To the Editor:
Adverse cardiovascular outcomes have been associated with radiation therapy (RT) and adjuvant chemotherapy in patients with breast cancer. Long‐term effects may be increased among patients with left‐sided tumors who receive unwanted irradiation to cardiac segments that lie within the treatment field. 1
Ionizing radiation damages healthy heart tissue via microvascular changes, inflammation, and edema, followed by reduced perfusion, fibrosis, and depressed ventricular systolic and diastolic function. 2 , 3 Myocardial edema, fibrosis, and myocyte atrophy have been demonstrated using T1 and T2 relaxation times (T1 and T2) acquired with cardiovascular magnetic resonance imaging (MRI) in a spectrum of cardiac disorders, including breast cancer patients late after anthracycline or trastuzumab treatment. 4 , 5 , 6 , 7 , 8
The aim of this study is to investigate whether T1 and T2, measured in a single breath hold with three‐dimensional (3D)‐QALAS techniques (3D‐quantification using an interleaved Look‐Locker acquisition sequence with T2 preparation pulse), 9 can detect myocardial changes early in RT treatment in breast cancer patients, and assess the impact of cardiac radiation dose and concomitant chemotherapy on the evolution of those changes during and early following RT.
Materials and Methods
Ten female breast cancer patients aged 55 ± 9 years with stage I–III disease undergoing 3D conformal RT were studied. Radiation burden to the left anterior descending artery and heart were measured as mean and near maximum dose, D2%. 10 Seven patients had left‐sided and three had right‐sided breast cancer. Five patients received RT alone; five patients received RT plus chemotherapy. Four of five chemotherapy patients also received trastuzumab before, during, and after RT (Table 1).
TABLE 1.
Patient Characteristics and Radiation Dose
| Patient | Cancer Site | Chemotherapy | Trastuzumab | Target Dose (Gy) | LAD Dose (% of Target) | Heart Dose (% of Target) | ||
|---|---|---|---|---|---|---|---|---|
| D2% Max | Mean | D2% Max | Mean | |||||
| 1 | Left | FEC + Tax | Yes | 42.56 | 93.8 | 69.7 | 80.3 | 6.7 |
| 2 | Right | FEC + Tax | No | 50 | 0.4 | 0.1 | 8.2 | 1.1 |
| 3 | Left | No | No | 39.9 | 20.1 | 11.8 | 9.4 | 2.1 |
| 4 | Right | FEC + Tax | Yes | 50 | 0.6 | 0.3 | 10.7 | 1.4 |
| 5 | Left | No | No | 42.56 | 93.5 | 66.9 | 28.7 | 3.8 |
| 6 | Right | No | No | 42.56 | 0.4 | 0.3 | 9.7 | 1.1 |
| 7 | Left | FEC + Tax | Yes | 42.56 | 96.9 | 80.1 | 99.6 | 7.3 |
| 8 | Left | Tax | Yes | 42.56 | 93.6 | 42.6 | 96.4 | 4.3 |
| 9 | Left | No | No | 42.56 | 103.1 | 76.2 | 102 | 5 |
| 10 | Left | No | No | 42.56 | 79.7 | 31 | 13.9 | 1.8 |
FEC = fluorouracil, epirubicin, and cyclophosphamide; Tax = Taxotere; Gy = Gray units; LAD = left anterior descending artery; D2% Max = maximal dose to 2% of the myocardium.
Patients were recruited at Linköping University, Sweden, between January 2015 and October 2016 and gave written informed consent to participate in the study. Approval was granted from the Regional Ethical Review Board in Linköping.
Patients were examined within 1 week prior to start of RT (examination 1), 2–3 weeks after RT initiation (examination 2), and 1 and 6 months following RT completion (examinations 3 and 4). Myocardial T1 and T2, left ventricular mass (LVM), and ejection fraction (LVEF) were measured with MRI. Global LV longitudinal strain (GLS) and mitral valve E/é ratio were measured with speckle‐tracking and Doppler echocardiography.
Cardiac MRI examinations were performed using a Philips Ingenia 3 T system and included a short‐axis cine steady‐state free precession (SSFP) acquisition and a native 3D‐QALAS 9 acquisition at end diastole. The 3D‐QALAS acquisition had a resolution of 2.0 mm × 2.0 mm in‐plane, a slice thickness of 12.0 mm (reconstructed to 2.0 mm × 2.0 mm × 6.0 mm), flip angle of 5°, and SENSE factor of 2 and 1.2 in phase and slice directions. Echo time was 1.2 msec and repetition time was 2.6 msec. The 3D‐QALAS acquisition provided 13 short‐axis LV slices. An experienced observer used commercially available software (Segment version 2.1 R6274) to analyze cine data. LVM and LVEF were calculated from manually drawn LV contours.
Quantitative T1 and T2 maps from the 3D‐QALAS acquisitions were generated using SyMRI (SyntheticMR, Sweden). For each 3D‐QALAS acquisition, epi‐ and endocardial borders were manually contoured on T1 and T2 maps from 13 LV slices using Segment version 1.9 R3644. T1 and T2 were measured in each segment, and values from all segments averaged to represent the overall T1 and T2 for each examination.
T1 and T2 were compared between examinations using IBM SPSS Statistics version 25 (Armonk, NY). Linear mixed models with repeated measures analysis were used to investigate differences in each myocardial segment over time. The model included time and myocardial segment as fixed classification factors and patient as a random classification factor. Changes in LVM, LVEF, GLS, and E/é ratio between examinations 1 and 4 were assessed using Student's t‐test. Statistical significance was set as P < 0.05.
Results
Eight patients completed four examinations; two patients completed three and two examinations each.
T1 increased and T2 decreased significantly between examination 4 and earlier examinations (Fig. 1). T1 increases were most apparent between examinations 3 and 4 and occurred in left‐sided but not in right‐sided breast cancer patients (average increase 7.6% vs. 0.1%, respectively). T2 decreases were more evident between examinations 1 and 2, and among right‐ compared to left‐sided patients (average decrease 13.4% vs. 0.6%). Between examinations 1 and 4, LVM increased by 2.1 g, GLS fell by 3.0%, and E/é increased by 0.8 (P < 0.05 for all three). LVEF did not change.
FIGURE 1.
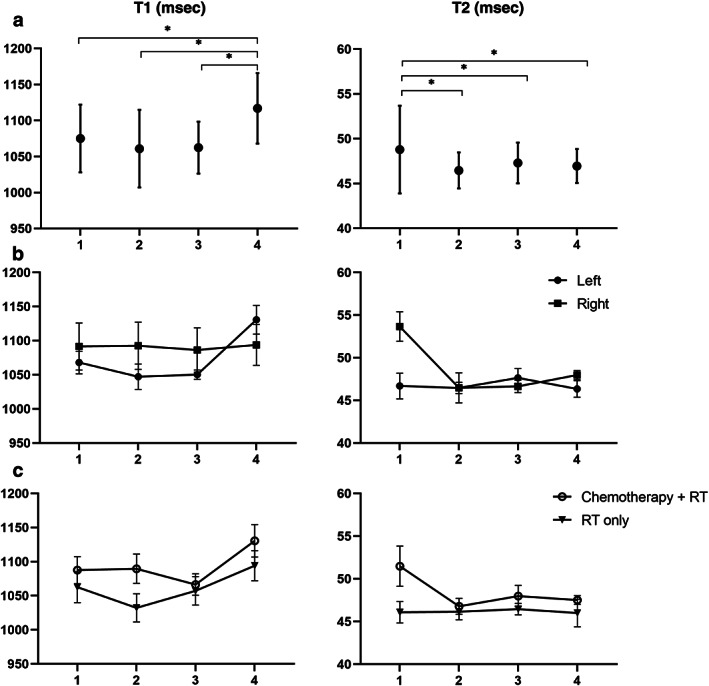
T1 (left) and T2 (right) myocardial relaxation times for patients at four examinations. (a) Average and confidence intervals for full cohort, n = 10. (b) Comparison of patients treated for left‐sided (n = 7) and right‐sided (n = 3) breast cancer. (c) Comparison of patients treated with chemotherapy + radiation therapy (RT) (n = 5) or RT alone (n = 5)
Patients receiving chemotherapy had larger changes in T1, T2, and LVM between examinations 1 and 4 than those undergoing RT alone (T1: +6.0 vs. +3.4%; T2: −9.2 vs. +0.1%; LVM: +6.0% vs. −2.2%).
Discussion
T1 and T2 relaxation times detected myocardial changes during and up to 6 months after RT which were associated with deterioration of LV systolic and diastolic function.
A significant increase in T1 occurred after RT completion among patients with left‐sided cancers and higher cardiac radiation doses, but not in patients with right‐sided cancers, suggesting a causal role for RT.
T2 decreased significantly from examination 1 to 4, with a notable early decrease among the right‐sided patients who had the lowest cardiac radiation exposure, suggesting that early T2 decline may not be an RT effect. The early T2 fall was seen in patients who had received chemotherapy, raising the possibility that preceding myocardial edema or inflammation due to chemotherapy may have also impacted the early T2 fall. 8
T1 and T2 relaxation times detect RT effects on the myocardium early in the treatment of breast cancer patients that are associated with decreased systolic and diastolic LV function. Changes in T1, T2, and LVM suggest myocardial edema or fibrosis developed in patients receiving RT, and that the temporal evolution of those changes might be influenced by both the cardiac radiation dose and exposure to concomitant chemotherapy and trastuzumab. T1 and T2 relaxation times may assist early detection of tissue changes that presage longer‐term cardiovascular risk in patients undergoing RT for breast cancer treatment. Confirmation merits larger and longer‐term studies.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
SK contributed to the study design, analyzed the results, performed the statistical analysis, and drafted the manuscript. AMF recruited the patients and contributed to the study design. JEE contributed to analyze the results and to the study design. AD and TE contributed to the study design. AFB edited the manuscript. All authors have critically read and revised the manuscript and approved the final version.
Acknowledgments
The authors thank statistician Mats G. Fredriksson, PhD, for valuable advice on the statistical methods used in this study. This study was partially financed through ALF Grants, Region Ostergotland LIO‐284291, LIO‐284411, and LIO‐448281, and LIU Cancer Projects Grants 2012.
Level of Evidence: 2
Technical Efficacy: Stage 2
References
- 1. Darby SC, Ewertz M, McGale P, Bennet AM, Blom‐Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. [DOI] [PubMed] [Google Scholar]
- 2. Taylor CW, Kirby AM. Cardiac side‐effects from breast cancer radiotherapy. Clin Oncol (R Coll Radiol). 2015;27(11):621–9. [DOI] [PubMed] [Google Scholar]
- 3. Stewart FA. Mechanisms and dose‐response relationships for radiation‐induced cardiovascular disease. Ann ICRP. 2012;41(3–4):72–9. [DOI] [PubMed] [Google Scholar]
- 4. Puntmann VO, Peker E, Chandrashekhar Y, Nagel E. T1 mapping in characterizing myocardial disease: a comprehensive review. Circ Res. 2016;119(2):277–99. [DOI] [PubMed] [Google Scholar]
- 5. Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jordan JH, Vasu S, Morgan TM, et al. Anthracycline‐associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging. 2016;9(8):e004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferreira de Souza T, Quinaglia AC, Silva T, Osorio Costa F, et al. Anthracycline therapy is associated with cardiomyocyte atrophy and preclinical manifestations of heart disease. JACC Cardiovasc Imaging. 2018;11(8):1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Copeland‐Halperin RS, Liu JE, Yu AF. Cardiotoxicity of HER2‐targeted therapies. Curr Opin Cardiol. 2019;34(4):451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kvernby S, Warntjes MJ, Haraldsson H, Carlhäll CJ, Engvall J, Ebbers T. Simultaneous three‐dimensional myocardial T1 and T2 mapping in one breath hold with 3D‐QALAS. J Cardiovasc Magn Reson. 2014;16(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grégoire V, Mackie TR. State of the art on dose prescription, reporting and recording in intensity‐modulated radiation therapy (ICRU report no. 83). Cancer Radiother. 2011;15(6–7):555–9. [DOI] [PubMed] [Google Scholar]


