Abstract
The protein histidine phosphatase PHPT1 is implicated in a variety of cellular signaling pathways. However, little is known about the precise biological roles of this enzyme and a dearth of chemical tools for studying histidine phosphorylation and dephosphorylation has hampered progress in the field. With the goal of identifying the first inhibitors of PHPT1 activity, we carried out an inhibitor screen using a facile fluorogenic assay for PHPT1 activity recently developed in our laboratory. From a panel of approximately 4000 compounds obtained from the Microsource Spectrum Collection and the NCI Diversity Set IV, we identified several potential hits with significant selectivity for inhibiting PHPT1 activity over other phosphatases. Of these, norstictic acid was the most potent inhibitor of PHPT1 activity with an IC50 value of 7.9 ± 0.8 μM under our assay conditions. Norstictic acid is a time-dependent, covalent inhibitor of PHPT1 activity with KI = 90 ± 20 μM and kinact = 1.7 ± 0.1 min–1.
Keywords: Histidine phosphatase, enzyme assay, enzyme inhibitor, PHPT1
Reversible protein phosphorylation is a widely recognized post-translational modification occurring in nature that regulates numerous cellular processes.1 Although histidine phosphorylation is suggested to account for ∼6% of protein phosphosites, the real proportion may be higher, and recent evidence indicates that the importance of phosphohistidine (pHis) in mammalian cellular pathways has been underestimated.2−4 Because of the relative instability of pHis as compared to pTyr, pSer, and pThr, it was long assumed that dedicated enzymes for dephosphorylating pHis were not needed in mammalian systems.3 However, several mammalian enzymes with histidine phosphatase activity have been identified,3 and in 2002 the first dedicated protein histidine phosphatase, PHPT1, was discovered.5 PHPT1 has been shown to dephosphorylate a variety of cellular targets including the ion channels TRPV56 and KCa3.1,7 EGFR,8 the G protein beta subunit,9 ATP citrate lyase,10 and histones H1 and H4.11,12 These diverse biological targets suggest that PHPT1 is involved in numerous biological pathways including DNA regulation, cell signaling, and fatty acid catabolism. PHPT1 is also an intriguing therapeutic target, as increased expression of this enzyme has been implicated in lung cancer,13 hepatocarcinoma,14 and renal cancer.15
Although interest in the biological roles of histidine phosphorylation continues to grow,3,16,17 the lack of chemical tools available to study pHis and the enzymes that regulate it is a significant roadblock in the field.3,16 Critically, no inhibitors of PHPT1 activity have been developed aside from a recent report that divalent zinc and copper inhibit enzyme activity.18 Potent and selective PHPT1 inhibitors would be invaluable as tools for interrogating PHPT1 activity, identifying potential substrates, and elucidating the roles of this intriguing enzyme in biological cellular signaling pathways. In the work reported here, we identify the first inhibitors of PHPT1 activity and provide a thorough characterization of the enzyme–inhibitor interaction. The results obtained provide insight into key features of PHPT1-targeted inhibitor design as well as the first hit compounds for future optimization.
Our laboratory recently developed a facile, fluorogenic assay for monitoring PHPT1 activity.18,19 Building upon this work, we validated the assay for use in high throughput screening (HTS). A robust HTS assay is defined as having a Z′ and signal window greater than 0.4 and 2, respectively.20,21 Using ZnCl2 as the inhibitor,18 our assay was determined to have an average Z′ of 0.82 and a signal window of 14, assuming that only a single replicate will be used during screening (see Figure S1 for representative data).
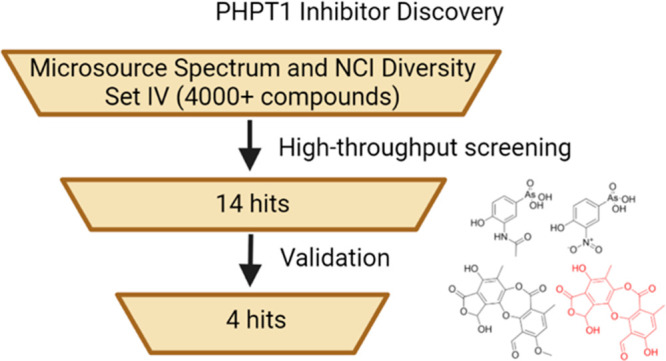
With a fully validated assay in hand, we carried out a screen of over 4000 compounds obtained from the Microsource Spectrum Collection and the NCI Diversity Set IV in conjunction with the Drug Discovery Core Facility, a part of the Health Sciences Cores at the University of Utah. Approximately 20 compounds that inhibited PHPT1 activity more than 45% at a concentration of 50 μM were identified as potential hits, for a hit rate of approximately 0.5%. These potential hits were further screened to remove structures that were likely to be pan-assay interference compounds (PAINs) using an online screening tool.22,23 Independent authentic samples of the remaining potential hits (Figure 1) were obtained from commercial sources and each compound was screened again in triplicate at 50 μM, resulting in the loss of a few potential hits that were not commercially available (highlighted in the blue box in Figure 1) and the identification of a few false positive hits from the initial screen (highlighted in the red box in Figure 1). The four remaining hits were investigated as dose-dependent inhibitors of PHPT1 activity and all had IC50 values at or below 100 μM (Table 1, Figure S2).
Figure 1.
Structures of the potential hits identified during inhibitor screening. Compounds in the blue box (top) were not available commercially and not investigated further. Compounds in the red box (middle) were identified as false positives or compounds with IC50 values of greater than 200 μM, while those in the black box (bottom) had IC50 values of less than or equal to 100 μM.
Table 1. IC50 Values of the Top Four Hit Compounds against PHPT1.
| inhibitor | IC50 (μM) |
|---|---|
| acetarsol | 100 ± 30 |
| roxarsone | 80 ± 10 |
| stictic acid | 22 ± 2 |
| norstictic acid | 7.9 ± 0.8 |
To investigate the mechanism of PHPT1 inhibitory activity for the hit compounds, we investigated the time-dependence of inhibition, as shown in Figure 2. Norstictic acid and stictic acid inhibited PHPT1 activity in a time-dependent manner, while acetarsol and roxarsone did not. Time-dependent inhibition is frequently a hallmark of covalent or pseudoirreversible inhibition and indicates that further characterization of these compounds is necessary as IC50 values do not provide an accurate measure of the potency of irreversible inhibitors. Further kinetic analysis of norstictic acid mediated PHPT1 inhibition suggests that this compound is a modestly fast inhibitor of PHPT1 with a KI value of 90 ± 20 μM and a kinact value of 1.7 ± 0.1 min–1 (Figure 2C). Experiments in which PHPT1 inhibition was measured pre- and postdialysis reveal that acetarsol inhibition is readily reversible while norstictic acid showed little change in activity, consistent with the above data (Figure S3). Further mechanistic analysis of acetarsol and roxarsone inhibition reveals that below 150 μM these compounds show changes in KM and Vmax consistent with a competitive mechanism of action, while concentrations above this show a mixed inhibition mechanism (Figure S4, Table S1).
Figure 2.
Investigating the mechanism of inhibition of the four hit compounds. (A) Roxarsone and acetarsol are not time-dependent inhibitors. (B) Norstictic acid and stictic acid both show time-dependent inhibition. (C) The kinact and KI values for norstictic acid mediated inhibition of PHPT1 activity can be obtained by plotting kobs vs concentration of norstictic acid: kinact = 1.7 ± 0.1 min–1 and KI = 90 ± 20 μM. These compounds showed no evidence of insolubility by visual inspection up to 500 μM under the assay conditions.
Finally, in order to gain insight into the possible selectivity of these inhibitors for PHPT1, three of the hit compounds (acetarsol, roxarsone, and norstictic acid) were counterscreened against a small panel of enzymes with known histidine phosphatase activity (PP2C)2 or promiscuous phosphatase activity (AlkP). Stictic acid was not included due to limited availability. The assay conditions for the counterscreen are shown in Table S2 with the results in Figure 3. We also attempted to counterscreen against the histidine phosphatase LHPP.24 Although the assay described in Table S2 was not robust enough to give satisfactorily reproducible data, none of the compounds appeared to inhibit LHPP activity (data not shown). Taken together, these data indicate that these compounds have some selectivity for PHPT1 inhibition over other related enzymes.
Figure 3.
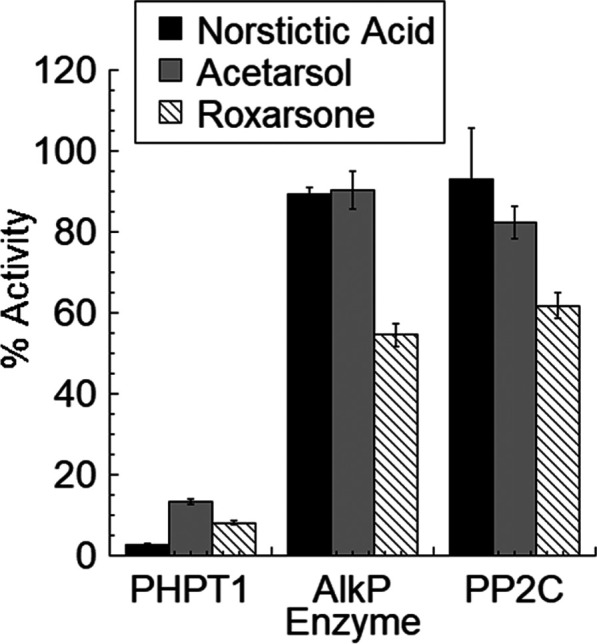
Counterscreen of norstictic acid, acetarsol, and roxarsone against PP2C, and AlkP at 100 μM norstictic acid and 500 μM acetarsol and roxarsone. PHPT1 is included for reference.
Norstictic acid and related depsidone natural products have been identified as inhibitors in other key biological pathways in addition to the PHPT1 inhibitory activity presented here. For example, norstictic acid blocks transcriptional activation by binding covalently to the transcriptional coactivator Med25 with an IC50 of 2.3 ± 0.1 μM.25 Depsidones have demonstrated antimicrobial and cytotoxic activity, though the mechanisms of action are not well understood.26 Related compounds have been shown to inhibit the activity of the tyrosine phosphatase, PTP1B with low and submicromolar potency.27,28 However, in our hands, norstictic acid did not inhibit PTP1B activity at 10 μM (see Figure S5).
The data presented here indicate that norstictic acid is a reasonably potent and selective inhibitor of PHPT1 activity as compared with selected protein phosphatase enzymes. Inhibition is time-dependent, suggesting that a covalent or pseudoirreversible adduct is formed between norstictic acid and PHPT1. This work provides the first inhibitors of PHPT1 activity aside from divalent copper and zinc, reported previously.18 Further optimization and validation of these initial hits will provide valuable chemical tools for studying the biological roles of histidine phosphorylation.
Acknowledgments
This work was supported by NIH Grant R21GM127970 to A.M.B. B.S.M. and H.W. both gratefully acknowledge funding from Kuramoto and Skaggs Graduate Research Fellowship Awards. Compound screening was performed in collaboration with the Drug Discovery Core Facility at the University of Utah.
Glossary
Abbreviations
- PHPT1
protein histidine phosphatase T1
- TRPV5
transient receptor potential cation channel subfamily V member 5
- KCa3.1
calcium activated potassium channel 3.1
- EGFR
epidermal growth factor receptor
- pNPP
para-nitrophenyl phosphate
- DiFMUP
6,8-difluoromethyl umbelliferyl phosphate
- AlkP
alkaline phosphatase
- PP2C
protein phosphatase 2C
- LHPP
phospholysine phosphohistidine inorganic pyrophosphate phosphatase
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00053.
Experimental details, HTS assay validation data, IC50 curves of the top four hits, dialysis experiments for acetarsol, kinetic analysis of acetarsol and roxarsone inhibition, and full conditions for the counterscreens (PDF)
Author Present Address
† Molecular and Cell Biology Laboratory, Salk Institute for Biological Studies, La Jolla CA 92037, USA
Author Contributions
A.M.B. and B.S.M. conceptualized and designed the experiments. B.S.M. and H.W. performed the experiments and data analysis. All authors contributed to the preparation of the manuscript and approved of the final version.
The authors declare no competing financial interest.
Supplementary Material
References
- Walsh C.Posttranslational Modification of Proteins : Expanding Nature’s Inventory; Roberts and Co. Publishers, 2006. [Google Scholar]
- Kim Y.; Huang J.; Cohen P.; Matthews H. R. Protein Phosphatases 1, 2A, and 2C Are Protein Histidine Phosphatases. J. Biol. Chem. 1993, 268 (25), 18513–18518. 10.1016/S0021-9258(17)46657-9. [DOI] [PubMed] [Google Scholar]
- Fuhs S. R.; Hunter T. pHisphorylation: The Emergence of Histidine Phosphorylation as a Reversible Regulatory Modification. Curr. Opin. Cell Biol. 2017, 45, 8–16. 10.1016/j.ceb.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.; Lee H.; Kwon O. K.; Cheng Z.; Tan M.; Kim K.; Lee S. Profiling of Histidine Phosphoproteome in Danio Rerio by TiO2 Enrichment. Proteomics 2019, 19 (9), 1800471. 10.1002/pmic.201800471. [DOI] [PubMed] [Google Scholar]
- Ek P.; Pettersson G.; Ek B.; Gong F.; Li J.-P.; Zetterqvist Ö. Identification and Characterization of a Mammalian 14-KDa Phosphohistidine Phosphatase. Eur. J. Biochem. 2002, 269 (20), 5016–5023. 10.1046/j.1432-1033.2002.03206.x. [DOI] [PubMed] [Google Scholar]
- Cai X.; Srivastava S.; Surindran S.; Li Z.; Skolnik E. Y. A Highlights from MBoC Selection: Regulation of the Epithelial Ca2+ Channel TRPV5 by Reversible Histidine Phosphorylation Mediated by NDPK-B and PHPT1. Mol. Biol. Cell 2014, 25 (8), 1244. 10.1091/mbc.e13-04-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S.; Zhdanova O.; Di L.; Li Z.; Albaqumi M.; Wulff H.; Skolnik E. Y. Protein Histidine Phosphatase 1 Negatively Regulates CD4 T Cells by Inhibiting the K+ Channel KCa3.1. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (38), 14442–14446. 10.1073/pnas.0803678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke J.Regulation of EGF Receptor Activation by Phosphohistidine Phosphatase PHPT1. PhD Thesis, Technische Universität Dortmund, 2017. 10.17877/DE290R-1804. [DOI] [Google Scholar]
- Mäurer A.; Wieland T.; Meissl F.; Niroomand F.; Mehringer R.; Krieglstein J.; Klumpp S. The β-Subunit of G Proteins Is a Substrate of Protein Histidine Phosphatase. Biochem. Biophys. Res. Commun. 2005, 334 (4), 1115–1120. 10.1016/j.bbrc.2005.06.200. [DOI] [PubMed] [Google Scholar]
- Klumpp S.; Bechmann G.; Mäurer A.; Selke D.; Krieglstein J. ATP-Citrate Lyase as a Substrate of Protein Histidine Phosphatase in Vertebrates. Biochem. Biophys. Res. Commun. 2003, 306 (1), 110–115. 10.1016/S0006-291X(03)00920-3. [DOI] [PubMed] [Google Scholar]
- Ek P.; Ek B.; Zetterqvist Ö. Phosphohistidine Phosphatase 1 (PHPT1) Also Dephosphorylates Phospholysine of Chemically Phosphorylated Histone H1 and Polylysine. Ups. J. Med. Sci. 2015, 120 (1), 20–27. 10.3109/03009734.2014.996720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood P. V.; Ludwig K.; Bergander K.; Besant P. G.; Adina-Zada A.; Krieglstein J.; Klumpp S. Chemical Phosphorylation of Histidine-Containing Peptides Based on the Sequence of Histone H4 and Their Dephosphorylation by Protein Histidine Phosphatase. Biochim. Biophys. Acta - Proteins Proteomics 2010, 1804 (1), 199–205. 10.1016/j.bbapap.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Xu A.; Hao J. J.; Zhang Z.; Tian T.; Jiang S.; Hao J. J.; Liu C.; Huang L.; Xiao X.; He D. 14-KDa Phosphohistidine Phosphatase and Its Role in Human Lung Cancer Cell Migration and Invasion. Lung Cancer 2010, 67 (1), 48–56. 10.1016/j.lungcan.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Han S.-X.; Wang L.-J.; Zhao J.; Zhang Y.; Li M.; Zhou X.; Wang J.; Zhu Q. 14-KDa Phosphohistidine Phosphatase Plays an Important Role in Hepatocellular Carcinoma Cell Proliferation. Oncol. Lett. 2012, 4 (4), 658–664. 10.3892/ol.2012.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H.; Yang P.; Liu Q.; Tian Y. Nuclear Expression and Clinical Significance of Phosphohistidine Phosphatase 1 in Clear-Cell Renal Cell Carcinoma. J. Int. Med. Res. 2015, 43 (6), 747–757. 10.1177/0300060515587576. [DOI] [PubMed] [Google Scholar]
- Kee J.-M.; Muir T. W. Chasing Phosphohistidine, an Elusive Sibling in the Phosphoamino Acid Family. ACS Chem. Biol. 2012, 7 (1), 44–51. 10.1021/cb200445w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sanchez M.-B.; Lanucara F.; Helm M.; Eyers C. E. Attempting to Rewrite His Tory: Challenges with the Analysis of Histidine-Phosphorylated Peptides. Biochem. Soc. Trans. 2013, 41 (4), 1089–1095. 10.1042/BST20130072. [DOI] [PubMed] [Google Scholar]
- McCullough B. S.; Barrios A. M. Facile, Fluorogenic Assay for Protein Histidine Phosphatase Activity. Biochemistry 2018, 57 (18), 2584–2589. 10.1021/acs.biochem.8b00278. [DOI] [PubMed] [Google Scholar]
- McCullough B. S.; Barrios A. M.. In Vitro Assays for Measuring Protein Histidine Phosphatase Activity. In Methods in Molecular Biology; Eyers C. E., Ed.; Springer: New York, 2020; pp 109–120. 10.1007/978-1-4939-9884-5_8. [DOI] [PubMed] [Google Scholar]
- Iversen P. W.; Beck B.; Chen Y.-F.; Dere W.; Devanarayan V.; Eastwood B. J.; Farmen M. W.; Iturria S. J.; Montrose C.; Moore R. A.; Weidner J. R.; Sittampalam G. S.. HTS Assay Validation. In Assay Guidance Manual; Markossian S., Grossman A., Brimacombe K., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, 2012. http://www.ncbi.nlm.nih.gov/books/NBK83783/ (accessed 2016-03-03).
- Zhang J. H.; Chung T. D.; Oldenburg K. R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4 (2), 67–73. 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Baell J. B.; Holloway G. a. New Substructure Filters for Removal of Pan Assay Interference Compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J. Med. Chem. 2010, 53 (7), 2719–2740. 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- Lagorce D.; Sperandio O.; Baell J. B.; Miteva M. A.; Villoutreix B. O. FAF-Drugs3: A Web Server for Compound Property Calculation and Chemical Library Design. Nucleic Acids Res. 2015, 43 (W1), W200–W207. 10.1093/nar/gkv353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindupur S. K.; Colombi M.; Fuhs S. R.; Matter M. S.; Guri Y.; Adam K.; Cornu M.; Piscuoglio S.; Ng C. K. Y.; Betz C.; Liko D.; Quagliata L.; Moes S.; Jenoe P.; Terracciano L. M.; Heim M. H.; Hunter T.; Hall M. N. The Protein Histidine Phosphatase LHPP Is a Tumour Suppressor. Nature 2018, 555 (7698), 678–682. 10.1038/nature26140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick J. M.; Sturlis S. M.; Bruno P. A.; Yates J. A.; Peiffer A. L.; Liu Y.; Goo L.; Bao L.; De Salle S. N.; Tamayo-Castillo G.; Brooks C. L.; Merajver S. D.; Mapp A. K. Norstictic Acid Is a Selective Allosteric Transcriptional Regulator. J. Am. Chem. Soc. 2021, 143 (25), 9297–9302. 10.1021/jacs.1c03258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña-Vacas I.; González-Burgos E.; Divakar P. K.; Gómez-Serranillos M. P. Lichen Depsidones with Biological Interest. Planta Med. 2021, 10.1055/a-1482-6381. [DOI] [PubMed] [Google Scholar]
- Vu T. H.; Delalande O.; Lalli C.; Reider S.; Ferron S.; Boustie J.; Waltenberger B.; Lohézic-Le Dévéhat F. Inhibitory Effects of Secondary Metabolites from the Lichen Stereocaulon Evolutum on Protein Tyrosine Phosphatase 1B. Planta Med. 2021, 87 (9), 701–708. 10.1055/a-1334-4480. [DOI] [PubMed] [Google Scholar]
- Seo C.; Sohn J. H.; Ahn J. S.; Yim J. H.; Lee H. K.; Oh H. Protein Tyrosine Phosphatase 1B Inhibitory Effects of Depsidone and Pseudodepsidone Metabolites from the Antarctic Lichen Stereocaulon Alpinum. Bioorg. Med. Chem. Lett. 2009, 19 (10), 2801–2803. 10.1016/j.bmcl.2009.03.108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




