaloid nucleus region. I. Cytology and involution of amygdaleum profundum. J. Hirnforsch. 1957;3:56–77. [PubMed] [Google Scholar]
- Saygin Z.M., et al. Structural connectivity of the developing human amygdala. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin Z.M., et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage. 2017;155:370–382. doi: 10.1016/j.neuroimage.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Smyth J.M., Delgado M.R. The amygdala: an agent of change in adolescent neural networks. Horm. Behav. 2013;64:298–313. doi: 10.1016/j.yhbeh.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann C.M., Bauman M.D., Amaral D.G. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49:745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B.B., Gardner T., Ji N., Fee M.S., Lois C. Wandering neuronal migration in the postnatal vertebrate forebrain. J. Neurosci. 2012;32:1436–1446. doi: 10.1523/JNEUROSCI.2145-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T., Arai Y. Expression of highly polysialylated NCAM in the neocortex and piriform cortex of the developing and the adult rat. Anat. Embryol. 1991;184:395–401. doi: 10.1007/BF00957900. [DOI] [PubMed] [Google Scholar]
- Sheppard P.A.S., Choleris E., Galea L.A.M. Structural plasticity of the hippocampus in response to estrogens in female rodents. Mol. Brain. 2019;12:22. doi: 10.1186/s13041-019-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims K.S., Williams R.S. The human amygdaloid complex: a cytologic and histochemical atlas using Nissl, myelin, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate diaphorase staining. Neuroscience. 1990;36:449–472. doi: 10.1016/0306-4522(90)90440-f. [DOI] [PubMed] [Google Scholar]
- Snyder J.S. Recalibrating the relevance of adult neurogenesis. Trends Neurosci. 2019;42:164–178. doi: 10.1016/j.tins.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Sorrells S.F. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S.F. Immature excitatory neurons develop during adolescence in the human amygdala. Nat. Commun. 2019;10:2748. doi: 10.1038/s41467-019-10765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S.F. Positive controls in adults and children support that very few, if any, new neurons are born in the adult human hippocampus. J. Neurosci. 2021;41:2554–2565. doi: 10.1523/JNEUROSCI.0676-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding K.L. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer M.D., Galea L.A.M. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev. Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Sripada R.K. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J. Psychiatry Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L., Suzuki W.A., Amaral D.G. Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. J. Comp. Neurol. 1996;375:552–582. doi: 10.1002/(SICI)1096-9861(19961125)375:4<552::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Sylvester C.M. Individual-specific functional connectivity of the amygdala: a substrate for precision psychiatry. Proc. Natl. Acad. Sci. USA. 2020;117:3808–3818. doi: 10.1073/pnas.1910842117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura N.U. Evidence for neurogenesis within the white matter beneath the temporal neocortex of the adult rat brain. Neuroscience. 2005;134:121–132. doi: 10.1016/j.neuroscience.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Tang S. Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: a comparative meta-analysis. eBioMedicine. 2018;36:436–445. doi: 10.1016/j.ebiom.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N., Schinder A.F. Maturation and functional integration of new granule cells into the adult hippocampus. Cold Spring Harb. Perspect. Biol. 2015;8:a018903. doi: 10.1101/cshperspect.a018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y., Fukuda S., Namba T., Seki T., Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Tyszka J.M., Pauli W.M. In vivo delineation of subdivisions of the human amygdaloid complex in a high-resolution group template. Hum. Brain Mapp. 2016;37:3979–3998. doi: 10.1002/hbm.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N., Chan W.Y. Expression patterns of PSA-NCAM in the human ganglionic eminence and its vicinity: role of PSA-NCAM in neuronal migration and axonal growth? Cells Tissues Organs. 2004;177:229–236. doi: 10.1159/000080136. [DOI] [PubMed] [Google Scholar]
- Vadodaria K.C. Noradrenergic regulation of plasticity marker expression in the adult rodent piriform cortex. Neurosci. Lett. 2017;644:76–82. doi: 10.1016/j.neulet.2017.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem M., et al. Longitudinal changes in amygdala, hippocampus and cortisol development following early caregiving adversity. Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varea E., et al. Differential evolution of PSA-NCAM expression during aging of the rat telencephalon. Neurobiol. Aging. 2009;30:808–818. doi: 10.1016/j.neurobiolaging.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Pfeifer J.H., Flournoy J.C., Hernandez L.M., Dapretto M. Affective reactivity during adolescence: associations with age, puberty and testosterone. Cortex. 2019;117:336–350. doi: 10.1016/j.cortex.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., et al. Human and monkey striatal interneurons are derived from the medial ganglionic eminence but not from the adult subventricular zone. J. Neurosci. 2014;34:10906–10923. doi: 10.1523/JNEUROSCI.1758-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Andersson S., Warner M., Gustafsson J.-Åke. Estrogen receptor (ER)β knockout mice reveal a role for ERβ in migration of cortical neurons in the developing brain. Proc. Natl. Acad. Sci. 2003;100:703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen A., Yoon K.L. Depression and affective flexibility: a valence-specific bias. Behav. Res. Ther. 2019;123 doi: 10.1016/j.brat.2019.103502. [DOI] [PubMed] [Google Scholar]
- Wiesel T.N. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Williams S., Leventhal C., Lemmon V., Nedergaard M., Goldman S.A. Estrogen promotes the initial migration and inception of NgCAM-dependent calcium-signaling by new neurons of the adult songbird brain. Mol. Cell. Neurosci. 1999;13:41–55. doi: 10.1006/mcne.1998.0729. [DOI] [PubMed] [Google Scholar]
- Xiong K., et al. Doublecortin-expressing cells are present in layer II across the adult guinea pig cerebral cortex: partial colocalization with mature interneuron markers. Exp. Neurol. 2008;211:271–282. doi: 10.1016/j.expneurol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachnis A.T., Roper S.N., Love A., Fancey J.T., Muir D. Bcl-2 immunoreactive cells with immature neuronal phenotype exist in the nonepileptic adult human brain. J. Neuropathol. Exp. Neurol. 2000;59:113–119. doi: 10.1093/jnen/59.2.113. [DOI] [PubMed] [Google Scholar]
- Yasinski C., et al. Processes of change in cognitive behavioral therapy for treatment-resistant depression: psychological flexibility, rumination, avoidance, and emotional processing. Psychother. Res. 2020;30:983–997. doi: 10.1080/10503307.2019.1699972. [DOI] [PubMed] [Google Scholar]
- Zeidman P., Maguire E.A. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat. Rev. Neurosci. 2016;17:173–182. doi: 10.1038/nrn.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-M., et al. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front. Neuroanat. 2009;3:17. doi: 10.3389/neuro.05.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., et al. Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology. 2012;37:959–967. doi: 10.1038/npp.2011.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Li B. Population coding of valence in the basolateral amygdala. Nat. Commun. 2018;9:5195. doi: 10.1038/s41467-018-07679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., et al. Multiplexing of theta and alpha rhythms in the amygdala-hippocampal circuit supports pattern separation of emotional information. Neuron. 2019;102(887–898) doi: 10.1016/j.neuron.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B., John Y.J., García-Cabezas M.Á., Bunce J.G., Barbas H. The intercalated nuclear complex of the primate amygdala. Neuroscience. 2016;330:267–290. doi: 10.1016/j.neuroscience.2016.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]