Abstract
Strong inhibitory effects of the anionic surfactant linear alkylbenzene sulfonate (LAS) on four strains of autotrophic ammonia-oxidizing bacteria (AOB) are reported. Two Nitrosospira strains were considerably more sensitive to LAS than two Nitrosomonas strains were. Interestingly, the two Nitrosospira strains showed a weak capacity to remove LAS from the medium. This could not be attributed to adsorption or any other known physical or chemical process, suggesting that biodegradation of LAS took place. In each strain, the metabolic activity (50% effective concentration [EC50], 6 to 38 mg liter−1) was affected much less by LAS than the growth rate and viability (EC50, 3 to 14 mg liter−1) were. However, at LAS levels that inhibited growth, metabolic activity took place only for 1 to 5 days, after which metabolic activity also ceased. The potential for adaptation to LAS exposure was investigated with Nitrosomonas europaea grown at a sublethal LAS level (10 mg liter−1); compared to control cells, preexposed cells showed severely affected cell functions (cessation of growth, loss of viability, and reduced NH4+ oxidation activity), demonstrating that long-term incubation at sublethal LAS levels was also detrimental. Our data strongly suggest that AOB are more sensitive to LAS than most heterotrophic bacteria are, and we hypothesize that thermodynamic constraints make AOB more susceptible to surfactant-induced stress than heterotrophic bacteria are. We further suggest that AOB may comprise a sensitive indicator group which can be used to determine the impact of LAS on microbial communities.
Autotrophic ammonia-oxidizing bacteria (AOB) have been considered ideal microbial indicators of perturbations caused by pollutants in natural environments (20, 54, 58). At least three reasons for this can be formulated: (i) AOB perform a vital bottleneck role in N cycling in many natural environments because of their unique ability to oxidize NH4+ to NO2− (1, 45, 51); (ii) AOB are generally sensitive to pollutants and often require a long time for recovery (20, 25, 33, 54); and (iii) there is a simple and cheap method for measuring NH4+ oxidation activities in environmental samples (25). In addition, the AOB constitute a very promising model group for studies of microbial diversity and activity, since the methods used for studying these bacteria have been drastically improved recently (9). Given the impressive development of methods, it is likely that AOB will continue to be considered attractive indicators of environmental perturbations in the years to come.
Inhibitory effects of various compounds on AOB in various environments have been extensively reported. However, due to the difficulties involved in cultivation of AOB, there is still a general lack of data concerning the effects of toxic chemicals on the general physiology of these bacteria. Previous studies have dealt mostly with specific nitrification inhibitors targeting the NH4+ monooxygenase enzyme (5, 42), and very little is known about other targets of toxicants in AOB cells.
Xenobiotic surfactants comprise a very important group of potentially toxic compounds that are believed to be harmful due to disruption of the function and structure of bacterial membranes (11, 13, 14, 43, 64). The linear alkylbenzene sulfonates (LAS) constitute the quantitatively most important group of synthetic surfactants used today, and the use of these compounds will likely increase in the years to come (12). Concern has been raised about possible toxic effects of LAS on susceptible biota in agricultural soils receiving high loads of these anionic surfactants from recycled sewage sludge or contaminated irrigation water (38, 61). Indeed, recent research has demonstrated that autotrophic NH4+ oxidation in agricultural soil may be affected at LAS concentrations occasionally encountered in soils after application of municipal sewage sludge (19, 60, 61; L. Elsgaard, S. O. Petersen, and K. Debosz, submitted for publication). Here we describe a detailed study of the effects of LAS on four strains of AOB belonging to different phylogenetic clusters in the β subgroup of the class Proteobacteria. Surfactant-induced toxic effects on various essential physiological parameters, such as metabolic activity, specific growth rate, and microcolony formation, were investigated. Our aims were to obtain toxicological information for each of these parameters and to compare the overall physiological status of nonstressed and LAS-stressed cultures of ammonia-oxidizing cells.
MATERIALS AND METHODS
Bacterial strains.
Three soil isolates, Nitrosomonas europaea NCIMB 11850 (63), Nitrosospira multiformis (formerly Nitrosolobus multiformis) NCIMB 11849 (59), and Nitrosospira sp. strain AV (41), and one marine isolate, “Nitrosococcus mobilis” NC2, of AOB belonging to the β subclass of the Proteobacteria were used. Based on its 16S ribosomal DNA sequence, “N. mobilis” should be reclassified as a member of the genus Nitrosomonas (27), and below we refer to the “N. mobilis” strain as a Nitrosomonas strain. The marine strain and the soil isolates were kindly supplied by H.-P. Koops (University of Hamburg, Hamburg, Germany) and J. I. Prosser (University of Aberdeen, Aberdeen, United Kingdom), respectively.
Culture conditions.
An autotrophic growth medium modified from the medium of MacDonald and Spokes (40) was used for all experiments. This medium contained (per liter of membrane-filtered water) 0.5 g of (NH4)2SO4, 0.2 g of KH2PO4, 20 mg of CaCl2 · 2H2O, 40 mg of MgSO4 · 7H2O, 3.8 mg of FeNaEDTA, 1 μg of phenol red, 4.77 g of HEPES, and 1 ml of a trace element solution (16). The pH of the medium was adjusted to 7.5 with 10 M NaOH, and the medium was autoclaved at 121°C for 20 min. For all growth experiments with suspended or filter-grown cells (see below), sterile filtered NaHCO3 was added as a carbon source to a final concentration of 1.5 mM. The purity of the strains used was routinely tested on tryptic soy agar plates and by epifluorescense microscopy (Zeiss Axioscope or Axioplan). Only AOB cultures that produced no visible colonies after 5 days of incubation on tryptic soy agar plates were used for experiments. Prior to individual experiments, an LAS was added to each experimental flask or petri dish from a stock solution in which methanol was the solvent. The methanol was subsequently allowed to evaporate in a sterile hood (Holten LaminAir, Allerød, Denmark) before fixed volumes of medium were added to each container. The LAS used was a C12 homologue [4-(2-dodecyl)benzenesulfonic acid, sodium salt; molecular mass, 348 g mol−1; purity, >97%; impurities consisted mainly of sodium salts)] that was synthesized and supplied by Risø National Laboratory (Roskilde, Denmark). All other chemicals were analytical grade and were obtained from commercial suppliers. Acid-washed glassware was used for all experiments.
Growth rates in liquid batch cultures.
Growth experiments were carried out in triplicate in sterile 120-ml serum vials containing 20 ml of newly inoculated growth medium (0.1%[vol/vol] from an early-stationary-phase culture) and different concentrations of LAS. The vials were capped with Teflon-coated rubber septa and shaken to resolubilize the surfactant added. Incubation was performed at 30°C in the dark on a rotary shaker (200 rpm), and subsamples were taken at regular intervals by means of a sterile syringe. To measure NO2− production, NO2− samples were frozen (−18°C) and examined within a few days. Cell counts were determined directly by using acridine orange-stained black polycarbonate membrane filters (pore size, 0.2 μm; diameter, 25 mm; Poretics Products, Livermore, Calif.) as described previously (28). Specific growth rates and generation times were calculated from log-transformed growth curves based on the accumulation of NO2−, since this approach has been shown to give more precise estimates of specific growth rates than direct cell counting in batch cultures of AOB (52). When cultures were challenged with surfactant, a slow initial exponential growth phase was sometimes followed by a faster exponential growth phase; in these cases the steepest part of the log-transformed growth curve was used to determine the specific growth rate. Cultures showing no or only weak NO2− accumulation (<200 μM) were monitored for 3 months to confirm that they were negative for growth.
Biodegradation of LAS during growth was monitored in 20-ml liquid cultures containing 3 mg of LAS liter−1. Growth was terminated 1 h or 11 days after inoculation, by adding 60 ml of methanol. Cultures containing no AOB cells and either 0 or 3 mg of LAS liter−1 served as controls. Before LAS analysis, all samples were filtered (pore size, 0.45 μm).
Microcolony formation by early-stationary-phase cells exposed to LAS.
The viability of AOB was determined by using a modified version of the microcolony technique previously used as a viability index for AOB in our laboratory (28, 63). The assay measures the proportion of cells that are able to initiate growth and form microcolonies consisting of four or more cells when they are incubated on a membrane filter floating on autotrophic growth medium. However, instead of using naked membrane filters as in the original protocol (28), the cell-inoculated filters were mounted on a circular glass coverslip coated with a thin layer of silicone oil as described previously (29). The modification was essential because surfactant-containing medium disrupted microcolony formation on naked filters. Briefly, early-stationary-phase cells from the four AOB cultures were filtered onto white polycarbonate membrane filters (pore size, 0.2 μm; diameter, 25 mm; Poretics Products). The filters were then transferred to the surface of 10 ml of sterile filtered autotrophic growth medium without (NH4)2SO4 by using a sterile petri dish with blotting paper at the bottom. The membrane filters were then mounted with the bacterial side towards a silicone oil-coated glass coverslip. The mounted filters were finally transferred (filter side down) to the surface of sterile growth medium in a glass petri dish divided into four wells (each containing 4 ml of medium with the same LAS concentration; i.e., there were four replicates). Optimal incubation times were determined for each strain by determining the time necessary to maximize microcolony formation for counting; the strain-specific optimal incubation times (between 5 and 10 days) were subsequently used for all the LAS exposure studies. Incubation was performed in moist chambers at 30°C in the dark. Incubation was terminated by acridine orange staining and subsequent mounting of the filters on microscope slides as described elsewhere (63). It was important to wipe off any drops of LAS-containing medium from the filters and to rinse the filters with water in order to prevent nonspecific staining of the filters exposed to LAS. The filters were inspected with an epifluorescense microscope (Zeiss Axioscope). Finally, NO2− levels were measured (see below) in the filter-containing wells at the end of incubation.
Experiments to study the growth recovery of cells based on their ability to form microcolonies (viability index) (28) were performed with N. europaea. Early-stationary-phase cells were applied to membrane filters (see above) and incubated for 5 days in the presence of LAS concentrations just above the threshold concentrations for growth and microcolony formation (15 and 18 mg liter−1, respectively). After 5 days, one-half of the filter incubations were terminated (negative controls), and the remaining filters were incubated for another 5 days on fresh medium without LAS.
NH4+ oxidation and CO2 fixation rates in early-stationary-phase cells exposed to LAS.
The effects of LAS on NH4+ oxidation and CO2 fixation rates were determined with early-stationary-phase cultures of all four AOB. Cells were grown in 500-ml batch cultures (1,500-ml batch cultures for Nitrosospira sp. strain AV) and harvested when the NO2− level had increased to 5 to 6 mM. The cultures were added to 300-ml centrifuge bottles and centrifuged (Beckman J2-21 M/E; 9,000 × g, 20°C, 30 min). The pellets were resuspended in NH4+-free growth medium by using 25% (strain AV) or 50% (other strains) of the original culture volume.
NH4+ oxidation and CO2 fixation assays were carried out in triplicate in 26-ml serum vials containing 5-ml cell suspensions and different LAS concentrations. Radiolabelled H14CO3− (54 mCi μmol of C−1; Amersham Life Science, Little Chalfont, United Kingdom) was added to vials used for CO2 fixation measurements at a concentration of 1 μM (600,000 dpm or 0.27 μCi per vial), while the same amount of unlabelled HCO3− was added to parallel vials used for NH4+ oxidation measurements. Immediately after HCO3− was added, incubation was initiated by adding 2.5 mM (NH4)2SO4. The two parallel assays were terminated after 6 h of incubation at 25°C on a shaker (120 rpm). The NH4+ oxidation rates were calculated from the amounts of accumulated NO2− in samples taken every 1 to 1.5 h, while the CO2 fixation rates were determined from the amounts of radiolabel incorporated into bacterial biomass after the 6-h incubation period. Subsamples (2 ml) were collected from the vials containing H14CO3− initially and again after 6 h of incubation. The samples were transferred to 20-ml polyethylene scintillation vials (Packard, Groningen, The Netherlands), and incubation was terminated by adding 4 ml of 0.1 M HCl. The acidified samples were flushed with air for 1.5 h to remove inorganic 14C as 14CO2. The cell-specific CO2 fixation rate could be calculated from the amount of 14C assimilated during the 6-h incubation period. In the two metabolic assays just described, a low HCO3− concentration (approximately 50 μM as calculated from headspace gas chromatographic measurements of acidified medium) was used to obtain a high 14C/12C ratio and hence ensure high sensitivity of the CO2 fixation assay. To test the effect of the HCO3− concentration on the NH4+ oxidation rate, we repeated the experiments with LAS-free incubation mixtures containing a high HCO3− concentration (1 mM) in the medium. The cell-specific NH4+ oxidation rates ranged between 4.5 × 10−15 and 9.7 × 10−15 mol cell−1 h−1 for the four AOB and were not significantly affected by a change in the HCO3− concentration in the incubation medium (data not shown). Hence, we assumed that the conditions for the cells were optimal even at the relatively low HCO3− concentration used in the 14CO2 fixation assay.
Adaptability of N. europaea after exposure to LAS.
To investigate the short-term adaptability of AOB to LAS, the physiological stress parameter tests described above were repeated with N. europaea cells which had been cultured for about 20 generations in medium containing a sublethal concentration of LAS. The N. europaea strain used for these experiments was first inoculated (0.1%, vol/vol) into fresh medium containing 10 mg of LAS liter−1. After a second transfer to LAS-containing medium, the cells (in the early stationary phase) were used for activity, growth, and microcolony formation assays as described above, except that the NH4+ oxidation experiments were performed in medium with a high HCO3− concentration (1 mM).
Analytical techniques.
LAS contents were measured by reverse-phase high-performance liquid chromatography by using a 25-cm Nucleosil 100-5 C18 column (Macherey-Nagel, Düren, Germany) and UV detection (Dionex spectral array detector; Dionex, Sunnyvale, Calif.) as described previously (50). NO2− contents were measured spectrophotometrically by using a plate reader (EL312e; Bio-Tek Instruments, Winooski, Vt.) as described previously (63). The radioactivity associated with radiolabelled AOB cells was determined by liquid scintillation counting by using 10 ml of Ultima Gold XR (Packard) as the scintillation cocktail. Samples were stored for 24 h in the dark to reduce quenching and then counted with a Packard 1600 TR liquid scintillation counter.
Estimation of key toxicological parameters.
The lowest-observed-effect concentrations and highest-no-effect concentrations were determined by Dunnett's t test by using an SAS analysis of variance procedure (57; Elsgaard et al., submitted), and 50% effective concentrations were estimated by nonlinear regression of nontransformed data as described by Nyholm et al. (44).
RESULTS
Effects of LAS on growth in liquid cultures.
It was important for data interpretation if LAS could be degraded by the AOB strains. When N. europaea and “N. mobilis” were cultivated in the presence of a sublethal LAS concentration, 3 mg liter−1, all of the LAS was recovered in the fully grown cultures. In contrast, the two Nitrosospira strains removed significant amounts of LAS from the medium, as only 88% (N. multiformis) and 58% (Nitrosospira sp. strain AV) of the added LAS remained in the fully grown cultures. Removal of LAS by adsorption to the AOB cells was negligible, however, because the LAS levels measured in the liquid phase were similar in flasks with and without added cells (data not shown).
Figure 1 shows that growth of all four AOB strains was progressively inhibited as the LAS concentration was increased. In the absence of LAS or at low concentrations of LAS, N. europaea and “N. mobilis” both showed monophasic, exponential growth from inoculation until the stationary phase was reached after approximately 1 week (Fig. 1A and B). At higher LAS concentrations, however, growth was in some cases inhibited initially and there was a long lag phase of several days before growth was observed. Nevertheless, such populations subsequently exhibited growth rates similar to those recorded at low LAS levels. By comparison, the two Nitrosospira strains also showed immediate, exponential growth until the stationary phase both in the absence of LAS and at lower concentrations of LAS (Fig. 1C and D). Also in these strains, the higher LAS concentrations resulted in complete inhibition of growth or at least in reduced initial growth rates. At intermediate LAS levels, the initial exponential growth rates increased markedly after 1 to 2 weeks, indicating that there was biphasic exponential growth.
FIG. 1.
Representative growth curves for the four AOB strains investigated grown in liquid medium containing different LAS concentrations. (A) N. europaea; (B) “N. mobilis”; (C) N. multiformis; (D) Nitrosospira sp. strain AV. Different symbols indicate different concentrations of LAS (in milligrams per liter). d, day.
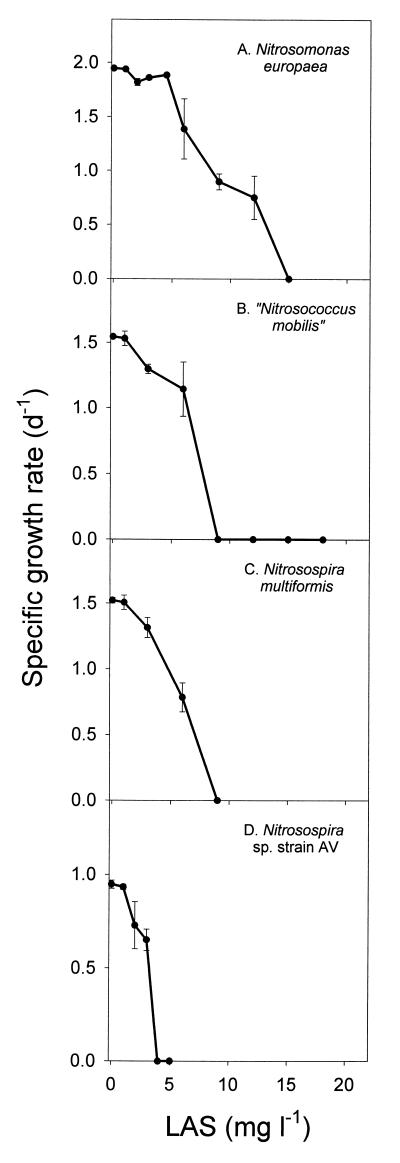
Figure 2 shows the calculated specific growth rates of the four AOB strains when they were exposed to different LAS concentrations. Typical dose-response curves showing that growth was progressively inhibited as the LAS concentration was increased were observed for all four AOB strains. The specific growth rates were calculated directly from NO2− accumulation data. This approach relies on the assumption that specific metabolic activity (the amount of NH4+-oxidizing activity per cell) and specific cell yield are constant during balanced, exponential growth (52). As shown by the almost perfect (R2 > 0.99) exponential nitrite accumulation curves, our use of NO2− accumulation data to estimate specific growth rates was justified. Specific cell yields (in number of cells per mole of N transformed) were also found to be unaffected at LAS levels that allowed for long-term growth with one exception, when the specific cell yield was reduced by approximately 50% for N. multiformis grown in the presence of 6 mg of LAS liter−1.
FIG. 2.
Influence of LAS on the specific growth rates of the four AOB strains investigated. d−1, day−1; l−1, liter−1.
Effects of LAS on microcolony formation.
Early-stationary-phase cells of all four AOB strains were used to test the toxic effect of LAS on cell viability, as measured by the ability of cells to form microcolonies on a membrane filter surface. It was previously suggested that this assay provides a useful viability index for AOB in cultures (28), and we have used it to study specific physiological responses to osmotic stress in N. europaea (63). Formation of colonies containing four or more cells, corresponding to at least two cell divisions on the filters, was taken to indicate that AOB cells were viable by the standard protocol (28). In the present study, however, we supplemented the standard protocol for enumeration of viable cells in N. europaea and “N. mobilis” cultures by also recording the formation of microcolonies containing 16 more cells, corresponding to at least four cell divisions on the filters.
Figure 3 shows the results of the microcolony formation assay when early-stationary-phase cells from the cultures were exposed to different LAS concentrations. The results are expressed as percentages of the inoculated cells that formed microcolonies. The shape of the dose-response curves for microcolony formation was similar to the shapes recorded for LAS effects on the growth rates in liquid cultures. Again, N. europaea was the least sensitive organism, followed by “N. mobilis,” N. multiformis, and Nitrosospira sp. strain AV. For the two least sensitive organisms, N. europaea and “N. mobilis,” formation of both small microcolonies (4 or more cells) and large microcolonies (16 or more cells) indicated that LAS also had a specific effect on continued microcolony formation after the first two cell divisions. Hence, when the LAS concentration was increased, the fraction of cells that formed large microcolonies became smaller than the fraction of cells that formed small microcolonies. This trend was also observed in experiments in which an extended incubation time (10 days) was used and thus did not result from inadequate incubation time for microcolony formation (data not shown). A subpopulation of cells recorded as viable in the standard protocol for microcolony formation apparently lost the ability to divide continuously and eventually form large microcolonies on the filters.
FIG. 3.
Influence of LAS on microcolony formation (MCFU) (i.e., the percentage of membrane-immobilized cells forming microcolonies). Symbols: ○, microcolonies consisting of four or more cells; ●, microcolonies consisting of 16 or more cells. l−1, liter−1.
Effects of LAS on NH4+ oxidation and CO2 fixation rates.
Early-stationary-phase cells of all four AOB strains were used to test the acute toxic effects of LAS on NH4+ oxidation and CO2 fixation activities in short-term experiments performed with resting cells from stationary-phase cultures. Figure 4 shows that both NH4+ oxidation and CO2 fixation rates progressively decreased as a function of LAS amendment, but N. europaea and “N. mobilis” were generally less susceptible than the two Nitrosospira strains. In general, LAS inhibited both NH4+ oxidation and CO2 fixation rates to the same degree, but an exception was observed with N. europaea, where NH4+ oxidation was markedly more sensitive than CO2 fixation in the 3- to 18-mg liter−1 range. Repeating the experiments resulted in almost perfect reproduction of the dose-response curves, indicating that the apparent differences in the shapes of the dose-response curves for the NH4+ oxidation and CO2 fixation rates in N. europaea were real.
FIG. 4.
Influence of LAS on CO2 fixation (A to D) and NH4+ oxidation (E to H) rates (relative numbers) in the four AOB strains investigated. The absolute rates of NH4+ oxidation are given in Table 1. l−1, liter−1.
Estimation of physiological and toxicological test parameters.
Table 1 shows that the generation times, specific cell yields, and specific NH4+ oxidation activities varied within a factor of 4 for the four AOB strains. In contrast, the percentages of cells forming microcolonies varied by more than 2 orders of magnitude. Stationary-phase cells of the two Nitrosomonas strains (including “N. mobilis”) clearly exhibited a much better ability to form microcolonies than cells of the two Nitrosospira strains.
TABLE 1.
Physiological parameters for the AOB used in this study
| Organism | Generation time (h)a | Growth yield (1012 cells mol−1)a | Microcolony formation (%)b | NH4+ oxidation (10−15 mol cell−1 h−1)c |
|---|---|---|---|---|
| N. europaea | 8.5 ± 0.1d | 10.2 ± 0.2 | 55.2 ± 4.6 | 9.7 ± 0.1 |
| “N. mobilis” | 10.8 ± 0.1 | 4.9 ± 0.2 | 22.9 ± 2.0 | 4.7 ± 0.3 |
| N. multiformis | 10.9 ± 0.2 | 2.5 ± 0.6 | 0.3 ± 0.1 | 8.6 ± 0.2 |
| Nitrosospira sp. strain AV | 17.5 ± 0.4 | 7.6 ± 1.6 | 5.6 ± 1.0 | 9.5 ± 0.4 |
Organisms were grown in liquid medium (batch culture).
Early-stationary-phase cells were grown on membrane filters, and the percentages of microcolony-forming units were determined.
Activity of early-stationary-phase cells after transfer to fresh medium (based on total cell counts).
Mean ± standard deviation.
Table 2 shows that the estimated toxicological parameters varied significantly when we compared the effects of LAS on the specific growth rates of the four strains. Generally, sensitivity to LAS increased in the following order: N. europaea, “N. mobilis,” N. multiformis, Nitrosospira sp. strain AV. For each strain, sensitivity to LAS also differed for the different physiological parameters tested. Generally, sensitivity increased in the following order: CO2 fixation, NH4+ oxidation, microcolony formation, growth rate. Hence, certain processes involved in growth and growth initiation (microcolony formation) were apparently inhibited at a lower LAS level than the metabolic activities NH4+ oxidation and CO2 fixation were. This conclusion was supported by continued NO2− production in suspended or filter-grown cells exposed to LAS concentrations just above the threshold concentration for growth inhibition. Despite the absence of growth, NO2− was produced for up to 5 days in these cells, before NH4+ oxidation activity eventually became completely inhibited (data not shown). Interestingly, these experiments also showed that membrane-immobilized cells of all strains produced NO2− at slightly higher LAS concentrations than suspended cells, suggesting that the metabolic activity in the immobilized cells were less sensitive to LAS inhibition than that in the suspended cells was (data not shown).
TABLE 2.
Toxicological test parameters estimated for the AOB used in this study
| Organism | LAS concn (mg liter−1)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth rate
|
Microcolony formation
|
NH4+ oxidation
|
CO2 fixation
|
|||||||||
| NOECa | LOECb | EC50c | NOEC | LOEC | EC50 | NOEC | LOEC | EC50 | NOEC | LOEC | EC50 | |
| N. europaea | 5 | 6 | 9 | 9 | 12 | 14 | 3 | 6 | 16 | 12 | 18 | 21d |
| “N. mobilis” | 3 | 6 | 7 | 6 | 9 | 9 | 6 | 9 | 38d | 9 | 12 | 28d |
| N. multiformis | 1 | 3 | 6 | 3 | 6 | 5 | 0 | 3 | 6 | 3 | 6 | 8 |
| Nitrosospira sp. strain AV | 1 | 2 | 3 | 1 | 2 | 3 | 0 | 3 | 8 | 0 | 3 | 7 |
NOEC, no-observed-effect concentration (highest concentration tested that had no significant effect), calculated by using Dunnett's t test.
LOEC, lowest-observed-effect concentration (lowest concentration tested that had a significant effect), calculated by using Dunnett's t test.
EC50, effective concentration causing 50% inhibition, estimated by using nonlinear regression of nontransformed data unless indicated otherwise.
Based on visual inspection of dose-response curves.
Effect of LAS preexposure in N. europaea.
Although spontaneously inhibited by LAS treatment, growth occasionally resumed in some AOB cultures after long lag periods, and the subsequent growth rates were similar to those of the untreated controls. This is most clearly shown in Fig. 1 for the N. europaea and “N. mobilis” cultures grown at an LAS concentration slightly above the lowest observed effect concentration. Based on these observations, we examined whether physiological parameters in N. europaea, such as specific metabolic activity (NH4+ oxidation rate), lag period prior to growth, specific growth rate, and microcolony formation, were reversibly or irreversibly affected by preexposure to LAS. We compared cultures by using untreated inocula (controls) and inocula which had been preexposed for approximately 20 cell generations (two culture transfers) to a sublethal LAS level, 10 mg liter−1. The comparison failed to reveal any difference in the lengths of the lag phases or the subsequent growth rates (data not shown). We did observe, however, that when the length of exposure to LAS was extended by repeated culturing (tested up to approximately 100 cell generations) in the presence of 10 mg of LAS liter−1, cultures frequently stopped growing, while this was never the case during routine cultivation of N. europaea without LAS (data not shown). The observations indicated that long-term exposure to LAS, even at a sublethal level (10 mg liter−1), eventually led to compromised cell functions that resulted in irreversible cessation of growth.
Figure 5A shows that the ability of cells to form microcolonies was negatively affected in inocula of stationary-phase cells which had been pregrown with 10 mg of LAS liter−1. Although the ability of preexposed stationary-phase cells to form microcolonies on membrane filters was significantly reduced (P < 0.05) at some of the lower LAS concentrations, the differences were much more pronounced at the higher LAS concentrations. Exposure of filter-incubated cells to 15 mg of LAS liter−1 thus resulted in almost complete loss of viability in preexposed cells. By comparison, the viability was reduced only by approximately 50% when untreated control cells were subsequently exposed to 15 mg of LAS liter−1. Neither of the filters with untreated control cells or preexposed cells incubated in the presence of 15 mg of LAS liter−1 developed new, additional microcolonies when they were subsequently transferred to LAS-free medium for extended incubation (5 days) (data not shown). These results thus confirmed that preexposure to LAS affected some AOB cell functions and led to irreversible loss of viability.
FIG. 5.
Effect of preexposure for approximately 20 generations to a sublethal LAS level (10 mg liter−1) on microcolony formation (A) and the cell-specific NH4+ oxidation rate (B) of N. europaea. Symbols: ○, nontreated control cells; ●, preexposed inoculant cells. MCFU, microcolony formation; l−1, liter−1.
Figure 5B shows that cell-specific activity (NH4+ oxidation) was also affected in inocula containing resting stationary-phase cells of N. europaea which had been pre grown in the presence of 10 mg of LAS liter−1. When incubated in the presence of LAS levels ranging from 0 to 12 mg liter−1, the preexposed cells clearly demonstrated lower (by approximately 50%) NH4+ oxidation activity than the controls. It was noticed that preexposed cells remained affected during subsequent incubation without LAS (Fig. 5B). In contrast, NH4+ oxidation activities were strongly inhibited for both types of inocula at very high LAS levels (24 to 48 mg liter−1) (data not shown).
DISCUSSION
Physiological characteristics of the AOB strains.
The generation times, specific cell yields, and NH4+ oxidation rates of the four strains investigated (Table 1) were generally within the ranges of values reported previously for pure cultures of AOB (7, 51), although each strain grew quite rapidly in the present study. In earlier reports (3, 47–49) generation times of 11.6 to 15.4 h were recorded for N. europaea NCIMB 11850, while the same strain grew with generation times of only 7.8 to 9.6 h under similar conditions in our laboratory (63) (Table 1). Likewise, longer generation times have been reported for N. multiformis (18 h) (59), Nitrosospira sp. strain AV (21 to 40 h) (6, 7), and “N. mobilis” NC2 (12 to 13 h) (36) compared to those reported in this study (Table 1). This comparison supports the hypothesis that AOB strains may adapt to higher growth rates when they are cultured repeatedly in laboratory media, as suggested previously (2, 48).
The ability of stationary-phase cells of the four AOB strains to form microcolonies varied by more than 2 orders of magnitude, from about 0.3% to more than 50% of the cells applied to the membrane filters (Table 1). In agreement with previous observations (28, 63), the two Nitrosomonas strains (including “N. mobilis”) showed a much greater ability to form microcolonies (20 to 60%) than the two Nitrosospira strains showed (0.3 to 6%). In two previous studies (28, 63) rather freshly isolated Nitrosospira strains were studied, and it could be speculated that this resulted in poor growth performance on filters. In the present study, however, the two Nitrosospira strains had already been cultured intensively in laboratory media, and it seems more likely that their poor ability to form microcolonies was actually a characteristic of this group of AOB. Despite the low proportion of cells that formed microcolonies, the LAS dose-response experiments with all four AOB strains supported the view that microcolony formation can be used as a rapid and reliable viability index, as proposed previously (28, 63).
LAS interactions with the AOB strains.
The two Nitrosomonas strains did not degrade LAS, while N. multiformis and Nitrosospira sp. strain AV removed 12 and 42% of the LAS added, respectively. Our study thus represents the first report of LAS removal by autotrophic AOB. We found no evidence that physical or chemical processes (adhesion, volatilization, or precipitation) explain the observed removal of LAS, and we therefore suggest that the two Nitrosospira strains have a limited capacity for LAS degradation. It has recently been shown that some methanotrophic bacteria are able to cometabolize LAS by means of a methane monooxygenase (31, 32), which functionally resembles the ammonia monooxygenase of AOB. Thus, our finding was not totally unexpected, but it was surprising that only the two Nitrosospira strains were able to remove LAS. Attempts to promote degradation of LAS by N. europaea by using higher LAS concentrations (6, 12, or 18 mg liter−1) were all unsuccessful (data not shown). The biodegradation data did not affect interpretation of our LAS toxicity experiments, however, since constant or almost constant LAS levels would have been present throughout the incubation periods. Only in the long-term experiments performed with Nitrosospira sp. strain AV may significant LAS degradation have obscured interpretation of the levels of toxicity. Degradation products of LAS are less toxic than the parent compound (46), and the toxicity of LAS might thus have been slightly underestimated in these experiments. Finally, the observed variability among AOB strains in terms of degradation potential strongly suggests that use of N. europaea as a model organism for studies of the bioremediation potential of AOB (17, 18, 30) should be complemented with studies of Nitrosospira strains.
All four AOB strains showed high sensitivity to LAS, but the two Nitrosospira strains were clearly the most sensitive organisms (Table 2). Furthermore, all of the AOB were able to metabolize (NH4+ oxidation and CO2 fixation) in the presence of LAS concentrations higher than those that inhibited growth and microcolony formation (Fig. 2 to 4; Table 2). The lower sensitivity of the metabolic activities was not surprising, because activity and growth may not always be tightly coupled (56). A lag period before inhibition of NH4+ oxidation was effective at least partially explained the decreased sensitivity of metabolic activity compared to growth. Hence, a delay in the inhibitory effect was observed for NH4+ oxidation at LAS levels greater than the upper threshold level for growth. Similarly, at least N. europaea and “N. mobilis” showed a gradual loss of microcolony formation during incubation in the presence of increasing LAS levels.
It is generally thought that cell-specific CO2 fixation and NH4+ oxidation are rather tightly coupled by a C/N mole ratio of CO2 fixed to NH4+ oxidized in the 0.01 to 0.1 range (6, 21, 22, 36, 37, 59). In some cases, however, the ratio may decrease (e.g., in cells exposed to metabolic inhibitors ([6, 21]), and the C/N mole ratio may potentially be used as a sensitive, short-term index of toxicity. To our surprise, we observed that NH4+ oxidation was actually more sensitive than CO2 fixation in N. europaea at certain intermediate LAS concentrations (Fig. 4A and E). This result was surprising, since CO2 fixation is generally thought to be limited by the availability of reducing power derived from NH4+ oxidation (8). Whatever the reason, the apparent uncoupling of NH4+ oxidation and CO2 fixation is likely to have been a transient phenomenon, since the specific cell yields (number of cells per mole of N) obtained from the long-term growth experiments were found to be largely unaffected by LAS (data not shown). In the three other AOB strains, NH4+ oxidation and CO2 fixation were generally affected to the same degree by LAS (Fig. 4), suggesting that the C/N mole ratio was unaffected. Overall, the data show that the C/N mole ratio cannot easily be used as a short-term index of toxicity in AOB.
The experiments with N. europaea cells preexposed to LAS clearly showed that this organism was unable to adapt to this compound. The ability of single cells to develop microcolonies and the NH4+ oxidation activity of preexposed, stationary-phase cells were thus significantly reduced compared to the activities of control cells that were not preexposed, suggesting that preexposure irreversibly damaged important cell functions. Accumulated injuries may also have caused the complete cessation of growth that we frequently observed in cultures containing 10 mg of LAS liter−1. On the other hand, the subpopulations of surviving cells in inocula preexposed to LAS always grew at the same rate as control cells in LAS-free medium. As reported previously for injuries induced in ageing bacterial cultures (4), cell division in a small subpopulation of surviving cells in liquid cultures may dilute the cells with surfactant-induced injuries.
Although the present study was not designed to study the detailed mechanism of LAS toxicity for AOB, we can rule out surfactant-micelle interactions (11), since the upper threshold concentrations of LAS that allowed AOB growth were far below the critical micelle concentration reported for LAS (approximately 410 mg liter−1) (24). Furthermore, by adding viscosine (a surfactant known to strongly decrease surface tension [15]) to cultures of N. europaea, we were able to demonstrate that surface tension per se was not responsible for the LAS toxicity (Brandt, unpublished data). As a result, we suggest that LAS toxicity is due to direct interaction of LAS monomers with the cell wall structure. One mechanism could be an increase in membrane permeability that causes dissipation of ion gradients and membrane potential or leakage of essential cell constituents. Such a mechanism has previously been suggested to explain the cellular effects in Bacillus subtilis challenged with LAS (64). We thus propose that LAS is a nitrification inhibitor belonging to postulated class 4 of Rasche et al. (53); i.e., it is a compound which is highly toxic to AOB but is not a substrate for ammonia monooxygenase activity. However, the significant LAS removal observed with the two Nitrosospira strains could indicate that turnover-dependent inactivation of ammonia monooxygenase activity also contributed to the relatively high LAS sensitivity in these strains. In this case, LAS would be a combined class 3 (a compound that is cooxidized and causes ammonia monooxygenase turnover-dependent inactivation of NH4+ oxidation) and class 4 nitrification inhibitor (53).
The four AOB strains were much more sensitive to LAS than any of the heterotrophic bacteria studied so far (23, 26, 39, 64). In our laboratory, growth experiments with four heterotrophic soil isolates of Pseudomonas fluorescens and Bacillus cereus showed that these strains were not affected or even stimulated by LAS at concentrations up to 300 mg liter−1 (data not shown). This finding is in accordance with studies performed with agricultural soils that showed that respiration in AOB was much more sensitive to LAS than respiration in heterotrophic microorganisms (10, 19; Elsgaard et al., submitted). The reason for the high sensitivity of AOB to LAS compared to the sensitivity of heterotrophic bacteria is unknown. The cell membranes of AOB do not seem to be fundamentally different from those of many other gram-negative bacteria; the lipid composition is quite similar (55, 62), and even if AOB have a larger specific membrane area than most other bacteria (51), it is difficult to see how this could contribute to their greater sensitivity to LAS. However, monomers of LAS and other surfactants are known to increase the permeability of biological membranes (13, 14, 43, 64), and this could lead to uncoupling reactions during energy metabolism. Due to the low energy yield from NH4+ oxidation (8, 34) and the high energy requirement for CO2 fixation (34, 35), it is likely that AOB are particularly sensitive to such uncoupling surfactants.
ACKNOWLEDGMENTS
This work was financed by the Centre for Sustainable Land Use and Management of Contaminants, Carbon and Nitrogen (Denmark).
We thank Gwen Abrill for helping with the analysis of dissolved inorganic carbon in the cultivation media, Tommy Harder Nielsen for advice on LAS analysis, and Gerda Krog Mortensen for providing information on the purity of the C12 LAS used. Finally, Bente O/stergaard and Christina Mogensen are acknowledged for excellent technical assistance.
REFERENCES
- 1.Abeliovich A. Transformations of ammonia and the environmental impact of nitrifying bacteria. Biodegradation. 1992;3:255–264. [Google Scholar]
- 2.Allison S M, Prosser J I. Survival of ammonia oxidising bacteria in air-dried soil. FEMS Microbiol Lett. 1991;79:61–68. [Google Scholar]
- 3.Armstrong E F, Prosser J I. Growth of Nitrosomonas europaea on ammonia-treated vermiculite. Soil Biol Biochem. 1988;20:409–411. [Google Scholar]
- 4.Barer M R, Harwood C R. Bacterial viability and culturability. Adv Microb Physiol. 1999;41:93–137. doi: 10.1016/s0065-2911(08)60166-6. [DOI] [PubMed] [Google Scholar]
- 5.Bédard C, Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belser L W. Bicarbonate uptake by nitrifiers: effects of growth rate, pH, substrate concentration, and metabolic inhibitors. Appl Environ Microbiol. 1984;48:1100–1104. doi: 10.1128/aem.48.6.1100-1104.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belser L W, Smith E L. Growth and oxidation kinetics of three genera of ammonia oxidizing nitrifiers. FEMS Microbiol Lett. 1980;7:213–216. [Google Scholar]
- 8.Bock E, Koops H-P, Ahlers B, Harms H. Oxidation of inorganic nitrogen compounds as energy source. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1991. pp. 414–430. [Google Scholar]
- 9.Bothe H, Jost G, Schloter M, Ward B B, Witzel K-P. Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiol Rev. 2000;24:673–690. doi: 10.1111/j.1574-6976.2000.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 10.Brandt K K, Krogh P H, Cassani G, Sørensen J. Proceedings of the 5th World Surfactants Congress. Florence, Italy: CESIO; 2000. Does LAS affect the soil ecosystem in sludge-amended soil? Results from a field trial with well-defined strings of LAS-amended sludge in soil; pp. 1590–1597. [Google Scholar]
- 11.Cabral J-P S. Mode of antibacterial action of dodine (dodecylguanidine monoacetate) in Pseudomonas syringae. Can J Microbiol. 1992;38:115–123. doi: 10.1139/m92-019. [DOI] [PubMed] [Google Scholar]
- 12.Cavalli L, Clerici R, Radici P, Valtorta L. Update on LAB/LAS. Tens Surf Deterg. 1999;36:254–258. [Google Scholar]
- 13.de la Maza A, Parra J L. Alterations in phospholipid bilayers caused by oxyethylenated nonylphenol surfactants. Arch Biochem Biophys. 1996;329:1–8. doi: 10.1006/abbi.1996.0184. [DOI] [PubMed] [Google Scholar]
- 14.de la Maza A, Sanchez-Leal J, Parra J L, Garcia M T, Ribosa I. Permeability changes of phospholipid vesicles caused by surfactants. J Am Oil Chem Soc. 1991;68:315–319. [Google Scholar]
- 15.Desai J D, Banat I M. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev. 1997;61:47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaldson J M, Henderson G S. A dilute medium to determine population size of ammonium oxidizers in forest soils. Soil Sci Soc Am J. 1989;53:1608–1611. [Google Scholar]
- 17.Duddleston K N, Bottomley P J, Porter A, Arp D J. Effect of soil and water content on methyl bromide oxidation by the ammonia-oxidizing bacterium Nitrosomonas europaea. Appl Environ Microbiol. 2000;66:2636–2640. doi: 10.1128/aem.66.6.2636-2640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duddleston K N, Bottomley P J, Porter A, Arp D J. New insights into methyl bromide cooxidation by Nitrosomonas europaea obtained by experimenting with moderately low density cell suspensions. Appl Environ Microbiol. 2000;66:2726–2731. doi: 10.1128/aem.66.7.2726-2731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsgaard L, Petersen S O, Debosz K. Effect and risk assessment of linear alkylbenzene sulphonates (LAS) in agricultural soil. 2001. II. Effects on soil microbiology as influenced by sewage sludge and incubation time. Environ. Toxicol. Chem., in press. [DOI] [PubMed] [Google Scholar]
- 20.Fuller M E, Scow K M. Impact of trichloroethylene and toluene on nitrogen cycling in soil. Appl Environ Microbiol. 1997;63:4015–4019. doi: 10.1128/aem.63.10.4015-4019.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glover H E. Methylamine, an inhibitor of ammonium oxidation and chemoautotrophic growth in the marine nitrifying bacterium Nitrosococcus oceanus. Arch Microbiol. 1982;132:37–40. [Google Scholar]
- 22.Glover H E. The relationship between inorganic nitrogen oxidation and organic carbon production in batch and chemostat cultures of marine nitrifying bacteria. Arch Microbiol. 1985;142:45–50. [Google Scholar]
- 23.Goodnow R A, Harrison A P., Jr Bacterial degradation of detergent compounds. Appl Microbiol. 1972;24:555–560. doi: 10.1128/am.24.4.555-560.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haigh S D. A review of the interaction of surfactants with organic contaminants in soil. Sci Total Environ. 1996;185:161–170. [Google Scholar]
- 25.Hansson G B, Klemedtsson L, Stenström J, Torstensson L. Testing the influence of chemicals on soil autotrophic ammonium oxidation. Environ Toxicol Water Qual. 1991;6:351–360. [Google Scholar]
- 26.Hartmann L. Effect of surfactants on soil bacteria. Bull Environ Contam Toxicol. 1966;1:219–224. doi: 10.1007/BF01684063. [DOI] [PubMed] [Google Scholar]
- 27.Head I M, Hiorns W D, Embley T M, McCarthy A J, Saunders J R. The phylogeny of autotrophic ammonia-oxidizing bacteria as determined by analysis of 16S ribosomal RNA gene sequences. J Gen Microbiol. 1993;139:1147–1153. doi: 10.1099/00221287-139-6-1147. [DOI] [PubMed] [Google Scholar]
- 28.Hesselsøe M, Sørensen J. Microcolony formation as a viability index for ammonia-oxidizing bacteria: Nitrosomonas europaea and Nitrosospira sp. FEMS Microbiol Ecol. 1999;28:383–391. [Google Scholar]
- 29.Højberg O, Binnerup S J, Sørensen J. Growth of silicone-immobilized bacteria on polycarbonate membrane filters, a technique to study microcolony formation under anaerobic conditions. Appl Environ Microbiol. 1997;63:2920–2924. doi: 10.1128/aem.63.7.2920-2924.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hommes N G, Russell S A, Bottomley P J, Arp D J. Effects of soil on ammonia, ethylene, chloroethane, and 1,1,1-trichloroethane oxidation by Nitrosomonas europaea. Appl Environ Microbiol. 1998;64:1372–1378. doi: 10.1128/aem.64.4.1372-1378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hrsak D. Cometabolic transformation of linear alkylbenzenesulphonates by methanotrophs. Water Res. 1996;30:3092–3098. [Google Scholar]
- 32.Hrsak D, Begonja A. Growth characteristics and metabolic activities of the methanotroph-heterotrophic groundwater community. J Appl Microbiol. 1998;85:448–456. doi: 10.1046/j.1365-2672.1998.853505.x. [DOI] [PubMed] [Google Scholar]
- 33.Joye S B, Hollibaugh J T. Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science. 1995;270:623–625. [Google Scholar]
- 34.Kelly D P. The chemolithotrophic prokaryotes. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1991. pp. 331–343. [Google Scholar]
- 35.Kelly D P. Thermodynamic aspects of energy conservation by chemolithotrophic sulfur bacteria in relation to the sulfur oxidation pathways. Arch Microbiol. 1999;171:219–229. [Google Scholar]
- 36.Koops H-P, Harms H, Wehrmann H. Isolation of a moderate halophilic ammonia-oxidizing bacterium, Nitrosococcus mobilis nov. sp. Arch Microbiol. 1976;107:277–282. doi: 10.1007/BF00425339. [DOI] [PubMed] [Google Scholar]
- 37.Krümmel A, Harms H. Effect of organic matter on growth and cell yield of ammonia-oxidizing bacteria. Arch Microbiol. 1982;133:50–54. [Google Scholar]
- 38.Kuhnt G. Behavior and fate of surfactants in soil. Environ Toxicol Chem. 1993;12:1813–1820. [Google Scholar]
- 39.Ledent P, Michels H, Blackman G, Naveau H, Agathos S N. Reversal of the inhibitory effect of surfactants upon germination and growth of a consortium of two strains of Bacillus. Appl Microbiol Biotechnol. 1999;51:370–374. [Google Scholar]
- 40.MacDonald R M, Spokes J R. A selective and diagnostic medium for ammonia oxidising bacteria. FEMS Microbiol Lett. 1980;8:143–145. [Google Scholar]
- 41.McCaig A E, Embley T M, Prosser J I. Molecular analysis of enrichment cultures of marine ammonia oxidisers. FEMS Microbiol Lett. 1994;120:363–368. doi: 10.1111/j.1574-6968.1994.tb07059.x. [DOI] [PubMed] [Google Scholar]
- 42.McCarty G W. Modes of action of nitrification inhibitors. Biol Fertil Soils. 1999;29:1–9. [Google Scholar]
- 43.Müller M T, Zehnder A J B, Escher B I. Membrane toxicity of linear alcohol ethoxylates. Environ Toxicol Chem. 1999;18:2767–2774. doi: 10.1002/etc.5620181011. [DOI] [PubMed] [Google Scholar]
- 44.Nyholm N, Sørensen B S, Kusk K O. Statistical treatment of data from microbial toxicity tests. Environ Toxicol Chem. 1992;11:157–167. [Google Scholar]
- 45.Painter H A. Nitrification in the treatment of sewage and waste-waters. In: Prosser J I, editor. Nitrification. Oxford, United Kingdom: IRL Press; 1986. pp. 185–211. [Google Scholar]
- 46.Painter H A. Anionic surfactants. In: de Oude N T, editor. Detergents. Berlin, Germany: Springer-Verlag; 1992. pp. 1–88. [Google Scholar]
- 47.Powell S J, Prosser J I. The effect of nitrapyrin and chloropicolinic acid on ammonium oxidation by Nitrosomonas europaea. FEMS Microbiol Lett. 1985;28:51–54. [Google Scholar]
- 48.Powell S J, Prosser J I. Inhibition of ammonium oxidation by nitrapyrin in soil and liquid culture. Appl Environ Microbiol. 1986;52:782–787. doi: 10.1128/aem.52.4.782-787.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powell S J, Prosser J I. Protection of Nitrosomonas europaea colonizing clay minerals from inhibition by nitrapyrin. J Gen Microbiol. 1991;137:1923–1929. doi: 10.1099/00221287-137-8-1923. [DOI] [PubMed] [Google Scholar]
- 50.Prats D, Ruiz F, Vásquez B, Rodriguez-Pastor M. Removal of anionic and non-ionic surfactants in a wastewater treatment plant with anaerobic digestion. A comparative study. Water Res. 1997;31:1925–1930. [Google Scholar]
- 51.Prosser J I. Autotrophic nitrification in bacteria. Adv Microb Physiol. 1989;30:125–181. doi: 10.1016/s0065-2911(08)60112-5. [DOI] [PubMed] [Google Scholar]
- 52.Prosser J I. Mathematical modeling of nitrification processes. Adv Microb Ecol. 1989;11:263–304. [Google Scholar]
- 53.Rasche M E, Hyman M R, Arp D J. Factors limiting aliphatic chlorocarbon degradation by Nitrosomonas europaea: cometabolic inactivation of ammonia monooxygenase and substrate specificity. Appl Environ Microbiol. 1991;57:2986–2994. doi: 10.1128/aem.57.10.2986-2994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Remde A, Hund K. Response of soil autotrophic nitrification and soil respiration to chemical pollution in long-term experiments. Chemosphere. 1994;29:391–404. [Google Scholar]
- 55.Roslev P, Iversen N. Radioactive fingerprinting of microorganisms that oxidize atmospheric methane in different soils. Appl Environ Microbiol. 1999;65:4064–4070. doi: 10.1128/aem.65.9.4064-4070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell J B, Cook G M. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol Rev. 1995;59:48–62. doi: 10.1128/mr.59.1.48-62.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.SAS Institute. SAS/STAT user guide, release 6.03. SAS Institute Inc., Cary, N.C.
- 58.Siciliano S D, Roy R. The role of soil microbial tests in ecological risk assessment: differentiating between exposure and effects. Hum Ecol Risk Assess. 1999;5:671–682. [Google Scholar]
- 59.Watson S W, Graham L B, Remsen C C, Valois F W. A lobular, ammonia-oxidizing bacterium, Nitrosolobus multiformis nov. gen. nov. sp. Arch Microbiol. 1971;76:183–203. doi: 10.1007/BF00409115. [DOI] [PubMed] [Google Scholar]
- 60.Welp G, Brümmer G W. Toxicity of increased amounts of chemicals and the dose-response curves for heterogeneous microbial populations in soil. Ecotoxicol Environ Saf. 1997;37:37–44. doi: 10.1006/eesa.1997.1520. [DOI] [PubMed] [Google Scholar]
- 61.Wilke B M. Effects of non-pesticide organic pollutants on soil microbial activity. Adv Geoecol. 1997;30:117–132. [Google Scholar]
- 62.Wilkinson S G. Gram-negative bacteria. In: Ratledge C, Wilkinson S G, editors. Microbial lipids. London, United Kingdom: Academic Press; 1988. pp. 299–488. [Google Scholar]
- 63.Wood N J, Sørensen J. Osmotic stimulation of microcolony development by Nitrosomonas europaea. FEMS Microbiol Ecol. 1998;27:175–183. [Google Scholar]
- 64.Yamada J. Antimicrobial action of sodium laurylbenzenesulfonate. Agric Biol Chem. 1979;43:2601–2602. [Google Scholar]