Abstract
Neuroblastoma (NB) is an extracranial pediatric tumor with highly invasive growth of cancer biomass and frequent metastases. During the differentiation process in embryonic development, altered epigenetic modifications lead to dysregulated expression of pluripotency markers, resulting in epithelial–mesenchymal transition (EMT) progression. Currently, available chemotherapies have provided a limited solution to this problem due to systemic toxicities and drug resistance. Epigenetic therapeutic molecules like histone deacetylase inhibitors are still in the initial stages of development. We have developed a retinoid (N-(4-hydroxyphenyl) retinamide, 4HPR) loaded acetylated human serum albumin (HSA) nanoformulation to address the epigenetic imbalance and chemoresistance in NB. The idea was conceived to deliver an acetyl pool along with a chemotherapeutic drug, 4HPR, to restrict the invasiveness of NB by maintaining the balance between histone acetylation and trimethylation. The therapeutic efficacy of the formulation was successfully evaluated in the in vitro and in vivo xenograft mouse model system of neuroblastoma. The synthesized nanoparticles show high biocompatibility and therapeutic efficacy in treating neuroblastoma subcutaneous xenografts in nude mice.
Keywords: Fenretinide, Acetylated human serum albumin nanoparticles, Epigenetic rebalancing, Epithelial-mesenchymal transition, Neuroblastoma
Neuroblastoma is a form of extracranial solid tumor in children in the age group of 0–14 years with a median age of 19 months at the time of diagnosis.1 The majority of neuroblastoma cases are diagnosed as high-risk disease with overall survival of 50%.2,3 The clinical behavior and therapeutic outcomes of NB are highly variable. Patients with high-risk, aggressive, metastatic NB are reported each year. These patients require multimodal therapies, including chemotherapy, surgery, radiotherapy, biologics, and immunotherapeutic maintenance therapy to improve their survival odds, although long-term survival depends primarily on the tumor growth patterns. A more primitive type of tumor has a less favorable outcome than well-differentiated ones.4
A higher incidence of cancer relapse and associated deaths have raised concern about the currently available treatments in recent times. The past decade has witnessed effective treatments of NB patients with minimal residual disease using retinoid-based therapy. These differentiation inducers represent critical therapeutic options in high-risk NB treatment; however, an increase in drug resistance limits their use in clinical practice.5 Therefore, the development of new formulations is vital for improvement in treatment efficacy. In the past decade, protein-based nanoformulations have provided treatment diversity; several human serum albumin (HSA) based nanoformulations are in clinical trials for multiple cancer types (NCT00629499, NCT00736619, NCT02646319). In this work, we have synthesized a novel HSA nanoformulation of fenretinide (N-(4-hydroxyphenyl) retinamide; 4HPR), a synthetic cytotoxic retinoid with clinical activity in recurrent neuroblastoma.6 The current nanoformulation is inspired by the post-translational modifications (PTMs) of histone (acetylation and methylation), causing epigenetic changes in the cells.
Histone acetylation and methylation play crucial roles in gene regulation in every cell type. Alterations in the patterns of these PTMs have been extensively reported in many cancer types, including NB. Increased expression of histone deacetylases HDAC8 and HDAC10 is reported in high-risk NB. Interestingly, inhibition of these HDACs reduced cell proliferation in vitro and in vivo.7,8 In addition to the histone deacetylases, increased expression of histone methyltransferases, like EZH2, has been linked to poor prognosis and differentiation and increased invasiveness in NB.9,10
Several epigenetic regulatory compounds have already been approved to treat certain tumors or are currently in preclinical stages.11 In NB, three histone deacetylase inhibitors (HDACi), two DNA methyltransferase inhibitors (DNMTi), and one bromodomain and extraterminal (BET) inhibitor (iBET) compound have reached clinical trials; among these, only two inhibitors, genistein and vorinostat, have reached phase II trials.12
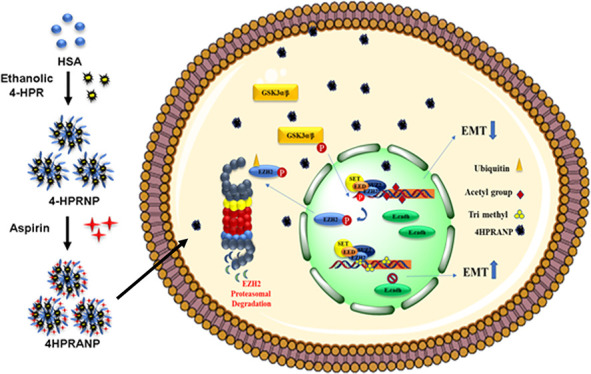
It is interesting to understand that PTMs are reversible and highly dependent on cells’ metabolic states and metabolite availability.13 There are several critical metabolites, including acetate and acetyl coenzyme A (acetyl-CoA), that play a crucial role in dysregulated gene expression in cancer cells.14 The availability of acetyl-CoA has been shown to correlate with global histone acetylation and deacetylation levels.15,16 In addition, the extent of histone acetylation and deacetylation is tightly regulated by changes in the activity of HAT (histone acetyltransferase) and HDAC (histone deacetylase) enzymes. We hypothesized that nutrient limitation in solid cancers restricts the adequate supply of acetyl-CoA to the cancer cells, resulting in increased expression of HDACs, causing deacetylation of histones and maintenance of acetate levels to fuel acetyl-CoA in the cancer cells. This hypoacetylated state of histones might be leading to hypermethylation events in cancer cells through methyltransferases like EZH2 and could be a driving force for epigenome alterations and cancer invasiveness. In the current study, we focus on developing and studying an acetyl-modified N-(4-hydroxyphenyl) retinamide (4HPR)-loaded HSA nanoformulation that could potentially restructure the balance between histone methylation and acetylation signatures to overcome epigenetic changes responsible for epithelial–mesenchymal transition (EMT) and simultaneously induce apoptosis in NB.
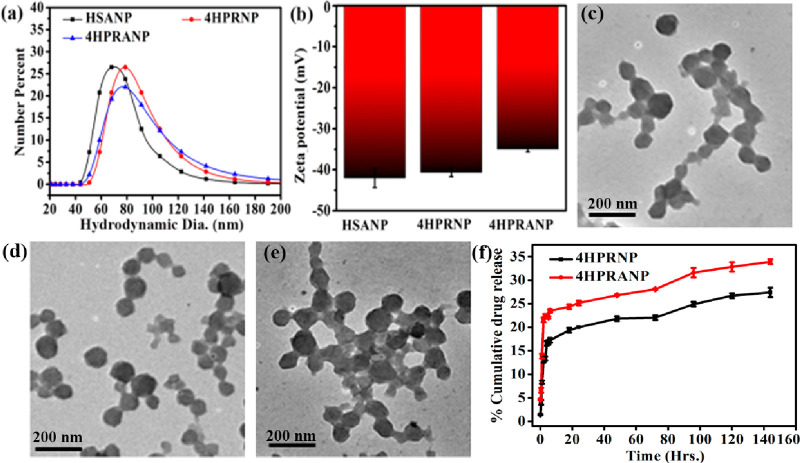
HSA nanoparticles (HSANPs) were synthesized using a well-established ethanol precipitation and glutaraldehyde cross-linking method. The synthesized HSANPs showed a mean diameter of 80–100 nm. Precisely, average sizes of 72, 84, and 78 nm were recorded for HSANPs, 4HPR-loaded HSA nanoparticles (4HPRNPs), and 4HPR-loaded acetylated nanoparticles (4HPRANPs)(Figure 1a) respectively. The 4HPR drug loading results in a small increase in the size of 4HPRNPs and 4HPRANPs. All of the nanoformulations have shown excellent polydispersity index (PDI) values of <0.1, which shows considerable dispersibility of the polymeric nanoparticles. The ζ potentials of HSANPs, 4HPRNPs, and 4HPRANPs, were recorded to be −41 mV, −39 mV, and −37 mV, respectively (Figure 1b). These ζ values indicated the excellent stability of the nanoparticles in the solvent system. The TEM images revealed that the nanoformulations exhibit very similar size and spherical morphology (Figure 1c–e).
Figure 1.
(a) Hydrodynamic size and (b) ζ potential of nanoparticles, (c–e) TEM images of (c) HSANPs, (d) 4HPRNPs, and (e) 4HPRANPs, and (f) percent cumulative drug release for 4HPRNPs and 4HPRANPs.
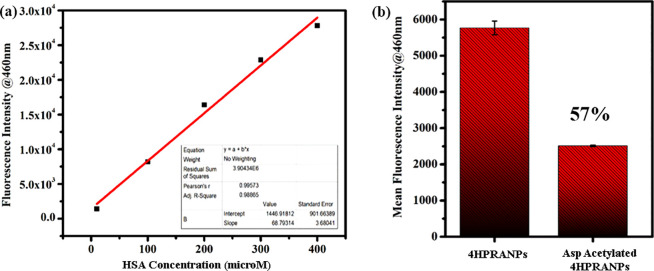
The synthesized 4HPRNPs and 4HPRANPs were further subjected to in vitro drug release analysis. The drug release patterns of both formulations show an initial burst release for a few hours, which could be due to the swelling effect and leaching of surface-bound drug molecules. After the initial burst release, 4HPRNPs and 4HPRANPs showed sustained release patterns (Figure 1f). Percentage cumulative release for 4HPRNPs and 4HPRANPs at 150 h was 26% ± 0.43% and 34% ± 0.11%, respectively. The acetyl variant, 4HPRANPs, has shown a better release profile than the 4HPRNPs. This improved drug release behavior could be due to the hydrogen bonding of the acetyl group to the water molecules, which may result in better dispersibility of the nanoformulation and better drug release from the nanoparticles. The presence of the acetyl group on the surface of 4HPRANPs was calculated using a fluorescamine assay. The aspirin mediated acetylation (Scheme 1) of the 4HPRNPs resulted in 57% modification of the available amine groups compared to the non-acetylated nanoparticles (Figure 2a,b).
Scheme 1. Schematic Showing Acetyl Salicylic Acid Mediated Acetylation of HSA Nanoparticles (HSANPs) and Acetate Release Mechanism in Cancer Cells.
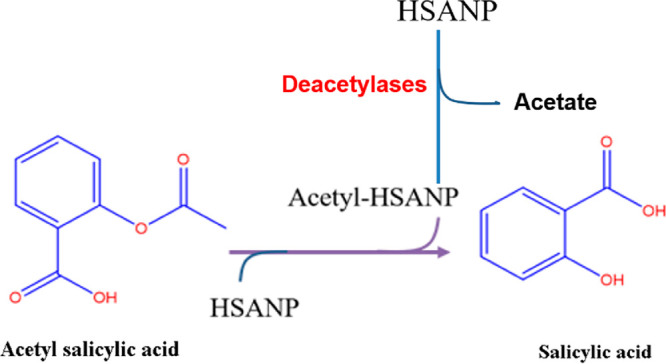
Figure 2.
(a) Standard curve of fluorescamine with HSA, (b) quantitative estimation of aspirin mediated acetylation in 4HPRANPs.
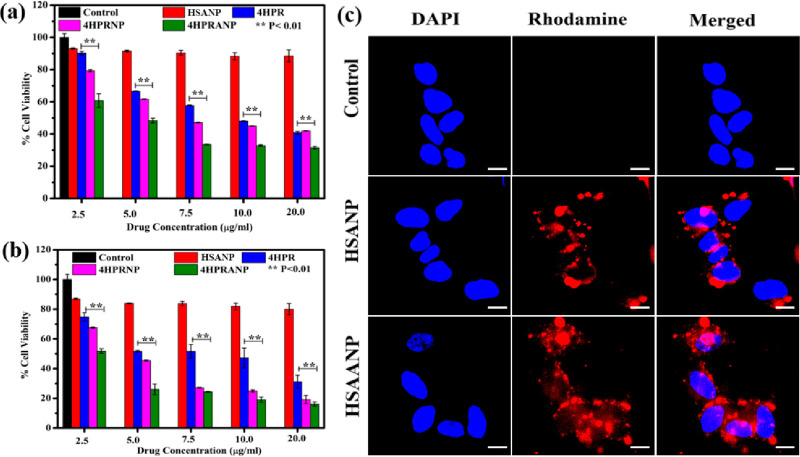
The prepared nanoparticles were evaluated for their cytotoxicity in the SH-SY-5Y in vitro model of neuroblastoma. All the formulations, including HSANPs, 4HPR, 4HPRANPs, and 4HPRANPs, were screened to determine the concentration inducing 50% growth inhibition (IC50) at 48 and 72 h treatment duration (Figure 3a,b).
Figure 3.
(a, b) In vitro cytotoxicity analysis showing MTT-determined viability at (a) 48 h and (b) 72 h. (c) Cellular uptake studies of HSANPs and HSAANPs in monolayer cells; scale bar represents 10 μm.
The IC50 value of 4HPR was 10.15 ± 0.8 μg mL–1 after 48 h of treatment and significantly improved to 5.2 ± 0.32 μg mL–1 at 72 h. 4HPRNPs and 4HPRANPs showed better therapeutic efficacy at 48 and 72 h of therapy than the 4HPR drug. The IC50 values of 4HPRNPs and 4HPRANPs significantly improved to 7.63 ± 0.41 μg mL–1 and 5.14 ± 0.13 μg mL–1, respectively, after 48 h of treatment. After 72 h of treatment, the IC50 values of 4HPRNPs and 4HPRANPs were 4.8 ± 0.2 μg mL–1 and 2.6 ± 0.16 μg mL–1, respectively. The available reports support the therapeutic potential of the 4HPR drug in restricting the growth of neuroblastoma cells. However, a pleiotropic effect of the drug was observed among several neuroblastoma cell lines.
We have analyzed the toxicity of these nanoparticles in normal human cells (HEK293 cells) as has been reported in the literature17−19 and found that these nanoparticles are nontoxic to the noncancerous cells (Figure S1), which is an essential parameter for accessing the associated systemic toxicities of chemotherapeutic drugs.
We have further studied the nanoparticle uptake in the monolayer culture of the cancer cell. The cells significantly internalized the nanoparticles, and the NPs were widely distributed and accumulated in cytoplasm and nucleoplasm (Figure 3c). It is unclear how acetyl groups release from the acetylated nanoparticles; however, higher expression of deacetylases in cancer cells might be a prime mechanism for the deacetylation of nanoparticles and acetate release in the cells. We have further performed nanoparticle uptake studies in the 3D spheroid model of SH-SY-5Y to mimic the tumor environment and found that acetylated HSANPs (HSAANPs) had higher internalization than the non-acetylated HSANPs (Figure S2). There is evidence to support acetate uptake in colon cancer cell lines, which shows that acetate enters these cells by the secondary active transporters MCT1 or MCT2 and SMCT1 and by facilitated diffusion via aquaporins.20 To understand the role of acetate in cellular uptake, we replaced glucose with acetate in the cell culture medium and monitored how it affected the cellular uptake of the particles (Figure S3). No significant changes were observed in the cellular uptake of the nanoparticles, except their absence in the nucleoplasm compared to the cells in the glucose supplemented medium.
We have further studied the mechanism of therapeutic action by monitoring the effect of 4HPR, 4HPRNPs, and 4HPRANPs on cell cycle arrest. Interestingly, no cell cycle arrest was reported in the SH-SY-5Y cells in any treatment group at the IC50 values (Figure S4) after 48 h of treatment. The cell cycle arrest results agree with earlier reports. These reports support the notion that 4HPR has a pleiotropic effect on different neuroblastoma cell lines, where apoptosis was achieved in neuroblastoma cell lines without cell cycle arrest.21
We evaluated the in vitro therapeutic potential of the nanoformulation at the transcriptional and translational level of expression. The gene expression analysis of the SH-SY-5Y cells after treatment with 4HPR, HSANPs, 4HPRNPs, and 4HPRANPs revealed that 4HPRNPs and 4HPRANPs are potentially very effective in regulating the metastasis-related genes like Slug, E-cadherin, fibronectin, MMP9, GSK3β, and β-catenin and epigenetic regulatory markers like EZH2 and Bmi-1.
E-cadherin is the prime target in epithelial–mesenchymal transition (EMT) of the cells. A significantly lower expression of E cadherin is the hallmark of the mesenchymal phenotype. The gene expression of E cadherin is epigenetically controlled by histone methyltransferase EZH2 through H3K27me3. A better strategy to stop the disease progression is either to decrease H3K27me3 expression or to improve histone H3 acetylation. In some earlier studies on colon cancer, it was reported that the H3K27me3 downregulation is controlled by downregulating EZH2 expression or improving demethylase UTX expression. UTX recruits histone acetyltransferases (HATs) and induces histone acetylation at residue lysine 27, leading to the chromatin open conformation and increased E-cadherin expression.22
In this study, 4HPRNPs and 4HPRANPs successfully downregulated EZH2 expression and improved the ratio of Ac-histone3 (Ac-H3)/H3K27me3 in the in vitro assay. 4HPRANPs did not show any added advantage in enhancing the expression of Ac-H3; however, they significantly downregulated HDAC3 expression compared to the HPRNPs (Figure S5). Fibronectin is another critical protein expressed in the mesenchymal phenotype of the cells and represents the metastasis niche of cancer cells.23 The mRNA expression analysis revealed that 4HPRANPs significantly reduced the expression of fibronectin, showing better therapeutic potential of the nanoformulation in inhibiting the metastasis phenotype of SH-SY-5Y. In addition to these vital molecular markers, reduced expression of slug, Gsk3β, β-catenin, and MMP-9 strongly supports the therapeutic potential of 4HPRANPs in restricting metastasis in NB.
We have studied the effect of nanoformulations on restricting the metastatic potential of SH-SY-5Y cells through scratch assay model and immunofluorescence studies in monolayer cells. The 4HPRANP treatment in SH-SY-5Y monolayer cells resulted in migration arrest across the wound boundaries (Figure S6) and in downregulation of fibronectin, ICAM, and SNAIL expression (Figure S7). These molecular markers are highly expressed in metastatic cancer cells and regulate several molecular pathways that induce invasiveness and resistance to therapies.24,25 We further observed that 4HPRNPs and 4HPRANPs successfully induced caspase 3 activation in the cancer cells showing an active apoptotic process (Figure 4c,d).
Figure 4.
In vitro therapeutic efficacy of nanoformulations showing (a, b) relative expression of mRNA in fold change and (c, d) relative protein expression of several molecular markers in different treatment groups of SH-SY-5Y cells. SH-SY-5Y cells without any treatment were considered as control group. Data are presented as mean ± SEM, n = 3 biological replicates; level of significance is indicated as ***p < 0.001, **p < 0.01, or *p < 0.05 compared to the control group.
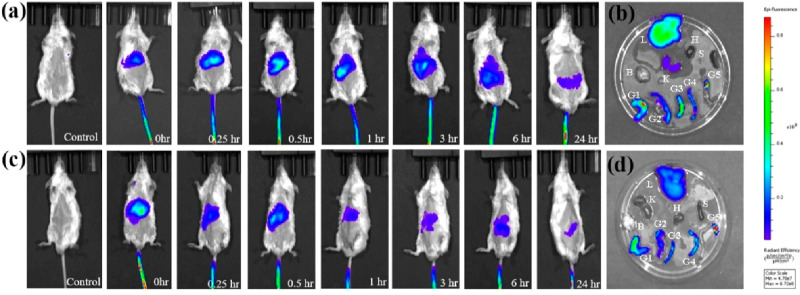
The HSANPs and acetylated HSA nanoparticles (Ac-HSANPs) were tagged with NIR dye indocyanine green.26 The whole-body biodistribution of nanoparticles was studied in the in vivo mouse model system. The tagged particles were injected through the tail vein, and time-dependent epifluorescence imaging was performed to monitor the whole body biodistribution. The epifluorescence results showed time dependent decline in the signal intensity, indicating gradual clearance of the nanoparticles from the system. However, ex vivo imaging performed on harvested body organs 24 h after administration of the nanoparticles showed some accumulation in the liver, kidney, and gut (Figure 5a,c). This accumulation of nanoparticles could be associated with the complex excretory machinery of the organs (Figure 5b,d). Contrary to the HSANPs, Ac-HSANPs showed less accumulation in the body organs and better clearance from the system; this faster clearance could be due to the improved blood circulation in the mice after HSAANPs treatment, very similar to the acetylation effects of aspirin in humans.27
Figure 5.
(a) Time dependent in vivo biodistribution of HSANPs, (b) ex vivo distribution in organs, (c) in vivo biodistribution of acetylated HSANPs, and (d) ex vivo distribution in organs. (L = liver, H = heart, K = kidney, S = spleen, B = brain, G1–G5 = gut).
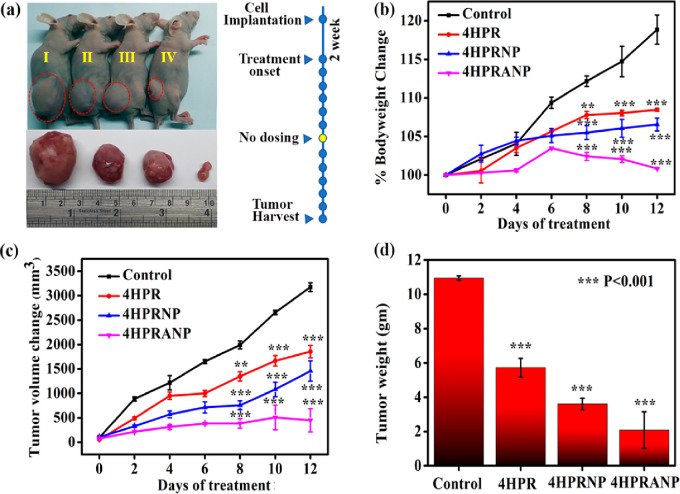
The in vitro therapeutic results were further evaluated in the in vivo subcutaneous xenograft mouse model of NB developed in the nude mice (Figure S8). The xenograft bearing mice were treated with a 5 (mg/kg)/day equivalent dose of 4HPR, 4HPRNPs, and 4HPRANPs. A daily dose of the formulation was given for 13 days, with a day off after six consecutive doses. The body weight of the mice was monitored on alternate days. The humane point was determine to be when tumor volume reached 4000 mm3 or tumor diameter was 20 mm. The drug and nanoformulation treated mice showed a significant difference in tumor volume compared to the control. The difference in tumor size and volume was most significant in the 4HPRANP group, followed by 4HPRNP and 4HPR groups (Figure 6a,c). The percent body weight of the 4HPRANP group was constant throughout the treatment phase, showing good treatment efficacy and biocompatibility of the nanoformulations. 4HPRNP and 4HPR groups also showed percent body weight maintenance compared to the control groups (Figure 6b). The tumors were harvested after completion of treatment and weighed for the actual difference in weight compared to the control (Figure 6d). The result indicated a successful reduction of tumor weight compared to the control group. We have further analyzed survival with Kaplan–Meier curves (Figure S9). The first day of treatment is day 0, and the study was terminated on day 12 when all the mice were euthanized due to the tumor burden. Statistical significance of differences in the survival curves between the groups was evaluated using the log-rank trend test. We found a significant trend (p-value = 0.0203) in the survival pattern in the treatment groups. These results show that 4HPRANPs have maximum therapeutic potential in treating NB in the in vivo model system at the 5 (mg/kg)/day IP dose.
Figure 6.
(a) In vivo therapeutic efficacy of nanoformulation in xenograft nude mouse model of control (I), 4HPR (II), 4HPRNP (III), and 4HPRANP, (b) percent body weight change during days of treatment, (c) tumor volume change during days of treatment, and (d) tumor weight after completion of treatment (n = 3; **p < 0.01 or ***p < 0.001 considered significant compared to the control group).
We hypothesized that metastasis and cancer growth of NB could be addressed by simultaneously targeting apoptotic and epigenetic pathways. 4HPR is a vitamin A derivative known to induce apoptosis and cellular differentiation of neuroblastoma cells.28,29 The nanoformulation of 4HPR showed better bioavailability of the drug inside the tumor, therefore imparting better therapeutic potential.
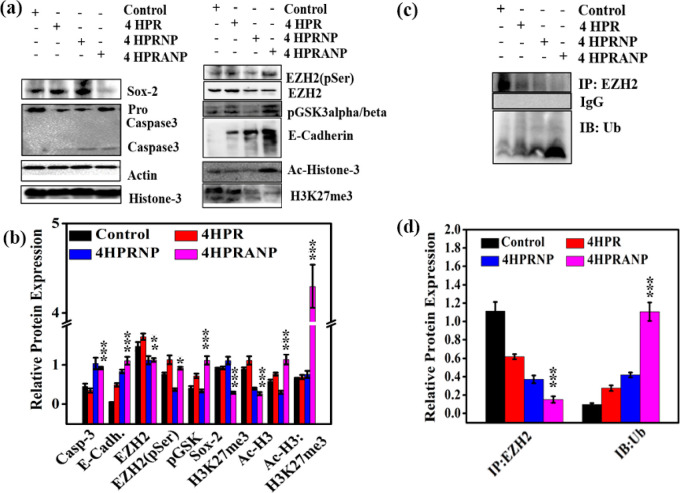
Western blot analysis (Figure 7a–d) revealed that 4HPRNPs and 4HPRANPs significantly induced apoptosis in the tumor tissues by activating caspase-3 protease. The overexpression of PRC2 complex EZH2 in NB patients signifies epigenetic gene repression by the formation of the H3K27me3 mark and hypoacetylation of histone at the promoter site of epithelial marker E-cadherin. The alteration in HAT and HDAC activity in NB leads to an imbalance in the acetylation–deacetylation equilibrium, which induces epigenetic modifications and aberrant gene expression.30 In the present study, we primarily focused on delivering acetyl moieties in the form of 4HPRANPs along with retinoic acid derivative 4HPR to induce apoptosis. The expression of EZH2 is highly upregulated in several cancer types, including NB, resulting in the vigorous and invasive phenotype of cancer.9,31 Increased expression of EZH2 is known to silence E-cadherin expression and therefore induce EMT.10
Figure 7.
(a) Relative protein expression of several molecular markers in different treatment groups, (b) quantitative estimation of the relative protein expression, (c) pull down assay showing EZH2 and Ub interaction, and (d) quantification of pull down assay showing increased expression of ubiquitin in 4HPRNP treated group (n = 3; *p < 0.05, **p < 0.01, and ***p < 0.001 considered significant compared to the control group).
The treatment results showed that 4HPRNPs and 4HPRANPs successfully downregulated EZH2 expression in the tumors, resulting in a decrease in the H3K27me3 mark, although improvement in the histone H3 acetylation profile was achieved only in the 4HPRANP treated group, complementing the highest increase in the E-cadherin expression. These results suggest that 4HPRANP has improved therapeutic potential to address NB. In addition to these molecular pathways, we studied the expression of pluripotency markers (SOX2, Nanog, and Oct3/4).32,33 These transcriptional factors regulate E-cadherin protein expression through distinct molecular pathways. The protein expression analysis confirmed that 4HPRANPs also downregulate the protein expression of SOX2, which is highly elevated in the cancer cells and contributes to EMT.34,35 We did not find any significant changes in the expression of Nanog and Oct3/4 among the treatment groups (data not included).
In this study, 4HPRNPs and 4HPRANPs downregulated EZH2, which may subsequently cause a decrease in the expression of H3K27me3 on the E cadherin promoter. Several mechanisms are reported in the literature through which EZH2 expression is regulated inside the cell; among those mechanisms is the role of upstream molecule pGSK3α/β. pGSK3α/β is known to interact with the EZH2 and induce phosphorylation of the molecule at serine and threonine residues, causing conformational changes and directing it to proteasomal degradation.36 We have found increased expression level of pGSK3α/β in the 4HPRANP treatment group, along with increased expression of phospho-EZH2 (pSerEZH2). These results were further validated by increased expression of ubiquitin in 4HPRANP treatment groups inducing its proteasomal degradation.
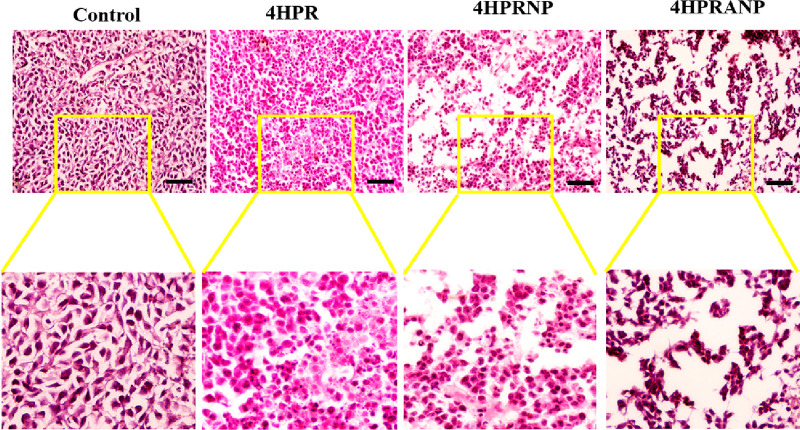
All the treatment groups were subjected to histopathological evaluation (Figure 8) and immunohistochemistry to study the physiological changes and expression of selective biomarkers in the harvested tumor sections. The histology of the tumor sections confirmed that nanoparticle treatment had induced cancer cell death with visible loss of cellular morphology and nuclear condensation.
Figure 8.
Histopathological evaluation using H&E staining showing changes in tumor tissues during treatment; scale bars represent 200 μm.
The immunofluorescence studies were performed on harvested tumor tissue after the completion of treatment. The results of immunofluorescence (Figure S10a–f) were consistent with the protein expression results obtained by Western blot analysis. The 4HPRANP treatment group successfully showed caspase3 activation, downregulation of fibronectin and increased expression of E-cadherin. Further evaluation of the histological sections of major body organs confirmed that 4HPR produced mild liver toxicity at the treatment dose of 5 (mg/kg)/day (Figure S11). The nanoformulation treatment did not produce any associated liver toxicities, demonstrating high biocompatibility and therapeutic potential for treating NB. This toxicity is probably due to the drug accumulation in the liver tissues. We have performed Nissl staining to stain neurons in brain tissues (Figure S12), and no neuronal loss was observed due to nanoformulation treatment. Similarly, periodic acid-Schiff staining was performed to detect the glomerular damage; no pathological distinction was observed in the nanoformulation treated groups (Figure S13). All other major organs, including the spleen and heart, showed no observable toxicity in any treatment group.
In conclusion, we have shown that 4HPR nanoformulations have significant therapeutic efficacy in the in vitro and in vivo tumor xenograft models of NB. 4HPRANPs showed maximum potential in the initial preclinical assessment for restricting the neuroblastoma xenografts. We have successfully shown the reestablishment of the high ratios of Ac-H3/H3K27me3, repression of H3K27 methyltransferase EZH2, followed by downregulation of HDAC3, and subsequent upregulation of H3K27ac could provide a solution to the EMT progression in NB. The current novel nanoformulation 4HPRANP promises potential in NB therapeutics.
Acknowledgments
The authors acknowledge ICMR (35/4/2020-Nano/BMS and R11013/09/2021-GIA/HR) and DBT (BT/PR40647/MED/31/436/2020) for providing partial funding support to the PIs. The Facilities of Indian Institute of Science Education and Research, Mohali (IISER, Mohali), National Agri-Food Biotechnology Institute (NABI, Mohali), and Institute of Nano Science and Technology (INST, Mohali) are duly acknowledged. We are also thankful to Dr. Nitin Singhal of National Agri-Food Biotechnology Institute (NABI, Mohali) for providing support to use the in vivo imaging facility of NABI, Mohali.
Glossary
Abbreviations
- Bmi-1
B lymphoma Mo-MLV insertion region-1 homologue
- EZH2
enhancer of zeste homologue 2
- NB
neuroblastoma
- HAT
histone acetyl transferase
- HDAC
histone deacetylase
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00135.
Materials, methods, MTT analysis, nanoparticle uptake studied in 3D spheroid model, cellular uptake studies in acetate supplemented medium, cell cycle analysis, Western blot analysis, scratch assay, immunofluorescence studies in SH-SY-5Y monolayer, development of SH-SY-5Y neuroblastoma xenograft model in nude mice, Kaplan–Meier curves for survival, immunofluorescence studies of tumor sections, histopathological evaluation of major organs, toxicity studied in brain sections, toxicity studied in kidney, Western blot of H3K27me3 in SH-SY-5Y cells, and primer sequences (PDF)
Author Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Medicinal Chemistry Letters special issue “Epigenetics 2022”.
Supplementary Material
References
- London W. B.; Castleberry R. P.; Matthay K. K.; Look A. T.; Seeger R. C.; Shimada H.; Thorner P.; Brodeur G.; Maris J. M.; Reynolds C. P.; Cohn S. L. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2005, 23, 6459–65. 10.1200/JCO.2005.05.571. [DOI] [PubMed] [Google Scholar]
- Strother D. R.; London W. B.; Schmidt M. L.; Brodeur G. M.; Shimada H.; Thorner P.; Collins M. H.; Tagge E.; Adkins S.; Reynolds C. P.; Murray K.; Lavey R. S.; Matthay K. K.; Castleberry R.; Maris J. M.; Cohn S. L. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: results of Children’s Oncology Group study P9641. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2012, 30, 1842–8. 10.1200/JCO.2011.37.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay K. K.; Reynolds C. P.; Seeger R. C.; Shimada H.; Adkins E. S.; Haas-Kogan D.; Gerbing R. B.; London W. B.; Villablanca J. G. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2009, 27, 1007–13. 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredlund E.; Ringner M.; Maris J. M.; Pahlman S. High Myc pathway activity and low stage of neuronal differentiation associate with poor outcome in neuroblastoma. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 14094–9. 10.1073/pnas.0804455105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masetti R.; Biagi C.; Zama D.; Vendemini F.; Martoni A.; Morello W.; Gasperini P.; Pession A. Retinoids in pediatric onco-hematology: the model of acute promyelocytic leukemia and neuroblastoma. Advances in therapy 2012, 29, 747–62. 10.1007/s12325-012-0047-3. [DOI] [PubMed] [Google Scholar]
- Nguyen T. H.; Koneru B.; Wei S. J.; Chen W. H.; Makena M. R.; Urias E.; Kang M. H.; Reynolds C. P. Fenretinide via NOXA Induction, Enhanced Activity of the BCL-2 Inhibitor Venetoclax in High BCL-2-Expressing Neuroblastoma Preclinical Models. Molecular cancer therapeutics 2019, 18, 2270–2282. 10.1158/1535-7163.MCT-19-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehme I.; Linke J. P.; Bock B. C.; Milde T.; Lodrini M.; Hartenstein B.; Wiegand I.; Eckert C.; Roth W.; Kool M.; Kaden S.; Grone H. J.; Schulte J. H.; Lindner S.; Hamacher-Brady A.; Brady N. R.; Deubzer H. E.; Witt O. Histone deacetylase 10 promotes autophagy-mediated cell survival. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, E2592–E2601. 10.1073/pnas.1300113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig I.; Koeneke E.; Trippel F.; Mueller W. C.; Burhenne J.; Kopp-Schneider A.; Fabian J.; Schober A.; Fernekorn U.; von Deimling A.; Deubzer H. E.; Milde T.; Witt O.; Oehme I. Selective inhibition of HDAC8 decreases neuroblastoma growth in vitro and in vivo and enhances retinoic acid-mediated differentiation. Cell death & disease 2015, 6, e1657 10.1038/cddis.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Liu Z.; Woo C. W.; Li Z.; Wang L.; Wei J. S.; Marquez V. E.; Bates S. E.; Jin Q.; Khan J.; Ge K.; Thiele C. J. EZH2Mediates epigenetic silencing of neuroblastoma suppressor genes CASZ1, CLU, RUNX3, and NGFR. Cancer research 2012, 72, 315–24. 10.1158/0008-5472.CAN-11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q.; Yu J.; Dhanasekaran S. M.; Kim J. H.; Mani R. S.; Tomlins S. A.; Mehra R.; Laxman B.; Cao X.; Yu J.; Kleer C. G.; Varambally S.; Chinnaiyan A. M. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 2008, 27, 7274–84. 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishina O.; Schmoor C.; Dohner K.; Hackanson B.; Lubrich B.; May A. M.; Cieslik C.; Muller M. J.; Lubbert M. DECIDER: prospective randomized multicenter phase II trial of low-dose decitabine (DAC) administered alone or in combination with the histone deacetylase inhibitor valproic acid (VPA) and all-trans retinoic acid (ATRA) in patients > 60 years with acute myeloid leukemia who are ineligible for induction chemotherapy. BMC cancer 2015, 15, 430. 10.1186/s12885-015-1432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubierre L.; Jimenez C.; Rovira E.; Soriano A.; Sabado C.; Gros L.; Llort A.; Hladun R.; Roma J.; Toledo J. S.; Gallego S.; Segura M. F. Targeting of epigenetic regulators in neuroblastoma. Exp. Mol. Med. 2018, 50, 1. 10.1038/s12276-018-0077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitosugi T.; Chen J. Post-translational modifications and the Warburg effect. Oncogene 2014, 33, 4279–85. 10.1038/onc.2013.406. [DOI] [PubMed] [Google Scholar]
- Schug Z. T.; Vande Voorde J.; Gottlieb E. The metabolic fate of acetate in cancer. Nature reviews. Cancer 2016, 16, 708–717. 10.1038/nrc.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell E.; Crown S. B.; Fox D. B.; Kitir B.; Ilkayeva O. R.; Olsen C. A.; Grimsrud P. A.; Hirschey M. D. Lipids Reprogram Metabolism to Become a Major Carbon Source for Histone Acetylation. Cell reports 2016, 17, 1463–1472. 10.1016/j.celrep.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet C.; Feron O. Cancer cell metabolism and mitochondria: Nutrient plasticity for TCA cycle fueling. Biochimica et biophysica acta. Reviews on cancer 2017, 1868, 7–15. 10.1016/j.bbcan.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Marona P.; Górka J.; Jura J.; Miękus K. Abstract 898: Clear cell renal cell carcinoma resistance to RTKs inhibitors is mediated by c-Met receptor and MCPIP1. Cancer Res. 2018, 78, 898–898. 10.1158/1538-7445.AM2018-898. [DOI] [Google Scholar]
- Horibe T.; Torisawa A.; Akiyoshi R.; Hatta-Ohashi Y.; Suzuki H.; Kawakami K. Transfection efficiency of normal and cancer cell lines and monitoring of promoter activity by single-cell bioluminescence imaging. Luminescence 2014, 29, 96–100. 10.1002/bio.2508. [DOI] [PubMed] [Google Scholar]
- Amuthan A.; Devi V.; Shreedhara C. S.; Rao V.; Lobo R. Cytoprotective Activity of Neichitti (Vernonia cinerea) in Human Embryonic Kidney (HEK293) Normal Cells and Human Cervix Epitheloid Carcinoma (HeLa) Cells against Cisplatin Induced Toxicity: A Comparative Study. J. Clin. Diagn. Res. 2019, KC01. 10.7860/JCDR/2019/40242.12624. [DOI] [Google Scholar]
- Ferro S.; Azevedo-Silva J.; Casal M.; Corte-Real M.; Baltazar F.; Preto A. Characterization of acetate transport in colorectal cancer cells and potential therapeutic implications. Oncotarget 2016, 7, 70639–70653. 10.18632/oncotarget.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperus R.; Tytgat G. A.; Leen R.; Brites P.; Bras J.; Caron H. N.; Van Kuilenburg A. B. Pleiotropic effects of fenretinide in neuroblastoma cell lines and multicellular tumor spheroids. International journal of oncology 2008, 32, 1011–9. [PubMed] [Google Scholar]
- Zha L.; Cao Q.; Cui X.; Li F.; Liang H.; Xue B.; Shi H. Epigenetic regulation of E-cadherin expression by the histone demethylase UTX in colon cancer cells. Medical oncology 2016, 33, 21. 10.1007/s12032-016-0734-z. [DOI] [PubMed] [Google Scholar]
- Wang J. P.; Hielscher A. Fibronectin: How Its Aberrant Expression in Tumors May Improve Therapeutic Targeting. Journal of Cancer 2017, 8, 674–682. 10.7150/jca.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Fei F.; Li S.; Zhao Y.; Yang Z.; Qu J.; Zhang X.; Yin Y.; Zhang S. The role of beta-catenin in the initiation and metastasis of TA2 mice spontaneous breast cancer. Journal of Cancer 2017, 8, 2114–2123. 10.7150/jca.19723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoopathi P.; Pradhan A. K.; Bacolod M. D.; Emdad L.; Sarkar D.; Das S. K.; Fisher P. B. Regulation of neuroblastoma migration, invasion, and in vivo metastasis by genetic and pharmacological manipulation of MDA-9/Syntenin. Oncogene 2019, 38, 6781–6793. 10.1038/s41388-019-0920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L.; Frommel S. C.; Oakes C. C.; Simon R.; Grupp K.; Gerig C. Y.; Bar D.; Robinson M. D.; Baer C.; Weiss M.; Gu Z.; Schapira M.; Kuner R.; Sultmann H.; Provenzano M.; Yaspo M. L.; Brors B.; Korbel J.; Schlomm T.; Sauter G.; Eils R.; Plass C.; Santoro R. BAZ2A (TIP5) is involved in epigenetic alterations in prostate cancer and its overexpression predicts disease recurrence. Nature genetics 2015, 47, 22–30. 10.1038/ng.3165. [DOI] [PubMed] [Google Scholar]
- Mekaj Y. H.; Daci F. T.; Mekaj A. Y. New insights into the mechanisms of action of aspirin and its use in the prevention and treatment of arterial and venous thromboembolism. Ther Clin Risk Manag 2015, 11, 1449–56. 10.2147/TCRM.S92222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson Q. D.; Lovat P. E.; Corazzari M.; Catterall J. B.; Redfern C. P. The NF-kappaB pathway mediates fenretinide-induced apoptosis in SH-SY5Y neuroblastoma cells. Apoptosis: an international journal on programmed cell death 2005, 10, 493–8. 10.1007/s10495-005-1878-z. [DOI] [PubMed] [Google Scholar]
- Chen S.; Samuel W.; Fariss R. N.; Duncan T.; Kutty R. K.; Wiggert B. Differentiation of human retinal pigment epithelial cells into neuronal phenotype by N-(4-hydroxyphenyl)retinamide. Journal of neurochemistry 2003, 84, 972–81. 10.1046/j.1471-4159.2003.01608.x. [DOI] [PubMed] [Google Scholar]
- Phimmachanh M.; Han J. Z. R.; O’Donnell Y. E. I.; Latham S. L.; Croucher D. R. Histone Deacetylases and Histone Deacetylase Inhibitors in Neuroblastoma. Frontiers in cell and developmental biology 2020, 8, 578770. 10.3389/fcell.2020.578770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Takenobu H.; Setyawati A. N.; Akita N.; Haruta M.; Satoh S.; Shinno Y.; Chikaraishi K.; Mukae K.; Akter J.; Sugino R. P.; Nakazawa A.; Nakagawara A.; Aburatani H.; Ohira M.; Kamijo T. EZH2 regulates neuroblastoma cell differentiation via NTRK1 promoter epigenetic modifications. Oncogene 2018, 37, 2714–2727. 10.1038/s41388-018-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S.; Isobe T.; Ariyama H.; Nakano M.; Kikushige Y.; Takaishi S.; Kusaba H.; Takenaka K.; Ueki T.; Nakamura M.; Akashi K.; Baba E. Ecadherin regulates proliferation of colorectal cancer stem cells through NANOG. Oncology reports 2018, 40, 693–703. 10.3892/or.2018.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancakli Usta C.; Turan G.; Bulbul C. B.; Usta A.; Adali E. Differential expression of Oct-4, CD44, and E-cadherin in eutopic and ectopic endometrium in ovarian endometriomas and their correlations with clinicopathological variables. Reproductive Biol. Endocrinol. 2020, 18, 116. 10.1186/s12958-020-00673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H.; Teng C.; Huang W.; Peng J.; Wang C. SOX2 Promotes the Epithelial to Mesenchymal Transition of Esophageal Squamous Cells by Modulating Slug Expression through the Activation of STAT3/HIF-alpha Signaling. International journal of molecular sciences 2015, 16, 21643–57. 10.3390/ijms160921643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Qiao B.; Zhao T.; Hu F.; Lam A. K.; Tao Q. Sox2 promotes tumor aggressiveness and epithelial-mesenchymal transition in tongue squamous cell carcinoma. Int. J. Mol. Med. 2018, 42, 1418–1426. 10.3892/ijmm.2018.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H. W.; Lee H. H.; Huo L.; Xia W.; Yang C. C.; Hsu J. L.; Li L. Y.; Lai C. C.; Chan L. C.; Cheng C. C.; Labaff A. M.; Liao H. W.; Lim S. O.; Li C. W.; Wei Y.; Nie L.; Yamaguchi H.; Hung M. C. GSK3beta inactivation promotes the oncogenic functions of EZH2 and enhances methylation of H3K27 in human breast cancers. Oncotarget 2016, 7, 57131–57144. 10.18632/oncotarget.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.