Abstract
Prognosis of canine oral malignant melanoma encompasses clinical, histological and immunohistochemical parameters. The aim of this study was to evaluate the prognostic impact of bone invasion in oral canine melanoma. Sixty‐eight dogs bearing oral melanoma staged II and III that underwent surgery and anti‐CSPG4 electrovaccination, with available histological data and a minimum follow up of minimum 1 year, were retrospectively selected. Bone invasion was detected on imaging and/or histology. Median survival time of dogs with evidence of bone invasion (group 1) was 397 days and significantly shorter compared with dogs with oral melanomas not invading the bone (group 2, 1063 days). Dogs with tumours localised at the level of the cheek, lip, tongue and soft palate (soft tissue ‐ group 3) lived significantly longer compared with dogs having tumours within the gingiva of the maxilla or mandible (hard tissue ‐ group 4) with a median survival time of 1063 and 470 days, respectively. Within group 4, the subgroup of dogs with tumours not invading the bone (group 5) showed a significant prolonged survival time (972 days) in comparison with dogs of group 1 (bone invasion group). Similar results were obtained for the disease‐free intervals amongst the different groups. Statistical analysis showed that Ki67 and mitotic count were correlated with shorter survival in patients of group 1 (with bone invasion). Bone invasion should always be assessed since it appears to be a negative prognostic factor.
Keywords: anti‐CSPG4 electrovaccination, bone invasion, dog, oral melanoma, prognosis, surgery
1. INTRODUCTION
Malignant melanoma is the most frequent oral malignant tumour in dogs accounting for 30%–40% of all canine oral malignancies. 1 , 2 , 3 Sites of primary growth are gingiva, internal lip/cheek, palate, tongue and tonsil. Canine oral malignant melanoma (OMM) is an aggressive tumour and it has been reported that its behaviour can be predicted by evaluating several clinical factors such as site of growth, size and clinical stage, leukocytes ratio, 2 , 3 , 4 , 5 and histological and immunohistochemical factors such as Ki67 expression, mitotic count, degree of pigmentation, nuclear atypia and platelet‐derived growth factor receptor expression. 6 , 7 , 8 The reported metastatic rate to regional lymph nodes and distant sites such as lungs and other organs ranges from 30.3% to 74.0% 3 , 9 and from 14.0% to 92.0%, respectively. 3
The treatment of choice for local tumour control, if feasible, is wide surgical excision, regardless of whether or not there is bone invasion at presentation; the feasibility of an en bloc excision is influenced by both the tumour location and the size of dog, as a minimum of 1.5–2 cm up to 3 cm of macroscopically normal tissue all around the OMM should be excised. 1 , 2 , 3 Local tumour control is then surgically reached by also performing a neck lymphadenectomy (mandibular and medial retropharyngeal lymph nodes), ipsilaterally or bilaterally. 10 , 11 The removal of lymph nodes followed by histological evaluation also allows for complete tumour staging, as lymph nodes with metastatic OMM may appear clinically and cytologically normal. 12 , 13 Radiotherapy should be considered as an adjuvant treatment for OMMs that are incompletely excised, as a primary treatment in combination with medical treatment for those cases deemed inoperable or when the owners refuse surgery. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 An alternative to radiotherapy for local tumour control is electrochemotherapy, which may be contraindicated when bone invasion is already evident. 22 , 23 , 24 The results derived from the addition of adjuvant chemotherapy (especially platinum‐based agents), to control the metastatic spread, has been disappointing if compared to local tumour control only. 16 , 17 , 18 , 25 , 26 , 27
Thanks to the immunogenic features of melanoma, several studies dealing with immunotherapy have been recently carried out. Melanoma‐associated antigens have been identified (e.g., tyrosinase and chondroitin sulphate proteoglycan 4 [CSPG4]) and utilized in producing vaccines capable of evoking an immune response against canine OMM. 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 In particular, the authors' focus has been on CSPG4, a cellular membrane antigen, characterised by restricted distribution in normal healthy tissues and high expression on neoplastic cells in both human and canine malignant melanoma. It coordinates several intracellular pathways regulating different cell functions (i.e., proliferation, migration and survival), thus being involved in tumourigenesis at multiple levels. 36 , 37 , 38 , 39 In addition, CSPG4 has also been shown to be overexpressed in human melanoma cancer stem cells and has been associated with poorer prognosis. 32 , 40 All these features make CSPG4 an ideal antigen to safely and effectively target. A recent paper has shown the advantage of the combination of en bloc excision plus adjuvant anti‐CSPG4 vaccination in dogs with OMM. 13
Bone invasion, as detected by advanced imaging and/or histologically, has been reported to occur in up to 57.0% of cases. 3 , 41 However, its influence on prognosis remains to be clearly defined. The aim of this study is to retrospectively evaluate the prognostic impact of bone invasion in a population of dogs affected by stage II‐III OMMs locally controlled by surgery and treated adjuvantly with anti‐CSPG4 DNA electrovaccination.
2. MATERIALS AND METHODS
2.1. Patient selection and data collection
All dogs of this retrospective study were treated at the Teaching Veterinary Hospital of Grugliasco (Turin) from 2010 to 2020. Dogs with confirmed OMM on histopathology, staged II‐III, that underwent surgery and adjuvant CSPG4 electrovaccination were eligible for entry into the study. Specifically, only dogs bearing an OMM with an immunohistochemical CSPG4 score ≥3/8 were considered as suitable candidates for vaccination. 40 Good Clinical Practise guidelines for animal clinical studies were observed and both the Ethics Committee of the University of Turin and the Italian Ministry of Health approved the study (0004230‐20/02/2018‐DGSAF‐MDS‐P and 0015537‐28/06/2017‐DGSAF‐MDS‐P). A written consent form was signed by the owners before dogs' recruitment in the study.
Additional criteria for inclusion were a minimum follow‐up of 1 year on 1 April 2021, no concurrent life threatening disease and information on the presence/absence of bone invasion based on imaging and/or histology. Information retrieved from medical records for each dog included age, sex, breed, body weight, tumour localization within the oral cavity, tumour size and type of surgery performed. Pre‐treatment work‐up consisted of physical examination, blood count, serum biochemistry, cardiologic evaluation and urinalysis. Complete tumour staging was achieved by means of total body computed tomography (CT scan) for the majority of cases. Alternatively, skull, three view chest radiographs and abdominal ultrasound were obtained. The primary tumour was resected by performing an en‐bloc excision (mandibulectomy, maxillectomy, lip‐cheek excision followed by reconstruction) with regional (ipsilateral or bilateral) lymphadenectomy. 1 , 2 , 3 Dogs were staged according to the World Health Organisation tumour/node/distant metastases (TNM) guidelines as illustrated in Table 1. 2 , 42
TABLE 1.
World Health Organization staging system for canine oral melanoma
| Stage I | Stage II | Stage III | Stage IV |
|---|---|---|---|
| ≤2 cm diameter | 2–4 cm diameter | >4 cm diameter | Any size |
| Negative lymph nodes | Negative lymph nodes | +/−Metastatic lymph node | Distant metastasis |
2.2. Histological and immunohistochemical analyses
Formalin‐fixed paraffin‐embedded (FFPE) samples of OMM were stained with haematoxylin‐eosin for diagnosis according to the tumour pathology guidelines. 43 The following histological and immunohistochemical data were recorded for all OMM samples: Ki67 expression (polyclonal Ki67 antibody A‐047; DAKO; cut‐off of 19.5), mitotic count in 10 high‐power fields (MC; <4/10 high‐power fields [hpf] or ≥4/10 hpf), nuclear atypia (quantification < or ≥ 30%), surgical margins infiltration status, presence of bone invasion, lymph node evaluation and CSPG4 score.
Immunohistochemistry (IHC) was performed on 4 mm thick paraffin sections. After blocking peroxidase activity (0.3% H2O2 in deionised water for 30 min) and heat‐induced antigen retrieval (30 min with citrate buffer at 98°C, pH 6), sections were incubated with anti‐Ki67 polyclonal antibody (Dako A‐047; diluted 1:50) and anti‐CSPG4 polyclonal antibody (Sigma Aldrich; diluted 1:40). Detection was performed using the Vector VIP Substrate kit for peroxidase (Vector Laboratories). In case of amelanotic neoplasms, IHC with both Melan‐A and PNL‐2 antibodies was also performed. Ki67 index was assessed according to the methods previously reported by Bergin and colleagues. The previously published threshold of 19.5 was used to predict prognosis. 6 A total score ranging from 0 to 8 was assigned to each melanoma sample by adding the value that represented the proportion of CSPG4 positively stained tumour cells (score from 0 to 5) and the average staining intensity of CSPG4‐positive tumour cells (score from 0 to 3). 31 , 40
The presence of bone within the resection margins was not considered as an inclusion criterium as only the detection of bone invasion at imaging and/or histology was evaluated in the study. Specifically, bone infiltration at imaging was defined as minimal cortical disruption up to advanced destruction of cortex involving the medullary bone in some cases. Bone infiltration at histology was reported when tumoural cells were found within the bone, together with a variable degree of bone destruction. Simple periosteal reaction at imaging was not considered as bone invasion unless bone invasion was detected at histological examination.
2.3. Patients' groups
First, all dogs were divided into two groups: group 1 (OMMs with bone invasion) and group 2 (OMMs without bone invasion), regardless of localization. Additionally, the total population was divided into two other groups based on the site of growth and statistically evaluated. Patients with OMMs at the level of cheek, lip, tonsil, soft palate and tongue were included in group 3 (soft tissue group); patients with OMMs attached to the gingiva of either the lower or upper dental arcade were included in group 4 (hard tissue group). A subgroup of group 4, called group 5, consisted of OMMs that were localised to the gingiva but did not invade the bone. Thus, group 4 consisted of OMMs of group 1 (OMMs with bone invasion) and OMMs of group 5 (no bone invasion). The DNA electrovaccination procedure was performed in all the dogs of this study as only OMMs characterised by a CSPG4 immunohistochemical expression ≥3/8 (cut‐off value chosen for enrollment in the immunisation group) were included. 40 Dogs, under brief general anaesthesia, were vaccinated with plasmids coding for the CSPG4 antigen. The vaccination was started 1–3 weeks after surgery and was repeated after 2 weeks and then monthly for a minimum of 6 and a maximum of 24 immunizations. The CSPG4‐coding plasmids (500 μg in 200 μl of 0.03% NaCl) were injected into the muscles of the caudal thigh and, 2 min later, nine electric pulses were applied to the injection site using the CLINIPORATOR (Igea), an instrument approved for veterinary application. The dogs were monitored for acute, late local or systemic side effects. 30 , 31 , 32
2.4. Statistical analysis
The analyses were carried out using GraphPad Prism (version 9.0.0 for Windows, GraphPad Software, San Diego, California, www.graphpad.com), with statistical significance set at a p < .05. For statistical purposes, the Shapiro–Wilk test was used to assess normality of distribution of the variables.
The DFI was calculated from the day of surgery to the first tumour recurrence or metastasis while the MST was calculated as the period from the day of surgery to the patient's death. DFI and MST were analysed through generation of Kaplan–Meier curves; log‐rank test was used to compare DFI and MST of patients amongst different groups. Dogs which died from non‐COMM‐related causes, those lost to follow‐up and those still alive at the end of the study were censored. Spearman's correlation was used to look for association between MST and MC or Ki67 of patients with and without bone invasion. Fisher's exact test was used to test for possible association between different groups and the probability of local recurrence and/or metastasis.
3. RESULTS
3.1. Signalment
Sixty‐eight dogs fulfilled the inclusion criteria. There were 39 males (23 intact, 16 castrated) and 29 females (5 intact, 24 spayed). The mean and median age were 11.1 and 12 years (range, 6–14 years), respectively; mean and median weight were 19.8 and 18 kg (range, 3–40 kg), respectively. Twenty‐one breeds were represented: 5 Cocker Spaniels, 5 Golden Retrievers, 5 German Sheperd dogs, 4 Pinschers, 3 Pekingese, 3 Yorkshire Terriers, 2 Beagles, 2 Labrador Retrievers, 2 English bulldogs, 2 Dwarf Poodles, 2 Setters, 2 Shih Tzu dogs and one of each of Jack Russell Terrier, Hovawart, Alaskan Malamute, Shar Pei, Dwarf Schnauzer, Rottweiler, Spitz, Amstaff and West Highland White Terrier. The remaining 22 dogs were crossbreeds.
3.2. Staging and treatment
Clinical staging identified 38 stage II and 30 stage III OMMs; total body CT scan was performed in 54 cases (79.4%) and chest and skull radiographs in conjunction with abdominal ultrasound in 14 cases (20.6%). Tumour localization within the oral cavity of all patients is shown in Table 2.
TABLE 2.
Tumour localization of the OMMs included in the study
| Localization | Overall population |
|---|---|
| (n = 68) | |
| Mandible | 24 (35.3%) |
| Maxilla | 18 (26.5%) |
| Cheek | 10 (14.7%) |
| Lip | 10 (14.7%) |
| Soft palate | 1 (1.5%) |
| Tongue | 3 (4.4%) |
| Tonsils | 2 (2.9%) |
Abbreviation: OMM, oral malignant melanoma.
Twenty‐four dogs underwent mandibulectomy (35.3%) and 14 maxillectomy (20.6%); en‐bloc excision was performed in 14 dogs (20.6%, 7 OMMs of the cheek and 7 OMMs of the lips) with or without mucosal or skin flap reconstruction or a combination of both. Two dogs (2.9%) underwent both mandibulectomy and en‐bloc excision of the cheek at the same time. One patient (1.5%) underwent tonsillectomy; a simple excision was performed in 13 patients (19.1%) by the referring veterinarian followed in two cases by a revision surgery. In the remaining cases, surgical revision was not done due to the absence of macroscopic and/or residual disease at clinical examination and staging.
Ipsilateral medial retropharyngeal lymphadenectomy was performed in 55 dogs (80.9%), ipsilaterally in 39 dogs (70.9%) and bilateral lymphadenectomy was performed in 16 dogs (29.1%). Ipsilateral medial retropharyngeal lymph node was removed in 3 (7.7%) of the 39 dogs and bilaterally in 2 (1.2%) of the 16 dogs. In 13 dogs (19.1%) the lymph node status was assessed for staging purposes only cytologically after fine needle aspiration. In addition to surgery and immunotherapy, 5 dogs (7.3%) received electrochemotherapy (with bleomycin intravenous injection) while 26 (38.2%) received metronomic chemotherapy. This treatment consisted of low dose oral administration of cyclophosphamide, piroxicam and thalidomide.
3.3. Characterisation of the groups of dogs
The characterisation of the five groups of dogs is summarized in Tables 3, 4, 5.
TABLE 3.
Characterisation of dogs in group 1 and group 2 based on bone invasion
| Overall patients | Group 1 | Group 2 |
|---|---|---|
| (n = 68) | (n = 28) | (n = 40) |
| Presence of bone invasion | 28 | 0 |
| Absence of bone invasion | 0 | 40 |
| Lymph nodes metastasis |
15 (15/28) (53.6%) |
19 (19/40) (47.5%) |
TABLE 4.
Characterisation of dogs in group 3 and group 4 based on localization of OMMs'
| Overall patients | Group 3 | Group 4 |
|---|---|---|
| (n = 68) | (n = 26) | (n = 42) |
| Hard tissue OMMs (gingiva of mandible or maxilla) | 0 | 42 |
| Soft tissue OMMs (lip, cheek, tongue tonsils and soft palate) | 26 | 0 |
| Lymph nodes metastasis |
14 (14/26) (53.8%) |
20(20/42) (47.6%) |
Abbreviation: OMM, oral malignant melanoma.
TABLE 5.
Characterisation of dogs in group 1 and group 5 based on bone invasion
| Group 4 | Group 1 | Group 5 |
|---|---|---|
| (n = 42) | (n = 28) | (n = 14) |
| Hard tissue OMMs (gingiva of mandible or maxilla) | ||
| Presence of bone invasion | 28 | 0 |
| Absence of bone invasion | 0 | 14 |
| Metastatic lymph nodes |
15 (15/28) (53.6%) |
5 (5/14) (37.5%) |
Abbreviation: OMM, oral malignant melanoma.
Local bone invasion was detected in 28 out of 68 OMMs (41.2%, group 1). Of the entire population 34 out of 68 dogs (50%) had lymph node metastasis. The lymph node metastatic rate in dogs of group 1 was 53.6% (15/28) while it was 47.5% (19/40) in group 2. Twenty‐six dogs were included in group 3 and 42 in group 4. The lymph node metastatic rate was 53.8% (14/26) and 47.6% (20/42), respectively. Fourteen dogs were included in group 5 and the lymph node metastatic rate was 35.7% (5/14).
Regarding histological and immunohistochemical parameters, Ki67 was <19.5 in 15/68 (22.1%) OMMs, >19.5 in 49/68 (72.1%) OMMs, unknown in 3/68 (4.4%) and not detectable in one case, 1/68 (1.4%) because of the high pigmentation of the sample. The MC was <4/10 hpf in 10/68 (14.7%) OMMs and ≥4/10 hpf in 58/68 (85.3%) OMMs. Nuclear atypia was <30% in 11/68 (16.1%) OMMs, ≥30% in 52/68 (76.5%) OMMs and not available in 5/68 (7.4%) cases. Based on histopathological of surgical margins, 38/68 (55.9%) OMMs were determined to be completely excised, 21/68 (30.9%) were incompletely excised, and the margin status could not be determined in 9/68 (13.2%) OMMs. Histological and immunohistochemical parameters for every group are summarized in Table 6.
TABLE 6.
Histological and immunohistochemical parameters of OMMs in each group
| Threshold | Overall population | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|---|---|
| (n = 68) | (n = 28) | (n = 40) | (n = 26) | (n = 42) | (n = 14) | ||
| Mitotic count | <4/10 hpf | 10 | 5 | 5 | 2 | 8 | 3 |
| ≥4/10 hpf | 58 | 23 | 45 | 24 | 34 | 11 | |
| Ki67 | <19.5 | 15 | 4 | 11 | 7 | 8 | 4 |
| ≥19.5 | 49 | 23 | 26 | 18 | 31 | 8 | |
| Unknown | 4 | 1 | 3 | 1 | 3 | 2 | |
| <30% | 11 | 3 | 8 | 6 | 5 | 2 | |
| Nuclear atypia | ≥30% | 52 | 25 | 27 | 17 | 35 | 10 |
| Unknown | 5 | 0 | 5 | 3 | 2 | 2 | |
| Margins | Clear | 38 | 17 | 21 | 16 | 22 | 7 |
| Infiltrated | 21 | 9 | 12 | 7 | 14 | 18 | |
| Unknown | 9 | 2 | 7 | 3 | 6 | 1 |
Abbreviation: OMM, oral malignant melanoma.
3.4. Outcome and prognostic factors
Kaplan Meier curves were analysed for survival times and DFI. At the end of the study, out of the 68 dogs, 7 dogs (10.3%) were still alive (range 544–2815 days) and only one of these dogs (14.3%, 1/7) had an OMM with bone invasion; 60 (88.2%) dogs were dead (range 78–2155 days) and one (1.5%) was lost to follow up at 1178 days. Thirty‐seven dogs out of these 60 dogs (61.6%, 37/60) died of OMM‐related disease with 19 (51.3%, 19/37) of these displaying bone invasion.
When groups' MSTs were compared, MST of group 1 (OMMs with bone invasion) was 397 days (range 78–1951 days) while it was 1063 days (range 172–2815 days) in group 2 (OMMs without bone invasion), with a significant difference (p = .0006) (Figure 1A). The same significant result was evident for DFI; the DFI of group 1 was 193 days (range 29–782 days) and 470 days (range 13–2815 days) for group 2 (p = .004) (Figure 1B). When group 3 (soft tissue group) and group 4 (hard tissue group) were compared, MST of group 3 was 1063 days (range 227–2815 days) and was significantly longer (p = .004) than that of group 4 (470 days, range 78–2155 days) (Figure 2A). Similar to MST, the DFI of group 3 (470 days, range 13–2815 days) was longer than in group 4 (202 days, range 21–1681 days), although no statistical significance was found (p = .115). When comparing dogs with OMMs of group 1 (bone invasion) and group 5 (hard tissue OMMs without bone invasion), dogs of group 5 had longer MSTs (972 days, range 172–2155 days, p = .035) and DFIs (261 days, range 21–1681 days, p = .058) compared with dogs of group 1 (MST 397 days, DFI 193 days) (Figure 2B). The MST of the entire population was 653 days while DFI was 230 days.
FIGURE 1.
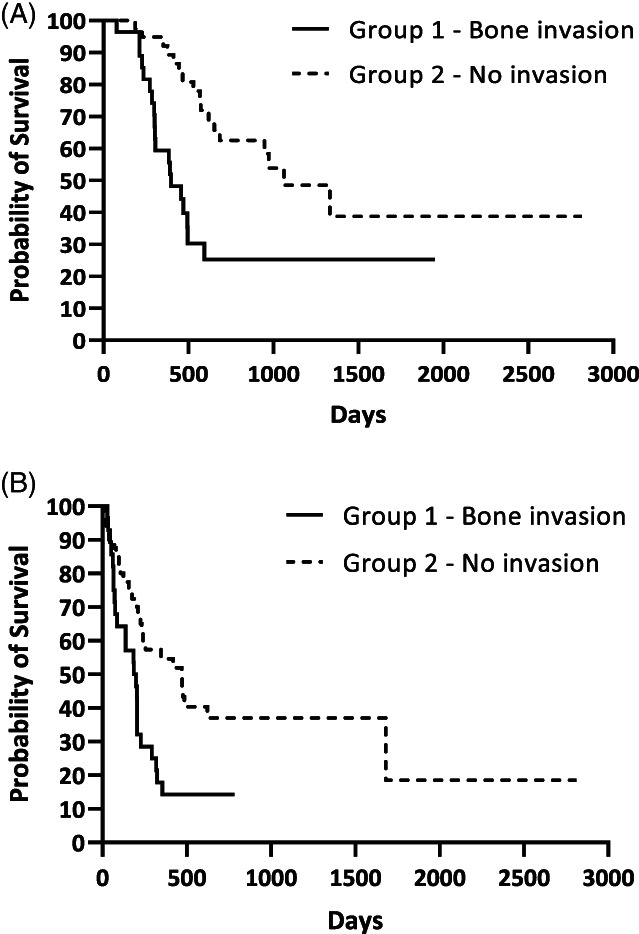
Kaplan Meier analysis of (A) median survival time (p = .0006) and (B) disease free interval (p = .004) of group 1 and group 2
FIGURE 2.
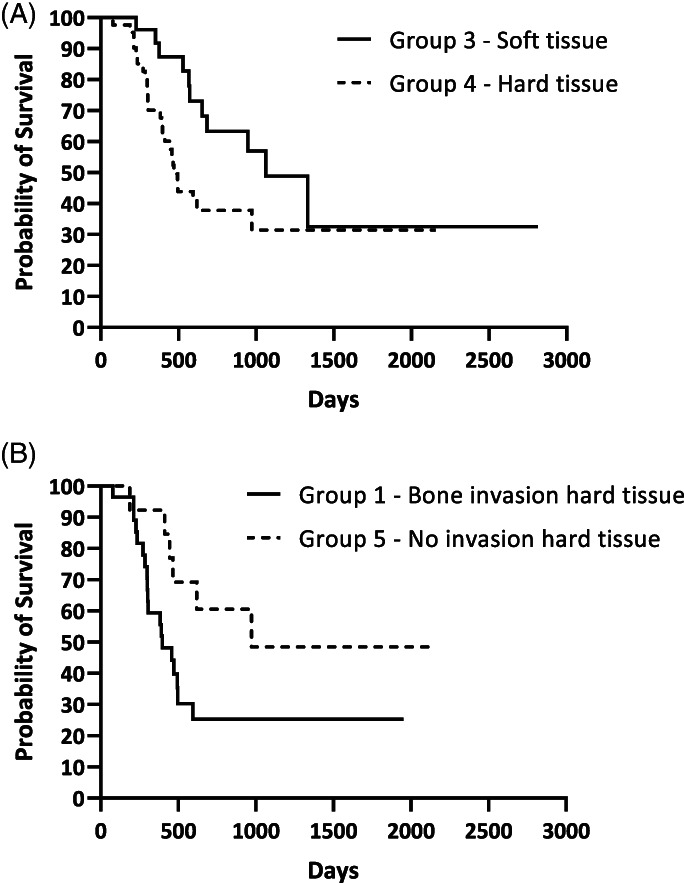
Kaplan Meier analysis of (A) median survival time of group 3 and group 4 (p = .0433) and (B) median survival time of group 1 and group 5 (p = .0357)
No statistical association was found between groups 1 and 2, 3 and 4 or 1 and 5 in regard to occurrence of metastatic distant disease and the incidence of local recurrence. Additionally, MST and Ki67 (p = .02, r = −0.43) and MC (p = .04, r = −0.39) were only significantly correlated in group 1 (OMMs with bone invasion).
4. DISCUSSION
In this study authors evaluate the impact of bone invasion in dogs with canine OMMs in terms of DFI and MST amongst different groups based on the presence/absence of bone invasion and OMM's localization (hard vs. soft tissue). The presence/absence of bone invasion was assessed only in dogs of the hard tissue group. All dogs were treated with a multimodal approach by means of surgery and adjuvant anti‐CSPG4 vaccination. Some dogs also received metronomic chemotherapy in addition to electrochemotherapy, in few cases. In addition, the prognostic value of bone invasion, MC and Ki67 were evaluated in this study. 7
In recent years, several studies have aimed to identify prognostic markers for melanocytic neoplasms. The prognostic impact of bone invasion in canine OMM has been reported in very few studies and its role remains to be clearly defined. Early studies found that the presence of bone lysis observed on skull radiographs in dogs with oral melanoma did not significantly influence the survival time. 44 , 45 In contrast, in another study evidence of bone lysis, recorded in 28% of the cases, was significantly associated with a worse prognosis and time to first event, which included tumour recurrence, regional or distant metastatic spread and death. 15 In the Smedley et al. review, several prognostic factors of melanocytic neoplasms were discussed, and the level of invasion was divided into shallow infiltration with absence of bone lysis associated with a favourable prognosis, and deep invasion with bone lysis that negatively affected the patients' outcome 7 ; the propensity of OMMs to invade locally, involving the bone in some cases, might be due to the high vascular supply and lymphatic network of the oral cavity that provides an optimal microenvironment for tumour growth and metastatic invasion. 46
Most of the dogs of this study underwent a curative intent surgery to entirely remove the tumour, including at least 1.5 cm up to 3 cm of macroscopically normal bone, soft tissues or both (depending on tumour localization) 13 ; OMMs with bone invasion (group 1) were characterised by reduced MST and DFI compared with dogs bearing OMMs without bone invasion (group 2), with a significant difference for both end points. Patients of group 3 (soft tissue) exhibited a significantly longer MST when compared with group 4 (hard tissue); the same was true for the DFI, despite the lack of a significant difference.
The results of this study showed that when only considering dogs with gingival OMMs (group 4, hard tissue), the subgroup that consisted of dogs bearing OMMs without bone invasion (group 5) displayed a significantly prolonged MST compared with those of group 1 (bone invasion). However, there was no significant difference regarding the DFI. The reason behind this group division was to evaluate the impact of bone invasion more precisely in terms of prognosis. It should also be noted that the evaluation of DFI of group 4 and group 5, during the vaccination protocol, may not have been as accurate, considering that these dogs were restaged with radiographs instead of CT. The different diagnostic accuracy of the two techniques could make the DFI a less reliable and precise end point. Additionally, based on previous anti‐CSPG4 electrovaccination studies, authors reckon that the immunity induced by the vaccine is more effective in reducing the development of distant metastatic disease rather than local recurrence. 31
A significant correlation was observed between Ki67, MC and MST exclusively in dogs of group 1. This result might corroborate the recognized prognostic value of Ki67 and MC, especially in OMMs with bone invasion. As no specific numerical value was attributed to nuclear atypia (which was ≥30% in 76.5% of samples) other than the cut off < or ≥ 30%, 7 no statistical correlation was assessed.
In this study, authors decided to include OMMs with evidence of bone lysis detected on CT and/or histology; however, imaging and histology might have some limitations in identifying this feature. On one side, if the lysis process is in the early phase, CT scan might not capture it because of the very superficial and slight remodelling at the level of the bone surface. Additionally, 20% of our patients were staged using radiographs which are less accurate compared with CT in detecting bone invasion; in fact, it has been reported that bone lysis is not evident on radiographs until 40% of the cortex is destroyed. This suggests that CT scan is preferable for oral tumour staging. 3 , 47 , 48 On the other side, tumours including bone are trimmed for histological evaluation through a cross‐sectioning technique. In the case of wide surgical margins, as they are in case of mandibulectomy or maxillectomy, the cross‐sectioning is performed through the cranial, caudal, and central part of bone specimen. 49 This may miss some areas where bone lysis may be present; this problem should be overcome with further sections, but this is not always feasible. Considering these limitations, the bone invasion may have been underestimated in this study.
Nevertheless, MST was prolonged compared with historically reported survival times. 18 , 50 This result may be partially attributable to the effect of the anti‐CSPG4 electrovaccination. 12 , 27 , 28 Several clinical trials have been geared towards the use of DNA vaccination as a fundamental step in the management of malignant melanoma. 13 , 28 , 29 , 51 , 52 Our protocol involves the electrovaccinaion against CSPG4, a class one oncoantigen involved in several oncogenic pathways such as melanoma tumour cell progression, survival and metastasis, and is poorly expressed in healthy tissue. Therefore, CSPG4 has gained value as an ideal immunotherapeutic target 37 , 38 , 39 , 40 ; in recent and ongoing clinical trials, anti‐CSPG4 electrovaccination has revealed its potential therapeutic impact, being safe, immunogenic in inducing a significant humoral response and effective in prolonging survival in OMM bearing dogs. 13 , 30 , 31 Based on previous and current results, authors reckon that anti‐CSPG4 electrovaccination is worthwhile to consider as adjunct treatment considering its positive role in terms of outcome.
The lymph node metastatic rate did not significantly differ between groups 1 and 2, although it was slightly higher in group 1. However, it is difficult to make any conclusions as different procedures were used to stage the lymph node status. Some dogs had their lymph nodes evaluated only cytologically while others underwent ipsilateral lymphadenectomy of mandibular and/or medial retropharyngeal lymph nodes, and only a few had a bilateral neck lymphadenectomy. This limitation, together with the retrospective nature of the study, may have led to underestimation of the actual lymph node status in some patients. Recently, new surgical procedures and methods have been proposed to improve lymph node staging as part of the clinical staging based on the TNM system. 10 , 11 , 53 , 54 , 55
Regarding the localization of OMMs, the literature reports that rostral tumours may be associated with a longer survival time. 2 , 56 , 57 This can be explained by the fact that rostral tumours have a better chance of being completely excised when compared to caudal tumours. Additionally, because of their location, caudal malignant tumours are often detected later in the course of the disease, having already progressed to an advanced stage at the time of diagnosis. Unfortunately, it was not feasible to evaluate OMMs of the present canine population based on this detail because of the incompleteness of data derived from both the retrospective nature of the study and the different diagnostic procedures (CT scan vs. radiographs) used to stage and therefore to detect bone invasion. Additionally, another shortcoming of this study is the limited number of dogs included in each group.
According to our data, bone invasion was significantly associated with a shorter MST and a shorter DFI. The negative impact of bone invasion was also evident when authors evaluated the MST and DFI of dogs with OMMs of soft tissue and dogs with OMMs of hard tissue, the latter group having a reduced MST and DFI.
In conclusion, several prognostic markers should be carefully evaluated for prognosis and treatment of OMM and this study further supports that bone invasion is one of these factors. Because of its negative impact on prognosis, bone invasion caused by canine OMM should be assessed, if feasible, through an advanced diagnostic imaging procedure and evaluated via histopathology.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Torino within the CRUI‐CARE Agreement.
Camerino M, Giacobino D, Manassero L, et al. Prognostic impact of bone invasion in canine oral malignant melanoma treated by surgery and anti‐CSPG4 vaccination: A retrospective study on 68 cases (2010–2020). Vet Comp Oncol. 2022;20(1):189-197. doi: 10.1111/vco.12761
Buracco Paolo and Morello Emanuela contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in [repository name] at [DOI], reference number [reference number].
REFERENCES
- 1. Liptak JM, Lascelles BDX. Oral tumors. In: Kudnig ST, Séguin B, eds. Veterinary Surgical Oncology. 1st ed. Ames, IA: Wiley‐Blackwell; 2012:119‐177. [Google Scholar]
- 2. Bergman PJ, Laura ES, Kent MS. Melanoma. In: Vail DM, Thamm DH, Liptak JM, eds. Withrow & mac Ewen'sSmall Animal Clinical Oncology. 6th ed. St. Louis, MO: Elsevier; 2019:367‐381. [Google Scholar]
- 3. Liptak JM. Oral tumors. In: Vail DM, Thamm DH, Liptak JM, eds. Withrow and MacEwen's Small Animal Clinical Oncology. 6th ed. St. Louis, MO: Elsevier; 2020:432‐448. [Google Scholar]
- 4. Spangler WL, Kass PH. The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Vet Pathol. 2006;43(2):136‐149. [DOI] [PubMed] [Google Scholar]
- 5. Camerino M, Giacobino D, Iussich S, et al. Evaluation of prognostic impact of pre‐treatment neutrophil to lymphocyte and lymphocyte to monocyte ratios in dogs with oral malignant melanoma treated with surgery and adjuvant CSPG4‐antigen electrovaccination: an explorative study. Vet Comp Oncol. 2021;19(2):353‐361. [DOI] [PubMed] [Google Scholar]
- 6. Bergin IL, Smedley RC, Esplin DG, Spangler WL, Kiupel M. Prognostic evaluation of Ki67 threshold value in canine oral melanoma. Vet Pathol. 2011;48(1):41‐53. [DOI] [PubMed] [Google Scholar]
- 7. Smedley RC, Spangler WL, Esplin DG, et al. Prognostic markers for canine melanocytic neoplasms: a comparative review of the literature and goals for future investigation. Vet Pathol. 2011;48(1):54‐72. [DOI] [PubMed] [Google Scholar]
- 8. Iussich S, Maniscalco L, Di Sciuva A, et al. PDGFRs expression in dogs affected by malignant oral melanomas: correlation with prognosis. Vet Comp Oncol. 2017;15(2):462‐469. [DOI] [PubMed] [Google Scholar]
- 9. Williams LE, Packer RA. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987‐2001). J Am Vet Med Assoc. 2003;222(9):1234‐1236. [DOI] [PubMed] [Google Scholar]
- 10. Skinner OT, Boston SE, Souza CHM. Patterns of lymph node metastasis identified following bilateral mandibular and medial retropharyngeal lymphadenectomy in 31 dogs with malignancies of the head. Vet Comp Oncol. 2017;15(3):881‐889. [DOI] [PubMed] [Google Scholar]
- 11. Grimes JA, Mestrinho LA, Berg J, et al. Histologic evaluation of mandibular and medial retropharyngeal lymph nodes during staging of oral malignant melanoma and squamous cell carcinoma in dogs. J Am Vet Med Assoc. 2019;254(8):938‐943. [DOI] [PubMed] [Google Scholar]
- 12. Grimes JA, Matz BM, Christopherson PW, et al. Agreement between cytology and histopathology for regional lymph node metastasis in dogs with melanocytic neoplasms. Vet Pathol. 2017;54(4):579‐587. [DOI] [PubMed] [Google Scholar]
- 13. Giacobino D, Camerino M, Riccardo F, et al. Difference in outcome between curative intent vs marginal excision as a first treatment in dogs with oral malignant melanoma and the impact of adjuvant CSPG4‐DNA electrovaccination: A retrospective study on 155 cases. Vet Comp Oncol. 2021. 10.1111/vco.12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freeman KP, Hahn KA, Harris FD, King GK. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum‐based chemotherapy (1987‐1997). J Vet Intern Med. 2003;17(1):96‐101. [DOI] [PubMed] [Google Scholar]
- 15. Proulx DR, Ruslander DM, Dodge RK, et al. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet Radiol Ultrasound. 2003;44(3):352‐359. [DOI] [PubMed] [Google Scholar]
- 16. Boria PA, Murry DJ, Bennett PF, et al. Evaluation of cisplatin combined with piroxicam for the treatment of oral malignant melanoma and oral squamous cell carcinoma in dogs. J Am Vet Med Assoc. 2004;224(3):388‐394. [DOI] [PubMed] [Google Scholar]
- 17. Murphy S, Hayes AM, Blackwood L, Maglennon G, Pattinson H, Sparkes AH. Oral malignant melanoma ‐ the effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Vet Comp Oncol. 2005;3(4):222‐229. [DOI] [PubMed] [Google Scholar]
- 18. Boston SE, Lu X, Culp WT, et al. Efficacy of systemic adjuvant therapies administered to dogs after excision of oral malignant melanomas: 151 cases (2001‐2012). J Am Vet Med Assoc. 2014;245(4):401‐407. [DOI] [PubMed] [Google Scholar]
- 19. Kawabe M, Mori T, Ito Y, et al. Outcomes of dogs undergoing radiotherapy for treatment of oral malignant melanoma: 111 cases (2006‐2012). J Am Vet Med Assoc. 2015;247(10):1146‐1153. [DOI] [PubMed] [Google Scholar]
- 20. Cancedda S, Rohrer Bley C, Aresu L, et al. Efficacy and side effects of radiation therapy in comparison with radiation therapy and temozolomide in the treatment of measurable canine malignant melanoma. Vet Comp Oncol. 2016;14(4):e146–e157. [DOI] [PubMed] [Google Scholar]
- 21. Turek M, LaDue T, Looper J, et al. Multimodality treatment including ONCEPT for canine oral melanoma: a retrospective analysis of 131 dogs. Vet Radiol Ultrasound. 2020;61(4):471‐480. [DOI] [PubMed] [Google Scholar]
- 22. Milevoj N, Tratar UL, Nemec A, et al. A combination of electrochemotherapy, gene electrotransfer of plasmid encoding canine IL‐12 and cytoreductive surgery in the treatment of canine oral malignant melanoma. Res Vet Sci. 2019;122:40‐49. [DOI] [PubMed] [Google Scholar]
- 23. Nemec A, Milevoj N, Lampreht Tratar U, Serša G, Čemažar M, Tozon N. Electroporation‐based treatments in small animal veterinary oral and maxillofacial oncology. Front Vet Sci. 2020;7:575911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tellado MN, Maglietti FH, Michinski SD, Marshall GR, Signori E. Electrochemotherapy in treatment of canine oral malignant melanoma and factors influencing treatment outcome. Radiol Oncol. 2020;54(1):68‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rassnick KM, Ruslander DM, Cotter SM, et al. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989‐2000). J Am Vet Med Assoc. 2001;218(9):1444‐1448. [DOI] [PubMed] [Google Scholar]
- 26. Brockley LK, Cooper MA, Bennett PF. Malignant melanoma in 63 dogs (2001‐2011): the effect of carboplatin chemotherapy on survival. N Z Vet J. 2013;61(1):25‐31. [DOI] [PubMed] [Google Scholar]
- 27. Dank G, Rassnick KM, Sokolovsky Y, et al. Use of adjuvant carboplatin for treatment of dogs with oral malignant melanoma following surgical excision. Vet Comp Oncol. 2014;12(1):78‐84. [DOI] [PubMed] [Google Scholar]
- 28. Bergman PJ, McKnight J, Novosad A, et al. Long‐term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. 2003;9(4):1284‐1290. [PubMed] [Google Scholar]
- 29. Grosenbaugh DA, Leard AT, Bergman PJ, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. 2011;72(12):1631‐1638. [DOI] [PubMed] [Google Scholar]
- 30. Riccardo F, Iussich S, Maniscalco L, et al. CSPG4‐specific immunity and survival prolongation in dogs with oral malignant melanoma immunized with human CSPG4 DNA. Clin Cancer Res. 2014;20:3753‐3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Piras LA, Riccardo F, Iussich S, et al. Prolongation of survival of dogs with oral malignant melanoma treated by en bloc surgical resection and adjuvant CSPG4‐antigen electrovaccination. Vet Comp Oncol. 2017;15(3):996‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tarone L, Barutello G, Iussich S, et al. Naturally occurring cancers in pet dogs as pre‐clinical models for cancer immunotherapy. Cancer Immunol Immunother. 2019;68(11):1839‐1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ottnod JM, Smedley RC, Walshaw R, Hauptman JG, Kiupel M, Obradovich JE. A retrospective analysis of the efficacy of oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Vet Comp Oncol. 2013;11(3):219‐229. [DOI] [PubMed] [Google Scholar]
- 34. McLean JL, Lobetti RG. Use of the melanoma vaccine in 38 dogs: the south African experience. J S Afr Vet Assoc. 2015;86(1):1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Treggiari E, Grant JP, North SM. A retrospective review of outcome and survival following surgery and adjuvant xenogeneic DNA vaccination in 32 dogs with oral malignant melanoma. J Vet Med Sci. 2016;78(5):845‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight‐melanoma‐associated antigen (HMW‐MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24(4):267‐296. [DOI] [PubMed] [Google Scholar]
- 37. Yang J, Price MA, Neudauer CL, et al. Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms. J Cell Biol 2004;21;165(6):881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Price MA, Colvin Wanshura LE, Yang J, et al. CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment Cell Melanoma Res. 2011;24(6):1148‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rolih V, Barutello G, Iussich S, et al. CSPG4: a prototype oncoantigen for translational immunotherapy studies. J Transl Med. 2017;15(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mayayo SL, Prestigio S, Maniscalco L, et al. Chondroitin sulfate proteoglycan‐4: a biomarker and a potential immunotherapeutic target for canine malignant melanoma. Vet J. 2011;190(2):e26–e30. [DOI] [PubMed] [Google Scholar]
- 41. Nishiya AT, Massoco CO, Felizzola CR, et al. Comparative aspects of canine melanoma. Vet Sci. 2016;3(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bergman PJ. Canine oral melanoma. Clin Tech Small Anim Pract. 2007;22(2):55‐60. [DOI] [PubMed] [Google Scholar]
- 43. Ramos‐Vara JA, Borst LB. Immunohistochemistry: fundamentals and application in oncology. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: John Wiley & Sons; 2017:44‐47. [Google Scholar]
- 44. Kosovsky JK, Matthiesen DT, Marretta SM, Patnaik AK. Results of partial mandibulectomy for the treatment of oral tumors in 142 dogs. Vet Surg. 1991;20(6):397‐401. [DOI] [PubMed] [Google Scholar]
- 45. Wallace J, Matthiesen DT, Patnaik AK. Hemimaxillectomy for the treatment of oral tumors in 69 dogs. Vet Surg. 1992;21(5):337‐341. [DOI] [PubMed] [Google Scholar]
- 46. Mukaratirwa S, Chikafa L, Dliwayo R, Moyo N. Mast cells and angiogenesis in canine melanomas: malignancy and clinicopathological factors. Vet Dermatol. 2006;17(2):141‐146. [DOI] [PubMed] [Google Scholar]
- 47. Gendler A, Lewis JR, Reetz JA, Schwarz T. Computed tomographic features of oral squamous cell carcinoma in cats: 18 cases (2002–2008). J Am Vet Med Assoc. 2010;236(3):319‐325. [DOI] [PubMed] [Google Scholar]
- 48. Ghirelli CO, Villamizar LA, Pinto AC. Comparison of standard radiography and computed tomography in 21 dogs with maxillary masses. J Vet Dent. 2013;30(2):72‐76. [DOI] [PubMed] [Google Scholar]
- 49. Kamstock DA, Ehrhart EJ, Getzy DM, et al. American College of Veterinary Pathologists' Oncology Committee. Recommended guidelines for submission, trimming, margin evaluation, and reporting of tumor biopsy specimens in veterinary surgical pathology. Vet Pathol. 2011;48(1):19‐31. [DOI] [PubMed] [Google Scholar]
- 50. Tuohy JL, Selmic LE, Worley DR, Ehrhart NP, Withrow SJ. Outcome following curative‐intent surgery for oral melanoma in dogs: 70 cases (1998‐2011). J Am Vet Med Assoc. 2014;245(11):1266‐1273. [DOI] [PubMed] [Google Scholar]
- 51. Bergman PJ, Camps‐Palau MA, McKnight JA, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the animal medical center. Vaccine. 2006;24(21):4582‐4585. [DOI] [PubMed] [Google Scholar]
- 52. Impellizeri JA, Ciliberto G, Aurisicchio L. Electro‐gene‐transfer as a new tool for cancer immunotherapy in animals. Vet Comp Oncol. 2014;12(4):310‐318. [DOI] [PubMed] [Google Scholar]
- 53. Green K, Boston SE. Bilateral removal of the mandibular and medial retropharyngeal lymph nodes through a single ventral midline incision for staging of head and neck cancers in dogs: a description of surgical technique. Vet Comp Oncol. 2017;15(1):208‐214. [DOI] [PubMed] [Google Scholar]
- 54. Skinner OT, Boston SE, Giglio RF, Whitley EM, Colee JC, Porter EG. Diagnostic accuracy of contrast‐enhanced computed tomography for assessment of mandibular and medial retropharyngeal lymph node metastasis in dogs with oral and nasal cancer. Vet Comp Oncol. 2018;16(4):562‐570. [DOI] [PubMed] [Google Scholar]
- 55. Wainberg SH, Oblak ML, Giuffrida MA. Ventral cervical versus bilateral lateral approach for extirpation of mandibular and medial retropharyngeal lymph nodes in dogs. Vet Surg. 2018;47(5):629‐633. [DOI] [PubMed] [Google Scholar]
- 56. Hahn KA, DeNicola DB, Richardson RC, Hahn EA. Canine oral malignant melanoma: prognostic utility of an alternative staging system. J Small Anim Pract. 1994;35(5):251‐256. [Google Scholar]
- 57. Schwarz PD, Withrow SJ, Curtis CR. Partial maxillary resection as a treatment for oral cancer in 61 dogs. J Am Anim Hosp Assoc. 1991;27:617‐624. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in [repository name] at [DOI], reference number [reference number].


