Abstract
Purpose
The present study was designed to investigate the electromyographic (EMG) response in leg muscles to whole-body vibration while using different body positions and vibration amplitudes.
Methods:
An experimental study with repeated measures design involved a group of community-dwelling middle-aged and older women (n = 15; mean age=60.8 ± 4.18 years). Muscle activity of the gluteus maximus (GM), rectus femoris (RF), vastus medialis (VM), vastus lateralis (VL), biceps femoris (BF), and gastrocnemius (GS) was measured by surface electromyography, which participants were performing three different body positions during three WBV amplitudes. The body positions included static semi-squat, static semi-squat with elastic band loading, and dynamic semi-squat. Vibration stimuli tested were 0 mm, 2 mm, and 4 mm amplitude and 30 Hz frequencies. And the maximum accelerations produced by vibration stimuli with amplitudes of 2 mm and 4 mm are approximately 1.83 g and 3.17 g.
Results:
Significantly greater muscle activity was recorded in VL, BF, and GS. When WBV was applied to training, compared with the same training without WBV (P < .05). There were significant main effects of body positions on EMGrms for the GM, RF, and VM (P < .05). Compared to static semi-squat, static semi-squat with elastic band significantly increased the EMGrms of GM, and dynamic semi-squat significantly increased the EMGrms of GM, RF and VM (P < .05). And there were significant main effects of amplitudes on EMGrms for the GM, RF, and VM (P < .05). The EMGrms of the VL, BF, and GS at 4 mm were significantly higher than 0 mm, and the EMGrms of the VL and BF at 4 mm were significantly higher than 2 mm. There was no significant body interaction between body positions and amplitudes (P > .05).
Conclusions:
The EMG amplitudes of most leg muscles tested were significantly greater during WBV exposure than in the no-WBV condition. The dynamic semi-squat 4 mm whole-body vibration training is recommended for middle-aged and older women to improve lower limb muscle strength and function.
Keywords: whole-body vibration, surface electromyography, middle-aged and older women, amplitude, body position, root mean square amplitude (EMGrms)
Introduction
Older individuals typically experience impairments in physical functioning and an increasing incidence of chronic health problems such as osteoporosis, frailty, sarcopenia, or cardiovascular disease. The aging process is associated with the loss of muscle mass, reduced strength, and impairment of physical functioning. 1 The most physiologic means to fight this decline of muscle mass and function is a physically active lifestyle or physical exercise.2,3 Indeed, many exercise studies proved favorable changes in muscle mass, power, and strength parameters. 4 However, to realize relevant changes in muscle mass, strength, and power, exercise has to be performed regularly with moderate exercise frequency (≥2 sessions/week) and moderate to high levels of intensity.5,6 Due to physical limitations or lack of motivation, many elderly subjects seem to be either unable or unwilling to perform (intense) corresponding resistance exercise programs. In this context, exercise technologies that increase the impact of low-level exercise on the musculoskeletal system are of high relevance.
Whole-body vibration (WBV) is a relatively new exercise modality gaining significant interest in geriatric rehabilitation and fitness. This exercise typically involves individuals performing traditional resistance exercises on the platform with their body mass as resistance. It has been shown that mechanical vibrations applied to the muscle or tendon stimulate sensory receptors, mainly length-detecting muscle spindles. 7 The activation of these muscle spindles facilitates the activation of alpha-motoneurons, leading to reflex muscle contractions (tonic vibration reflex). 7 This response is mediated by monosynaptic and polysynaptic pathways and increases motor unit activation. 8 Several studies showed that whole-body vibration (WBV) training, in which subjects perform unloaded exercises on a vibrating platform, improved muscle strength or performance.9,10
Several experimental studies have demonstrated the effect of WBV on muscle activation,6,11-16 and virtually all reported an increase in electromyographic (EMG) amplitude upon adding vibration to various exercises.6,13-16 However, all existing WBV studies on EMG responses were conducted in either the healthy young population (mean age ≤32.7 years)6,11,15,16or in people with stroke.17,18 Skeletal formation and muscle adaptation mechanisms are different in older and younger people, and the same mechanical stimuli do not have the same effect on different groups. 19 Furthermore, middle-aged and older women experience a faster decline in muscle mass, muscle strength and bone density than men of the same age after menopause. 20 They are also more likely to suffer from chronic diseases such as osteoporosis and sarcopenia, and have higher rates of disability from falls. 21 Therefore, we should carry out further research on target groups of middle-aged and older women.
Recently, electromyography has been used to characterize muscle activation, and previous literature has demonstrated that exposure to WBV results in an increase in leg muscle EMG activity. Furthermore, from a biomechanical perspective, WBV exposure increases exercise intensity by increasing the accelerations of the body and because force = mass × acceleration, increasing the amplitudes or frequency at a fixed mass the resultant should be an increase in force. Although EMG is not a direct measure of force, they are strongly correlated, so any increase in force should be represented by an increase in EMG.
The intensity of the required muscle contraction also changes, when the posture is changed or when a different posture is maintained. Therefore, the choice of body position during vibration training can influence the intensity of muscle strength training to some extent. 22 Lam, F. M et al. 23 compared the effects of static erect stand, static deep squat, static semi-squat and single-legged static squat on the activation of lower limb muscles and showed that static erect stand and single-leg squat induced maximal activation of lower limb muscles. Roelants, M. et al. 16 also demonstrated that single-leg squats significantly increased lower limb muscle activity compared to double leg squats. Due to the age of the subjects, it is difficult to ensure the standardized and safety of the movement during the single-leg squat. Static erect stand was prone to adverse reactions such as dizziness, and heel raises reduce the shock to the head to a certain extent. Therefore, this study excluded the static erect stand and single-leg squat, and instead of using a static semi-squat and dynamic semi-squat for vibration training. In addition to these 2 positions, the intensity of the vibration training was increased by adding additional load using a mini elastic band to compare the effects of the three-body positions on lower limb muscle activation.
This study aimed to quantify and analyze the effects of variations in the body positions and amplitudes on the neuromuscular responses of the lower limbs to exercise. A better insight into muscle activation while performing standard exercises on a WBV platform is undoubtedly helpful to determine the potential of WBV programs in training.
Methods
Subjects
Among the 20 women belonging to communities in Beijing, 15 middle-aged and older women who were at least 3 years postmenopausal and not affected by conditions that contraindicated the vibration training were enrolled in the study population. Assessment of eligibility and health status for participation was based on screening by questionnaire. Women had to be between 55 and 70 years of age, non-institutionalized, and free from hormones or medications that are known to affect muscle strength or bone metabolism. And none of the subjects had any experience with whole-body vibration training prior to participating in this experiment. Their average age, year of menopause, height, and weight were respectively (mean ±SD): 60.8 ± 4.18 years, 10.11 ± 5.67 years,1.58 ± .04 m, 61.96 ± 9.42 kg. The subjects were aware of the purpose of the study, and they all provided written informed consent. This study was reviewed and approved by the Ethics Committee of Capital University of Physical Education and Sports. The exclusion criteria included a history of back pain, acute inflammation in the pelvis or lower extremities, acute thrombosis, recent fractures, recent implants, gallstones, kidney or bladder stones, any disease of the spine, peripheral vascular disease, and severe delayed onset of muscle soreness in leg muscles.
Experimental Design
Prior to the experimental protocol, subjects underwent a familiarization session to acclimate to the sensation of WBV. This session consisted of standing with feet shoulder-width apart on the vibration platform in a comfortable static semi-squat with their knees flexed at an angle of approximately 45°. Subjects were given a demonstration of proper technique for the squat position and were allowed to practice until they performed the squats correctly and verbal instruction was provided to complete the squats at a constant pace of 2 s down, 1s up and 1s interval.
The subjects were exposed to multidimensional whole-body vibration using a vibratory platform (USA, Power Plate Pro5 AIR daptive). In a single-group, the repeated measures study design was used in which the EMGrms of 2 leg muscles were the dependent variables. The independent variables were the 3 different amplitudes of whole-body vibration (0 mm, 2 mm, and 4 mm) and 3 different body positions (static semi-squat, static semi-squat with elastic band loading, and dynamic semi-squat). And the vibration training platform with a frequency of 30 Hz, separated by 5 min pause between trials,24,25 and each trial lasted 35s. The order of the trials for each subject was randomized across the frequencies and the positions.
The design of the vibration protocol was obtained from a review of the literature. The frequencies of 15–35 Hz were commonly used by previous researchers to maximize access to the conduction of mechanical stimuli. 26 The results of the Lienhard K. et al. 27 study showed that an increase in vibration frequency did not necessarily lead to sEMG enhancements in the calf and thigh muscles. The results of Lam FM et.al 23 concluded that there was no significant difference in the activation effect of 30 Hz and 40 Hz vibration stimulation on the rectus femoris, lateral femoris, and gastrocnemius muscles. And he suggests that the optimal vibration frequency is 35 Hz for young women and 30 Hz for middle-aged and older women. 25 This is generally consistent with the results of my other study. In addition, subjects need to keep their heels off the vibration platform in order to reduce the vibration to the head. And Di Giminiani R. et al. 12 suggest that the half-squat position with the heels raised is more conducive to improving muscle activity in the lateral femoral and gastrocnemius muscles than the heels on the ground. Therefore, a semi-squat mode intermittent vibration training program with a frequency of 30 Hz and heels off the ground was used in this study. And the subjects wore uniform floor socks during the study to prevent attenuation of shoe vibration.
The three-body positions included: (1) static semi-squat: The subject squats on the vibration platform without shoes, lifting both heels off the vibration platform, holding the handrails lightly with both hands, keeping the knees flexed at an angle of approximately 45°;(2) static semi-squat with elastic band loading: A green mini elastic band (Joinfit brand, 40lbs) is placed 3 cm above the knee in the static semi-squat position;(3) dynamic semi-squat: The subject stands on the vibration platform, feet shoulder-width apart, both heels raised slightly, squatting with knee flexion between 5° and 45°. The rhythm of the half squat is 4s/rep, 2s down, 1s up, 1s interval. Subjects followed a laptop video to complete 8 dynamic semi-squat whole-body vibration training.
Joint angle measurements were collected with a goniometer to ensure subjects maintained the required position; joint angles were not used in the analytical measurements. However, subjects were provided verbal feedback for the joint position throughout the protocol and the movement position was self-corrected. Generally, subjects were consistent in maintaining the required joint angle.
The acceleration of the vibration stimuli was controlled and recorded with the help of a tri-axial accelerometer (Actigraph, Ft.Walton Beach, USA) that was placed on the platform in alignment with the third toe. 28 Analysis of the acceleration signal revealed that the acceleration stimulus was mainly in the vertical plane. The results of the accelerometer tests show that 30 Hz, 2 mm and 30 Hz 4 mm correspond to maximum accelerations of approximately 1.83 g and 3.17 g.
EMG Measurements and Processing
The recordings of the myoelectric activity were performed through POCKET EMG (BTS FreeEMG300, Italy), which is a portable unit for collecting and transmitting data wirelessly to the computer. The sampling frequency of 1000 Hz was used. The surface EMG signals from the gluteus maximus (GM), rectus femoris (RF), vastus medialis (VM), vastus lateralis (VL), biceps femoris (BF), and gastrocnemius (GS) (medial) muscle of both legs were recorded bipolarly by disposable 20-mm disc electrodes (Blue Sensor Ag/AgCl). The electrodes were fixed lengthwise over the middle of the muscle belly with an interelectrode (center-to-center) distance of 20 mm. Before the electrodes were attached, the subjects' skin was cleaned of the dead epidermis with abrasive paste and then degreased.
After the acquisition, the raw data was further processed utilizing the EMG-Analyzer software. There were the following steps undertaken in the process of EMG signal analysis: filtering with a bandpass filter (3th order Butterworth filter of 100–480 Hz); the root mean square method (RMS) 29 was used with moving time window (100 ms) for the whole 35s trial. For the 2 static exercises, the middle twenty seconds of the data were used to calculate the EMG root mean square (EMGrms) value, and the EMGrms values of the 2 trials were averaged to obtain a mean value. For the dynamic squat exercises, the middle 5 repetitions were used to calculate the EMGrms.
Statistical Analysis
Statistical analyses were conducted with IBM SPSS software (version 20.0; IBM, Armonk, NY). The normality of the samples was calculated according to the Shapiro–Wilk test. Two-way ANOVA with repeated measures (3[amplitudes]×3[body positions]) was conducted to examine the EMGrms data across different conditions separately for each muscle tested. Greenhouse–Geisser epsilon adjustment was used when the sphericity assumption was violated (P < .05). The stated ANOVA model would provide information on: 1) the main effect of amplitudes; 2) the main effect of body positions; 3) the effect of the amplitudes×body positions. If the main effect of amplitudes or body positions were significant, an LSD post hoc test was used to determine which changes in factors contributed to the overall significant results. The significance level was set at P < .05.
Results
The repeated measures ANOVA applied to compare the effects of different body positions and amplitudes on lower limb EMGrms. The results of the study revealed no significant interaction between body positions and amplitudes (P > .05) (Table 1). However, the results showed that there were significant main effects of body position on EMGrms for the GM, RF, and VM (P < .05), and there were no significant main effects on VL, BF, and GS (P > .05); There were significant main effects of amplitudes on EMGrms for the VL, BF and GS (P < .05) and no significant main effects of amplitudes on GM, RF, and VM (P > .05).
Table 1.
Repeated measures ANOVA results for the main effects and interaction effects of different body positions and different amplitudes.
| Body positions | amplitudes | Body positions*amplitudes | ||
|---|---|---|---|---|
| gluteus maximus (GM) | F | 2.056 | 2.821 | .562 |
| P | .023* | .102 | .576 | |
| Rectus femoris (RF) | F | 6.882 | .009 | .829 |
| P | .013* | .962 | .421 | |
| Vastus medialis (VM) | F | 5.887 | 3.540 | 1.485 |
| P | .022* | .068 | .239 | |
| Vastus lateralis (VL) | F | .927 | .687 | .761 |
| P | .407 | .016* | .456 | |
| Biceps femoris (BF) | F | .546 | 54.694 | .409 |
| P | .499 | .000* | .580 | |
| gastrocnemius (GS) | F | 2.411 | 3.143 | .312 |
| P | .105 | .042* | .464 |
Asterisk indicates a significant difference (P < .05).
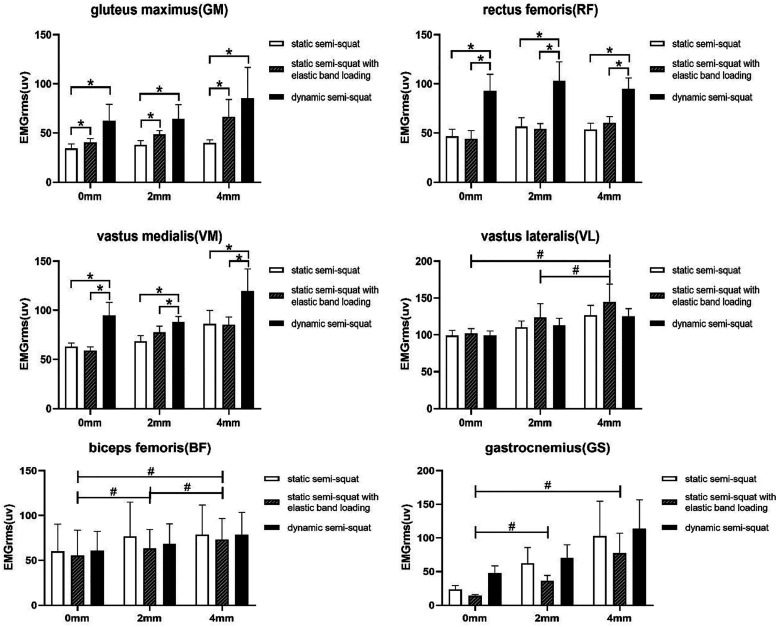
After we have clarified the main effects of body position and amplitude, we should perform post hoc tests on the EMGrms values of muscles with significant main effects of body positions and amplitudes (see Table 2 and Figure 1). Post hoc tests on EMGrms of lower limb muscles in different body positions showed that the EMGrms for the GM was significantly higher in the static semi-squat with elastic band loading and the dynamic semi-squat than in the static semi-squat (P < .05), but no significant difference between static semi-squat with elastic band loading and dynamic semi-squats (P > .05). And the EMGrms for the RF and VM were significantly higher in the dynamic semi-squat than in the static semi-squat, and the static semi-squat with elastic band applied (P <.05), but no significant difference between static semi-squats and static semi-squat with elastic band loading (P > .05). Post hoc tests on EMGrms of lower limb muscles in different amplitudes showed that the VL, BF and GS EMGrms at 4 mm were significantly higher than 0 mm. And the BF and GS EMGrms at 2 mm were significantly higher than 0 mm. The VL and BF EMGrms at 4 mm were significantly higher than 2 mm.
Table 2.
LSD-t post hoc test results for lower limb muscles EMGrms of different body postures and amplitudes.
| Body positions | amplitudes | |||||
|---|---|---|---|---|---|---|
| Static semi-squat/static semi-squat with elastic band loading | Static semi-squat/dynamic semi-squat | Static semi-squat with elastic band loading/dynamic semi-squat | 0mm/2 mm | 0mm/4 mm | 2mm/4 mm | |
| gluteus maximus (GM) | .024* | .021* | .329 | .283 | .095 | .114 |
| Rectus femoris (RF) | .399 | .009* | .018* | .941 | .972 | .837 |
| Vastus medialis (VM) | .733 | .011* | .038* | .155 | .047 | .120 |
| Vastus lateralis (VL) | .313 | .925 | .292 | .051 | .016* | .009* |
| Biceps femoris (BF) | .425 | .827 | .122 | .000* | .000* | .001* |
| gastrocnemius (GS) | .266 | .331 | .038 | .016* | .018* | .164 |
The LSD-t post hoc test summary on 15 subjects of the electromyography root mean square (EMGrms) in case of three different body positions and three different amplitudes. P-values were reported.
Asterisk indicates a significant difference (P < .05).
Figure 1.
Electromyography root mean square (EMGrms) of the gluteus maximus muscle (GM), restus femoris (RF), vastus medialis (VM), vastus lateralis (VL), biceps femoris (BF) and gastrocnemius (GS) in different body positions and amplitudes conditions. Values are mean ±SD. *P < .05 represent the different body postures at the same amplitude. #P < .05 represent the different amplitude at the same body postures.
Discussion
Using various combinations of vibration parameters, we could provide important insight into the WBV body positions and amplitude on the effect of lower limb muscle activity induced.
Influence of Body Positions
Numerous studies have been conducted to increase the intensity of whole-body vibration training by increasing the external body load. Considering the safety of middle-aged and older women and the limitations of their fitness level on their ability to exercise, they often train by resisting their body weight. 30 The sequence of force generation and tension distribution in the muscles varies due to different movement patterns. Variations in damping coefficients and joint angles in various body parts directly affect the efficiency of physical vibration absorption and transmission in various parts of the body. 31 This reason ultimately leads to differences in the effect of vibration on the strength training of each muscle group. So the choice of body position influences to some extent, the intensity of muscle strength training. 32
The results of this study showed a significant main effect of body position on EMGrms for the gluteus maximus, rectus femoris, and vastus medialis (P < .05). Pairwise comparisons of the EMGrms of the gluteus maximus in different body positions showed that the static semi-squat with elastic bands loading and dynamic semi-squat significantly increased muscle activity of the gluteus maximus compared to the static semi-squat. The dynamic semi-squat increased muscle activity in the rectus femoris and vastus medialis compared to the static semi-squat and static semi-squats with elastic bands loading. This suggests that dynamic semi-squat may be more beneficial to the development of lower limb muscle group strength in middle-aged women than static semi-squats and static semi-squats with elastic bands.
Although many studies have been conducted on vibration training body positions, relatively few studies compare whole-body vibration training protocols in static and dynamic semi-squat positions. Two studies by Hazell TJ and Lam, F. M. on the effects of both static and dynamic semi-squat positions on lower limb electromyography. The Hazell TJ study concluded that the more intense vibration frequencies of 35–45 Hz with 4 mm amplitude elicited the greatest EMG responses in the upper-and lower-body muscles measured during both static and dynamic contractions. The study by Lam, F. M. 23 focused on different postures on muscle activation in the lower limbs. His study concluded that muscle activation in the lower limb was stronger in static upright and static single-leg stances than in static and dynamic semi-squats. However, neither of their studies compared the significance of the difference between the effects of static and dynamic half-squat positions on lower limb muscle activation.
External loading is a particularly interesting strategy as the intensity of the WBV stimulus can be boosted without increasing the extent of the acceleration. Chronic exposure to occupational vibration has been linked to many side effects such as spinal degeneration, low back pain, and visual impairment. 33 Thus, external loading is a better alternative to increase the WBV stimulus than increasing the magnitude of the acceleration. Elderly individuals have the option to use elastic belt as an alternative to weight bars, to improve the muscle activity of the gluteus maximus and avoid mechanical stress at the spine level.
Previous studies have used vibration training body positions that resist their own body weight including static semi-squat,15,16,34 dynamic semi-squat, 23 forefoot stands,12,15 static single-leg-standing, 23 etc. Middle-aged and older women have more difficulty completing single-leg squats or adding extra weight to the vibration platform, and the quality of the movement is easily compromised. In addition, whole-body vibration training in the static upright is likely to cause head discomfort in older adults. 35 Therefore, static upright and single-leg squats were excluded from the selection of vibration training positions. The study aimed to discuss the effects of whole-body vibration training on lower limb muscle activity in middle-aged and older women with different forms of contraction against self-gravity or with elastic band loading. It is hoped that this will provide a more objective and reliable theoretical reference for developing and applying whole-body vibration training exercise prescriptions for middle-aged and older women.
Influence of Amplitudes
Amplitude is the maximum value at which the physical quantity of vibration deviates from its equilibrium position when an object undergoes periodic vibration. It is measured in millimeters (mm). The biomechanical mechanisms of whole-body vibration training determine the important role that amplitude plays in controlling the intensity of vibration training. 36 The higher the amplitude of whole-body vibration training, the better. When the frequency of vibration is fixed, high amplitude leads to excessive gravitational acceleration, which tends to resonate with different parts of the body, posing a risk to the health and safety of the subject. 37 Therefore, previous studies have established a relatively safe and effective amplitude range of 2 mm–10 mm. 6 In this paper, through a review of the literature, the safety of vibration training and its adaptability to middle-aged and older people were considered. The amplitudes were set at 0 mm, 2 mm, and 4 mm.
The study results show that WBV can effectively induce leg muscle activity in the three modes of exercise, especially the VL, BF, and GS. Our VL, BF, and GS results are generally in line with those reported in the young adult population.12,37-39 When compared with the no-WBV condition, the results of this study found a significant increase in muscle activity in VL (by 11.1%–42.15%), GS (46.84%–453.00%), and BF (12.4%–32.3%) in WBV conditions. The degree of muscle activation induced by WBV was higher than that in the young healthy population. For instance, the healthy young subjects in the study by Abercromby et al. 40 assumed a similar squatting position while being exposed to a vibration intensity up to 7.24 g. The acceleration of the vibrating platform he applied was much higher than the acceleration of 1.83 g and 3.17 g applied in this study. However, he reported increases in EMG were 132% and 9% in the GS and BF, respectively, which were lower than the values reported here. Compared with healthy young adults, the basal activation of lower limb muscles during vibration-free half squats was relatively low in middle-aged and older women. This may partially explain why the WBV-induced increase in leg muscle activity was more substantial among middle-aged and older women.
The high peak-to-peak displacement of the platform (4 mm) increased EMGrms in VL and BF to a higher extent than with the low peak-to-peak displacement (2 mm). This is in general agreement with the findings of Mester J., 41 Krol P, 42 Lienhard K. 27 Perchthaler D, 34 and Simsek D. 43 They all confirmed that superimposed vibration stimulation on top of simple semi-squat training could significantly increase the activation of lower limb muscles. The higher amplitude (4 mm) was more effective than, the lower amplitude (2 mm) whole-body vibration training in activating the lower limb muscles. Henceforth, and according to the percentage increases in muscle activity between the displacements of 0 mm and 4 mm (i.e., +32.39% for the VL, and +30.74% for the BF), we suggest that selecting the highest WBV peak-to-peak displacement is of high importance. Therefore, increasing the displacement of the platform during WBV is a successful strategy to enhance muscle activity in lower limb muscles. This is particularly valuable when designing WBV protocols. As an example, a recent chronic WBV study has given evidence to believe that the use of a high displacement could also lead to a leaner body mass compared to the low displacement. 44 As mentioned in the Introduction, increased activity of tensed muscles during the vibration training is likely to be related to the appearance of the stretch reflex. 7 The increased signal of EMGrms, which was obtained while the higher amplitude of vibration at the same frequency was applied, may be associated with faster and bigger stretching of the muscle.
Interaction Effects of Body positions ×amplitudes
The results of this paper examining the interaction effect of body position and amplitude showed that they had no significant interaction effect on the neuromuscular activation of the lower limb muscle groups in middle-aged and older women (P > .05). This is in general agreement with the findings of Lam L.R. 23 Therefore, the body position and amplitude parameters can be adjusted individually to control the intensity of the vibration training when developing a whole-body vibration training program for middle-aged women.
Conclusions
The EMG amplitude of most leg muscles tested was significantly greater during WBV exposure than in the no-WBV condition. The results indicate that higher vibration amplitudes (4 mm) result in maximal VL, BF, and GS activation. Dynamic semi-squats effectively increase muscle activity in GM, RF, and VM compared to static semi-squats and elastic band static semi-squats. The dynamic semi-squat 4 mm whole-body vibration training is recommended for middle-aged and older women to improve lower limb muscle strength and function.
Limitations
This study provides a comprehensive analysis of the activation effect of whole-body vibration training on lower limb muscles with different body positions and amplitudes and finds that whole-body vibration training can effectively improve the activation effect of lower limb muscles in middle-aged and older women. The study found that whole-body vibration training effectively improved the activation of lower limb muscles in middle-aged and older women and that the choice of body position and amplitude of vibration was site-specific. However, the following limitations of the study remain: firstly, only healthy middle-aged and older women were studied in this study. Future studies could expand the range of subjects to clarify the muscle activation characteristics of whole-body vibration training in different target groups. Secondly, this study only analyzed the immediate electromyographic characteristics of whole-body vibration training with different protocols. The long-term effects of whole-body vibration training on the muscle health of older people need to be further investigated in the future.
Footnotes
Author Contributions: YXL and XHC designed the research study and performed the research. YZF provided help and advice on the whole-body vibration experiments. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the National Key Research and Development Program of China (No. 2020YFC2004900).
Ethical Approval: The ethical committee ethically approved the study of the Capital University of Physical Education and Sports and all study procedures were under relevant guidelines.
Informed Consent: All the study participants signed an informed consent form.
ORCID iD
Yongzhao Fan https://orcid.org/0000-0002-9073-8361
References
- 1.Abellan van Kan G. Epidemiology and consequences of sarcopenia. J Nutr Health Aging. 2009;13(8):708-712. [DOI] [PubMed] [Google Scholar]
- 2.Abercromby AF, Amonette WE, Layne CS, Mcfarlin BK, Hinman MR, Paloski WH. Vibration exposure and biodynamic responses during whole-body vibration training. Med Sci Sports Exerc. 2007;39(10):1794-1800. [DOI] [PubMed] [Google Scholar]
- 3.Bogaerts A, Delecluse C, Claessens AL, Coudyzer W, Boonen S, Verschueren SMP. Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men: A 1-year randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2007;62(6):630-635. [DOI] [PubMed] [Google Scholar]
- 4.Chou CH, Hwang CL, Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: A meta-analysis. Arch Phys Med Rehabil. 2012;93(2):237-244. [DOI] [PubMed] [Google Scholar]
- 5.Bogaerts A, Verschueren S, Delecluse C, Claessens AL, Boonen S. Effects of whole body vibration training on postural control in older individuals: A 1 year randomized controlled trial. Gait Posture. 2007;26(2):309-316. [DOI] [PubMed] [Google Scholar]
- 6.Cardinale M, Lim J. Electromyography activity of vastus lateralis muscle during whole-body vibrations of different frequencies. J Strength Cond Res. 2003;17(3):621-624. [DOI] [PubMed] [Google Scholar]
- 7.Hagbarth KE, Eklund G. Tonic vibration reflexes (TVR) in spasticity. Brain Res. 1966;2(2):201-203. [DOI] [PubMed] [Google Scholar]
- 8.Park HS, Martin BJ. Contribution of the tonic vibration reflex to muscle stress and muscle fatigue. Scand J Work Environ Health. 1993;19(1):35-42. [DOI] [PubMed] [Google Scholar]
- 9.Delecluse C, Roelants M, Verschueren S. Strength increase after whole-body vibration compared with resistance training. Med Sci Sports Exerc. 2003;35(6):1033-1041. [DOI] [PubMed] [Google Scholar]
- 10.Roelants M, Delecluse C, Goris M, VerSchueren S. Effects of 24 weeks of whole body vibration training on body composition and muscle strength in untrained females. Int J Sports Med. 2004;25(1):1-5. [DOI] [PubMed] [Google Scholar]
- 11.Avelar NC, Ribeiro VG, Mezêncio B, et al. Influence of the knee flexion on muscle activation and transmissibility during whole body vibration. J Electromyogr Kinesiol. 2013;23(4):844-850. [DOI] [PubMed] [Google Scholar]
- 12.Di Giminiani R, Masedu F, Tihanyi J, Scrimaglio R, Valenti M. The interaction between body position and vibration frequency on acute response to whole body vibration. J Electromyogr Kinesiol. 2013;23(1):245-251. [DOI] [PubMed] [Google Scholar]
- 13.Di Giminiani R, Masedu F, Padulo J, Tihanyi J, Valenti M. The EMG activity-acceleration relationship to quantify the optimal vibration load when applying synchronous whole-body vibration. J Electromyogr Kinesiol. 2015;25(6):853-859. [DOI] [PubMed] [Google Scholar]
- 14.Lienhard K, Vienneau J, Nigg S, Meste O, Colson SS, Nigg BM. Relationship between lower limb muscle activity and platform acceleration during whole-body vibration exercise. J Strength Cond Res. 2015;29(10):2844-2853. [DOI] [PubMed] [Google Scholar]
- 15.Ritzmann R, Gollhofer A, Kramer A. The influence of vibration type, frequency, body position and additional load on the neuromuscular activity during whole body vibration. Eur J Appl Physiol. 2013;113(1):1-11. [DOI] [PubMed] [Google Scholar]
- 16.Roelants M, Verschueren SM, Delecluse C, Levin O, Stijnen V. Whole-body-vibration-induced increase in leg muscle activity during different squat exercises. J Strength Cond Res. 2006;20(1):124-129. [DOI] [PubMed] [Google Scholar]
- 17.Liao LR, Lam FM, Pang MY, Jones AYM, Ng GYF. Leg muscle activity during whole-body vibration in individuals with chronic stroke. Med Sci Sports Exerc. 2014;46(3):537-545. [DOI] [PubMed] [Google Scholar]
- 18.Liao LR, Ng GY, Jones AY, Chung RC, Pang MY. Effects of vibration intensity, exercise, and motor impairment on leg muscle activity induced by whole-body vibration in people with stroke. Phys Ther. 2015;95(12):1617-1627. [DOI] [PubMed] [Google Scholar]
- 19.Prisby RD, Lafage-Proust MH, Malaval L, Belli A, Vico L. Effects of whole body vibration on the skeleton and other organ systems in man and animal models: What we know and what we need to know. Ageing Res Rev. 2008;7(4):319-329. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira LC, Oliveira RG, Pires-Oliveira DA. Effects of whole body vibration on bone mineral density in postmenopausal women: A systematic review and meta-analysis. Osteoporos Int. 2016;27(10):2913-2933. [DOI] [PubMed] [Google Scholar]
- 21.Di Z. An epidemiological study on the occurrence and factors influencing falls among the elderly in a rural community in Miyun, Beijing: Chinese People's Liberation Army Medical College; 2016. [Google Scholar]
- 22.Yan Y. Progress in the study of the effect of vibration training on muscle H-reflex, T-reflex and surface EMG signals. Chinese Journal of Sports Medicine. 2016;35(06):581-587. [Google Scholar]
- 23.Lam FM, Liao LR, Kwok TC, Pang MY. The effect of vertical whole-body vibration on lower limb muscle activation in elderly adults: Influence of vibration frequency, amplitude and exercise. Maturitas. 2016;88:59-64. [DOI] [PubMed] [Google Scholar]
- 24.Pujari AN, Neilson RD, Cardinale M. Effects of different vibration frequencies, amplitudes and contraction levels on lower limb muscles during graded isometric contractions superimposed on whole body vibration stimulation. J Rehabil Assist Technol Eng. 2019;6:2055668319827466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlucci F, Orlando G, Haxhi J, et al. Older age is associated with lower optimal vibration frequency in lower-limb muscles during whole-body vibration. Am J Phys Med Rehabil. 2015;94(7):522-529. [DOI] [PubMed] [Google Scholar]
- 26.Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: Determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine. 2003;28(23):2621-2627. [DOI] [PubMed] [Google Scholar]
- 27.Lienhard K, Cabasson A, Meste O, Colson SS. Determination of the optimal parameters maximizing muscle activity of the lower limbs during vertical synchronous whole-body vibration. Eur J Appl Physiol. 2014;114(7):1493-1501. [DOI] [PubMed] [Google Scholar]
- 28.Lorenzen C, Maschette W, Koh M, Wilson C. Inconsistent use of terminology in whole body vibration exercise research. J Sci Med Sport. 2009;12(6):676-678. [DOI] [PubMed] [Google Scholar]
- 29.Soderberg GL, Knutson LM. A guide for use and interpretation of kinesiologic electromyographic data. Phys Ther. 2000;80(5):485-498. [PubMed] [Google Scholar]
- 30.Yuan Y. Characteristics and mechanism of neuromuscular adaptation to weight whole-body vibration training. Shang Hai: Shanghai University of Sport; 2013. [Google Scholar]
- 31.Huang Y. Mechanism of nonlinear biodynamic response of the human body exposed to whole-body vibration[D]. University of Southampton; 2008. [Google Scholar]
- 32.Yuan Y, Su Y, wu Y. Progress in the study of the effects of vibration training on muscle H-reflexes, T-reflexes and surface EMG signals. Chinese Journal of Sports Medicine. 2016;35(06):581-587. [Google Scholar]
- 33.Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31(1):3-7. [DOI] [PubMed] [Google Scholar]
- 34.Perchthaler D, Horstmann T, Grau S. Variations in neuromuscular activity of thigh muscles during whole-body vibration in consideration of different biomechanical variables. J Sports Sci Med. 2013;12(3):439-446. [PMC free article] [PubMed] [Google Scholar]
- 35.Caryn RC, Dickey JP. Transmission of acceleration from a synchronous vibration exercise platform to the head during dynamic squats. Dose Response. 2019;17(1):1559325819827467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng L. Effect of different whole -body frequency filtration training on blood pressure and bone mineral density of the lower extremities in postmenopausal women. Chinese Journal of Osteoporosis. 2018;24(3):305331-306310. [Google Scholar]
- 37.Tankisheva E, Jonkers I, Boonen S, et al. Transmission of whole-body vibration and its effect on muscle activation. J Strength Cond Res. 2013;27(9):2533-2541. [DOI] [PubMed] [Google Scholar]
- 38.Runge M, Hunter G. Determinants of musculoskeletal frailty and the risk of falls in old age. J Musculoskelet Neuronal Interact. 2006;6(2):167-173. [PubMed] [Google Scholar]
- 39.Hazell TJ, Kenno KA, Jakobi JM. Evaluation of muscle activity for loaded and unloaded dynamic squats during vertical whole-body vibration. J Strength Cond Res. 2010;24(7):1860-1865. [DOI] [PubMed] [Google Scholar]
- 40.Abercromby AF, Amonette WE, Layne CS, Mcfarlin BK, Hinman MR, Paloski WH. Variation in neuromuscular responses during acute whole-body vibration exercise. Med Sci Sports Exerc. 2007;39(9):1642-1650. [DOI] [PubMed] [Google Scholar]
- 41.Mester J, Kleinöder H, Yue Z. Vibration training: benefits and risks. Journal of biomechanics. 2006;39(6):1056-1065. [DOI] [PubMed] [Google Scholar]
- 42.Krol P, Piecha M, Slomka K, Sobota G, Polak A, Juras G. The effect of whole-body vibration frequency and amplitude on the myoelectric activity of vastus medialis and vastus lateralis. J Sports Sci Med. 2011;10(1):169-174. [PMC free article] [PubMed] [Google Scholar]
- 43.Simsek D. Different fatigue-resistant leg muscles and EMG response during whole-body vibration. J Electromyogr Kinesiol. 2017;37:147-154. [DOI] [PubMed] [Google Scholar]
- 44.Marín PJ, Bunker D, Rhea MR, Ayllon FN. Neuromuscular activity during whole-body vibration of different amplitudes and footwear conditions: implications for prescription of vibratory stimulation. J Strength Cond Res. 2009;23(8):2311-2316. [DOI] [PubMed] [Google Scholar]