Abstract
Objective:
Palpitations during peri- and post-menopause are common. It is unclear what variables are related to palpitations in peri- and post-menopausal women. The purpose of this scoping review was to summarize potential correlates of palpitations in women transitioning through menopause.
Methods:
The review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR). Authors included English-language, full-length, peer-reviewed, cross-sectional research articles on palpitations in menopausal women published through December 18, 2021, from PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and PsycINFO searches. Following de-duplication, screening of titles and abstracts, and review of full-texts, independent reviewers extracted data on variables studied in relationship to palpitations from 84 articles and resolved discrepancies. Authors extracted data on (1) demographic, clinical, biomarker, and symptom/quality of life variables and (2) data analysis method (bivariate, multivariate). Authors classified each variable as a likely, unlikely, or unclear correlate of palpitations.
Results:
Articles were diverse in region of origin, sample sizes, and variables assessed in relationship to palpitations. Evidence for any one variable was sparse. Likely correlates of palpitations included race/ethnicity, lower physical activity, worse vasomotor symptoms (VMSs), worse sleep, and worse quality of life. Unlikely correlates included age, employment, education, marital status, socioeconomic status, comorbidities, body mass index, and sexual difficulties. Unclear correlates due to equivocal evidence were menopausal status, smoking, and depression. Unclear correlates due to insufficient evidence (less than three articles) included all of the assessed biomarkers, anxiety, and stress.
Conclusion:
Likely correlates were identified including race/ethnicity, physical activity, VMS, sleep, and quality of life. However, additional research is needed to better understand potential correlates of palpitations.
Keywords: cardiology, menopause, perimenopause, postmenopause, review
Introduction
About 21 million women living in the United States today, and 1.1 billion women worldwide by 2025 will experience menopause symptoms. 1 Menopause symptoms can begin in perimenopause (when menses become irregular) and last into the postmenopause (when menses stop for 12 or more months). Women’s individual experiences vary, 2 but up to 75% of women report vasomotor symptoms (VMSs, hot flashes, night sweats). 1 Studies suggest VMS and other menopause symptoms, such as sleep and mood disturbances, can last 10 years (range = 0–15+ years) during the transition from peri- to post-menopause.1,3
Many menopause symptom checklists include palpitations (e.g. rapid, irregular, and/or exaggerated heartbeats), 4 and research suggests palpitations are relatively common. Up to 42% of perimenopausal women and 54% of postmenopausal women report having palpitations. 5 However, although 44%–87% of women aged 40–59 years believed their palpitations required treatment, 6 a systematic review found no Level 1 evidence for managing menopause palpitations. 7 This and other evidence suggests palpitations in peri- and post-menopausal women are common yet relatively understudied in comparison to VMS,4,5,7 may be normalized and/or trivialized like other menopause symptoms, 8 and have been mostly attributed to psychosomatic (e.g. anxiety, stress) rather than cardiac causes. 9
Understanding more about palpitations in peri- and post-menopausal women is important. In the general population, palpitations account for 16% of primary care visits and are the second leading reason for cardiologist visits. 10 Women are more likely than men to report palpitations during outpatient visits, 11 emergency department visits, 11 and during acute cardiac events.12,13 However, there has been a historical and well-documented bias against women in cardiology with poor understanding and/or trivialization of symptoms, such as palpitations, that results in missed or delayed diagnosis of cardiac events. 14
It is unclear what demographics, clinical variables, biomarkers, and/or symptom/quality of life (QOL) outcomes are related to palpitations. Our team found no review of correlates of palpitations in peri- and post-menopausal women. Understanding correlates that are related to palpitations can help researchers control potential confounding variables and potentially identify avenues for mechanistic research to understand the pathophysiological underpinnings of palpitations. Furthermore, understanding such mechanisms as well as understanding modifiable correlates can help guide the development of interventions to alleviate the symptom. A logical first step to understand correlates of palpitations is to review the literature and map or synthesize evidence.
Therefore, the purpose of this scoping review was to systematically map what is currently known and unknown about correlates of palpitations in peri- and post-menopausal women to guide future research and clinical practice. We focused on correlates in cross-sectional studies rather than risk factors or predictors because of the scarcity of longitudinal studies on the topic (n = 2 articles). 5 Our goal was to fully map the current state of the science related to potential correlates of palpitations in peri- and post-menopausal women. Our questions were as follows: (1) what demographics, clinical variables, biomarkers, and/or symptom/QOL outcomes have been studied in relation to palpitations in peri- and post-menopausal women? (2) What is the evidence for these associations from bivariate and multivariate statistical tests? (3) What variables are likely, unlikely, or unclear correlates of palpitations based on review findings regarding the number of articles assessing each association, type of statistical tests performed, and article results?
Methods
Protocol
The scoping review methodology was the review methodology most closely matching the goal and research questions. Scoping reviews can be thought of “reconnaissance” to determine boundaries of what is known and unknown on a topic and are most useful when other literature reviews are lacking. 15 They are intentionally broad and differ from systematic reviews that focus on subsets of articles that are narrowly defined and pass quality filters. 16 Scoping reviews aim to visually map available evidence using tables or figures.15–17 Thus, scoping reviews are useful for identifying gaps in the field for future research and may or may not be used as a stepping stone in conducting future systematic reviews or meta-analyses.15,17
This scoping review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Review (PRISMA-ScR, see Supplementary A). 17 The authors did not register the review protocol as this step is not required for scoping reviews. 17 Ethics board approval was not applicable because the review did not meet criteria for human subjects’ research. 18
Eligibility criteria
Articles included in the review needed to be English-language, full-length, peer-reviewed, cross-sectional, descriptive research on palpitations (or similar symptoms such as racing heart), in menopausal women (per accepted nomenclature for defining perimenopause and postmenopause),19–21 and reported results of testing at least one variable in relation to palpitations. Studies that included pre-menopausal women as a comparison group were included but those that focused solely on premenopausal women were excluded.
Articles excluded from the review were not full-length (e.g. abstracts), not data-based (e.g. editorials), studies of other populations (i.e. adolescents or other premenopausal women, transgender or gender transitioning populations, men, or animals), studies where populations were labeled as “menopausal women” or “symptomatic women” without further clarification or definition of menopausal status, and studies that provided data on palpitations but did not assess at least one variable in relation to palpitations. Regarding factor analytic studies, we excluded those where the only relationships assessed were inter-item correlations from a single scale (e.g. factor analyses of a symptom checklist in psychometric studies) and included those that assessed at least one other variable.
Information sources
An experienced librarian performed the searches. The librarian drafted the initial search strategies and further refined them after team discussion. The final literature searches included all articles published through December 18, 2021. The librarian used three gold standard biomedically focused databases selected for their breadth: PubMed, Cumulated Index to Nursing and Allied Health Literature (CINAHL), and PsycINFO. Because of the health-related focus of the review, authors did not search outside the biomedical literature (e.g. Scopus which contains general science journals as well as textbooks, abstracts, and other non-full-length writings, or the Education Resources Information Center (ERIC)).
Search strategy
The search strategy relied on the National Library of Medicine Medical Subject Headings terms and keywords: (“Menopause” OR menopaus*) AND (palpitation* OR heart racing OR heart pounding OR irregular heart). The word “palpitations” was too specific to locate pertinent articles, and therefore, we searched for the articles that used standard menopause symptom assessment tools known to assess palpitations. 4 These included the Blatt–Kupperman Index,22–24 the Greene Climacteric Scale, 25 the Heinemann Menopause Rating Scale, 26 the Holte/Mikkelsen Menopause Checklist, 27 Hunter’s Women’s Health Questionnaire, 28 the Menopausal-Specific Quality of Life (MENQOL) scale, 29 the Menopause Symptom Checklist, 30 the Midlife Women’s Symptom Index, 31 Neugarten and Kraines’ Symptom Checklist, 32 and the Study of Women’s Health Across the Nation menopausal symptom scale. 33 The final electronic search strategies are shown in Supplementary B.
Selection of sources of evidence
Authors used a structured program available at Covidence.org to de-duplicate articles and track progress on the screening and full-text review processes. After de-duplication, four reviewers working in pairs screened article titles and abstracts to determine if they met criteria for inclusion or exclusion. The reviewers discussed screening disagreements and achieved consensus through group discussion. Reviewers were overly inclusive at this screening stage and retained articles that referred to menopausal symptoms in general, as we could not determine whether menopausal symptoms included palpitations. Next, four reviewers working in pairs performed full-text review. At this stage, reviewers were more selective and excluded articles that did not include data on palpitations or did not assess at least one potential variable in relation to palpitations. The reviewers discussed disagreements on full-text review and reached consensus after group discussion.
Data charting methods
Two reviewers developed a data-extraction form in Microsoft Excel. Four reviewers working in pairs extracted data from approximately 10 articles, met to discuss the process, and updated the data charting form with additional details. For all articles, one author performed extraction and at least one additional author verified accuracy. Each row represented an article, and each column contained data about the article.
Data items
There were four types of data extracted. First, authors extracted general information about the articles including author, title, country of origin, and sample size. Second, authors extracted information on variables assessed in relationship to palpitations within articles. Authors applied a simple four-category taxonomy to organize correlates during the data extraction process: (1) demographics, (2) clinical variables, (3) biomarkers, and (4) symptoms/QOL. The list of variables under each of these headings can be found in the results’ tables. Third, authors extracted whether evidence was based on bivariate or multivariate test statistics. Bivariate tests for association included chi-square tests, Mann–Whitney U tests, t-tests, analysis of variance, or univariate logistic regression. Multivariate or multivariable tests for association included multiple logistic or multiple regressions that controlled for confounders. If bivariate and multivariate results differed within a study, we report only the higher level adjusted multivariate results. Fourth, during data extraction, authors noted some articles provided data on tests of interactions between variables, and therefore extracted data on those interactions. Significant interactions indicated that a relationship between two variables depended on a third variable. Reviewers met to resolve disagreements and achieved consensus through discussion. Our data extraction table is available upon request.
Critical appraisal and summarization
To determine the amount of evidence available, authors counted the overall number of articles that assessed an association between palpitations and each variable. They then determined the number of articles that did and did not show evidence of association at the bivariate and multivariate levels. At this stage, to facilitate communication among authors and ease summarization, all variables were listed in terms of what worsens palpitations (e.g. older age, lower physical activity).
We then developed criteria to classify each variable as a likely, unlikely, or unknown correlate of palpitations as we were unable to find published criteria. Likely correlates were those supported by significant associations in at least 60% of three or more studies. Unlikely correlates were those assessed but not statistically significant in at least 60% of three or more studies. Conversely, unclear correlates were those where there was either (1) equivocal evidence (less than 60% evidence in three or more studies) or (2) an insufficient number of articles (less than three articles).
Risk of bias
PRISMA-ScR does not require risk of bias assessments in scoping reviews. 17 Other scoping review guidelines discourage inclusion of literature based on risk of bias as the nature of a scoping review is to review and map available evidence, rather than a limited number of studies meeting bias criteria. 16 For these reasons, risk of bias was not assessed.
Results
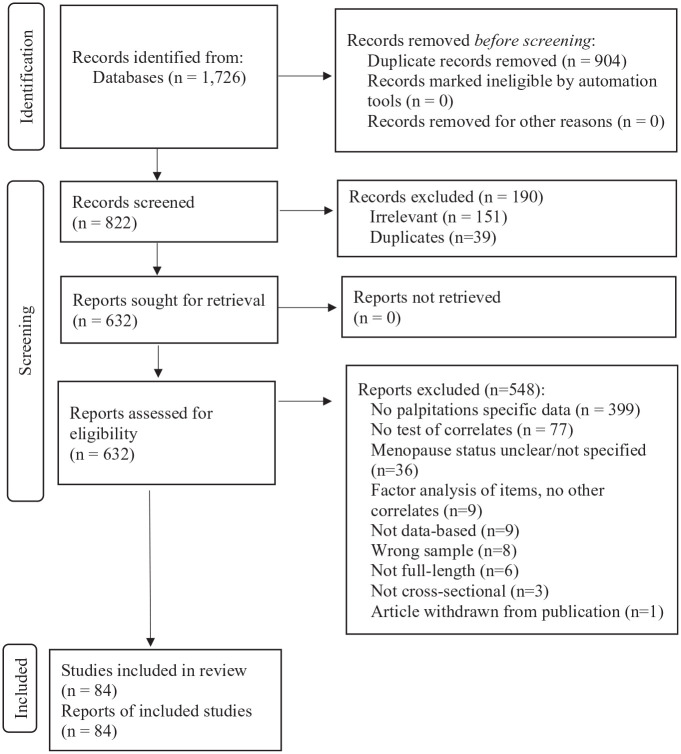
The PRISMA in Figure 1 shows the flow of records. Of 822 records screened, 632 proceeded to full-text review. Of the 632, a total of 84 articles met criteria for inclusion and were included in this review.33–116 Most articles were excluded for not reporting palpitations-specific data (n = 399). Consistent with inclusion criteria and the dearth of longitudinal studies discussed earlier, all of the included articles (100%) were based on cross-sectional data analysis.
Figure 1.
PRISMA flow diagram of records.
The number of records identified, numbers excluded at title/abstract screening and full-text screening, reasons excluded, and the final number of unique articles included in the review. Despite de-duplication, additional duplicates were found during screening as a result of incorrect article metadata being included in the searched databases.
Table 1 shows descriptive information about the articles. Only 2% of articles included the word “palpitations” in the title.47,58 Articles originated from multiple regions, most frequently from Asia (East, North, South East, South total n = 31, 37%). Only one article included participants from multiple world regions. Sample sizes varied from less than 200 to over 5000, with between 200 and 500 participants being most common (43%). The upper age range of participants was between 50 and 65 years old in most articles (n = 58, 69%), was greater than 65 in 15 articles (18%), and was not specified in 11 articles (13%) (data not shown). Clinical variables were most frequently studied in relationship to palpitations (77%) followed by demographics (20%), symptom/QOL variables (19%), and least commonly biomarkers (6%). No studies (0%) included all four categories of variables.
Table 1.
Descriptive information about the reviewed articles (n = 84).
| Descriptor and citations | n (%) |
|---|---|
| Title contains word palpitations47,58 | 2 (2) |
| Region | |
| Africa36,88,103 | 3 (4) |
| Asia, East50,51,60,65,73,76,93,105,113–116 | 12 (14) |
| Asia, North58,67,74,86,94,100 | 6 (7) |
| Asia, South41,52,54,70,85,91,92,111 | 8 (10) |
| Asia, South East34,90,102,106,107 | 5 (6) |
| Australia64,89 | 2 (2) |
| Europe, Continental39,63,71,72,81,96,101 | 7 (8) |
| Europe, Eastern and Central37,42,49,55,56,61,79,80,99,104,108,110 | 12 (14) |
| Latin America43–46,48,53,59,62,75,77,82–84,87,95,109 | 16 (19) |
| Middle East35,38,57,68,69 | 5 (6) |
| North America33,47,66,78,97,112 | 6 (7) |
| Scandinavia 40 | 1 (1) |
| Multiple regions 98 | 1 (1) |
| Sample size | |
| 69–19938,39,50,53,55,62,71–74,77,78,88,96,115 | 15 (18) |
| 200–50034,35,37,41,46,51,54,56–59,61,63,65,66,68–70,79,80,90,92,97,99–101,103–110,113,114 | 36 (43) |
| 501–99936,47–49,52,81–83,86,87,91,95,102,111,112,116 | 16 (19) |
| 1000–499940,42,60,64,67,75,76,85,93,94,98 | 11 (13) |
| 5000–12,42533,43–45,84,89 | 6 (7) |
| Variables evaluated as potential correlates a | |
| Demographic33,34,47,58,65,66,68,82,83,85–87,97,98,103,110,115 | 17 (20) |
| Clinical33–42,44–47,49,50,52,54–59,62–64,67–76,78–81,84–86,88–96,99–102,104,106–108,111–114,116 | 65 (77) |
| Biomarkers77,79,80,86,112 | 5 (6) |
| Symptom/QOL40,41,43,47,48,51,53,56,58,60,61,67,76,105,108,109 | 16 (19) |
| All four categories | 0 (0) |
QOL: quality of life.
Exceeds 100% because articles could contain more than one category of correlates.
Likely correlates of palpitations
As shown in Table 2, five variables emerged as likely correlates of palpitations. They included one demographic (race/ethnicity), one clinical (less physical activity), and three symptom/QOL variables (worse VMSs, worse sleep, poorer QOL). The number of studies testing each variable ranged from 3 (VMS) to 11 (race/ethnicity). Among studies testing a given correlate, the percentages of studies showing evidence of association were 73% for race/ethnicity, 63% for lower physical activity, 67% for VMS, 75% for worse sleep, and 67% for poorer QOL. Most evidence of association was based on bivariate statistical tests for race/ethnicity, physical activity, and sleep and on multivariate tests for VMS and QOL.
Table 2.
Likely correlates of palpitations in peri- and post-menopausal women based on number of articles, type of statistical tests, and article results with citations.
| n | Association | No Association | |||||
|---|---|---|---|---|---|---|---|
| n (%) | Citations | n (%) | Citations | ||||
| Bi | Multi | Bi | Multi | ||||
| Demographic | |||||||
| Race/ethnicity | 11 | 8 (73) | 34,82,83,87,97,98,110 | 33 | 3 (27) | 47,115 | 66 |
| Clinical | |||||||
| Less physical activity | 8 | 5 (63) | 39,45,46 | 33,67 | 3 (37) | 54,104 | 58 |
| Symptom/QOL | |||||||
| Worse VMS | 3 | 2 (67) | 43 | 58 | 1 (33) | 47 | |
| Worse sleep | 4 | 3 (75) | 40,105 | 47 | 1 (25) | 48 | |
| Poorer QOL | 6 | 4 (67) | 47,53,56,61 | 2 (33) | 51,61 | ||
Bi: bivariate tests for association such as chi-square tests, Mann–Whitney U tests, t-tests, analysis of variance, or univariate logistic regression; multi: multivariate or multivariable tests for association such as logistic or multiple regressions that controlled for confounders; QOL: quality of life; VMS: vasomotor symptom.
Superscripted numbers are the article reference citations.
Race/ethnicity
Greater versus lesser palpitations were found in Hispanic versus White women (p < .05),33,97 Black Columbian versus non-Black Columbian women (p = .003), 82 Black versus Hispanic Ecuadorian women (p < .001), 83 Quechua versus Zenu women (p < .0001), 87 Lebanese versus Spanish, Lebanese, or American women (p < .01), 98 and Turkish versus German women (p < .001). 110 There was a significant difference in palpitations across women of Malay, Indian, and Chinese descent living in Malaysia, but the details were not specified. 34 In contrast, no differences in palpitations were reported between Mosuo versus Han Chinese women in a bivariate analysis 115 or non-Hispanic Whites versus other racial groups in the United States in bivariate 47 or multivariate analysis. 66
Physical activity
Greater palpitations were associated with lower physical activity in five studies.33,39,45,46,67 Greater palpitations were related to somewhat and much less physical activity versus much more physical activity, 33 a sedentary versus active lifestyle, 45 less physical activity overall, 46 and seldom versus daily or one to three times a week exercise. 67 Similarly, lesser palpitations were associated with greater moderate-to-vigorous physical activity. 39 In contrast, in three articles, there were no significant differences in palpitations across low, moderate, and high physical activity groups54,104 or between those who did and did not report regular exercise. 58
VMS and sleep
Greater palpitations were associated with having VMS43,58 and sleep problems (poor sleep including having nightmares at least once a week, 40 greater insomnia, 47 and overall sleep quality and disturbances). 105 In contrast, palpitations were related to VMS bother (but not VMS frequency) in bivariate but not multivariate models. 47 Similarly, although palpitations were associated with more sleep complaints in bivariate analyses, findings did not hold in multivariate analyses. 48
QOL
Greater palpitations were associated with poorer menopausal QOL, 47 poorer physical (but not mental) component QOL, 53 poorer sleep-related QOL, 56 and poorer occupational QOL. 56 In contrast, palpitations were not related to general 51 or sexual 61 QOL.
Unlikely correlates of palpitations
As shown in Table 3, eight variables were not likely related to palpitations. They included five demographic (age, employment/occupation, education, marital status, socioeconomic status (SES)/income), two clinical (comorbidities, body mass index (BMI)), and one symptom/QOL variable (sexual dysfunction/inactivity). The number of studies testing each ranged from 3 (SES) to 11 (comorbidities). The percentages of studies showing no evidence of association were 100% for age, 100% for employment, 80% for education, 80% for marital status, 67% for SES, 100% for comorbidities, 63% for BMI, and 75% for sexual dysfunction. Tests of association were evenly split between bivariate and multivariate tests for education and marital status; were predominantly bivariate for age, comorbidities, and BMI; and were predominantly multivariate for employment, SES, and sexual function.
Table 3.
Unlikely correlates of palpitations in peri- and post-menopausal women based on number of articles, type of statistical tests, and article results with citations.
| n | Association | No association | |||||
|---|---|---|---|---|---|---|---|
| n (%) | Citations | n (%) | Citations | ||||
| Bi | Multi | Bi | Multi | ||||
| Demographic | |||||||
| Older age | 7 | 0 (0) | 7 (100) | 47,58,86,103 | 33,68,85 | ||
| Employment or occupation | 5 | 0 (0) | 5 (100) | 34,65 | 33,68,85 | ||
| Lower education | 5 | 1 (20) | 33 | 4 (80) | 47,65 | 68,85 | |
| Being divorced | 5 | 1 (20) | 68 | 4 (80) | 34,47 | 33,85 | |
| Lower SES/income | 3 | 1 (33) | 33 | 2 (67) | 68,85 | ||
| Clinical | |||||||
| Comorbidities a | 11 | 0 (0) | 11 (100) | 36,37,49,58,59,62,74,78,84,86,96 | |||
| BMI | 8 | 3 (37) | ↑ 67 | ↓, 47 ↑ 76 | 5 (63) | 58,72,95,104 | 33 |
| Symptom/QOL | |||||||
| Sexual dysfunction or inactivity | 4 | 1 (25) | 108 | 3 (75) | 61 | 76,109 | |
Bi: bivariate tests for association such as chi-square tests, Mann–Whitney U tests, t-tests, analysis of variance, or univariate logistic regression; multi: multivariate or multivariable tests for association such as multiple logistic or multiple regressions that controlled for confounders; SES: socioeconomic status; BMI: body mass index; QOL: quality of life.
Superscripted numbers are the article reference citations.
Age, employment/occupation, education, marital status, and SES/income
Greater palpitations were consistently not related to age33,47,58,68,85,86,103 or employment/occupation.33,34,65,68,85 Greater palpitations were not related to lower education in four of five studies.47,65,68,85 Greater palpitations were not related to marital status in bivariate34,47 or multivariate33,85 analyses. However, being divorced was associated with higher palpitations severity in one multivariate analysis. 68 In two of three studies, palpitations were not related to lower socioeconomic status 85 or income. 68 In the third article, greater palpitations were associated with greater difficulty paying for basics when controlling for other variables. 33
Comorbidities
For clinical variables, greater palpitations were consistently not related to the presence or absence of the following comorbidities: human immunodeficiency virus,36,59,78 osteopenia/osteoporosis, 37 metabolic syndrome,49,74 atherosclerosis, 58 arrhythmias, 58 risk of sarcopenia, 62 diabetes, 84 thyroid tumor, 86 and Celiac disease. 96
BMI
Greater palpitations were not associated with BMI in four articles33,58,72,95,104 and in the remaining three articles, findings were contradictory. One study showed reduced odds of palpitations with higher BMI 47 whereas other studies showed increased odds of palpitations with higher BMI (e.g. BMI greater than 23 (vs less than 20 or 20–23) 67 or obese BMI (vs underweight or normal or overweight)). 76
Sexual activity and function
Greater palpitations were not related to sexual activity61,76 or sexual function. 109 In another article, greater palpitations were significantly related to sexual dysfunction (total scores and subscales of infrequency, non-communication, dissatisfaction, avoidance, non-sensuality, vaginismus, and anorgasmia) in surgically menopausal women but not naturally menopausal women. 108
Unclear correlates of palpitations
Equivocal evidence
As shown in Table 4, a sufficient number of articles showed equivocal evidence for two clinical variables (menopause status, smoking) and one symptom (depression).
Table 4.
Unclear correlates of palpitations in peri- and post-menopausal women based on equivocal evidence as determined by number of articles, type of statistical tests, and article results with citations.
| n | Association | No association | |||||
|---|---|---|---|---|---|---|---|
| n (%) | Citations | n (%) | Citations | ||||
| Bi | Multi | Bi | Multi | ||||
| Clinical | |||||||
| Menopause status | 40 | 20 (50) | Higher in: Post vs pre41,42,44,52,94,113,114 Post/peri vs pre75,79,88 Post vs peri44,52,93 Peri vs pre 44,52,76,90,91,113,114,116 Surg post vs nat post55,63 Peri vs post90,91 |
Higher in: Post/peri vs pre 80 Late peri vs pre 33 Early peri vs pre 33 Peri vs pre 85 Surg post vs pre 33 |
20 (50) | groups compared: post, pre92,111
Post, peri/pre 102 Post, peri56,70,99,111 Post, peri, pre38,57,58,64,101,106,107 Late post, early post, peri, pre73,86 Surg post, nat post 108 |
groups compared: post, peri, pre67,68
Post, peri 47 Post, peri/pre 40 |
| Smoking | 3 | 2 (67) | ↑, 33 ↓ 47 | 1 (33) | 58 | ||
| Symptom/QOL | |||||||
| Worse depression | 4 | 2 (50) | 47,60 | 2 (50) | 58 | 41 | |
Bi: bivariate tests for association such as chi-square tests, Mann–Whitney U tests, t-tests, ANOVA, or univariate logistic regression; multi: multivariate or multivariable tests for association such as multiple logistic or multiple regressions that controlled for confounders; post: postmenopausal; peri: perimenopausal; pre: premenopausal; surg: surgically; nat: naturally; QOL: quality of life.
Past smoking increased odds of palpitations (↑), and past and current smoking decreased odds of palpitations (↓).
Superscripted numbers are the article reference citations.
Menopause status
Menopause status was the most commonly studied variable, and evidence was split 50/50 in favor of and against an association with palpitations. The evidence favoring an association generally showed greater palpitations with advanced menopausal stage (e.g. postmenopausal more symptomatic than premenopausal,41,42,44,52,94,113,114 or perimenopausal,44,52,93 post-/peri-menopausal combined more symptomatic than premenopausal,75,79,80,88 perimenopausal more symptomatic than premenopausal33,44,52,76,85,90,91,113,114,116). However, in other studies, evidence for association was reversed (e.g. perimenopausal more symptomatic than postmenopausal) or absent.38,40,47,56–58,64,67,68,70,73,86,92,99,101,102,106,107,111 In addition, three of four studies showed greater palpitations in surgically menopausal women compared to naturally postmenopausal55,63 or premenopausal women, 33 whereas a fourth study showed no association. 108
Smoking
Findings for smoking were equivocal. Past smoking versus never smoking increased the odds of palpitations, 33 both past and current smoking reduced the odds of palpitations, 47 and smoking more than 20 cigarettes per day was not associated with palpitations. 58
Depression
Findings for depression were equivocal. Depression was associated with greater palpitations in two articles47,60 but not two others.41,58
Insufficient number of articles
As shown in Table 5, there were an insufficient number of articles (less than three) for multiple clinical variables, biomarkers, and some symptoms.
Table 5.
Unclear correlates of palpitations in peri- and post-menopausal women based on insufficient evidence as determined by number of articles, type of statistical tests, and article results with citations.
| n | Citations | ||||
|---|---|---|---|---|---|
| Association | No association | ||||
| Bi | Multi | Bi | Multi | ||
| Clinical | |||||
| Higher parity | 2 | 33 | 34 | ||
| Hyperthyroidism a | 2 | 86,99 | |||
| Bone density or fracture | 2 | 37,69 | |||
| Using estrogen/hormone therapy | 2 | 50,76 | |||
| Higher gravidity | 1 | 68 | |||
| Older age at second abortion | 1 | 71 | |||
| Diet (high animal fat) | 1 | 67 | |||
| Diet (low soy) | 1 | 100 | |||
| Chinese herbal medicine | 1 | 89 | |||
| Chinese medical diagnosis | 1 | 114 | |||
| Poorer fitness, flexibility, or balance | 2 | 39 | 58 | ||
| Alcohol use | 2 | 47,58 | |||
| Anthropometrics | 2 | 58,72 | |||
| Older age at menopause | 1 | 34 | |||
| Body composition b | 1 | 58 | |||
| Metabolism c | 1 | 58 | |||
| Overall health rating | 1 | 47 | |||
| Caffeine intake | 1 | 58 | |||
| Diet (low antioxidants) | 1 | 35 | |||
| Coronary artery calcium | 1 | 112 | |||
| Carotid intima-media thickness | 1 | 112 | |||
| Cardio-ankle vascular index | 1 | 58 | |||
| Ankle brachial pulse index | 1 | 58 | |||
| Heart rate, blood pressure | 1 | 58 | |||
| Sympathetic/parasympathetic tone | 1 | 58 | |||
| Biomarkers | |||||
| Genotype (CYP1B1 Leu432Val) | 2 | 79,80 | |||
| Genotype (CYP17 A1 rs743572) | 1 | 77 | |||
| Estradiol levels | 1 | 112 | |||
| Hashimoto’s antibody present | 1 | 86 | |||
| Symptom/QOL | |||||
| Worse anxiety | 2 | 58,67 | |||
| Worse stress d | 2 | 47,76 | |||
Bi: bivariate tests for association such as chi-square tests, Mann–Whitney U tests, t-tests, ANOVA, or univariate logistic regression; multi: multivariate or multivariable tests for association such as multiple logistic or multiple regressions that controlled for confounders; CYP: cytochrome P450; QOL: quality of life.
Superscripted numbers are the article reference citations.
Grave’s disease, low thyroid-stimulating hormone (TSH), and high thyroxine (T4).
Included % body fat, muscle mass, water mass, waist–hip ratio, and visceral fat level.
Resting energy expenditure and body temperature.
Perceived stress or occurrence of upsetting events and being pessimistic.
Clinical variables
There was evidence for 33 and against parity 34 and some evidence of association for hyperthyroidism,86,99 lumbar 1–2 bone density or fracture,37,69 use of estrogen or hormone therapy,50,76 higher gravidity, 68 older age at second abortion, 71 diet (e.g. high animal fat, 67 low soy 100 ), use of Chinese herbal medicine, 89 and having a Chinese medical diagnosis of Yang-xu. 114 In contrast, there was no evidence of association for poorer fitness, flexibility, or balance,39,58 alcohol use,47,58 anthropometrics,58,72 older age at menopause, 34 body composition, 58 metabolism, 58 overall health rating, 47 caffeine intake, 58 diet (low antioxidants 35 ), coronary artery calcium, 112 carotid intima-media thickness, 112 cardio-ankle vascular index, 58 ankle brachial pulse index, 58 heart rate, blood pressure, 58 and sympathetic/parasympathetic tone. 58
Biomarkers
There was no evidence of association between palpitations and genotypes cytochrome P450 CYP1B1 Leu432Val79,80 or CYP17 A1 rs743572, 77 estradiol concentration, 112 or presence of Hashimoto’s antibody. 86
Symptoms
For symptoms, there was some evidence that greater palpitations were associated with anxiety58,67 and stress.47,76
Discussion
This scoping review summarizes evidence surrounding what is known and unknown about demographic, clinical, biomarker, and symptom/QOL variables in relationship to palpitations in peri- and post-menopausal women to guide future research and clinical practice. The scoping review was appropriate for this task as there were a plethora of variables with varying degrees of evidence for and against associations. By scoping (or mapping) the number of articles, type of statistics used to assess association, and article results, we were able to summarize variables that were likely, unlikely, or unclear correlates of palpitations in this population of women. There were limitations in the reviewed articles, including sparse evidence for any one variable and statistical testing at the bivariate level without control for potential confounders.
The reviewed articles were diverse in country of origin, sample size, and variables assessed. Although palpitations were rarely the main focus (as evidenced by lack of inclusion of the word palpitations in the title), there appears to be global interest in assessing palpitations in peri- and post-menopausal women. However, evidence from one part of the world may not generalize to others. Geographical and racial/ethnic differences may have contributed to variability in findings related to correlates other than race/ethnicity across studies. Although sample sizes varied, most studies (80%) included 200 or more subjects suggesting there was sufficient statistical power to find significant associations between variables and palpitations. Finally, differences across variables assessed suggest there is little consensus on which variables are important when conducting research on palpitations in peri- and post-menopausal women. The fact that no study assessed all four categories of variables suggests there may be a need for a comprehensive analysis of demographic, clinical, biomarker, and symptom/QOL correlates of palpitations in this population.
Findings that palpitations varied by race/ethnicity are not surprising, given that individual differences in other menopausal symptoms exist across racial/ethnic groups. In many of the reviewed articles, palpitations were rarely the only menopausal symptom noted to vary by race/ethnicity. For example, findings from the Study of Women Across the Nation showed multiple symptoms varied by race/ethnicity: more frequent reports of VMS in Black women compared to White women, more forgetfulness in Hispanic women compared to non-Hispanic White women, and more sleep difficulties in White women compared to all other groups. 33
Less physical activity was a likely correlate of palpitations, yet other indicators of physical health tended to show no association with palpitations (e.g. BMI, poorer fitness, flexibility, balance, anthropometrics, or presence of various comorbidities). These differences could be true or due to variations across sample inclusion criteria or ceiling effects on measures in healthy women.
The relationships between palpitations and VMS and sleep problems may be due to greater symptom reporting in some women and/or a shared underlying mechanism. Some authors describe palpitations as part of what is experienced during VMS 117 and VMS are well-known to disrupt sleep. Given the dearth of biomarkers studied in the reviewed articles, additional research is needed to tease apart any potential shared mechanism underlying this trio of symptoms.
The relationship between palpitations and QOL is not surprising. Many menopausal symptoms, including VMS, disrupt a woman’s QOL. The association between palpitations and QOL was similar across different QOL constructs and measures (e.g. menopause QOL, 47 physical component QOL, 53 sleep-related QOL, 56 and occupational QOL), 56 yet there was only one study for each of these measures. Review findings suggest palpitations may be important to women’s QOL; however, additional research to confirm the association is needed.
The lack of information about palpitations in relation to subclinical or overt cardiovascular disease is notable. In the general population, palpitations that affect sleep and work 118 and occur more frequently 119 are more likely to be associated with serious, life-threatening arrhythmias. Only two articles tested associations between palpitations and indicators of cardiac health.58,112 Only one study examined palpitations and arrhythmias and found no association. 58 Much more research will be needed to understand if palpitations experienced during peri- and/or post-menopause are associated with electrocardiographic abnormalities, subclinical cardiovascular disease, or life-threatening cardiac events.
The lack of information about palpitations in relation to biomarkers is also notable. There is little evidence mapped in this review that is relevant to potential physiological mechanisms of palpitations. This scoping review suggests that the field has not yet advanced in the direction of understanding pathophysiological mechanisms of palpitations. Further research on mechanisms of palpitations seems to be warranted.
There were some strengths and limitations to this review. For strengths, the review was based on multiple search engines and pre-specified inclusion criteria to locate pertinent articles. A team of authors carefully screened, reviewed, and extracted data. Two to three individuals were involved at all stages of the review. For limitations, only English-language articles were included. There may be additional evidence in articles written in languages other than English. “Palpitations” is not yet a standard MeSH term, and this could have led to the omission of some articles. The word’s omission as a standard search term is unfortunate and may reflect the historical trivialization and/or understudied nature of palpitations at menopause. VMS are another historically trivialized menopause symptom, 8 and an MeSH keyword “hot flashes” was not added until the late 1990s 120 after decades of research on VMS had been conducted. Finally, the classification as likely, unlikely, or unknown correlate was based on author-developed criteria as we were unable to find a published reference using a similar standard.
Conclusion
In summary, this review mapped and summarized a wide variety of demographic, clinical, biomarker, and symptom/QOL variables in relationship to palpitations in peri- and post-menopausal women. The review highlights the need for additional research to understand cross-sectional and longitudinal relationships between variables identified in this review and menopausal palpitations to guide future research and clinical practice.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057221112267 for Correlates of palpitations during menopause: A scoping review by Janet S Carpenter, Ying Sheng, Caitlin Pike, Charles D Elomba, Jennifer S Alwine, Chen X Chen and James E Tisdale in Women’s Health
Supplemental material, sj-docx-2-whe-10.1177_17455057221112267 for Correlates of palpitations during menopause: A scoping review by Janet S Carpenter, Ying Sheng, Caitlin Pike, Charles D Elomba, Jennifer S Alwine, Chen X Chen and James E Tisdale in Women’s Health
Footnotes
Author contribution(s): Janet S Carpenter: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Ying Sheng: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing.
Caitlin Pike: Data curation; Methodology; Writing – original draft; Writing – review & editing.
Charles D Elomba: Formal analysis; Writing – review & editing.
Jennifer S Alwine: Formal analysis; Validation; Writing – review & editing.
Chen X Chen: Conceptualization; Validation; Writing – review & editing.
James E Tisdale: Conceptualization; Validation; Writing – review & editing.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr J.S.C. wishes to disclose personal fees from the University of Wisconsin Milwaukee, Simumetrix, and Mapi Research Trust. Ms C.P., Mr C.D.E., Ms J.S.A., and Drs Y.S., C.X.C., and J.E.T. have no conflicts to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This publication was made possible with support of: (1) an Indiana University Ethel Clarke Fellowship, (2) a Collaboration in Translational Research Grant from the Indiana Clinical and Translational Sciences Institute (Carpenter/Tisdale, multiple principal investigator) funded, in part, by grant no. UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (S. Moe, principal investigator), and (3) Dr Sheng’s postdoctoral fellowship under 5T32CA117865 (V. Champion, principal investigator). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iDs: Janet S Carpenter  https://orcid.org/0000-0003-3988-8855
https://orcid.org/0000-0003-3988-8855
Chen X Chen  https://orcid.org/0000-0002-6902-1227
https://orcid.org/0000-0002-6902-1227
Supplemental material: Supplemental material for this article is available online.
References
- 1. North American Menopause Society. Menopause practice: a clinician’s guide. 6th ed. Mayfield Heights, OH: North American Menopause Society, 2019, 266 pp. [Google Scholar]
- 2. Sievert LL. Variation in sweating patterns: implications for studies of hot flashes through skin conductance. Menopause 2007; 14(4): 742–751. [DOI] [PubMed] [Google Scholar]
- 3. Dennerstein L, Lehert P, Burger HG, et al. New findings from non-linear longitudinal modeling of menopausal hormone changes. Hum Reprod Update 2007; 13: 551–557. [DOI] [PubMed] [Google Scholar]
- 4. Sheng Y, Carpenter JS, Elomba CD, et al. Review of menopausal palpitations measures. Womens Midlife Health 2021; 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carpenter JS, Sheng Y, Elomba C, et al. A systematic review of palpitations prevalence by menopausal status. Curr Obstet Gynecol Rep 2021; 10: 7–13. [Google Scholar]
- 6. Blümel JE, Arteaga E, Parra J, et al. Decision-making for the treatment of climacteric symptoms using the Menopause Rating Scale. Maturitas 2018; 111: 15–19. [DOI] [PubMed] [Google Scholar]
- 7. Sheng Y, Carpenter JS, Elomba CD, et al. Effect of menopausal symptom treatment options on palpitations: a systematic review. Climacteric 2022; 25(2): 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pinkerton JV, Zion AS. Vasomotor symptoms in menopause: where we’ve been and where we’re going. J Womens Health (Larchmt) 2006; 15(2): 135–145. [DOI] [PubMed] [Google Scholar]
- 9. Muskin PR. Panics, prolapse, and PVCs. Gen Hosp Psychiatry 1985; 7(3): 219–223. [DOI] [PubMed] [Google Scholar]
- 10. Raviele A, Giada F, Bergfeldt L, et al. Management of patients with palpitations: a position paper from the European Heart Rhythm Association. Europace 2011; 13(7): 920–934. [DOI] [PubMed] [Google Scholar]
- 11. Misiri J, Candler S, Kusumoto FM. Evaluation of syncope and palpitations in women. J Womens Health (Larchmt) 2011; 20(10): 1505–1515. [DOI] [PubMed] [Google Scholar]
- 12. Mujtaba SF, Rizvi SN, Talpur A, et al. Gender based differences in symptoms of acute coronary syndrome. J Coll Physicians Surg Pak 2012; 22(5): 285–288. [PubMed] [Google Scholar]
- 13. O’Donnell S, McKee G, O’Brien F, et al. Gendered symptom presentation in acute coronary syndrome: a cross sectional analysis. Int J Nurs Stud 2012; 49(11): 1325–1332. [DOI] [PubMed] [Google Scholar]
- 14. Stehli J, Duffy SJ, Burgess S, et al. Sex disparities in myocardial infarction: biology or bias? Heart Lung Circ 2021; 30: 18–26. [DOI] [PubMed] [Google Scholar]
- 15. Peters MD, Godfrey CM, Khalil H, et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015; 13: 141–146. [DOI] [PubMed] [Google Scholar]
- 16. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8: 19–32. [Google Scholar]
- 17. Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–473. [DOI] [PubMed] [Google Scholar]
- 18. Indiana University. Protocol decision tree, https://research.iu.edu/compliance/human-subjects/review-levels/protocol-decision-tree/index.html (2022, accessed 5 December 2022).
- 19. Soules MR, Sherman S, Parrott E, et al. Executive summary: stages of reproductive aging workshop (STRAW). Menopause 2001; 8: 402–407. [DOI] [PubMed] [Google Scholar]
- 20. Utian WH. Semantics, menopause-related terminology, and the STRAW reproductive aging staging system. Menopause 2001; 8(6): 398–401. [DOI] [PubMed] [Google Scholar]
- 21. Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause 2012; 19: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Delaplaine RW, Bottomy JR, Blatt M, et al. Effective control of the surgical menopause by estradiol pellet implantation at the time of surgery. Surg Gynecol Obstet 1952; 94(3): 323–333. [PubMed] [Google Scholar]
- 23. Blatt MH, Wiesbader H, Kupperman HS. Vitamin E and climacteric syndrome; failure of effective control as measured by menopausal index. AMA Arch Intern Med 1953; 91(6): 792–799. [DOI] [PubMed] [Google Scholar]
- 24. Kupperman HS, Wetchler BB, Blatt MH. Contemporary therapy of the menopausal syndrome. J Am Med Assoc 1959; 171: 1627–1637. [DOI] [PubMed] [Google Scholar]
- 25. Greene JG. Constructing a standard climacteric scale. Maturitas 1998; 29: 25–31. [DOI] [PubMed] [Google Scholar]
- 26. Heinemann LA, DoMinh T, Strelow F, et al. The Menopause Rating Scale (MRS) as outcome measure for hormone treatment? A validation study. Health Qual Life Outcomes 2004; 2: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holte A, Mikkelsen A. The menopausal syndrome: a factor analytic replication. Maturitas 1991; 13(3): 193–203. [DOI] [PubMed] [Google Scholar]
- 28. Hunter M. The south-east England longitudinal study of the climacteric and postmenopause. Maturitas 1992; 14(2): 117–126. [DOI] [PubMed] [Google Scholar]
- 29. Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas 1996; 24: 161–175. [DOI] [PubMed] [Google Scholar]
- 30. Perz JM. Development of the menopause symptom list: a factor analytic study of menopause associated symptoms. Women Health 1997; 25(1): 53–69. [DOI] [PubMed] [Google Scholar]
- 31. Im EO. The Midlife Women’s Symptom Index (MSI). Health Care Women Int 2006; 27: 268–287. [DOI] [PubMed] [Google Scholar]
- 32. Neugarten BL, Kraines RJ. “Menopausal symptoms” in women of various ages. Psychosom Med 1965; 27: 266–273. [DOI] [PubMed] [Google Scholar]
- 33. Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol 2000; 152: 463473. [DOI] [PubMed] [Google Scholar]
- 34. Abdullah B, Moize B, Ismail BA, et al. Prevalence of menopausal symptoms, its effect to quality of life among Malaysian women and their treatment seeking behaviour. Med J Malaysia 2017; 72(2): 94–99. [PubMed] [Google Scholar]
- 35. Abshirini M, Siassi F, Koohdani F, et al. Dietary total antioxidant capacity is inversely related to menopausal symptoms: a cross-sectional study among Iranian postmenopausal women. Nutrition 2018; 55–56: 161–167. [DOI] [PubMed] [Google Scholar]
- 36. Agaba PA, Meloni ST, Sule HM, et al. Prevalence and predictors of severe menopause symptoms among HIV-positive and -negative Nigerian women. Int J STD AIDS 2017; 28(13): 1325–1334. [DOI] [PubMed] [Google Scholar]
- 37. Alay I, Kaya C, Cengiz H, et al. The relation of body mass index, menopausal symptoms, and lipid profile with bone mineral density in postmenopausal women. Taiwan J Obstet Gynecol 2020; 59(1): 61–66. [DOI] [PubMed] [Google Scholar]
- 38. AlDughaither A, AlMutairy H, AlAteeq M. Menopausal symptoms and quality of life among Saudi women visiting primary care clinics in Riyadh, Saudi Arabia. Int J Womens Health 2015; 7: 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aparicio VA, Borges-Cosic M, Ruiz-Cabello P, et al. Association of objectively measured physical activity and physical fitness with menopause symptoms. Climacteric 2017; 20(5): 456–461. [DOI] [PubMed] [Google Scholar]
- 40. Asplund R, Aberg HE. Nightmares, cardiac symptoms and the menopause. Climacteric 2003; 6(4): 314–320. [PubMed] [Google Scholar]
- 41. Bashar M, Ahmed K, Uddin MS, et al. Depression and quality of life among postmenopausal women in Bangladesh: a cross-sectional study. J Menopausal Med 2017; 23(3): 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Biri A, Bakar C, Maral I, et al. Women with and without menopause over age of 40 in Turkey: consequences and treatment options. Maturitas 2005; 50: 167–176. [DOI] [PubMed] [Google Scholar]
- 43. Blümel JE, Chedraui P, Baron G, et al. A large multinational study of vasomotor symptom prevalence, duration, and impact on quality of life in middle-aged women. Menopause 2011; 18(7): 778–785. [DOI] [PubMed] [Google Scholar]
- 44. Blümel JE, Chedraui P, Baron G, et al. Menopausal symptoms appear before the menopause and persist 5 years beyond: a detailed analysis of a multinational study. Climacteric 2012; 15(6): 542–551. [DOI] [PubMed] [Google Scholar]
- 45. Blümel JE, Fica J, Chedraui P, et al. Sedentary lifestyle in middle-aged women is associated with severe menopausal symptoms and obesity. Menopause 2016; 23(5): 488–493. [DOI] [PubMed] [Google Scholar]
- 46. Canário AC, Cabral PU, Spyrides MH, et al. The impact of physical activity on menopausal symptoms in middle-aged women. Int J Gynaecol Obstet 2012; 118(1): 34–36. [DOI] [PubMed] [Google Scholar]
- 47. Carpenter JS, Tisdale JE, Chen CX, et al. A Menopause Strategies-Finding Lasting Answers for Symptoms and Health (MsFLASH) investigation of self-reported menopausal palpitation distress. J Womens Health (Larchmt) 2021; 30(4): 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Castro AM, Beltrán-Barrios T, Mercado-Lara M. Assessment of the frequency of sleep complaints and menopausal symptoms in climacteric women using the Jenkins Sleep Scale. Sleep Sci 2021; 14(2): 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cengiz H, Kaya C, Suzen Caypinar S, et al. The relationship between menopausal symptoms and metabolic syndrome in postmenopausal women. J Obstet Gynaecol 2019; 39(4): 529–533. [DOI] [PubMed] [Google Scholar]
- 50. Chen RJ, Chang TC, Chow SN. Perceptions of and attitudes toward estrogen therapy among surgically menopausal women in Taiwan. Menopause 2008; 15(3): 517–523. [DOI] [PubMed] [Google Scholar]
- 51. Chou MF, Wun YT, Pang SM. Menopausal symptoms and the menopausal rating scale among midlife Chinese women in Macau, China. Women Health 2014; 54: 115–126. [DOI] [PubMed] [Google Scholar]
- 52. Chuni N, Sreeramareddy CT. Frequency of symptoms, determinants of severe symptoms, validity of and cut-off score for Menopause Rating Scale (MRS) as a screening tool: a cross-sectional survey among midlife Nepalese women. BMC Womens Health 2011; 11: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Conde DM, Pinto-Neto AM, Santos-Sá D, et al. Factors associated with quality of life in a cohort of postmenopausal women. Gynecol Endocrinol 2006; 22(8): 441–446. [DOI] [PubMed] [Google Scholar]
- 54. D’Souza CJ, Haripriya S, Krishna HS. The association between physical activity and menopause-related quality of life. Int J Ther Rehabil 2021; 28: 1–11. [Google Scholar]
- 55. Demir O, Ozalp M, Sal H, et al. The relationship of menopausal symptoms with the type of menopause and lipid levels. Prz Menopauzalny 2020; 19(1): 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dotlic J, Kurtagic I, Nurkovic S, et al. Factors associated with general and health-related quality of life in menopausal transition among women from Serbia. Women Health 2018; 58: 278–296. [DOI] [PubMed] [Google Scholar]
- 57. El Shafie K, Al Farsi Y, Al Zadjali N, et al. Menopausal symptoms among healthy, middle-aged Omani women as assessed with the Menopause Rating Scale. Menopause 2011; 18(10): 1113–1119. [DOI] [PubMed] [Google Scholar]
- 58. Enomoto H, Terauchi M, Odai T, et al. Independent association of palpitation with vasomotor symptoms and anxiety in middle-aged women. Menopause 2021; 28: 741–747. [DOI] [PubMed] [Google Scholar]
- 59. Ferreira CE, Pinto-Neto AM, Conde DM, et al. Menopause symptoms in women infected with HIV: prevalence and associated factors. Gynecol Endocrinol 2007; 23(4): 198–205. [DOI] [PubMed] [Google Scholar]
- 60. Fu JX, Luo Y, Chen MZ, et al. Associations among menopausal status, menopausal symptoms, and depressive symptoms in midlife women in Hunan Province, China. Climacteric 2020; 23(3): 259–266. [DOI] [PubMed] [Google Scholar]
- 61. Gazibara T, Nurkovic S, Kovacevic N, et al. Factors associated with sexual quality of life among midlife women in Serbia. Qual Life Res 2017; 26(10): 2793–2804. [DOI] [PubMed] [Google Scholar]
- 62. Gómez-Tabares G, García W, Bedoya-Dorado E, et al. Screening sarcopenia through SARC-F in postmenopausal women: a single-center study from South America. Climacteric 2019; 22: 627–631. [DOI] [PubMed] [Google Scholar]
- 63. Hartmann BW, Kirchengast S, Albrecht A, et al. Hysterectomy increases the symptomatology of postmenopausal syndrome. Gynecol Endocrinol 1995; 9(3): 247–252. [DOI] [PubMed] [Google Scholar]
- 64. Hickey M, Riach K, Kachouie R, et al. No sweat: managing menopausal symptoms at work. J Psychosom Obstet Gynaecol 2017; 38(3): 202–209. [DOI] [PubMed] [Google Scholar]
- 65. Huseth-Zosel A, Strand M, Perry J. Socioeconomic differences in the menopausal experience of Chinese women. Post Reprod Health 2014; 20(3): 98–103. [DOI] [PubMed] [Google Scholar]
- 66. Im EO, Ham OK, Chee E, et al. Racial/ethnic differences in cardiovascular symptoms in four major racial/ethnic groups of midlife women: a secondary analysis. Women Health 2015; 55: 525–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ishizuka B, Kudo Y, Tango T. Cross-sectional community survey of menopause symptoms among Japanese women. Maturitas 2008; 61: 260–267. [DOI] [PubMed] [Google Scholar]
- 68. Jaber RM, Khalifeh SF, Bunni F, et al. Patterns and severity of menopausal symptoms among Jordanian women. J Women Aging 2017; 29(5): 428–436. [DOI] [PubMed] [Google Scholar]
- 69. Jaber RM, Khalil IA, Almohtasib YS, et al. Association of menopausal symptom severity and osteoporotic fractures: a case-control study. J Women Aging 2022; 34(1): 93–100. [DOI] [PubMed] [Google Scholar]
- 70. Kalhan M, Singhania K, Choudhary P, et al. Prevalence of menopausal symptoms and its effect on quality of life among rural middle aged women (40–60 Years) of Haryana, India. Int J Appl Basic Med Res 2020; 10: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kirchengast S. Relations between fertility, body shape and menopause in Austrian women. J Biosoc Sci 1992; 24(4): 555–559. [DOI] [PubMed] [Google Scholar]
- 72. Kirchengast S. Relations between anthropometric characteristics and degree of severity of the climacteric syndrome in Austrian women. Maturitas 1993; 17(3): 167–180. [DOI] [PubMed] [Google Scholar]
- 73. Kong F, Wang J, Zhang C, et al. Assessment of sexual activity and menopausal symptoms in middle-aged Chinese women using the Menopause Rating Scale. Climacteric 2019; 22(4): 370–376. [DOI] [PubMed] [Google Scholar]
- 74. Lee SW, Jo HH, Kim MR, et al. Association between menopausal symptoms and metabolic syndrome in postmenopausal women. Arch Gynecol Obstet 2012; 285(2): 541–548. [DOI] [PubMed] [Google Scholar]
- 75. Legorreta D, Montaño JA, Hernández I, et al. Age at menopause, motives for consultation and symptoms reported by 40–59-year-old Mexican women. Climacteric 2013; 16(4): 417–425. [DOI] [PubMed] [Google Scholar]
- 76. Liu P, Yuan Y, Liu M, et al. Factors associated with menopausal symptoms among middle-aged registered nurses in Beijing. Gynecol Endocrinol 2015; 31(2): 119–124. [DOI] [PubMed] [Google Scholar]
- 77. Loja-Chango R, Pérez-López FR, Simoncini T, et al. Increased mood symptoms in postmenopausal women related to the polymorphism rs743572 of the CYP17 A1 gene. Gynecol Endocrinol 2016; 32(10): 827–830. [DOI] [PubMed] [Google Scholar]
- 78. Looby SE, Shifren J, Corless I, et al. Increased hot flash severity and related interference in perimenopausal human immunodeficiency virus-infected women. Menopause 2014; 21(4): 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Luptáková L, Sivaková D, Srámeková D, et al. The association of cytochrome P450 1B1 Leu432Val polymorphism with biological markers of health and menopausal symptoms in Slovak midlife women. Menopause 2012; 19(2): 216–224. [DOI] [PubMed] [Google Scholar]
- 80. Luptáková L, Sivtáková D, Cernanová V, et al. Menopausal complaints in Slovak midlife women and the impact of CYP1B1 polymorphism on their incidence. Anthropol Anz 2012; 69(4): 399–415. [PubMed] [Google Scholar]
- 81. McKinlay SM, Jefferys M. The menopausal syndrome. Br J Prev Soc Med 1974; 28: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Monterrosa A, Blumel JE, Chedraui P. Increased menopausal symptoms among Afro-Colombian women as assessed with the Menopause Rating Scale. Maturitas 2008; 59: 182–190. [DOI] [PubMed] [Google Scholar]
- 83. Monterrosa A, Blumel JE, Chedraui P, et al. Quality of life impairment among postmenopausal women varies according to race. Gynecol Endocrinol 2009; 25(8): 491–497. [DOI] [PubMed] [Google Scholar]
- 84. Monterrosa-Castro A, Blümel JE, Portela-Buelvas K, et al. Type II diabetes mellitus and menopause: a multinational study. Climacteric 2013; 16(6): 663–672. [DOI] [PubMed] [Google Scholar]
- 85. Nisar N, Sikandar R, Sohoo NA. Menopausal symptoms: prevalence, severity and correlation with sociodemographic and reproductive characteristics. A cross sectional community based survey from rural Sindh Pakistan. J Pak Med Assoc 2015; 65(4): 409–413. [PubMed] [Google Scholar]
- 86. Oi N, Ohi K. Comparison of the symptoms of menopause and symptoms of thyroid disease in Japanese women aged 35–59 years. Climacteric 2013; 16(5): 555–560. [DOI] [PubMed] [Google Scholar]
- 87. Ojeda E, Monterrosa A, Blümel JE, et al. Severe menopausal symptoms in mid-aged Latin American women can be related to their indigenous ethnic component. Climacteric 2011; 14(1): 157–163. [DOI] [PubMed] [Google Scholar]
- 88. Otolorin EO, Adeyefa I, Osotimehin BO, et al. Clinical, hormonal and biochemical features of menopausal women in Ibadan, Nigeria. Afr J Med Med Sci 1989; 18(4): 251–255. [PubMed] [Google Scholar]
- 89. Peng W, Sibbritt DW, Hickman L, et al. Association between use of self-prescribed complementary and alternative medicine and menopause-related symptoms: a cross-sectional study. Complement Ther Med 2015; 23(5): 666–673. [DOI] [PubMed] [Google Scholar]
- 90. Rahman SA, Zainudin SR, Mun VL. Assessment of menopausal symptoms using modified Menopause Rating Scale (MRS) among middle age women in Kuching, Sarawak, Malaysia. Asia Pac Fam Med 2010; 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rahman S, Salehin F, Iqbal A. Menopausal symptoms assessment among middle age women in Kushtia, Bangladesh. BMC Res Notes 2011; 4: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rathnayake N, Lenora J, Alwis G, et al. Prevalence and severity of menopausal symptoms and the quality of life in middle-aged women: a study from Sri Lanka. Nurs Res Pract 2019; 2019: 2081507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ruan X, Cui Y, Du J, et al. Prevalence of climacteric symptoms comparing perimenopausal and postmenopausal Chinese women. J Psychosom Obstet Gynaecol 2017; 38(3): 161–169. [DOI] [PubMed] [Google Scholar]
- 94. Ryu KJ, Park H, Kim YJ, et al. Comparison of various menopausal symptoms and risk factor analysis in Korean women according to stage of menopause. Maturitas 2020; 140: 41–48. [DOI] [PubMed] [Google Scholar]
- 95. Saccomani S, Lui-Filho JF, Juliato CR, et al. Does obesity increase the risk of hot flashes among midlife women? A population-based study. Menopause 2017; 24(9): 1065–1070. [DOI] [PubMed] [Google Scholar]
- 96. Santonicola A, Iovino P, Cappello C, et al. From menarche to menopause: the fertile life span of celiac women. Menopause 2011; 18(10): 1125–1130. [DOI] [PubMed] [Google Scholar]
- 97. Schnatz PF, Serra J, O’Sullivan DM, et al. Menopausal symptoms in Hispanic women and the role of socioeconomic factors. Obstet Gynecol Surv 2006; 61(3): 187–193. [DOI] [PubMed] [Google Scholar]
- 98. Sievert LL, Obermeyer CM. Symptom clusters at midlife: a four-country comparison of checklist and qualitative responses. Menopause 2012; 19(2): 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Slopien R, Owecki M, Slopien A, et al. Climacteric symptoms are related to thyroid status in euthyroid menopausal women. J Endocrinol Invest 2020; 43(1): 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Somekawa Y, Chiguchi M, Ishibashi T, et al. Soy intake related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet Gynecol 2001; 97(1): 109–115. [DOI] [PubMed] [Google Scholar]
- 101. Suarez-García I, Alejos B, Pérez-Elías M-J, et al. How do women living with HIV experience menopause? Menopausal symptoms, anxiety and depression according to reproductive age in a multicenter cohort. BMC Womens Health 2021; 21: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sukwatana P, Meekhangvan J, Tamrongterakul T, et al. Menopausal symptoms among Thai women in Bangkok. Maturitas 1991; 13(3): 217–228. [DOI] [PubMed] [Google Scholar]
- 103. Sweed HS, Elawam AE, Nabeel AM, et al. Postmenopausal symptoms among Egyptian geripausal women. East Mediterr Health J 2012; 18(3): 213–220. [DOI] [PubMed] [Google Scholar]
- 104. Tan MN, Kartal M, Guldal D. The effect of physical activity and body mass index on menopausal symptoms in Turkish women: a cross-sectional study in primary care. BMC Womens Health 2014; 14: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tao MF, Sun DM, Shao HF, et al. Poor sleep in middle-aged women is not associated with menopause per se. Braz J Med Biol Res 2016; 49: e4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thapa R, Yang Y, Bekemeier B. Menopausal symptoms and associated factors in women living with HIV in Cambodia. J Women Aging 2019; 32: 517–536. [DOI] [PubMed] [Google Scholar]
- 107. Thapa R, Yang Y. Menopausal symptoms and related factors among Cambodian women. Women Health 2020; 60(4): 396–411. [DOI] [PubMed] [Google Scholar]
- 108. Topatan S, Yıldız H. Symptoms experienced by women who enter into natural and surgical menopause and their relation to sexual functions. Health Care Women Int 2012; 33(6): 525–539. [DOI] [PubMed] [Google Scholar]
- 109. Trento S, Madeiro A, Rufino AC. Sexual function and associated factors in postmenopausal women. Rev Bras Ginecol Obstet 2021; 43: 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Vural PI, Yangin HB. Assessing menopausal symptoms among Turkish and German women with the Menopause Rating Scale: a cross-cultural study. Int J Caring Sci 2017; 10: 979–987. [Google Scholar]
- 111. Waidyasekera H, Wijewardena K, Lindmark G, et al. Menopausal symptoms and quality of life during the menopausal transition in Sri Lankan women. Menopause 2009; 16(1): 164–170. [DOI] [PubMed] [Google Scholar]
- 112. Wolff EF, He Y, Black DM, et al. Self-reported menopausal symptoms, coronary artery calcification, and carotid intima-media thickness in recently menopausal women screened for the Kronos early estrogen prevention study (KEEPS). Fertil Steril 2013; 99(5): 1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wu HC, Wen SH, Hwang JS, et al. Validation of the traditional Chinese version of the Menopausal Rating Scale with WHOQOL-BREF. Climacteric 2015; 18(5): 750–756. [DOI] [PubMed] [Google Scholar]
- 114. Wu HC, Chen KH, Hwang JS. Association of menopausal symptoms with different constitutions in climacteric women. Complement Med Res 2018; 25(6): 398–405. [DOI] [PubMed] [Google Scholar]
- 115. Zhang Y, Zhao X, Leonhart R, et al. A cross-cultural comparison of climacteric symptoms, self-esteem, and perceived social support between Mosuo women and Han Chinese women. Menopause 2016; 23(7): 784–791. [DOI] [PubMed] [Google Scholar]
- 116. Zhao G, Wang L, Yan R, et al. Menopausal symptoms: experience of Chinese women. Climacteric 2000; 3: 135–144. [DOI] [PubMed] [Google Scholar]
- 117. Lugo T, Tetrokalashvili M. Hot flashes. StatPearls. Treasure Island, FL: StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 118. Thavendiranathan P, Bagai A, Khoo C, et al. Does this patient with palpitations have a cardiac arrhythmia? JAMA 2009; 302: 2135–2143. [DOI] [PubMed] [Google Scholar]
- 119. Clementy N, Fourquet A, Andre C, et al. Benefits of an early management of palpitations. Medicine (Baltimore) 2018; 97(28): e11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. National Library of Medicine. Hot flashes: MeSH descriptor data 2022, https://meshb.nlm.nih.gov/record/ui?ui=D019584 (2022, accessed 13 May 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057221112267 for Correlates of palpitations during menopause: A scoping review by Janet S Carpenter, Ying Sheng, Caitlin Pike, Charles D Elomba, Jennifer S Alwine, Chen X Chen and James E Tisdale in Women’s Health
Supplemental material, sj-docx-2-whe-10.1177_17455057221112267 for Correlates of palpitations during menopause: A scoping review by Janet S Carpenter, Ying Sheng, Caitlin Pike, Charles D Elomba, Jennifer S Alwine, Chen X Chen and James E Tisdale in Women’s Health