Abstract
Background:
Menstrual disorders were not reported as a possible secondary effect in any of the clinical trials for the SARS-CoV-2 vaccines.
Aim:
To describe the prevalence of perceived premenstrual and menstrual changes after COVID-19 vaccine administration.
Design:
Cross-sectional study.
Methods:
A total of 14,153 women (mean age 31.5 ± 9.3 years old) who had received the full course of vaccination at least three months earlier were included in this cross-sectional study. Data including the type of vaccine administered, perceived changes in the amount and duration of menstrual bleeding, presence of clots, cycle length, and premenstrual symptoms were collected through a retrospective online survey from June to September 2021.
Results:
Of the women who participated in this study, 3136 reported no menstrual changes and 11,017 (78% of the study sample) reported experiencing menstrual cycle changes after vaccination. In summary, women who reported menstrual changes after vaccination were older (overall p < 0.001) and slightly more smokers (p = 0.05) than women who did not report any changes. The most prevalent changes in relation to premenstrual symptoms were increased fatigue (43%), abdominal bloating (37%), irritability (29%), sadness (28%), and headaches (28%). The most predominant menstrual changes were more menstrual bleeding (43%), more menstrual pain (41%), delayed menstruation (38%), fewer days of menstrual bleeding (34.5%), and shorter cycle length (32%).
Conclusion:
Women vaccinated against COVID-19 usually perceive mild menstrual and premenstrual changes. Future studies are warranted to clarify the physiological mechanisms behind these widely reported changes.
Keywords: fatigue, immunization, menstrual bleeding, menstrual cycle, pain, SARS-CoV-2
Introduction
For decades, the importance of biological sex in relation to health and disease has been recognized. In 1993, a federal law was implemented requiring researchers funded by the United States National Institutes of Health (NIH) to ensure that women were represented in clinical trials, allowing for subsequent analysis of results by sex. 1 Although significant progress has been made in increasing the representation of women in scientific studies, sex disparity is still maintained even in clinical trials in animal models. 2 These differences may lead to a limited understanding of female pathophysiology, which could affect women’s healthcare. In this context, the COVID-19 pandemic has recently shown sex differences even in response to this infectious disease. In this regard, SARS-CoV-2 virus infection rates have been similar in both sexes but with higher rates of hospital admissions and mortality in men. 3 Nevertheless, a recent study explored the inclusion of sex variables in COVID-19 clinical trials registered on ClinicalTrials.gov. The results showed that only 4% of the 4420 registered trials included sex as an analytical variable. 4 Given this data, it is not surprising that none of the clinical trials for the vaccines developed against COVID-19 specifically reported significant health effects specific to women, such as menstrual disorders.5–8
The menstrual cycle is one of the most important physiological processes for female health. It involves a complex hormonal process and it may be affected by external factors including lifestyle, stress, energy deficiency, and drug use. 9 Menstrual disorders can affect the quality of life of women who suffer from them and often increase the need for medical consultations or tests. 10 Nevertheless, menstrual health is also underrepresented in basic and translational research. 11 Although menstrual changes have previously been reported following the administration of other vaccines such as the human papillomavirus vaccine, 12 the COVID-19 vaccine has been administered for the first time to millions of menstruating women around the world in a short space of time. This situation may be associated with the fact that a few months after the vaccination campaign, a large number of women reported on social media that they had noticed premenstrual or menstrual changes. 13 However, this reporting of menstrual-related adverse events on official platforms remains anecdotal. 14 In fact, to date, the Spanish Agency for Medicines and Health Products states that there is no proven causal relationship between the COVID-19 vaccine and menstrual disorders. 15
Therefore, this study aims to describe the prevalence of perceived changes in premenstrual and menstrual symptoms after administration of the COVID-19 vaccine.
Methods
Design
A cross-sectional study was conducted through an online survey. This retrospective study is part of a larger project entitled “The Effect of Vaccination against SARS-CoV-2 on the Menstrual Cycle (EVA Project)” which aims to explore the prevalence and etiology of premenstrual and menstrual changes after COVID-19 vaccine administration.
Participants and data collection
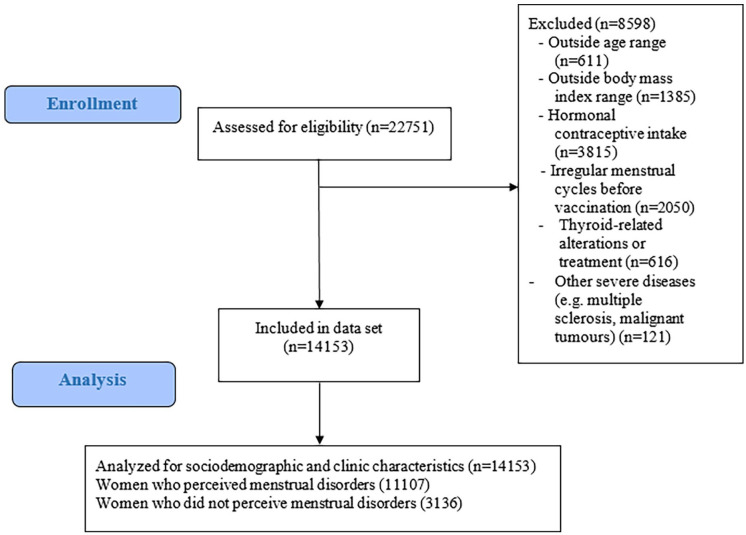
Data were collected retrospectively from women who had received the full vaccination course at least three months earlier with one of the vaccines approved by the European Medicines Agency (EMA).5–8 The online survey was open from June to September 2021. For the present descriptive study aims, the inclusion criteria were (1) age range between 18 and 55 years old; (2) body mass index (BMI) range between 18.5 and 39.9; (3) not presenting thyroid-related alterations or treatment; (4) not presenting polycystic ovarian syndrome; (5) not presenting endometriosis; and (6) not presenting severe diseases (e.g. multiple sclerosis or malignant tumors). In addition, for the present study aims, menopausal individuals, women who were taking hormonal contraceptives and those who received other non-EMA approved vaccines were excluded from the study. Therefore, the vaccine types included were Pfizer-BioNTech (Pfizer), Moderna, Oxford/AstraZeneca, and Johnson&Johnson/Janssen (J&J/Janssen). Women were asked about perceived menstrual changes in relation to pre-vaccination periods through Google Surveys. The flow chart of study participants after applying inclusion and exclusion criteria is shown in Figure 1. Sociodemographic (age, educational status, and daily hours of sun exposure) and clinical (weight and height and history of specific diseases) data were assessed using a self-reported online survey. Age was categorized as 18–24, 25–34, 35–44, and 45–55 years old. Body mass index was calculated as weight (kg)/height (m2) (both self-reported).
Figure 1.
Flowchart of participants for the specific study aims.
The e-survey
For the specific aims of this study, an online survey was designed. Before starting the survey, participants accessed an informative text about the study aims and the average response time for the entire questionnaire, which stated that participation was completely anonymous and voluntary. A final statement on informed consent for using the data exclusively for research purposes was included, which must be signed before continuing. In addition, an email address was provided to allow participants to ask any questions before starting the survey.
This survey was composed of 45 questions divided into different sections: (a) sociodemographic and clinical data (15 questions) such as age, cohabitation data, weight, height, previously diagnosed diseases, and work status; (b) lifestyle habits and drug intake (10 questions) such as smoking habits, alcohol use, and medication; (c) data related to COVID-19 and the administration of the vaccine (7 questions); and (d) perceived premenstrual changes and changes in menstruation after vaccine administration compared to the six previous menstruations (13 questions) (Supplementary Appendix S1).
Ethical considerations
The present cross-sectional study forms part of the EVA project, which was approved by the Clinical Research Ethics Committee of Granada, Government of Andalusia, Spain (code: 130290). Moreover, as mentioned above, participants completed an informed consent statement before starting the survey.
Data analysis
Descriptive statistics are shown as the mean (standard deviation, SD) for quantitative variables and the number of women (%) for categorical variables. Comparisons between women who reported premenstrual or menstrual changes and those who did not report any changes were performed using Student’s t-test. A chi-square test was performed to determine the differences among qualitative variables.
The statistical analysis was conducted with the Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp) and statistical significance was set at p < 0.05.
Results
The sociodemographic and clinical characteristics of study participants are shown in Table 1. Among the 22,751 women who answered the questionnaire, a total sample of 14,153 participants was included for analysis after applying the inclusion and exclusion criteria. Of these, 3136 women did not perceive menstrual changes and 11,017 perceived menstrual changes (78% of the sample). In summary, most of the women who reported more symptoms related to vaccination were older than 35 years (overall p < 0.001) and there were slightly more smokers. Regarding the type of vaccine administrated, women vaccinated with Pfizer or Moderna (ARN-m design/technology based) reported less premenstrual and menstrual-related symptomatology than those vaccinated with Astra-Zeneca or Janssen (adenovirus vectored design/technology based) (overall p = 0.004).
Table 1.
Sociodemographic and clinical characteristics of the study sample.
| All women (n = 14,153) | Women who did not perceive menstrual changes (n = 3136) | Women who perceived menstrual changes (n = 11,017) | Difference between groups p | |
|---|---|---|---|---|
| Age, years, mean, SD | 31.1 (9.17) | 29.3 (8.6) | 31.52 (9.3) | <0.001 |
| 18–24 | 2992 (21.1) | 834 (26.6) | 2158 (19.6) | <0.001 |
| 25–34 | 5575 (39.4) | 1311 (41.8) | 4264 (38.7) | |
| 35–44 | 4189 (29.6) | 763 (24.3) | 3426 (31.1) | |
| 45–55 | 1397 (9.9) | 228 (7.3) | 1169 (10.6) | |
| Current smoker (yes) | 3465 (24.5) | 733 (23.4) | 2732 (24.8) | 0.053 |
| Educational status | Mean (SD) | Mean (SD) | Mean (SD) | |
| Primary or high-school | 2382 (16.9) | 543 (17.3) | 1839 (16.7) | 0.755 |
| Specialized training | 2807 (19.8) | 613 (19.5) | 2194 (19.9) | |
| University degree | 8964 (63.3) | 1980 (63.1) | 6984 (63.4) | |
| Vaccine administered | ||||
| Pfizer-BioNTech (Pfizer) | 8727 (61.7) | 1999 (63.7) | 6728 (61.1) | 0.004 |
| Oxford/AstraZeneca | 2224 (15.7) | 433 (13.8) | 1791 (16.3) | |
| Moderna | 2476 (17.5) | 563 (18) | 1913 (17.4) | |
| Johnson&Johnson/Janssen (J&J/Janssen) | 725 (5.1) | 141 (4.5) | 584 (5.3) | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Body mass index (kg/m2) | 24.13 (4.24) | 24.0 (4.2) | 24.2 (4.2) | 0.060 |
| Exposure to sun (min/day) | 71.2 (77.6) | 72.22 (80.05) | 69.42 (74.9) | 0.079 |
SD: standard deviation.
Values shown as n (percentage) unless otherwise indicated.
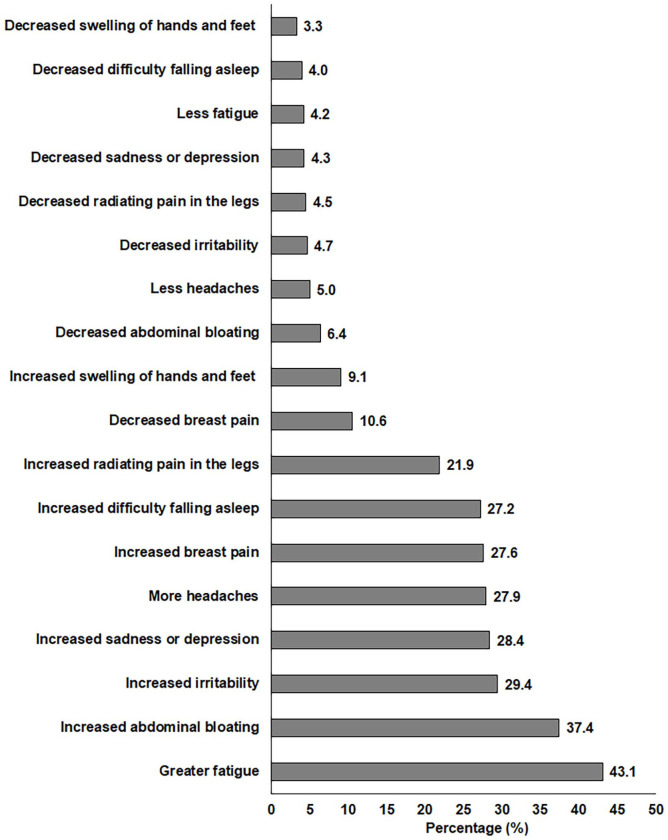
The main premenstrual changes reported by women after vaccination are shown in Figure 2. The most prevalent changes in relation to premenstrual symptoms were increased fatigue (43%), abdominal bloating (37%), emotional irritability (29%), sadness or depression (28%), headaches (28%), breast pain (28%), and difficulty falling asleep (27%).
Figure 2.
Main premenstrual changes reported by women after COVID-19 vaccination.
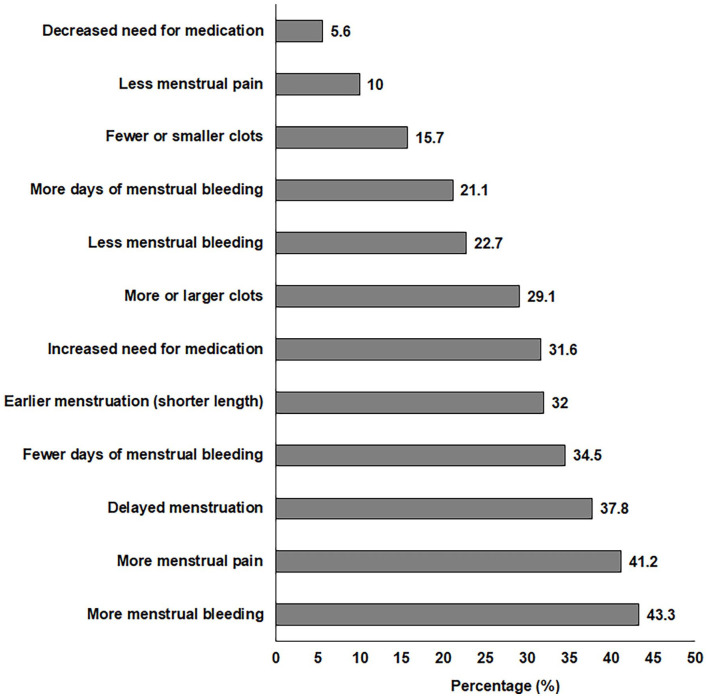
The main menstrual changes reported by women are shown in Figure 3. The most predominant menstrual changes were more menstrual bleeding (43%), more menstrual pain (41%), and delayed menstruation (38%).
Figure 3.
Main menstrual changes reported by women after COVID-19 vaccination.
Discussion
This is one of the first studies describing the prevalence of premenstrual and menstrual changes associated with the COVID-19 vaccine. A major finding of this study is that almost 78% of the women surveyed reported changes in their menstrual cycle. In relation to premenstrual symptoms, women reported increased fatigue, abdominal bloating, irritability, sadness or depression, headaches, and greater difficulty falling asleep compared with pre-vaccination menstruation. The most frequent menstrual changes were: more menstrual bleeding and pain, delayed menstrual cycle, fewer days of menstrual bleeding, shorter cycle length, increased need for medication, and more or larger clots.
To date, no study has explored the potential influence of the COVID-19 vaccination on premenstrual changes,16,17 therefore, we cannot properly compare our results. Increased premenstrual symptoms may strongly affect women’s quality of life, as has been demonstrated in the study by Mushtaq et al. 18 where premenstrual somatic symptoms like headaches, fatigue, backaches, and abdominal bloating negatively affected all aspects of women’s lives: their psychological and physical wellbeing, social life, and work life. 18
Regarding menstrual changes, a recent study found an association between the COVID-19 vaccine and a slight increase in the menstrual cycle length, 16 which is consistent with our findings, where 38% of our study sample reported delayed menstruation. In a sample of 2400 vaccinated women, the authors observed that, overall, the COVID-19 vaccine was associated with a less than 1-day change in cycle length. 16 In order to avoid recall bias, our survey was conducted shortly after the end of the vaccination campaign, so we were unable to collect the duration of reported menstrual changes. Nevertheless, other relevant changes related to menstruation were reported by our study sample, such as more menstrual bleeding and pain by over 40% of the women asked. These results are consistent with those described in a recent study performed on a cohort of 4000 Norwegian women. 17 In that study sample, the most common menstrual changes following vaccine administration were increased bleeding, cycle shortening, and increased menstrual pain. 17 These findings are consistent with those described in a recent study carried out after the administration of the second dose of the vaccine in 164 women. 19 As with our participants, their results show that the most frequently self-reported menstrual change was increased menstrual bleeding. 19 In addition, the participants in our study who perceived menstrual changes after vaccination were more likely to be smokers compared to those who reported no changes. This is consistent with the results of a UK survey of women vaccinated against COVID-19 20 and other studies which have previously shown that smoking worsens both premenstrual and menstrual symptoms.21,22
In our study sample, adenovirus vectored COVID-19 vaccines seem to be more associated with changes in the menstrual cycle than mRNA vaccines. Although there is no data to understand the origin of these disorders, it has previously been suggested that they are due to the immunological reaction induced by vaccination. 23 Accordingly, in a retrospective study of women who had been infected by the SARS-CoV-2 virus, a fifth of them experienced menstrual disorders. 24 Nevertheless, the authors stated that these changes did not respond to sex hormone concentrations or ovarian reserve changes, 24 which strengthens the hypothesis of a possible immunological pathway. However, although the vaccine may be associated with mild menstrual disorders, it should be noted that SARS-CoV-2 infection may not only cause menstrual cycle disturbances but can also severely affect a wide range of organs and systems in the human body. 25 It should be noted that the pandemic has affected reproductive women’s health in several areas, such as menstrual disorders during lockdown, 26 the decrease in gynecological oncology screening tests, 27 and the difficulty in training women’s health specialists. 27 Therefore, further studies are needed to confirm or contrast the present findings and to better explain the potential physiological or psychosomatic mechanisms behind these premenstrual and menstrual-related changes.
Limitations and strengths
Some limitations of this novel descriptive study must be highlighted. The present results are based on self-reported data provided by volunteers, which can result in a bias error (i.e. women who perceived changes in their menstrual cycle might have been more prone to participate). Therefore, the study sample was of convenience (i.e. women who voluntarily wanted to complete the survey), which could have affected the representativeness of the sample. Moreover, the assessment of objective biochemical markers would have been helpful for a better interpretation of the present findings and the discussion of a potential mechanism that might explain these reported changes. This study also has several strengths to note. As far as we know, this is the first study to establish a relationship between the COVID-19 vaccination and premenstrual changes along with other relevant menstrual disturbances not previously reported, and to do so with a large study sample (i.e. >14,000 participants). Moreover, the survey was conducted immediately after the second dose of the vaccine was administered, so there is a lower risk of recall bias in our participants compared to other similar studies. Finally, we strictly excluded women taking hormonal contraceptives or with other clinical circumstances that may affect the menstrual cycle.
Conclusion
Women vaccinated against COVID-19 perceive mild menstrual and premenstrual changes. The most frequently reported premenstrual changes were increased fatigue, abdominal bloating, emotional irritability, sadness, headaches, and breast pain. The most frequently reported menstrual changes were more menstrual bleeding, pain, and delayed menstruation. This study confirms that menstrual status is under-represented in basic and translational research. Future studies are warranted to clarify the current prevalence of these disorders and the physiological mechanisms behind these widely reported changes after COVID-19 vaccination in order to provide a better preventive or therapeutic approach. The results of the present study could help properly inform women who are going to be vaccinated against COVID-19 about the most frequent changes in the menstrual cycle. In this way, the fear of experiencing severe menstrual disorders after vaccine administration can be mitigated, and unnecessary medical consultations and tests could be avoided.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057221112237 for Premenstrual and menstrual changes reported after COVID-19 vaccination: The EVA project by Laura Baena-García, Virginia A Aparicio, Ana Molina-López, Pilar Aranda, Laura Cámara-Roca and Olga Ocón-Hernández in Women’s Health
Acknowledgments
The authors thank Charlotte Bower for editing assistance. For financial support, we thank the Antonio Chamorro–Alejandro Otero Research Chair, University of Granada.
Footnotes
Author contribution(s): Laura Baena-García: Conceptualization; Formal analysis; Funding acquisition; Supervision; Writing – original draft.
Virginia A Aparicio: Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Ana Molina-López: Conceptualization; Data curation; Investigation; Writing – review & editing.
Pilar Aranda: Conceptualization; Investigation; Methodology; Supervision; Writing – review & editing.
Laura Cámara-Roca: Conceptualization; Investigation; Methodology; Writing – review & editing.
Olga Ocón-Hernández: Conceptualization; Funding acquisition; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was partially funded by the Antonio Chamorro–Alejandro Otero Research Chair, University of Granada.
ORCID iDs: Laura Baena-García  https://orcid.org/0000-0002-4895-567X
https://orcid.org/0000-0002-4895-567X
Ana Molina-López  https://orcid.org/0000-0002-6211-9408
https://orcid.org/0000-0002-6211-9408
Supplemental material: Supplemental material for this article is available online.
References
- 1. National Institutes of Health. Inclusion of women and minorities in clinical research. Revitalization Act, Subtitle B, Part 1 1993; 131–133. [Google Scholar]
- 2. Clayton JA, Collins FS. NIH to balance sex in cell and animal studies. Nature 2014; 509: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scully EP, Haverfield J, Ursin RL, et al. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol 2020; 20(7): 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brady E, Nielsen MW, Andersen JP, et al. Lack of consideration of sex and gender in COVID-19 clinical studies. Nat Commun 2021; 12: 4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oliver SE, Gargano JW, Marin M, et al. The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 vaccine – United States, December 2020. MMWR Morb Mortal Wkly Rep 2021; 69: 1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021; 384: 2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farage MA, Neill S, MacLean AB. Physiological changes associated with the menstrual cycle a review. Obstet Gynecol Surv 2009; 64(1): 58–72. [DOI] [PubMed] [Google Scholar]
- 10. Matteson KA, Raker CA, Clark MA, et al. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Womens Health 2013; 22(11): 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Critchley HOD, Babayev E, Bulun SE, et al. Menstruation: science and society. Am J Obstet Gynecol 2020; 223(5): 624–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gong L, Ji H, Tang X, et al. Human papillomavirus vaccine-associated premature ovarian insufficiency and related adverse events: data mining of vaccine adverse event reporting system. Sci Reports 2020; 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. A midwife collects data on menstrual disorders after receiving COVID-19 vaccines: don’t be taken for fools, https://www.eldiario.es/andalucia/alteraciones-menstruacion-vacunacion-covid-19_1_7324107.html (accessed 24 April 2021).
- 14. Spanish Agency of Medicines Health Products. 2nd pharmacovigilance report on COVID-19 vaccines, https://www.aemps.gob.es/informa/boletines-aemps/boletin-fv/2021-boletin-fv/11o-informe-de-farmacovigilancia-sobre-vacunas-covid-19/ (2021, accessed 21 January 2022).
- 15. Spanish Agency of Medicines Health Products. 9th pharmacovigilance report on COVID-19 vaccines, https://www.aemps.gob.es/informa/boletines-aemps/boletin-fv/2021-boletin-fv/9o-informe-de-farmacovigilancia-sobre-vacunas-covid-19/?lang=en (2021, accessed 21 January 2022).
- 16. Edelman A, Boniface ER, Benhar E, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination. Obstet Gynecol 2022; Publish Ah: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trogstad L. Increased occurrence of menstrual disturbances in 18- to 30-year-old women after COVID-19 vaccination. SSRN Electron J. Epub ahead of print 1 January 2022. DOI: 10.2139/SSRN.3998180. [DOI] [Google Scholar]
- 18. Mushtaq A, Arif S, Sabih F. Premenstrual symptoms as predictor of quality of life in reproductive-aged women of Rawalakot, Azad Kashmir: a cross sectional study. J Pak Med Assoc 2020; 70(12(B)): 2394–2397. [DOI] [PubMed] [Google Scholar]
- 19. Laganà AS, Veronesi G, Ghezzi F, et al. Evaluation of menstrual irregularities after COVID-19 vaccination: results of the MECOVAC survey. Open Med 2022; 17(1): 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alvergne A, Kountourides G, Argentieri MA, et al. COVID-19 vaccination and menstrual cycle changes: a United Kingdom (UK) retrospective case-control study. Medrxiv, 2021, https://www.medrxiv.org/content/10.1101/2021.11.23.21266709v1 [DOI] [PMC free article] [PubMed]
- 21. del Mar Fernández M, Montes-Martínez A, Piñeiro-Lamas M, et al. Tobacco consumption and premenstrual syndrome: a case-control study. PLoS ONE 2019; 14(6): e0218794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakai H, Kawamura C, Cardenas X, et al. Premenstrual and menstrual symptomatology in young adult Japanese females who smoke tobacco. J Obstet Gynaecol Res 2011; 37(4): 325–330. [DOI] [PubMed] [Google Scholar]
- 23. Male V. Menstrual changes after covid-19 vaccination. BMJ 2021; 374: 1–2. [DOI] [PubMed] [Google Scholar]
- 24. Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online 2021; 42: 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 2020; 14: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haile L, van de Roemer N, Gemzell-Danielsson K, et al. The global pandemic and changes in women’s reproductive health: an observational study. Eur J Contracept Reprod Health Care 2022; 27(2): 102–106. [DOI] [PubMed] [Google Scholar]
- 27. Lukanović D, Laganà AS. The impact of Covid-19 on simulation-based learning of gynecology and obstetrics skills. Minim Invasive Ther Allied Technol 2022; 31: 684–689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057221112237 for Premenstrual and menstrual changes reported after COVID-19 vaccination: The EVA project by Laura Baena-García, Virginia A Aparicio, Ana Molina-López, Pilar Aranda, Laura Cámara-Roca and Olga Ocón-Hernández in Women’s Health