Abstract
Thermal degradation of verbascoside (VB) in Acanthus ebracteatus Vahl (AE) always affects its health benefit. Here the temperature effect on VB in both AE extract and solid lipid nanoparticles (SLNs)-encapsulated AE extract was demonstrated using the Arrhenius plot. The reaction rate constants were calculated for shelf life and plotted to obtain pH–rate profiles. VB degradation was a first-order reaction. The reaction rate in a neutral to alkaline solution was faster than in an acidic solution. VB in AE extract-loaded SLNs was more stable than in uncapped AE extract. The shelf life of VB in SLNs was 153 days with activation energy (Ea) of 76.16 kJ mol–1, whereas those of VB in AE extract and in AE extract solution were 75 days with Ea = 78.03 kJ mol–1 and 12 days with Ea = 49.24 kJ mol–1, respectively. Therefore, we anticipate that the AE extract-loaded SLNs will be beneficial for product development.
Keywords: Verbascoside, Stability, Arrhenius equation, Solid lipid nanoparticles, Acanthus ebracteatus
Plants and their chemical constituents have gained attention and been studied for their biological activities both in vitro and in the clinic.1−4 Verbascoside (VB), or acteoside, is a phenylpropanoid glycoside found in some medicinal plants such as Verbena officinalis, Syringa vulgaris, Clerodendrum trichotomum Thunb., Orobanche rapum-genistae Thuill., and Acanthus ebracteatus Vahl (AE),5−9 Many reports document the beneficial bioactivities of VB, which include antioxidant, anti-inflammatory, antimicrobial, and anti-androgen activities.5,8,10,11 VB’s bioactivities have inspired the development of these plants into health applications. Previous reports have identified AE as containing high amounts of VB, and AE has been traditionally used as the main ingredient in an anti-inflammation recipe for treating skin and musculoskeletal inflammation.12,13 Thus, AE is a promising natural ingredient for the development of health products.
Unfortunately, instability is an important problem of bioactive compounds in plant extracts and herbal preparations. Thermal degradation kinetics and shelf life evaluation using Arrhenius’ theory can be used for predicting and controlling the quality of plant extracts and products.14,15 However, the stability of VB has not yet been fully explored, and no instability problem of AE extract has been identified. VB is degraded by the hydrolysis reaction of its ester bond (Figure 1),10 which is induced by high temperatures or by being contained in a neutral solution.10,16 Liposomes were used to entrap VB, which improved its stability.17,18 Although VB was successfully stabilized in a lipid vesicle, stability testing under elevated temperatures and for extended shelf life has not been reported.
Figure 1.
Chemical structure of verbascoside.
The objectives of our study were to evaluate the effects of pH and temperature on the degradation of VB in a semi-solid form of AE extract, in a solution form of AE extract, and in AE extract-loaded solid lipid nanoparticles (SLNs). Arrhenius’s theory was used to determine the thermal degradation kinetic parameters, such as the order of reaction and reaction rate constant. These parameters were then used to predict the shelf life of VB.
The SLN is a delivery system created from Compritol 888 ATO (glyceryl dibehenate), which was used for encapsulating AE extract. The thermal degradation behavior of VB in the AE extract-loaded SLNs was evaluated. Finally, the thermal degradation behaviors of VB in the AE extract in three different forms, e.g., liquid, semi-solid, and AE-loaded SLNs, were evaluated and compared.
Chemicals
Verbascoside (VB) was purchased from Abcam (Cambridgeshire, UK). Sodium dihydrogen orthophosphate and potassium chloride were purchased from Ajax Finechem (NSW, Australia). Glacial acetic acid, hydrochloric acid, and sodium hydroxide were purchased from RCI Labscan (Bangkok, Thailand). Acetonitrile was HPLC grade (Mallinckrodt Baker Inc., Phillipsburg, NJ, USA). All reagents were of analytical grade. Compritol ATO 888 (glyceryl dibehenate) was purchased from PT Intertrade Co., Ltd. (Nonthaburi, Thailand). Water was produced from a Milli-Q water purification system (Millipore, Billerica, MA, USA).
Plant Extraction
Fresh AE leaves were purchased from Khaokhoherbary organic farm (Phetchaboon, Thailand). The plant collection method and experimental use were in accordance with all the relevant guidelines. The specimens were identified by Assistant Professor Dr. Pranee Nangngam of the Faculty of Science at Naresuan University. The voucher specimen, collection no. 004163, was deposited at the PNU herbarium, also located at the Faculty of Science. The fresh leaves were dried at 50 °C for 24 h. Five-hundred gram samples of the dried leaves were weighed and then macerated with 2000 mL of 95% ethanol for 3 days. The extract solutions were filtered, and the solvent was removed using a rotary evaporator at 40 °C. The extract was then kept at −20 °C and protected from light until use.
High-Performance Liquid Chromatography (HPLC) Analysis of VB
HPLC analysis of VB was performed using a Shimadzu HPLC (Bara Scientific, Japan) with a Luna Phenomenex analytical column (250 mm × 7 mm, 5-μm particle size) (Phenomenex, Deerfield, IL, USA). The mobile phase consisted of 23% acetonitrile (vol%) buffered with 50 mM sodium phosphate at pH 2.5. Elution was performed with a flow rate of 1.5 mL/min, detection wavelength at 332 nm, and injection volume of 20 μL. The system was validated to achieve the following parameters: linearity, limit of detection (LOD), limit of quantification (LOQ), precision, and accuracy.
Preparation of AE Extract-Loaded Solid Lipid Nanoparticles
AE extract was encapsulated into SLNs using the hot melt homogenization technique. The lipid carrier, Compritol ATO 888 (HLB = 2), was used to formulated the lipid particles, which were stabilized by using Tween 80 (HLB = 15) and Span 80 (HLB = 4) as the co-surfactants (HLB = hydrophilic–lipophilic balance). The formulation of the particle preparation is shown in Table 1. Briefly describing the preparation process, the lipid phase containing Compritol 888 ATO and Span 80 was melted at 80 °C. AE extract was then added to the melted lipid, and the mixture was stirred until it was homogeneous. Distilled water containing Tween 80 was separately heated to 90 °C, and the lipid part was poured into the aqueous phase. The mixture was then emulsified by using a high-speed homogenizer (Silverson L5M, Silverson Machines Ltd., Waterside, UK) at 10 000 rpm for 15 min. The particle was solidified at room temperature and then dried using a freeze-dryer. A free-flowing powder was achieved using maltodextrin as a cryoprotectant. No unexpected or unusually high safety hazards were encountered. The particle size was evaluated by using a dynamic light scattering (DLS) particle size analyzer (ZetaPals, Brookhaven, New York, USA). Encapsulation efficacy was calculated as the amount of VB remaining encapsulated in the particles as a percentage of the original amount of VB in the extract, calculated from the HPLC chromatogram. The influential parameters affecting the particle characteristics were the amount of Compritol ATO 888 and concentration of surfactant, and these were varied and optimized.
Table 1. Formulations for Preparing AE Extract-Loaded SLNs and Particle Characteristicsa.
| parameters |
particle
characteristics |
||||||
|---|---|---|---|---|---|---|---|
| formula | Compritol ATO 888 (g) | Tween 80 (%) | Span 80 (%) | PS (nm) ± SD | PDI ± SD | ZP (mV) ± SD | entrapment efficiency (%) ± SD |
| F1 | 10.00 | 1.00 | 0.00 | 1240.05 ± 365.44 | 0.564 ± 0.08 | –23.10 ± 4.55 | 4.21 ± 2.01 |
| F2 | 10.00 | 0.50 | 0.00 | 3546.77 ± 548.78 | 0.321 ± 0.01 | –19.75 ± 3.78 | 40.84 ± 1.42 |
| F3 | 1.50 | 0.50 | 0.25 | 2692.93 ± 218.86 | 0.293 ± 0.04 | –31.42 ± 2.06 | 13.51 ± 0.27 |
| F4 | 1.50 | 0.50 | 0.50 | 2606.76 ± 125.83 | 0.248 ± 0.03 | –31.09 ± 0.61 | 18.24 ± 1.07 |
| F5 | 1.50 | 0.50 | 1.00 | 2056.5 ± 407.20 | 0.393 ± 0.07 | –33.07 ± 3.33 | 15.87 ± 2.22 |
| F6 | 1.50 | 0.50 | 2.00 | 1372.3 ± 100.36 | 0.120 ± 0.05 | –37.48 ± 0.90 | 21.73 ± 1.79 |
| F7 | 1.50 | 0.50 | 3.00 | 628.06 ± 159.36 | 0.210 ± 0.04 | –37.65 ± 0.90 | 31.28 ± 0.98 |
| F8 | 1.50 | 0.50 | 4.00 | 1450.96 ± 66.10 | 0.324 ± 0.08 | –39.77 ± 0.86 | 24.99 ± 0.44 |
| F9 | 1.50 | 0.50 | 5.00 | 1129.16 ± 61.88 | 0.355 ± 0.02 | –44.46 ± 1.12 | 25.37 ± 0.56 |
PS = particle size, PDI = polydispersity index, ZP = zeta potential, SD = standard deviation.
Effect of pH on VB in AE Extract
The effect of pH on the stability of VB was determined by dissolving AE extract in buffer solutions at various pH values—pH 2.0 (0.2 M HCl + 0.2 KCl), pH 5.5 (0.1 M citric acid and 0.1 M trisodium citrate), pH 7.4 (0.2 M HCl and 0.2 M KCl), pH 8 (0.1 M trimethylamine and 0.1 M HCl), and pH 11.0 (0.05 M NaHCO3 + 0.1 M NaOH)—to produce 10 mg/mL solutions of each, which were then kept in a saturated sodium chloride solution box at 25 °C for 30 days. The collection of samples for analysis was done on days 0, 3, 7, 14, 21, and 28. The remaining VB was evaluated by using a HPLC.
Thermal Degradation Kinetic Analysis of VB in AE Extract
Ten milligrams of AE extract (semi-solid), 1 mL of AE extract solution (10 mg of AE extract dissolved in 10 mL of pH 7.4 phosphate buffer solution), and 50 mg of the AE extract-loaded SLNs were separately put into microcentrifuge tube (Thermo Fisher Scientific, Waltham, MA, USA) that were then sealed with laboratory parafilm tape. These tubes were then kept in saturated sodium chloride solution environments in an incubator (Memmert GmbH, Selecta Biosciences, Germany) at temperatures ranging from 50 to 80 °C, 16 days for AE extract, and 60 to 90 °C, 28 days for AE extract solution, while the AE extract-loaded SLNs were stored between 40 and 70 °C, 28 days. Samples were collected at days 0, 4, 8, 12, and 16 for the AE extract and AE extract solution, while the AE extract-loaded SLNs were collected at days 0, 7, 14, 21, and 28. All samples were kept at −20 °C until quantification of the remaining VB by using the HPLC method. The remaining VB was used to identify the reaction order and calculate the rate of reaction constant (K) and shelf life (T90).
The ethanolic extract of AE was successfully prepared with a percentage yield of 18.19% ± 0.75. VB content in this extract was quantitated by the HPLC technique that was validated to achieve the parameters of linearity, limit of detection (LOD), limit of quantification (LOQ), precision, and accuracy. Achieving linearity was defined as having R2 greater than or equal to 0.9997 using concentrations ranging from 19.53 to 1000.00 μg/mL. The LOD value of VB was 0.04 pg/mL, while the LOQ was 0.12 pg/mL. We found that this method was precise, with intraday and interday relative standard deviations (%RSD) of less than 5.84% and 5.05%, respectively. We also observed high accuracy of our procedure, with percentage recovery ranging from 90% to 110%. The evaluation results indicated that the HPLC method was accurate and reliable for the determination of the amount of VB in AE extract. The chromatogram of VB in ethanolic extract of AE is shown in Figure 2. VB content in AE extract was 9.58 ± 0.65% (w/w).
Figure 2.

Chromatogram of VB in AE extract.
AE extract-loaded SLNs were successfully prepared by using the hot melt homogenization technique. The particle characteristics are presented in Table 1. The ratios of co-surfactant and lipid content were varied, and the particle properties were observed. The properties of lipid particles were dependent on several factors, particularly surfactant concentrations. Formulas F1 and F2 indicated that the high content of Tween 80 caused the particles to be smaller in size, while the entrapment efficiency also distinctly decreased. Formulas F2 and F3 showed that an increased amount of Compritol ATO 888 increased both particle size and entrapment efficiency. A co-surfactant system is usually recommended for effectively inducing and stabilizing lipid particles. This study optimized the concentrations of Tween 80 and Span 80 for forming lipid particles of the appropriate size and entrapment efficiency. Particle entrapment efficiency was increased by increasing the amount of Span 80. However, the entrapment efficiency tended to decrease when the Span 80 concentration was higher than 3.00%. In addition, the zeta potential (ZP) of particles tended to increase the value of negative charge when the concentration of Span 80 was increased. The results in Table 1 also show that optimized proportions of 0.50% of Tween 80 and 3.00% of Span 80 along with 1.50 g of Compritol ATO 888 formed lipid particles with the highest entrapment efficiency of 31.28% and a particle size of approximately 628 nm. With this formulation, smooth and spherical particles were obtained (Figure 3).
Figure 3.
Morphology of AE extract-loaded SLNs observed under the scanning electron microscope.
AE extracts were dissolved in various pH buffer solutions. The remaining VB was quantitated at designated times to determine the degradation reaction. The order of the VB degradation rate was predicted by the graphical method.19 Zero-order, first-order, and second-order profiles were plotted for each temperature. The reaction rate constant (K) of chemical reaction at each temperature was calculated from 1% the slope of the curve of remaining VB concentration versus time for zero-order, 2% natural logarithm of remaining VB versus time for first order, and 3% inverse of remaining VB versus time for second order. In addition, the correlation coefficient (R2) of each graph was calculated to find the best linearity that correlated with the order of a chemical reaction. The degradation of VB at each pH was found to be a first-order reaction. The reaction rate constant (K) of VB was calculated from the slope and was then plotted against the pH values to generate the degradation pH–rate profile of VB (Figure 4). VB was more stable in acidic solutions than in alkaline solutions, with the fastest decrease at pH 8.
Figure 4.
pH–rate profile of VB in solution form of AE extract at 25 °C (n ≥ 3).
The pH–rate profile of VB can be used to optimize VB stability in a solution. However, valid estimates of shelf life of VB in solution and other forms are needed for the development of VB products. This was an important aspect of our study, to evaluate the thermal and chemical kinetics stability for VB in the semi-solid form of an AE extract, solution form of an AE extract, and AE extract-loaded SLNs by using Arrhenius’ theory. The amount of VB remaining in all sample forms after being stored at various temperatures for 16–28 days is presented in Figure 5.
Figure 5.

Thermal degradation kinetics for VB in (a) AE extract, (b) AE extract solution, and (c) AE extract-loaded SLNs as fitted to a first-order kinetic model.
These results indicated that the rate of VB decomposition was faster at elevated temperatures. The correlation between the natural logarithm of remaining VB in each sample form versus time was linear, which indicates a first-order reaction of VB in thermal degradation. The degradation rate constants (K) of VB at each tested temperature were calculated from the slope of the curve plotting the natural logarithm of remaining VB concentration versus time elapsed. The reaction rate constants are presented in Table 2. The slope of the semi-logarithmic curve multiplied by the gas constant (R = 8.3145 J/mol·K) represents the activation energy (Ea). The Arrhenius frequency factor (A) for the accelerated breakdown over the tested temperature of each compound can be calculated from eq 1. The natural logarithm of each rate reaction constant (ln K) was plotted against the inverse of absolute temperature (1/T). The reaction rate constant at 25 °C was calculated from the linear equation.
Table 2. Effect of Temperature on the Reaction Rate Constant (K) of VB in AE Extract, AE Extract Solution, and AE Extract-Loaded SLNsa.
|
K (×102 day–1) |
R2 |
|||||
|---|---|---|---|---|---|---|
| temp (°C) | AE extract (semi-solid form) | AE extract (solution form) | AE extract-loaded SLNs | AE extract (semi-solid form) | AE extract (solution form) | AE extract-loaded SLNs |
| 40 | – | – | 0.270 ± 0.078 | – | – | 0.8936 |
| 50 | 0.691 ± 0.044 | 10.778 ± 0.078 | 0.910 ± 0.014 | 0.9410 | – | 0.9421 |
| 60 | 3.086 ± 0.879 | 4.514 ± 0.085 | 1.570 ± 0.096 | 0.9963 | 0.9954 | 0.9138 |
| 70 | 5.573 ± 0.975 | 9.488 ± 1.779 | 2.140 ± 0.034 | 0.9967 | 0.9786 | 0.9068 |
| 80 | 8.982 ± 1.879 | 11.216 ± 3.451 | – | 0.9446 | 0.9821 | – |
| 90 | – | 20.957 ± 2.441 | – | – | 0.9853 | – |
K = degradation rate constants ± standard deviation (n = 3), R2 = coefficient of determination.
| 1 |
As shown in Table 2, the temperature played a crucial role in the degradation rate constants of VB. The rate constants increased with increasing temperatures. In addition, the reaction rate constant of VB in AE extract solution was faster than those of VB in AE extract and AE extract-loaded SLNs, respectively. This implies that VB in AE extract solution was less stable than the forms. The shelf life of VB at 25 °C was predicted by using eq 2, where the reaction rate constant (K) for this temperature was obtained by utilizing the linear equation expressing the relationship between the natural logarithm of rate constant (ln K) and the inverse of absolute temperature (1/T) (Figure 6).
| 2 |
Figure 6.
Arrhenius plot for thermal degradation of VB in (a) AE extract, (b) AE extract solution, and (c) AE extract-loaded SLNs over the temperature range 40–90 °C.
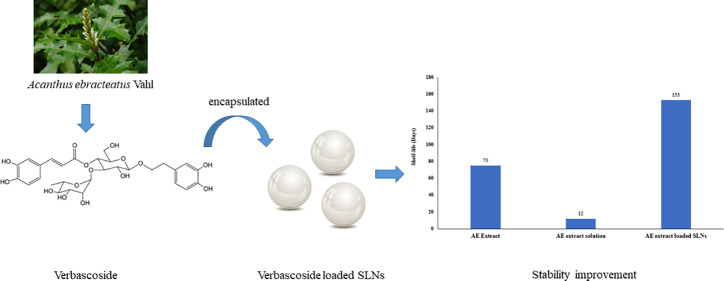
The estimated reaction rate constants and shelf life at 25 °C of VB in three forms, as well as activation energy (Ea), are shown in Table 3. The results indicated that VB in AE extract solution showed the highest rate constant, followed by VB in AE extract and VB in AE extract-loaded SLNs. The VB in AE extract-loaded SLNs showed the highest shelf life (153 days), followed by the VB in AE extract (75 days) and the VB in AE extract solution (12 days).
Table 3. Reaction Rate Constant and Shelf Life of VB at 25 °C: Comparison among AE Extract, AE Extract Solution, and AE Extract-Loaded SLNs.
| forms | K (×10–2 day–1) | Ea (kJ mol–1) | shelf life (days) |
|---|---|---|---|
| AE extract | 0.1398 | 78.03 | 75 |
| AE extract solution | 0.8189 | 49.24 | 12 |
| AE extract-loaded SLNs | 0.0048 | 76.16 | 153 |
VB is a naturally occurring water-soluble phenylpropanoid glycoside that is widely distributed in the plant kingdom. Most commonly, VB has been isolated from medicinal plants.6,8,20,21 Several biological properties have been documented including anti-inflammatory, antimicrobial, antitumor, and antioxidant activities.8,10,20 Although VB is a promising compound due to its bioactivities, its usage is limited because of chemical degradation. Previous studies have shown that the hydrolysis reaction is the cause of the instability of VB10,16 and that the glycosidic bond is hydrolyzed in an alkaline solution.22 Similarly, VB in AE extract is further degraded by basic hydrolysis. However, the degradation rate of VB tends to decrease, implying an increase in VB stability, when the pH of the solution is more than 8. This increase in VB stability in a strongly alkaline solution is not fully understood. In addition to the pH of the aqueous solution, temperature also shows a major effect on VB stability. Elevated temperatures of 50 °C or more induce more degradation of VB in both AE extract and AE extract solution. At these elevated temperatures, the degradation rate constant of VB tends to be higher. Increasing pH in an aqueous preparation also increases the degradation rate constant of VB. Therefore, since VB is water-soluble, it can be degraded by hydrolysis and oxidation reactions. Thus, increasing the hydrophobicity or preventing water exposure are alternative ways for increasing VB’s stability. Some reports have revealed that derivatized VB10 or encapsulated VB17,18 can decrease water exposure and increase VB’s stability.
In our study, we encapsulated AE extract into lipid carriers to make SLNs loaded with VB. Some previous studies had reported that high amounts of lipid carrier significantly affect the particle size.23,24 We found that increasing the concentration of Compritol ATO 888, and thus increasing the amount of lipid in the formulation, resulted in increased particle size. High entrapment efficiency was obtained from the high amount of lipid due to the increased particle capacity. However, some reports revealed that the increase in lipid quantity made the particles become unstable because of drug expulsion induced by the lipid recrystallization process.24,25 We also found that doubling the concentration of Tween 80 decreased the entrapment efficiency 10 times because the high amount of Tween 80 increased the amount of VB solubilized into the outer phase. Although a decrease of Tween 80 increases VB encapsulation, a low concentration of surfactant causes instability of the lipid particles.26 Co-surfactants are normally recommended for improving the stability and entrapment efficiency of particles.27,28 Previous studies reported that Span 80 and Tween 80 were effective in forming and stabilizing SLNs.27,29 Span 80 influences the entrapment efficiency, where an increase in the amount of Span 80 results in an increase in the entrapment efficiency of the particles. However, the entrapment efficiency is decreased when the concentration of Span 80 is more than 3%. It can be concluded, based on our study results, that an increase of Span 80 over 3% causes the entrapment efficiency to decrease because Span 80 alters the layer formation of Tween 80.28 The layer alteration causes VB to be solubilized in the outer phase.30 Moreover, the increased amount of Span 80 also decreased the HLB value of the system; therefore, VB’s solubility and miscibility in the lipid matrix decreased. On the other hand, a decrease of Span 80 to less than 3% also reduced VB’s entrapment efficiency, because the decrease of Span 80 allows the HLB value to increase. In this scenario, VB tends to be solubilized in the aqueous phase.28 In the optimization process for preparing AE extract-loaded SLNs, the percentage ratio of Compritol ATO 888, Tween 80, and Span 80 was 15.00:0.50:3.00.
We also examined the thermal degradation kinetics of VB in AE extract-loaded SLNs using Arrhenius’s theory. We used the reaction rate constant to infer VB’s degradation trend. The reaction rate constant of VB in AE extract-loaded SLNs presented the lowest value when compared with the reaction rate constants of VB in AE extract and AE extract solution. The shelf life times of VB in AE extract-loaded SLNs, AE extract solution, and AE extract were 153 days, 12 days, and 75 days, respectively. These results indicate that VB in AE extract-loaded SLNs is more stable than VB in the two other forms. The improvement of VB’s stability might be due to the SLN preventing encapsulated active substances from undergoing chemical reactions induced by environmental conditions.31 Although lipid particles can protect VB from being influenced by some external factors, the elevated temperature still obviously affects encapsulated VB. High temperatures can induce lipid particles to become gelled and encapsulated compounds to be expelled from the SLN.32,33 Recrystallization can also occur due to a change of temperature;33 the lipid structure alteration allows more of the encapsulated compound to be liberated from lipid particles.25,33 Hence, the degradation of encapsulated VB is exacerbated at high temperatures.
Although VB is a promising component of Acanthus ebracteatus Vahl. (AE) for health applications due to its bioactivities, its degradation is a limitation to potential uses. Our thermal degradation kinetics study reveals that the degradation of VB is a first-order reaction. The chemical reaction parameters according to Arrhenius’s theory indicate that VB is unstable when it dissolves in aqueous solution. The reaction rate in neutral to alkaline solutions was faster than in acidic solution. The stability of VB is successfully improved by encapsulating VB into SLNs that can substantially extend VB’s shelf life. The shelf life of VB in SLNs was 153 days with Ea = 76.16 kJ mol–1, whereas those of VB in AE extract and in AE extract solution were 75 days with Ea = 78.03 kJ mol–1 and 12 days with Ea = 49.24 kJ mol–1, respectively. Therefore, we anticipate that the AE extract-loaded SLNs will be beneficial for product development. However, they should be further examined in both in vitro and clinical studies to evaluate their safety and efficacy.
Acknowledgments
This study was supported by Thailand Center of Excellence for Life Sciences (TCELS), and the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation, Thailand. We thank Mr. Roy I. Morien of the Naresuan University Graduate School for his assistance in editing the English expression and grammar in this document.
Glossary
Abbreviations
- AE
Acanthus ebracteatus
- Ea
activation energy
- HLB
hydrophilic–lipophilic balance
- LOD
limit of detection
- LOQ
limit of quantification
- PS
particle size
- PDI
polydispersity index
- SLN
solid lipid nanoparticle
- VB
verbascoside
- ZP
zeta potential
Author Contributions
Conceptualization, V.W., N.W., P.C., and K.I.; methodology and experimental design, V.W., K.I., W.W., E.W., and N.W.; validation, V.W., E.W., and N.W.; formal analysis, V.W. and N.W.; investigation, V.W., K.I., and N.W.; resources, V.W. and N.W.; data curation and interpretation, V.W., W.W., and N.W.; writing - original draft preparation, V.W., W.W., and N.W.; writing - review and editing, V.W., K.I., and N.W.; visualization, V.W. and N.W.; supervision, V.W., P.C., K.I., and N.W.; project administration, N.W.; funding acquisition, N.W. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
References
- Srivilai J.; Phimnuan P.; Jaisabai J.; Luangtoomma N.; Waranuch N.; Khorana N.; Wisuitiprot W.; Scholfield C. N.; Champachaisri K.; Ingkaninan K. Curcuma aeruginosa Roxb. essential oil slows hair-growth and lightens skin in axillae; a randomised, double blinded trial. Phytomedicine 2017, 25, 29–38. 10.1016/j.phymed.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Therdphapiyanak N.; Jaturanpinyo M.; Waranuch N.; Kongkaneramit L.; Sarisuta N. Development and assessment of tyrosinase inhibitory activity of liposomes of Asparagus racemosus extracts. Asian Journal of Pharmaceutical Sciences 2013, 8 (2), 134–142. 10.1016/j.ajps.2013.07.017. [DOI] [Google Scholar]
- Viyoch J.; Buranajaree S.; Grandmottet F.; Robin S.; Binda D.; Viennet C.; Waranuch N.; Humbert P. Evaluation of the effect of Thai breadfruit’s heartwood extract on the biological functions of fibroblasts from wrinkles. J. Cosmet Sci. 2010, 61 (4), 311–324. [PubMed] [Google Scholar]
- Khunkitti W.; Veerapan P.; Hahnvajanawong C. In vitro bioactivities of clove buds oil (Eugenia caryophyllata) and its effect on dermal fibroblast. International Journal of Pharmacy and Pharmaceutical Sciences 2012, 4, 556–560. [Google Scholar]
- Kubica P.; Szopa A.; Kokotkiewicz A.; Miceli N.; Taviano M. F.; Maugeri A.; Cirmi S.; Synowiec A.; Gniewosz M.; Elansary H. O.; Mahmoud E. A.; El-Ansary D. O.; Nasif O.; Luczkiewicz M.; Ekiert H. Production of Verbascoside, Isoverbascoside and Phenolic Acids in Callus, Suspension, and Bioreactor Cultures of Verbena officinalis and Biological Properties of Biomass Extracts. Molecules 2020, 25 (23), 5609. 10.3390/molecules25235609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchanapoom T.; Kasai R.; Picheansoonthon C.; Yamasaki K. Megastigmane, aliphatic alcohol and benzoxazinoid glycosides from Acanthus ebracteatus. Phytochemistry 2001, 58 (5), 811–817. 10.1016/S0031-9422(01)00306-5. [DOI] [PubMed] [Google Scholar]
- Paola R. D.; Oteri G.; Mazzon E.; Crisafulli C.; Galuppo M.; Toso R. D.; Pressi G.; Cordasco G.; Cuzzocrea S. Effects of verbascoside, biotechnologically purified by Syringa vulgaris plant cell cultures, in a rodent model of periodontitis. J. Pharm. Pharmacol. 2011, 63 (5), 707–17. 10.1111/j.2042-7158.2011.01262.x. [DOI] [PubMed] [Google Scholar]
- Kim K. H.; Kim S.; Jung M. Y.; Ham I. H.; Whang W. K. Anti-inflammatory phenylpropanoid glycosides from Clerodendron trichotomum leaves. Arch. Pharm. Res. 2009, 32 (1), 7–13. 10.1007/s12272-009-1112-6. [DOI] [PubMed] [Google Scholar]
- Andary C.; Wylde R.; Laffite C.; Privat G.; Winternitz F. Structures of verbascoside and orobanchoside, caffeic acid sugar esters from Orobanche rapum-genistae. Phytochemistry 1982, 21 (5), 1123–1127. 10.1016/S0031-9422(00)82429-2. [DOI] [Google Scholar]
- Vertuani S.; Beghelli E.; Scalambra E.; Malisardi G.; Copetti S.; Dal Toso R.; Baldisserotto A.; Manfredini S. Activity and stability studies of verbascoside, a novel antioxidant, in dermo-cosmetic and pharmaceutical topical formulations. Molecules (Basel, Switzerland) 2011, 16 (8), 7068–7080. 10.3390/molecules16087068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisuitiprot V.; Ingkaninan K.; Chakkavittumrong P.; Wisuitiprot W.; Neungchamnong N.; Chantakul R.; Waranuch N. Effects of Acanthus ebracteatus Vahl. extract and verbascoside on human dermal papilla and murine macrophage. Sci. Rep 2022, 12 (1), 1491. 10.1038/s41598-022-04966-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebayo S. A.; Dzoyem J. P.; Shai L. J.; Eloff J. N. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complementary and Alternative Medicine 2015, 15, 159. 10.1186/s12906-015-0669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupattarakasem P.; Houghton P. J.; Hoult J. R. S.; Itharat A. An evaluation of the activity related to inflammation of four plants used in Thailand to treat arthritis. Journal of Ethnopharmacology 2003, 85 (2–3), 207–215. 10.1016/S0378-8741(02)00367-7. [DOI] [PubMed] [Google Scholar]
- Jaidee W.; Siridechakorn I.; Nessopa S.; Wisuitiprot V.; Chaiwangrach N.; Ingkaninan K.; Waranuch N. Kinetics of CBD, Δ(9)-THC Degradation and Cannabinol Formation in Cannabis Resin at Various Temperature and pH Conditions. Cannabis Cannabinoid Res. 2021, 10.1089/can.2021.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongwad E.; Ingkaninan K.; Wisuitiprot W.; Sritularak B.; Waranuch N. Thermal Degradation Kinetics and pH-Rate Profiles of Iriflophenone 3,5-C-β-d-diglucoside, Iriflophenone 3-C-β-d-Glucoside and Mangiferin in Aquilaria crassna Leaf Extract. Molecules 2020, 25 (21), 4898. 10.3390/molecules25214898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.; Zhao Y.; Li M.; Xu T.; Zhang L.; Lu B.; Wu X.; Ge Z. Degradation of phenylethanoid glycosides in Osmanthus fragrans Lour. flowers and its effect on anti-hypoxia activity. Sci. Rep. 2017, 7 (1), 10068. 10.1038/s41598-017-10411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacchi B.; Bergonzi M. C.; Iacopi R.; Ghelardini C.; Galeotti N.; Bilia A. R. Liposomal Formulation to Increase Stability and Prolong Antineuropathic Activity of Verbascoside. Planta Med. 2017, 83 (5), 412–419. 10.1055/s-0042-106650. [DOI] [PubMed] [Google Scholar]
- Sinico C.; Caddeo C.; Valenti D.; Fadda A. M.; Bilia A. R.; Vincieri F. F. Liposomes as carriers for verbascoside: stability and skin permeation studies. J. Liposome Res. 2008, 18 (1), 83–90. 10.1080/08982100801894067. [DOI] [PubMed] [Google Scholar]
- Sinko P. J.Chemical Kinetics and Stability. In Martin’s Physical Pharmacy and Pharmaceutical Sciences; Sinko P. J., Ed.; Lippincot Williams & Wilns: China, 2006; Vol. 6, p 318. [Google Scholar]
- Akdemir Z.; Kahraman C.; Tatlı I. I.; Küpeli Akkol E.; Süntar I.; Keles H. Bioassay-guided isolation of anti-inflammatory, antinociceptive and wound healer glycosides from the flowers of Verbascum mucronatum Lam. J. Ethnopharmacol 2011, 136 (3), 436–43. 10.1016/j.jep.2010.05.059. [DOI] [PubMed] [Google Scholar]
- Jiménez C.; Villaverde M. C.; Riguera R.; Castedo L.; Stermitz F. Phenylpropanoid Glycosides from Mussatia hyacinthina. J. Nat. Prod. 1989, 52 (2), 408–410. 10.1021/np50062a036. [DOI] [Google Scholar]
- Brito-Arias M., Hydrolysis of Glycosides. In Synthesis and Characterization of Glycosides; Brito-Arias M., Ed.; Springer US: Boston, MA, 2007; pp 304–313. [Google Scholar]
- Madan J. R.; Khude P. A.; Dua K. Development and evaluation of solid lipid nanoparticles of Mometasone furoate for topical delivery. International journal of pharmaceutical investigation 2014, 4 (2), 60–4. 10.4103/2230-973X.133047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R. H.; Mäder K.; Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50 (1), 161–177. 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Aburahma M. H.; Badr-Eldin S. M. Compritol 888 ATO: a multifunctional lipid excipient in drug delivery systems and nanopharmaceuticals. Expert opinion on drug delivery 2014, 11 (12), 1865–83. 10.1517/17425247.2014.935335. [DOI] [PubMed] [Google Scholar]
- Helgason T.; Awad T. S.; Kristbergsson K.; McClements D. J.; Weiss J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J. Colloid Interface Sci. 2009, 334 (1), 75–81. 10.1016/j.jcis.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Salminen H.; Helgason T.; Aulbach S.; Kristinsson B.; Kristbergsson K.; Weiss J. Influence of co-surfactants on crystallization and stability of solid lipid nanoparticles. J. Colloid Interface Sci. 2014, 426, 256–63. 10.1016/j.jcis.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Rostamkalaei S. S.; Akbari J.; Saeedi M.; Morteza-Semnani K.; Nokhodchi A. Topical gel of Metformin solid lipid nanoparticles: A hopeful promise as a dermal delivery system. Colloids Surf., B 2019, 175, 150–157. 10.1016/j.colsurfb.2018.11.072. [DOI] [PubMed] [Google Scholar]
- Bhalekar M.; Upadhaya P.; Madgulkar A. Formulation and characterization of solid lipid nanoparticles for an anti-retroviral drug darunavir. Applied Nanoscience 2017, 7 (1), 47–57. 10.1007/s13204-017-0547-1. [DOI] [Google Scholar]
- Ngwuluka N. C.; Kotak D. J.; Devarajan P. V. Design and Characterization of Metformin-Loaded Solid Lipid Nanoparticles for Colon Cancer. AAPS PharmSciTech 2017, 18 (2), 358–368. 10.1208/s12249-016-0505-3. [DOI] [PubMed] [Google Scholar]
- Singh H.; Bhandari R.; Kaur I. P. Encapsulation of Rifampicin in a solid lipid nanoparticulate system to limit its degradation and interaction with Isoniazid at acidic pH. International journal of pharmaceutics 2013, 446 (1–2), 106–11. 10.1016/j.ijpharm.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Makoni P. A.; Wa Kasongo K.; Walker R. B. Short Term Stability Testing of Efavirenz-Loaded Solid Lipid Nanoparticle (SLN) and Nanostructured Lipid Carrier (NLC) Dispersions. Pharmaceutics 2019, 11 (8), 397. 10.3390/pharmaceutics11080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C.; Muller R. H. Correlation between long-term stability of solid lipid nanoparticles (SLN) and crystallinity of the lipid phase. Eur. J. Pharm. Biopharm 1999, 47 (2), 125–32. 10.1016/S0939-6411(98)00074-5. [DOI] [PubMed] [Google Scholar]