Summary
Background
Post-COVID syndrome (PCS) is an important sequela of COVID-19, characterised by symptom persistence for >3 months, post-acute symptom development, and worsening of pre-existing comorbidities. The causes and public health impact of PCS are still unclear, not least for the lack of efficient means to assess the presence and severity of PCS.
Methods
COVIDOM is a population-based cohort study of polymerase chain reaction (PCR) confirmed cases of SARS-CoV-2 infection, recruited through public health authorities in three German regions (Kiel, Berlin, Würzburg) between November 15, 2020 and September 29, 2021. Main inclusion criteria were (i) a PCR confirmed SARS-CoV-2 infection and (ii) a period of at least 6 months between the infection and the visit to the COVIDOM study site. Other inclusion criteria were written informed consent and age ≥18 years. Key exclusion criterion was an acute reinfection with SARS-CoV-2. Study site visits included standardised interviews, in-depth examination, and biomaterial procurement. In sub-cohort Kiel-I, a PCS (severity) score was developed based upon 12 long-term symptom complexes. Two validation sub-cohorts (Würzburg/Berlin, Kiel-II) were used for PCS score replication and identification of clinically meaningful predictors. This study is registered at clinicaltrials.gov (NCT04679584) and at the German Registry for Clinical Studies (DRKS, DRKS00023742).
Findings
In Kiel-I (n = 667, 57% women), 90% of participants had received outpatient treatment for acute COVID-19. Neurological ailments (61·5%), fatigue (57·1%), and sleep disturbance (57·0%) were the most frequent persisting symptoms at 6–12 months after infection. Across sub-cohorts (Würzburg/Berlin, n = 316, 52% women; Kiel-II, n = 459, 56% women), higher PCS scores were associated with lower health-related quality of life (EQ-5D-5L-VAS/-index: r = -0·54/ -0·56, all p < 0·0001). Severe, moderate, and mild/no PCS according to the individual participant's PCS score occurred in 18·8%, 48·2%, and 32·9%, respectively, of the Kiel-I sub-cohort. In both validation sub-cohorts, statistically significant predictors of the PCS score included the intensity of acute phase symptoms and the level of personal resilience.
Interpretation
PCS severity can be quantified by an easy-to-use symptom-based score reflecting acute phase disease burden and general psychological predisposition. The PCS score thus holds promise to facilitate the clinical diagnosis of PCS, scientific studies of its natural course, and the development of therapeutic interventions.
Funding
The COVIDOM study is funded by the Network University Medicine (NUM) as part of the National Pandemic Cohort Network (NAPKON).
Keywords: COVID-19; Post-COVID-Syndrome (PCS); LongCOVID, Post-acute sequelae of SARS-CoV-2 infection (PASC); SARS-CoV-2 infection; Fatigue; Resilience
Research in context.
Evidence before this study
The COVIDOM study was conceptualised in March 2020 when the first cases of acute COVID-19 appeared in Germany and when research on the long-term sequelae of the disease, subsumed under the term ‘Post-COVID Syndrome’ (PCS), was non-existent. Per design, the aims of COVIDOM are (I) to estimate the frequency of persistent symptoms and late complications of SARS-CoV-2 infection, (II) to assess and quantify the lasting health burden of COVID-19, including the development of chronic (non-communicable) disease and signs of accelerated ageing, and (III) to identify predictors of PCS development. Before the present COVIDOM-embedded analysis, we searched PubMed for the terms ‘post-covid’ OR ‘long-covid’ OR ‘longcovid’ OR ‘longhaul covid’, followed by the identification of other relevant articles from the reference lists of the initial hits. Our search covered original articles, reviews, comments, editorials, and meta-analyses published between January 1st 2020 and January 30th 2022. The results highlighted the very heterogenous scope and quality of PCS studies, leading to the conclusion that accurate estimation of PCS prevalence and severity in unselected populations has hardly been possible so far.
To our knowledge, only three population-based studies of symptom persistence after COVID-19 have been published to date, and these studies were small, lacked functional assessments beyond symptom self-reporting, recruited only participants infected during the first wave of the pandemic, and included many acutely hospitalised patients. Moreover, one of the studies followed participants for <3 months, thereby contradicting current recommendations for PCS diagnosis.
Added value of this study
In COVIDOM, we recruited PCR-confirmed cases with COVID-19 through local public health authorities, who are legally required to document all SARS-CoV-2 infections in a given region in Germany irrespective of course and severity of the acute disease. Our study protocol includes detailed functional assessments, in-depth on-site examinations, and standardised interviews, all scheduled ≥6 months post infection. For the purpose of the present study, we developed and validated an easy-to-use PCS severity score. The score was found to be inversely correlated with impaired quality of life, thereby providing evidence that it is a good marker of PCS. Notably, severity of acute COVID-19 symptoms and low resilience were the strongest predictors of a high PCS score at 9 months post infection. The PCS score also correlated with specific psychosocial characteristics, functional measures, and inflammatory and cardiovascular blood biomarkers.
Implications of all the available evidence
The PCS severity score presented here may serve not only as a simple decision-making tool in clinical practice, helping guide and prioritise post-COVID patient care, but also as a meaningful outcome parameter in interventional and prevention trials for PCS.
Alt-text: Unlabelled box
Introduction
The clinical presentation of acute SARS-CoV-2 infection is multi-faceted, including mild oligosymptomatic disease, acute respiratory distress syndrome (ARDS), and potentially lethal complications in multiple other organs.1 In many patients, clinical recovery from infection-associated COVID-19 is delayed by the persistence of symptoms from the acute phase of the infection, worsening of pre-existing comorbidities, or the development of post-acute phase symptoms attributable to the infection.2,3 The most frequent long-term sequelae of COVID-19 are fatigue, dyspnoea, exercise intolerance, loss of smell and taste, cognitive impairments, headache, and general pain.4,5 Collectively, the persistence of these symptoms beyond 12 weeks after infection has been labelled, for instance, “Long COVID”, “Post-acute Sequelae of SARS-CoV-2 Infection (PASC)”, or “Post-COVID Syndrome (PCS)”.2,4,6 While clear definitions of PCS are still missing, the need for a better scientific understanding of this health impairment has been expressed repeatedly by patient advocacy groups, in social media, and in the general press.6
Most studies of PCS undertaken so far were conducted in single-centre settings, or were enriched with patients with severe COVID-19 requiring hospital or intensive care treatment.4 Moreover, the latter study type in particular may not have allowed to differentiate sufficiently well between PCS and post-intensive care syndrome, meaning the generally delayed recovery from intensive care treatment or ARDS.7
Population-based studies in geographically confined regions, or of frequency-stratified samples, potentially provide more accurate estimates of PCS prevalence in the general population because such studies are usually less subject to recruitment bias. Previously published examples from the Faroe Islands, the Bergen area (Norway), and specific regions of Michigan (USA) were, however, small, involved follow-up periods of <3 months and lacked functional assessments other than symptom self-reporting.8, 9, 10 Against this background, we initiated a prospective population-based multi-centre study (COVIDOM) to investigate PCS in three regions across Germany, recruiting unselected PCR-confirmed cases of SARS-CoV-2 infection, and using standardised questionnaires as well as in-depth functional assessments.
Scores hitherto proposed for grading PCS severity lacked precision although a reliable scoring system is still one of the most pressing needs in post-COVID research and patient care.11,12 Therapeutic interventions targeting the long-term sequelae of COVID-19 urgently require simple and clinically meaningful means of capturing and quantifying their effectiveness through ‘measuring’ PCS severity. Especially for previously healthy individuals with subtle albeit complex post-COVID phenotypes, existing scoring systems such as the Post-Covid Functional Status (PCFS) may be unsuitable to resolve subtle health impairments. The PCFS, for instance, only covers functional limitations in everyday life but does not take symptom- or organ-related impairments into account.11 Other scoring systems may be useful for PCS prediction but not for PCS severity grading.13 In addition to reporting early insights from the ongoing COVIDOM study, the main objective of the present work was therefore the development and validation of an easy-to-use PCS score drawing upon 12 key long-term symptom complexes of COVID-19.
Materials and methods
Study samples
In April 2020, the German government established the ‘Network University Medicine (NUM)’ to coordinate and sustain COVID-19-related research at a national level, including the ‘National Pandemic Cohort Network (NAPKON)’. COVIDOM is a prospective, population-based cohort study of the long-term health sequelae of SARS-CoV-2 infection, embedded into the population-based NAPKON platform (NAPKON-POP). Details about COVIDOM and NAPKON have been reported elsewhere.14,15
COVIDOM participants were recruited in catchment areas around Kiel (Northern Germany) and Würzburg (Southern Germany), and in the Neukölln district of Berlin (Eastern Germany). Main inclusion criteria were (i) a polymerase chain reaction (PCR) confirmed SARS-CoV-2 infection and (ii) a period of at least 6 months between the infection and the visit to the COVIDOM study site.14 Other inclusion criteria were ≥18 years of age and written informed consent. Key exclusion criterion was an acute reinfection with SARS-CoV-2. Eligible individuals were identified through local public health authorities so as to address an unbiased subpopulation regarding age, sex, hospitalisation, and media literacy. Participation was additionally incentivised financially (i.e. reimbursement of travel and other participation costs) in order to reduce recruitment bias favouring more symptomatic participants. A subset of responders, representative of the local infected population regarding basic demographics (i.e. age and sex) and severity of acute COVID-19 (i.e. hospitalisation frequency), was invited to a study site visit (for details, see Supplementary Material, page 3). Study site visits took place between November 15, 2020 and September 29, 2021
Since COVIDOM requirements stipulated a minimum local sample size of 300, the Würzburg and Berlin samples were combined into one sub-cohort for the present work. By contrast, the Kiel samples were divided into two sub-cohorts (Kiel-I and Kiel-II) primarily for the purpose of independent validation of the PCS score developed in Kiel-I (see below) but also for technical reasons of data curation.
COVIDOM was approved by the local ethic committees of the university hospitals of Kiel (No. D 537/20), and Würzburg (No. 236/20_z). According to the professional code of the Berlin Medical Association, approval by the Kiel ethics committee was also valid for the Berlin study site. All participants gave written informed consent. The study was registered at clinicaltrials.gov (NCT04679584) and at the German Registry for Clinical Studies (DRKS, DRKS00023742). All reporting of procedures and results adheres to the STROBE guidelines for cohort studies.
Study procedures
Initial survey
Before the study site visit, participants received a questionnaire to complete at home or online (see Supplementary Material, page 4). The questionnaire, which was based upon previous experience with local and national epidemiological projects,16,17 covered basic demographic characteristics, general lifestyle, course of disease, circumstances of acute SARS-CoV-2 infection, pre-existing comorbidities, healthcare utilisation, and symptom persistence.
Symptom assessment
Participants were asked to remember the presence, and to retrospectively rate the severity (on a 4-point Likert scale: mild/moderate/severe/life-threatening), of 23 possible symptoms during the acute phase of COVID-19. In addition, they were asked about the subsequent development of post-acute symptoms, including fatigue, sleep disturbance, and neurological ailments such as concentration deficits or sensory deficits. The persistence of acute or post-acute symptoms into the long-term phase of COVID-19 (>12 weeks according to Nalbandian et al.2) was assessed during the study site visit approximately 9 months post infection. Symptoms still present around that time are henceforth referred to as ‘long-term symptoms’, irrespective of the time of onset. Long-term symptoms were grouped into 12 clinically meaningful symptom complexes (Table 1).
Table 1.
Long-term symptom complexes underlying PCS score definition.
| No. | Symptom complex | Self-reported sub-symptomsa |
|---|---|---|
| 1 | Chemosensory deficits | Smelling disturbance, impaired sense of taste |
| 2 | Fatigue | Fatigue |
| 3 | Exercise intolerance | Shortness of breath, reduced exercise capacity |
| 4 | Joint or muscle pain | Muscle pain, joint pain |
| 5 | Ear-Nose-Throat (ENT) ailments | Hoarseness, sore throat, running nose |
| 6 | Coughing, wheezing | Coughing, wheezing |
| 7 | Chest pain | Chest pain |
| 8 | Gastrointestinal ailments | Stomach pain, diarrhoea, vomiting, nausea |
| 9 | Neurological ailments | Confusion, vertigo, headache, motor deficits, sensory deficits, numbness, tremor, deficits of concentration, cognition or speech |
| 10 | Dermatological ailments | Hair loss, rash, itchiness |
| 11 | Infection signs | Chills, fever, general sickness/flu-like symptoms |
| 12 | Sleep disturbance | Insomnia, unrestful sleep |
All self-reported sub-symptoms were ascertained in standardised interviews at approximately 6–12 months post infection. Whenever at least one sub-symptom was present, the binary indicator of the corresponding symptom complex was encoded as 1 (present), otherwise the indicator was set equal to 0 (absent).
On-site examination
The COVIDOM examination programme comprised additional questionnaires focusing on depression (PHQ-8), anxiety (GAD-7), fatigue (FACIT-F; Canadian Criteria for chronic fatigue syndrome/myalgic encephalomyelitis, CFS/ME), cognitive function (MoCA), stress (PSS), resilience (BRS), dyspnoea (mMRC; MDP), and quality of life (EQ-5D-5L). General anxiousness was rated with regard to nine health or catastrophic events, and participants were grouped as generally more or less anxious by way of a median split (for details, see Horn et al.14 and Supplementary Material, pages 4 to 7).
Functional measures included standardised assessment of lung function and echocardiography, as well as anthropometry, and vital signs. Assessments of lung function comprised forced spirometry and diffusing capacity of the lung for carbon monoxide (DLCO; for details, see Supplementary Material, page 4). All examinations were carried out by trained study staff following standard operating procedures (SOPs).
Laboratory measurements were performed in certified laboratories of the participating university hospitals in compliance with established quality control standards.
Statistical analysis
All statistical analyses were carried out with IBM SPSS software version 21.0 for Windows (released 2012; IBM Corp. Armonk, NY), adopting a two-sided significance level of 0·05 throughout. p values were Bonferroni-adjusted if and when appropriate (for details, see legends to Tables 4 and 5). For categorical and dichotomous variables, absolute frequencies and percentages are reported. Group differences were assessed for statistical significance with a chi-squared test or Fisher's exact test, as appropriate. For metric variables, mean and standard deviation are reported, and analysis of variance (ANOVA) or Student's t-test was used for inter-group comparisons, as appropriate (for additional statistical analyses, see below). Graphs were created with either SigmaPlot v. 8.0 or Microsoft Excel.
Table 4.
Univariate clinical and functional correlates of the PCS score in the Kiel-I sub-cohort.
| Characteristic | PCS score |
p valued |
|||
|---|---|---|---|---|---|
| ≤10·75 (none/mild, n = 191) |
>10·75 to ≤26·25 (moderate, n = 280) |
>26·25 (severe, n = 109) |
unadjusted | adjusted | |
| Age [years] | 46·5 (16·4) | 47·0 (14·3) | 52·1 (16·1) | 0·0045 | 0·018 |
| Women, n (%) | 90 (47·4) | 169 (60·4) | 75 (68·8) | 0·00068 | 0·0027 |
| BMI [kg/m2] | 26·0 (4·4) | 27·2 (5·4) | 27·4 (5·8) | 0·018 | 0·073 |
| Smoker, n (%) | 46 (24·9) | 93 (33·9) | 33 (31·4) | 0·11 | n.a. |
| Vital signs at rest | |||||
| Oxygen saturation [%] | 98·9 (1·3) | 98·7 (1·2) | 98·6 (1·4) | 0·062 | n.a. |
| Heart rate [min−1] | 60·2 (10·2) | 62·3 (10·1) | 65·0 (12·2) | 0·00081 | 0·0032 |
| Systolic blood pressure [mmHg] | 141·7 (17·4) | 139·9 (18·7) | 142·6 (18·2) | 0·35 | n.a. |
| Diastolic blood pressure [mmHg] | 88·4 (9·8) | 89·5 (10·0) | 89·5 (9·2) | 0·44 | n.a. |
| Lung function: forced spirometrya | |||||
| FEV1 [L] | 3·51 (0·84) | 3·38 (0·85) | 2·96 (0·74) | <0·0001 | <0·0001 |
| FEV1 z score | -0·24 (0·93) | -0·30 (0·88) | -0·47 (0·98) | 0·14 | n.a. |
| FVC [L] | 4·48 (1·07) | 4·30 (1·03) | 3·79 (0·94) | <0·0001 | <0·0001 |
| FVC z score | -0·10 (0·85) | -0·15 (0·83) | -0·32 (0·87) | 0·10 | n.a. |
| FEV1/FVC | 0·79 (0·06) | 0·79 (0·06) | 0·78 (0·06) | 0·76 | n.a. |
| FEV1/FVC<0·7b, n (%) | 11 (6·2) | 18 (7·1) | 9 (9·1) | 0·65 | n.a. |
| Lung function: diffusing capacity of the lung for carbon monoxidea | |||||
| DLCO [mmol/kPa/min] | 8·75 (2·08) | 8·13 (2·01) | 7·35 (1·70) | <0·0001 | <0·0001 |
| DLCO z score | -0·19 (0·89) | -0·44 (0·88) | -0·49 (0·98) | 0·0093 | 0·056 |
| DLCO <80% predicted, n (%) | 12 (7·4) | 34 (12·7) | 12 (13·0) | 0·19 | n.a. |
| KCO [mmol/kPa/min/L] | 1·45 (0·22) | 1·42 (0·21) | 1·40 (0·25) | 0·14 | n.a. |
| KCO z score | -0·17 (0·99) | -0·26 (0·93) | -0·29 (1·10) | 0·57 | n.a. |
| KCO <80% predicted, n (%) | 11 (6·8) | 21 (7·9) | 12 (13·0) | 0·20 | n.a. |
| Echocardiographyc | |||||
| Left ventricular stroke volume [mL] | 58·5 (15·6) | 55·3 (16·6) | 48·9 (13·7) | <0·0001 | <0·0001 |
| Left ventricular ejection fraction [%] | 64·8 (5·4) | 65·1 (5·6) | 63·5 (6·8) | 0·053 | n.a. |
| MAPSE septal [mm] | 14·1 (2·4) | 14·0 (2·4) | 13·3 (2·3) | 0·018 | 0·14 |
| MAPSE lateral [mm] | 16·6 (3·1) | 16·3 (3·1) | 15·9 (3·1) | 0·16 | n.a. |
| TAPSE [mm] | 24·4 (3·6) | 24·0 (3·6) | 23·8 (3·8) | 0·33 | n.a. |
| Diastolic dysfunction grade 1 or higher, n (%) | 63 (33·2) | 87 (31·5) | 45 (41·3) | 0·18 | n.a. |
| TR Pmax [mmHg] | 18·7 (5·1) | 19·8 (5·4) | 19·3 (7·0) | 0·28 | n.a. |
| Clinically relevant valve dysfunction, n (%) | 30 (17·5) | 49 (19·6) | 20 (20·8) | 0·78 | n.a. |
All participant characteristics are given as mean (SD) unless otherwise specified.
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in the first second; FVC, forced expiratory volume; DLCO, diffusing capacity of the lung for carbon monoxide; KCO, DLCO/ alveolar volume (VA); MAPSE, mitral annular plane systolic excursion; TAPSE, tricuspid annular plane systolic excursion; TR Pmax, maximal pressure gradient over tricuspid valve.
All three study sites used the same lung function device and followed harmonised standard operating procedures (SOPs) based upon international guidelines. Reference values from the Global Lung Function Initiative (GLI) were used for deriving characteristics of forced spirometry and diffusing capacity of the lung for carbon monoxide (accessed via http://gli-calculator.ersnet.org/index.html).
FEV1/FVC<0·7 was used to indicate airflow limitation.
Echocardiography was performed by trained sonographers according to harmonised SOPs and supervised by a board-certified cardiologist.
dNominally significant p values were Bonferroni-adjusted by multiplication with the size of the respective group of characteristics (i.e., 4, 5, 6, or 8). n.a.: not applicable because the unadjusted p value already exceeded 0·050.
Table 5.
Predictors of PCS score class in the Würzburg/Berlin and Kiel-II sub-cohorts (final ordinal logistic regression models).
| Predictor variable | Level | Regression coefficient |
Odds ratio |
p valuee |
||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Standard error | 95% confidence interval | Estimate | 95% confidence interval | unadjusted | adjusted | ||
| Würzburg/Berlin (n = 277) | ||||||||
| No. serious or life-threatening symptomsa | 0 | -2·509 | 0·472 | [-3·434; -1·584] | 0·081 | [0·032; 0·205] | <0·0001 | <0·0001 |
| 1-3 | -1·792 | 0·412 | [-2·599; -0·985] | 0·167 | [0.074; 0·373] | <0·0001 | <0·0001 | |
| 4-6 | -1·240 | 0·448 | [-2·117; -0·363] | 0·289 | [0·120; 0·696] | 0·0056 | 0·039 | |
| Pre-existing neurologic or psychiatric disease | Yes | 1·387 | 0·301 | [0·798; 1·976] | 4·003 | [2·221; 7·214] | <0·0001 | <0·0001 |
| Pre-existing cardiovascular disease | Yes | 0·947 | 0·282 | [0·394; 1·499] | 2·578 | [1·483; 4·477] | 0·00078 | 0·0055 |
| General anxiousness | less anxious | -0·803 | 0·277 | [-1·346; -0·261] | 0·448 | [0·260; 0·770] | 0·0037 | 0·026 |
| Resilience (BRS) | Scale | -0·606 | 0·213 | [-1·024; -0·187] | 0·546 | [0·359; 0·829] | 0·0045 | 0·032 |
| Kiel-II (n = 399) | ||||||||
| No. symptomsb | 0-2 | -2·288 | 0·677 | [-3·615; -0·961] | 0·101 | [0·027; 0·383] | 0·00073 | 0·0087 |
| 3-5 | -1·741 | 0·377 | [-2·480; -1·002] | 0·175 | [0·084; 0·367] | <0·0001 | <0·0001 | |
| 6-8 | -1·238 | 0·295 | [-1·817; -0·659] | 0·290 | [0·163; 0·517] | <0·0001 | 0·00034 | |
| No. serious or life-threatening symptomsa | 0 | -1·922 | 0·443 | [-2·791; -1·054] | 0·146 | [0·061; 0·349] | <0·0001 | 0·00017 |
| 1-3 | -1·707 | 0·351 | [-2·395; -1·019] | 0·181 | [0·091; 0·361] | <0·0001 | <0·0001 | |
| 4-6 | -1·368 | 0·353 | [-2·060; -0·676] | 0·255 | [0·127; 0·509] | <0·0001 | 0·00019 | |
| Resilience (BRS) | Scale | -0·707 | 0·145 | [-0·991; -0·423] | 0·493 | [0·371; 0·655] | <0·0001 | <0·0001 |
| Age | Scale | 0·022 | 0·007 | [0·007; 0·036] | 1·022 | [1·007; 1·037] | 0·0031 | 0·037 |
| Body weight change after infectionc | Gain | 0·745 | 0·250 | [0·256; 1·234] | 2·106 | [1·292; 3·435] | 0·0028 | 0·034 |
| Loss | 0·428 | 0·297 | [0·154; 1·009] | 1·534 | [1·166; 2·743] | 0·15 | n.a. | |
| Serious post-acute complications | Yes | 0·636 | 0·282 | [0·086; 1·187] | 1·889 | [1·090; 3·277] | 0·023 | 0·28 |
| Educationd | university entrance | -0·427 | 0·217 | [-0·851; -0·003] | 0·652 | [0·427; 0·997] | 0·049 | 0·58 |
Significant predictors of the PCS score class were identified by multivariate ordinal logistic regression analysis with backward selection (threshold p < 0·05). Only participants with complete data for all 12 symptom complexes underlying the PCS score definition were included (Würzburg/Berlin: n = 277; Kiel-II: n = 399). Missing predictor variables were imputed by multiple imputation.
Reference levels:
≥7 symptoms.
≥9 symptoms.
no weight change.
other school degree.
p values were Bonferroni-adjusted by multiplication with the total number of proband characteristic levels present in each sub-cohort-specific regression model (i.e., 7, or 12).
Post-COVID syndrome (PCS) score development
The development of a PCS score was based upon the binary indicators of 12 non-overlapping long-term symptom complexes of COVID-19, chosen a priori to cover the likely spectrum of infection-related health complications (Table 1).
Members of the Kiel-I cohort with complete data for all 12 indicators were repeatedly subjected to k-means clustering with SPSS function K-Means Cluster. K-means clustering is an unsupervised machine learning algorithm aimed to group n observations into k clusters such that, on average, all observations are as close as possible to the mean (or ‘centre’) of their own cluster. Each cluster number k led to a k-specific PCS score, SPCS(k), for each cohort member (see below). Starting from k = 2, the cluster number was increased successively until the score became stable in the sense that the Pearson correlation coefficient between SPCS(k) and SPCS(k+1) was approximately 0·95. We deliberately chose not to employ an elbow (or comparable) method for determining the optimal number of clusters, but to use the described correlation-based criterion instead. This is because the focus of the corresponding stopping rule had to be on the stability of the ensuing PCS score, not of the underlying clustering.
For each value of k, the resulting clusters were ranked according to phenotype severity, measured by the component sum of the respective cluster centres. Next, the 12 indicators were included as predictor variables in an ordinal logistic regression analysis of the cluster affiliation of participants. The estimated regression coefficients then served as weights of the corresponding indicator in the definition of the PCS score, i.e. SPCS (k) was set equal to the linear combination of the indicators included in the final regression model.
The incremental alternation of k-means clustering and PCS score definition was terminated, at k0, when the abovementioned Pearson correlation criterion was met. For ease of practical implementation, the final score was slightly modified by rounding all weights in SPCS(k0) to the nearest half-integer.
Multivariate identification of PCS score predictors
The relevance of different acute-phase and general characteristics for the long-term health status of participants was assessed by way of multivariate ordinal logistic regression analysis with backward selection (threshold p < 0·05), treating PCS score class (for definition, see Results) as the outcome variable. A total of 20 potential clinically meaningful predictor variables, chosen by expert agreement from the available COVIDOM data, were jointly considered in the analysis (see Supplementary Table 14). Symptoms with post-acute onset (i.e. fatigue, sleep disturbances, and most neurological sub-symptoms) were not included. Missing values of predictor variables were imputed from available data by multiple imputation, assuming that missing was completely at random. Usually, this type of analysis is repeated a number of times, and confidence intervals and p values are pooled so as to account for the randomness introduced by the imputation step. However, since single rounds of mean imputation combined with either forward or backward selection yielded virtually identical results, we concluded that such resource-intense and difficult to implement repetition was not required here.
Replication of nominally significant results from the multivariate analysis of Kiel-I was sought in the two other COVIDOM sub-cohorts. To this end, the PCS score was calculated and classified for all remaining participants for whom complete data on the 12 symptom complexes were available (280 of 316 participants in Würzburg/Berlin, 407 of 459 participants in Kiel-II). Goodness-of-fit of the final logistic regression models obtained in the replication analyses was quantified by Nagelkerke pseudo-R2.
Role of the funding source
The funder of the study (Network University Medicine, NUM) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. TB, CB, WL, TK, PH, MKr, and SSch had access to the data and are finally responsible for the decision to submit the current work for publication.
Results
Characteristics of COVIDOM sub-cohorts
The Kiel-I (training) sub-cohort used to develop a PCS score comprised 667 middle-aged inhabitants from Schleswig-Holstein who were infected by SARS-CoV-2, mostly with mild acute disease (Table 2). Sex distribution and frequency of in-patient treatment for acute COVID-19 in Kiel-I were comparable to the local infected population, matching for the date of SARS-CoV-2 infection (all p > 0·05). The age distribution in Kiel-I and in the local infected population was similar for the 3rd, 4th, 5th, 7th, and 8th decade of life, but not for the other age categories (for details, see Supplementary Figures 1–3). In sub-cohorts Würzburg/Berlin and Kiel-II, used to replicate the PCS score and to identify its predictors, the sex ratio was similar to Kiel-I whereas the age distribution and hospitalisation frequency were slightly different (Table 2). During the acute phase of COVID-19, most participants in COVIDOM in all three sub-cohorts experienced ≥9 of 23 pre-defined symptoms. The frequency of serious or life-threatening symptoms differed only slightly between sub-cohorts (Table 2).
Table 2.
Characteristics of COVIDOM sub-cohorts and disease severity during the acute phase of COVID-19.
| Kiel-I (n = 667) |
Würzburg/ Berlin (n = 316) |
Kiel-II (n = 459) |
p valueh | |
|---|---|---|---|---|
| Age [years], mean (SD)a | 48·2 (15·9) | 47·2 (16·7) | 45·3 (15·1) | 0·0089 |
| Women, n (%)b | 376 (56·5) | 164 (52·1) | 256 (55·8) | 0·42 |
| Men, n (%)b | 290 (43·5) | 151 (47·8) | 203 (44·2) | |
| Caucasian ethnicity, n (%)b | 644 (96·6) | 302 (98·1) | 438 (95·8) | 0·24 |
| BMI [kg/m2], mean (SD)a | 26·9 (5·2) | 26·5 (5·8) | 27·7 (5·8) | 0·0097 |
| Smokera,b,c, n (%) | 189 (30·0) | 88 (29·5) | 134 (31·1) | 0·87 |
| Pre-existing comorbidities | ||||
| Respiratory diseases, n (%)b,d | 118 (17·9) | 52 (16·7) | 100 (22·2) | 0·11 |
| Cardiovascular diseases, n (%)b,d | 205 (31·0) | 94 (30·5) | 129 (28·6) | 0·70 |
| Neurological diseases, n (%)b,d | 131 (19·6) | 44 (13·9) | 57 (12·4) | 0·0027 |
| Psychiatric diseases, n (%)b,d | 92 (13·8) | 31 (9·8) | 56 (12·2) | 0·21 |
| Gastrointestinal diseases, n (%)b,d | 72 (10·8) | 31 (9·8) | 39 (8·5) | 0·037 |
| Diabetes, n (%)b,d | 33 (5·2) | 14 (4·5) | 13 (2·8) | <0·0001 |
| Rheumatologic or immunologic diseases, n (%)b,d | 67 (10·2) | 26 (8·4) | 44 (9·6) | 0·68 |
| Nephrological diseases, n (%)b,d | 2 (0·3) | 5 (1·6) | 0 | 0·0056 |
| ENT diseases, n (%)b,d | 251 (37·6) | 60 (19·0) | 34 (7·4) | <0·0001 |
| Allergies, n (%)b,d | 266 (39·9) | 112 (35·4) | 169 (36·8) | 0·28 |
| Cancer, n (%)b,d | 12 (1·8) | 7 (2·2) | 6 (1·3) | 0·59 |
| Organ transplantation, n (%)b,d | 1 (0·1) | 0 | 0 | 0·33 |
| Date of SARS-CoV-2 infection | ||||
| PCR proof of SARS-CoV-2 infection before symptom onset, n (%)b | 91 (15·3) | 129 (40·8) | 82 (17·9) | <0·0001 |
| Time between infection and study site visit [days], mean (SD) | 288·6 (69·3) | 356·1 (46·1) | 232·7 (52·0) | <0·0001 |
| Disease severity during the acute phase of COVID-19 | ||||
| No. of symptomse | ||||
| 0-2, n (%) | 56 (8·8) | 26 (8·4) | 32 (7·2) | |
| 3-5, n (%) | 93 (14·5) | 45 (14·5) | 62 (14·0) | 0·31 |
| 6-8, n (%) | 152 (23·8) | 71 (22·5) | 82 (18·5) | |
| 9 or more, n (%) | 339 (53·0) | 168 (54·2) | 267 (60·3) | |
| No. of symptoms rated serious or life-threateningf | ||||
| 0, n (%) | 128 (20·0) | 93 (30·0) | 85 (19·2) | |
| 1-3, n (%) | 296 (46·3) | 125 (40·3) | 197 (44·5) | 0·017 |
| 4-6, n (%) | 139 (21·1) | 56 (18·1) | 99 (22·3) | |
| 7 or more, n (%) | 77 (12·0) | 36 (11·6) | 62 (14·0) | |
| Hospitalisation frequency | ||||
| Inpatient treatmentb,g, n (%) | 66 (10·3) | 13 (6·5) | 22 (4·8) | 0·0024 |
Age, body mass index (BMI), and smoking status as per date of study site visit (i.e., ≥9 months post infection).
Percentages relate to the number of participants with available data (missing data: Caucasian ethnicity 0 [Kiel-I], 8 [Würzburg/Berlin], 2 [Kiel-II]; smoker 46, 18, 28; time between infection and site visit 5, 1, 1; respiratory diseases 10, 4, 8; cardiovascular diseases 5, 8, 8; diabetes 30, 7, 20; rheumatologic/immunologic diseases 11, 6, 3; nephrological diseases 1, 5, 0; ENT diseases 15, 5, 13; allergies 25, 7, 19; cancer 0, 3, 1; no. of symptoms 27, 6, 16; no of symptoms rated serious or life-threatening 27, 6, 16; hospitalisation frequency 26, 117, 0).
current smoker, or former smoker with >5 pack-years.
All information on pre-existing comorbidities was self-reported, assisted by standardised questionnaires and a study physician. ‘Pre-existing’ refers to the time before SARS-CoV-2 infection. The total list of comorbidities underlying the corresponding categorization was derived from the German Corona Consensus Dataset (GECCO-83), the common core data set of the NAPKON project.18
Participants were asked for the presence of the following 23 symptoms during the acute phase of COVID-19: smell distortion, taste distortion, stomach pain, disturbed consciousness or confusion, diarrhea, vomiting, nausea, dizziness, cough, hoarseness, sore throat, runny nose, chills, muscle pain, body aches, dyspnoea, wheezing, chest pain, skin rash, fever, headache, hair loss, other symptoms (for further details, see Supplementary Table 1).
Each symptom was rated by the participant as either mild, moderate, severe, or life-threatening.
A total of 17 participants (Kiel-I, 2·5%), 5 participants (Würzburg/Berlin, 1·6%), and 2 participants (Kiel-II, 0·4%), respectively, had received intensive care treatment for acute COVID-19.
Since no formal statistical testing of parameter differences was involved, p values are to interpreted as informal measures of sub-cohort comparability that need not be multiplicity-adjusted.
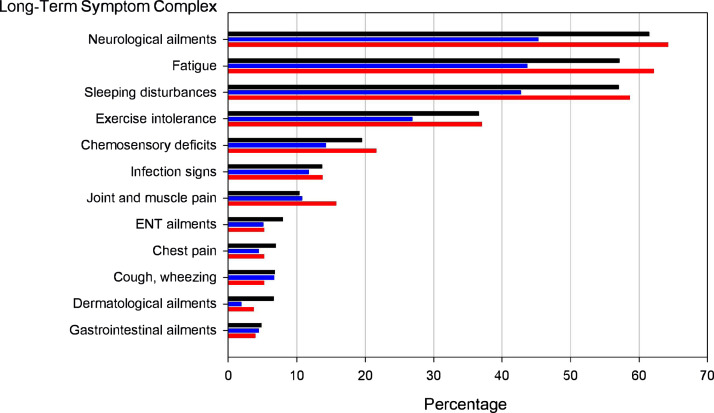
The most frequently reported long-term symptoms at 9 months post infection were neurological ailments, fatigue, and sleeping disturbances (Figure 1), all of them characterised by post-acute symptom onset. In 338 members of the Kiel-I cohort (50·7%), at least one of the 23 possible symptoms from the acute phase of COVID-19 was still present during the study site visit. Only 91 participants (15·7%) from the Kiel-I sub-cohort reported complete absence of any long-term symptom. The other two COVIDOM sub-cohorts showed similar patterns albeit at a generally lower level of overall symptom load in Würzburg/Berlin than in Kiel-I and Kiel-II (Figure 1).
Figure 1.
Frequency of symptom complexes in COVIDOM sub-cohorts. Bar lengths correspond to the percentage prevalence, in the respective COVIDOM sub-cohort, of one of the 12 long-term symptom complexes of COVID-19 upon which the Post-COVID syndrome (PCS) score definition is based (Kiel-I, black; Würzburg/Berlin, blue; Kiel-II, red). Symptom complexes are ordered according to their prevalence in the Kiel-I sub-cohort.
Development of PCS score
Complete data on all 12 long-term symptom complexes of COVID-19 were available for 580 of 667 participants in the Kiel-I cohort (87·0%). The Pearson correlation coefficient between the cluster number-specific intermediate PCS scores SPCS(k) and SPCS(k+1) was r = 0·756 (p < 0·0001) for k = 2, and r = 0·948 (p < 0·0001) for k = 3. Therefore, the alternation of k-means clustering and PCS score definition was terminated at k0 = 3 (Supplementary Figure 4). Based upon the component sum of their centres, the three clusters could unambiguously be classified by phenotype severity as ‘none/low’ (I), ‘moderate’ (II), or ‘severe’ (III; Table 3).
Table 3.
Post-COVID Syndrome (PCS) score development by k-means clustering and ordinal logistic regression analysis.
| No. | Symptom complex | Cluster centre |
Regression coefficient | PCS score weight | ||
|---|---|---|---|---|---|---|
| I (n = 198) |
II (n = 287) |
III (n = 95) |
||||
| 2 | Fatigue | 0·07 | 0·89 | 0·97 | 7·234 | 7 |
| 6 | Cough, wheezing | 0·02 | 0·02 | 0·38 | 6·881 | 7 |
| 9 | Neurological ailments | 0·15 | 0·86 | 0·96 | 6·434 | 6·5 |
| 4 | Joint and muscle pain | 0·02 | 0·04 | 0·57 | 6·366 | 6·5 |
| 5 | ENT ailments | 0·02 | 0·02 | 0·46 | 5·455 | 5·5 |
| 8 | Gastrointestinal ailments | 0·00 | 0·01 | 0·29 | 5·064 | 5 |
| 12 | Sleeping disturbance | 0·18 | 0·81 | 0·85 | 4·828 | 5 |
| 3 | Exercise intolerance | 0·05 | 0·50 | 0·93 | 4·033 | 4 |
| 11 | Infection signs | 0·02 | 0·14 | 0·47 | 3·372 | 3·5 |
| 1 | Chemosensory deficits | 0·17 | 0·16 | 0·53 | 3·318 | 3·5 |
| 7 | Chest pain | 0·02 | 0·05 | 0·28 | 3·259 | 3·5 |
| 10 | Dermatological ailments | 0·03 | 0·03 | 0·29 | 1·782 | 2 |
The 12 long-term symptom complexes are ordered by their PCS score weight of the corresponding indicators, starting with the highest PCS score weight.
ROC analysis confirmed that the PCS score almost fully reproduced the original k-means clustering. The area-under-curve was 0·996 for distinguishing between cluster I and clusters II+III, and 0·994 for distinguishing between clusters I+II and cluster III. Maximisation of the sum of sensitivity and specificity resulted in optimal thresholds for the PCS score of 10·75 and 26·25, respectively, for classifying a participant as either none/mildly, moderately, or severely affected by PCS. Only 27 of 580 participants (4·7%) were classified discordantly (Cohen's kappa: 0·925).
Ordinal logistic regression analysis confirmed that all but one complex indicator had a highly significant influence (Wald test p < 0·0001) upon the cluster affiliation of participants, except for symptom complex 10 (dermatology; p = 0·013). The logistic regression coefficients, which varied between 1·782 (dermatology) and 7·234 (fatigue), were next transformed into PCS score weights by rounding them to the nearest half-integer (Table 3), resulting in a range of possible score values from zero (all indicators equal to 0) to 59 (all indicators equal to 1). The median PCS score of Kiel-I participants equalled 17·5 (interquartile range: 6·5 to 26·0).
In order to internally validate the PCS score as a measure of phenotype severity, the score was related to the self-reported quality of life of participants, measured by EQ-5D-5L Index and VAS. Both parameters exhibited a significant inverse correlation with the PCS score (EQ-5D-5L Index: r = -0·54, 95%CI: [-0·48; -0·60]; EQ-5D-5L VAS: r = -0·56, 95%CI: [-0·50; -0·61]; both p < 0·0001), confirming that a higher PCS score was associated with a significantly lower quality of life (see Supplementary Figure 5).
Clinical and functional correlates of the PCS score
When the Kiel-I sub-cohort was divided according to the PCS score into ‘none/mild’, ‘moderate’, and ‘severe’ cases (for details, see legend to Table 3), significant inter-class differences became apparent with respect to age, sex, and body mass index (BMI), where the latter finding would not however withstand Bonferroni adjustment for multiple testing (Table 4). In addition, participants with higher PCS scores had been hospitalised more often during the acute phase (none/mild: 8, 4·2%; moderate: 30, 10·8%; severe: 17, 15·6%; p = 0·0022) and had experienced a greater number of, as well as more severe, acute symptoms (both p < 0·0001). Furthermore, pre-existing respiratory, cardiovascular, neurologic, psychiatric, rheumatologic/immunologic, and allergic comorbidities were more frequent in Kiel-I cohort members with high PCS scores (all p < 0·05; see Supplementary Table 3). While all functional measurements taken during the site visit were within non-pathological reference ranges, resting heart rate as well as some lung function and echocardiography parameters were different between PCS score-defined classes (Table 4).
Of the laboratory measurements taken during the study site visit, white blood cell count, neutrophil cell count, CRP levels, D-dimer, creatinine levels, as well as vitamin D and iron levels differed significantly between PCS score-defined severity classes in the Kiel-I sub-cohort. All laboratory measurements in all classes fell within the normal ranges provided by the local laboratory, except for ferritin (for details, see Supplementary Table 4). Participants in the three severity classes also differed significantly in terms of dyspnoea (mMRC, MDP) and fatigue (FACIT-F, CFS) as well as prevalence of anxiety, depression, stress, and resilience (GAD-7, PHQ-8, PSS, BRS), all assessed via standardised questionnaires (Supplementary Table 5). Participants with higher PCS scores tended to have experienced more dyspnoea, more severe fatigue and more anxiety, depression, and stress while their resilience was lower.
Distribution and validation of PCS score in independent sub-cohorts
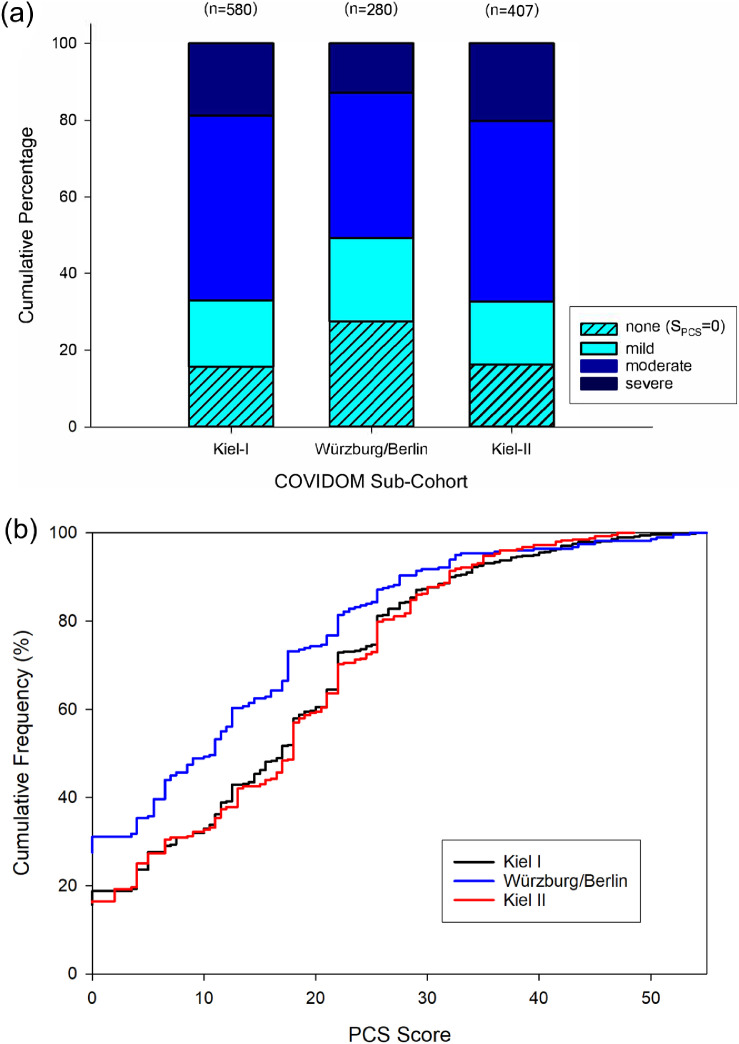
The PCS score was lower, on average, in the Würzburg/Berlin sub-cohort than in the two Kiel sub-cohorts mainly because the complete absence of long-term symptoms was rarer in the latter (Kiel-I: 15·7%, Kiel-II: 16·2%) than the former (24·4%; Figure 2a). Other than that, the general distribution characteristics of the PCS score were similar across sub-cohorts (Figure 2b).
Figure 2.
Distribution of the Post-COVID syndrome (PCS) score in COVIDOM sub-cohorts. (a) Frequency of PCS score-defined severity classes. A PCS score of 0 (light blue, hatched) means complete absence of long-term symptoms, which was observed in 91 (15·7%, Kiel-I), 77 (27·5%, Würzburg/Berlin), and 66 (16·2%, Kiel-II) of the participants in COVIDOM, respectively. Severe PCS, defined as a PCS score ≥26·25 (dark blue), was present in 109 (18·8%, Kiel-I), 36 (12·9%, Würzburg/Berlin), and 82 (20·1%, Kiel-II) participants, respectively. (b) Cumulative distribution function of PCS score. The PCS score distribution in Würzburg/Berlin was notably different from those in the two Kiel sub-cohorts owing to a larger proportion in the former sub-cohort of participants with a PCS score equal to zero. Consequently, the mean PCS score equalled 13·0 (SD: 12·6) in Würzburg/Berlin, which was significantly lower than in both Kiel-I I (17·0, SD: 12·4) and Kiel-II (17·0, SD: 12·1; both p < 0·0001).
The correlation in Kiel-II between the PCS score and quality of life equalled r = -0·53 for EQ-5D-5L Index (95%CI: [-0·46; -0·60]) and r = -0·58 for EQ-5D-5L VAS (95%CI: [-0·51; -0·64]; both p < 0·0001), similar to Kiel-I. In the Würzburg/Berlin sub-cohort, the correlation was even stronger with r = -0·61 for EQ-5D-5L Index (95%CI: [-0·53; -0·68]), and r = -0·62 for EQ-5D-5L VAS (95%CI: [-0·54; -0·69]; both p < 0·0001), thereby adding to the validity of the PCS score as a measure of long-term disease severity. Correlations between PCS score and laboratory parameters (Supplementary Tables 9 and 13), functional measures (Supplementary Tables 8 and 12), standardized questionnaires (i.e. dyspnoea, cognition, fatigue, stress, resilience, anxiety, depression; Supplementary Tables 7 and 11) and clinical information (Supplementary Tables 6 and 10) were largely consistent across sub-cohorts.
There were some minor differences between sub-cohorts in terms of age, BMI, and the frequency of some pre-existing comorbidities. In contrast, sex, ethnicity, smoking status, and most comorbidities were similarly distributed, and general disease severity of acute COVID-19 was also largely comparable between sub-cohorts (Table 2).
Association of PCS score class with acute-phase and general patient characteristics
Significant predictors of the PCS score class of participants were identified in the Würzburg/Berlin and Kiel-II sub-cohorts from 20 predefined characteristics, including severity and treatment of acute COVID-19, as well as pre-existing comorbidities, general demographics, and personal resources. Notably, in addition to the differences alluded to above, the three COVIDOM sub-cohorts differed in terms of education level, weight gain or loss after COVID-19 infection, visits to general practitioners or specialised physicians, the presence of some pre-existing comorbidities, as well as resilience (BRS) and anxiety towards catastrophic events (for details, see Supplementary Table 14).
In a multivariate ordinal logistic regression analysis, both resilience and the number of acute-phase symptoms rated serious or life-threatening were found to be significant predictors of the PCS score class in both Würzburg/Berlin and Kiel-II (Table 5). The actual number of acute phase symptoms, age, a gain in body weight after the infection, and pre-existing cardiovascular as well as neurological diseases were statistically significant predictors in one sub-cohort only even after Bonferroni adjustment for multiple testing (Table 5). The final regression models explained the variation of the PCSS scores quite well in both sub-cohorts, as was indicated by Nagelkerke pseudo-R2 values of 0·390 in Würzburg/Berlin and 0·440 in Kiel-II.
Discussion
The German population-based COVIDOM study represents a nation-wide effort to systematically investigate the long-term sequelae of SARS-CoV-2 infection, widely subsumed under the term ‘Post-COVID Syndrome’ (PCS). So far, the main concern of healthcare officials, politicians, and the general public in Germany and many other countries has been to avoid the short-term exhaustion of healthcare systems due to COVID-19. However, the long-lasting consequences of the pandemic, such as widespread PCS, may cause far greater economical and psychosocial damage than the temporary overuse of healthcare resources and infrastructure by acute cases of the disease.
Both, gauging the consequences of the SARS-CoV-2 pandemic and promoting the diagnosis, treatment, and prevention of PCS require simple and efficient means of measuring the latter in individual patients. Although functional limitations of elderly or multi-morbid patients, or of patients with severe acute COVID-19, may also be rated with the Post-COVID-19 Functional Status (PCFS) devised by Klok et al.,11 the latter is likely unsuitable for resolving the more subtle health problems of the vast majority of patients at risk of PCS, namely those of young to middle age or without pre-existing health impairments. In the context of COVIDOM, we therefore developed an easy-to-use tool for assessing PCS presence and severity in the general population. The resulting PCS score encompasses a wide range of 35 long-term symptoms, grouped into 12 clinically meaningful symptom complexes, thereby representing an important and clinically useful complementation of the more confined PCFS. Asking the questions necessary to calculate the PCS score should take no longer than a few minutes and is therefore easy to implement in clinical practice. Proof of the PCS score's suitability to characterise PCS was provided by its comparatively strong correlation, not only with an impaired quality of life, but also with specific psychosocial characteristics, and by some more subtle associations with functional measures and inflammatory and cardiovascular blood phenotypes. What is more, the time span between infection and study site visit in COVIDOM and its general design allowed proper differentiation between PCS and both prolonged natural recovery from COVID-19 and post-intensive-care syndrome.
Severe PCS, defined as a PCS score >26·25, occurred in 13% to 20% of participants across COVIDOM sub-cohorts. Despite our registry-based recruitment strategy, however, selection bias hindering accurate estimation of the general prevalence of PCS cannot be completely ruled out for COVIDOM. This notwithstanding, we may surmise that the above frequencies give a good hint towards the health care resources required to deal with this novel long-term health issue.
Previous population-based studies, such as those in the Faroe Islands, Norway (Bergen area), and the US (Michigan) differed from COVIDOM in various methodological aspects and covered only the first wave of the pandemic.8, 9, 10 In contrast to these studies’ findings, the number of participants with complete absence of any long-term symptoms was rather low in COVIDOM, at least in the two Kiel sub-cohorts. This might be due to particularly detailed symptom assessment in COVIDOM, but since all study participants were recruited up until September 29th 2021, another explanation may be that 1·5 years of unparalleled social and economic challenges and disruptions during the pandemic had already put serious psychological strain on the general population. Therefore, it may be hypothesised that some of the long-term symptoms may not only be attributable to the original SARS-CoV-2 infection but also to the pandemic in general. That participants in COVIDOM from Würzburg/Berlin were less often affected by severe PCS may be due in part to their generally less severe course of acute COVID-19 and by the 3-months-longer time span between infection and local study site visit, allowing the natural resolution of PCS symptoms. This notwithstanding, the general pattern of PCS as captured by the PCS score distribution was found to be similar between the training and replication sub-cohorts, suggesting that the PCS score may work well in other regions, or even countries, with different SARS-CoV-2 testing strategies and health care settings.
Previous studies reported disease severity during the acute phase of COVID-19 as one of the strongest prognostic factors of PCS.8, 9, 10,18,19 However, there is increasing evidence that at least two types of patients with PCS can be distinguished.3,20 The first category comprises patients with severe acute COVID-19 and/or previous health problems who present with persistent symptoms and require physicians to take detailed medical histories and make functional assessments. Accordingly, our study revealed significant differences between participants with high and low PCS score regarding lung function (DLCO), inflammatory blood markers (e.g., CRP), hemodynamic stress (NT-proBNP), markers of thrombosis (D-Dimer), and renal function (eGFR). Moreover, those with a high PCS score also did have more severe acute COVID-19 and were more frequently affected by pre-existing respiratory, cardiovascular, neurological, psychiatric, and rheumatologic diseases. Nevertheless, similar to another German study embedded into an ongoing cardiovascular cohort project,21 all functional and laboratory parameters in COVIDOM fell within the normal range, suggesting that PCS comprises mostly minor albeit long-lasting instances of organ involvement.
For a second category of patients, PCS symptoms may only develop in the post-acute phase of COVID-19.3 Even after thorough clinical work-up, the complaints reported by these patients often do not match the results of laboratory or functional diagnostics, which lack abnormalities, or the reports of a usually mild course of acute COVID-19. Interestingly, factors associated with an increased PCS score in our study also included impaired personal resources in terms of low resilience and increased anxiousness. These findings corroborate a recent French population-based study investigating symptom persistence after self-reported COVID-19 in 26,000 randomly chosen indivuduals.22 Surprisingly, the frequency of predominant post-COVID conditions such as fatigue, breathing difficulties, and pain was very similar in individuals believing they had suffered from COVID-19, irrespective of the SARS-CoV-2 serology test result or other confirmatory diagnosis. These observations imply that strengthening personal resources and patient education may be important preventive or interventional measures against PCS which should be easy to incorporate into structured rehabilitation programmes for patients with PCS. Furthermore, our findings underline the necessity to further study the pathogenesis and course of fatigue in the context of PCS as well as other communicable and non-communicable diseases because the causes of this phenomenon are still elusive and its importance might have been underestimated in the past.23, 24, 25
Strengths of our study include its multi-centre setting, the use of stratified study samples from different catchment areas across Germany, and its recruitment schedule allowing the distinction between PCS and prolonged natural recovery. In addition, COVIDOM pursues a sustainable study plan approved by federal funding authorities, and additional funding is available for the continued longitudinal evaluation of the PCS score.
Our study also has some weaknesses. First, the symptom spectrum underlying the PCS score might be incomplete even though, to the best of our knowledge, we took all clinically relevant symptoms into account. Second, symptoms from the acute phase of COVID-19 were assessed and rated retrospectively, potentially introducing recall bias. In particular, the significant association observed between early disease severity and the PCS score may thus be due to both physiological and psychological mechanisms. The two are however difficult to disentangle in a study like ours where the majority of participants had not received intense medical attention. Third, COVIDOM included only individuals infected before March 27th 2021, when vaccination was not widely available in Germany. This could limit the study's relevance if PCS presents as a different phenotype in vaccinated and unvaccinated patients, a question to be addressed in future COVIDOM research. Finally, like any other invitation-based study, COVIDOM may have been subject to selection bias favouring the inclusion of participants who were either more severely, or less severely, affected by PCS. Both directions of such bias are plausible which also implies, however, that the two effects may have balanced out to some extent. At a global level, another source of bias might have been the ethnic and cultural homogeneity of the COVIDOM sample. Even although both urban and rural catchment areas with different levels of migration background were selected, Germany still provides comparatively high levels of general education and healthcare quality, which may have affected both the statistical properties and the clinical relevance of the PCS score.
In conclusion, we developed a clinically meaningful and easy-to-use scoring system to study the course and causes of PCS in the large population-based COVIDOM study. The PCS score is the first validated measure for grading PCS severity and may serve as a valuable tool and outcome parameter in preventive and interventional trials. Moreover, the score captures different types of PCS, either resulting from severe acute COVID-19 or developing in the post-acute phase of COVID-19, with the latter type potentially congruent with psychological and psychosomatic issues. The PCS score developed may thus assist the clinical management of the long-term effects of COVID-19, help prioritise PCS care, and better resolve the clinical picture of PCS.
Contributors
Conceptualization: TB, WL, AHo, LK, JF, CS, KFR, WM, ML, DF, AHe, MW, JJV, CMo, SStö, JPR, TK, PH, MKr, SSch
Data curation: TB, CB, WL, AHo, LK, JF, CMa, SB, AHe, OM, FAM, MKo, CMo, SStö, JPR
Formal Analysis: TB, CB, WL, MKr, SSch
Funding acquisition: TB, WL, MW, JJV, DK, JPR, TK, PH, MKr, SSch
Investigation: TB, AHo, WM, ML, DF, DP, KGH, FAM, CMo, SStö
Methodology: TB, CB, WL, LK, JF, CS, KFR, WM, ML, DF, AHe, OM, KGH, NEEM, MW, JJV, DK, FAM, MKo, CMo, SStö, JPR, TK, PH, MKr, SSch
Project administration: TB, WL, AHo, LK, JF, CMa, SB, AHe, OM, KGH, MW, JJV, DP, FAM, JPR, TK, PH, MKr, SSch
Resources: TB, WL, WM, ML, DF, KGH, NEEM, MW, JJV, DK, DP, JPR, TK, PH, MKr, SSch
Software: CB, CMa, OM, DK, MKo, JPR, MKr
Supervision: TB, WL, AHo, LK, JF, KFR, WM, ML, DF, SB, AHe, OM, KGH, NEEM, MW, JJV, CMo, SStö, JPR, TK, PH, MKr, SSch
Validation: TB, CB, WL, AHo, LK, JF, CS, WM, ML, DF, AHe, KGH, DP, MKo, JPR, PH, MKr, SSch
Visualization: CB, MKr
Wrtiting – original draft: TB, CB, WL, MKr, SSch
Writing – review & editing: TB, CB, WL, AHo, LK, JF, CS, KFR, WM, CMa, ML, DF, SB, AHe, OM, KGH, NEEM, MW, JJV, DK, DP, FAM, MKo, CMo, SStö, JPR, TK, PH, MKr, SSch
The data underlying the present work were verified by TB, CB, WL, PH, MKr, SSch.
All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
All authors had full access to all data and accept responsibility to submit the manuscript for publication.
Data sharing statement
All data of this study may be shared upon request to the NAPKON data use and access committee. Interested parties can access relevant data governance information and submit their research proposal to the NAPKON use and access committee at https://proskive.napkon.de.
Declaration of interests
TB reports support for the present manuscript by Network University Medicine (NUM)/ German Federal Ministry of Education and Research (BMBF); grants or contracts from the German Center for Lung Research (DZL); consulting fees from AstraZeneca, GlaxoSmithKline; honoraria from AstraZeneca, GlaxoSmithKline, Novartis, Roche, Chiesi, Boeringer-Ingelheim, Merck; Support for attending meetings and/or travel from Chiesi, AstraZeneca; participation on a Data Safety Monitoring Board or Advisory Board for CoVit-2 (NCT04751604); CB has nothing to disclose; WL has nothing to disclose; AHo has nothing to disclose; LK has nothing to disclose; JF has nothing to disclose; CS reports consulting fees from Celtrend; honoraria from MSD, Novartis, Astra Zeneca, Boeringer Ingelheim, Bayer, Fresenius, Octapharma, and Oberberg; payment for expert testimony from IQWIG, support for attending meetings from Octapharma and Fresenius; patents for ß2 rececptor antibodies for diagnosis of ME/CFS (Charité); participation on a data safety monitoring board for Curevac, HDIT, and Vaccibody; being a board member of EUROMEDE, and DGMECFS; KFR reports honoraria from AstraZeneca, BoeringerIngelheim, Chiesi Pharmaceuticals, Novartis, Sanofi & Regeneron, GlaxoSmithKline, BerlinChemie, Roche; participation on a Data Safety Monitoring Board or Advisory Boards for AstraZeneca, BoeringerIngelheim, Sanofi & Regeneron; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for German Center for Lung Research (DZL), German Chest Society (DGP), American Thoracic Society (ATS); WM has nothing to disclose; CM has nothing to disclose; ML reports support for the present manuscript by the German Federal Ministry for Education and Research (BMBF) and by a state fund of Schleswig-Holstein; honoraria by Olympus, Novartis and Sanofi; participation on a Data Safety Monitoring Board or Advisory Board for GSK; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for board of the working group Rhinology/ Rhino-Surgery DGHNO and advisory board John Grube Foundation; DF reports grants or contracts from DFG Sachbeihilfe, DZHK Standortprojekt, Edwards Lifesciences grant; honoraria from AstraZeneca, Bayer, Edwards LifeSciences, Medtronic, Pfizer, Daiichi Sankyo, BoehringerIngelheim, Novartis, Janssen; SB reports BioNTech stocks from 11/20 to 5/21; AHe has nothing to disclose; OM has nothing to disclose; KGH reports grants or contracts from Bayer Healthcare; honoraria from Abbott, Bayer, Biotronik, BoeringerIngelheim, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, Sanofi-Aventis, SUN Pharma, W.L. Gore & Associates; participation on a Data Safety Monitoring Board or Advisory Boards for AMARIN, Alexion, AstraZeneca, Bayer, BoeringerIngelheim, Daiichi Sankyo, EIP Pharma, Edwards Lifesciences, Medtronic, Pfizer, Portola, Premier Research; NEEM has nothing to disclose; MW reports grants or contracts from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, Deutsche Gesellschaft für Pneumologie, European Respiratory Society, Marie Curie Foundation, Else Kröner Fresenius Stiftung, Capnetz Stiftung, International Max Planck Research School, Vaxxilon, Actelion, Bayer Health Care, Biotest, Boehringer Ingelheim; consulting fees from Noxxon, Pantherna, Vaxxilon, Aptarion, Glaxo Smith Kline, Sinoxa, Biotest, Thieme; honoraria from Astra Zeneca, Berlin Chemie, Chiesi, Novartis, Actelion, Boehringer Ingelheim, Glaxo Smith Kline, Biotest, Bayer Health Care; patents, issued or pending for EPO 12181535.1 : IL-27 for modulation of immune response in acute lung injury, WO/2010/094491: Means for inhibiting the expression of Ang-2; JJV reports grants from Merck/MSD, Gilead, Pfizer, Astellas Pharma, Basilea, German Center for Infection Research (DZIF), German Federal Ministry of Education and Research (BMBF), DLR (Deutsches Zentrum für Luft- und Raumfahrt), University of Bristol, Righospital Copenhagen; consulting fees from Pfizer, Gilead and Shiongi, honoraria from MSD/ Merck, Gilead, Pfizer, Astellas Pharma, Basilea, German Center for Infection Research (DZIF), University Hopsital of Freiburg/ Congress and Communication, Academy for Infectious Medicine, University Manchester, German Society for Infectious Diseases (DGI), Ärztekammer Nordrhein, University Hospital Aachen, BackRay Strategies, German Society for Internal Medicine (DGIM), Shionogi, Molecular Health, Netzwerk Univerisity Medicine (NUM), Janssen, NordForsk; DK has nothing to disclose; DP reports support for attending meetings from Advanz Pharma Germany; FAM reports grants from the German Research Council (Deutsche Forschungsgemeinschaft, DFG); MKo has nothing to disclose; CMo has nothing to disclose; SStö reports research support in form of case payments covering for study staff and consumables by a grant of the German Federal Ministry of Education and Research; JPR reports grants from the German Ministry of Research and Education during the conduct of the study; grants from the German Ministry of Research and Education, grants from Bavarian State (ministry for science and the arts), grants from Federal Joint Committee (G-BA) within the Innovationfond, grants from the German Center for Lung Research, personal fees from the Landesaerztekammer Hessen, outside the submitted work; TK reports support for the present manuscript by the Federal Ministry of Education and Research, Germany; PH reports grants from the German Ministry of Research and Education during the conduct of the study; research grants from the German Ministry of Research and Education, European Union, Charité – Universitätsmedizin Berlin, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert Koch Institute, German Heart Foundation, Federal Joint Committee (G-BA) within the Innovationfond, German Research Foundation, Bavarian State (ministry for science and the arts), German Cancer Aid, Charité – Universitätsmedizin Berlin (within Mondafis; supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AF randomized; supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo), outside the submitted work; MKr has nothing to disclose; SSch reports consulting fees from Abbvie, Allergosan Amgen, Arena, BMS, Biogen, Celltrion, Celgene, Ferring, Fresenius, Galapagos, Gilead, IMAB, Janssen, Lilly, MSD, Mylan, Pfizer, Protagonist, Provention Bio, Sandoz/Hexal Takeda, Theravance, UCB; honoraria from Abbvie, Allergosan Amgen, Arena, BMS, Biogen, Celltrion, Celgene, Falk, Ferring, Fresenius, Galapagos, Gilead, IMAB, Janssen, Lilly, MSD, Mylan, Pfizer, Protagonist, Provention Bio, Sandoz/Hexal Takeda, Theravance, UCB; payment for expert testimony from Allergosan; support for attending meetings and/ or travel from Abbvie, Allergosan Amgen, Arena, BMS, Biogen, Celltrion, Celgene, Falk, Ferring, Fresenius, Galapagos, Gilead, IMAB, Janssen, Lilly, MSD, Mylan, Pfizer, Protagonist, Provention Bio, Sandoz/Hexal Takeda, Theravance, UCB; participation on a Data Safety Monitoring Board or Advisory Boards for Abbvie, Allergosan Amgen, Arena, BMS, Biogen, Celltrion, Celgene, Ferring, Fresenius, Galapagos, Gilead, IMAB, Janssen, Lilly, MSD, Mylan, Pfizer, Protagonist, Provention Bio, Sandoz/Hexal Takeda, Theravance, UCB.
Acknowledgements
The COVIDOM study is part of the National Pandemic Cohort Network (NAPKON). NAPKON is funded by COVID-19-related grants from the Network University Medicine (NUM; NAPKON grant number: 01KX2021). Parts of the infrastructure of the Kiel and Würzburg study sites were funded by the federal states of Schleswig-Holstein and Bavaria.
We thank the COVIDOM study teams at Kiel, Würzburg, and Berlin for contributing to the study logistics, contact management, and data acquisition.
We also thank the NAPKON infrastructural units for making COVIDOM possible, including the interaction core unit (ICU), epidemiological core unit (ECU), and biosample core unit (BCU), as well as the NAPKON steering committee and the NAPKON Use and Access Committee (see the NAPKON study group list for a full disclosure of NAPKON and COVIDOM collaborators who contributed to the project).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101549.
Contributor Information
Thomas Bahmer, Email: thomas.bahmer@uksh.de.
Stefan Schreiber, Email: s.schreiber@mucosa.de.
Appendix. Supplementary materials
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 rapid guideline: managing the long-term effects of COVID-19 . 2020. NICE Guideline [NG188]https://www.nice.org.uk/guidance/NG188 published online December 18. Accessed 18 February 2022. [PubMed] [Google Scholar]
- 4.Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes LD, Ingram J, Sculthorpe NF. More than 100 persistent symptoms of SARS-CoV-2 (Long COVID): a scoping review. Front Med. 2021;8 doi: 10.3389/fmed.2021.750378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callard F, Perego E. How and why patients made Long Covid. Soc Sci Med. 2021;268 doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikkelsen ME, Still M, Anderson BJ, et al. Society of critical care medicine's international consensus conference on prediction and identification of long-term impairments after critical illness. Crit Care Med. 2020;48:1670–1679. doi: 10.1097/CCM.0000000000004586. [DOI] [PubMed] [Google Scholar]
- 8.Petersen MS, Kristiansen MF, Hanusson KD, et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis. 2021;73:e4058–e4063. doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blomberg B, Mohn KG-I, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirschtick JL, Titus AR, Slocum E, et al. Population-based estimates of post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) prevalence and characteristics. Clin Infect Dis. 2021;73:2055–2064. doi: 10.1093/cid/ciab408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klok FA, Boon GJAM, Barco S, et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56 doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adeloye D, Elneima O, Daines L, et al. The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease. Lancet Respir Med. 2021;9:1467–1478. doi: 10.1016/S2213-2600(21)00286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cervia C, Zurbuchen Y, Taeschler P, et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat Commun. 2022;13:446. doi: 10.1038/s41467-021-27797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn A, Krist L, Lieb W, et al. Long-term health sequelae and quality of life at least 6 months after infection with SARS-CoV-2: design and rationale of the COVIDOM-study as part of the NAPKON population-based cohort platform (POP) Infection. 2021;49:1277–1287. doi: 10.1007/s15010-021-01707-5. published online October 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schons MJ, Pilgram L, Reese J-P, et al. The German National Pandemic Cohort Network (NAPKON): rationale, study design and baseline characteristics. In Review [Accepted for Publication], 2022. 10.21203/rs.3.rs-1249111/v1. [DOI] [PMC free article] [PubMed]
- 16.German National Cohort (GNC) Consortium The German National Cohort: aims, study design and organization. Eur J Epidemiol. 2014;29:371–382. doi: 10.1007/s10654-014-9890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieb W, Jacobs G, Wolf A, et al. Linking pre-existing biorepositories for medical research: the PopGen 2.0 network. J Community Genet. 2019;10:523–530. doi: 10.1007/s12687-019-00417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daugherty SE, Guo Y, Heath K, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373(n1098):1–12. doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koczulla AR, Ankermann T, Behrends U, et al. S1-Leitlinie Post-COVID/Long-COVID. Pneumologie. 2021;75:869–900. doi: 10.1055/a-1551-9734. [DOI] [PubMed] [Google Scholar]
- 21.Petersen EL, Goßling A, Adam G, et al. Multi-organ assessment in mainly non-hospitalized individuals after SARS-CoV-2 infection: the Hamburg City Health Study COVID programme. Eur Heart J. 2022;43(11):1124–1137. doi: 10.1093/eurheartj/ehab914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matta J, Wiernik E, Robineau O, et al. Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Kleijn WPE, De Vries J, Lower EE, Elfferich MDP, Baughman RP, Drent M. Fatigue in sarcoidosis: a systematic review. Curr Opin Pulm Med. 2009;15:499–506. doi: 10.1097/MCP.0b013e32832d0403. [DOI] [PubMed] [Google Scholar]
- 24.Rasa S, Nora-Krukle Z, Henning N, et al. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) J Transl Med. 2018;16:268. doi: 10.1186/s12967-018-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz-Pablos M, Paiva B, Montero-Mateo R, Garcia N, Zabaleta A. Epstein-Barr virus and the origin of myalgic encephalomyelitis or chronic fatigue syndrome. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.656797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.